Abstract
Myasthenia gravis (MG) is a heterogeneous autoimmune disease, which is characterized by a postsynaptic neuromuscular transmission defect, with antibodies directly targeting the acetylcholine receptor (AChR) or other structural proteins of the neuromuscular junction. The majority of MG cases are associated with thymic pathologies, including thymoma, thyroiditis, autoimmune diseases or malignant hematologic neoplasia. The present study reported a rare case of AChR-positive and late-onset ocular MG, which rapidly progressed to a generalized myasthenic syndrome as an initial presentation of a pancreatic neuroendocrine neoplasia (pNEN). Following complete surgical resection of the pNEN, the myasthenic syndrome was improved and the anti-AChR antibody titers were reduced. It has been reported that MG is a paraneoplastic syndrome in thymic neoplasms and less common in hematologic malignancies. However, currently, only few cases of MG as initial presentation of a solid tumor, and more particular of a neuroendocrine neoplasm, have been reported in the literature. In conclusion, surveillance for extrathymic solid malignancies in newly diagnosed patients with MG could promote the early diagnosis of associated tumor diseases.
Keywords: myasthenia gravis, acetylcholine receptor, solid tumor, pancreatic neuroendocrine neoplasia, paraneoplastic syndrome, chromogranin A
Introduction
Neuroendocrine neoplasms (NENs), originating from neuroendocrine cells, represent a rare heterogenic group of solid tumors, which can be involved in hormone homeostasis via the release of bioactive peptides (1,2). NENs can develop in several organs and more common in the lungs, the intestine and pancreas (1). Depending on the primary tumor, the occurrence of metastasis, histological grading, hormone production in case of secreting NENs and their association with hereditary syndromes, such as multiple endocrine neoplasia or von Hippel-Lindau syndrome, the clinical presentation of NENs varies from asymptomatic patients to patients with specific and non-specific symptoms (3,4). Pancreatic NENs (pNENs) are commonly non-secreting. However, when hormones are produced, peptides, such as insulin, glucagon, vasoactive intestinal peptide (VIP) and pancreatic polypeptide (PP) are the most common, eventually resulting in hormone-specific symptoms (3,5). The treatment options for pNENs include the surgical resection of the primary tumor, administration of somatostatin analogues, targeted therapy with tyrosine kinase inhibitors and the mammalian target of rapamycin (mTOR) inhibitor everolimus, peptide-receptor radiotherapy (PRRT) and chemotherapy in metastasized disease (6-8). Chromogranin A (CgA) and neuron specific enolase (NSE) are the most commonly used established biomarkers for therapy monitoring and clinical management of patients with pNEN (9,10). Myasthenia gravis (MG) is an autoimmune neuromuscular junction (NMJ) disorder, which is associated with the secretion of autoantibodies directly targeting key molecules at the NMJ, including acetylcholine receptor (AChR) in ~85% of all patients with MG, muscle specific kinase (MuSK), titin and LDL receptor related protein 3 (LRP3) (11-13). In ~10% of patients with MG no autoantibodies are detected (seronegative MG) (12). It has been reported that in AChR antibody-positive MG, the thymus affects the development of autoreactive T cells targeting AChR and the induction of AChR-antibody producing B cells, which are involved in the symptoms of MG (13,14). Therefore, in the majority of cases, MG is associated with thymic pathologies, such as thymoma or thymic hyperplasia. Less frequent other autoimmune diseases, such as thyroiditis, lupus erythematosus, rheumatic arthritis and hematologic neoplasia are also associated with MG (15,16). Previous studies also demonstrated that several MG cases were associated with extrathymic solid tumors, while the association between MG and pNEN has only been described in three cases worldwide (17-20). The clinical presentation of ocular MG (OMG) commonly includes weakness of the extraorbital muscle, accompanied by fluctuating ptosis and diplopia (21). A previous study also showed that generalization of MG could lead to exercise-induced fatigue and muscle weakness in 50-60% of MG cases within the first two years (21). Therapy schemes for OMG include symptomatic treatment with acetylcholine esterase inhibition, long-term immunosuppression for generalized MG and thymectomy in younger adults with thymic pathologies (22,23).
Case presentation
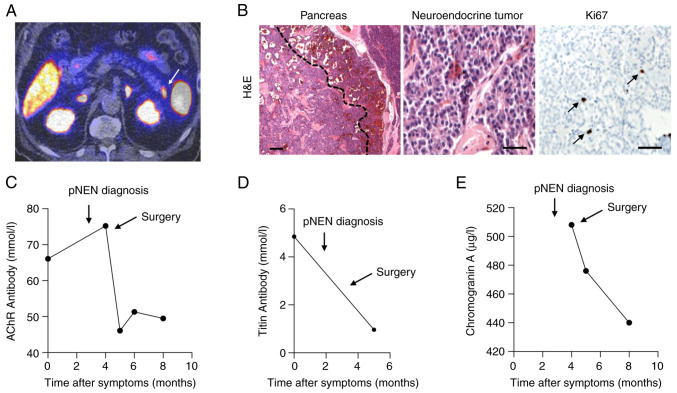
The present study presents a case of a 76-year-old patient with a history of age-related macular degeneration was presented. Physical examination at the Ophthalmologic Department of University Hospital Tuebingen revealed an asymmetric ptosis of the left eye and an anamnestic progressive weakness of the left eyelid over the course of one week. Besides recurrent thromboembolic events in the past, treated by anticoagulation with rivaroxaban, no underlying diseases were detected. The initial cerebral computed tomography (CT) and magnetic resonance imaging (MRI) scan revealed no evidence of thromboembolic events. In addition, no endocardial thromboembolic vegetations or persistent foramen ovale were diagnosed. Interestingly, during the Simpson's test, accentuating ptosis in upward gaze (after one minute), as well as horizontal non-exhaustive nystagmus of the left eye in leftward gaze were observed. The patient showed no signs of muscle weakness or autonomic dysfunction. Deep tendon reflexes were within normal ranges. Further neurological examination, including electrophysiological testing (ENG) of facial nerve/orbicularis oculi muscle showed a significant decrement in low frequency repetitive nerve stimulation, thus indicating a neuromuscular transmission defect (Table I). In addition, serological tests revealed high levels of anti-AChR autoantibodies (66 nmol/l) and the presence of anti-titin antibodies (Fig. 1C and D), supporting the diagnosis of OMG.
Table I.
Electroneurography of repetitive facial nerve stimulation at first diagnosis.
| 3 Hz frequency stimulation (facial nerve/left orbicularis oculi muscle) | Amplitude CMAP 1 vs. 5 (%) |
|---|---|
| Decrement prior to stimulation | -14.2 |
| Decrement 5 sec after muscle stimulation (duration, 60 sec) | -23.8 |
| Decrement 1 min after muscle stimulation (duration, 60 sec) | -14.8 |
| Decrement 3 min after muscle stimulation (duration, 60 sec) | -22.1 |
CMAP, compound muscle action potential.
Figure 1.
PET imaging, histopathological examination of pNEN and serum levels of particular markers. (A) Preoperative PET-computed tomography scan showing a somatostatin receptor-positive lesion at the tail of the pancreas (white arrow). (B) Histopathological examination of the resected pNEN using H&E staining revealed a well circumscribed tumor within the pancreas with hemorrhage in the periphery and small sclerotic areas in the center (magnification, 25x; left) and monotonous tumor cells with eosinophilic cytoplasm, oval nuclei and small nucleoli (magnification, 200x; middle). The cell proliferation rate was very low (Ki67 staining; black arrows; magnification, 200x; right). (C) The serum levels of anti-AChR antibody were notably decreased after the surgical resection of the pNEN. (D) The serum levels of anti-titin antibody after surgery are shown. (E) The serum levels of the tumor marker chromogranin A were reduced after the surgical resection of pNEN. PET, positron emission tomography; pNEN, pancreatic neuroendocrine neoplasia; AChR, acetylcholine receptor; H&E, hematoxylin and eosin.
After starting a symptomatic treatment with pyridostigmine (180 mg/daily), the patient underwent diagnostic tests for thymic pathologies, autoimmune diseases and malignant neoplasms. A CT scan showed a 11x9-mm lesion in the pancreatic cauda. At three weeks after initial presentation, the patient reported progressive diplopia, difficulty in maintaining head posture, as well as progressive weakening of the limbs. Furthermore, dysarthria worsened throughout the day. Therefore, the patient was treated with an additional immunosuppressive therapy with 500 mg/three days methylprednisolone (following gradually reduction) and azathioprine (2 mg/kg/day). Following further escalation of the pyridostigmine dose (220 mg/daily), the progressive symptoms declined. Furthermore, the somatostatin receptor (SSR)/positron emission tomography (PET)/computed tomography (CT) scan showed that the lesion in the pancreas intensively expressed SSR (Fig. 1A; indicated by arrow). However, extrapancreatic lesions were not identified. Interestingly, the levels of CgA (Fig. 1E) and NSE were moderately elevated, with values of 508 µg/l and 13 µg/l, respectively. However, the serum levels of the bioactive peptides, insulin, gastrin, glucagon, VIP and PP, and the urine levels of 5-hydroxy-indole acetic acid (5-HIAA) were within physiological ranges. Therefore, the SSR-positive pancreatic tumor was surgically resected. The histopathology results revealed a neuroendocrine tumor of 1.3 cm in diameter with a proliferation rate (Ki67 staining) of 0.7% (Fig. 1B). The initial tumor stage was pT1 pN0 cM0 L0 V0 Pn0 R0 G1.
After the surgical resection, the patient experienced a myasthenic crisis with worsening dyspnea. Therefore, the patient received symptomatic treatment that required pyridostigmine dose escalation, combined with neostigmine perfusion and a 5-day course of intravenous immunoglobulins (0.4 mg/kg body weight). Following three days of intensive care monitoring, the patient rapidly improved. Of note, the anti-myasthenic therapy was continued with pyridostigmine and azathioprine. Post-interventional SSR/PET/CT scan did not display any SSR-positive lesions. Furthermore, the clinical symptoms in terms of ptosis and dysarthria improved quickly. At three months after the surgical resection, the patient showed no residual symptoms. Additionally, the antibody titers, as well as the levels of the tumor marker CgA were steeply declined immediately after surgery (Fig. 1C-E).
Discussion
In the present case report the patient was diagnosed with MG based on the typical clinical presentation of fluctuating oculo-bulbar weakness, elevated anti-AChR antibody titer and neurophysiological tests. The response to myasthenic therapy with pyridostigmine and immunosuppression led to the diagnosis of MG over other NMJ diseases, such as the Lambert-Eaton myasthenic syndrome (LEMS). However, the differentiation between MG and LEMS proves to be challenging, since several coexisting and overlapping syndromes have been reported in the literature (24,25).
The majority of neuroendocrine neoplasms of the pancreas are non-secreting and patients are often asymptomatic or present with unspecific clinical symptoms (26). This poses a limiting factor for timely diagnosis and, therefore, tumors are usually diagnosed at advanced stages (26).
The present study reported a rare case of late-onset MG, which led to the early diagnosis of an underlying pancreatic malignancy. Due to early diagnosis and lack of metastasis, the effective treatment of the pNEN by surgical resection was possible.
Furthermore, the underlying diseases of MG are commonly thymic pathologies or autoimmune diseases, and significantly less often, extrathymic solid tumors (15-18). With the increasing improvement of the diagnostic approaches, the presence of extrathymic malignancies should be sought in all patients with MG without evidence of thymic pathology or autoimmune diseases.
Furthermore, ~90% of MG cases are seropositive, thus indicating that autoantibodies, such as anti-AChR or anti-titin, are detectable in the blood serum of patients (11-13). The present study detected anti-AChR antibodies, which were significantly reduced after the surgical resection of the pNEN. Therefore, anti-AChR antibodies, combined with established tumor markers in NENs, such as CgA and NSE, could be prospectively considered as additional biomarkers in patients with NENs and seropositive MG. However, there is currently no other available evidence to support this hypothesis.
Paraneoplastic MG may often lead to a distinctive clinical pattern characterized by more severe demonstration and commonly involves bulbar, respiratory and neck muscles (27), as shown in the current case. Paraneoplastic syndromes are associated with substances secreted by the tumor. However, these substances are not directly specific to their tissue of origin (28). The surgical resection of the pNEN improved the clinical symptoms and notably attenuated the titers of anti-AChR antibodies in the present study. Therefore, the therapy of the underlying diseases should also be considered when planning the treatment approach for MG.
In conclusion, the present case study reported a rare case of MG as an initial paraneoplastic syndrome associated with pNEN. The early diagnosis of both pNEN and MG enabled the curative targeted treatment of the tumor and led to the efficient treatment of MG. In addition to early diagnosis, monitoring of the paraneoplastic syndrome could be a useful tool to manage the clinical course and treatment response of the underlying neoplasm. Since the efficient treatment of NENs is instrumental in achieving a complete remission of MG, screening for rare neuroendocrine malignancies, including pNEN, could be considered in the diagnostic evaluation.
Acknowledgements
The authors wish to thank the study nurses Mrs Kristina MacMillan, Mrs Simone Braun and Mrs Ines Hildebrand of the Center for Neuroendocrine Tumors Tuebingen (Tuebingen, Germany) for their assistance in conducting various investigations and collecting medical findings.
Funding Statement
Funding: The present study was funded by the German Research Foundation under Germany's Excellence Strategy (grant no. EXC 2180-390900677).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
CH and MHi conceived and designed the study. ES, JD, PB, DN, SS, MHo, UML and LZ analyzed the patient data and medical records. ES, PB, CH and MHi analyzed the data. ES and MHi confirm the authenticity of all the raw data. ES, CH and MHi prepared the figure. ES, JD, CH and MHi wrote the first draft of the manuscript. CH and MHi contributed to the interpretation of the data and edited the manuscript. All authors critically reviewed the manuscript. All authors have read and approved the final version of the manuscript.
Ethics approval and consent to participate
The present case study was performed in accordance with the Declaration of Helsinki. The patient provided written informed consent.
Patient consent for publication
The patient provided their consent for the publication of their data.
Competing interest
The authors declare that they have no competing interests.
References
- 1.Perren A, Couvelard A, Scoazec JY, Costa F, Borbath I, Delle Fave G, Gorbounova V, Gross D, Grossma A, Jense RT, et al. ENETS consensus guidelines for the standards of care in neuroendocrine tumors: Pathology: Diagnosis and prognostic stratification. Neuroendocrinology. 2017;105:196–200. doi: 10.1159/000457956. [DOI] [PubMed] [Google Scholar]
- 2.Rickman DS, Beltran H, Demichelis F, Rubin MA. Biology and evolution of poorly differentiated neuroendocrine tumors. Nat Med. 2017;23:1–10. doi: 10.1038/nm.4341. [DOI] [PubMed] [Google Scholar]
- 3.Cives M, Strosberg JR. Gastroenteropancreatic neuroendocrine tumors. CA Cancer J Clin. 2018;68:471–487. doi: 10.3322/caac.21493. [DOI] [PubMed] [Google Scholar]
- 4.Soczomski P, Jurecka-Lubieniecka B, Krzywon A, Cortez AJ, Zgliczynski S, Rogozik N, Oczko-Wojciechowska M, Pawlaczek A, Bednarczuk T, Jarzab B. A direct comparison of patients with hereditary and sporadic pancreatic neuroendocrine tumors: Evaluation of clinical course, prognostic factors and genotype-phenotype correlations. Front Endocrinol (Lausanne) 2021;12(681013) doi: 10.3389/fendo.2021.681013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guilmette JM, Nosé V. Neoplasms of the neuroendocrine pancreas: An update in the classification, definition, and molecular genetic advances. Adv Anat Pathol. 2019;26:13–30. doi: 10.1097/PAP.0000000000000201. [DOI] [PubMed] [Google Scholar]
- 6.Krug S, Schrader J, Rinke A. Updates on Diagnostic and therapeutic management of gastrointestinal and pancreatic NET. Cancers (Basel) 2022;14(2628) doi: 10.3390/cancers14112628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurita Y, Kobayashi N, Hara K, Mizuno N, Kuwahara T, Okuno N, Haba S, Tokuhisa M, Hasegawa S, Kubota K, et al. Clinical outcomes of everolimus rechallenge in patients with pancreatic neuroendocrine neoplasms with no other treatment options. Cancers (Basel) 2022;14(5669) doi: 10.3390/cancers14225669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Camus B, Cottereau AS, Palmieri LJ, Dermine S, Tenenbaum F, Brezault C, Coriat R. Indications of peptide receptor radionuclide therapy (PRRT) in gastroenteropancreatic and pulmonary neuroendocrine tumors: An updated review. J Clin Med. 2021;10(1267) doi: 10.3390/jcm10061267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu F, Fu J, Zhang C, Wu W, Ai S, Yao X, Meng Q, Huang Y, Lu G, Wang F, Qu W. Use of chromogranin a for monitoring patients with pancreatic neuroendocrine neoplasms. Pancreas. 2021;50:882–889. doi: 10.1097/MPA.0000000000001852. [DOI] [PubMed] [Google Scholar]
- 10.Fuksiewicz M, Kowalska M, Kolasinska-Cwikla A, Kotowicz B. Serum levels of neuron-specific enolase as a prognostic factor for disease progression in patients with GET/NEN in the pancreas and the small intestine. Endocr Connect. 2022;11(e210647) doi: 10.1530/EC-21-0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koneczny I, Herbst R. Myasthenia gravis: Pathogenic effects of autoantibodies on neuromuscular architecture. Cells. 2019;8(671) doi: 10.3390/cells8070671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lazaridis K, Tzartos SJ. Myasthenia gravis: Autoantibody specificities and their role in MG management. Front Neurol. 2020;11(596981) doi: 10.3389/fneur.2020.596981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lazaridis K, Tzartos SJ. Autoantibody specificities in myasthenia gravis; Implications for improved diagnostics and therapeutics. Front Immunol. 2020;11(212) doi: 10.3389/fimmu.2020.00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yi JS, Guptill JT, Stathopoulos P, Nowak RJ, O'Connor KC. B cells in the pathophysiology of myasthenia gravis. Muscle Nerve. 2018;57:172–184. doi: 10.1002/mus.25973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shelly S, Agmon-Levin N, Altman A, Shoenfeld Y. Thymoma and autoimmunity. Cell Mol Immunol. 2011;8:199–202. doi: 10.1038/cmi.2010.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ali M, Riad M, Adhikari P, Bhattarai S, Gupta A, Ali E, Mostafa JA. Association between myasthenia gravis and systemic lupus erythematosus as a comorbid state. Cureus. 2021;13(e14719) doi: 10.7759/cureus.14719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanovska N, Novotni G, Sazdova-Burneska S, Kuzmanovski I, Boshkovski B, Kondov G, Jovanovski-Srceva M, Kokareva A, Isjanovska R. Myasthenia gravis and associated diseases. Open Access Maced J Med Sci. 2018;6:472–478. doi: 10.3889/oamjms.2018.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verwijst J, Westerberg E, Punga AR. Cancer in myasthenia gravis subtypes in relation to immunosuppressive treatment and acetylcholine receptor antibodies: A Swedish nationwide register study. Eur J Neurol. 2021;28:1706–1715. doi: 10.1111/ene.14730. [DOI] [PubMed] [Google Scholar]
- 19.Hermans MA, Stelten BM, Haak HR, de Herder WW, Dercksen MW. Two patients with a neuroendocrine tumour of the small intestine and paraneoplastic myasthenia gravis. Endocrinol Diabetes Metab Case Rep. 2014;2014(140013) doi: 10.1530/EDM-14-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gay SA, Nawras SA. Myasthenia gravis with coexisting primary pancreatic neuroendocrine tumor in a young female patient. Am J Gastroenterol. 2011;106(S235) [Google Scholar]
- 21.Wong SH, Huda S, Vincent A, Plant GT. Ocular myasthenia gravis: Controversies and updates. Curr Neurol Neurosci Rep. 2014;14(421) doi: 10.1007/s11910-013-0421-9. [DOI] [PubMed] [Google Scholar]
- 22.Alhaidar MK, Abumurad S, Soliven B, Rezania K. Current treatment of myasthenia gravis. J Clin Med. 2022;11(1597) doi: 10.3390/jcm11061597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farrugia ME, Goodfellow JA. A practical approach to managing patients with myasthenia gravis-opinions and a review of the literature. Front Neurol. 2020;11(604) doi: 10.3389/fneur.2020.00604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim JA, Lim YM, Jang EH, Kim KK. A patient with coexisting myasthenia gravis and lambert-eaton myasthenic syndrome. J Clin Neurol. 2012;8:235–237. doi: 10.3988/jcn.2012.8.3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oh SJ. Myasthenia gravis Lambert-Eaton overlap syndrome. Muscle Nerve. 2016;53:20–26. doi: 10.1002/mus.24921. [DOI] [PubMed] [Google Scholar]
- 26.Falconi M, Eriksson B, Kaltsas G, Bartsch DK, Capdevila J, Caplin M, Kos-Kudla B, Kwekkeboom D, Rindi G, Klöppel G, et al. ENETS consensus guidelines update for the management of patients with functional pancreatic neuroendocrine tumors and non-functional pancreatic neuroendocrine tumors. Neuroendocrinology. 2016;103:153–171. doi: 10.1159/000443171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Skeie GO, Romi F. Paraneoplastic myasthenia gravis: Immunological and clinical aspects. Eur J Neurol. 2008;15:1029–1033. doi: 10.1111/j.1468-1331.2008.02242.x. [DOI] [PubMed] [Google Scholar]
- 28.Graus F, Delattre JY, Antoine JC, Dalmau J, Giometto B, Grisold W, Honnorat J, Smitt PS, Vedeler Ch, Verschuuren JJ, et al. Recommended diagnostic criteria for paraneoplastic neurological syndromes. J Neurol Neurosurg Psychiatry. 2004;75:1135–1140. doi: 10.1136/jnnp.2003.034447. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.