Abstract
Background
Dementia is a progressive neurodegenerative syndrome that has no cure. Although a significant proportion of people with dementia progress into the severe stages of the disease, evidence on the clinical effectiveness of treatments for people with severe dementia remains limited.
Aims
To systematically review the effectiveness of pharmacological and non-pharmacological treatments for people living with severe dementia and assess the quality of the evidence.
Method
We searched MEDLINE, EMBASE, PsycINFO, CINAHL and online clinical trial registers up to January 2022, for Randomised Controlled Trials (RCT) in people living with severe dementia. Quality and risk of bias were assessed independently by two authors.
Results
A total of 30 trials met our inclusion criteria of which 14 evaluated the effectiveness of pharmacological treatments, and 16 evaluated a non-pharmacological intervention. Pharmacological treatments: Meta-analyses indicated that pharmacological treatments (donepezil: 10 mg, 5 mg; galantamine: 24 mg; memantine: 10 mg) are associated with better outcomes compared to placebo for: severity of symptoms (standardized mean difference (SMD) 0.37, 95% CI 0.26–0.48; 4 studies; moderate-certainty evidence), activities of daily living (SMD 0.15, 95% CI 0.04–0.26; 5 studies; moderate-certainty evidence), and clinical impression of change (Relative Risk (RR) 1.34, 95% CI 1.14–1.57; 4 studies; low-certainty evidence). Pharmacological treatments were also more likely to reduce mortality compared to placebo (RR 0.60, 95% CI 0.40–0.89; 6 studies; low-certainty evidence). Non-pharmacological treatments: Five trials were included in the meta-analyses of non-pharmacological interventions (multi-sensory stimulation, needs assessment, and activities-based interventions); results showed that non-pharmacological interventions may reduce neuropsychiatric symptoms of dementia compared to usual care (SMD −0.33, 95% CI −0.59 to −0.06; low certainty evidence).
Conclusions
There is moderate-certainty evidence that pharmacological treatments may decrease disease severity and improve function for people with severe dementia. Non-pharmacological treatments are probably effective in reducing neuropsychiatric symptoms but the quality of evidence remains low. There is an urgent need for high-quality evidence for other outcomes and for developing service-user informed interventions for this under-served group.
Keywords: Severe dementia, Pharmacological treatments, Non-pharmacological treatments, Randomized controlled clinical trials
Highlights
-
•
Moderate-certainty evidence shows that pharmacological treatments may decrease disease severity and improve function in severe dementia.
-
•
Non-pharmacological interventions may reduce neuropsychiatric symptoms but evidence remains of low-quality.
-
•
The evidence-base of effectiveness of treatments for people with severe dementia remains small and should be a priority for future research.
1. Introduction
Dementia is a progressive neurodegenerative syndrome affecting approximately 50 million people worldwide (Livingston et al., 2020). Although significant progress has been made in the development and evaluation of interventions for people living with mild and moderate dementia, evidence-base of treatments for people living with severe dementia remains substantially limited (Livingston et al., 2017). Prevalence figures estimate that around 12.5 % of people above the age of 60, and 17.3 % of those aged over 85 years are affected by severe dementia (Prince et al., 2013), with both of these numbers expected to rise sharply in the next 25 years (Wittenberg et al., 2020). Despite this large projected increase, and that over 20 % of the population of people with dementia currently live with severe dementia (Farlow, 2005), this group remains under-treated (Edvardsson et al., 2008), is often excluded by research evaluating treatments (Clare et al., 2010), and remains a highly under-served population (Shepherd et al., 2019).
Progression to severe dementia is characterised by a marked loss in cognition and the ability to carry out activities of daily living (ADL), leading to earlier care home admission (Jonsson et al., 2006). Living with severe dementia is associated with the highest community and informal care costs, which double from the milder to the more advanced stages of the disease (Ku et al., 2016). People with severe dementia are also at greater risk of experiencing frequent and severe behavioural and psychological symptoms, which can be very distressing, and constitute the largest contributor of increased costs of care (Lacey et al., 2017).
Current clinical guidelines on the pharmacological management of severe dementia recommend the use of cholinesterase inhibitors in severe Alzheimer’s disease (AD) with more recent recommendations of the addition of memantine to anticholinesterase inhibitors for severe AD (O'Brien et al., 2017, Schmidt et al., 2015). Although several systematic reviews exist reporting on the clinical effectiveness of pharmacological interventions for people with moderate to severe dementia (Birks and Harvey, 2018), most are now out of date (McShane et al., 2019), and neither has focused on effectiveness of pharmacological treatments specifically for people with severe dementia. Therefore, estimates of clinical effectiveness of pharmacological treatments for this under-represented group remain largely unknown.
Although recent clinical guidelines recommend the use of psychological interventions for preventing or reducing neuropsychiatric symptoms in severe dementia (NICE, 2018), a recent Cochrane review identified no such trials in people living with severe dementia (Orgeta et al., 2022). Despite the multiple reviews in the literature examining the effectiveness of non-pharmacological interventions in dementia, most provide only a narrative synthesis, and are focused on people living with moderate to severe dementia (Hui et al., 2021). As a result, effectiveness of these treatments for people with severe dementia remains unknown with no quantitative estimates available. An important aim of the present review therefore was to evaluate the effectiveness of all types of non-pharmacological interventions for this group, which is typically excluded from both psychosocial and psychological clinical trials research (Orgeta et al., 2022).
This systematic review and meta-analysis aimed to evaluate current evidence of the clinical effectiveness of both pharmacological and non-pharmacological treatments for people living with severe dementia in all settings, in order to inform clinical guidelines, and clinical practice. A secondary objective was to rate the quality of the evidence to date and to make recommendations for future research in the area.
2. Methods
This systematic review was conducted based on the guidelines of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (Moher et al., 2009), and was registered with PROSPERO (CRD42021193086). The titles, abstracts and full text of articles were screened by two reviewers independently.
2.1. Search strategy
We searched relevant terms to severe dementia and randomised controlled trials (RCTs) (see Appendix in the Supplement) up to January 2022 in major health databases (MEDLINE, EMBASE, PsycINFO, CINAHL; the Cochrane Dementia and Cognitive Improvement Group’s Specialized Register and clinical trials registers). We additionally added the terms ‘moderate’ and ‘moderate to severe dementia’ in our search to avoid missing any mixed sample studies that provided separate data for people with severe dementia (see Supplement for details of the search terms). We additionally hand searched reference lists of identified articles to ensure no studies were missed.
2.2. Inclusion criteria
We included: a) RCTs of interventions b) in people with severe dementia, with a diagnosis of any type, c) living in any setting (community, nursing homes, hospitals, inpatient settings). Multicomponent trials, and studies reporting on combinations of pharmacological and non-pharmacological interventions were also eligible. Our judgements of definitions of severe dementia were based on cut-off scores of tools used widely in the published literature, which included the Mini Mental State Examination (MMSE) (Folstein et al., 1975), the Global Deterioration Scale (GDS) (Reisberg et al., 1982), the Functional Assessment Staging Tool (FAST) (Reisberg, 1988), and the Clinical Dementia Rating Scale (CDR) (Hughes et al., 1982). Inclusion criteria for severity of dementia were based on the use of a cognitive screening tool, and/or global assessment scales that measured overall function and disease severity.
Studies that did not provide separate data for people with severe dementia were excluded (e.g., studies reporting on a mixed sample or studies reporting data on people with moderate-to-severe dementia). We additionally excluded studies that did not use a screening tool to assess dementia severity, and studies on the effectiveness of palliative care or end of life care interventions. Studies assessing effectiveness of continuation and discontinuation of treatments were also excluded.
2.3. Statistical analysis and quality assessment
We used a fixed-effects model to represent overall estimate effects, which assumes that all studies are estimating the same (fixed) treatment effect, and quantified heterogeneity by using the I2 statistic. All calculations were conducted using Review Manager (RevMan) 5.2 for Windows (Cochrane Collaboration, Oxford UK; www.cc-ims.net/RevMan). Where data were available, we collected the number of participants for whom the outcome was measured in each group, means and standard deviations (SDs). We used change from baseline scores for all analyses reported and, if necessary, calculated the change scores. We used Cochrane's tool for assessing risk of bias (Higgins et al., 2011), and the GRADE approach to summarize overall certainty of evidence (Guyatt et al., 2011).
3. Results
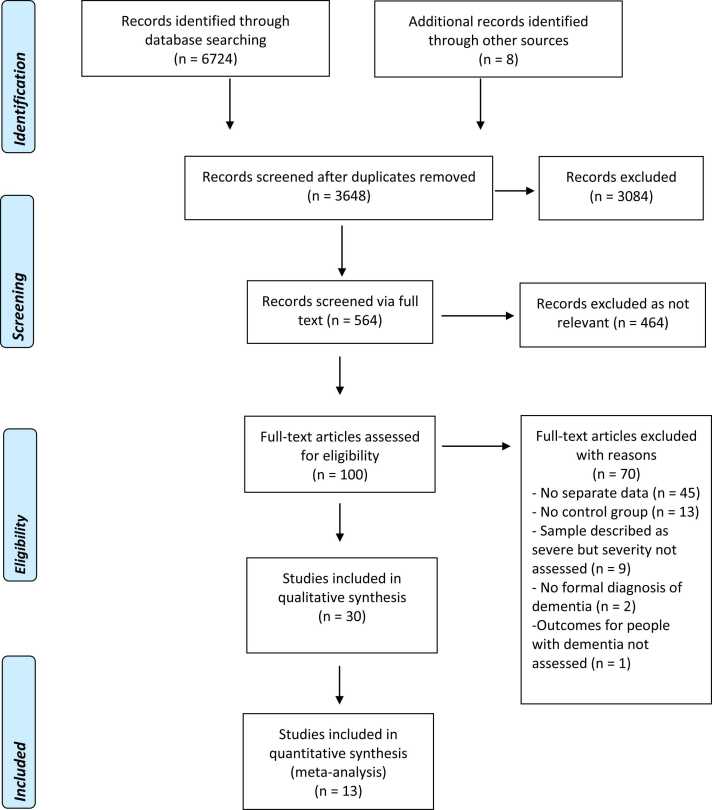
A total of 6724 journal articles were identified by the search (see Fig. 1 for details of the search process), with 8 additional articles identified via hand search. After removing duplicates 3648 articles remained, of which 564 were screened via full text. After removing studies that were not relevant 100 articles were assessed for eligibility. Of these studies, 70 were excluded with reasons (see Appendix in the Supplement) and 30 met inclusion criteria. There were fourteen studies evaluating the clinical effectiveness of pharmacological treatments, of which one tested the effectiveness of a plasma infusion treatment, and sixteen trials of non-pharmacological interventions (see Table 1, Table 2, Table 3 for Characteristics of Included studies). We identified two trials that had multiple treatment groups; for these studies, we combined the two control conditions and then divided the combined group in line with the methods recommended by Cochrane. We were not able to perform any sensitivity analyses due to the small number of studies. We assessed publication bias by producing funnel plots and inspecting them visually for all analyses combining six studies or more (Egger et al., 1997). Due to the small number of studies combined in meta‐analyses, we did not conduct statistical tests for funnel plot asymmetry.
Fig. 1.
Flowchart of the search and study selection process.
Table 1.
Characteristics of Included studies of pharmacological treatments (donepezil, galantamine, and memantine) for dementia severity and global function.
| Study | Sample and setting | Outcomes | Treatment | Follow-up time points |
|---|---|---|---|---|
| Black 2007 | n = 343 (F=241; M=102) Mean age: 78.0; Mean MMSE: 7.45 Diagnosis: AD |
Primary outcomes
|
Donepezil 10 mg/day (n=176) Placebo (n=167) |
|
| Inclusion criteria: | ||||
| ||||
| Definition of severe dementia: MMSE ≤ 12 and FAST score ≥ 6 | ||||
| Community | ||||
| ||||
| Burns 2009 (SERAD study) | n = 407 (F=329; M=78) Mean age: 83.6; Mean MMSE: 8.95 Diagnosis: AD and AD with CVD |
Primary outcomes
|
Galantamine 24 mg/day (n=207) Placebo (n=200) |
|
| Inclusion criteria: | ||||
| ||||
| Definition of severe dementia: MMSE 5-12 | ||||
| 57 Nursing homes | ||||
| ||||
| Feldman 2005 (MSAD study) | n = 145 (F=86; M=59) Mean age: 73; Mean MMSE: 8.94 Diagnosis: AD |
Primary outcomes
|
Donepezil 10 mg/day (n = 72) Placebo (n = 73) |
|
| Inclusion criteria: | ||||
| ||||
| Definition of severe dementia: sMMSE 5-12 | ||||
| Community/assisted living facilities | ||||
| ||||
| Hannestad 2021 | n = 26 (F=16; M=10) Mean age= 73.5; Mean MMSE: 5.4 Diagnosis: AD Inclusion criteria:
|
Primary outcomes
|
GRF6019 intravenous infusions of 250 mL IV over 5 days (n=18) Placebo (n=8) |
|
| Definition of severe dementia: MMSE 0 -10 | ||||
| Community and 4 nursing homes/ long-term care facilities | ||||
| ||||
| Homma 2008b | n = 302 (F=245; M=57) Mean age: 78.2; Mean MMSE: 7.76 Diagnosis: AD |
Primary outcomes
|
Donepezil 5 mg/day (n = 101) Donepezil 10 mg/day (n =96) Placebo (n = 105) |
|
| Inclusion criteria: | ||||
| ||||
| Definition of severe dementia: MMSE 1-12 and FAST score ≥ 6 | ||||
| Community | ||||
| ||||
| Jia 2017 | n = 313 (F=266; M=110) Mean age: 70.8; Mean MMSE: 7.3 Diagnosis: AD |
Primary outcomes
|
Donepezil 10 mg/day (n=157) Placebo (n=156) |
|
| Inclusion criteria: | ||||
| ||||
| Definition of severe dementia: MMSE 1-12 and SIB scores of 10 – 90 | ||||
| Hospitals | ||||
| ||||
| Reisberg 2003ª | n = 252 (F=170; M=82) Mean age: 76.1; Mean MMSE: 7.9 Diagnosis: AD |
Primary outcomes
|
Memantine 20mg (n=126) Placebo (n=126) |
|
| Inclusion criteria: | ||||
| ||||
| Definition of severe dementia: MMSE 1-10 | ||||
| Community | ||||
| ||||
| Winblad 1999 | n = 166 (F=96; M=70) Mean age: 68.4; Mean MMSE: 6.3 Diagnosis: Dementia (not further specified) |
Primary outcomes
|
Memantine 10mg (n=82) Placebo (n=84) |
|
| Inclusion criteria: | ||||
| ||||
| Definition of severe dementia: MMSE <10 and GDS score 5-7 | ||||
| 1 psychiatric hospital and 6 nursing homes | ||||
| ||||
| Winblad 2006 | n = 248 (F=190; M=58) Mean age: 84.9; Mean MMSE: 6.1 Diagnosis: AD |
Primary outcomes
|
Donepezil 10 mg/day (n=128) Placebo (n=120) |
|
| Inclusion criteria: | ||||
| ||||
| Definition of severe dementia: MMSE 1 -10 and FAST score 5-7c | ||||
| 50 nursing homes | ||||
|
Note: MMSE: Mini-Mental State Examination; FAST: Functional Assessment Staging; HIS: Hachinski Ischemic Score; SIB: Severe Impairment Battery; CIBIC-Plus: Clinician’s Interview-Based Impression of Change-Plus; ADCS-ADL-sev: Alzheimer's Disease Cooperative Study-Activities of Daily Living inventory modified for Severe Patients; NPI: Neuropsychiatric Inventory; CBQ: Caregiver Burden Questionnaire; RUSP: Resources Utilisation for Severe Alzheimer's Disease; CVD: Cerebrovascular disease; MDS-ADL: Minimum Data Set Activities of Daily Living; sMMSE: Severe Mini-Mental State Examination; DAD: Disability Assessment for Dementia; IADL: Instrumental Activities of Daily Living; PSMS: Physical Self-Maintenance Scale; BEHAVE-AD: Behavioral pathology in Alzheimer's disease; GDS: Global Deterioration Scale; GCI-C: Clinical Global Impression of Change; BGP: Behavioral Rating Scale for Geriatric Patients
ªData in people with severe dementia are reported but are not extractable from the primary paper
bData extracted were based on the safety population as reported in the original paper
Table 2.
Characteristics of Included studies of pharmacological treatments for people with dementia and neuropsychiatric symptoms.
| Study | Sample and setting | Outcomes | Treatment | Follow-up time points |
|---|---|---|---|---|
| Deberdt 2008 *combined data from three studies |
Study 1; n = 99 Studies 2 & 3; n = 72 Diagnosis: AD, vascular dementia, or mixed dementia |
Primary outcome
|
Olanzapine for cognition Study 1 Olanzapine (5, 10, or 15 mg/d) over 6 weeks (n=80) Placebo (n=19) Studies 2 & 3 Olanzapine (1.0, 2.5, 5.0, or 7.5 mg/d & 2.5–10 mg/d) over 10 weeks (n=52) Placebo (n=20) |
|
| Inclusion criteria: | ||||
| ||||
| Definition of severe dementia: MMSE 0-6 | ||||
| Community, nursing homes, or assisted living centres | ||||
| ||||
| De Deyn 2005 **combined data from three studies |
Sample across all studies n = 530 Diagnosis: AD, vascular dementia, or mixed dementia |
Primary outcomes
|
Risperidone for agitation, aggression, and psychosis Risperidone (n=333) Placebo (n=201) Study 1 Risperidone (fixed 0.25, 0.5, and 1 mg/d) over 12 weeks Study 2: Risperidone (flexible 0,25 – 2.0 mg oral solution) over 12 weeks Study 3: Risperidone (flexible 0,25 – 1.0 mg oral solution) over 12 weeks |
|
| Inclusion criteria: | ||||
| ||||
| Definition of severe dementia: MMSE 0-5 | ||||
| Nursing homes | ||||
| ||||
| Erdal 2018 (DEP.PAIN.DEM study) |
n = 92 Diagnosis: AD |
Primary outcomes
|
Analgesic treatment for depression Active analgesic treatment comprising of paracetamol or buprenorphine for depression over 13 weeks Placebo |
|
| Inclusion criteria: | ||||
| ||||
| Definition of severe dementia: MMSE from 0-10 | ||||
| 47 Nursing homes | ||||
| ||||
| Magai 2000 | n = 31 (F=31; M=0) Mean age= 89.2 Diagnosis: AD |
Primary outcomes
|
Sertraline for depression Sertraline 100 mg over 8 weeks (n=17) Placebo (n=14) |
|
| Inclusion criteria: | ||||
| ||||
| Definition of severe dementia: GDS 6 or 7 | ||||
| Nursing homes | ||||
| ||||
| Mintzer 2006 | n = 119 (demographics for severe group not reported) Diagnosis: AD with or without vascular dementia |
Primary outcomes
|
Risperidone for psychosis Risperidone 1.0-1.5 mg over 8 weeks (n=57) Placebo (n=62) |
|
| Inclusion criteria: | ||||
| ||||
| Definition of severe dementia: MMSE 5-9 | ||||
| Nursing homes | ||||
|
Note: MMSE: Mini-Mental State Examination; BEHAVE-AD: Behavioral pathology in Alzheimer's disease; CMAI: Cohen-Mansfield Agitation Inventory; CSDD: Cornell Scale for Depression in Dementia; MOBID-2: Mobilization-Observation-Behavior-Intensity-Dementia-2; GDS: Global Deterioration Scale; GS: Gestalt Scale; AFBS: Aversive Behaviour Feeding Scale; CGI-S: Clinical Global Impressions scale-Severity
*Study 1 - Street 2000; Study 2 - De Deyn 2004; Study 3 - Deberdt 2005
** Study 1 - Katz 1999; Study 2 - De Deyn 1999; Study 3 - Brodaty 2003
Table 3.
Characteristics of Included studies of non-pharmacological treatments.
| Study | Sample and setting | Outcomes | Treatment | Follow-up time points |
|---|---|---|---|---|
| Baker 2003ª | n = 136 (no further details) Diagnosis: AD, vascular or mixed dementia |
Primary outcomes
|
Multi-sensory stimulation over 4 weeks (n=65) Control group (simple activities such as quizzes and playing cards) (n=71) |
|
| Inclusion criteria: | ||||
| ||||
| Definition of severe dementia: MMSE 0-9 Day hospitals and psycho-geriatric wards
| ||||
| Ballard 2002ª | n = 72 (F=43; M=29) Mean age: 78.5 Diagnosis: Dementia (not further specified) |
Primary outcomes
|
Melissa essential oil over 4 weeks (n=36) Placebo oil (n=36) |
|
| Inclusion criteria: | ||||
| ||||
| Definition of severe dementia: CDR score of 3 | ||||
| 8 NHS nursing homes | ||||
| ||||
| Ballard 2018 ª (WHELD study) | n = 180 (no further details) Diagnosis: Dementia (not further specified) |
Primary outcomes 1. DEMQOL-Proxy Secondary outcomes 2. CMAI 3. NPI-MH 4. Antipsychotic use 5. GDS 6. CDR 7. CSDD 8. CANE 9. QuIS 10. Abbey Pain Scale |
Person-centered care training for staff, structured tailored activities, and anti-psychotic review over 8 months (n=77) Treatment as usual (n=103) |
|
| Inclusion criteria: | ||||
| ||||
| Definition of severe dementia: FAST score of 7 | ||||
| 69 NHS nursing homes | ||||
| ||||
| Clare 2013 | n = 65 (F=51; M=14) Mean age: 83.4 Diagnosis: AD, vascular dementia, mixed dementia, unspecified dementia, and Pick’s disease |
Primary outcomes
|
Communication skills training and supervision over 8 weeks (n = 32) Treatment as usual (n =33) |
|
| Inclusion criteria: | ||||
| ||||
| Definition of severe dementia: FAST score of 6 or 7 with limited or no verbal communication | ||||
| 8 privately owned care homes | ||||
| ||||
| Hjetland 2021 ª (DEM.LIGHT study) |
n = 69 (F=68; M=1) Mean age: 84; Mean MMSE: 4 Diagnosis: AD, vascular dementia, Lewy-body dementia, and unspecified dementia |
Primary outcomes
|
Ambient bright light treatment over 24 weeks (n = 33) Placebo control condition (n =36) |
|
| Inclusion criteria: | ||||
| ||||
| Definition of severe dementia: FAST score of 6 or 7 and MMSE scores 10 to 1 | ||||
| 8 nursing home units | ||||
| ||||
| Hutson 2014 | n = 39 (F=34; M=5) Mean age: 86.6; Mean MMSE: 4.9 Diagnosis: AD, vascular dementia, Lewy body dementia, mixed dementia, and unspecified dementia |
Primary outcomes
|
Multisensory stimulation, reminiscence, and light physical activity over 8 weeks (n=21) Treatment as usual (n=18) |
|
| Inclusion criteria: | ||||
| ||||
| Definition of severe dementia: MMSE; 80% of the sample is described as having severe dementia | ||||
| 4 care homes | ||||
| ||||
| Kovach 2004 ª | n = 78 (F=71; M=7) Mean age: 86.6; Median MMSE: 4.64 Diagnosis: Dementia (not further specified) |
Primary outcomes
|
Controlling and implementing activity schedules to ensure balance between high and low arousal (n=36) Treatment as usual (n=42) |
|
| Inclusion criteria: | ||||
| ||||
| Definition of severe dementia: MMSE ≤ 9 | ||||
| 13 long-term care facilities | ||||
| ||||
| Kovach 2006 | n = 114 (F=86; M=28) Mean age: 86.5; Mean MMSE: 7.8 Diagnosis: Dementia (not further specified) |
Primary outcomes
|
Assessment of needs, administering non-pharmacological treatments, analgesics, and consultation with practitioners (n=57) Treatment as usual (n=57) |
|
| Inclusion criteria: | ||||
| ||||
| Definition of severe dementia: MMSE and FAST 6 or 7 95% of this sample has a FAST score 6 or 7 | ||||
| 14 nursing homes | ||||
| ||||
| Liu 2017 ª | n = 128 (F=107; M=21) Mean age: 88.6; Mean MMSE: 3.41 Diagnosis: Dementia (not further specified) |
Primary outcomes
|
Pain management over 16 weeks (n=64) Treatment as usual (n=64) |
|
| Inclusion criteria: | ||||
| ||||
| Definition of severe dementia: Score of > 5 on interRAI HC | ||||
| 7 nursing homes | ||||
| ||||
| Olsen 2016 | n = 24 (no further details) Diagnosis: Dementia (not further specified) |
Primary outcomes
|
Animal assisted activities over 12 weeks (n=12) Treatment as usual (n=12) |
|
| Inclusion criteria: | ||||
| ||||
| Definition of severe dementia: CDR score of 3 (subsample) | ||||
| 3 nursing homes | ||||
| ||||
| Pieper 2018 ª | n = 288 (F=207; M=81) Mean age: 83.8 91.5% of the sample had a GDS score of 6 or 7 Diagnosis: Dementia (not further specified) |
Primary outcomes
|
Multidisciplinary intervention for pain management over 12 weeks (n=148) Treatment as usual (n=140) |
|
| Inclusion criteria: | ||||
| ||||
| Definition of severe dementia: GDS score of 6 or 7 | ||||
12 Nursing homes
| ||||
| Reisberg 2017 | n =20 (F=15; M=5) Mean age: 78.9; Mean MMSE: 8 Diagnosis: AD |
Primary outcomes
|
Comprehensive person-centered management over 28 weeks (n=10) Treatment as usual (n=10) |
|
| Inclusion criteria: | ||||
| ||||
| Definition of severe dementia: GDS 6 and FAST score ≥ 6a | ||||
| Community | ||||
| ||||
| Sakamoto 2013 | n =39 (F=32; M=7) Diagnosis: AD |
Primary outcomes
|
Interactive music therapy over 10 weeks (n=13) Passive music therapy over 10 weeks (n=13) Treatment as usual (n=13) |
10 (primary endpoint), and 13 weeks |
| Inclusion criteria: | ||||
| ||||
| Definition of severe dementia: CDR score of 3 | ||||
| Care homes and dementia hospital | ||||
| ||||
| Sánchez 2016 ª | n =32 (F=25; M=7) Mean age= 85.4 Diagnosis: Dementia (not further specified) |
Primary outcomes
|
Multisensory stimulation over 16 weeks (n=11) One-to-one activity sessions over 16 weeks (n=11) Treatment as usual (n=10) |
|
| Inclusion criteria: | ||||
| ||||
| Definition of severe dementia: GDS score 6 or 7 | ||||
| Specialized dementia centers for older people | ||||
| ||||
| Stenvall 2012 ª | n = 64 Mean age = 82.1 MMSE = 7.7 Diagnosis: Dementia (not further specified) |
Primary outcomes
|
Multidisciplinary post-operative intervention of rehabilitation (n=28) Treatment as usual (n=36) |
|
| Inclusion criteria: | ||||
| ||||
| Definition of severe dementia: MMSE ≤ 9 | ||||
| Hospitals | ||||
| ||||
| Strøm 2017 ª | n = 63 (no further details) Diagnosis: Dementia (not further specified) |
Primary outcomes
|
Multi-sensory stimulation over 24 weeks (n=29) Reading group (n=15) Treatment as usual (n=19) |
|
| Inclusion criteria: | ||||
| ||||
| Definition of severe dementia: MMSE score of 0-10 | ||||
| 6 nursing homes | ||||
|
Note: MMSE: Mini-Mental State Examination; REHAB: Rehabilitation Evaluation Hall and Baker tool; GIP: Behavior Rating Scale for Psychogeriatric Inpatients; BMD: Behaviour and Mood Disturbance Scale; BRS, Behaviour Rating Scale; CMAI: Cohen-Mansfield Agitation Inventory; NPI: Neuropsychiatric Inventory; CDR: Clinical Dementia Rating; FAST: Functional Assessment Staging; NPI-NH: Neuropsychiatric Inventory–Nursing Home Version; GDS: Global Deterioration Scale; CSDD: Cornell Scale for Depression in Dementia; CANE: Camberwell Assessment of Need for the Elderly; QuIS: Quality of Interactions Schedule; QUALID: Quality of Life in Late-stage Dementia scale; PRS: Positive Response Schedule; GADS: Guy’s Advanced Dementia Schedule; BASOLL: Behavioural Assessment Scale of Later Life; SDI: Sleep Disorder Inventory; RAID: Rating Anxiety in Dementia Scale; QoL-AD: Quality of Life-Alzheimer’s Disease Scale; HCS: Holden Communication Scale; ASD: Arousal States in Dementia Scale; Discomfort-DAT: Discomfort Dementia of the Alzheimer’s Type; BEHAVE-AD: Behavioral pathology in Alzheimer's disease; interRAI HC: interRAI-Home Care Assessment; MQS III: Medication Quantification Scale version III; C-PAINAD: Chinese-Pain Assessment in Advanced Dementia; BARS: Brief Agitation Rating Scale; PACSLAC-D: Pain Assessment Checklist for Seniors with Limited Ability to Communicate; MDS-RAI: Minimum Dataset of the Resident Assessment Instrument Pain scale; CIBIC-Plus: Clinician’s Interview-Based Impression of Change-Plus; ADCS-ADL-sev: Alzheimer's Disease Cooperative Study-Activities of Daily Living inventory modified for Severe Patients; SIB: Severe Impairment Battery; FAST-DS: Fast Disability Score; RMBPC: Revised Memory and Behavior Problems Checklist; sMMSE: Severe Mini-Mental State Examination; BANS-S: Bedford Alzheimer Nursing Severity Scale; S-COS: Clinical Outcome Variables; Katz ADL Index: Katz Index of Independence in Activities of Daily Living;
ª Study not included in meta-analysis
3.1. Pharmacological treatments for severity of dementia and global function
Five studies evaluated the effectiveness and safety of donepezil versus placebo (Black et al., 2007, Feldman et al., 2005, Homma et al., 2008, Jia et al., 2017, Winblad et al., 2006), two studies effectiveness of memantine (Reisberg et al., 2003, Winblad and Poritis, 1999), and one study the efficacy of galantamine (Burns et al., 2009) (see Table 1). Four of these trials were conducted in community settings, three in nursing homes, and one study in a hospital setting. We were able to conduct meta-analyses by extracting data for eight outcomes in total.
3.1.1. Severity of symptoms
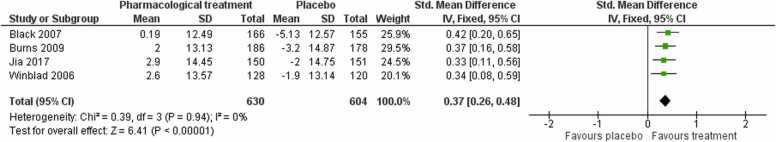
There was moderate‐certainty evidence from four studies that pharmacological treatments may be superior to placebo at improving severity of dementia symptoms at end of treatment (Standardized Mean Difference (SMD) 0.37, 95% Confidence Interval (CI) 0.26–0.48; I² = 0%; 1234 participants; Fig. 2).
Fig. 2.
Forest plot of comparison of pharmacological treatments versus placebo for severity of dementia symptoms at post-treatment.
3.1.2. Activities of daily living
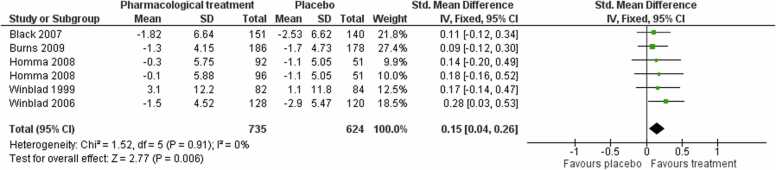
Pooling data from five studies showed that pharmacological treatments probably improve patient function compared to placebo at the end of treatment (SMD 0.15, 95 % CI 0.04–0.26; moderate‐certainty evidence; I² = 0 %; one study contributed two independent comparisons; 1359 participants; Fig. 3), representing a small effect.
Fig. 3.
Forest plot of comparison of pharmacological treatments versus placebo for activities of daily living at post-treatment.
3.1.3. Global impression of change
Pooling data from four studies (Black et al., 2007, Jia et al., 2017, Winblad et al., 2006, Winblad and Poritis, 1999) showed that pharmacological treatments were probably favoured compared to placebo at improving global impression of change measured as a dichotomous outcome at end of treatment (Risk ratio (RR) 1.34, 95 % CI 1.14–1.57; low-certainty evidence; I² = 0 %; 1009 participants). There was very-low certainty evidence that pharmacological treatments were no different to placebo at end of treatment for global impression of change measured as a continuous outcome (SMD −0.11, 95 % CI −0.25 to 0.02; I² = 34 %; 3 studies (Black et al., 2007, Jia et al., 2017, Winblad et al., 2006); 864 participants).
3.1.4. Cognition
We found low-certainty evidence that pharmacological treatments are probably better than placebo at improving cognition at end of treatment (mean difference (MD) 0.78, 95 % CI 0.33–1.23; I2 = 0 %; 3 studies (Black et al., 2007, Jia et al., 2017, Winblad et al., 2006); 832 participants).
3.1.5. Neuropsychiatric symptoms
We pooled four studies to assess the effectiveness of pharmacological treatments on neuropsychiatric symptoms, of which one contributed two independent comparisons. There was low‐certainty evidence that pharmacological treatments may not differ from placebo in their effect on neuropsychiatric symptoms at the end of treatment (SMD −0.06, 95 % CI −0.19 to 0.06; I² = 38 %; 4 studies (Black et al., 2007, Homma et al., 2008, Winblad et al., 2006, Winblad and Poritis, 1999); 1001 participants).
3.1.6. Adverse events
The meta-analyses of the total number of participants experiencing at least one adverse event showed differences in favour of placebo at end of treatment (RR 1.09, 95 % CI 1.03–1.15; I² = 37 %; moderate-certainty evidence; 7 studies, 1924 participants; see Appendix in the Supplement), and no evidence of publication bias (see Appendix in the Supplement).
3.1.7. Serious adverse events
We pooled data from seven studies to examine differences between pharmacological treatments and placebo on the total number of participants experiencing a serious adverse event at end of treatment; there were no differences between the two groups (RR 0.83, 95 % CI 0.67–1.03; I² = 0 %; low-certainty evidence; 7 studies, 1924 participants; see Appendix in the Supplement), and no evidence of publication bias (see Appendix in the Supplement).
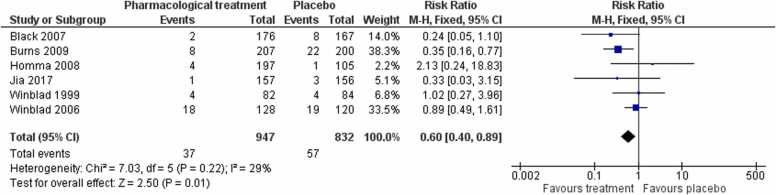
3.1.8. Mortality
We found low-certainty evidence that for number of deaths pharmacological treatments were favoured in comparison to placebo at end of treatment (RR 0.60, 95 % CI 0.40–0.89; I² = 29 %; 6 studies, 1779 participants; Fig. 4). There was no evidence of publication bias (see Appendix in the Supplement). We were not able to extract data for the Reisberg et al. (2003) study as these were not available from the primary paper; the authors reported that analyses favoured treatment for all outcomes tested (data not reported). Hannestad et al. (2021) examined the safety, tolerability, and preliminary efficacy of GRF6019 in people with severe dementia living in both the community and nursing home care; overall results indicated that GRF6019 was safe, with good feasibility and tolerability.
Fig. 4.
Forest plot of comparison of pharmacological treatments versus placebo for number of deaths at post-treatment.
3.2. Pharmacological treatments for people living with severe dementia and neuropsychiatric symptoms
Five trials tested the effectiveness of pharmacological treatments for people living with severe dementia and neuropsychiatric symptoms. Four studies were conducted in nursing homes, with one study recruiting participants from both community and long-term care settings. Two studies examined the effectiveness of risperidone (De Deyn et al., 2005, Mintzer et al., 2006), and one study (Deberdt et al., 2008) the effectiveness of olanzapine on cognition. Magai et al. (2000) assessed the clinical effectiveness of sertraline in patients with severe AD and depression, and Erdal et al. (2018) an active analgesic treatment versus placebo in people with severe AD and clinically significant symptoms of depression. Due to the small number of studies, we were not able to conduct any meta-analyses, and therefore discuss findings narratively.
3.2.1. Neuropsychiatric symptoms
The study by De Deyn et al. (2005) reported significant differences favouring risperidone at end of treatment; for both agitation (mean change from baseline −13.1 for the risperidone group vs −4.9 for the placebo group; p < 0.001), and overall neuropsychiatric symptoms (mean change from baseline −6.0 for the risperidone group vs −3.1 for the placebo group; p < 0.001). Mintzer et al. (2006) found no differences between groups on psychosis (estimated group difference of risperidone minus placebo at endpoint was −1.2 95% CI: −2.5 to 0.1), but a significant effect favouring treatment on global impression of change (χ² [1] = 5.11, p = 0.024).
3.2.2. Cognition
In the study by Deberdt et al. (2008) olanzapine was no different to placebo at end of treatment for cognition in people with severe dementia and neuropsychiatric symptoms (p = 0.78).
3.2.3. Depression
Magai et al. (2000) reported no differences between sertraline versus placebo at post-treatment on both depression scores (sertraline mean at endpoint 3.53 vs 4.43 for placebo), and clinical response at end of treatment (47 % showed ≥ 50 % improvement for sertraline vs 36 % for placebo). Similarly in the study by Erdal et al. (2018) active analgesic treatment comprised of paracetamol or buprenorphine transdermal system was no different to placebo at post-treatment (mean change on depression scores −0.66 in the treatment group vs −3.30 in the placebo group).
3.3. Non-pharmacological treatments
A total of sixteen trials evaluated non-pharmacological interventions versus usual care; 13 studies were conducted in nursing homes, with the remaining three trials conducted in day hospitals, general hospitals, or people living with severe dementia at home. There were four trials evaluating multi-sensory stimulation (Baker et al., 2003, Hutson et al., 2014, Sánchez et al., 2016, Strøm et al., 2017); four studies evaluating person-centered care (Ballard et al., 2018, Clare et al., 2013, Kovach et al., 2006, Reisberg et al., 2017); three trials assessing effectiveness of activities-based interventions (Kovach et al., 2004, Olsen et al., 2016, Sakamoto et al., 2013), and two studies evaluating pain management (Liu and Lai, 2017, Pieper et al., 2018). The remaining trials evaluated bright light treatment (Hjetland et al., 2021), aromatherapy (Ballard et al., 2002), and hip fracture rehabilitation (Stenvall et al., 2012). We were able to extract data for two outcomes.
3.3.1. Neuropsychiatric symptoms
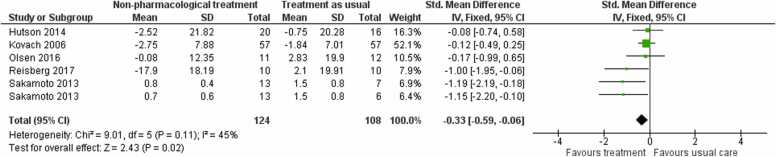
There was low-certainty evidence that nonpharmacological interventions may reduce neuropsychiatric symptoms at end of treatment (SMD −0.33, 95 % CI −0.59 to −0.06; I² = 45 %; 5 studies; of which one contributed two independent comparisons; 232 participants; Fig. 5).
Fig. 5.
Forest plot of comparison of non-pharmacological treatments versus treatment as usual for neuropsychiatric symptoms at post-treatment.
3.3.2. Quality of life
There was very-low certainty evidence that non-pharmacological interventions may not differ from treatment as usual for patient quality of life at end of treatment (SMD 0.31, 95 % CI ‐0.10–0.71; I² = 0 %; 3 studies (Clare et al., 2013, Hutson et al., 2014, Olsen et al., 2016); 95 participants).
3.3.3. Studies not included in the meta-analyses
3.3.3.1. Multi-sensory stimulation
In the study by Baker et al. (2003) multi-sensory stimulation was associated with lower apathy scores compared to control at end of treatment (mean improvement of −0.4 points for the treatment group vs 0.6 points for the control group). Sánchez et al. (2016) evaluated the effectiveness of multi-sensory stimulation versus one-to-one activity sessions versus a control intervention; multi-sensory stimulation was associated with lower agitation compared to control (p < 0.001; η2 =0.30), and greater improvement on severity of symptoms compared to both control and activity sessions (p < 0.001; η2 =0.33). Strøm et al. (2017) assessed the effect of multi-sensory stimulation versus usual care versus reading sessions; this study found that those randomised in multi-sensory stimulation had higher communication scores compared to those randomised in the reading sessions (p = 0.044).
3.3.3.2. Person-centered care interventions
Ballard et al. (2018) evaluated the effectiveness of a person-centered care intervention incorporating antipsychotic review for people living with both moderate and severe dementia in nursing homes; although scores favoured the intervention in reducing neuropsychiatric symptoms for people with severe dementia, results were not significant (p = 0.05), and there was no effect on quality of life (p = 0.97).
3.3.3.3. Activities-based interventions
Kovach et al. (2004) compared an activities-based intervention to usual care; the study reported lower agitation scores favouring the intervention group with no interaction effect on whether the person had moderate or severe dementia (p = 0.488).
3.3.3.4. Pain management
Liu and Lai (2017) evaluated pain management for people with severe dementia living in nursing homes versus usual care; although no differences were observed on the use of pain medications, there was a significant reduction on observational pain scores favouring treatment (p < 0.001). Similarly, in the study by Pieper et al. (2018), pain management decreased overall observed pain but not estimated pain compared to usual care (B = −1.21 points 95 % −2.35 to −0.06; p = 0.020).
3.3.3.5. Other non-pharmacological interventions
Hjetland et al. (2021) tested the effectiveness of bright light treatment for people living with severe dementia in nursing homes; sleep was significantly improved in the intervention group compared to control (p < 0.05). In the study by Ballard et al. (2002) aromatherapy reduced overall levels of agitation for people receiving the intervention compared to those receiving placebo (p < 0.0001). Stenvall et al. (2012) examined the effects of a multidisciplinary hip fracture rehabilitation intervention; those randomised to receive the intervention had less post-operative complications such as falls (p = 0.005), and were more likely to regain overall premorbid ADL levels (p = 0.027).
3.4. Quality of evidence
None of the studies meeting our inclusion criteria were classified as having low risk of bias in all domains of risk assessment. We considered 9 studies to be at unclear risk of bias, and two studies at high risk, for the domain of random sequence generation. Most studies were judged to be at unclear risk of bias for the allocation concealment domain, due to insufficient information provided, and all but two studies had unclear risk in the domain of performance bias, given that personnel and/or participants were not blinded. For the domain of detection bias we assessed eight studies as having unclear risk, and four studies as having high risk in this domain. Sixteen studies were judged to be at unclear risk in the domain of incomplete outcome data, and in eight studies there was evidence of selective reporting indicative of unclear risk in this domain. Other potential biases were identified only in one study. Detailed ratings for each individual study and across all included studies can be seen in Appendix in the Supplement. Given the small number of studies, we tested for publication bias only for three outcomes.
4. Discussion
4.1. Summary of findings
To our knowledge, this is the first high-quality systematic review evaluating the clinical effectiveness of treatments for people with severe dementia. Despite several decades of research, our review finds that systematic evaluation of treatments for people with severe dementia remains largely under-developed, with very few large-scale randomised controlled trials in the area. Nevertheless, our review found 30 studies meeting our inclusion criteria indicating that research in this area is growing. Key findings of our review are that moderate-certainty evidence shows that pharmacological treatment (donepezil, memantine, and galantamine) has benefits for people with severe dementia by improving severity of symptoms and activities of daily living. There was also moderate-certainty evidence that pharmacological treatments were more likely to be associated with adverse effects. Results consistent with benefits in terms of improvement of severity of symptoms and a reduction in functional decline in this group are important as they may translate to reduced costs of care (Lacey et al., 2017), delays in care home admission, and reductions in caregiver burden (Ku et al., 2016).
Despite evidence indicating that pharmacological treatments may potentially benefit people with severe dementia on several other outcomes such as global impression of change, cognition, and mortality, certainty of evidence for these outcomes remains low. We are also very uncertain about the effectiveness of pharmacological treatments in reducing neuropsychiatric symptoms in people with severe dementia, which is generally based on very-low certainty evidence. In addition, most of the studies tested effectiveness of donepezil 10 mg/day, so our knowledge of the specific effects of galantamine and memantine for this group, or effect interactions of dosage of treatments remains limited.
A further important limitation in terms of the completeness and applicability of the evidence is the lack of long-term data of effectiveness of pharmacological treatments beyond 26 weeks, and very limited data reporting on patient quality of life, and caregiver outcomes. Future long-term effectiveness trials therefore of the different pharmacological treatments would be important in order to establish whether any benefits persist beyond 6 months. These studies will also be very informative in terms of the effect of incidence of serious adverse events of these treatments and their long-term safety, and whether effect sizes achieve minimum clinically important differences (Andrews et al., 2019). Nevertheless, our findings do support current clinical guidelines recommending continuation of pharmacological treatments for people at more advanced stages of AD (O'Brien et al., 2017, Schmidt et al., 2015). We also found a small number of studies evaluating the effectiveness of pharmacological treatments for people living with severe dementia and neuropsychiatric symptoms. Due however to the small evidence base we are unable to make any conclusions about these treatments.
An important contribution of our review is that it is the first to systematically examine the clinical effectiveness of non-pharmacological treatments for people with severe dementia. We found sixteen studies evaluating the effectiveness of non-pharmacological interventions, which included mostly interventions aimed at multi-sensory stimulation, supporting person-centered care, and activities-based interventions. Our meta-analyses showed that interventions focusing on person-centered care and activities may also reduce neuropsychiatric symptoms compared to usual care, although these results were based on low-certainty evidence. Additionally, the variation between studies in terms of the nature of the interventions, makes the interpretation of these effects less straightforward. Despite these limitations, however, these results compare favourably with minimal or no benefits of pharmacological treatments and the potential for harm from these treatments. Our review additionally identified two trials evaluating the effectiveness of pain management, and although preliminary, both of these studies suggested that pain management may provide important clinical benefits in terms of reducing observational pain in severe dementia (Liu and Lai, 2017, Pieper et al., 2018).
Although finding several studies testing effectiveness of non-pharmacological interventions across many different countries is encouraging, most studies to date tend to be feasibility and acceptability studies as opposed to large-scale clinical effectiveness trials. Although we did identify one large-scale clinical effectiveness trial (Ballard et al., 2018), this study included people with both moderate and severe dementia, and results indicated that the benefits reported for people with moderate dementia did not extend to people with severe dementia. An important gap therefore to be addressed by future research is the development and evaluation of interventions that directly address needs of people with severe dementia, and those contributing to their care. Similarly to pharmacological treatments data on long-term effectiveness of interventions was lacking, and there were limited data on effects of these interventions on carers. Contrary to pharmacological trials however most of the non-pharmacological interventions were conducted in long-term care settings, therefore results may not be applicable to the increasing number of people living with severe dementia at home (Wittenberg et al., 2020).
4.2. Implications
The evidence to date indicates that further small trials reporting on the effectiveness of pharmacological treatments would not add to the current evidence base, and that future trials should test the efficacy of these treatments long-term as well as investigating their cost-effectiveness across care settings. Evidence of improvements in severity of symptoms and activities of daily living suggest that pharmacological management of people with severe dementia should be closely monitored as it may prevent further disease progression and delay care home admission. An important finding of our review is the observation that many of the studies meeting our inclusion criteria did not follow Consolidated Standards of Reporting Trials, and for a large number of trials data were not extractable. Addressing this methodological issue will be key for future development in this area. There was also evidence of selective reporting which may introduce bias, and for many outcomes such as global impression of change, cognition, quality of life, and neuropsychiatric symptoms evidence remains of low or very-low certainty.
An important finding of our review is that pharmacological treatments were associated with a small effect in reducing risk of mortality for people with severe dementia. This is an important and original finding highlighting a potential survival advantage for people with severe dementia being prescribed pharmacological treatments, however this conclusion remains uncertain due to low-certainty evidence. Similarly, in regards to tolerability although most trials monitored adverse events, reporting of serious adverse events was not detailed, and the long-term safety of pharmacological treatments remains unknown.
An important observation across all studies meeting our inclusion criteria was that people with severe dementia had limited exposure to medication and access to non-pharmacological treatments, indicating that this group remains largely under-treated (Black et al., 2007). We also found that large-scale trials based on well-defined non-pharmacological interventions were generally missing with many studies including multimodal approaches which combined a variety of treatments, with limited input in terms of lived experiences. Initiatives aimed at harmonising and standardising interventions in the area would be important for pooling data in future meta‐analyses. It will be important for future work in the area to develop dementia care interventions that have been developed alongside people with dementia, family carers, and professionals involved in their care. There is also no evidence base on interventions in other types of dementia such as Parkinson's disease dementia, dementia with Lewy bodies, and frontotemporal dementia.
4.3. Limitations
Despite the originality of our findings our review has several limitations. While we employed a systematic approach in identifying studies, we may have still missed trials reporting on outcomes for people with severe dementia. Not all studies used the same ‘definition’ of severe dementia, and although heterogeneity was low in most of our analyses, it is likely that the population differed across studies. Selection bias may have also influenced our results whereby healthier patients with severe dementia may be recruited in these trials. Further longitudinal observational studies are needed to investigate the effects of selection bias in recruiting people living with severe dementia across settings.
Although we extracted data on several outcomes, we could be moderately certain only for the effects of pharmacological treatments on severity of dementia, activities of daily living, and incidence of adverse events. For the remaining analyses, evidence was of low or very-low certainty. Most of the studies included had unclear risk of bias in several domains, and intervention effects were measured at end of treatment, so we are unable to comment on the long-term effectiveness of these treatments. We were also not able to extract data on adverse events considered to be related to treatment as this information was missing from the majority of trials.
5. Conclusion
Our review provides the first comprehensive summary estimate of the clinical effectiveness of both pharmacological and non-pharmacological interventions for people with severe dementia. Our findings highlight an important gap in dementia care research for people with severe dementia. Although our results are consistent with clinical guidelines, they nevertheless show a lack of high-quality research in the area and highlight an urgent need to develop evidence-based interventions supporting and improving care for people with advanced dementia.
To conclude, our review finds moderate-certainty evidence that pharmacological treatments may improve severity of symptoms and activities of daily living at end of treatment for people with severe dementia. There is also evidence that non-pharmacological interventions may reduce neuropsychiatric symptoms, however this finding is less certain. It is likely that integrated care where a combination of both pharmacological and psychosocial care is being provided, may result in the greatest clinical benefits for this under-served and under-represented vulnerable population.
Conflict of interest
The authors have no conflicts of interest to report.
Acknowledgments
This review did not receive any specific funding. Vasiliki Orgeta is supported by the UCLH BRC (Biomedical Research Centre). Jonathan Huntley is funded by a Wellcome Clinical Research Career Development Fellowship. This research was funded in whole, or in part, by the Wellcome Trust [Grant number 214547/Z/18/Z]. For the purpose of open access, the author has applied a CC BY public copyright licence to any Author Accepted Manuscript version arising from this submission (214547/Z/18/Z). Correspondence for this article should be addressed to Vasiliki Orgeta, Associate Professor, Faculty of Brain Sciences, Division of Psychiatry, University College London, Division of Psychiatry 6th Floor, Maple House, 149 Tottenham Court Road, London W1T 7NF. E-mail: v.orgeta@ucl.ac.uk.
Author contribution
Elena Profyri: conceptualisation of the review; selection of studies; data extraction; data analysis; data quality; writing, review and editing of manuscript.
Phuong Leung: conceptualisation of the review; selection of studies; data extraction; data analysis; data quality; review and editing of final draft.
Jonathan Huntley: conceptualisation of the review; data extraction; data analysis; review and editing of final draft.
Vasiliki Orgeta: conceptualisation of the review; selection of studies; data extraction; data analysis; data quality; writing, review and editing of manuscript.
Footnotes
PROSPERO registration (CRD42021193086).
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.arr.2022.101758.
Appendix A. Supplementary material
Supplementary material
.
Data Availability
Data will be made available on request. The data that support the findings of this study are available on request from the corresponding author (VO); please email v.orgeta@ucl.ac.uk.
References
- Andrews J.S., Desai U., Kirson N.Y., Zichlin M.L., Ball D.E., Matthews B.R. Disease severity and minimal clinically important differences in clinical outcome assessments for Alzheimer's disease clinical trials. Alzheimers Dement. 2019;5:354–363. doi: 10.1016/j.trci.2019.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker R., Holloway J., Holtkamp C.C.M., Larsson A., Hartman L.C., Pearce R., Scherman B., Johansson S., Thomas P.W., Wareing L.A., Owens M. Effects of multi-sensory stimulation for people with dementia. J. Adv. Nurs. 2003;43:465–477. doi: 10.1046/j.1365-2648.2003.02744.x. [DOI] [PubMed] [Google Scholar]
- Ballard C., Corbett A., Orrell M., Williams G., Moniz-Cook E., Romeo R., Woods B., Garrod L., Testad I., Woodward-Carlton B., Wenborn J., Knapp M., Fossey J. Impact of person-centred care training and person-centred activities on quality of life, agitation, and antipsychotic use in people with dementia living in nursing homes: a cluster-randomised controlled trial. PLoS Med. 2018:15. doi: 10.1371/journal.pmed.1002500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard C.G., O'Brien J.T., Reichelt K., Perry E.K. Aromatherapy as a safe and effective treatment for the management of agitation in severe dementia: the results of a double-blind, placebo-controlled trial with Melissa. J. Clin. Psychiatry. 2002;63:553–558. doi: 10.4088/jcp.v63n0703. [DOI] [PubMed] [Google Scholar]
- Birks, J.S., Harvey, R.J., 2018. Donepezil for dementia due to Alzheimer's disease. Cochrane Db Syst Rev.
- Black S.E., Doody R., Li H., McRae T., Jambor K.M., Xu Y., Sun Y., Perdomo C.A., Richardson S. Donepezil preserves cognition and global function in patients with severe Alzheimer disease. Neurology. 2007;69:459–469. doi: 10.1212/01.wnl.0000266627.96040.5a. [DOI] [PubMed] [Google Scholar]
- Burns A., Bemabei R., Bullock R., Jentoft A.J.C., Frolich L., Hock C., Raivio M., Triou E., Vandewoude M., Wima A., Came E., Van Baelen B., Hammond G.L., van Gene J.C., Schwalen S. Safety and efficacy of galantamine (Reminyl) in severe Alzheimer's disease (the SERAD study): a randomised, placebo-controlled, double-blind trial. Lancet Neurol. 2009;8:39–47. doi: 10.1016/S1474-4422(08)70261-8. [DOI] [PubMed] [Google Scholar]
- Clare L., Woods R.T., Whitaker R., Wilson B.A., Downs M. Development of an awareness-based intervention to enhance quality of life in severe dementia: trial platform. Trials. 2010:11. doi: 10.1186/1745-6215-11-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clare L., Whitaker R., Woods R.T., Quinn C., Jelley H., Hoare Z., Woods J., Downs M., Wilson B.A. AwareCare: a pilot randomized controlled trial of an awareness-based staff training intervention to improve quality of life for residents with severe dementia in long-term care settings. Int. Psychogeriatr. 2013;25:128–139. doi: 10.1017/S1041610212001226. [DOI] [PubMed] [Google Scholar]
- De Deyn P.P., Katz I.R., Brodaty H., Lyons B., Greenspan A., Burns A. Management of agitation, aggression, and psychosis associated with dementia: A pooled analysis including three randomized, placebo-controlled double-blind trials in nursing home residents treated with risperidone. Clin. Neurol. Neurosurg. 2005;107:497–508. doi: 10.1016/j.clineuro.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Deberdt W.G., Siegal A., Ahl J., Meyers A.L., Landbloom R. Effect of olanzapine on cognition during treatment of behavioral and psychiatric symptoms in patients with dementia: a post-hoc analysis. Int. J. Geriatric Psychiatry. 2008;23:364–369. doi: 10.1002/gps.1885. [DOI] [PubMed] [Google Scholar]
- Edvardsson D., Winblad B., Sandman P. Person-centred care of people with severe Alzheimer's disease: current status and ways forward. Lancet Neurol. 2008;7:362–367. doi: 10.1016/S1474-4422(08)70063-2. [DOI] [PubMed] [Google Scholar]
- Egger M., Davey Smith G., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdal A., Flo E., Aarsland D., Ballard C., Slettebo D.D., Husebo B.S. Efficacy and safety of analgesic treatment for depression in people with advanced dementia: randomised, multicentre, double-blind, placebo-controlled trial (DEP.PAIN.DEM) Drug Aging. 2018;35:545–558. doi: 10.1007/s40266-018-0546-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farlow M.R. Moderate to severe Alzheimer disease - fefinition and clinical relevance. Neurology. 2005;65:S1–S4. [Google Scholar]
- Feldman H., Gauthier S., Hecker J., Vellas B., Xu Y.K., Ieni J.R., Schwam E.M., Grp D.M.S.I. Efficacy and safety of donepezil in patients with more severe Alzheimer's disease: a subgroup analysis from a randomized, placebo-controlled trial. Int. J. Geriatric Psychiatry. 2005;20:559–569. doi: 10.1002/gps.1325. [DOI] [PubMed] [Google Scholar]
- Folstein M.F., Folstein S.E., McHugh P.R. Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Guyatt G., Oxman A.D., Akl E.A., Kunz R., Vist G., Brozek J., Norris S., Falck-Ytter Y., Glasziou P., DeBeer H., Jaeschke R., Rind D., Meerpohl J., Dahm P., Schunemann H.J. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol. 2011;64:383–394. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- Hannestad J., Duclos T., Chao W., Koborsi K., Klutzaritz V., Beck B., Patel A.K., Scott J., Thein S.G., Cummings J.L., Kay G., Braithwaite S., Nikolich K. Safety and tolerability of GRF6019 infusions in severe Alzheimer's disease: a phase II double-blind placebo-controlled trial. J. Alzheimers Dis. 2021;81:1649–1662. doi: 10.3233/JAD-210011. [DOI] [PubMed] [Google Scholar]
- Higgins J.P., Altman D.G., Gotzsche P.C., Juni P., Moher D., Oxman A.D., Savovic J., Schulz K.F., Weeks L., Sterne J.A., Cochrane Bias Methods G., Cochrane Statistical Methods G. The COCHRANE COLLABORation's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjetland G.J., Kolberg E., Pallesen S., Thun E., Nordhus I.H., Bjorvatn B., Flo-Groeneboom E. Ambient bright light treatment improved proxy-rated sleep but not sleep measured by actigraphy in nursing home patients with dementia: a placebo-controlled randomised trial. BMC Geriatr. 2021:21. doi: 10.1186/s12877-021-02236-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homma A., Imai Y., Tago H., Asada T., Shigeta M., Iwamoto T., Takita M., Arimoto I., Koma H., Ohbayashi T. Donepezil treatment of patients with severe Alzheimer's disease in a Japanese population: results from a 24-week, double-blind, placebo-controlled, randomized trial. Dement. Geriatr. Cogn. 2008;25:399–407. doi: 10.1159/000122961. [DOI] [PubMed] [Google Scholar]
- Hughes C.P., Berg L., Danziger W.L., Coben L.A., Martin R.L. A new clinical scale for the staging of dementia. Br. J. Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- Hui E.K., Tischler V., Wong G.H.Y., Lau W.Y.T., Spector A. Systematic review of the current psychosocial interventions for people with moderate to severe dementia. Int. J. Geriatric Psychiatry. 2021;36:1313–1329. doi: 10.1002/gps.5554. [DOI] [PubMed] [Google Scholar]
- Hutson C., Orrell M., Dugmore O., Spector A. Sonas: a pilot study investigating the effectiveness of an intervention for people with moderate to severe dementia. Am. J. Alzheimers Dis. 2014;29:696–703. doi: 10.1177/1533317514534756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia J.P., Wei C.B., Jia L.F., Tang Y., Liang J.H., Zhou A.H., Li F.Y., Shi L., Doody R.S. Efficacy and safety of donepezil in chinese patients with severe Alzheimer's disease: a randomized controlled trial. J. Alzheimers Dis. 2017;56:1495–1503. doi: 10.3233/JAD-161117. [DOI] [PubMed] [Google Scholar]
- Jonsson L., Jonhagen M.E., Kilander L., Soininen H., Hallikainen M., Waldemar G., Nygaard H., Andreasen N., Winblad B., Wimo A. Determinants of costs of care for patients with Alzheimer's disease. Int. J. Geriatric Psychiatry. 2006;21:449–459. doi: 10.1002/gps.1489. [DOI] [PubMed] [Google Scholar]
- Kovach C.R., Taneli Y., Dohearty P., Schlidt A.M., Cashin S., Silva-Smith A.L. Effect of the BACE intervention on agitation of people with dementia. Gerontologist. 2004;44:797–806. doi: 10.1093/geront/44.6.797. [DOI] [PubMed] [Google Scholar]
- Kovach C.R., Logan B.R., Noonan P.E., Schlidt A.M., Smerz J., Simpson M., Wells T. Effects of the serial trial intervention on discomfort and behavior of nursing home residents with dementia. Am. J. Alzheimers Dis. 2006;21:147–155. doi: 10.1177/1533317506288949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku L.J.E., Pai M.C., Shih P.Y. Economic impact of dementia by disease severity: exploring the relationship between stage of dementia and cost of care in Taiwan. PLoS One. 2016:11. doi: 10.1371/journal.pone.0148779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey L., Bobula J., Niecko T., Leibman C. Informal care time and cost in a large clinical trial sample of patients with mild to moderate Alzheimer's disease: determinants and level of change observed. Neurol. Ther. 2017;6:11–23. doi: 10.1007/s40120-016-0056-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J.Y.W., Lai C.K.Y. Implementation of observational pain management protocol for residents with dementia: a cluster-RCT. J. Am. Geriatr. Soc. 2017;65:e56–e63. doi: 10.1111/jgs.14763. [DOI] [PubMed] [Google Scholar]
- Livingston G., Sommerlad A., Orgeta V., Costafreda S.G., Huntley J., Ames D., Ballard C., Banerjee S., Burns A., Cohen-Mansfield J., Cooper C., Fox N., Gitlin L.N., Howard R., Kales H.C., Larson E.B., Ritchie K., Rockwood K., Sampson E.L., Samus Q., Schneider L.S., Selbaek G., Teri L., Mukadam N. Dementia prevention, intervention, and care. Lancet. 2017;390:2673–2734. doi: 10.1016/S0140-6736(17)31363-6. [DOI] [PubMed] [Google Scholar]
- Livingston G., Huntley J., Sommerlad A., Ames D., Ballard C., Banerjee S., Brayne C., Burns A., Cohen-Mansfield J., Cooper C., Costafreda S.G., Dias A., Fox N., Gitlin L.N., Howard R., Kales H.C., Kivimaki M., Larson E.B., Ogunniyi A., Orgeta V., Ritchie K., Rockwood K., Sampson E.L., Samus Q., Schneider L.S., Selbaek G., Teri L., Mukadam N. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396:413–446. doi: 10.1016/S0140-6736(20)30367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magai C., Kennedy G., Cohen C.I., Gomberg D. A controlled clinical trial of sertraline in the treatment of depression in nursing home patients with late-stage Alzheimer's disease. Am. J. Geriatric Psychiatry. 2000;8:66–74. doi: 10.1097/00019442-200002000-00009. [DOI] [PubMed] [Google Scholar]
- McShane, R., Westby, M.J., Roberts, E., Minakaran, N., Schneider, L., Farrimond, L.E., Maayan, N., Ware, J., Debarros, J., 2019. Memantine for dementia. Cochrane Db Syst Rev. [DOI] [PMC free article] [PubMed]
- Mintzer J., Greenspan A., Caers I., Van Hove I., Kushner S., Weiner M., Gharabawi G., Schneider L.S. Risperidone in the treatment of psychosis of Alzheimer disease: Results from a prospective clinical trial. Am. J. Geriatric Psychiatry. 2006;14:280–291. doi: 10.1097/01.JGP.0000194643.63245.8c. [DOI] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D.G., Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6 [PMC free article] [PubMed] [Google Scholar]
- NICE, 2018. Dementia: assessment, management and support for people living with dementia and their carers. NICE guideline [NG97]. [PubMed]
- O'Brien J.T., Holmes C., Jones M., Jones R., Livingston G., McKeith I., Mittler P., Passmore P., Ritchie C., Robinson L., Sampson E.L., Taylor J.P., Thomas A., Burns A. Clinical practice with anti-dementia drugs: a revised (third) consensus statement from the British Association for Psychopharmacology. J. Psychopharmacol. 2017;31:147–168. doi: 10.1177/0269881116680924. [DOI] [PubMed] [Google Scholar]
- Olsen C., Pedersen I., Bergland A., Enders-Slegers M.J., Patil G., Ihlebaek C. Effect of animal-assisted interventions on depression, agitation and quality of life in nursing home residents suffering from cognitive impairment or dementia: a cluster randomized controlled trial. Int J. Geriatric Psychiatry. 2016;31:1312–1321. doi: 10.1002/gps.4436. [DOI] [PubMed] [Google Scholar]
- Orgeta V., Leung P., Del-Pino-Casado R., Qazi A., Orrell M., Spector A.E., Methley A.M. Psychological treatments for depression and anxiety in dementia and mild cognitive impairment. Cochrane Database Syst. Rev. 2022;4 doi: 10.1002/14651858.CD009125.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieper M.J.C., van der Steen J.T., Francke A.L., Scherder E.J.A., Twisk J.W.R., Achterberg W.P. Effects on pain of a stepwise multidisciplinary intervention (STA OP!) that targets pain and behavior in advanced dementia: A cluster randomized controlled trial. Palliat. Med. 2018;32:682–692. doi: 10.1177/0269216316689237. [DOI] [PubMed] [Google Scholar]
- Prince M., Bryce R., Albanese E., Wimo A., Ribeiro W., Ferri C.P. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement. 2013;9:63–75. doi: 10.1016/j.jalz.2012.11.007. [DOI] [PubMed] [Google Scholar]
- Reisberg B. Functional assessment staging (FAST) Psychopharmacol. Bull. 1988;24:653–659. [PubMed] [Google Scholar]
- Reisberg B., Ferris S.H., de Leon M.J., Crook T. The global deterioration scale for assessment of primary degenerative dementia. Am. J. Psychiatry. 1982;139:1136–1139. doi: 10.1176/ajp.139.9.1136. [DOI] [PubMed] [Google Scholar]
- Reisberg B., Doody R., Stoffler A., Schmitt F., Ferris S., Mobius H.J., Memantine Study G. Memantine in moderate-to-severe Alzheimer's disease. New Engl. J. Med. 2003;348:1333–1341. doi: 10.1056/NEJMoa013128. [DOI] [PubMed] [Google Scholar]
- Reisberg B., Shao Y., Golomb J., Monteiro I., Torossian C., Boksay I., Shulman M., Heller S., Zhu Z., Atif A., Sidhu J., Vedvyas A., Kenowsky S. Comprehensive, individualized, person-centered management of community-residing persons with moderate-to-severe Alzheimer disease: a randomized controlled trial. Dement. Geriatr. Cogn. Disord. 2017;43:100–117. doi: 10.1159/000455397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto M., Ando H., Tsutou A. Comparing the effects of different individualized music interventions for elderly individuals with severe dementia. Int. Psychogeriatr. 2013;25:775–784. doi: 10.1017/S1041610212002256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez A., Marante-Moar M.P., Sarabia C., de Labra C., Lorenzo T., Maseda A., Millan-Calenti J.C. Multisensory stimulation as an intervention strategy for elderly patients with severe dementia: a pilot randomized controlled trial. Am. J. Alzheimers Dis. 2016;31:341–350. doi: 10.1177/1533317515618801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt R., Hofer E., Bouwman F.H., Buerger K., Cordonnier C., Fladby T., Galimberti D., Georges J., Heneka M.T., Hort J., Laczo J., Molinuevo J.L., O'Brien J.T., Religa D., Scheltens P., Schott J.M., Sorbi S. EFNS-ENS/EAN guideline on concomitant use of cholinesterase inhibitors and memantine in moderate to severe Alzheimer's disease. Eur. J. Neurol. 2015;22:889–898. doi: 10.1111/ene.12707. [DOI] [PubMed] [Google Scholar]
- Shepherd V., Wood F., Griffith R., Sheehan M., Hood K. Protection by exclusion? The (lack of) inclusion of adults who lack capacity to consent to research in clinical trials in the UK. Trials. 2019;20:474. doi: 10.1186/s13063-019-3603-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenvall M., Berggren M., Lundstrom M., Gustafson Y., Olofsson B. A multidisciplinary intervention program improved the outcome after hip fracture for people with dementia-Subgroup analyses of a randomized controlled trial. Arch. Gerontol. Geriatrics. 2012;54:E284–E289. doi: 10.1016/j.archger.2011.08.013. [DOI] [PubMed] [Google Scholar]
- Strøm B.S., Engedal K., Benth J.S., Grov E.K. Effect of the sonas programme on communication in people with dementia: a randomized controlled trial. Dement Ger. Cogn. Disor. Extra. 2017;7:122–135. doi: 10.1159/000468147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winblad B., Poritis N. Memantine in severe dementia: Results of the M-9-BEST study (benefit and efficacy in severly demented patients during treatment with memantine) Int J. Geriatr. Psychiatry. 1999;14:135–146. doi: 10.1002/(sici)1099-1166(199902)14:2<135::aid-gps906>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Winblad, B., Kilander, L., Eriksson, S., 2006. Donepezil in patients with severe Alzheimer's disease: double-blind, parallel-group, placebo-controlled study (vol 367, pg 1057, 2006). Lancet 368, 1650–1650. [DOI] [PubMed]
- Wittenberg R., Hu B., Jagger C., Kingston A., Knapp M., Comas-Herrera A., King D., Rehill A., Banerjee S. Projections of care for older people with dementia in England: 2015 to 2040. Age Ageing. 2020;49:264–269. doi: 10.1093/ageing/afz154. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
Data will be made available on request. The data that support the findings of this study are available on request from the corresponding author (VO); please email v.orgeta@ucl.ac.uk.