Severe RPE after MIMVS.
Central Message.
With the increasing number of MICS, surgeons should be aware of and prepared to manage the uncommon complication of RPE.
Major cardiac operations can now be performed safely through minimally invasive cardiac surgery (MICS). Despite the improvement in technique and technology of MICS, one risk that is still almost exclusively observed in MICS is re-expansion pulmonary edema (RPE).1 We report a case of RPE requiring venovenous extracorporeal membrane oxygenation (vvECMO) after minimally invasive mitral valve surgery (MIMVS).
Case Report
A 63-year-old Chinese man with congestive heart failure related to chronic mitral regurgitation was referred for mitral valve surgery. Preoperative chest x-ray (CXR) revealed cardiac failure with pulmonary hypertension (PHTN). Transesophageal echocardiography showed severe mitral regurgitation with P3 chordal rupture with dilated mitral annulus, ejection fraction of 57%, and mean pulmonary pressure of 30 mm Hg. After multiple counseling sessions, the patient consented for elective MIMVS. Informed consent was obtained from the patient to include the information in this article. Institutional Review Board approval was not required.
MIMVS was performed with a right minithoracotomy made on the fourth intercostal space and peripheral cardiopulmonary bypass (CPB). Left-lung ventilation through a double-lumen endotracheal tube was used briefly before and after establishing CPB. Repair was attempted with the insertion of neochords to P3, but the result was unsatisfactory. Subsequently, the decision was made to replace the valve with a 29-mm Epic valve (Abbott) using Cor-Knot (LSI Solutions) to preserve all chords. The period of aortic crossclamp was 4.5 hours, with CPB lasting 5.5 hours. Total cardioplegia given was 3.7 L, and the lowest hemoglobin level during CPB was 9.2 with no transfusion given. Mean arterial pressure was maintained at 60 mm Hg throughout, and the lowest temperature recorded was 32°C. At the end of CPB, the fluid balance was positive at 520 mL. After coming off bypass with a satisfactory transesophageal echocardiography result, the aortic root cannula was removed, and bilateral lung ventilation was resumed. The patient was weaned off CPB easily.
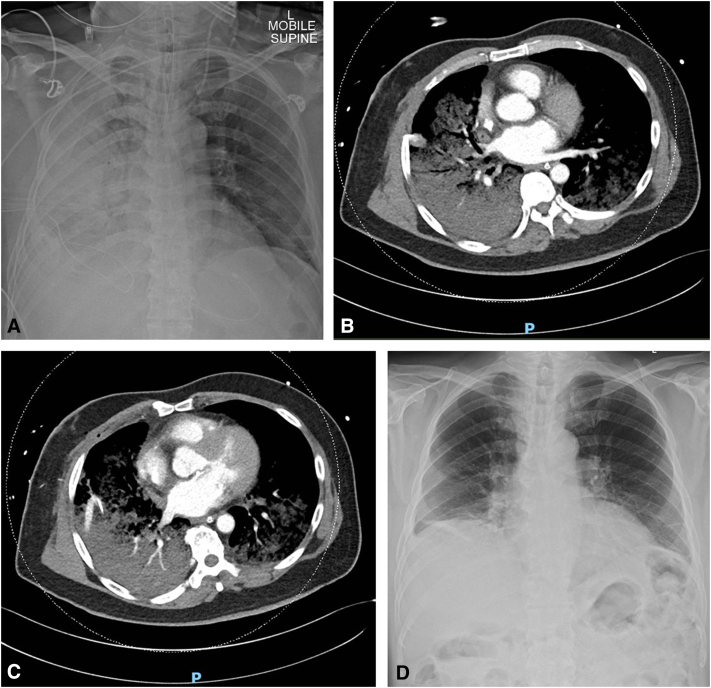
Upon reaching the intensive care unit, the patient’s oxygen saturation was noted to be 78% to 82% despite escalating to 100% fraction of inspired oxygen, and arterial blood gas showed PaO2 of 47 mm Hg. CXR (Figure 1, A) showed right lung RPE. vvECMO was swiftly established with a rapid return of optimal oxygen saturation. Computed tomography (Figure 1, B and C) showed no evidence of pulmonary vein obstruction. Bedside transthoracic echocardiography showed satisfactory mitral valve function with an ejection fraction of 60% to 65%. During the patient’s stay, he also developed acute kidney injury requiring continuous venovenous hemofiltration (total of 5 days), postoperative atrial fibrillation, ischemic hepatitis, and critical illness polymyopathy.
Figure 1.
A, CXR showing extensive right lung opacification, consistent with RPE of the right lung. B and C, Computed tomography scan of the thorax shows pulmonary edema in the right lung and acute respiratory distress syndrome in the left lung. D, CXR before discharge showing resolution of right lung opacification.
He remained stable and continued to improve clinically during vvECMO support, with kidney and liver function normalized within days of supportive care. On postoperative day 10, vvECMO was removed and the patient was weaned off mechanical ventilation. The remainder of the patient's hospital stay was unremarkable. CXR (Figure 1, C) before discharge revealed complete resolution of right lung opacity.
Discussion
RPE after MICS is a potentially catastrophic complication if not managed appropriately. Radiographic evidence of RPE after MICS has been reported to be 5.0% to 7.9%, and symptomatic RPE after MICS has been reported to be 1.5%.2 Patients with RPE after MICS have a 30-day mortality rate of 12.5%.3
Etiology and Risk Factors
Despite ambiguity in pathogenesis and risk factors, it has been hypothesized that RPE after MICS occurs primarily due to ischemic-reperfusion injury of the lung and the inflammatory response that ensues.3
During single-lung ventilation, the deflated lung is susceptible to ischemia due to shunting of blood away from unventilated alveoli. During reperfusion, reactive oxygen species and other inflammatory mediators are produced, which increases permeability of the pulmonary microvessels.4 This inflammatory response is aggravated with prolonged durations of ischemia and the use of CPB.5 In addition, prolonged collapse of the lungs can lead to hardening of the pulmonary microvessels and decreased vessel flexibility, which can cause injury to the microvessels during re-expansion.3,4 Possible risk factors for RPE include comorbidities such as PHTN,2 chronic obstructive pulmonary disease and diabetes,2 preoperative factors such as use of steroids or immunosuppressants,3 increased preoperative C-reactive protein, intraoperative factors such as prolonged aortic crossclamp3 and CPB time, and intraoperative blood transfusion.2
Our patient has a background of PHTN, and long intraoperative aortic crossclamp and CPB time predisposed him to develop RPE. Learning points from this case includes better case selection (do not take PHTN lightly when one does not have extensive MIS experience) and better clinical judgment and decision making (consider early conversion to sternotomy or proceed to mitral replacement when the attempt to repair is taking too long).
Management
Although RPE has a high mortality rate and few cases of fatal RPE after MICS have been reported, there has yet to be any definitive treatment. Most reported cases have generally been supportive, including oxygen supplementation and noninvasive and invasive ventilation, reserving ECMO as a last resort.
A literature review yielded a total of 11 reported cases of RPE requiring vvECMO after MICS (Table 1). Case reports of RPE requiring vvECMO after MICS when supportive therapy has failed are limited. In our case, the early recognition and use of vvECMO allowed our patient's multiorgan function to recover steadily. Through better understanding of the pathophysiology behind RPE, MICS surgeons may have better insights on how to prevent RPE and administer appropriate early intervention.
Table 1.
Reported cases of re-expansion pulmonary edema requiring venovenous extracorporeal membrane oxygenation after minimally invasive cardiac surgery
| Reference | Procedure | Age/sex | Duration | Time before induction of vvECMO | Duration of vvECMO (d) |
|---|---|---|---|---|---|
| Madershahian and colleaguesE1 | Minimally invasive mitral valve repair | 62/M | AXC - 2.5 h CPB - 3 h |
Nil | Despite initial improvement, patient died 1 wk later of septic shock and multiple organ failure |
| Shires and colleaguesE2 | Minimally invasive aortic valve replacement | 57/M | AXC - 184 min CPB - 151 min |
POD 1 | 1 |
| Kanemitsu and colleaguesE3 | Minimally invasive mitral valve repair | 56/M | AXC - 192 min CPB - 340 min |
6 h | 10 |
| Fujita and colleagues4 | Minimally invasive mitral valve repair | 45/M | AXC - 270 min | Immediately postoperation | 4 |
| Hsu and colleaguesE4 | Minimally invasive mitral valve repair | 46/M | AXC - 215 min CPB - 405 min OLV - 180 min |
POD 1 | 3 |
| Kim and colleaguesE5 | Robot-assisted mitral valve repair and Maze procedure | 49/M | AXC - 165 min CPB - 250 min OLV - 100 min |
5 h | 3 |
| Kitahara and colleaguesE6 | Minimally invasive mitral valve repair, tricuspid valve repair and left atrial MAZE procedure | 60/M | AXC - 236 min CPB - 359 min |
POD 1 | 4 |
| Goyal and colleaguesE7 | Minimally invasive mitral valve repair | 48/F | AXC - 166 min CPB - 321 min |
POD 1 | 6 |
| 40/M | AXC - 135 min CPB - 272 min |
Intraoperative | 5 | ||
| Jung and colleaguesE8 | Minimally invasive resection of recurrent left atrial myxoma | 46/F | ACX - 166 min CPB - 321 min |
Intraoperative | 2.5 |
| Viox and colleaguesE9 | Robot-assisted mitral valve repair, tricuspid valve repair, left atrial cryoMaze procedure and LA appendage ligation | 56/F | CPB - 289 min | Intraoperative | 10 |
vvECMO, Venovenous extracorporeal membrane oxygenation; AXC, Aortic crossclamp; CPB, cardiopulmonary bypass; POD, postoperative day; OLV, 1-lung ventilation; LA, left atrial.
Conclusions
Although RPE is a rare complication, MICS surgeons need to have raised awareness of its predisposing factors and pathophysiology. vvECMO is likely useful in extreme cases of RPE.
Conflict of Interest Statement
The authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
References
- 1.Inoue K., Hiraoka A., Chikazawa G., Totsugawa T., Nakajima K., Masuda M., et al. Preventive strategy for reexpansion pulmonary edema after minimally invasive cardiac surgery. Ann Thorac Surg. 2020;109:e375–e377. doi: 10.1016/j.athoracsur.2019.10.073. [DOI] [PubMed] [Google Scholar]
- 2.Keyl C., Staier K., Pingpoh C., Pache G., Thoma M., Günkel L., et al. Unilateral pulmonary oedema after minimally invasive cardiac surgery via right anterolateral minithoracotomy. Eur J Cardiothorac Surg. 2015;47:1097–1102. doi: 10.1093/ejcts/ezu312. [DOI] [PubMed] [Google Scholar]
- 3.Irisawa Y., Hiraoka A., Totsugawa T., Chikazawa G., Nakajima K., Tamura K., et al. Re-expansion pulmonary oedema after minimally invasive cardiac surgery with right mini-thoracotomy. Eur J Cardiothorac Surg. 2015;49:500–505. doi: 10.1093/ejcts/ezv089. [DOI] [PubMed] [Google Scholar]
- 4.Fujita N., Miyasaka K., Okada O., Katayama M., Miyasaka K. Localized pulmonary edema in the middle and inferior lobes of the right lung after one-lung ventilation for minimally invasive mitral valve surgery. J Cardiothorac Vasc Anesth. 2015;29:1009–1012. doi: 10.1053/j.jvca.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 5.Kim H.C., Suh K.H., Lee Y.C. Severe bilateral re-expansion pulmonary edema successfully managed with extracorporeal membrane oxygenation after robot-assisted mitral valve repair surgery. J Cardiothorac Vasc Anesth. 2016;30:1038–1041. doi: 10.1053/j.jvca.2015.10.001. [DOI] [PubMed] [Google Scholar]
E-References
- Madershahian N., Wippermann J., Sindhu D., Wahlers T. Unilateral re-expansion pulmonary edema: a rare complication following one-lung ventilation for minimal invasive mitral valve reconstruction. J Card Surg. 2009;24:693–694. doi: 10.1111/j.1540-8191.2009.00813.x. [DOI] [PubMed] [Google Scholar]
- Shires A.L., Green T.M., Owen H.L., Hansen T.N., Iqbal Z., Markan S., et al. Case 4--2009. Severe reexpansion pulmonary edema after minimally invasive aortic valve replacement: management using extracorporeal membrane oxygenation. J Cardiothorac Vasc Anesth. 2009;23:549–554. doi: 10.1053/j.jvca.2009.04.009. [DOI] [PubMed] [Google Scholar]
- Kanemitsu N., Yamanaka K., Nishina T., Hirose K., Mizuno A., Nakatsuka D., et al. Re-expansion pulmonary edema after mitral valve plasty via small right thoracotomy. Jpn J Cardiovasc Surg. 2014;43:213–217. [Google Scholar]
- Hsu L.-C., Ting C.-K., Lin S.-P., Tsou M.-Y., Lin S.-M. Successful management of unilateral re-expansion pulmonary edema following one-lung ventilation for robot-assisted mitral valve repair. Resusc Intensive Care Med. 2016;1:142–146. [Google Scholar]
- Kim H.C., Suh K.H., Lee Y.C. Severe bilateral re-expansion pulmonary edema successfully managed with extracorporeal membrane oxygenation after robot-assisted mitral valve repair surgery. J Cardiothorac Vasc Anesth. 2016;30:1038–1041. doi: 10.1053/j.jvca.2015.10.001. [DOI] [PubMed] [Google Scholar]
- Kitahara H., Okamoto K., Kudo M., Yoshitake A., Hayashi K., Inaba Y., et al. Successful management of severe unilateral re-expansion pulmonary edema after mitral valve repair with mini-thoracotomy using extracorporeal membrane oxygenation. Gen Thorac Cardiovasc Surg. 2017;65:164–166. doi: 10.1007/s11748-015-0592-1. [DOI] [PubMed] [Google Scholar]
- Goyal S., Dashey S., Zlocha V., HannaJumma S. The successful use of extra-corporeal membrane oxygenation as rescue therapy for unilateral pulmonary edema following minimally invasive mitral valve surgery. Perfusion. 2020;35:356–359. doi: 10.1177/0267659119874696. [DOI] [PubMed] [Google Scholar]
- Jung E.Y., Kang H.J., Min H.K. Unilateral pulmonary edema after minimally invasive cardiac surgery: a case report. J Chest Surg. 2022;55:98–100. doi: 10.5090/jcs.21.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viox D., Dhawan R., Balkhy H.H., Cormican D., Bhatt H., Savadjian A., et al. Unilateral pulmonary edema after robotically assisted mitral valve repair requiring veno-venous extracorporeal membrane oxygenation. J Cardiothorac Vasc Anesth. 2022;36:321–331. doi: 10.1053/j.jvca.2021.03.051. [DOI] [PubMed] [Google Scholar]