This cohort study investigates the effectiveness of monovalent SARS-CoV-2 mRNA vaccines against Omicron infection in children younger than 5 years.
Key Points
Question
What is the effectiveness of monovalent SARS-CoV-2 mRNA vaccines against Omicron infection in children younger than 5 years?
Findings
In this population-wide cohort study including all Singaporean children aged 1 through 4 years (N = 121 628; 21 015 956 person-days) during an Omicron XBB surge, mRNA vaccine effectiveness against confirmed infection was 63.3% in fully vaccinated, infection-naive children and 74.6% against reinfections in previously infected children with at least 1 vaccine dose.
Meaning
Study results suggest that completion of a primary mRNA vaccine series provided protection against SARS-CoV-2 infection in children younger than 5 years, even during community circulation of the Omicron XBB variant.
Abstract
Importance
Literature on vaccine effectiveness of SARS-CoV-2 messenger RNA (mRNA) vaccines for children younger than 5 years is limited.
Objective
To report the effectiveness of monovalent mRNA vaccines against SARS-CoV-2 infection among Singaporean children aged 1 through 4 years during a COVID-19 pandemic wave of the Omicron XBB variant.
Design, Setting, and Participants
This was a population-based cohort study, conducted over a 6-month study period from October 1, 2022, through March 31, 2023, after the implementation of community vaccination among all Singaporean children aged 1 through 4 years. The study period was dominated by the Omicron XBB subvariant.
Exposure
Receipt of SARS-CoV-2 mRNA vaccines.
Main Outcome Measure
Vaccine effectiveness against confirmed SARS-CoV-2 infection. The adjusted incidence rate ratio for confirmed infections using Poisson regression was reported, with the reference group being those who were unvaccinated. Analyses were stratified by prior documented SARS-CoV-2 infection.
Results
A total of 121 628 children (median [IQR] age, 3.1 [2.2-3.9] years; 61 925 male [50.9%]) were included in the study, contributing 21 015 956 person-days of observation. The majority of children (11 294 of 11 705 [96.5%]) received the mRNA-1273 COVID-19 vaccine (Moderna). Vaccine effectiveness against confirmed infection was 45.2% (95% CI, 24.7%-60.2%) in partially vaccinated, infection-naive children and 63.3% (95% CI, 40.6%-77.3%) in fully vaccinated, infection-naive children compared with the unvaccinated group. Among previously infected children, vaccine effectiveness against reinfections in those with at least 1 vaccine dose was estimated at 74.6% (95% CI, 38.7%-89.5%).
Conclusions and Relevance
Study results suggest that completion of a primary mRNA vaccine series provided protection against SARS-CoV-2 infection in children aged 1 through 4 years. Although incidence of hospitalization and severe illness is low in this age group, there is potential benefit of vaccination in preventing infection and potential sequelae.
Introduction
Evaluations have demonstrated vaccine effectiveness against SARS-CoV-2 in the adult population, even in the Omicron variant era,1,2 albeit with varying effectiveness depending on prevailing variant and time since last dose. However, the effectiveness of vaccines in reducing the incidence of infection and COVID-19–related hospitalization among children younger than 5 years is much less certain, in part due to the delay in eligibility for vaccination. On June 17, 2022, the US Food and Drug Administration (FDA) extended emergency use authorizations for the monovalent BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna) COVID-19 vaccines to children aged 6 months through 4 years,3 coinciding with a spike in COVID-19 infections among children during the Omicron variant surge.4
Estimates of monovalent mRNA vaccine efficacy against infection in this age group, however, have varied widely in clinical trials.5,6 Vaccine efficacy for the mRNA-1273 (Moderna) vaccine (2-dose primary series) was estimated at 36.8% (95% CI, 12.5%-54.0%) among 2- to 5-year-old children and 50.6% (95% CI, 21.4%-68.6%) among children 6 to 23 months old,5 whereas overall vaccine efficacy of the BNT162b2 (Pfizer-BioNTech) vaccine (3-dose primary series) against COVID-19 infection in children aged 6 months to 4 years was estimated at 73.2% (95% CI, 43.8%-87.6%).6 Evidence of vaccine effectiveness in children younger than 5 years is sparse. Postauthorization estimates of monovalent mRNA COVID-19 vaccine effectiveness against infection in children aged 3 to 5 years range from 36% to 60% for the mRNA-1273 vaccine, and 31% for the BNT162b2 vaccine.7 Inactivated SARS-CoV-2 vaccines show similarly modest estimates of effectiveness. Among Chilean children aged 3 to 5 years, the inactivated Sinovac vaccine (Sinovac Biotech) was estimated to be approximately 40% effective in protecting against infection8; similar estimates were obtained for Hong Kong children aged 3 to 11 years during Omicron transmission.9,10
Higher transmissibility of Omicron has resulted in a surge in COVID-19 hospitalizations among children younger than 5 years and a corresponding increase in COVID-19–related pediatric intensive care unit admissions,11,12 despite lower frequency of severe outcomes.13 Most studies5,6,8,9,10 on vaccine effectiveness in children younger than 5 years were conducted before the emergence of the Omicron XBB subvariant. In Singapore, the monovalent mRNA-1273 (Moderna) and BNT162b2 (Pfizer-BioNTech) vaccines were approved for children aged 6 months to 4 years in August 2022 and September 2022, respectively.14 From October 2022, community transmission in Singapore surged due to the emergence of Omicron XBB.15 Omicron XBB remained the predominant subvariant (≥50% of community cases) throughout the end of 2022 and early 2023.15 In March 2023, another surge in community transmission was attributed to a mix of XBB sublineages (XBB 1.5/XBB 1.9/XBB 1.16) that accounted for 90% or more of cases on genomic surveillance.16 Schools remained open throughout these surges and mask wearing was optional for preschool staff and children, with close-contacts of those infected with SARS-CoV-2 allowed to return to preschool if well.17 This study investigated the effectiveness of mRNA vaccines against COVID-19 infection in Singaporean children aged 1 through 4 years during Omicron XBB transmission.
Methods
Study Population and Study Period
This study was done as part of national public health research. Under the Infectious Diseases Act, Singapore, ethics review by an institutional review board was not required, and requirement for individual written informed consent from the children’s guardians was waived. We assessed vaccine effectiveness against confirmed SARS-CoV-2 infection (either positive polymerase chain reaction [PCR] or rapid antigen test [RAT] result) in Singaporean children aged 1 through 4 years, using a population-based cohort study design. Anonymized data on all Singapore citizens and permanent residents aged 1 through 4 years (before 5 years of age) were extracted on May 9, 2023, for the study period of October 1, 2022, through to March 31, 2023, when the Omicron XBB subvariant and its sublineages predominated community transmission.15,16 During the study period, few children younger than 12 months had received vaccination; hence, children younger than 1 year were excluded. Children who had received non–mRNA COVID-19 vaccines, those who received vaccination doses before the date of rollout to the general population, and those with incomplete sociodemographic or clinical information were also excluded. Singapore children from the following ethnic groups were included in the study: Chinese, Indian, Malay, and other (ie, individuals of other ethnicities or mixed ethnicities). Participant ethnicity was included as it was considered an important sociodemographic covariate; ethnicity was defined based on self-designation in national identification records. Data extracted included vaccination dates, age, sex, ethnic group, housing type as a proxy for socioeconomic status, and documented prior SARS-CoV-2 infection. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines.
Data were extracted from national databases, including records of all confirmed SARS-CoV-2 infections, hospitalizations, and vaccines administered. Full vaccination was defined as having completed a primary vaccination series of 2 doses of mRNA-1273 (Moderna) or 3 doses of BNT162b2 (Pfizer-BioNTech) vaccine, with a recommended interval of 8 weeks apart.14 Children were recommended to complete the same number of vaccine doses regardless of prior infection status.14 To obtain additional information on coexisting comorbidity burden and preexisting immunocompromised status, these records were fused with the national health claims database. In Singapore, inpatient care is predominantly provided by public hospitals, which account for 83.8% of beds and 77.8% of acute inpatient admissions; inpatient care is financed by reimbursement claims against a national medical savings scheme (Medisave).18 Claims data included information on diagnoses; comorbidity was defined as the presence of a pediatric complex chronic condition, using International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) diagnosis codes for cardiovascular, respiratory, kidney, gastrointestinal, neurologic, metabolic, congenital, and hematologic/immunologic conditions and malignancies.19 Immunocompromised status was defined as the presence of solid malignancy, hematologic malignancy, rheumatologic/inflammatory disorders, immunodeficiency, or organ or stem-cell transplant, using ICD-10 diagnosis codes.19
Study Outcomes
Vaccine effectiveness against confirmed SARS-CoV-2 infection (PCR/RAT-positive test results) was the primary study outcome. SARS-CoV-2 infection status was determined based on information extracted from the national testing registry, including test date, result, and test type (PCR/RAT). The national testing registry holds records of all SARS-CoV-2 tests performed by any health care professional in Singapore since the start of the COVID-19 pandemic. SARS-CoV-2 testing was compulsory for all individuals presenting with symptoms of acute respiratory infection, such as cough, runny nose, fever, or sore throat, to a health care professional; individuals with severe symptoms or those at higher risk of progression underwent PCR, whereas low-risk individuals underwent RAT.20 All positive results (PCR/RAT) confirmed on health care professional–administered testing were legally mandated to be notified.20 Although self-administered RAT results were not reportable to the Ministry of Health, during the study period, symptomatic children younger than 2 years were highly encouraged to present to clinicians for diagnostic testing and confirmation and clinical assessment.20 Free SARS-CoV-2 testing was offered at all public primary care clinics (polyclinics) and a national network of more than 1000 public health preparedness clinics designated for subsidized treatment and investigations during public health emergencies.20 Lower-risk children were subsequently monitored at home, whereas mildly symptomatic children at higher risk of severe COVID-19 infection (eg, those immunocompromised, with chronic heart or respiratory conditions, neurodevelopmental conditions, or receiving dialysis) were placed on a home recovery program with access to telemedicine clinicians to facilitate subsequent hospitalization in the event of clinical deterioration.20 We reported the incidence of COVID-19–related hospitalization as a secondary outcome, defined as all-cause hospitalizations occurring within 30 days from a positive COVID-19 test result (PCR/RAT). Reporting hospitalizations to the Ministry of Health was mandatory.20
Statistical Analysis
We used a Poisson regression model to estimate the incidence rate ratio (IRR) of reported infections adjusting for age, sex, ethnicity, housing type, vaccination status, time elapsed since last vaccination, Omicron XBB viral lineages as imputed by time period (Omicron XBB prior to March 2023; XBB 1.5/XBB 1.9/XBB 1.16 from March 2023 onward), comorbidities, and immunocompromised status, following methodology used in previously published studies.21,22 The main analysis was stratified by presence or absence of previous documented SARS-CoV-2 infection, with unvaccinated individuals chosen as the reference group. Throughout the study period, individuals were continuously introduced into the study population for prior SARS-CoV-2 infection 90 days after the reported date of prior infection. In secondary analyses, the IRR of confirmed SARS-CoV-2 infection among infection-naive children was stratified by age group and unvaccinated status. Infection-naive children were chosen as the reference category, and time elapsed since last infection was additionally controlled (eMethods 1-4 in Supplement 1) Vaccine effectiveness was calculated as (1 − IRR) × 100%; point estimates and 95% CIs were reported. Although we did not differentiate by vaccine manufacturer in the main analysis, as a secondary analysis, we provided estimates of vaccine effectiveness for mRNA-1273 (Moderna), given that the majority were vaccinated with mRNA-1273 (eMethods 3 in Supplement 1). We calculated the contribution of person-time risks to the unvaccinated, partially vaccinated, and vaccinated groups on the basis of each child’s vaccination status and vaccination dates. Children were considered to be partially vaccinated 7 days after they received the first dose up to 6 days after they received the final dose, and they were considered to be fully vaccinated 7 days or more after they received the final dose. We chose an interval of 7 days or more to facilitate comparisons with previously reported studies of vaccine efficacy among older Singaporean children.21 However, as an additional sensitivity analysis, we used an alternative definition of vaccination status where individuals were considered partially vaccinated 14 days after receipt of the first dose up until 13 days after receiving the final dose and fully vaccinated 14 days or more after they received the final dose. All P values were 2-sided; the cutoff for statistical significance was set as P < .05. Analysis was performed using Stata, version 17.0 (StataCorp).
Results
A total of 121 628 children in Singapore aged 1 through 4 years (median [IQR] age, 3.1 [2.2-3.9] years; 61 925 male [50.9%]; 59 703 female [49.1%]) were included in the analysis after filtering due to various exclusion criteria (Figure), of which 75 935 (62.4%) had no prior SARS-CoV-2 infection. Children identified with the following ethnicity categories: 79 613 Chinese (65.5%), 10 228 Indian (8.4%), 27 278 Malay (22.4%), and 4509 other (3.7%). A total of 45 693 children (37.6%) had prior SARS-CoV-2 infection, with the majority having had prior infection during the earlier Omicron BA.1/2 wave (eFigure in Supplement 1). These 121 628 children contributed 21 015 956 person-days of observation, of which 19 741 062 person-days (93.9%) were unvaccinated, 965 424 person-days (4.6%) were partially vaccinated, and 309 470 person-days (1.5%) were fully vaccinated (Table 1). The majority of vaccinated children (11 294 of 11 705 [96.5%]) received the mRNA-1273 (Moderna) vaccine (eTable 1 in Supplement 1). During the study period, 3888 children developed PCR/RAT-confirmed SARS-CoV-2 infection, of whom 138 (3.5%) required hospitalization (Table 2). Six children (0.2%) progressed to severe COVID-19 infection; none were vaccinated. There were no hospitalizations among fully vaccinated children. There were no fatalities; 2 cases of multisystem inflammatory syndrome in children (MIS-C) were reported among unvaccinated individuals.
Figure. Flowchart of Study Cohort Construction and Breakdown by Vaccination Status.
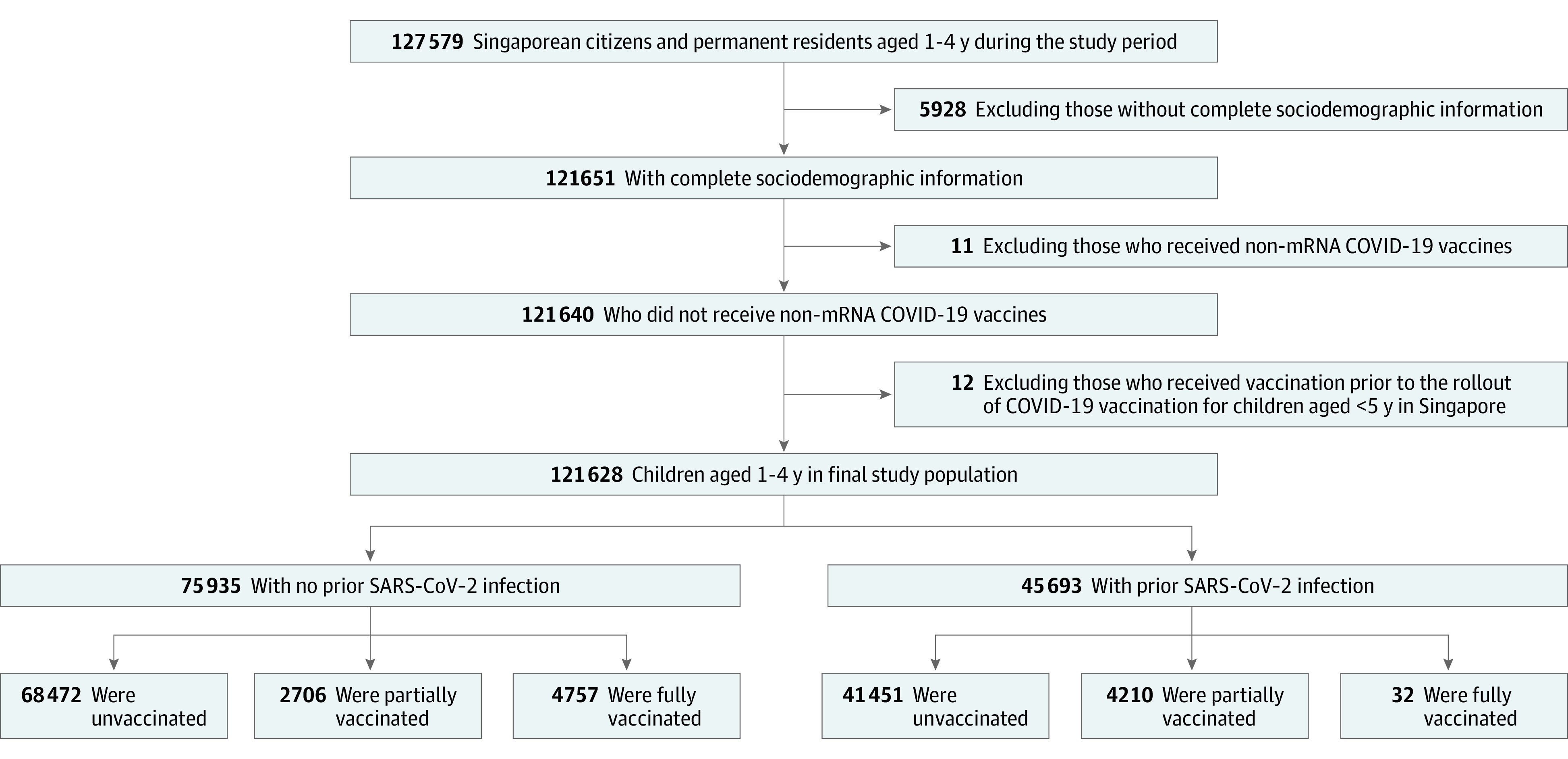
mRNA indicates messenger RNA.
Table 1. Demographic Characteristics of the Study Cohort (Person-Days) Stratified by Prior SARS-CoV-2 Infection and Vaccination Status.
| Variable | No. (%) | |||||
|---|---|---|---|---|---|---|
| No prior SARS-CoV-2 infection (13 360 044 person-days of observation) | Prior SARS-CoV-2 infection (7 655 912 person-days of observation)a | |||||
| Unvaccinated person-days at risk | Partially vaccinated person-days at riskb | Fully vaccinated person-days at riskc | Unvaccinated person-days at risk | Partially vaccinated person-days at riskb | Fully vaccinated person-days at riskc | |
| Total person-days at risk | 12 532 687 (100) | 519 177 (100) | 308 180 (100) | 7 208 375 (100) | 446 247 (100) | 1290 (100) |
| Sex | ||||||
| Female | 6 169 824 (49.2) | 256 348 (49.4) | 147 530 (47.9) | 3 518 229 (48.8) | 219 704 (49.2) | 742 (57.5) |
| Male | 6 362 863 (50.8) | 262 829 (50.6) | 160 650 (52.1) | 3 690 146 (51.2) | 226 543 (50.8) | 548 (42.5) |
| Age distribution, y | ||||||
| 1 | 2 366 119 (18.9) | 58 068 (11.2) | 26 020 (8.4) | 1 456 023 (20.2) | 41 467 (9.3) | 96 (7.4) |
| 2 | 3 500 080 (27.9) | 119 581 (23.0) | 63 922 (20.7) | 2 010 471 (27.9) | 104 421 (23.4) | 347 (26.9) |
| 3 | 3 874 142 (30.9) | 176 482 (34.0) | 101 962 (33.1) | 2 099 851 (29.1) | 144 256 (32.3) | 528 (40.9) |
| 4 | 2 792 346 (22.3) | 165 046 (31.8) | 116 276 (37.7) | 1 642 030 (22.8) | 156 103 (35.0) | 319 (24.7) |
| Ethnicity | ||||||
| Chinese | 8 213 824 (65.5) | 356 068 (68.6) | 224 567 (72.9) | 4 654 521 (64.6) | 282 032 (63.2) | 992 (76.9) |
| Indian | 1 140 756 (9.1) | 36 615 (7.1) | 20 271 (6.6) | 552 636 (7.7) | 29 126 (6.5) | 0 (0.0) |
| Malay | 2 624 369 (20.9) | 106 157 (20.4) | 50 872 (16.5) | 1 803 338 (25.0) | 122 788 (27.5) | 256 (19.8) |
| Otherd | 553 738 (4.4) | 20 337 (3.9) | 12 470 (4.0) | 197 880 (2.7) | 12 301 (2.8) | 42 (3.3) |
| Housing type | ||||||
| 1-3 Room public housing | 7 765 136 (62.0) | 313 009 (60.3) | 184 567 (59.9) | 4 744 535 (65.8) | 289 807 (64.9) | 706 (54.7) |
| 4-5 Room public housing | 2 065 845 (16.5) | 85 003 (16.4) | 47 159 (15.3) | 1 338 389 (18.6) | 86 955 (19.5) | 323 (25.0) |
| Private housing | 2 701 706 (21.6) | 121 165 (23.3) | 76 454 (24.8) | 1 125 451 (15.6) | 69 485 (15.6) | 261 (20.2) |
| Time since last vaccination dose | ||||||
| Mean time since last dose, median (IQR), d | NA | 37 (19-61) | 41 (23-63) | NA | 62 (34-95) | 46 (25-66) |
| ≤5 wk | NA | 246 824 (47.5) | 131 265 (42.6) | NA | 119 317 (26.7) | 485 (37.6) |
| >5 wk | NA | 272 353 (52.5) | 176 915 (57.4) | NA | 326 930 (73.3) | 805 (62.4) |
| Comorbidities | ||||||
| None | 12 317 576 (98.3) | 509 404 (98.1) | 301 786 (97.9) | 7 049 235 (97.8) | 437 582 (98.1) | 1121 (86.9) |
| ≥1 Pediatric CCCe | 215 111 (1.7) | 9773 (1.9) | 6394 (2.1) | 159 140 (2.2) | 8665 (1.9) | 169 (13.1) |
| Immunocompromised statusf | ||||||
| No | 12 531 298 (100) | 519 079 (100) | 307 980 (99.9) | 7 206 095 (100) | 446 105 (100.0) | 1239 (96.0) |
| Yes | 1389 (0) | 98 (0) | 200 (0.1) | 2280 (0) | 142 (0) | 51 (4.0) |
Abbreviations: CCC, complex chronic condition; ICD-10, International Statistical Classification of Diseases and Related Health Problems, Tenth Revision; mRNA, messenger RNA; NA, not applicable.
Prior SARS-CoV-2 infection was defined as either positive polymerase chain reaction or rapid antigen test result in national records before study onset.
Partial vaccination was defined as having received at least 1 dose of mRNA vaccines but not having completed a full vaccination regimen.
Full vaccination was defined as having a primary vaccination series of 2 doses of mRNA-1273 (Moderna), or 3 doses of BNT162b2 (Pfizer-BioNTech) vaccine, at a recommended interval of 8 weeks apart, with 7 or more days having elapsed since the final dose.
Includes individuals of other ethnicities or mixed ethnicities.
Comorbidity was defined as the presence of a pediatric CCC, using ICD-10 diagnosis codes for cardiovascular, respiratory, kidney, gastrointestinal, neurologic, metabolic, congenital, and hematologic/immunologic conditions and malignancies.
Immunocompromised status was defined as the presence of solid malignancy, hematologic malignancy, rheumatologic or inflammatory disorders, other immunodeficiency, or organ or stem-cell transplant.
Table 2. Vaccine Effectiveness Against Confirmed SARS-CoV-2 Infection Among Singaporean Children Aged 1 Through 4 Years, Stratified by Prior SARS-CoV-2 Infection and Vaccination Status (N = 121 628).
| Vaccination status | Total person-days at risk | Hospitalization for COVID-19 during study period | Confirmed SARS-CoV-2 infection during study period | ||||
|---|---|---|---|---|---|---|---|
| No. of children | Incidence, per million person-days | No. of children | Incidence, per million person-days | Adjusted IRR (95% CI)a | Vaccine efficacy (1 − IRR) × 100%, (95% CI) | ||
| No prior SARS-CoV-2 infection (n = 75 935) | |||||||
| Unvaccinated | 12 532 687 | 121 | 10.0 | 3261 | 260.2 | 1 [Reference] | NA |
| Partially vaccinatedb | 519 177 | 6 | 12.0 | 69 | 132.9 | 0.55 (0.40-0.75) | 45.2% (24.7%-60.2%) |
| Fully vaccinatedc | 308 180 | 0 | 0 | 21 | 68.1 | 0.37 (0.23-0.59) | 63.3% (40.6%-77.3%) |
| Prior SARS-CoV-2 infection (n = 45 693) d | |||||||
| Unvaccinated | 7 208 375 | 11 | 1.6 | 532 | 73.8 | 1 [Reference] | NA |
| Partially vaccinatedb | 446 247 | 0 | 0.0 | 5 | 11.2 | 0.25 (0.10-0.61) | 74.6% (38.7%-89.5%) |
| Fully vaccinatedc | 1290 | 0 | 0 | 0 | 0 | NAe | NA |
Abbreviations: IRR, incidence rate ratio; mRNA, messenger RNA; NA, not applicable.
Adjusted with Poisson regression for age, sex, ethnicity, housing type, vaccination status, viral lineage as imputed by time period (before or after March 2023), time elapsed since last vaccination dose, comorbidities, and immunocompromised status.
Partial vaccination was defined as having received at least 1 dose of mRNA vaccines but not having completed a full vaccination regimen.
Full vaccination was defined as having a primary vaccination series of 2 doses of mRNA-1273 (Moderna) or 3 doses of BNT162b2 (Pfizer-BioNTech) vaccine, at a recommended interval of 8 weeks apart, with 7 or more days having elapsed since the final dose.
Prior SARS-CoV-2 infection was defined as either positive polymerase chain reaction or rapid antigen test result in national records.
Not computed due to an absence of cases.
Among unvaccinated, infection-naive children aged 1 through 4 years, the incidence rates of PCR/RAT-confirmed infections, hospitalizations, and severe COVID-19 infection were 260.2, 10.0, and 0.4 per 1 million person-days, respectively (Table 2). The corresponding rates were 132.9, 12.0, and 0 per 1 million person-days, respectively, in the partially vaccinated group and 68.1, 0, and 0 per 1 million person-days, respectively, in the fully vaccinated group. For unvaccinated children aged 1 through 4 years with prior infection, the incidence rates of all PCR/RAT-confirmed reinfections, hospitalizations, and severe COVID-19 infection were 73.8, 1.6, and 0.1 per 1 million person-days, respectively (Table 2). The corresponding rates for PCR/RAT-confirmed reinfections were 11.2 per 1 million person-days in those who had received at least 1 vaccine dose; there were no hospitalizations or cases of severe COVID-19 infection among children aged 1 through 4 years with prior infection who had received at least 1 vaccine dose. After adjustment for covariates, among infection-naive children, vaccine effectiveness against all PCR/RAT-confirmed infections in partially vaccinated children as compared with unvaccinated children was 45.2% (95% CI, 24.7%-60.2%) at 7 to 157 days since the last dose (median [IQR] time interval, 37 [19-61] days). In fully vaccinated children, we estimated vaccine effectiveness against all PCR/RAT-confirmed infections to be 63.3% (95% CI, 40.6%-77.3%) at 7 to 128 days since last dose (median [IQR] time interval, 41 [23-63] days). (Table 2) The IRR of PCR/RAT-confirmed infection in infection-naive children was fairly uniform across all age groups (eTable 2 in Supplement 1).
Among children aged 1 through 4 years with prior infection, vaccine effectiveness against all PCR/RAT-confirmed reinfections in those who had received at least 1 vaccine dose was estimated at 74.6% (95% CI, 38.7%-89.5%) at 7 to 157 days since last dose (median [IQR] time interval, 62 [34-95] days), using unvaccinated children with prior SARS-CoV-2 infection as the reference category(Table 2). Compared with unvaccinated, infection-naive children, the IRR of all PCR/RAT-confirmed reinfections in previously infected children who had received at least 1 vaccine dose was estimated at 0.05 (95% CI, 0.02-0.12) at 7 to 157 days since last dose (median [IQR] time interval, 62 [34-95] days) (eTable 3 in Supplement 1). Data were insufficient to assess vaccine effectiveness in fully vaccinated children with prior SARS-CoV-2 infection (only 1290 person-days of observation). We assessed vaccine efficacy for mRNA-1273 alone (eTable 4 in Supplement 1) and controlled for time elapsed since prior infection when estimating the IRR of reinfections (eTable 5 in Supplement 1); there were no substantial differences in estimates. Use of an alternative interval of 14 days postdose to define vaccination status did not result in substantially different estimates (eTable 6 in Supplement 1).
Discussion
Our estimates of the effectiveness of mRNA vaccines in children aged 1 through 4 years suggest that complete primary series vaccination, predominantly with mRNA-1273, provides a substantial degree of protection (63.3%) in infection-naive children. In those with documented prior infection, efficacy against reinfections among those who had received at least 1 vaccine dose was estimated at 74.6%. These estimates were derived against a background of Omicron XBB circulation including sublineages (XBB 1.5/XBB 1.9/XBB 1.16). In terms of COVID-19 hospitalizations, the incidence rate was nil for fully vaccinated children compared with 10.0 and 1.6 per million person-days for unvaccinated-naive and previously infected children, respectively.
A large test-negative case-control study7 among children aged 3 to 5 years who participated in a nationwide community-based testing program in the US during circulation of a mix of Omicron subvariants (XBB/BQ 1.1/BA.5) reported estimates of vaccine effectiveness against infection ranging from 31% to 60%, although analysis was not adjusted for prior infection. Vaccine effectiveness can potentially be underestimated using a test-negative case-control study design because vaccinated children with positive SARS-CoV-2 test results may be overrepresented, given that parents of vaccinated children may be more highly motivated to test their children and access health care compared with less motivated parents.23 Estimates of vaccine effectiveness against Omicron infection ranged from 36.8% to 65.3% in a cohort study of older Singaporean children (aged 5-11 years)21; in contrast, estimates of vaccine effectiveness among Omicron infection in US children aged 5 to 11 years using test-negative case-control study designs ranged from 28.9% to 50%.24,25 However, other population-based differences, such as the recommended interval of 8 weeks between vaccine doses used in our population and the extent of prior infection conferring additional immunity, may also exist. Although seroprevalence data among Singaporean children was not available for direct comparison, lower seroprevalence estimates have generally been reported for children in Western Pacific countries (eg, China, Australia, and Japan) compared with US children, potentially attributed to public health measures (eg, nationwide or citywide lockdowns, school closures, mandatory masking) instituted at earlier stages of the COVID-19 pandemic.26 Additional studies in other populations are needed to examine vaccine effectiveness against infection in this age group and the durability of vaccine protection.
Demonstration of effective protection against infection is important as part of public health surveillance, especially with the emergence of variants with potential to evade vaccine-induced immunity, such as Omicron XBB. Whether children younger than 5 years should be vaccinated against COVID-19 infection remains debatable, given that children are much less likely to have severe disease requiring hospitalization.27 Indeed, rates of hospitalization and severe COVID-19 infection in our cohort were extremely low. However, rapid increases in pediatric COVID-19 infections coinciding with periods of high community transmission may still place health care systems under strain. Among previously uninfected and unvaccinated children, Omicron infection may not be mild; when compared with influenza or parainfluenza infection, infection with Omicron BA.2 had higher risk of pediatric intensive care unit admission, with increased risk of neurologic complications.28 Fatal fulminant cerebral edema has been reported as a rare but severe neurologic complication among unvaccinated children.29 Additionally, COVID-19 vaccination is associated with a lower incidence of MIS-C.30,31 In a cohort of children with MIS-C in Singapore, all were not age eligible for vaccination at the time of their infection. With the subsequent inclusion of children aged 6 months to 4 years in vaccination guidelines, 90% or more children with MIS-C would have been eligible for vaccination.32 Long-term sequelae after acute SARS-CoV-2 infection remains a concern even in the pediatric age group.33,34 The potential benefit of COVID-19 vaccination in minimizing acute and long-term sequelae of SARS-CoV-2 infection needs to be considered in tandem with safety data, with no serious adverse events associated with vaccination.35 Higher COVID-19 vaccine hesitancy has been reported for pediatric vaccination, compared with adult vaccination programs.36 Acceptance of pediatric vaccination depends on parental beliefs and perceptions. Around 15.9% of Singaporean parents had vaccine hesitancy36; the results of this study may enable parents to weigh benefits against the potential risks of vaccination. In Singapore, 8 adverse events after vaccination (0.05% of all doses administered) among children younger than 5 years were reported to the Health Sciences Authority as of December 31, 2022; in contrast, the overall rate of adverse events after vaccination across all age groups was 0.11%.37 To date, there have been 3 fatalities from COVID-19 infection reported among children younger than 5 years in Singapore; all of whom were unvaccinated.38
Limitations
Although this study used data extracted from a high-quality registry covering an entire national population, there are limitations to consider. First, vaccination coverage was low; vaccinated children may differ in terms of their COVID-19 risk, which could be a reason for vaccination and thus bias estimates of vaccine effectiveness. Second, residual confounding from other unmeasured factors such as behaviors affecting COVID-19 risk (eg, preschool attendance) could not be excluded. Third, variant type was assumed on the basis of case-reporting date. Fourth, the results reflect vaccine effectiveness for the mRNA-1273 rather than BNT162b2 vaccine, given that the vast majority received mRNA-1273. Finally, classification of SARS-CoV-2 infection was based on PCR/RAT performed by health care professionals; results of self-administered RAT were not included in our national database, which could potentially bias estimates of vaccine effectiveness upward. However, throughout the entire study period, COVID-19–related health care costs including testing, consultation, and treatment were free; testing was mandatory for all children presenting to clinicians with symptoms of acute respiratory infection , and public health messaging encouraged pediatric patients to proceed to primary care practitioners for confirmatory PCR/RAT testing and clinical assessment.20 Although our national testing protocol did not incorporate additional PCR testing in individuals with RAT-negative test results, overall sensitivity and specificity of RAT were reasonably high, with estimates of 83.3% and 97.5%, respectively, among hospitalized Singaporean children during the Omicron wave.39
Conclusions
In this cohort study, among Singaporean children aged 1 through 4 years during an Omicron XBB wave, vaccine effectiveness against PCR/RAT-confirmed infection was 45.2% in partially vaccinated, infection-naive children and 63.3% in fully vaccinated, infection-naive children. Among those with prior infection, vaccine effectiveness against reinfections in those who had received at least 1 vaccine dose was estimated at 74.6%. The potential benefit in minimizing acute and long-term sequelae of SARS-CoV-2 infection in this age group may outweigh the minimal adverse effects arising from vaccination.
eMethods 1. Secondary Analysis 1—Incidence Rate Ratio (IRR) of Confirmed SARS-CoV-2 Infection Among Infection-Naive Singaporean Children Aged 1 Through 4 Years, by Age Group
eMethods 2. Secondary Analysis 2—Incidence Rate Ratio of Confirmed SARS-CoV-2 Infection Among Singaporean Children Aged 1 Through 4 Years, Using Unvaccinated, Infection-Naive Children as the Reference Category
eMethods 3. Secondary Analysis 3—Analysis of mRNA-1273 (Moderna) Vaccine Effectiveness Against Confirmed SARS-CoV-2 Infection Among Singaporean Children Aged 1 Through 4 Years, Stratified by Prior SARS-CoV-2 Infection and Vaccination Status
eMethods 4. Secondary Analysis 4—Incidence Rate Ratio of Confirmed SARS-CoV-2 Reinfection Among Singaporean Children Aged 1 Through 4 Years With Prior SARS-CoV-2 Infection, Controlling for Time Elapsed Since Last Infection
eTable 1. COVID-19 Vaccine Combinations Received by Individuals in the Vaccinated Cohort (N=11,705)
eTable 2. Incidence Rate Ratio (IRR) of Confirmed SARS-CoV-2 Infection Among Infection-Naive Singaporean Children Aged 1 Through 4 Years, Stratified by Age Group (N= 75,935)
eTable 3. Incidence Rate Ratio of Confirmed SARS-CoV-2 Infection Among Singaporean Children Aged 1 Through 4 Years, Using Unvaccinated, Infection-Naive Children as the Reference Category (N= 121,628)
eTable 4. Vaccine Effectiveness of mRNA-1273 (Moderna) Against Confirmed SARS-CoV-2 Infection Among Singaporean Children Aged 1 Through 4 Years, Stratified by Prior SARS-CoV-2 Infection and Vaccination Status (N= 121,027)
eTable 5. Incidence Rate Ratio (IRR) of Confirmed SARS-CoV-2 Reinfection Among Singaporean Children Aged 1 Through 4 Years With Prior SARS-CoV-2 Infection, Controlled for Time Elapsed Since Last Infection (N= 45,693)
eTable 6. Vaccine Effectiveness Against Confirmed SARS-CoV-2 Infection and Hospitalization Among Singaporean Children Aged 1 Through 4 Years, Stratified by Prior SARS-CoV-2 Infection and Vaccination Status, Using an Interval of 14 Days After Receipt of Vaccination (N= 121,628)
eFigure. Date of Prior Recorded SARS-CoV-2 Infections in Study Cohort (N=45,693)
Data Sharing Statement
References
- 1.Abu-Raddad LJ, Chemaitelly H, Ayoub HH, et al. Effect of mRNA vaccine boosters against SARS-CoV-2 Omicron infection in Qatar. N Engl J Med. 2022;386(19):1804-1816. doi: 10.1056/NEJMoa2200797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrews N, Stowe J, Kirsebom F, et al. COVID-19 vaccine effectiveness against the Omicron (B.1.1.529) variant. N Engl J Med. 2022;386(16):1532-1546. doi: 10.1056/NEJMoa2119451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Food and Drug Administration . Coronavirus (COVID-19) update: FDA authorizes Moderna and Pfizer-BioNTech COVID-19 vaccines for children down to 6 months of age. Accessed August 8, 2023. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-moderna-and-pfizer-biontech-covid-19-vaccines-children
- 4.World Health Organization . Interim statement on COVID-19 vaccination for children. Accessed August 8, 2023. https://www.who.int/news/item/11-08-2022-interim-statement-on-covid-19-vaccination-for-children
- 5.Anderson EJ, Creech CB, Berthaud V, et al. ; KidCOVE Study Group . Evaluation of mRNA-1273 vaccine in children 6 months to 5 years of age. N Engl J Med. 2022;387(18):1673-1687. doi: 10.1056/NEJMoa2209367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muñoz FM, Sher LD, Sabharwal C, et al. ; C4591007 Clinical Trial Group . Evaluation of BNT162b2 COVID-19 vaccine in children younger than 5 years of age. N Engl J Med. 2023;388(7):621-634. doi: 10.1056/NEJMoa2211031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fleming-Dutra KE, Ciesla AA, Roper LE, et al. Preliminary estimates of effectiveness of monovalent mRNA vaccines in preventing symptomatic SARS-CoV-2 infection among children aged 3-5 years—increasing community access to testing program, US, July 2022-February 2023. MMWR Morb Mortal Wkly Rep. 2023;72(7):177-182. doi: 10.15585/mmwr.mm7207a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jara A, Undurraga EA, Zubizarreta JR, et al. Effectiveness of CoronaVac in children 3-5 years of age during the SARS-CoV-2 Omicron outbreak in Chile. Nat Med. 2022;28(7):1377-1380. doi: 10.1038/s41591-022-01874-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leung D, Rosa Duque JS, Yip KM, So HK, Wong WHS, Lau YL. Effectiveness of BNT162b2 and CoronaVac in children and adolescents against SARS-CoV-2 infection during Omicron BA.2 wave in Hong Kong. Commun Med (Lond). 2023;3(1):3. doi: 10.1038/s43856-022-00233-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yan VKC, Cheng FWT, Chui CSL, et al. Effectiveness of BNT162b2 and CoronaVac vaccines in preventing SARS-CoV-2 Omicron infections, hospitalizations, and severe complications in the pediatric population in Hong Kong: a case-control study. Emerg Microbes Infect. 2023;12(1):2185455. doi: 10.1080/22221751.2023.2185455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marks KJ, Whitaker M, Agathis NT, et al. ; COVID-NET Surveillance Team . Hospitalization of infants and children aged 0-4 years with laboratory-confirmed COVID-19—COVID-NET, 14 States, March 2020-February 2022. MMWR Morb Mortal Wkly Rep. 2022;71(11):429-436. doi: 10.15585/mmwr.mm7111e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ross CE, Burns JP, Grossestreuer AV, et al. Trends in disease severity among critically ill children with severe acute respiratory syndrome Coronavirus 2: a retrospective multicenter cohort study in the US. Pediatr Crit Care Med. 2023;24(1):25-33. doi: 10.1097/PCC.0000000000003105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang L, Berger NA, Kaelber DC, Davis PB, Volkow ND, Xu R. Incidence rates and clinical outcomes of SARS-CoV-2 infection with the Omicron and Delta variants in children younger than 5 years in the US. JAMA Pediatr. 2022;176(8):811-813. doi: 10.1001/jamapediatrics.2022.0945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ministry of Health Singapore . Child vaccination. Accessed March 5, 2023. https://www.moh.gov.sg/covid-19/vaccination/child#
- 15.Goh AXC, Chae SR, Chiew CJ, et al. Characteristics of the omicron XBB subvariant wave in Singapore. Lancet. 2023;401(10384):1261-1262. doi: 10.1016/S0140-6736(23)00390-2 [DOI] [PubMed] [Google Scholar]
- 16.Ministry of Health Singapore . Speech by Mr Ong Ye Kung, minister for health, at the National Healthcare Group’s population health collective annual work plan seminar for community partners and GPs, 14 April 2023, NG Teng Fong Centre for Healthcare Innovation. Accessed May 10, 2023. https://www.moh.gov.sg/news-highlights/details/speech-by-mr-ong-ye-kung-minister-for-health-at-the-national-healthcare-group-s-population-health-collective-(popcollect)-annual-work-plan-seminar-for-community-partners-and-gps-14-april-2023-ng-teng-fong-centre-for-healthcare-innovation
- 17.Early Childhood Development Agency Singapore . FAQ for parents. Accessed March 5, 2023. https://www.ecda.gov.sg/parents/faq/covid-19-faq
- 18.Tan CC, Lam CSP, Matchar DB, Zee YK, Wong JEL. Singapore’s health-care system: key features, challenges, and shifts. Lancet. 2021;398(10305):1091-1104. doi: 10.1016/S0140-6736(21)00252-X [DOI] [PubMed] [Google Scholar]
- 19.Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. doi: 10.1186/1471-2431-14-199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ministry of Health Singapore . Update on the management of COVID-19 with protocol 2 (primary care). Accessed August 8, 2023. https://www.cfps.org.sg/assets/1-Circular-for-GPs/10-UpdateontheManagementofCovid19withProtocol2PrimaryCare-C-04-2022-1.pdf
- 21.Tan SHX, Cook AR, Heng D, Ong B, Lye DC, Tan KB. Effectiveness of BNT162b2 vaccine against Omicron in children 5 to 11 years of age. N Engl J Med. 2022;387(6):525-532. doi: 10.1056/NEJMoa2203209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bar-On YM, Goldberg Y, Mandel M, et al. Protection of BNT162b2 vaccine booster against COVID-19 in Israel. N Engl J Med. 2021;385(15):1393-1400. doi: 10.1056/NEJMoa2114255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dean NE, Hogan JW, Schnitzer ME. COVID-19 vaccine effectiveness and the test-negative design. N Engl J Med. 2021;385(15):1431-1433. doi: 10.1056/NEJMe2113151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fleming-Dutra KE, Britton A, Shang N, et al. Association of prior BNT162b2 COVID-19 vaccination with symptomatic SARS-CoV-2 infection in children and adolescents during Omicron predominance. JAMA. 2022;327(22):2210-2219. doi: 10.1001/jama.2022.7493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khan FL, Nguyen JL, Singh TG, et al. Estimated BNT162b2 vaccine effectiveness against infection with Delta and Omicron variants among US children 5 to 11 years of age. JAMA Netw Open. 2022;5(12):e2246915. doi: 10.1001/jamanetworkopen.2022.46915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Naeimi R, Sepidarkish M, Mollalo A, et al. SARS-CoV-2 seroprevalence in children worldwide: a systematic review and meta-analysis. EClinicalMedicine. 2023;56:101786. doi: 10.1016/j.eclinm.2022.101786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cox D. What do we know about COVID-19 vaccines in under 5s? BMJ. 2022;378:o1892. doi: 10.1136/bmj.o1892 [DOI] [PubMed] [Google Scholar]
- 28.Tso WWY, Kwan MYW, Wang YL, et al. Severity of SARS-CoV-2 Omicron BA.2 infection in unvaccinated hospitalized children: comparison to influenza and parainfluenza infections. Emerg Microbes Infect. 2022;11(1):1742-1750. doi: 10.1080/22221751.2022.2093135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin JJ, Tu YF, Chen SJ, et al. Fatal fulminant cerebral edema in 6 children with SARS-CoV-2 Omicron BA.2 infection in Taiwan. J Pediatric Infect Dis Soc. 2023;12(2):99-103. doi: 10.1093/jpids/piac116 [DOI] [PubMed] [Google Scholar]
- 30.Levy M, Recher M, Hubert H, et al. Multisystem inflammatory syndrome in children by COVID-19 vaccination status of adolescents in France. JAMA. 2022;327(3):281-283. doi: 10.1001/jama.2021.23262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zambrano LD, Newhams MM, Olson SM, et al. ; Overcoming COVID-19 Investigators . BNT162b2 mRNA vaccination against Coronavirus Disease 2019 is associated with a decreased likelihood of multisystem inflammatory syndrome in children aged 5-18 years—US, July 2021 - April 2022. Clin Infect Dis. 2023;76(3):e90-e100. doi: 10.1093/cid/ciac637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nadua KD, Chong CY, Kam KQ, et al. Multisystem inflammatory syndrome in children in Singapore. Ann Acad Med Singap. 2022;51(11):669-676. doi: 10.47102/annals-acadmedsg.202283 [DOI] [PubMed] [Google Scholar]
- 33.Kikkenborg Berg S, Palm P, Nygaard U, et al. Long COVID symptoms in SARS-CoV-2-positive children aged 0-14 years and matched controls in Denmark (LongCOVIDKidsDK): a national, cross-sectional study. Lancet Child Adolesc Health. 2022;6(9):614-623. doi: 10.1016/S2352-4642(22)00154-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Behnood SA, Shafran R, Bennett SD, et al. Persistent symptoms following SARS-CoV-2 infection amongst children and young people: a meta-analysis of controlled and uncontrolled studies. J Infect. 2022;84(2):158-170. doi: 10.1016/j.jinf.2021.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hause AM, Marquez P, Zhang B, et al. COVID-19 mRNA vaccine safety among children aged 6 months to 5 years—US, June 18, 2022-August 21, 2022. MMWR Morb Mortal Wkly Rep. 2022;71(35):1115-1120. doi: 10.15585/mmwr.mm7135a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Griva K, Tan KYK, Chan FHF, et al. Evaluating rates and determinants of COVID-19 vaccine hesitancy for adults and children in the Singapore population: Strengthening Our Community’s Resilience Against Threats From Emerging Infections (SOCRATEs) cohort. Vaccines (Basel). 2021;9(12):1415. doi: 10.3390/vaccines9121415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Health Sciences Authority of Singapore . HSA’s COVID-19 vaccine safety update #14 (30 December 2020–31 December 2022). Accessed March 5, 2023. https://www.hsa.gov.sg/docs/default-source/hprg-vcb/safety-update-on-covid19-vaccines/hsa-safety-update-no-14-on-covid-19-vaccines-(31-december-2022).pdf
- 38.Channel News Asia . A 3-year-old girl dies from COVID-19 infection; third patient under 12 to die of the disease in Singapore. Accessed March 5, 2023. https://www.channelnewsasia.com/singapore/3-year-old-girl-dies-covid-19-third-patient-under-12-singapore-2938431
- 39.Kam KQ, Maiwald M, Chong CY, et al. SARS-CoV-2 antigen rapid tests and universal screening for COVID-19 Omicron variant among hospitalized children. Am J Infect Control. 2023;51(3):255-260. doi: 10.1016/j.ajic.2022.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods 1. Secondary Analysis 1—Incidence Rate Ratio (IRR) of Confirmed SARS-CoV-2 Infection Among Infection-Naive Singaporean Children Aged 1 Through 4 Years, by Age Group
eMethods 2. Secondary Analysis 2—Incidence Rate Ratio of Confirmed SARS-CoV-2 Infection Among Singaporean Children Aged 1 Through 4 Years, Using Unvaccinated, Infection-Naive Children as the Reference Category
eMethods 3. Secondary Analysis 3—Analysis of mRNA-1273 (Moderna) Vaccine Effectiveness Against Confirmed SARS-CoV-2 Infection Among Singaporean Children Aged 1 Through 4 Years, Stratified by Prior SARS-CoV-2 Infection and Vaccination Status
eMethods 4. Secondary Analysis 4—Incidence Rate Ratio of Confirmed SARS-CoV-2 Reinfection Among Singaporean Children Aged 1 Through 4 Years With Prior SARS-CoV-2 Infection, Controlling for Time Elapsed Since Last Infection
eTable 1. COVID-19 Vaccine Combinations Received by Individuals in the Vaccinated Cohort (N=11,705)
eTable 2. Incidence Rate Ratio (IRR) of Confirmed SARS-CoV-2 Infection Among Infection-Naive Singaporean Children Aged 1 Through 4 Years, Stratified by Age Group (N= 75,935)
eTable 3. Incidence Rate Ratio of Confirmed SARS-CoV-2 Infection Among Singaporean Children Aged 1 Through 4 Years, Using Unvaccinated, Infection-Naive Children as the Reference Category (N= 121,628)
eTable 4. Vaccine Effectiveness of mRNA-1273 (Moderna) Against Confirmed SARS-CoV-2 Infection Among Singaporean Children Aged 1 Through 4 Years, Stratified by Prior SARS-CoV-2 Infection and Vaccination Status (N= 121,027)
eTable 5. Incidence Rate Ratio (IRR) of Confirmed SARS-CoV-2 Reinfection Among Singaporean Children Aged 1 Through 4 Years With Prior SARS-CoV-2 Infection, Controlled for Time Elapsed Since Last Infection (N= 45,693)
eTable 6. Vaccine Effectiveness Against Confirmed SARS-CoV-2 Infection and Hospitalization Among Singaporean Children Aged 1 Through 4 Years, Stratified by Prior SARS-CoV-2 Infection and Vaccination Status, Using an Interval of 14 Days After Receipt of Vaccination (N= 121,628)
eFigure. Date of Prior Recorded SARS-CoV-2 Infections in Study Cohort (N=45,693)
Data Sharing Statement


