Abstract
Background:
Considering that metformin is widely used in the treatment of diabetes, and its protective role against various malignancies, the strength and validity of the available evidence from related systematic reviews and meta-analysis were evaluated.
Methods:
Scopus, PubMed, Embase, Cochrane, Web of science databases, and Google Scholar and manual screening of retrieved references were systematically searched from their inception dates to 24 March 2020 by extracting the effect size (Odds ratios (OR) and relative risk (RR) in each study. To present the forest plot of effect of metformin on each cancer, Stata version 14.2 was used.
Results:
This study included 36 meta-analysis studies and 620 original research studies (26 randomized control trials studies and 594 observational studies (cohort, case–control)) covering 15 different cancers. Overall, metformin medication prevented different cancers, including ovarian cancer (OR = 0.76, 95% CI: 0.62,0.93), cervical cancer (OR = 0.60, 95% CI: 0.43, 0.83), endometrial cancer (OR = 1.05, 95% CI: 0.82,1.35), liver cancer (OR = 0.59, 95% CI: 0.47,0.74), pancreatic cancer (OR = 0.59, 95%CI 0.50,0.69), head and neck cancer (OR = 0.71, 95% CI: 0.61,0.83), stomach cancer (OR = 0.72, 95% CI: 0.26,1.99), colorectal cancer (OR = 0.73, 95% CI: 0.59,0.91), colorectal adenoma cancer (OR = 0.75, 95% CI: 0.65,0.86), colon cancer (OR = 0.79, 95% CI: 0.69,0.91), esophagus cancer (OR = 0.90, 95% CI: 0.83,0.98), lung cancer (OR = 0.92, CI95%:0.85,0.99), breast cancer (OR = 0.93, 95% CI: 0.84,1.02), prostate cancer (OR = 0.94, 95% CI: 0.85-1.04), and bladder cancer (OR = 0.94 95% CI: 0.64,1.38).
Conclusions:
Treatment with metformin can significantly decrease the chance of all cancers with larger preventive effect on hepatocellular carcinoma and smaller preventive effect on lung and breast cancers.
Keywords: Diabetes mellitus, meta-analysis, metformin, neoplasms, review
Introduction
Diabetes comprises a major component of the global burden of disease.[1] Diabetes mellitus is a risk factor for cardiovascular diseases, retinopathy, chronic kidney disease, and neuropathy and causes other adverse health effects. Findings from a number of population-based studies have also shown that diabetic patients face an increased risk of various types of malignant tumors.[2] Therefore, physicians are interested in prescribing antidiuretic drugs for diabetic patients to reduce the risk of cancer.
The role of insulin resistance has recently been proved as a risk factor for cancer in diabetes.[3] Metformin is a biguanide drug that is mainly used as first-line drug to treat type II diabetes for improving insulin resistance.[4] The effect of anti-diabetic drugs on reducing the risk of cancer has recently attracted researchers' attention. Some documents show metformin medication may reduce the incidence of cancer, progression, and even cancer-related mortality.[5]
Cancer is the second leading cause of death across both developing and developed countries, approximately 9.6 million death was recorded because of cancer worldwide in 2018.[6] The cancer-related burden is expected to rise worldwide because of aging of population.[7]
The major anti-cancer mechanism of metformin relates to its ability to activate Liver Kinase B/AMP-activated protein kinase (AMPK)/mammalian target of rapamycin (mTOR) that blocks the tumor growth because of decreased circulating insulin levels.[8] Recent studies show that metformin can reduce the risk of various cancers in diabetic population, such as thyroid cancer,[9] oral cancer,[10] gastric cancer,[11] bladder cancer,[12] prostate cancer,[13] breast cancer,[14] endometrial cancer,[15] ovarian cancer,[16] and cervical cancer.[17]
An initial meta-analysis of studies in diabetic patients showed that compared to other diabetes treatments, the metformin medication can reduce the risk of all metformin-related cancers up to 30%.[18] Systematic review and meta-analyses on the effects of the metformin medication on incidence of various cancers have been carried out. Furthermore, a published umbrella review of the systematic review and meta-analyses has been searched up to 2018. However, some systematic review and meta-analysis studies after this date are controversial,[19,20] and in some cases, studies have reviewed a new outcome, such as colorectal adenoma[21] and cervical cancers.[22] In addition, in case of finding more than one meta-analysis regarding a certain cancer, authors selected the meta-analysis that has the most number of the original article. It is possible that the meta-analysis study that we exclude has several basic studies that are not present in the largest existing meta-analysis, and we miss those studies. Therefore, for a more precise estimation, the umbrella review needs to be updated.
Methods
Protocol and registration
This is an updated umbrella review study investigating meta-analyses that have examined the relationship between metformin consumption to treat diabetes and the risk of developing cancers. Our report follows the Preferred Reporting Items for Systematic Reviews and Meta-Analyzes protocol (www.prisma-statement.org).[23] The protocol for this study has been registered at PROSPERO (ID: 124229, Date: 02-02-2019).
Eligibility criteria
The following studies were excluded from this umbrella review: studies that examined (a) the effect of combination of metformin and another antidiabetic drug, (b) the effect of metformin on the mortality rate of cancer patients, (c) the effect of metformin on cancer recurrence among patients with history of cancer, and (d) the effect of metformin on the prognosis of cancer patients.
In this study, to achieve targeted studies, eligible meta-analyzes based on cancer site were separated. If there was only one meta-analysis study for a particular cancer site, then the same study was chosen as the most comprehensive study. Whenever more than one meta-analysis study examined the relationship between metformin consumption and the risk of a particular cancer, a meta-analysis that was more up-to-date and comprehensive than other meta-analysis studies was chosen. In this case, three conditions were considered for selecting the most comprehensive and up-to-date meta-analysis. In the first step, the timeframe of meta-analysis studies to find out which meta-analysis study is more up-to-date and has covered more years was compared. Second, the number of included studies of each meta-analysis was compared. In the third step, the quality of meta-analysis studies were examined using the AMSTAR checklist If a meta-analysis was found to meet all three conditions (more comprehensive timeframe, number of more basic studies, and higher quality level), that meta-analysis was chosen. But whenever more than one eligible meta-analysis study was selected for each cancer site, the remaining studies were re-analyzed by integrating each of those meta-analyzes and eliminating overlapping (duplicate) cases and a new meta-analysis was carried out in these cases,
Search strategy
Different databases were systematically searched: Scopus, PubMed, Embase, Cochrane, Web of Science and Google Scholar from inception by 24 March 2020. Limited the search to humans and no language or time restrictions were applied. Supplementary Table S1 in the appendix shows the search strategy. The References list of the eligible reviews were also reviewed.
Table S1.
Search strategy in some databases
| Databases | Search strategy |
|---|---|
| Search strategy in Scopus | (TITLE-ABS-KEY (cancer* OR neoplasia OR tumor* OR malignan*) AND TITLE-ABS-KEY (metformin) AND TITLE-ABS-KEY (diabetes AND mellitus) AND TITLE-ABS-KEY (meta-analysis OR eta-analyses)) |
| Search strategy in PubMed | ((((((neoplasms[MeSH Terms]) OR (cancer[Title/Abstract])) OR (malignan*[Title/Abstract])) OR (tumor*[Title/Abstract])) OR (neoplasm[Title/Abstract])) AND ((meta-analysis[Title/Abstract]) OR (Meta-Analysis[Publication Type]))) AND ((metformin[Title/Abstract]) OR (metformin[MeSH Terms])) |
| Search strategy in Web of Science | TOPIC: (metformin) AND TOPIC: (cancer OR neoplasm OR neoplasia OR tumor) AND TOPIC: (meta-analysis OR meta-analyses) AND TOPIC: (diabetes mellitus) |
| Search strategy in Cochrane | (“Cancer”):ti, ab, kw AND (“metformin”):ti, ab, kw AND (“diabetes mellitus”):ti, ab, kw AND (“meta analysis”):ti, ab, kw” |
| Search strategy in Embase | ‘malignant neoplasm’:ab, ti AND metformin: ab, ti AND ‘meta analysis’:ab, ti AND ‘diabetes mellitus’:ab, ti |
Main keywords or corresponding MeSH terms were as follows: cancer, carcinoma, neoplasia, tumor, neoplasm, Meta-analysis, Meta-analyses, Systematic review, Metformin, Diabetes Mellitus, and Malignancy. A manual search was also done for references cited in the selected articles, in selected reviews, or books.
Methodological quality assessment
Using the online version of assessing the Methodological Quality of Systematic Reviews (AMSTAR) (https://amstar.ca) the systematic reviews and meta-analyses graded into three levels of quality: “high,” “moderate,” and “low.” AMSTAR is an 11-item assessment tool that has been validated and is being increasingly used by health care policy makers, health technology assessment agencies, and some authors and journal editors.[24]
Data extraction
Two investigators carried out data extraction independently and then, the extracted data were compared and discrepancies were resolved with discussion. A third investigator arbitrated on any remaining differences. For each eligible article, the first author, year of publication, study design, cancer site, number of studies (by study design), and OR/RR with its confidence interval were extracted [Table 1].
Table 1.
Summary of retrieved meta-analyses
| Reference | Name of Author | Year of study | Country | Number of Study | Sample size | Type of control group | Type of cancer | OR/RR | LOW OR/RR | UP OR/RR | P | I2 (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [18] | Andrea Decensi | 1966-2009 | UK, Italy, Scotland, Netherlands, USA, and Canada | 11 (case-control (8), cohort (3)) | 35662 | Nonmetformin users SUs Exogenous insulin other hypoglycaemic drugs users insulin-based treatment | Breast, Colon | 0.69 | 0.61 | 0.79 | ---- | 64 | |
| [25] | Zhi-Jiang Zhang | 1966-2011 | Korea, China, and UK | 5 (case-control (2), cohort (3)) | 108161 | Non metformin users sulfonylurea use NSAID/aspirin use insulin, aspirin other drug use other oral anti-hyperglycemic | Colorectal | 0.63 | 0.5 | 0.79 | <0.001 | 18 | |
| [26] | Hiroshi Noto | Until 2011 | ---- | 10 (case-control (2), cohort (6), RCT (2)) | 210892 | Non metformin users | Hepatocellular, Lung, Colorectal, Prostate, Breast, Pancreatic, Gastric, Bladder | 0.67 | 0.53 | 0.85 | <0.001 | 93 | |
| [27] | Zhi-Jiang Zhang | 1966-2012 | Italy, France, Netherlands, USA, and China | 5 (case-control (2), cohort (3)) | 105495 | Non metformin users SUs Insulin | Liver | 0.38 | 0.24 | 0.59 | <0.001 | 78 | |
| [28] | Nananda F Col | 1966-2009 | Scotland, UK, Denmark, Netherlands, and USA | 7 (case-control (3), cohort (4)) | 418541 | Other drug used for diabetes therapy SUs insulin thiazolidinediones | Breast | 0.83 | 0.71 | 0.97 | ---- | 51 | |
| [29] | Monica Franciosi | 1966-2012 | ---- | 41 Observational | 1029389 | Non metformin users SUs Thiazolidinediones insulin other drug use | Liver, colorectal, Pancreas, stomach, Oesophagus, Breast, Prostate, Lung, Ovarian | 0.73 | 0.61 | 0.88 | 0.001 | 97 | |
| [29] | Monica Franciosi | 1966-2012 | ---- | RCT (12) | 1029389 | Liver, colorectal, Pancreas, stomach, Oesophagus, Breast, Prostate, Lung, Ovarian | 0.98 | 0.81 | 1.19 | 0.832 | 0 | ||
| [30] | Bindiya Thakkar | Until 2012 | USA, Canada, Europe, Australia, China, Japan, Taiwan, Germany, Italy, Netherlands, UK, and Denmark | RCT (2) | 415014 | Non metformin users | Overall | 1.01 | 0.81 | 1.26 | ---- | 10 | |
| [30] | Bindiya Thakkar | Until 2012 | USA, Canada, Europe, Australia, China, Japan, Taiwan, Germany, Italy, Netherlands, UK, and Denmark | Cohort (9) | 415014 | Overall | 0.7 | 0.67 | 0.73 | ---- | 97 | ||
| [30] | Bindiya Thakkar | Until 2012 | USA, Canada, Europe, Australia, China, Japan, Taiwan, Germany, Italy, Netherlands, UK, and Denmark | Case-control (13) | 415014 | Overall | 0.90 | 0.84 | 0.98 | ---- | 83.20 | ||
| [31] | Zhi-Jiang Zhang | 2009-2013 | USA, UK, Netherlands, and China | 6 (case-control (2), cohort (4)) | 566435 | Non metformin users | Lung and Respiratory | 0.85 | 0.75 | 0.96 | 0.01 | 56 | |
| [32] | Zhihang Nie | Until to 2014 | USA, UK, Netherlands, Scotland, Denmark, and Taiwan | 11 (case-control (3), cohort (8)) | 321306 | non metformin users SUs Insulin | Colorectal | 0.75 | 0.66 | 0.86 | ---- | 74.70 | |
| [33] | Lifeng Li | Until Jan 2016 | -???--- | 5 Observational | ---- | N on metformin users | Ovarian | 0.54 | 0.32 | 0.93 | ---- | 85.20 | |
| [34] | T. Rokkas | Until 2015 | USA, UK, Denmark, Netherlands, Taiwan, and Korea | 17 (RCT (1), Observational studies (16 (Cohort (13), Case-control (3))) | 709980 | Non metformin users | Colon | 0.75 | 0.65 | 0.87 | <0.001 | 86 | |
| [35] | Shujuan Ma | Until July 2016 | USA, Canada, Europe, China, Japan, Italy, Netherlands, UK, Spain, France, and Turkey | 19 (RCT (2), Cohort (10), Case-control (7)) | 550882 | Non metformin users | Liver | 0.52 | 0.4 | 0.68 | ---- | 83.70 | |
| [36] | Ping Wong | Until to 2011 | UK, Italy, Greece, USA, Canada, Taiwan, and Japan | 49 (case-control (17), cohort (32)) | ---- | Non metformin users SUs Insulin | Hepatocellular | 0.31 | 0.19 | 0.49 | ---- | ---- | |
| [37] | Siddharth Singh | Until to June 2012 | UK, Netherlands, USA, Taiwan, and Australia | 11 (case-control (3), cohort (6), RCT (2)) | 730664 | SUs Thiazolidinediones Insulin | Pancreatic | 0.76 | 0.57 | 1.03 | 0.073 | 86 | |
| [38] | Siddharth Singh | Until to Sep 2012 | UK, Scotland, Netherlands, USA, and Taiwan | 15 Observational | 840787 | SUs Thiazolidinediones Insulin | Colorectal | 0.89 | 0.81 | 0.99 | <0.010 | 62 | |
| [39] | Siddharth Singh | Until to Aug 2012 | USA, Europe, Japan, Italy, Netherlands, UK, France, and Australia | 10 (case-control (3), cohort (5), RCT (2)) | 334307 | SUs Thiazolidinediones Insulin | Hepatocellular | 0.5 | 0.34 | 0.73 | - --- | ---- | |
| [40] | Hui Zhang | 1966-2011 | Italy, France, USA, China, Japan, and Taiwan | 7 (case-control (4), cohort (3)) | 16549 | Non metformin users | Hepatocellular | 0.24 | 0.13 | 0.46 | <0.001 | 66.80 | |
| [41] | Zheng Wang | 1995-2013 | UK, Netherlands, USA, Taiwan, and China | 11 Observational | 766195 | Non metformin users SUs Insulin | Pancreatic | 0.63 | 0.46 | 0.86 | 0.003 | 86 | |
| [42] | Shu-ping Nie | Until to Aug 2013 | UK, Netherlands, USA, Taiwan, and China | 15 (case-control (4), cohort (11)) | ---- | Non metformin users SUs Thiazolidinediones Insulin | Lung | 0.99 | 0.87 | 1.12 | <0.001 | 80.40 | |
| [43] | Lang Wu | -???--- | -???--- | Case-control (39) | 7600000 | Non metformin users SUs Thiazolidinediones Insulin alpha glucosidase | Overall | 0.86 | 0.83 | 0.90 | <0.001 | 88.60 | |
| [44] | Contanza Saka Herran | 2012-2017 | USA, Brazil, UK, Italy, Switzerland, Taiwan, and Korea | 13 Observational | ---- | Non metformin users anti-inflammatory drugs NSAID/aspirin use | Head and Neck Cancer | 0.71 | 0.61 | 0.84 | <0.001 | 55 | |
| [45] | Bahareh Ghiasi | Until to 2018 | --- | 11 Observational | ---- | Non metformin users | Prostate | 0.89 | 0.67 | 1.17 | <0.001 | 99.6 | |
| [46] | Grace H. Tang | Inception to Nov 2016 | --- | 12 Observational | ---- | Non metformin users | Breast | 0.93 | 0.85 | 1.03 | 0.16 | 35 | |
| [20] | Christopher B. Chen | Inception to Aug 2015 | Asia Western | 26 (case-control (9), cohort (17)) | 1572307 | Non metformin users | Prostate | 1.01 | 0.86 | 1.18 | --- | 97 | |
| [47] | Long Yao | Until September 20, 2017 | UK, Netherlands, USA, Taiwan, China, Canada, France, and Germany | 13 (case-control (3), cohort (10)) | ---- | Non metformin users SUs Insulin | Lung | 0.89 | 0.83 | 0.96 | 0.002 | 66 | |
| [48] | Zhaohan Feng | Through July 2018 (these studies were published between 2011 and 2017) | UK, Netherlands, USA, Taiwan, Canada, France, Germany, Sweden, switzerlands, and Denmark | 18 (case-control (3), cohort (15)) | ---- | Non metformin users Other antidiabetic agents NSAID/aspirin use antihypertensive, antithrombotic agents | Prostate | 0.97 | 0.80 | 1.16 | <0.001 | 98.1 | |
| [49] | MohammadMoradi-Joo | Up to June 2015 | UK-Denmark- Spain-Germany-France- USA- Switzerland- Taiwan- Netherlands | 11(case-control (1), cohort (9), RCT (1)) | ---- | SU, metformin group | Breast | 0.63 | 0.56 | 0.70 | <0.001 | 94 | |
| [50] | Feifei Liu | Aug 31, 2016 | China - UK- Netherlands- Danish- USA- Germany | 17 (16 observational and 1 randomized controlled trial study) | --- | Non Metformin group | Colorectal | 0.73 | 0.62 | 0.86 | <0.0002 | ||
| [22] | Wen, Q | Last search was performed on August 15, 2018 | Asian and Caucasian | 7 (ovarian (4), cervical (2), endometrial (6)) | 1710080 | Non metformin users | Gynecological (ovarian, cervical, endometrial | 0.49 | 0.29 | 0.82 | 0.006 | 98 | |
| [21] | Deng, M | Jan 13, 2019 | Asian and non-Asian | 50 (Case-control (14), cohort (34), RCT (2)) | 238540 | Non metformin users or users of other antidiabetic agents | Colorectal adenoma, colorectal | Colorectal adenoma | 0.75 | 0.65 | 0.86 | 0.308 | 13.6 |
| colorectal | 0.73 | 0.58 | 0.90 | <0.001 | 90.4 | ||||||||
| [51] | Tain, J | Through Oct 2017 | USA, China, Europe, and England | 6(Case-control (4), cohort (2)) | 510344 | SUs, insulin | Endometrial | 1.29 | 1.16 | 1.44 | <0.001 | 8 | |
| [52] | Mekuria, AN | Until Dec 2018 | UK, Taiwan, Netherlands, Germany, and USA | 8 | 520106 | SUs | Overall | 0.76 | 0.54 | 1.07 | <0.001 | 98.12 | |
| [53] | Chu, D | Between 1980 and July 2016 | USA, UK, China, Finland, Poland, Italy, and Australia | 7 (2 case-control, 4 retrospective studies, one prospective study) | --- | Non-metformin users | Endometrial | 1.05 | 0.82 | 1.35 | 0.70 | 90.9 | |
| [54] | Hu, H | Until September 2016 | UK, Taiwan, Netherlands, and USA | 9(Case-control (2), cohort (7)) | 534699 | Other antidiabetic drugs (SUs, thiazolidinediones, or insulin) | Pancreatic | 0.61 | 0.55 | 0.67 | <0.001 | 31 | |
| [55] | Shi, J | Up to August 2018 | USA, UK, Germany, Finland, China, Canada, and Israel | 6 (Observational (5), RCT (1)) | --- | Non-metformin users or other hypoglycemic drug users | Ovarian | 0.76 | 0.62 | 0.93 | 0.008 | 32.2 | |
| [56] | Chai, S | Inception to 23 June 2017 | Network meta-analysis | 84 RCT | 101595 | Incretin-based drugs with placebo or other antidiabetic drugs | Overall | 0.32 | 0.07 | 1.38 | --- | --- | |
SUs=Sulfonylureas, RCT=randomized control trials
Statistical analysis
To evaluate the effect of metformin on the risk of cancer, the odds ratio (OR) and Relative Risk (RR) were used. To present the forest plot for the effect of metformin for each cancer, Stata version 14.2 (Stata Corp, College Station, Texas) was used.
Results
Description of meta-analyses
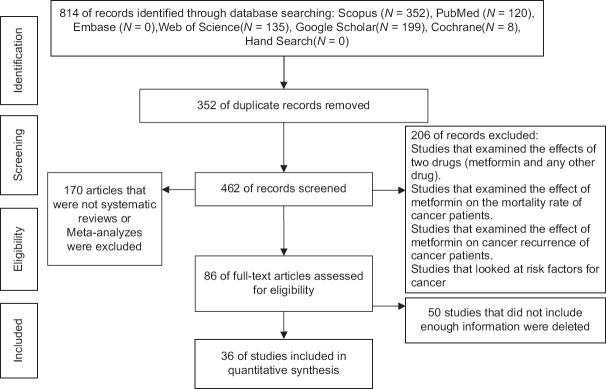
Using the search strategy outlined in the Materials and Methods Section, a total number of 814 articles were found in the reviewed databases. According to inclusion and exclusion criteria, 36 meta-analysis articles remained eligible that included a total of 620 articles (26 randomized control trial – RCT studies and 594 observational studies (cohort, case–control)) [Figure 1]. The largest sample size belonged to a meta-analysis by Lang Wu et al,[43] (sample size = 7,600,000, number of study = 265) and the smallest meta-analysis carried out by Hui Zhang et al.[40] (sample size = 16,549, number of study = 7) [Table 1].
Figure 1.
Diagram of selection of studies for inclusion in umbrella review
In all studies, taking metformin prevents the development of cancers (RR <1, OR <1) with the exception of four studies [Table 1].[20,30,51,53] Thakkar et al.[30] reviewed clinical trials (RCT) investigating the association between metformin consumption and cancers and concluded that metformin consumption increased the risk of cancer (RR >1); however, the result was not statistically significant. On the other hand, the same article had reported the protective effect of metformin consumption in cohort, case–control studies (RR <1), indicating that metformin consumption prevented the risk of cancer. Christopher B. Chen et al.[20] investigated the relationship between prostate cancer and metformin consumption in 26 included studies with no statistically and clinically important effect. Overall, in this study, out of all the reviewed articles, only 12 articles[20,29,30,37,42,45,46,48,51,52,53,56] indicated that metformin consumption had no statistically significant effect on cancer risk (with 77.6 of heterogeneity), but the effect of metformin consumption on cancer prevention was significant in other meta-analyses [Table 1].
In this umbrella review study, some studies focused on one specific type of cancer.[33,42,45] Others have investigated the association between metformin consumption and the risk of several different cancers that were reported by type of cancer.[26,29] To eliminate overlap between studies, the remaining 36 meta-analyses were separated by site of cancer. Of all reported meta-analyses on different sites of cancers, the most comprehensive and updated meta-analysis were retained. Finally, there were 15 different site of cancer [Table 2 and Figure 2].
Table 2.
The effect of metformin on the risk of cancers by type of cancer
| Type of cancer | Authors name | Number of study | Year of study | RR/OR | lower RR/OR | upper RR/OR | Study selection | AMSTAR score |
|---|---|---|---|---|---|---|---|---|
| Hepatocellular/Liver | Siddharth Singh | 10 | Update to Aug 2012 | 0.50 | 0.34 | 0.73 | Removed | --- |
| Hui Zhang | 7 | 1966-2011 | 0.24 | 0.13 | 0.46 | Removed | --- | |
| Ping Wong | 49 | Update to 2011 | 0.31 | 0.19 | 0.49 | Selected | Moderate | |
| Hiroshi Noto | 4 | Until 2011 | 0.20 | 0.07 | 0.59 | Removed | ||
| Shujuan Ma | 19 | Until July 2016 | 0.52 | 0.40 | 0.68 | Selected | Moderate | |
| Zhi-Jiang Zhang | 5 | 1966-2012 | 0.38 | 0.24 | 0.59 | Removed | --- | |
| Monica Franciosi | 8 | 1966-2012 | 0.34 | 0.19 | 0.60 | Removed | --- | |
| New meta-analysis (Ping Wong, Shujuan Ma) | 67 | Up to 2017 | 0.59 | 0.46 | 0.72 | Selected | --- | |
| Ovarian | Shi, J | 6 | Up to August 2018 | 0.76 | 0.62 | 0.93 | Selected | High |
| Wen, Q | 4 | Last search was performed on August 15, 2018 | 0.18 | 0.12 | 0.28 | Removed | --- | |
| Lifeng Li | 5 | Until Jan 2016 | 0.54 | 0.32 | 0.93 | Selected | --- | |
| Pancreatic | Hu, H | 9 | Until September 2016 | 0.61 | 0.55 | 0.67 | Selected | Moderate |
| Zheng Wang | 11 | 1995-2013 | 0.63 | 0.46 | 0.86 | Selected | Moderate | |
| Siddharth Singh | 11 | Update to June 2012 | 0.76 | 0.57 | 1.03 | Removed | --- | |
| Monica Franciosi | 9 | 1966-2012 | 0.56 | 0.36 | 0.86 | Removed | --- | |
| Hiroshi Noto | 6 | Until 2011 | 0.48 | 0.20 | 1.17 | Removed | --- | |
| New meta-analysis (Zheng Wang, Hu, H) | 13 | Up to 2016 | 0.59 | 0.50 | 0.69 | Selected | --- | |
| Head and Neck | Contanza Saka Herran | 13 | 2012-2017 | 0.71 | 0.61 | 0.84 | Selected | Moderate |
| Colorectal adenoma | Deng, M | 13 | Jan 13, 2019 | 0.75 | 0.65 | 0.86 | Selected | Moderate |
| Feifei Liu | 5 | Aug 31, 2016 | 0.80 | 0.71 | 0.90 | Removed | --- | |
| Colorectal | Deng, M | 14 | Jan 13, 2019 | 0.73 | 0.58 | 0.90 | Selected | Moderate |
| Feifei Liu | 12 | Aug 31, 2016 | 0.80 | 0.72 | 0.89 | Removed | --- | |
| Zhihang Nie | 11 | Update to 2014 | 0.75 | 0.66 | 0.86 | Removed | --- | |
| Hiroshi Noto | 6 | Until 2011 | 0.68 | 0.53 | 0.88 | Removed | --- | |
| Monica Franciosi | 12 | 1966-2012 | 0.83 | 0.74 | 0.92 | Removed | --- | |
| Zhi-Jiang Zhang | 5 | 1966-2011 | 0.63 | 0.47 | 0.84 | Removed | --- | |
| Siddharth Singh | 15 | Update to Sep 2012 | 0.89 | 0.81 | 0.99 | Removed | --- | |
| Colon | T. Rokkas | 17 | Until 2015 | 0.79 | 0.69 | 0.91 | Selected | High |
| Andrea Decensi | 11 | 1966-2009 | 0.64 | 0.38 | 1.08 | Removed | --- | |
| Stomach/ Gastric | Monica Franciosi | 2 | 1966-2012 | 0.83 | 0.76 | 0.91 | Removed | --- |
| Hiroshi Noto | 3 | Until 2011 | 0.72 | 0.26 | 1.98 | Selected | High | |
| Prostate | Zhaohan Feng | 18 | These studies were done between 2011 and 2017 | 0.97 | 0.80 | 1.16 | Selected | Moderate |
| Bahareh Ghiasi | 11 | 2009-2017 | 0.89 | 0.67 | 1.17 | Removed | --- | |
| Christopher B. Chen | 26 | Inception to Aug 2015 | 1.01 | 0.86 | 1.18 | Selected | High | |
| Hiroshi Noto | 7 | Until 2011 | 0.89 | 0.66 | 1.19 | Removed | --- | |
| New meta-analysis of (Christopher B. Chen, Zhaohan Feng) | 30 | Up to 2018 | 0.94 | 0.85 | 1.04 | Selected | --- | |
| Lung | Long Yao | 13 | Until September 20, 2017 | 0.89 | 0.83 | 0.96 | Selected | Moderate |
| Shu-ping Nie | 15 | Update to Aug 2013 | 0.99 | 0.87 | 1.12 | Selected | Moderate | |
| Zhi-Jiang Zhang | 6 | 2009-2013 | 0.71 | 0.55 | 0.95 | Removed | --- | |
| Hiroshi Noto | 3 | Until 2011 | 0.67 | 0.45 | 0.99 | Removed | --- | |
| Monica Franciosi | 4 | 1966-2012 | 0.83 | 0.64 | 1.06 | Removed | --- | |
| New meta-analysis of (Long Yao, Shu-ping Nie) | 20 | Up to 2018 | 0.92 | 0.85 | 0.99 | Selected | --- | |
| Breast | Grace H. Tang | 12 | Inception to Nov 2016 | 0.93 | 0.85 | 1.03 | Selected | High |
| MohammadMoradi-Joo | 11 | Up to June 2015 | 0.63 | 0.56 | 0.70 | Removed | --- | |
| Hiroshi Noto | 7 | Until 2011 | 0.98 | 0.80 | 1.20 | Removed | --- | |
| Andrea Decensi | 11 | 1966-2009 | 0.70 | 0.28 | 1.77 | Removed | --- | |
| Nananda F Col | 7 | 1966-2009 | 0.83 | 0.71 | 0.97 | Removed | --- | |
| Monica Franciosi.ob | 9 | 1966-2012 | 0.97 | 0.88 | 1.08 | Removed | --- | |
| Esophagus | Monica Franciosi | 2 | 1966-2012 | 0.90 | 0.83 | 0.98 | Selected | Moderate |
| Bladder | Hiroshi Noto | 3 | Until 2011 | 0.94 | 0.64 | 1.38 | Selected | High |
| Cervical | Wen, Q | 2 | Last search was performed on August 15, 2018 | 0.60 | 0.43 | 0.83 | Selected | High |
| Endometrial | Wen, Q | 6 | Last search was performed on August 15, 2018 | 0.71 | 0.29 | 1.74 | Removed | --- |
| Tain, J | 6 | Through Oct 2017 | 1.29 | 1.16 | 1.44 | Removed | --- | |
| Chu, D | 7 | Between 1980 and July 2016 | 1.05 | 0.82 | 1.35 | Selected | High | |
| All Cancer | Bindiya Thakkar | RCT (2) | Until 2012 | 1.01 | 0.81 | 1.26 | Removed | --- |
| Cohort (9) | 0.7 | 0.67 | 0.73 | Removed | --- | |||
| Case-control (13) | 0.90 | 0.84 | 0.98 | Removed | --- | |||
| Lang Wu | 39 | --- | 0.86 | 0.83 | 0.9 | Removed | --- | |
| Mekuria, AN | 8 | Until Dec 2018 | 0.76 | 0.54 | 1.07 | Removed | --- | |
| Chai, S | 84 | Inception to 23 June 2017 | 0.32 | 0.07 | 1.38 | Removed | --- |
RCT=randomized control trial
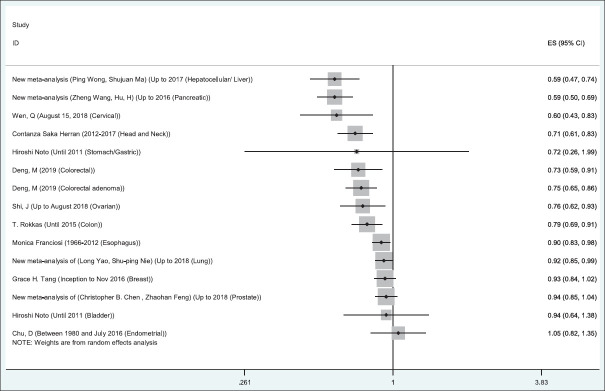
Figure 2.
Relationship between metformin use and the risk of cancer worldwide. The midpoint of each segment estimates the odds ratio and length of the segment, showing the 95% confidence interval in each study
Diabetic patients who received metformin medication had a lower risk for liver cancer, pancreatic cancer, cervical cancer, head and neck cancer, colorectal cancer, colorectal adenoma cancer, colon cancer, ovarian cancer, esophageal cancer, and lung cancer. Furthermore, a protective role also (but not significant) between metformin medication and incidence of prostate cancer, bladder cancer, gastric cancer, and breast cancer was observed. The largest protective effect of metformin was related to liver and pancreatic cancers and the least to lung cancer. However, metformin was a risk factor for incidence of endometrial cancer. Some of the meta-analyses evaluated all cancer incidence. They also revealed a protective role of the metformin [Table 2].
Discussion
This umbrella review showed that diabetic patients who received metformin treatment had a lower risk of cancer compared to diabetic patients who did not use metformin, with a non-significant effect on endometrial cancer. Metformin as an AMPK inhibitor exerts its anticancer effect by activating mTOR pathway. Metformin inhibits cancer cell mitosis by inducing activation of the activated protein kinase-adenosine monophosphate and consequently reducing growth factor signaling. Inhibition of GTPase and microRNA222 suppression induced by metformin administration leads to increased levels of p27 and p57 molecules and consequently disrupts cell cycle in tumor cells. Other possible mechanisms underlying the metformin potential anti-neoplasm effect could be the following: antagonizing effect on obesity or via the reduction of inflammation,[57] p-53 activation,[58] down regulation of cyclin D1,[59] and killing of cancer stem cells.[60]
Metformin consumption plays a protective role on cancer incidence, although it was not statistically significant in some meta-analyzes.[18,26] Previous studies have shown that metformin at lower doses can block HER2 activity. In addition, metformin can prevent drug resistance to targeted HER2 chemotherapy with drugs such as trastuzumab and lapatinib. Therefore, treatment with both metformin and HER2 may have a synergistic effect. These results confirmed that the risk of invasive breast cancer in metformin-treated diabetics is lower than in recipients of other antidiabetic drugs. There was also a significant effect of metformin treatment on reducing risk of both ovarian and cervical cancer, supported by high quality meta-analyses according to AMSTAR 2.
So far, two meta-analyses have been conducted to investigate the relationship between metformin consumption and colon cancer risk, both of them showed a protective effect on colon cancer, which was statistically significant in study by Rokkas et al.[34] [RR: 0.79 (95% CI: 0.69–0.91)]. Such protective effect of metformin consumption was not statistically significant in the study by Decensi et al.[18] Clinical and laboratory studies have shown that metformin inhibits cell growth in colorectal cancer. Results of a meta-analysis reviewing five studies (total sample size = 108,161 diabetic patients) showed that metformin significantly reduces the risk of colorectal cancer. This study reported that metformin reduced the relative risk of colorectal cancer by 39%. The same meta-analysis examined the effects of insulin and thiazolidinediones, both of them were shown to be unable to reduce the mortality rate of colorectal cancer.[25]
The results of six studies that examined the relationship between metformin and colorectal cancer showed that metformin consumption reduced the risk of colorectal cancer and this relationship was statistically significant [Table 2]. Metformin consumption had no effect on the risk of colorectal cancer in the meta-analysis performed on RCT studies by Franciosi et al.[29] [RR: 1.02 (95% CI: 0.41-2.50]. In contrast, analysis performed on observational studies had a preventive effect.
All five meta-analyses on the association between metformin consumption and prostate cancer showed no statistically significant association. However, three studies by Noto et al.,[26] Ghiasi et al.,[45] and Feng et al.[48] indicated its protective role. Low sex hormone-binding globulin levels may facilitate conversion of testosterone to estradiol, which in turn may increase the risk of hormone-dependent breast cancer. The duration of metformin treatment in diabetic patients was associated with a decrease in mortality from prostate cancer.[61]
Nie et al.[42] reported that metformin consumption had no effect on the risk of lung cancer (OR= 0.99 (95% CI: 0.87–1.12)). However, Zhang et al. showed metformin consumption reduced significantly the relative risk of lung cancer (RR= 0.71 (95% CI: 0.55–0.95)).[31] Noto et al.[26] also found that metformin consumption significantly reduced the risk of lung cancer (RR = 0.67, 95% CI: 0.45–0.99).
Studies have shown the protective role of metformin consumption against pancreatic cancer, although the effect was not statistically significant in some studies.[26,29,37,41] Metformin probably reduces inflammation and fibrosis, which is the most common cause of pancreatic cancer. Findings of cellular and animal models as well as in tumor specimens suggest that this positive effect may be observed in obese or overweight patients more frequently.[62]
In fact, metformin can reduce desmoplasia, an accumulation of dense connective tissue, and tumor-associated immune cells, and a key feature of pancreatic cancer. This function is accomplished by inhibiting the activity of pancreatic stellate cell (PaSCs). PaSCs produce extracellular matrix and reprogram immune cells to reduce inflammation. These effects are only visible in tumors found in obese and overweight people, as these tumors seem to be more fibrous in nature.
Review of previous studies showed that four meta-analyses were performed on the association between metformin consumption and the risk of hepatocellular cancer. All studies revealed that metformin consumption had a preventive effect on hepatocellular cancer and this relationship was statistically significant (Siddharth Singh,[38] RR = 0.50 (95% CI: 0.34–0.73), Hiroshi Noto[26] (RR = 0.20 (95% CI: 0.07–0.59), Ping Wang[36] (RR = 0.31 (95% CI: 0.19–0.49), and Hui Zhang[40] (RR = 0.24 (95% CI: 0.13–0.46).
Metformin not only inhibited proliferation and colony formation ability via (AMPK) in hepatocellular carcinoma cell[63] but also as an anti-hyperglycemic agent, it inhibited hepatic gluconeogenesis,[64] decreased serum concentrations of insulin and insulin growth factor,[65] improved the HbA1c levels, and reduced inflammatory response.[66] This process reduces the aggressive behavior of cancer cells. All previous relevant studies have shown that metformin consumption can prevent hepatocellular cancer.
Only three associations (between metformin and colon, ovarian, and cervical cancer) were supported by both high quality and statistically significant relationship. Patients who received metformin treatment have odds of 0.21, 0.24, and 0.40 to develop colon, ovarian, and cervical cancer, respectively.
Some of the reviews showed nonsignificant protective effect with a moderate to high quality. The possible reasons for the statistically insignificant results can be different, including the inadequate sample size and the study designs of included meta-analyses.
This study is an update of previous meta-analyses and umbrella reviews covering most common cancer sites. Because most included studies did not report the relative risk of cancers on consumption of metformin by study type (cohort, clinical trial, case–control, etc.), this report failed to perform analyses stratified by study type. In addition, some meta-analysis studies were based on medical or insurance data that are not specifically designed to evaluate the impact of metformin therapy on cancer. There were incomplete details on dose, duration, changes occurring in treatment over time, and potential confounders.
In fact, considerable heterogeneity among included studies in terms of population of the studies, diversity of the disease duration, type of cancer, and study design did not allow to pool the data for estimating an effect size. Unadjusted measures and some possible confounding factors in the original studies may have rendered the results of this study less valid. Overestimation of the effect of metformin may have occurred. In some studies, the characteristics of comparison group has been defined as “Non-metformin consumer,” which in turn may have received other glucose lowering drugs with synergistic effect with other medications, affecting the likelihood of cancer. The most commonly used drugs are insulin and sulfonylurea, which are associated with hyperinsulinemia which is associated with an increased risk of cancer. Therefore, hyperinsulinemia in comparison groups might overestimate the effect of metformin. On the other hand, the synergistic effect of metformin with some common medications in diabetic patients may have led to an overestimation of the effect of metformin on cancer.[67] Confounding by treatment indication such as using metformin medication in younger age with a lower risk of cancer also might overestimate the effect of metformin.
Conclusions
Metformin therapy in diabetic patients may be a reasonable prescription for the prevention of cancers if it has not been clinically contraindicated. Such effect was higher in hepatocellular carcinoma and lower in lung and breast cancers; however, it had no significant effect on some cancers, including prostate cancer, bladder cancer, endometrial cancer, gastric cancer, and breast cancer.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Khazaie H, Najafi F, Hamzeh B, Chehri A, Rahimi-Movaghar A, Amin-Esmaeili M, et al. Cluster analysis of psychiatric profile, its correlates, and using mental health services among the young people aged 15–34: Findings from thefirst phase of Iranian youth cohort in Ravansar. Soc Psychiatry Psychiatr Epidemiol. 2018;53:1339–48. doi: 10.1007/s00127-018-1580-4. [DOI] [PubMed] [Google Scholar]
- 2.Silidis KK, Kasimis JC, Lopez DS, Ntzani EE, Ioannidis JP. Type 2 diabetes andcancer: Umbrella review of meta-analyses of observational studies. BMJ. 2015;350:g7606. doi: 10.1136/bmj.g7607. doi: 10.1136/bmj.g7607. [DOI] [PubMed] [Google Scholar]
- 3.Sun W, Lu J, Wu S, Bi Y, Mu Y, Zhao J, et al. Association of insulin resistance with breast, ovarian, endometrial and cervical cancers in non-diabetic women. Am J Cancer Res. 2016;6:2334–44. [PMC free article] [PubMed] [Google Scholar]
- 4.Macaskill P, Walter SD, Irwig L. A comparison of methods to detect publication bias in meta-analysis. Stat Med. 2001;20:641–54. doi: 10.1002/sim.698. [DOI] [PubMed] [Google Scholar]
- 5.Evans JM, Donnelly LA, Emslie AM-Smith, Alessi DR, Morris AD. Metformin and reduced risk of cancer in diabetic patients. BMJ. 2005;330:1304–5. doi: 10.1136/bmj.38415.708634.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cho SM, Lee SG, Kim HS, Kim JH. Establishing pediatric reference intervals for 13 biochemical analytes derived from normal subjects in a pediatric endocrinology clinic in Korea. Clin Biochem. 2014;47:268–71. doi: 10.1016/j.clinbiochem.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 7.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 8.Sahra IB, Le Marchand-Brustel Y, Tanti JF, Bost F. Metformin reduces thyroid cancer risk in Taiwanese patients with type 2 diabetes. PLoS One. 2014;9:e109852. doi: 10.1371/journal.pone.0109852. doi: 10.1371/journal.pone. 0109852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tseng CH. Metformin reduces thyroid cancer risk in Taiwanese patients with type 2 diabetes. PLoS One. 2014;9:e109852. doi: 10.1371/journal.pone.0109852. doi: 10.1371/journal.pone. 0109852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tseng CH. Metformin may reduce oral cancer risk in patients with type 2 diabetes. Oncotarget. 2016;7:2000–8. doi: 10.18632/oncotarget.6626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tseng CH. Metformin reduces gastric cancer risk in patients with type 2 diabetes mellitus. Aging (Albany NY) 2016;8:1636–49. doi: 10.18632/aging.101019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tseng CH. Metformin may reduce bladder cancer risk in Taiwanese patients with type 2 diabetes. Acta Diabetol. 2014;51:295–303. doi: 10.1007/s00592-014-0562-6. [DOI] [PubMed] [Google Scholar]
- 13.Tseng CH. Metformin significantly reduces incident prostate cancer risk in Taiwanese men with type 2 diabetes mellitus. Eur J Cancer. 2014;50:2831–7. doi: 10.1016/j.ejca.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 14.Tseng CH. Metformin may reduce breast cancer risk in Taiwanese women with type 2 diabetes. Breast Cancer Res Treat. 2014;145:785–90. doi: 10.1007/s10549-014-2985-8. [DOI] [PubMed] [Google Scholar]
- 15.Tseng CH. Metformin and endometrial cancer risk in Chinese women with type 2 diabetes mellitus in Taiwan. Gynecol Oncol. 2015;138:147–53. doi: 10.1016/j.ygyno.2015.03.059. [DOI] [PubMed] [Google Scholar]
- 16.Tseng CH. Metformin reduces ovarian cancer risk in Taiwanese women with type 2 diabetes mellitus. Diabetes Metab Res Rev. 2015;31:619–26. doi: 10.1002/dmrr.2649. [DOI] [PubMed] [Google Scholar]
- 17.Tseng CH. Metformin use and cervical cancer risk in female patients with type 2 diabetes. Oncotarget. 2016;7:59548–55. doi: 10.18632/oncotarget.10934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Decensi A, Puntoni M, Goodwin P, Cazzaniga M, Gennari A, Bonanni B, et al. Metformin and cancer risk in diabetic patients: A systematic review and meta-analysis. Cancer Prev Res (Phila) 2010;3:1451–61. doi: 10.1158/1940-6207.CAPR-10-0157. [DOI] [PubMed] [Google Scholar]
- 19.Shuai Y, Li C, Zhou X. The effect of metformin on gastric cancer in patients with type 2 diabetes: A systematic review and meta-analysis. Clin Transl Oncol. 2020;22:1580–90. doi: 10.1007/s12094-020-02304-y. [DOI] [PubMed] [Google Scholar]
- 20.Chen CB, Eskin M, Eurich DT, Majumdar SR, Johnson JA. Metformin, Asian ethnicity and risk of prostate cancer in type 2 diabetes: A systematic review and meta-analysis. BMC Cancer. 2018;18:65. doi: 10.1186/s12885-017-3934-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deng M, Lei S, Huang D, Wang H, Xia S, Xu E, et al. Suppressive effects of metformin on colorectal adenoma incidence and malignant progression. Pathol Res Pract. 2020;216:152775. doi: 10.1016/j.prp.2019.152775. doi: 10.1016/j.prp. 2019.152775. [DOI] [PubMed] [Google Scholar]
- 22.Wen Q, Zhao Z, Wen J, Zhou J, Wu J, Lei S, et al. The association between metformin therapy and risk of gynecological cancer in patients: Two meta-analyses. Eur J Obstet Gynecol Reprod Biol. 2019;237:33–41. doi: 10.1016/j.ejogrb.2019.03.029. [DOI] [PubMed] [Google Scholar]
- 23.Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLOS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. doi: 10.1371/journal.pmed. 1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shea BJ, Grimshaw JM, Wells GA, Boers M, Andersson N, Hamel C, et al. Development of AMSTAR: A measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol. 2007;7:10. doi: 10.1186/1471-2288-7-10. doi: 10.1186/1471-2288-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang ZJ, Zheng ZJ, Kan H, Song Y, Cui W, Zhao G, et al. Reduced risk of colorectal cancer with metformin therapy in patients with type 2 diabetes: A meta-analysis. Diabetes Care. 2011;34:2323–8. doi: 10.2337/dc11-0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Noto H, Goto A, Tsujimoto T, Noda M. Cancer risk in diabetic patients treated with metformin: A systematic review and meta-analysis. PLoS One. 2012;7:e33411. doi: 10.1371/journal.pone.0033411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang ZJ, Zheng ZJ, Shi R, Su Q, Jiang Q, Kip KE. Metformin for liver cancer prevention in patients with type 2 diabetes: A systematic review and meta-analysis. J Clin Endocrinol Metab. 2012;97:2347–53. doi: 10.1210/jc.2012-1267. [DOI] [PubMed] [Google Scholar]
- 28.Col NF, Ochs L, Springmann V, Aragaki AK, Chlebowski RT. Metformin and breast cancer risk: A meta-analysis and critical literature review. Breast Cancer Res Treat. 2010;135:639–46. doi: 10.1007/s10549-012-2170-x. [DOI] [PubMed] [Google Scholar]
- 29.Franciosi M, Lucisano G, Lapice E, Strippoli GF, Pellegrini F, Nicolucci A. Metformin therapy and risk of cancer in patients with type 2 diabetes: Systematic review. PLoS One. 2013;8:e71583. doi: 10.1371/journal.pone.0071583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thakkar B, Aronis KN, Vamvini MT, Shields K, Mantzoros CS. Metformin and sulfonylureas in relation to cancer risk in type II diabetes patients: A meta-analysis using primary data of published studies. Metabolism. 2013;62:922–34. doi: 10.1016/j.metabol.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 31.Zhang ZJ, Bi Y, Li S, Zhang Q, Zhao G, Guo Y, et al. Reduced risk of lung cancer with metformin therapy in diabetic patients: A systematic review and meta-analysis. Am J Epidemiol. 2014;180:11–4. doi: 10.1093/aje/kwu124. [DOI] [PubMed] [Google Scholar]
- 32.Nie Z, Zhu H, Gu M. Reduced colorectal cancer incidence in type 2 diabetic patients treated with metformin: A meta-analysis. Pharm Biol. 2016;54:2636–42. doi: 10.1080/13880209.2016.1176057. [DOI] [PubMed] [Google Scholar]
- 33.Li L, Qi X, Xu M, Ding X, Zhou X, Zhang C, et al. The effects of metformin on ovarian cancer: An updated systematic review and meta-analysis. Int J Clin Exp Med. 2016;9:17559–68. [Google Scholar]
- 34.Rokkas T, Portincasa P. Colon neoplasia in patients with type 2 diabetes on metformin: A meta-analysis. Eur J Intern Med. 2016;33:60–6. doi: 10.1016/j.ejim.2016.05.027. [DOI] [PubMed] [Google Scholar]
- 35.Ma S, Zheng Y, Xiao Y, Zhou P, Tan H. Meta-analysis of studies using metformin as a reducer for liver cancer risk in diabetic patients. Medicine (Baltimore) 2017;96:e6888. doi: 10.1097/MD.0000000000006888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang P, Kang D, Cao W, Wang Y, Liu Z. Diabetes mellitus and risk of hepatocellular carcinoma: A systematic review and meta-analysis. Diabetes Metab Res Rev. 2012;28:109–22. doi: 10.1002/dmrr.1291. [DOI] [PubMed] [Google Scholar]
- 37.Singh S, Singh PP, Singh AG, Murad MH, McWilliams RR, Chari ST. Anti-diabetic medications and risk of pancreatic cancer in patients with diabetes mellitus: A systematic review and meta-analysis. Am J Gastroenterol. 2013;108:510–9. doi: 10.1038/ajg.2013.7. [DOI] [PubMed] [Google Scholar]
- 38.Singh S, Singh H, Singh PP, Murad MH, Limburg PJ. Antidiabetic medications and the risk of colorectal cancer in patients with diabetes mellitus: A systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2013;22:2258–68. doi: 10.1158/1055-9965.EPI-13-0429. [DOI] [PubMed] [Google Scholar]
- 39.Singh S, Singh PP, Singh AG, Murad MH, Sanchez W. Anti-diabetic medications and the risk of hepatocellular cancer: A systematic review and meta-analysis. Am J Gastroenterol. 2013;108:881–91. doi: 10.1038/ajg.2013.5. [DOI] [PubMed] [Google Scholar]
- 40.Zhang H, Gao C, Fang L, Zhao HC, Yao SK. Metformin and reduced risk of hepatocellular carcinoma in diabetic patients: A meta-analysis. Scand J Gastroenterol. 2013;48:78–87. doi: 10.3109/00365521.2012.719926. [DOI] [PubMed] [Google Scholar]
- 41.Wang Z, Lai ST, Xie L, Zhao JD, Ma NY, Zhu J, et al. Metformin is associated with reduced risk of pancreatic cancer in patients with type 2 diabetes mellitus: A systematic review and meta-analysis. Diabetes Res Clin Pract. 2014;106:19–26. doi: 10.1016/j.diabres.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 42.Nie SP, Chen H, Zhuang MQ, Lu M. Anti-diabetic medications do not influence risk of lung cancer in patients with diabetes mellitus: A systematic review and meta-analysis. Asian Pac J Cancer Prev. 2014;15:6863–9. doi: 10.7314/apjcp.2014.15.16.6863. [DOI] [PubMed] [Google Scholar]
- 43.Wu L, Zhu J, Prokop LJ, Murad MH. Pharmacologic therapy of diabetes and overall cancer risk and mortality: A meta-analysis of 265 studies. Sci Rep. 2015;5:10147. doi: 10.1038/srep10147. doi: 10.1038/srep10147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Herrán SC, Jané-Salas E, Estrugo Devesa A, López-López J. Protective effects of metformin, statins and anti-inflammatory drugs on head and neck cancer: A systematic review. Oral Oncol. 2018;85:68–81. doi: 10.1016/j.oraloncology.2018.08.015. [DOI] [PubMed] [Google Scholar]
- 45.Ghiasi B, Sarokhani D, Najafi F, Motedayen M, Dehkordi AH. The relationship between prostate cancer and metformin consumption: A systematic review and meta-analysis study. Curr Pharm Des. 2019;25:1021–9. doi: 10.2174/1381612825666190215123759. [DOI] [PubMed] [Google Scholar]
- 46.Tang GH, Satkunam M, Pond GR, Steinberg GR, Blandino G, Schünemann HJ, et al. Association of metformin with breast cancer incidence and mortality in patients with type II diabetes: A GRADE-assessed systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2018;27:627–35. doi: 10.1158/1055-9965.EPI-17-0936. [DOI] [PubMed] [Google Scholar]
- 47.Yao L, Liu M, Huang Y, Wu K, Huang X, Zhao Y, et al. Metformin use and lung cancer risk in diabetic patients: A systematic review and meta-analysis. Dis Markers. 2019;2019:6230162. doi: 10.1155/2019/6230162. doi: 10.1155/2019/6230162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Feng Z, Zhou X, Liu N, Wang J, Chen X, Xu X. Metformin use and prostate cancer risk: A meta-analysis of cohort studies. Medicine. 2019;98:e14955. doi: 10.1097/MD.0000000000014955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moradi-Joo M, Mohabbat-Bahar S, Heidari S, Davoodi SH, GarehSheyklo S, Akbari ME. Metforminversus sulfonylureain breast cancer risk of diabetic patients: A systematic review and meta-analysis. Iran J Cancer Prev. 2016;9:e5971. doi: 10.17795/ijcp-5971. [Google Scholar]
- 50.Liu F, Yan L, Wang Z, Lu Y, Chu Y, Li X, et al. Metformin therapy and risk of colorectal adenomas and colorectal cancer in type 2 diabetes mellitus patients: A systematic review and meta-analysis. Oncotarget. 2017;8:16017–26. doi: 10.18632/oncotarget.13762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tian J, Liang Y, Qu P. Antidiabetic medications and the risk of endometrial cancer in patients. Gynecol Invest. 2019;84:455–62. doi: 10.1159/000497202. [DOI] [PubMed] [Google Scholar]
- 52.Mekuria AN, Ayele Y, Tola A, Mishore KM. Monotherapy with metformin versus sulfonylureas and risk of cancer in type 2 diabetic patients: A systematic review and meta-analysis. J Diabetes Res. 2019;2019:7676909. doi: 10.1155/2019/7676909. doi: 10.1155/2019/7676909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chu D, Wu J, Wang K, Zhao M, Wang C, Li L, et al. Effect of metformin use on the risk and prognosis of endometrial cancer: A systematic review and meta-analysis. BMC Cancer. 2018;18:438. doi: 10.1186/s12885-018-4334-5. doi: 10.1186/s12885-018-4334-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hu H, Fang Y, Zhou X, Gong L, Liu L, Wang W, et al. Relationship of metformin with the risk of pancreatic cancer in patients with type 2 diabetes: A meta-analysis. Biomed Res. 2017;28:4439. [Google Scholar]
- 55.Shi J, Liu B, Wang H, Zhang T, Yang L. Association of metformin use with ovarian cancer incidence and prognosis: A systematic review and meta-analysis. Int J Gynecol Cancer. 2019;29:140–6. doi: 10.1136/ijgc-2018-000060. [DOI] [PubMed] [Google Scholar]
- 56.Chai S, Yu S, Yang Z, Wu S, Gao L, Wang H, et al. Effect of incretin-based therapies on cancers of digestive system among 101 595 patients with type 2 diabetes mellitus: A systematic review and network meta-analysis combining 84 trials with a median duration of 30 weeks. BMJ Open Diabetes Res Care. 2019;7:e000728. doi: 10.1136/bmjdrc-2019-000728. doi: 10.1136/bmjdrc-2019-000728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grenader T, Goldberg A, Shavit L. Metformin as an addition to conventional chemotherapy in breast cancer. J Clin Oncol. 2009;27:e259. doi: 10.1200/JCO.2009.25.4110. doi: 10.1200/JCO.2009.25.4110. [DOI] [PubMed] [Google Scholar]
- 58.Buzzai M, Jones RG, Amaravadi RK, Lum JJ, DeBerardinis RJ, Zhao F, et al. Systemic treatment with the antidiabetic drug metformin selectively impairs p53- deficient tumor cell growth. Cancer Res. 2007;67:6745–52. doi: 10.1158/0008-5472.CAN-06-4447. [DOI] [PubMed] [Google Scholar]
- 59.Zhuang Y, Miskimins WK. Cell cycle arrest in metformin treated breast cancer cells involves activation of AMPK, downregulation of cyclin D1, and requires p27Kip1 or p21Cip1. J Mol Signal. 2008;3:18. doi: 10.1186/1750-2187-3-18. doi: 10.1186/1750-2187-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hirsch HA, Iliopoulos D, Tsichlis PN, Struhl K. Metformin selectively targets cancer stem cells, and acts together with chemotherapy to block tumor growth and prolong remission. Cancer Res. 2009;69:7507–11. doi: 10.1158/0008-5472.CAN-09-2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li K, Si-Tu J, Qiu J, Lu L, Mao Y, Zeng H, et al. Statin and metformin therapy in prostate cancer patients with hyperlipidemia who underwent radiotherapy: A population-based cohort study. Cancer Manag Res. 2019;11:1189–97. doi: 10.2147/CMAR.S166638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gallagher EJ, LeRoith D. Obesity and diabetes: The increased risk of cancer and cancer-related mortality. Physiol Rev. 2015;95:727–48. doi: 10.1152/physrev.00030.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zheng LY, Zou DJ. Metformin inhibits proliferation and colony formation ability through AMP-activated protein kinase in hepatocellular carcinoma cells. Diabetes. 2012;61:A457. [Google Scholar]
- 64.Foretz M, Hébrard S, Leclerc J, Zarrinpashneh E, Soty M, Mithieux G, et al. Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. J Clin Invest. 2010;120:2355–69. doi: 10.1172/JCI40671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sośnicki S, Kapral M, Węglarz L. Molecular targets of metformin antitumor action. Pharmacol Rep. 2016;68:918–25. doi: 10.1016/j.pharep.2016.04.021. [DOI] [PubMed] [Google Scholar]
- 66.Fidan E, Onder Ersoz H, Yilmaz M, Yilmaz H, Kocak M, Karahan C, et al. The effects of rosiglitazone and metformin on inflammation and endothelial dysfunction in patients with type 2 diabetes mellitus. Acta Diabetol. 2011;48:297–302. doi: 10.1007/s00592-011-0276-y. [DOI] [PubMed] [Google Scholar]
- 67.Chen HH, Lin MC, Muo CH, Yeh SY, Sung FC, Kao CH. Combination therapy of metformin and statin may decrease hepatocellular carcinoma among diabetic patients in Asia. Medicine. 2015;94:e1013. doi: 10.1097/MD.0000000000001013. doi: 10.1097/MD.0000000000001013. [DOI] [PMC free article] [PubMed] [Google Scholar]