Abstract
The fetus can deploy a local or systemic inflammatory response when exposed to microorganisms or, alternatively, to non-infection-related stimuli (e.g., danger signals or alarmins). The term “Fetal Inflammatory Response Syndrome” (FIRS) was coined to describe a condition characterized by evidence of a systemic inflammatory response, frequently a result of the activation of the innate limb of the immune response. FIRS can be diagnosed by an increased concentration of umbilical cord plasma or serum acute phase reactants such as C-reactive protein or cytokines (e.g., interleukin-6). Pathologic evidence of a systemic fetal inflammatory response indicates the presence of funisitis or chorionic vasculitis. FIRS was first described in patients at risk for intraamniotic infection who presented preterm labor with intact membranes or preterm prelabor rupture of the membranes. However, FIRS can also be observed in patients with sterile intra-amniotic inflammation, alloimmunization (e.g., Rh disease), and active autoimmune disorders. Neonates born with FIRS have a higher rate of complications, such as early-onset neonatal sepsis, intraventricular hemorrhage, periventricular leukomalacia, and death, than those born without FIRS. Survivors are at risk for long-term sequelae that may include bronchopulmonary dysplasia, neurodevelopmental disorders, such as cerebral palsy, retinopathy of prematurity, and sensorineuronal hearing loss. Experimental FIRS can be induced by intra-amniotic administration of bacteria, microbial products (such as endotoxin), or inflammatory cytokines (such as interleukin-1), and animal models have provided important insights about the mechanisms responsible for multiple organ involvement and dysfunction. A systemic fetal inflammatory response is thought to be adaptive, but, on occasion, may become dysregulated whereby a fetal cytokine storm ensues and can lead to multiple organ dysfunction and even fetal death if delivery does not occur (“rescued by birth”). Thus, the onset of preterm labor in this context can be considered to have survival value. The evidence so far suggests that FIRS may compound the effects of immaturity and neonatal inflammation, thus increasing the risk of neonatal complications and long-term morbidity. Modulation of a dysregulated fetal inflammatory response by the administration of antimicrobial agents, anti-inflammatory agents, or cell-based therapy holds promise to reduce infant morbidity and mortality.
Keywords: Cerebral palsy, Chorioamnionitis, Congenital dermatitis, Cytokines, Fetal cytokine release syndrome, Fetal cytokine storm, Fetal hematophagocytic syndrome, Fetal macrophage activation-like syndrome, FIRS, Funisitis, Interleukin-6, Intra-amniotic infection, Intra-amniotic inflammation, Neonatal encephalopathy, Neonatal morbidity, Neonatal sepsis, Neuroinflammation perinatal morbidity, Prematurity, Premature birth, Preterm labor, Preterm prelabor rupture of the membranes (preterm PROM), Retinopathy of prematurity, Sensorineuronal hearing loss
1. The term “fetal inflammatory response syndrome” (FIRS)
The human fetus can deploy an inflammatory response when exposed to microbial invasion with bacteria [1], viruses [2,3], fungi [4, 5], and protozoa [6–8], or non-infection related stimuli. The inflammatory process can be localized to an organ (e.g., the lung following fetal aspiration of amniotic fluid) or become systemic when inflammatory mediators enter the circulation.
We coined the term “Fetal Inflammatory Response Syndrome” (FIRS) while studying the role of intra-amniotic infection in spontaneous preterm labor to describe the presence of systemic inflammation akin to that observed in adult patients with a systemic inflammatory response syndrome [9].
The term “Systemic Inflammatory Response Syndrome” (SIRS) emerged from the consensus conference of the American College of Chest Physicians and the Society for Critical Care Medicine in 1992, as participants recognized that clinical manifestations of sepsis could also be observed in patients without infection (e.g., burns, trauma, pancreatitis, ischemia, immune-mediated injury), and that they resulted from a systemic inflammatory process [10]. The group also proposed the term “Multiple Organ Dysfunction Syndrome” (MODS) to refer to the presence of altered organ function in an acutely ill patient, (e.g., homeostasis cannot be maintained without intervention) [10].
SIRS has been diagnosed in adults using the criteria displayed in Table 1. Yet, the definition could not be applied to the human fetus because vital signs (except for the fetal heart rate) and white blood cell counts cannot be readily determined before birth. For this reason, we elected to define FIRS as an elevated concentration of fetal plasma interleukin-6 (IL-6).
Table 1.
Criteria for systemic inflammatory response syndromea.
| Two or more of the following criteria should be met: • Temperature >38 °C or <36 °C • Heart rate >90 beats/min • Respiratory rate >20 breaths/min or PaCO2 <32 mm Hg • White blood cell count >12,000/mm3 or <4000/mm3 or >10% immature bands |
Modified with permission from American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992; 20:864–74.
In 2001, the American College of Chest Physicians and the Society of Critical Care Medicine reaffirmed the initial criteria for the diagnosis of SIRS. The group of experts noted that an elevation of plasma concentration of certain mediators, such as IL-6, could be associated with SIRS and speculated that this observation could bring about a new definition of the syndrome in adult patients. Concerns at the time were that the clinical and laboratory findings originally proposed to characterize SIRS were non-specific. The terms used to assess patients with suspected sepsis have continued to evolve [11]. Yet, it is clear that the host immune response is key in the recovery of patients as well as in the predisposition to secondary infections and morbidity/mortality.
The immunological response to infection or tissue injury varies over time and involves both pro- and anti-inflammatory responses. The initial concept was that SIRS was followed by compensatory anti-inflammatory response syndrome and that this process led to MODS and predisposed to death. In the early stages of the study of SIRS, it was believed that most patients died because of an excessive inflammatory response. However, subsequent observations uncovered the importance of the counter-inflammatory response that leads to progressive immune suppression and predisposition to secondary infection. It is now recognized that both pro- and anti-inflammatory responses are activated in early sepsis; however, the pro-inflammatory response is predominant [12–14]. As the disorder progresses, the anti-inflammatory limb of the immune response becomes predominant, and patients recovering from sepsis are more susceptible to secondary infections from bacteria, or even reactivation of latent viral infections (e.g., cytomegalvirus, herpes simplex virus) [15, 16]. Indeed, patients who have recovered from sepsis remain at risk of death for approximately one year after the sepsis episode, secondary to the prolonged period of immunosuppression [13,14]. The early pro-inflammatory phase has been attributed predominantly to activity of the innate immune system, while the counter-immune response has been attributed to a dysregulated adaptive immune system: this is probably an oversimplification of the complex nature of the evolution and interaction of different components of the immune system. However, the conceptual framework of an anti-inflammatory response in neonates is important because it may explain why some neonates are born with FIRS [17,18], improve clinically, and then become affected by late-onset neonatal sepsis [19].
2. Why focus on systemic fetal inflammation or FIRS?
In 1998 when the initial work in this field was reported, intra-amniotic infection in patients with preterm labor and intact membranes, as well as preterm prelabor rupture of the membranes (PROM), was known to be associated with impending delivery. However, it was not clear whether labor was associated with an intra-amniotic or a fetal systemic inflammatory response. Moreover, the frequency with which intraamniotic infection led to fetal infection and sepsis was unknown.
We had observed that of preterm neonates born to mothers with intra-amniotic inflammation/infection only a fraction had proven neonatal sepsis; yet, many of these neonates had morbidity which could be attributed at least in part to a systemic inflammatory process, but not necessarily to sepsis. Therefore, an important question emerged: does fetal inflammation predispose to multiple organ dysfunction and result in a higher rate of neonatal morbidity?
The operative definition of FIRS was an elevation in the fetal plasma concentration of IL-6. Since our group was studying pregnancy outcomes using cytokines to define the presence or absence of intra-amniotic inflammation [9], we chose IL-6 because this cytokine could be measured in both amniotic fluid and umbilical cord blood; therefore, we could ascertain the presence and intensity of the intra-amniotic and fetal inflammatory responses based on one analyte. Other cytokines (e.g., TNF-α and IL-1β) were not consistently detected in peripheral blood with the assays available at the time. We also chose IL-6 as a marker of inflammation because this cytokine is a major mediator of the acute phase response to infection or tissue injury (e.g., IL-6 induces production of C-reactive protein).
We used the term syndrome during the course of initial studies given our observation that both intra-amniotic inflammation and fetal systemic inflammation could be caused by infection and non-infection-related etiologies. We predicted that FIRS would: 1) be associated with the spontaneous onset of labor; 2) be associated with a higher rate of neonatal morbidity, since the fetuses were already affected in utero; and 3) lead to changes/dysfunction in multiple organ systems.
3. FIRS is followed by the spontaneous onset of preterm labor
Preterm labor in the setting of infection results from the action of pro-inflammatory cytokines secreted by the mother and/or fetus in response to intra-amniotic infection [20]. Delivery would allow the mother to maximize reproductive fitness and the fetus to exit a hostile intrauterine environment. The mechanisms of parturition require cooperation between the mother and the conceptus as the effector organs of parturition are maternal (myometrium, decidua, and cervix), but there is a substantial contribution of the conceptus (chorioamniotic membranes).
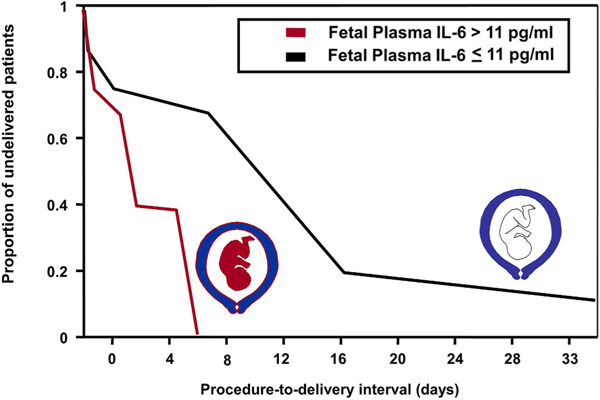
In a study of patients with preterm PROM who were not in labor upon admission, we found that FIRS was associated with a higher rate of spontaneous preterm delivery within 48 and 72 h of amniocentesis, compared to those without FIRS (48 h: 88% vs. 29.7%; and 72 h: 88% vs. 35%; p-value <0.05 for both) [20]. Moreover, patients with initiation of labor and delivery within 48 h of amniocentesis had a higher proportion of fetuses with plasma IL-6 values > 11 pg/mL than patients delivered >48 h [58% (7/12) vs. 8% (1/13), respectively, p-value <0.05] (Fig. 1). Multivariate analysis showed that plasma IL-6 was the only factor associated with pregnancy duration after adjusting for gestational age, amniotic fluid IL-6, and the microbiologic state of the amniotic cavity. The relationship between intra-amniotic inflammation and fetal systemic inflammation and the onset of preterm labor is displayed in Fig. 2. These findings led to the conclusion that FIRS was followed by the onset of preterm parturition in patients with preterm PROM.
Fig. 1.
Fetuses with fetal inflammatory response syndrome (FIRS) have a shorter intrauterine stay than those without FIRS. Mothers were admitted with preterm premature rupture of membranes and patients were not in labor at admission. The interval between the procedure and delivery reflects duration of pregnancy and spontaneous onset of labor. Fetuses with fetal plasma IL-6 concentrations greater than 11 pg/mL have a shorter procedure-to-delivery interval than those with plasma IL-6 concentrations of 11 pg/mL or less (median 0.8 days [range 0.1–5 days] vs. median 6 days [range 0.2–33.6 days]; respectively; P < 0.05). (Modified with permission from Romero R, Gomez R, Ghezzi F et al.: A fetal systemic inflammatory response is followed by the spontaneous onset of preterm parturition. Am J Obstet Gynecol 1998; 179:186–193.).
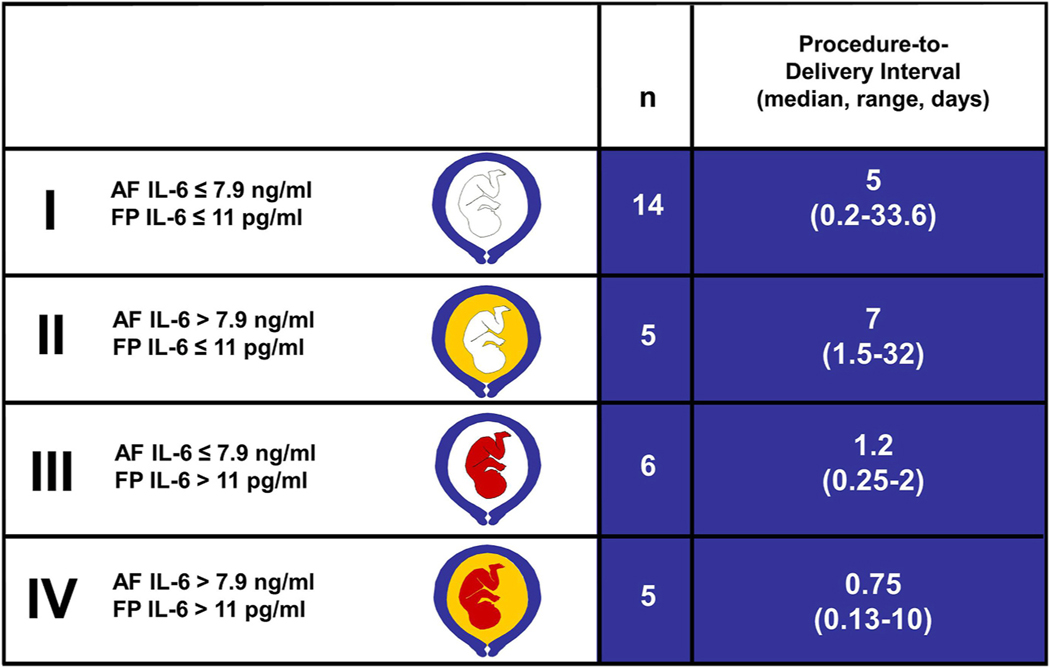
Fig. 2.
Duration of pregnancy according to whether or not there is intraamniotic inflammation and fetal systemic inflammation (FIRS). The key determinant of pregnancy duration is fetal systemic inflammation regardless of the inflammatory status of the amniotic cavity, as reflected by the procedure-to-delivery interval. The inflammatory status of the amniotic cavity and the fetus was assessed with interleukin-6 (IL-6) concentrations. A white color fetus represents no inflammation [defined as fetal plasma (FP) IL-6 less than 11 pg/mL]. A red color fetus represents fetal systemic inflammation (FP IL-6 greater than 11 pg/mL). The amniotic fluid compartment is either white (no intraamniotic inflammation) or yellow in color (intraamniotic inflammation present). The cut-off value of 7.9 ng/ml was used to define intra-amniotic inflammation. The number of patients in each group is depicted (n). (Reproduced with permission from Romero R, Gomez R, Ghezzi F et al: A fetal systemic inflammatory response is followed by the spontaneous onset of preterm parturition. Am J Obstet Gynecol 179:186–193, 1998.).
4. FIRS is associated with a higher rate of neonatal morbidity and mortality
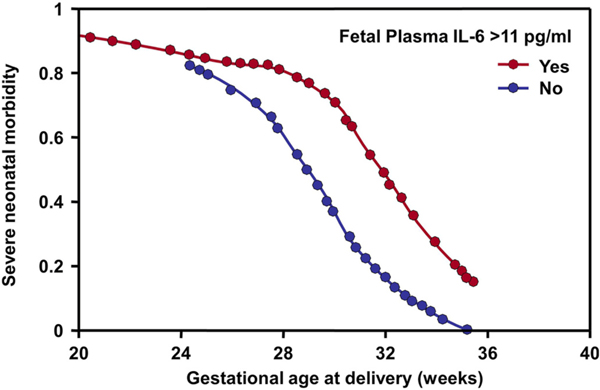
We then tested the hypothesis of whether fetuses with systemic inflammation would have a higher rate of neonatal morbidity. Just as adult patients with SIRS are critically ill, we reasoned that fetuses with FIRS were more likely to present morbidity after birth. To address this question, we studied patients with preterm labor and intact membranes as well as those with preterm PROM. Severe neonatal morbidity was defined as the presence of respiratory distress syndrome, suspected or proven neonatal sepsis, pneumonia, bronchopulmonary dysplasia, intraventricular hemorrhage, or necrotizing enterocolitis. The presence of FIRS (fetal plasma IL-6 >11 pg/mL) conferred a higher rate of neonatal morbidity than in those without FIRS (77.8% vs. 29%; p < 0.001) (Fig. 3) [9]. Multivariate analysis showed that FIRS was an independent predictor of severe neonatal morbidity after adjusting for gestational age, the obstetrical cause of preterm delivery (preterm labor or preterm PROM), clinical chorioamnionitis, presence of microorganisms in the amniotic cavity, and amniotic fluid IL-6 results. Importantly, the determination of the presence/absence of fetal systemic inflammation was done before delivery, thus the findings cannot be attributed to an intrapartum phenomenon.
Fig. 3.
Fetal inflammatory response syndrome (FIRS) was associated with a higher severe neonatal morbidity than the absence of FIRS. FIRS was defined as a fetal plasma IL-6 >11 pg/mL. This was calculated using logistic regression to adjust for gestational age and other covariates. (Reproduced and modified with permission from Gomez R, Romero R, Ghezzi F et al.: The fetal inflammatory response syndrome. Am J Obstet Gynecol 179:194–202, 1998.).
5. The clinical significance of FIRS
A systematic review and meta-analysis of observational studies including 1116 neonates has shown that FIRS was associated with a higher frequency of adverse outcomes – specifically, early-onset sepsis (RR = 3.1), bronchopulmonary dysplasia (RR = 5.9), intraventricular hemorrhage (RR = 4.9), periventricular leukomalacia (RR = 3.3), respiratory distress syndrome (RR = 2.4), and neonatal death (RR = 7.0), when compared to neonates without FIRS [21]. In preterm neonates, FIRS was significantly and independently associated with an increased risk of retinopathy of prematurity and its progression [22]. Moreover, FIRS is also associated with a neonatal systemic inflammatory response, which manifests as clinically suspected neonatal sepsis with negative blood and cerebrospinal fluid cultures [23–25]. Future studies using a uniform definition of FIRS would strengthen the accuracy of these observations.
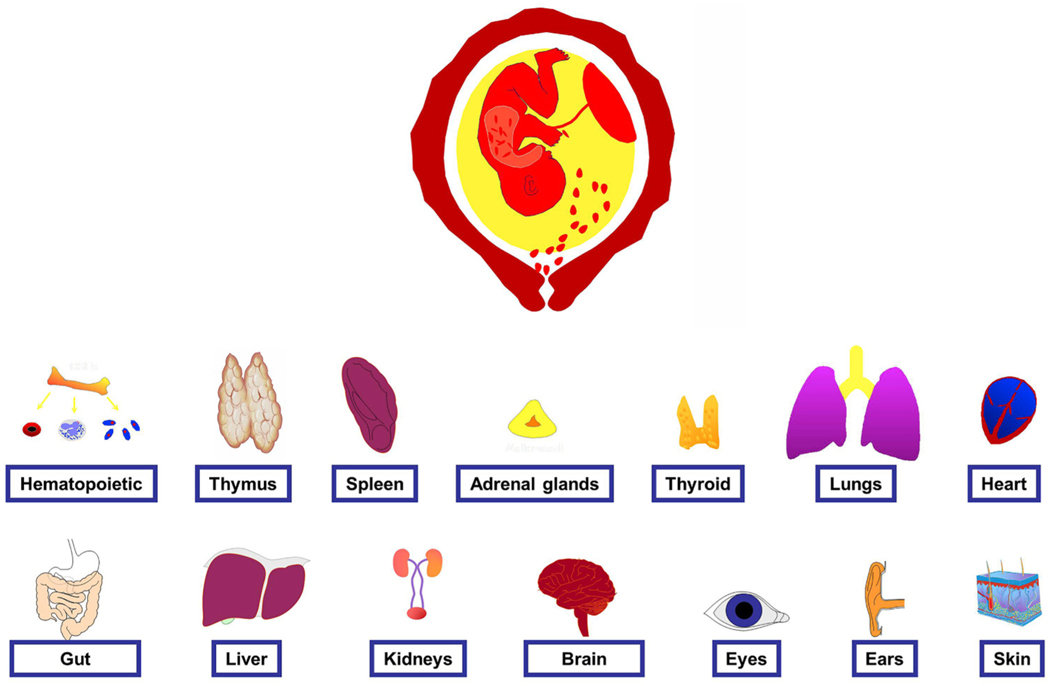
6. Evidence of multi-systemic involvement in FIRS
We had predicted that the fetus with FIRS will have evidence of multisystemic involvement in utero or in the immediate neonatal period (Fig. 4). However, there are important limitations to the study of this hypothesis in humans. Improved understanding of FIRS comes from studies in animal models—in particular, the pioneering contributions of the laboratories of Kallapur, Jobe, Adams, and Waldorf in the United States, Newnham in Australia, Kramer in the Netherlands, and Hallman in Finland.
Fig. 4.
The fetal inflammatory response syndrome (FIRS) is associated with multi-systemic involvement. Clinical and/or experimental evidence suggests that there is involvement of the organs displayed in the figure. The data to support this conclusion is reviewed in the article. (Modified with permission from Gotsch F, Romero R, Kusanovic JP, Mazaki-Tovi S, Pineles B, Erez O, Espinoza J, Hassan SS. Fetal Inflammatory Response Syndrome. Clinical Obstetrics and Gynecology 50:652–683; 2007).
6.1. Hematopoietic system
Neutrophils, a major component of the innate immune system, play a critical role in the host response against infection and other insults. During human fetal development, neutrophils first appear in the clavicular bone marrow at 12–13 weeks of gestation [26]. However, lymphocytes are the predominant circulating white blood cells in the preterm fetus [27]. After 32 weeks of gestation, the proportion of neutrophils increases in fetal blood to become the predominant leukocyte at term [27,28].
The initial study of the hematologic profile in FIRS reported that affected fetuses had a higher median corrected white blood cell count and corrected neutrophil count than unaffected fetuses. Neutrophilia (neutrophil count >95th percentile for gestational age) was found in 71% (30/42) of cases, while neutropenia (neutrophil count <5th percentile for gestational age) was present in 4.8% (2/42) [29]. No detectable changes in lymphocyte, monocyte, basophil or eosinophil counts were observed in the initial report.
In a subsequent study of preterm neonates born after spontaneous preterm labor and preterm PROM, Kim et al. reported the hematologic profiles in umbilical cord blood. FIRS was defined as the presence of funisitis, and the authors reported that preterm neonates with funisitis had significantly higher rates of neutrophilia (>95th percentile for gestational age) [93.2% (41/44) vs. 77.8% (119/153); p = 0.027] and monocytosis (>95th percentile for gestational age) [81.8% (36/44) vs. 64.1% (98/153); p = 0.026] than those without funisitis [30].
In addition to the change in number, FIRS (defined by the presence of funisitis) has also been associated with phenotypic evidence of monocyte and granulocyte activation [31]. Indeed, the umbilical cord blood of neonates with acute funisitis had a higher mean channel brightness for CD14 and CD64 on granulocytes and of CD64 on monocytes and a higher basal level of intracellular reactive oxygen species and oxidative burst in monocytes [32].
The mechanisms responsible for the quantitative and qualitative changes in neutrophils in the presence of FIRS have not been elucidated. However, the concentration of granulocyte colony stimulating factor (G-CSF), a cytokine and major physiologic regulator of neutrophil production released during stressful conditions, as well as the activation of neutrophils [33–35], is higher in fetuses with FIRS compared to those without FIRS [36].
Changes in the red blood cell lineage in FIRS (defined by an elevated fetal plasma IL-6 concentration) have also been observed. Affected fetuses have a slightly higher median nucleated red blood cell count (adjusted for gestational age) compared to those without FIRS [29]. An elevated nucleated red blood cell count has also been observed in neonates delivered from patients with prolonged (>24 h) rupture of the fetal membranes [37], histologic chorioamnionitis [38], a high acute placental inflammation score [39], and early-onset neonatal sepsis [40]. Since changes in nucleated red blood cells in FIRS are not associated with a lower pH or PO2 [41], metabolic acidemia/hypoxemia is unlikely to be the cause of the observed changes [42]. Given that the IL-6 concentration in umbilical cord blood correlates with the nucleated red blood cell count [43], IL-6 may mediate these changes [44,45], either directly or indirectly, through erythropoietin. The latter possibility must be considered since the serum erythropoietin concentration is higher in newborns with funisitis than in those without funisitis [46].
Experimental fetal systemic inflammation induced by the administration of IL-1α, an alarmin, into the amniotic cavity of pregnant sheep has been associated with a decreased number of circulating neutrophils within 24 h (early response), followed by an increase in neutrophil numbers at 3 and 7 days (late response) [47]. Fetal systemic inflammation induced by exposure to bacterial endotoxin in ewes is associated with an increased number of circulating neutrophils 7 days after exposure [48]. Consistent with these findings, preterm pigs exposed to intra-amniotic bacterial endotoxin for 3 days before delivery also demonstrated increased blood neutrophil counts at birth [49]. It is possible that the frequency of neutrophilia in human umbilical cord blood reflects at least, in part, the duration of exposure to an inflammatory stimulus.
6.2. Thymus
Thymic involution occurs after infection in the fetus and neonate [50–55]. Subclinical chorioamnionitis has been associated with a small thymus in very-low-birth-weight infants [50], possibly from acute fetal [51] and neonatal [51,52] involution. In neonates, thymic involution correlates with the duration of acute illness and with the percentage of lymphocytes in peripheral blood [52]. The proposed mechanism for thymic involution is thought to be stress (via glucocorticoid production) and the effects of proinflammatory cytokines [56–58].
A sonographically small thymus for gestational age has been reported by Di Naro et al. [59] and other investigators [53–55,60] in the presence of intra-amniotic infection and inflammation (preterm labor and intact membranes or preterm PROM). Another study reported that infants born preterm (<28 weeks of gestation) with sonographic signs of cerebral white matter damage have thymic involution more frequently than a control group matched for gestational age [61]. This has been interpreted as indicating that neurologic injury and thymic involution may have a common origin, namely fetal systemic inflammation.
Intra-amniotic administration of bacterial endotoxin in sheep results in both decreased thymic weight and corticomedullary ratio. The effects can be observed as early as 5 h after exposure to endotoxin [62,63]. Increased expression of IL-6 and type I interferons, as well as glucocorticoids, has been implicated in thymic involution [63–67]. In addition, an increase in the percentage of CD3+ cells, but a decrease in the percentage of CD8+ (cytotoxic lymphocytes) and FOXP3+ cells, occurs after exposure to endotoxin [62,63,68]. Such findings suggest that endotoxin exposure has an effect not only on the innate but also on the adaptive immune response. Taken together, FIRS can induce structural, functional, and immunologic changes in the thymus.
Activation of T cells (belonging to the adaptive limb of the immune response) has been observed in term and preterm infants born to mothers with clinical chorioamnionitis. Such infants demonstrate overexpression of CD25, HLA-DR, or CD69 markers in T cells [69], a higher number of memory T cells (CD45RO+), and a decreased number of naive T cells [69–72]. Further studies are required to comprehensively describe changes in cells involved in adaptive immunity in the presence of fetal systemic inflammation from different etiologies (e.g., bacterial and viral infections, non-infection related causes) and at varying gestational ages (i.e., preterm and term).
It is noteworthy that studies of fetal rhesus macaques report that the number of T regulatory cells (FOXP3+) is decreased, while there is an increase in the number of IL-17 (pro-inflammatory cytokine) producing cells in lymphoid tissues after intra-amniotic administration of IL-1β [73]. However, these changes were not observed in fetal blood [73].
6.3. Spleen
The spleen is the largest secondary lymphoid organ, and plays an important role in immunological processes, hematopoiesis, and red blood cell clearance [74]. The unique physical organization of the organ is thought to facilitate filtering of microorganisms and abnormal cells [74,75]. In adults affected by sepsis, there is a loss of B and CD4 T cells in the spleen, and similar findings have been reported in fetuses and neonates. In a study of 20 fetuses exposed to intra-amniotic infection/inflammation (diagnosed by acute histologic chorioamnionitis or funisitis), 10 neonates died of proven sepsis and showed splenic depletion [76]. Indeed, the percentage of red and white pulp areas occupied by CD45RA+, CD45RO+, and CD20+ cells was reduced [76].
Changes in the fetal splenic circulation have also been observed in the context of intra-amniotic infection/inflammation [77,78]. Under normal circumstances, the fetal splenic vein has a non-pulsatile Doppler waveform. However, Musilova et al. reported a pulsatile flow pattern in the fetal splenic vein in 84% (16/19) of cases with funisitis but in only 15% (9/60) of cases without fetal inflammation [79]. In addition, fetuses with a pulsatile splenic vein flow pattern exhibited a higher concentration of umbilical cord blood IL-6 than those with continuous flow [median 56.7 pg/mL versus 5.6 pg/mL; p < 0.0001] [77]. Collectively, this suggests that non-invasive interrogation of the fetal circulation may help to identify the fetus with systemic inflammation. The mechanism whereby pulsatile flow in the splenic vein is generated is thought to result from increased intrasplenic pressure secondary to microbial product-induced impairment of splenic drainage into the lymphatic circulation.
Several experimental studies have examined the effect of cytokines and bacterial endotoxin on the fetal spleen [48,80]. Kramer et al. demonstrated a two-fold increase in TNF-α expression in the fetal spleen 24 h after intra-amniotic injection of LPS [48]. A subsequent study showed that intra-amniotic LPS-induced changes in the splenic cytokine profile and increased CD3 expression, a marker for T cells [80]. Cytokine changes in the fetal spleen have been detected as early as 5 h after the injection of LPS and for up to 15 days after the onset of inflammation, indicating that intrauterine exposure to an inflammatory stimulus caused a rapid and sustained response in the fetal spleen [80]. Taken together, the available evidence suggests that intra-amniotic infection or intra-amniotic inflammation can induce changes in the developing fetal spleen. The long-term consequences of these changes in the spleen require investigation.
6.4. Adrenal glands
The adrenal glands are involved in the response to sepsis and systemic inflammation. Adult patients admitted to the intensive care unit with sterile inflammatory processes, such as burns or pancreatitis, present endocrine evidence of stress, as indicated by an elevation in the cortisol and dehydroepiandrosterone sulfate ratio [81]. The same has been reported in the human fetus [81] and has been implicated in the mechanisms for preterm labor. Specifically, Yoon et al. reported a significant association between the fetal plasma cortisol and dehydroepiandrosterone sulfate ratio and pregnancy duration in patients with preterm PROM (hazard ratio 2.9; 95% CI: 1 to 8.4) [81]. Patients with preterm PROM who went into spontaneous labor and delivered within 7 days of sampling had a significantly higher median fetal plasma concentration of cortisol than those who delivered after 7 days (p < 0.0001). Indeed, a fetal plasma cortisol, but not a maternal cortisol, concentration was an independent predictor of pregnancy duration after adjusting for both gestational age and the results of amniotic fluid cultures (hazard ratio 2.9; 95% CI 1.3 to 6.7). Interestingly, there is a significant correlation between fetal plasma cortisol and IL-6 concentrations (r = 0.3; p < 0.05) [81]. Such endocrine changes may have short- and long-term implications given observations about the effects of glucocorticoids in fetal programming of several metabolic functions [82–86].
6.5. Thyroid
Transient hypothyroxinemia of prematurity occurs in 30–80% of preterm infants (<30 weeks of gestation) and is characterized by a transient reduction in the serum concentrations of T4 and T3 as well as a low or normal TSH [87]. De Felice et al. reported that preterm neonates with acute histologic chorioamnionitis had a significantly lower T4 concentration on post-delivery day 4 in comparison to those without this placental lesion [88]. Low T4 concentrations in preterm infants have been linked to persistent neurodevelopmental deficits in cognitive and motor function at 2 years of age [89]. The mechanism responsible for these findings has not been established. Sepsis in adults has been associated with lower concentrations of T3 (“low T3 syndrome”), and this is thought to result from apoptosis of thyroid cells [90,91].
6.6. Lung
Amniotic fluid is inhaled by the fetus and reaches the airways, as demonstrated by color Doppler ultrasound imaging [92–95]. Congenital pneumonia, which is frequently diagnosed in cases of fetal death, is thought to be secondary to fetal aspiration of microorganisms. Meconium may be detected in the alveoli in stillbirths, indicating that amniotic fluid can reach the alveoli [96,97]. Tracheobronchial fluid obtained from neonates via an endotracheal tube placed shortly after birth often shows the presence of white blood cells and microorganisms in patients affected by intra-amniotic infection [98]. Interestingly, fetuses with intra-amniotic infection often have a dramatic decrease in fetal “breathing movements,” which may decrease the likelihood of bacterial entry into the lung [99–102].
The relationship between intra-amniotic inflammation/infection and the development of respiratory complications, such as respiratory distress syndrome (RDS) and chronic lung disease/bronchopulmonary dysplasia (BPD) has been the subject of intensive investigation [103–105]. The Watterberg hypothesis proposes that intra-amniotic inflammation/infection is associated with a decreased rate of RDS (early protective effect) but an increased rate of BPD [106]. A systematic review and meta-analysis, which included 158 studies and 244,096 preterm infants, showed that there is a significant association between clinical and histologic chorioamnionitis and subsequent development of BPD [107]. However, chorioamnionitis was not a risk factor for the development of RDS. A multivariate meta-regression revealed that a model combining the difference in gestational age and the odds of RDS explained 64% of the variance in the association between chorioamnionitis and BPD (36 weeks’ post-menstrual age) across studies [107].
Watterberg et al. reported that low-birth-weight infants exposed to chorioamnionitis had higher concentrations of IL-1β in tracheal lavage samples and a lower incidence of RDS, but a higher rate of BPD in comparison to the control group [106]. Ghezzi et al. reported that IL-8 concentrations in amniotic fluid were higher in women who presented with spontaneous preterm labor (intact or ruptured membranes) and delivered infants at 24–28 weeks of gestation who later developed BPD [108]. Indeed, preterm neonates born at 33 weeks of gestation or earlier who developed BPD had median amniotic fluid concentrations of IL-1β and IL-8 that were significantly higher than those in whom BPD did not develop [109]. Collectively, the evidence suggests that exposure to intra-amniotic inflammation is a risk factor for BPD.
Subsequently, Yoon et al. reported that neonates born between 25 and 34 weeks of gestation and in whom BPD developed had a significantly higher median umbilical cord plasma IL-6 concentration in comparison to matched preterm infants without BPD. This indicates that the risk for BPD is higher in the offspring of pregnancies in which there is intra-amniotic inflammation and fetal systemic inflammation [110].
This set of clinical observations led to systematic investigation of the effect of microbial products and pro-inflammatory cytokines on fetal lung development. The models include intra-amniotic administration of endotoxin, administration of IL-1β, or the inoculation of bacteria in the amniotic cavity. The major findings have been that inflammation stimulates surfactant protein production but alters lung development [111–113].
Bry et al. first reported that intra-amniotic administration of IL-1α to pregnant rabbits increased mRNA and protein expression of surfactant proteins A and B, as well as surfactant lipids [114]. This was accompanied by improved neonatal lung function. Injection of IL-1α also induced preterm labor and delivery; therefore, the effect of inflammation on surfactant production could be interpreted as promoting lung maturation in anticipation of preterm delivery.
The laboratories of Jobe and Newnham have systematically studied endotoxin-induced fetal lung injury in sheep and rhesus macaques [112, 115–128]. Intra-amniotic administration of LPS induces fetal lung inflammation, and this is accompanied by a dramatic increase in the number of mononuclear cells and granuocytes in bronchoalveolar lavage fluid and mRNA expression for IL-1, IL-8, and IL-6 in the lung tissue [112]. An increase in surfactant production, and structural changes in the developing fetal lungs, such as decreased alveolar number, thinning of alveolar septae, and increased alveolar size has been consistently reported [115]. In addition, there is down-regulation of the expression of elastin [127] and several genes involved in vascular development (vascular endothelial growth factor A, vascular endothelial growth factor receptor 2, and endothelial nitric oxide synthase) [119, 120]. The latter is thought to predispose to pulmonary hypertension [125]. The structural changes reported after endotoxin administration have also been observed after intra-amniotic inoculation with U. urealyticum in sheep and rhesus macques [113,129–132].
Fetal lung inflammation is characterized by robust expression of proinflammatory mediators such as IL-1β, IL-8, granulocyte-macrophage colony-stimulating factor, monocyte chemotactic protein 1, and serum amyloid A3 in both sheep and monkeys [73,112,122,124]. It is noteworthy that IL-1β is the major cytokine involved in fetal lung injury, as TNF-α and interferon-γ do not elicit the same degree of inflammation [73,133]. Moreover, pre-treatment with intra-amniotic injection of an IL-1α antagonist before the administration of bacterial endotoxin prevents lung inflammation and maturation [121].
In summary, exposure to microbial products and intra-amniotic inflammation induces fetal lung maturity, which favors survival in the context of preterm delivery [134]. However, acceleration of lung maturity is accompanied by dramatic changes in the anatomy of the lung (e.g., reduction in the number of alveoli, impaired microvascular development, and thickening of the arteriolar walls, which collectively resemble changes observed in infants with bronchopulmonary dysplasia) [135]. Therefore, the short-term gain in lung maturity appears to predispose to the development of chronic lung disease.
6.7. Heart
Cardiac dysfunction can occur during the course of a systemic inflammatory response and sepsis in adults [136–140]. Impaired cardiac performance results from a combination of systolic and diastolic dysfunction, which was originally attributed to the presence of circulating myocardial depressant factors and, more recently, to direct effects of pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6 [141]. The pattern of myocardial depression is characterized by left ventricular dilation, decreased left ventricular ejection fraction, and a normal or increased cardiac index [136]. Acute ventricular dilation within the first days of septic shock is more frequently observed among survivors, and this is believed to represent compensatory cardiac dilation to maintain stroke volume despite the loss of myocardial contractility [136].
In preterm PROM and intra-amniotic infection, human fetal echocardiographic studies have also reported evidence of cardiac diastolic dysfunction consistent with increased left ventricular compliance, when compared to those with uncomplicated pregnancies [142]. It is thought that these changes reflect a compensatory mechanism similar to that observed in adults with sepsis.
Another investigation has shown impairment in both fetal diastolic and systolic cardiac performance of the right ventricle through tissue Doppler and strain rate imaging in fetuses with preterm PROM and proven intra-amniotic infection [143]. These observations are consistent with the study of Yanowitz et al., who found that umbilical cord IL-6 concentrations inversely correlated with systolic, mean, and diastolic blood pressure, and that the presence of funisitis was also associated with changes in right ventricular cardiac output, consistent with lower systemic vascular resistance [144]. These hemodynamic abnormalities are thought to predispose infants to brain injury, thereby buttressing the case for the importance of multisystem organ involvement in the pathophysiology of neonatal complications in the context of FIRS.
Cardiotocography of fetuses exposed to intra-amniotic inflammation (defined as histologic chorioamnionitis) has shown an increased fetal heart baseline, a higher number of low variation episodes, and a higher number of short-term variations [145].
Several experimental studies conducted in mice, pregnant sheep, and rhesus macaques have shown that intra-amniotic administration of endotoxin or Candida albicans leads to functional abnormalities in the fetal heart and a change in the gene regulatory networks that program cardiac development [146–151]. Specifically, in non-human primates, intra-amniotic administration of group B streptococcus or E. coli led to increased concentrations of IL-6 and IL-8 in the fetal myocardium without significant evidence of histopathologic inflammation of the heart [151]. This was associated with changes in the gene set involving cardiac morphogenesis and vasculogenesis. This observation is important given that epidemiologic studies have shown a 17-fold increased risk of heart failure after preterm birth. Moreover, adults who were born prematurely have exhibited substantial changes in myocardial structure and function [152]. Thus, exposure to intra-amniotic inflammation can explain, at least in part, the predisposition of preterm neonates to develop long-term cardiovascular disorders and, specifically, heart failure [153–156].
6.8. Gut
The fetal intestines can be exposed to microorganisms and inflammatory mediators after amniotic fluid is swallowed. FIRS is a risk factor for necrotizing enterocolitis [23,157]. A systematic review and meta-analysis, inclusive of 22,601 patients from 12 studies, demonstrated that clinical chorioamnionitis (OR 1.24; 95% CI: 1.01–1.52) and histological chorioamnionitis with acute funisitis (OR 3.29; 95% CI: 1.87–5.78) were significantly associated with necrotizing enterocolitis [157]. The mechanism for these associations is thought to involve impaired gut barrier function and immune dysfunction [158].
Experimental evidence suggests that immaturity and prenatal inflammation alters maturation of the fetal intestine [49,159–162]. In a sheep model, Wolfs et al. reported that administration of intra-amniotic endotoxin prevented maturation of tight junctions in intestinal epithelial cells [159]. Abnormal tight junctional distribution predisposes to easy access of microorganisms and toxins to the mucosa and inner layers of the gut pre- and post-natally.
In a recent study, intraperitoneal administration of bacterial endotoxin to pregnant rats increased the frequency of necrotizing enterocolitis and neonatal mortality [163]. The proposed mechanism for this observation was fetal TNF-α-mediated impairment of intestinal microvasculature, which was associated with a decrease in vascular endothelial growth factor (VEGF) and VEGF receptor 2 protein expression [163]. This was ameliorated by a hypoxia-inducible factor-1α (HIF-1α) stabilizing agent (HIF-1α is a master regulator of VEGF function), and was abrogated by neutralizing TNF-α activity. This body of work indicates that prenatal inflammation increases the risk for necrotizing enterocolitis and that therapeutic approaches to maintain VEGF function may help prevent this complication.
Meconium-stained amniotic fluid, a common phenomenon (1 in 7 pregnancies), has traditionally been attributed to hypoxia [164]. Interestingly, such fluid frequently contains bacteria [165–167], endotoxin [167,168], and higher concentrations of inflammatory mediators, such as IL-1β, TNF-α [169], IL-8 [169,170], and phospholipase-A2. We have proposed that meconium passage in utero, in some cases, may represent accelerated bowel transit (i.e., fetal diarrhea). Most of the time, meconium-stained amniotic fluid is a benign finding and is not associated with adverse neonatal outcome. However, meconium aspiration syndrome is a serious neonatal complication that develops in 5% of cases having meconium-stained amniotic fluid.
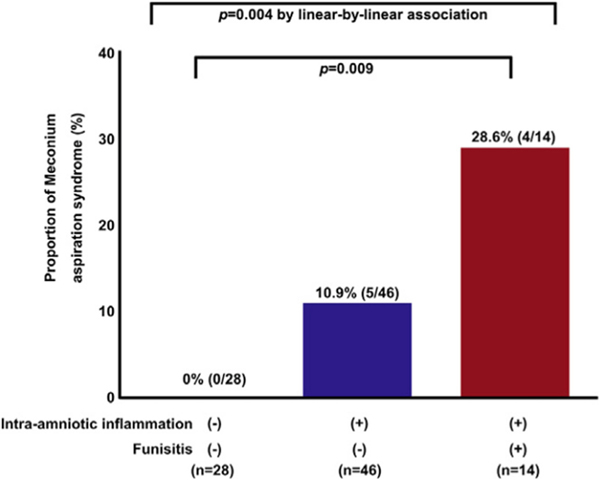
To explore why some infants with meconium-stained amniotic fluid develop this syndrome and others do not, we examined the role of fetal systemic inflammation. We reported that the combination of meconium-stained amniotic fluid and FIRS had a higher frequency of meconium aspiration syndrome than those without FIRS (Fig. 5) [164]. Thus, we proposed a chain of events in that meconium (with its proinflammatory properties), when aspirated before birth and combined with a fetal systemic inflammatory response involving the fetal lungs, can predispose to meconium aspiration syndrome [164,171,172]. In addition, elevated CRP concentrations, and low white blood cell and neutrophil counts in neonatal blood are associated with the severity of meconium aspiration syndrome during the early phases of the disease [171].
Fig. 5.
Meconium aspiration syndrome (MAS) is more likely to occur in patients with meconium-stained amniotic fluid if intraamniotic inflammation and fetal inflammatory response syndrome ascertained by funisitis are present. Frequency of MAS in the context of intraamniotic inflammation and funisitis. Neonates exposed to both intraamniotic inflammation and funisitis were at significantly greater risk of MAS than newborns exposed to neither of these 2 conditions [28.6% (4/14) vs 0% (0/28), P = 0.009]. In contrast, newborns exposed to only intraamniotic inflammation without funisitis were not at greater risk of MAS than newborns exposed to neither of these 2 conditions [10.9% (5/46) vs 0% (0/28); P = 0.15]. MAS did not occur in the absence of intraamniotic inflammation. (Reproduced with permission from Lee J, Romero R, Lee KA et al. Meconium aspiration syndrome: a role for fetal systemic inflammation. Am J Obstet Gynecol 214:366.e1–9, 2016.).
6.9. Liver
The liver is exposed to exogenous agents that reach the fetus either directly from the placenta (via the umbilical vein) or from the amniotic fluid, which is swallowed and resorbed in the intestine (via the portal vessels). Kupffer cells are macrophages lining the hepatic sinusoids, which are capable of recognition and phagocytosis of a wide range of bacteria as well as immune complexes [173]. Kupffer cells are able to produce cytokines (IL-6, IL-1β, TNF-α) [174,175], which can modify fetal hematopoiesis. During fetal development, hematopoiesis occurs mainly in the fetal liver, followed by localization to the bone marrow. Fetuses with funisitis exhibit a significant increase in fetal hepatic hematopoiesis and myelopoiesis during the second trimester [176–178], indicating that the fetal response to intra-amniotic infection involves the liver.
Experimental intra-amniotic inflammation induced by bacterial endotoxin in sheep fetuses led to hepatic inflammation and liver dysfunction in the neonatal period. Specifically, there was an increase in the number of CD3+ T lymphocytes as well as in IL-1β and TNFα mRNA in the fetal liver [179]. In addition, fetal hepatic inflammation was associated with metabolic disturbances, including increased umbilical cord plasma concentrations of total cholesterol, high-density lipoprotein, low-density lipoprotein, and triglycerides [179]. Two weeks after endotoxin injection, inflammation was still detected in the fetal liver, and there were higher glucose concentrations in the blood [179]. In a subsequent study focusing on the effects of bacterial endotoxin exposure during fetal life, Vlassaks et al. reported that liver triglycerides and plasma cholesterol concentrations were increased in LPS-exposed lambs compared to controls even though hepatic inflammation had resolved [180]. There was also evidence of increased lipid peroxidation, leptin receptor mRNA expression, and expression of cytochrome c oxidase subunit 4. This represents the first evidence that fetal inflammation has an effect on the liver and lipid metabolism. Whether this has long-term consequences by increasing the susceptibility to hepatic inflammation and insulin resistance is yet to be determined.
6.10. Kidneys
The fetal kidney is a potential target organ during FIRS [181–183]. Children and adult patients with sepsis can experience oliguria secondary to pre-renal failure (i.e., hypoperfusion) [184]. Since fetal urine is the main source of amniotic fluid, several investigators have examined the relationship between amniotic fluid volume and intra-amniotic and fetal inflammation. Yoon et al. reported that oligohydramnios in women with preterm PROM was associated with higher IL-6 concentrations in umbilical cord plasma at birth, higher concentrations of amniotic fluid proinflammatory cytokines (IL-6, IL-1β, and TNF-α), and a higher frequency of clinical and acute histologic chorioamnionitis compared to those without oligohydramnios [182]. Similarly, Lee et al. reported that patients with intra-amniotic infection and inflammation had a higher frequency of oligohydramnios than those with a negative amniotic fluid culture [183]. Carroll et al. reported that fetuses with bacteremia diagnosed by cordocentesis had a higher frequency of oligohydramnios than in those with a negative fetal blood culture [181]. Taken together, the data suggest that oligohydramnios is associated with intra-amniotic inflammation and FIRS.
In the context of preterm PROM, reduced amniotic fluid volume may be partly attributed to the loss of amniotic fluid through the site of membrane rupture. However, it is possible that fetal urine production is altered in FIRS, and this can result from redistribution of blood flow away from the kidneys to ensure adequate blood flow to critical organs and increase efficiency of oxygen utilization [185,186]. It is interesting that neonates delivered to mothers with intra-amniotic inflammation have higher serum blood urea nitrogen concentrations and that such concentrations correlate with amniotic fluid IL-6 [187].
In a murine model, maternal intraperitoneal endotoxin injection induced fetal leukocyte activation, recruitment, and infiltration of the fetal renal parenchyma [188]. Moreover, fetal sheep exposed to intra-amniotic endotoxin have a reduction in nephron numbers by 23% as well as low glomerular density [189]. Evidence of renal injury has also been reported in preterm pigs after intra-amniotic injection of endotoxin, which induced an altered postnatal renal biochemical profile (i.e., increased concentration of urinary microalbumin, microalbumin: creatinine ratio, and sodium) [49]. In summary, microbial products and inflammatory mediators in the amniotic fluid may predispose preterm fetuses to impaired renal function during the postnatal period and increased risk of hypertension and renal dysfunction in later life [190, 191].
6.11. Brain
A considerable body of evidence supports the concept that fetal systemic inflammation and often infection is causally related to fetal/neonatal neuroinflammation, brain injury, and neurodevelopmental disorders, including mental illness [192–197].
In 1955, Eastman and DeLeon reported that intrapartum maternal fever (often from intraamniotic infection with bacteria) conferred a seven-fold increased risk for cerebral palsy [198]. Subsequently, a solid body of evidence has accumulated over the last six decades showing that inflammation and infection play a role in the genesis of brain injury. In 1971, Leviton and Gilles [199] reported on the characteristics of perinatal telencephalic leucoencephalopathy (white matter abnormalities in the fetal brain after midgestation) and that there was a potential link to neonatal bacteremia and endotoxin exposure [200]. This original concept has gained considerable support and its evolution is described in another article in this issue [201]. Other articles in this issue describe the etiology, mechanisms of brain injury, short- and long-term neurologic outcome after fetal exposure to inflammation, and potential interventions to downregulate the inflammatory response [202–205]. The work of Nelson has supported the concept that maternal infection plays a role in the genesis of cerebral palsy in infants born at or close to term [206–208]. Other articles in this issue of Seminars in Fetal and Neonatal Medicine describe FIRS and its manifestations in term or late preterm gestation. Therefore, the role of intra-amniotic neuroinflammation does not seem to be limited to preterm neonates, a concept that is important given that most cases of cerebral palsy derive from infants born at term.
Briefly, the evidence in support of this concept indicates that 1) proven intra-amniotic infection is present in at least 25% of all preterm deliveries [209–215] and in 61% of patients with clinical chorioamnionitis at term [216]; 2) a fetal inflammatory response (diagnosed by the presence of funisitis, elevated concentrations of IL-6 or C-reactive protein in umbilical cord blood) is present in a large fraction of patients with intra-amniotic infection [9,217,218]; 3) there is a strong association between intra-amniotic infection/inflammation and the subsequent development of white matter lesions of the neonatal brain [219–237]; 4) a fetal inflammatory response (diagnosed by funisitis) increases the risk of both periventricular leukomalacia and cerebral palsy [9,20,192–195,238–262]; 5) intrauterine infection with bacteria or intra-amniotic administration of LPS can induce a fetal systemic inflammatory response, neuroinflammation, and lesions resembling periventricular leukomalacia with gliosis and neuronal injury [193, 223–225,228,263–265], and this neuroinflammatory process can be observed in animals delivered preterm as well as in those who delivered at term [233,266]; 6) intrauterine administration of bacterial endotoxin has been used to generate an animal model of cerebral palsy, in which there is neuroinflammation [267,268]; and 7) down-regulation of fetal neuroinflammation with the administration of anti-inflammatory agents (e.g. N-acetyl-cysteine) or stem cell-related products can reverse the neuroinflammatory process and the phenotype in animals [269–278].
The relative contributions of fetal inflammation and postnatal inflammation have been the subject of investigation. All available evidence suggests that both play a role and that sustained inflammation is particularly important.
Frontiers for clinical and experimental research are the non-invasive detection of fetal neuroinflammation in the early postnatal period, followed by treatment to prevent the sequelae. There are reasons to believe that both goals can be met with the use of molecular imaging and nanotechnology and cell-based therapies. A comprehensive discussion of the subject of neuroinflammation is available in other articles in this special issue.
6.12. Eyes
Approximately 2% of preterm birth survivors are diagnosed with blindness, and visual acuity is impaired compared to those born at term [279–282]. Moreover, among adolescents who were born preterm, 50% have vision-related problems [283]. These disorders have been traditionally attributed to either macular sequelae from severe retinopathy of prematurity (ROP) or cerebral visual impairment. Recent evidence suggests that ROP may be part of a spectrum that includes both disorders of the retina and the cerebrovascular interface [284]. ROP is considered to result from an arrest of neural and vascular development. Indeed, children affected with ROP are more likely to have cognitive and psychomotor development disorders at two years of age [284].
ROP is more frequent in infants born to mothers with histologic or clinical chorioamnionitis than in those without exposure to antenatal inflammation [229,285–290]. A recent systematic review and meta-analysis, which included 50 studies (38,986 infants and 9258 chorioamnionitis cases), showed a significant association between the presence of chorioamnionitis and any ROP stage (OR 1.39; 95% CI: 1.11 to 1.74), as well as severe ROP (stage ≥3) (OR 1.63; 95% CI: 1.41 to 1.89) [291]. The risk of severe ROP was substantially increased when histologic chorioamnionitis co-occurred with birth <29 weeks [287]. Of particular interest, infants who had a fetal inflammatory response (diagnosed by funisitis) had a higher risk of ROP than those with acute histologic chorioamnionitis in the absence of funisitis [291].
Further evidence of an association between perinatal inflammation and ROP derives from studies examining cytokine concentrations in neonatal and umbilical cord blood. For example, Sood et al. studied 877 infants and reported an association between ROP and cytokine concentrations measured serially in the first three weeks of life. Preterm neonates with ROP had higher concentrations of IL-6 and lower concentrations of IL-17 and IL-18 in the first three days of life [292]. Subsequently, such findings were confirmed, as serum umbilical cord concentrations of cytokines (IL-7, monocyte chemotactic protein-1, macrophage inflammatory protein 1 α, and macrophage inflammatory protein 1 β) were significantly higher in preterm neonates who developed ROP vs. healthy preterm neonates without ROP [293]. Recently, Park et al. reported that an elevated umbilical cord plasma concentration of IL-6 (>6.5 pg/mL) was an independent risk factor of severe ROP in a cohort of 110 preterm neonates [22].
There is also an association between ROP and neonatal sepsis. A meta-analysis including 16 studies (12,466 premature infants and 2494 cases of ROP) showed that neonatal sepsis was closely related to any stage of ROP (OR 1.57; 95% CI: 1.31 to 1.89) and severe stages of ROP (OR 2.33; 95% CI: 1.21 to 4.51) in premature infants [294]. Collectively, these results indicate that fetal systemic inflammation is associated with an increased risk of ROP.
6.13. Ears
Sensorineuronal hearing loss is more frequent in infants born preterm vs. term, and the earlier the gestational age at birth, the greater the risk. The proposed mechanisms for the increased risk of hearing loss have largely focused on postnatal factors, such as immaturity, administration of ototoxic medications (e.g., aminoglycosides, diuretics), environmental noise, hyperbilirubinemia, and hypoxia [295–298]. However, the role of congenital factors has been known since 1897 when Aschoff described the concept of otitis media neonatorium, which was thought to be a sterile middle ear inflammation related to aspiration of amniotic fluid cellular content based on microscopic study of the temporal bone in fetuses and neonates [299]. Subsequent studies have shown that the middle ear may contain both meconium and purulent material in cases of fetal death in patients with histologic chorioamnionitis [300]. Thus, intra-amniotic inflammation and infection has been implicated as a cause of otitis media for decades. This has been invoked as a cause of chronic otitis media and hearing loss. In addition, otitis media has been implicated as a cause of meningitis in the neonatal period [301,302].
An interest in this subject has been rekindled in recent years. Microbial invasion or inflammation of the middle ear in patients with intra-amniotic inflammation/infection, neuroinflammation, or a combination of both may lead to hearing loss. Experimental studies in guinea pigs have also shown that fluorescent bacterial endotoxin injected into the middle ear can reach the cochlea of the inner ear [303].
Investigators have examined whether exposure to antenatal inflammation is associated with an increased risk of hearing loss. When FIRS is defined as the presence of fetal vasculitis based on placental examination and/or umbilical cord serum IL-6 (>18.2 pg/mL), it was associated with an increased risk for hearing screen failure in preterm neonates (OR 3.6; 95% CI: 1.38 to 9.5) [297]. Moreover, Shim et al. reported that elevated umbilical cord plasma IL-6 and high CRP concentrations in the immediate neonatal period were significantly associated with neonatal hearing screening test failure in a cohort of 127 premature infants [304]. These results suggest that a fetal inflammatory response may increase the risk of hearing loss.
6.14. Skin
In the setting of intra-amniotic inflammation/infection, microorganisms and their inflammatory mediators are in direct contact with the fetal skin, an important part of the innate immune system. A newly described entity, congenital fetal dermatitis, has been observed in the context of intra-amniotic infection [305]. We found that the skin of fetuses exposed to intra-amniotic infection had the typical findings of dermatitis: an inflammatory infiltrate composed mainly of CD15+ neutrophils, CD3+ T lymphocytes, and CD68+ histiocytes; the overexpression of cytokines (such as IL-1α, TNF-α, and IL-8) and antimicrobial peptides (human defensins 2 and 3); and the overexpression of pattern recognition receptors, such as TLR-2, in the epidermis [305].
Experimental studies in an ovine pregnancy model have shown that exposure of the fetal skin to bacterial endotoxin results in the increased expression of IL-1β, IL-6, IL-8, and TNF-α [306]. On the other hand, intra-amniotic administration of U. parvum serovar 3 in an ovine pregnancy induced the increased expression of MCP-1, but not IL-1β, IL-6, IL-8, or TNF-α, in fetal skin [307]. Dermatitis is a local fetal response to microbial products or inflammatory mediators. The long-term effects of congenital dermatitis on the skin’s health and disease require further study.
7. Diagnosis of FIRS
The initial description of FIRS was based on an elevated concentration of IL-6 in the fetal blood obtained by cordocentesis. A cut-off value of IL-6 >11 pg/mL was used, as this was associated with severe perinatal morbidity [9]. The population subject to study included patients with preterm labor and preterm PROM, which are conditions frequently associated with intra-amniotic infection and inflammation. Nevertheless, funisitis is the histologic counterpart of elevated IL-6 levels [323–325].
Several factors need to be considered in defining FIRS when using the initial observations of other studies. IL-6 was quantitated using an enzyme-linked immunoassay developed for research purposes. At the time of our report, an international standard to calibrate immunoassays was not widely available. However, such a standard is now available from the National Institute of Biological Standards and Control of the U. K., called the WHO First International Standard for IL-6; details of this standard are available at https://www.nibsc.org [308]. Investigators should be aware of the need to calibrate the assays to be able to compare results among laboratories and studies.
The concentration of IL-6 in fetal blood appears to change with advancing gestational age [309,310] and with the presence or absence of labor [311–316]. Therefore, consideration should be given to the original findings that were based on preterm gestations and that the results and cutoff values may differ if sampling occurs after labor (i.e., umbilical cord blood obtained after spontaneous labor).
Many studies have used multiplex assays to measure several cytokines simultaneously. However, these are not the typical platforms used to quantitate analytes in clinical medicine. Most assays are targeted toward a particular protein. Therefore, it is important that studies report performance characteristics of assays, including sensitivity, cross-reactivity, inter- and intra-assay coefficients of variation, and the method of calibration. This will aid in meaningful comparison of results. Commercially available assays for clinical testing are now available.
The field of cytokine measurements has matured over the last several decades, and the time is now appropriate to undertake systematic determination to define normal ranges, not only for IL-6 but for other analytes that could be used to assess the cytokine profile at the time of birth.
C-reactive protein (CRP), an acute phase reactant widely used to detect the presence of systemic inflammation, is synthesized by the liver in response to IL-6. Given that assays for IL-6 have not been widely available, several investigators have used umbilical cord concentrations of CRP to identify FIRS because such assays are largely available in most hospitals [217,317]. CRP can be measured by ELISA (enzyme-linked immunosorbent assay), immunoturbidimetry, or antibody-based nephelometric assays. High-sensitivity assays for CRP that allow detection in the range of 0.5–10 mg/L are now available and have been used to assess the risk of cardiovascular disease. An international standard for human CRP is available (i.e. WHO 1st International Standard). A strong correlation exists between the umbilical cord plasma concentrations of IL-6 and CRP [217], and CRP determinations from umbilical cord blood (obtained for the purposes of blood and Rh typing) could allow an assessment of fetal systemic inflammation without venipuncture of the neonate. The diagnostic performance of umbilical cord plasma concentrations of IL-6 and CRP in the identification of funisitis, neonatal sepsis, and intra-amniotic infection are displayed in Table 2 [217]. The relative diagnostic performance of CRP, IL-6, and other analytes (e.g., procalcitonin) to identify neonatal sepsis has been the subject of multiple studies [318–320].
Table 2.
Diagnostic indices of cord plasma C-reactive protein (CRP) and interleukin-6 (IL-6) in the identification of a positive amniotic fluid culture, neonatal sepsis and funisitisa.
| Parameters | Sensitivity | Specificity |
|---|---|---|
|
| ||
| Amniotic fluid infection | ||
| CRP | 52% (23/44) | 76% (82/108) |
| IL-6 | 57% (25/44) | 73% (79/108) |
| Neonatal sepsis | ||
| CRP | 82% (9/11) | 74% (209/281) |
| IL-6 | 82% (9/11) | 69% (192/279) |
| Funisitis | ||
| CRP | 62% (47/76) | 83% (191/231) |
| I L-6 | 67% (51/76) | 76% (174/229) |
Modified with permission from Yoon BH, Romero R, Shim JY, Shim SS, Kim CJ, Jun JK. C-reactive protein in umbilical cord blood: a simple and widely available clinical method to assess the risk of amniotic fluid infection and funisitis. J Matern Fetal Neonatal Med. 2003; 14:85–90.
It is now clear that neonates can evince elevated concentrations of IL-6 and CRP yet show no evidence of funisitis, and the reverse is also true.
An elevation in proinflammatory cytokines or acute phase reactants may result from fetal inflammation unrelated to intra-amniotic inflammation, such as in cases of fetuses with anemia from alloimmunization [321] or maternal autoimmune disorders [322]. Given that such pathologic processes are unrelated to intra-amniotic inflammation, there is no chemotactic gradient in the amniotic cavity that would bring neutrophils from the fetal blood into the walls of the umbilical arteries and vein. The explanation for the absence of an elevated IL-6 or CRP in cases of funisitis is less clear at this time.
8. Funisitis and chorionic vasculitis: pathologic hallmarks of FIRS
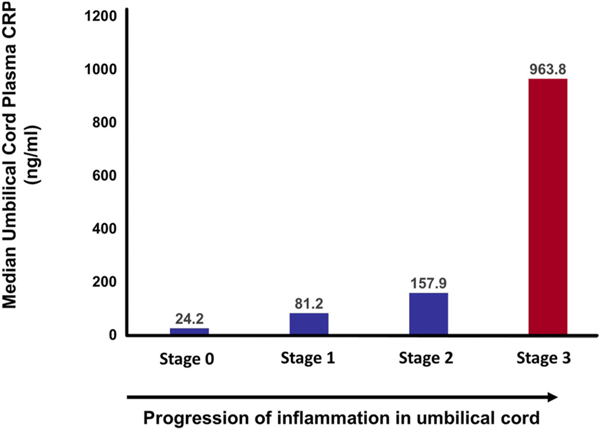
Inflammation of the umbilical vessels (acute funisitis) and/or inflammation of the chorionic vessels (chorionic vasculitis) strongly correlate with a fetal plasma IL-6 concentration >11 pg/mL [323–325] and, therefore, are considered the histological counterparts of FIRS. Funisitis is characterized by the presence of fetal neutrophils infiltrating into the umbilical vein wall (stage 1) and one or both umbilical artery walls (stage 2) with or without subsequent invasion into the Wharton’s jelly [325]. Necrotizing funisitis (stage 3) is distinguished by the presence of fetal neutrophils and/or cellular debris in a concentric ring around at least one of the umbilical vessels [325,326]. Chorionic vasculitis represents inflammation of the vessels on the surface of the chorionic plate of the placenta [325]. More advanced stages of funisitis are associated with a higher concentration of IL-6 or CRP in umbilical cord blood [327] (Fig. 6).
Fig. 6.
The higher the umbilical cord plasma concentration of C-reactive protein (CRP), the more severe the inflammatory process in the umbilical cord, assessed by the severity of funisitis by stage. (Reproduced with permission from Oh JW, Park CW, Moon KC et al. The relationship among the progression of inflammation in umbilical cord, fetal inflammatory response, early-onset neonatal sepsis, and chorioamnionitis. PLoS One 14:e0225328, 2019.).
The term “acute placental inflammation” is often used in discussions about FIRS. It is noteworthy that acute histologic chorioamnionitis is a maternal inflammatory response while funisitis and chorionic vasculitis are fetal inflammatory responses. Therefore, neutrophil inflammation of the extraplacental membranes or the chorionic plate, which is of maternal origin, should not be misconstrued to represent evidence of fetal inflammation.
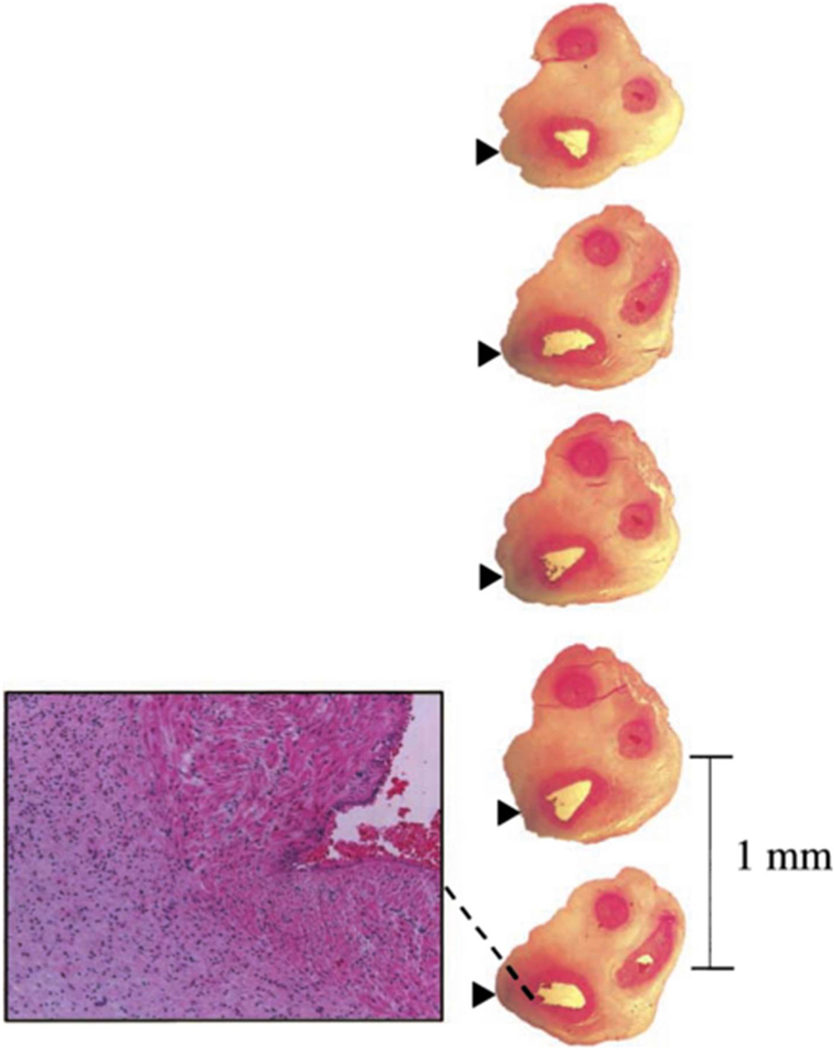
The availability of a placenta to make a histologic diagnosis of FIRS is unique, as there is no equivalent for children and adults. Studies in which the umbilical cord has been serially sectioned at 1 mm intervals suggest that the process begins as multiple discrete foci which then merge as the inflammatory process progresses (Fig. 7) [328].
Fig. 7.
Funisitis is inflammation of the umbilical vein, either or both umbilical arteries, and the Wharton’s jelly. Serial sections of the umbilical cord were taken at 1 mm intervals and showed neutrophil infiltration of the umbilical vein (dashed line). (Reproduced with permission from Kim CJ, Yoon BH, Kim M et al. Histo-topographic distribution of acute inflammation of the human umbilical cord. Pathol Int 51:861–5, 2001.).
9. FIRS, cytokine storm, cytokine release syndrome, and the macrophage-like activation syndrome
The current literature in sepsis uses the terms cytokine storm, cytokine release syndrome, macrophage-like activation syndrome, and hemophagocytic syndrome to refer to conditions in which hyperinflammation is responsible for morbidity and mortality in the context of infection or tissue injury. These terms denote an enhanced response of the innate immune system, generally diagnosed by increased concentrations of inflammatory mediators such as IL-6, IL-1β, TNF-α, and chemokines (e.g. MCP-1).
The term cytokine storm was originally introduced in the context of graft vs. host disease [329]; cytokine release syndrome was first used to describe changes in the concentration of cytokines in response to the administration of an anti-CD3 antibody (CD3 is a molecule expressed by T cells) [330,331]. The term hemophagocytic lymphohistiocytosis was employed to describe a hyperinflammatory state which could be familial (from mutations in the genes encoding for porphyrin or proteins required for the docking and fusion of granules in NK cells). The term macrophage-like activation syndrome overlaps with secondary hemophagocytic lymphohistiocytosis. It is interesting that these syndromes were identified often in the context of non-infectious conditions, such as cancer. However, these syndromes overlap with SIRS.
The phrase cytokine storm is often used to imply that there is dysregulation of the inflammatory response and that the inflammatory mediators could injure host cells and cause multi-organ dysfunction [332]. However, as of this writing, there is not a good way to differentiate an appropriate from a dysregulated inflammatory response. We envision a need to characterize the cellular and soluble profiles of pro- and anti-inflammatory mediators in umbilical cord blood to determine the level of the immune system at the time of birth, and to examine the clinical significance of these findings in terms of short- and long-term morbidity.
10. Transcriptome and proteome analyses in FIRS
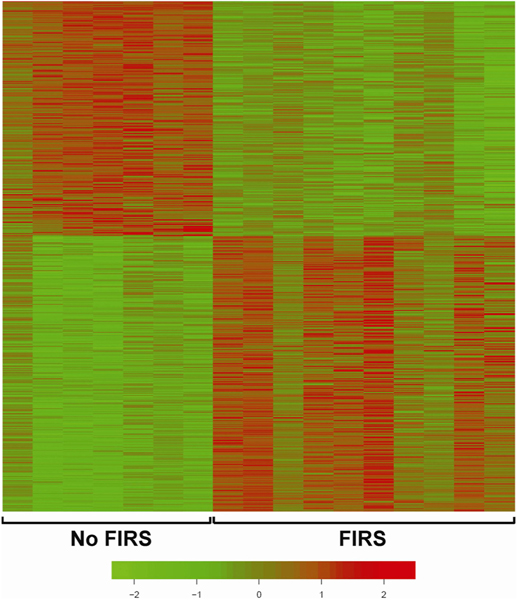
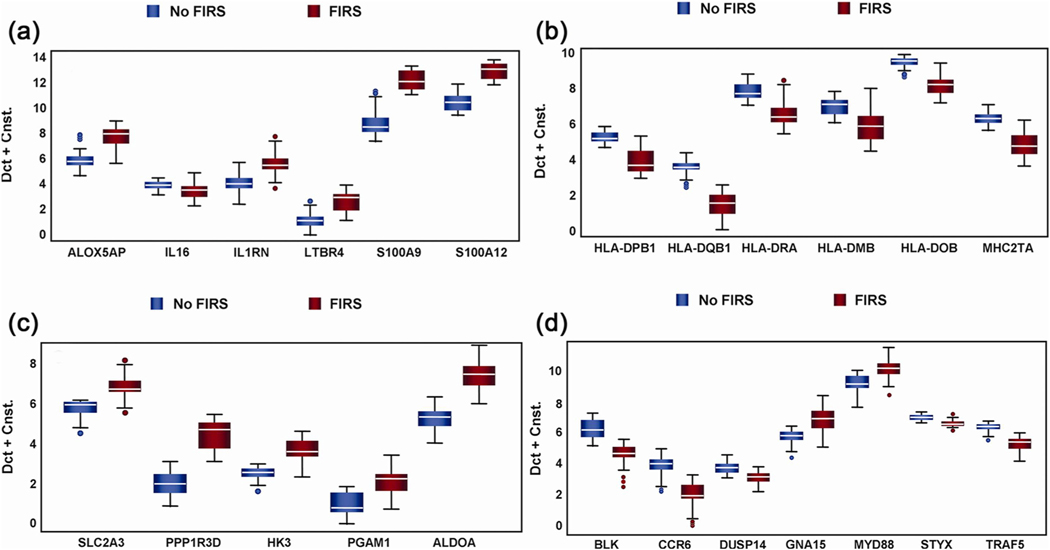
The transcriptome of umbilical cord leukocytes obtained from patients with FIRS (elevated umbilical cord IL-6 concentrations and funisitis) showed a dramatic upregulation of 296 genes and downregulation of 252 genes based on microarray analysis (Fig. 8) [333]. Differential gene expression in cord blood RNA with and without FIRS is displayed in Table 3 and Fig. 9. Ontological and pathway analyses showed the significant enrichment of biological processes, including antigen processing and presentation, immune response, and process critical to cellular metabolism. We compared the transcriptomes of FIRS, those of patients with pediatric sepsis, and volunteers with experimental endotoxin administration. The main observation was the remarkable similarity between FIRS and SIRS, despite the differences in maturity of the immune response. Our findings showed that in addition to the pro-inflammatory response, there was activation of the anti-inflammatory response (increased concentration of IL-10). Increased concentrations of IL-6, IL-8, IL-10, TNF-α, sIL-2Ra, CXCL-10, and MCP-1 (also known as CCL2) were observed in the serum of neonates born with FIRS [333].
Fig. 8.
The peripheral blood transcriptome of fetuses with fetal inflammatory response syndrome (FIRS) is dramatically different from that of fetuses with-outlammatory response syndrome (FIRS). Similar results have been reported in patients with pediatric sepsis and after volunteers have been given bacterial endotoxin. Transcriptome analysis of white blood cells collected from the umbilical cord in patients with preterm labor and preterm prelabor rupture of membranes was performed. Red color depicts upregulation, while green color depicts downregulation. Displayed are the 296 genes that were differentially upregulated in leukocytes from neonates with FIRS and the 252 that were decreased (False discovery rate <0.05). (Reproduced with permission from Madsen-Bouterse SA, Romero R, Tarca AL et al. The transcriptome of the fetal inflammatory response syndrome. Am J Reprod Immunol 63:73–92, 2010.).
Table 3.
Genes differentially expressed in fetal inflammatory response syndrome, pediatric sepsis, and endotoxin challenge models of sepsis.
| Gene Symbol | Fetal inflammatory response a | Systemic inflammatory response in humans b | Endotoxin challenge c | Pediatric septic shock d |
|---|---|---|---|---|
|
| ||||
| ADM | ↑ | ↑ | ↑ | ↑ |
| ALOX5AP | ↑ | ↑ | ↑ | ↑ |
| ALPL | ↑ | ↓ | ↑ | ↑ |
| ANXA3 | ↑ | ↑ | ↑ | ↑ |
| BAZ1A | ↑ | ↑ | ↑ | ↑ |
| CEACAM1 | ↑ | ↑ | ↑ | ↑ |
| CKAP4 | ↑ | ↑ | ↑ | ↑ |
| EMR1 | ↑ | ↓ | ↑ | ↑ |
| FGR | ↑ | ↓ | ↑ | ↑ |
| FLOT1 | ↑ | ↑ | ↑ | ↑ |
| HLA-DPA1 | ↓ | ↓ | ↓ | ↓ |
| HLA-DPB1 | ↓ | ↓ | ↓ | ↓ |
| HLA-DQB1 | ↓ | ↓ | ↓ | ↓ |
| HP | ↑ | ↑ | ↑ | ↑ |
| IL1RN | ↑ | ↑ | ↑ | ↑ |
| LIMK2 | ↑ | ↑ | ↑ | ↑ |
| MAPK14 | ↑ | ↑ | ↑ | ↑ |
| PGD | ↑ | ↑ | ↑ | ↑ |
| PGLYRP1 | ↑ | ↑ | ↑ | ↑ |
| S100A12 | ↑ | ↑ | ↑ | ↑ |
| SERPINB1 | ↑ | ↑ | ↑ | ↑ |
| SLC2A3 | ↑ | ↑ | ↑ | ↑ |
| SPOCK2 | ↓ | ↓ | ↓ | ↓ |
| UGCG | ↑ | ↑ | ↑ | ↑ |
| UPP1 | ↑ | ↑ | ↑ | ↑ |
Arrows indicate direction of change relative to healthy controls.
Modified with permission from Madsen-Bouterse SA, Romero R, Tarca AL et al. The transcriptome of the fetal inflammatory response syndrome. Am J Reprod Immunol. 2010 Jan; 63(1):73–92.
Calvano SE, Xiao W, Richards DR et al. A network-based analysis of systemic inflammation in humans. Nature 2005; 437:1032–1037.
Talwar S, Munson PJ, Barb J et al. Gene expression profiles of peripheral blood leukocytes after endotoxin challenge in humans. Physiol Genomics 2006; 25:203–215.
Wong HR, Shanley TP, Sakthivel B et al. Genome-level expression profiles in pediatric septic shock indicate a role for altered zinc homeostasis in poor outcome. Physiol Genomics 2007; 30:146–155.
Fig. 9.
Differentially expressed genes in umbilical cord blood according to the presence or absence of fetal inflammatory response syndrome (FIRS). The data were generated by qRT-PCR. Genes were selected after the results of microarray analysis. Altered abundance of genes within ontological categories of immune response and inflammation (a), MHC II receptor activity (b), carbohydrate metabolism (c) and signal transduction (d) was confirmed using qRT-PCR. Box-plots include 50% of the data with the middle line showing the median value; P < 0.05 for all genes is shown when modeled using GEE. Depending on the statistical method used, 93–95% of the genes tested confirmed the change observed by microarray analysis. (Reproduced with permission from Madsen-Bouterse SA, Romero R, Tarca AL et al. The transcriptome of the fetal inflammatory response syndrome. Am J Reprod Immunol 63:73–92, 2010.).
11. Heterogeneity of FIRS: types I and II
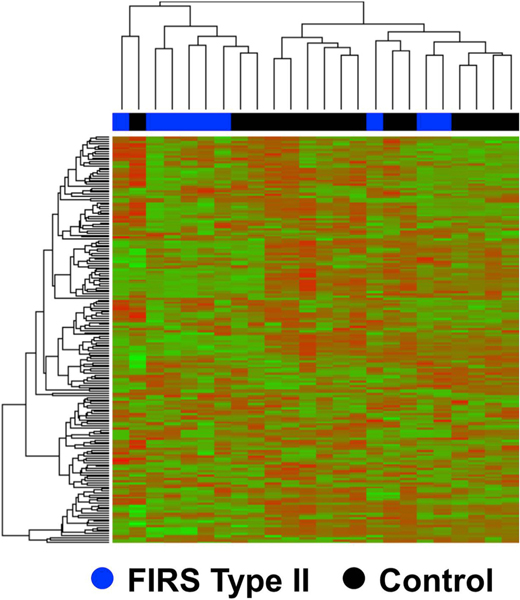
In the course of studying the mechanisms responsible for preterm labor, we identified a cluster of patients who had chronic inflammatory lesions of the placenta (i.e., chronic chorioamnionitis, villitis of unknown etiology, chronic deciduitis) rather than acute inflammatory lesions (e.g., acute chorioamnionitis, funisitis). These lesions were associated with a unique fetal systemic inflammatory response, which was characterized by the increased concentration of the cytokine CXCL-10 (a T-cell chemokine) rather than IL-6. We proposed that such placental lesions were related to maternal anti-fetal rejection rather than intra-amniotic infection/inflammation [334–341]. The umbilical cord transcriptome of these fetuses differed from that previously described in fetuses born to mothers with intra-amniotic infection/inflammation (FIRS Type I), consistent with the presence of an alloimmune reaction (Fig. 10). The serum proteome of umbilical cord blood was also different [340]. These findings suggest that FIRS may be present in patients without intra-amniotic infection/inflammation; hence, we proposed the term FIRS Type II [340]. Future investigation will determine the short- and long-term significance of this novel form of fetal systemic inflammation.
Fig. 10.
Transcriptome analysis of fetal blood using whole genome DASL® assay according to the presence or absence of fetal inflammatory response syndrome (FIRS) type II that is associated with maternal anti-fetal rejection. A clustered heat map based on the top 200 differentially expressed genes displays two main clusters: one dominated by samples of the fetal inflammatory response associated with maternal anti-fetal rejection group (FIRS II) (left) and one dominated by samples in the control group (right). (Reproduced with permission from Lee J, Romero R, Chaiworapongsa T et al. Characterization of the fetal blood transcriptome and proteome in maternal anti-fetal rejection: evidence of a distinct and novel type of human fetal systemic inflammatory response. Am J Reprod Immunol 70:265–84, 2013.).
12. Strategies for the management of FIRS
Several approaches can be used to manage the course of FIRS in the context of intra-amniotic inflammation: delivery of the fetus, antibiotic therapy, and immunomodulation of the inflammatory response.
Delivery of the fetus provides a rapid exit from a hostile environment. However, preterm delivery places the unborn child at risk for complications of prematurity. Therefore, the risks of prematurity and intra-amniotic infection/inflammation must be balanced. Antibiotic treatment can reduce the rate of intra-amniotic infection/inflammation, as well as funisitis in a subset of patients with spontaneous preterm delivery with intact membranes [342], cervical insufficiency [343], short cervix [344], and preterm PROM [345–347].
The use of immunomodulatory approaches in FIRS needs to be considered given recent successful approaches with the treatment of SARS-CoV-2, the virus responsible for COVID-19 (Coronavirus disease-19) [348–354]. The success of IL-1 blockade with high-dose anakinra and the blockade of the IL-6 pathway with a recombinant humanized monoclonal antibody suggests that these agents might be useful to manage hyper-inflammation in the perinatal period. The subject has been reviewed by Xiong and Wintermark [355] in this issue, and recently by Schuller [356]. A frontier is whether these interventions can be extended to the prenatal period. Recent studies suggest (1) that an anti-IL-6 receptor antibody inhibits preterm labor and delivery in a bacterial endotoxin model [357] and (2) that a similar intervention targeting IL-1 may reduce inflammation-induced fetal injury and improve neonatal and developmental outcomes in mice after exposure to bacterial endotoxin during pregnancy [358].
A combination of antibiotics and immunomodulatory agents (dexamethasone and indomethacin) has been shown to be effective in nonhuman pregnant primates to eradicate intra-amniotic infection, suppress the local inflammatory response, and prolong gestation in experimental premature labor induced by intra-amniotic inoculation with group B streptococci [359]. In addition, we recently showed that clarithromycin prevents preterm birth and reduces neonatal mortality in mice intra-amniotically injected with Ureaplasma parvum [360]. Collectively, this evidence indicates that immunomodulation may be an effective intervention in preventing fetal injury and prolonging gestation among patients with inflammation/infection-induced preterm labor.
Given that cells of the immune system are derived from pluripotent hematopoietic stem cells, stem cells have emerged as a promising potential neuroprotective and neuro-reparative treatment in the context of the fetal inflammatory response. Many immunomodulatory cells are present in umbilical cord blood, including mesenchymal stem cells and endothelial progenitor cells. In a mouse model of bacterial endotoxin-induced chorioamnionitis, maternal administration of mesenchymal stem cells resulted in reduced IL-6 concentration in the fetal brain, and improved neurobehavioral outcomes [361]. Umbilical cord blood cells have some benefits that support their therapeutic use for infants after birth who are at high risk for brain injury following antenatal or birth complications [362–368].
12.1. Practice points
Obstetricians should consider placental examination to determine whether preterm and term neonates have deployed a systemic inflammatory response, which can be identified by the presence of funisitis or chorionic vasculitis
An inflammatory process in the chorioamniotic membranes (i.e., acute chorioamnionitis) in the absence of funisitis or chorionic vasculitis is a maternal inflammatory response and should not be misconstrued as evidence of a fetal immune response
FIRS (defined as an elevation of cytokines in umbilical cord blood, presence of acute phase reactants, or severe funisitis) in preterm neonates is a risk factor for early neonatal sepsis, neuroinflammation, and chronic lung disease
FIRS can result not only from intra-amniotic infection but also sterile intra-amniotic inflammation. Efforts should be directed to identify the causes of fetal systemic inflammation before birth. This may require examination of amniotic fluid samples to ascertain the presence of microorganisms.
Evidence from clinical and experimental studies suggest that antimicrobial agents can eradicate intra-amniotic inflammation and intra-amniotic infection. Early preliminary evidence indicates that this approach may also reduce the rate of fetal systemic inflammation.
12.2. Research agenda
Determine the frequency and clinical significance of FIRS in each obstetrical syndrome leading to preterm birth, such as preterm labor with intact membranes, preterm PROM, pre-eclampsia, and fetal growth restriction
Develop biomarkers for rapid and practical diagnosis of FIRS that would allow ascertainment of this condition at the time of birth in high, as well as medium and low resource settings (e.g., dipstick to identify if a newborn has systemic inflammation by examining umbilical cord blood)
Test whether down-regulation of the fetal/neonatal inflammatory response with antimicrobial agents, anti-inflammatory agents, and cell-based therapies can improve the outcome of neonates and infants affected by FIRS
Acknowledgements
This review was supported, in part, by the Perinatology Research Branch, Division of Obstetrics and Maternal-Fetal Medicine, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, U.S. Department of Health and Human Services (NICHD/NIH/DHHS); and, in part, with Federal funds from NICHD/NIH/DHHS under Contract No. HHSN275201300006C.
Dr. Romero has contributed to this work as part of his official duties as an employee of the United States Federal Government.
Footnotes
Declaration of competing interest
The authors report no conflicts of interest.
References
- [1].Wendel GD Jr, Sanchez PJ, Peters MT, Harstad TW, Potter LL, Norgard MV. Identification of Treponema pallidum in amniotic fluid and fetal blood from pregnancies complicated by congenital syphilis. Obstet Gynecol 1991;78(5 Pt 2): 890–5. [PubMed] [Google Scholar]
- [2].Morgan-Capner P, Rodeck C, Nicolaides K, Cradock-Watson J. Prenatal detection of rubella-specific IgM in fetal sera. Prenat Diagn 1985;5(1):21–6. [DOI] [PubMed] [Google Scholar]
- [3].Thilaganathan B, Carroll SG, Plachouras N, Makrydimas G, Nicolaides KH. Fetal immunological and haematological changes in intrauterine infection. Br J Obstet Gynaecol 1994;101(5):418–21. [DOI] [PubMed] [Google Scholar]
- [4].Donders GG, Moerman P, Caudron J, Van Assche FA. Intra-uterine Candida infection: a report of four infected fetusses from two mothers. Eur J Obstet Gynecol Reprod Biol 1991;38(3):233–8. [DOI] [PubMed] [Google Scholar]
- [5].Carroll SG, Nicolaides KH. Fetal haematological response to intra-uterine infection in preterm prelabour amniorrhexis. Fetal Diagn Ther 1995;10(5): 279–85. [DOI] [PubMed] [Google Scholar]
- [6].Desmonts G, Forestier F, Thulliez P, Daffos F, Capella-Pavlovsky M, Chartier M. Prenatal diagnosis of congenital toxoplasmosis. Lancet 1985;325(8427):500–4. [DOI] [PubMed] [Google Scholar]
- [7].Pratlong F, Boulot P, Issert E, Msika M, Dupont F, Bachelard B, et al. Fetal diagnosis of toxoplasmosis in 190 women infected during pregnancy. Prenat Diagn 1994;14(3):191–8. [DOI] [PubMed] [Google Scholar]
- [8].Abrams ET, Kwiek JJ, Mwapasa V, Kamwendo DD, Tadesse E, Lema VM, et al. Malaria during pregnancy and foetal haematological status in Blantyre, Malawi. Malar J 2005;4:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gomez R, Romero R, Ghezzi F, Yoon BH, Mazor M, Berry SM. The fetal inflammatory response syndrome. Am J Obstet Gynecol 1998;179(1):194–202. [DOI] [PubMed] [Google Scholar]
- [10].American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med 1992;20(6):864–74. [PubMed] [Google Scholar]
- [11].Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). J Am Med Assoc 2016;315(8):801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med 2003;348(2):138–50. [DOI] [PubMed] [Google Scholar]
- [13].Hotchkiss RS, Monneret G, Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol 2013;13(12):862–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hotchkiss RS, Moldawer LL, Opal SM, Reinhart K, Turnbull IR, Vincent JL. Sepsis and septic shock. Nature reviews Disease primers 2016;2:16045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Luyt CE, Combes A, Deback C, Aubriot-Lorton MH, Nieszkowska A, Trouillet JL, et al. Herpes simplex virus lung infection in patients undergoing prolonged mechanical ventilation. Am J Respir Crit Care Med 2007;175(9):935–42. [DOI] [PubMed] [Google Scholar]
- [16].Limaye AP, Kirby KA, Rubenfeld GD, Leisenring WM, Bulger EM, Neff MJ, et al. Cytomegalovirus reactivation in critically ill immunocompetent patients. J Am Med Assoc 2008;300(4):413–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Stampalija T, Romero R, Korzeniewski SJ, Chaemsaithong P, Miranda J, Yeo L, et al. Soluble ST2 in the fetal inflammatory response syndrome: in vivo evidence of activation of the anti-inflammatory limb of the immune response. J Matern Fetal Neonatal Med 2013;26(14):1384–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gotsch F, Romero R, Kusanovic JP, Erez O, Espinoza J, Kim CJ, et al. The anti-inflammatory limb of the immune response in preterm labor, intra-amniotic infection/inflammation, and spontaneous parturition at term: a role for interleukin-10. J Matern Fetal Neonatal Med 2008;21(8):529–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hibbert JE, Currie A, Strunk T. Sepsis-induced immunosuppression in neonates. Front Pediatr 2018;6:357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Romero R, Gomez R, Ghezzi F, Yoon BH, Mazor M, Edwin SS, et al. A fetal systemic inflammatory response is followed by the spontaneous onset of preterm parturition. Am J Obstet Gynecol 1998;179(1):186–93. [DOI] [PubMed] [Google Scholar]
- [21].Tang Q, Zhang L, Li H, Shao Y. The fetal inflammation response syndrome and adverse neonatal outcomes: a meta-analysis. J Matern Fetal Neonatal Med 2019: 1–13. [DOI] [PubMed] [Google Scholar]
- [22].Park YJ, Woo SJ, Kim YM, Hong S, Lee YE, Park KH. Immune and inflammatory proteins in cord blood as predictive biomarkers of retinopathy of prematurity in preterm infants. Invest Ophthalmol Vis Sci 2019;60(12):3813–20. [DOI] [PubMed] [Google Scholar]
- [23].Andrews WW, Goldenberg RL, Faye-Petersen O, Cliver S, Goepfert AR, Hauth JC. The Alabama Preterm Birth study: polymorphonuclear and mononuclear cell placental infiltrations, other markers of inflammation, and outcomes in 23- to 32-week preterm newborn infants. Am J Obstet Gynecol 2006;195(3):803–8. [DOI] [PubMed] [Google Scholar]
- [24].Goldenberg RL, Andrews WW, Faye-Petersen OM, Cliver SP, Goepfert AR, Hauth JC. The Alabama preterm birth study: corticosteroids and neonatal outcomes in 23- to 32-week newborns with various markers of intrauterine infection. Am J Obstet Gynecol 2006;195(4):1020–4. [DOI] [PubMed] [Google Scholar]
- [25].Goldenberg RL, Andrews WW, Goepfert AR, Faye-Petersen O, Cliver SP, Carlo WA, et al. The Alabama Preterm Birth Study: umbilical cord blood Ureaplasma urealyticum and Mycoplasma hominis cultures in very preterm newborn infants. Am J Obstet Gynecol 2008;198(1):43 e1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Slayton WB, Li Y, Calhoun DA, Juul SE, Iturraspe J, Braylan RC, et al. The first-appearance of neutrophils in the human fetal bone marrow cavity. Early Hum Dev 1998;53(2):129–44. [DOI] [PubMed] [Google Scholar]
- [27].De Waele M, Foulon W, Renmans W, Segers E, Smet L, Jochmans K, et al. Hematologic values and lymphocyte subsets in fetal blood. Am J Clin Pathol 1988;89(6):742–6. [DOI] [PubMed] [Google Scholar]
- [28].Davies NP, Buggins AG, Snijders RJ, Jenkins E, Layton DM, Nicolaides KH. Blood leucocyte count in the human fetus. Arch Dis Child 1992;67(4 Spec No):399–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Romero R, Savasan ZA, Chaiworapongsa T, Berry SM, Kusanovic JP, Hassan SS, et al. Hematologic profile of the fetus with systemic inflammatory response syndrome. J Perinat Med 2011;40(1):19–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kim EN, Kim CJ, Park JW, Yoon BH. Acute funisitis is associated with distinct changes in fetal hematologic profile. J Matern Fetal Neonatal Med 2015;28(5): 588–93. [DOI] [PubMed] [Google Scholar]
- [31].Berry SM, Romero R, Gomez R, Puder KS, Ghezzi F, Cotton DB, et al. Premature parturition is characterized by in utero activation of the fetal immune system. Am J Obstet Gynecol 1995;173(4):1315–20. [DOI] [PubMed] [Google Scholar]
- [32].Kim SK, Romero R, Chaiworapongsa T, Kusanovic JP, Mazaki-Tovi S, Mittal P, et al. Evidence of changes in the immunophenotype and metabolic characteristics (intracellular reactive oxygen radicals) of fetal, but not maternal, monocytes and granulocytes in the fetal inflammatory response syndrome. J Perinat Med 2009; 37(5):543–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Nicola NA. Granulocyte colony stimulating factor. Methods Enzymol 1985;116: 600–19. [DOI] [PubMed] [Google Scholar]
- [34].Uzumaki H, Okabe T, Sasaki N, Hagiwara K, Takaku F, Tobita M, et al. Identification and characterization of receptors for granulocyte colony-stimulating factor on human placenta and trophoblastic cells. Proc Natl Acad Sci U S A 1989;86(23):9323–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Demetri GD, Griffin JD. Granulocyte colony-stimulating factor and its receptor. Blood 1991;78(11):2791–808. [PubMed] [Google Scholar]
- [36].Chaiworapongsa T, Romero R, Berry SM, Hassan SS, Yoon BH, Edwin S, et al. The role of granulocyte colony-stimulating factor in the neutrophilia observed in the fetal inflammatory response syndrome. J Perinat Med 2011;39(6):653–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Mandel D, Oron T, Mimouni GS, Littner Y, Dollberg S, Mimouni FB. The effect of prolonged rupture of membranes on circulating neonatal nucleated red blood cells. J Perinatol 2005;25(11):690–3. [DOI] [PubMed] [Google Scholar]
- [38].Leikin E, Garry D, Visintainer P, Verma U, Tejani N. Correlation of neonatal nucleated red blood cell counts in preterm infants with histologic chorioamnionitis. Am J Obstet Gynecol 1997;177(1):27–30. [DOI] [PubMed] [Google Scholar]
- [39].Salafia CM, Ghidini A, Pezzullo JC, Rosenkrantz TS. Early neonatal nucleated erythrocyte counts in preterm deliveries: clinical and pathologic correlations. J Soc Gynecol Invest 1997;4(3):138–43. [DOI] [PubMed] [Google Scholar]
- [40].Dulay AT, Buhimschi IA, Zhao G, Luo G, bdel-Razeq S, Cackovic M, et al. Nucleated red blood cells are a direct response to mediators of inflammation in newborns with early-onset neonatal sepsis. Am J Obstet Gynecol 2008;198(4): 426–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Romero R, Soto E, Berry SM, Hassan SS, Kusanovic JP, Yoon BH, et al. Blood pH and gases in fetuses in preterm labor with and without systemic inflammatory response syndrome. J Matern Fetal Neonatal Med 2012;25(7):1160–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Baschat AA, Gembruch U, Reiss I, Gortner L, Harman CR, Weiner CP. Neonatal nucleated red blood cell counts in growth-restricted fetuses: relationship to arterial and venous Doppler studies. Am J Obstet Gynecol 1999;181(1):190–5. [DOI] [PubMed] [Google Scholar]
- [43].Ferber A, Minior VK, Bornstein E, Divon MY. Fetal “nonreassuring status” is associated with elevation of nucleated red blood cell counts and interleukin-6. Am J Obstet Gynecol 2005;192(5):1427–9. [DOI] [PubMed] [Google Scholar]
- [44].Ulich TR, del Castillo J, Guo KZ. In vivo hematologic effects of recombinant interleukin-6 on hematopoiesis and circulating numbers of RBCs and WBCs. Blood 1989;73(1):108–10. [PubMed] [Google Scholar]
- [45].Spiropoulos A, Goussetis E, Margeli A, Premetis E, Skenderi K, Graphakos S, et al. Effect of inflammation induced by prolonged exercise on circulating erythroid progenitors and markers of erythropoiesis. Clin Chem Lab Med 2010;48(2): 199–203. [DOI] [PubMed] [Google Scholar]
- [46].Maier RF, Gunther A, Vogel M, Dudenhausen JW, Obladen M. Umbilical venous erythropoietin and umbilical arterial pH in relation to morphologic placental abnormalities. Obstet Gynecol 1994;84(1):81–7. [PubMed] [Google Scholar]
- [47].Kallapur SG, Kramer BW, Nitsos I, Pillow JJ, Collins JJ, Polglase GR, et al. Pulmonary and systemic inflammatory responses to intra-amniotic IL-1alpha in fetal sheep. Am J Physiol Lung Cell Mol Physiol 2011;301(3):L285–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Kramer BW, Moss TJ, Willet KE, Newnham JP, Sly PD, Kallapur SG, et al. Dose and time response after intraamniotic endotoxin in preterm lambs. Am J Respir Crit Care Med 2001;164(6):982–8. [DOI] [PubMed] [Google Scholar]
- [49].Nguyen DN, Thymann T, Goericke-Pesch SK, Ren S, Wei W, Skovgaard K, et al. Prenatal intra-amniotic endotoxin induces fetal gut and lung immune responses and postnatal systemic inflammation in preterm pigs. Am J Pathol 2018;188(11): 2629–43. [DOI] [PubMed] [Google Scholar]
- [50].De Felice C, Toti P, Santopietro R, Stumpo M, Pecciarini L, Bagnoli F. Small thymus in very low birth weight infants born to mothers with subclinical chorioamnionitis. J Pediatr 1999;135(3):384–6. [DOI] [PubMed] [Google Scholar]
- [51].Toti P, De Felice C, Stumpo M, Schürfeld K, Di Leo L, Vatti R, et al. Acute thymic involution in fetuses and neonates with chorioamnionitis. Hum Pathol 2000;31 (9):1121–8. [DOI] [PubMed] [Google Scholar]
- [52].Glavina-Durdov M, Springer O, Capkun V, Saratlija-Novaković Z, Rozić D, Barle M. The grade of acute thymus involution in neonates correlates with the duration of acute illness and with the percentage of lymphocytes in peripheral blood smear. Pathological study. Biol Neonate 2003;83(4):229–34. [DOI] [PubMed] [Google Scholar]
- [53].Yinon Y, Zalel Y, Weisz B, Mazaki-Tovi S, Sivan E, Schiff E, et al. Fetal thymus size as a predictor of chorioamnionitis in women with preterm premature rupture of membranes. Ultrasound Obstet Gynecol 2007;29(6):639–43. [DOI] [PubMed] [Google Scholar]
- [54].El-Haieg DO, Zidan AA, El-Nemr MM. The relationship between sonographic fetal thymus size and the components of the systemic fetal inflammatory response syndrome in women with preterm prelabour rupture of membranes. Bjog-an International Journal of Obstetrics and Gynaecology 2008;115(7):836–41. [DOI] [PubMed] [Google Scholar]
- [55].Musilova I, Hornychova H, Kostal M, Jacobsson B, Kacerovsky M. Ultrasound measurement of the transverse diameter of the fetal thymus in pregnancies complicated by the preterm prelabor rupture of membranes. J Clin Ultrasound 2013;41(5):283–9. [DOI] [PubMed] [Google Scholar]
- [56].Morrissey PJ, Charrier K, Alpert A, Bressler L. In vivo administration of IL-1 induces thymic hypoplasia and increased levels of serum corticosterone. J Immunol 1988;141(5):1456–63. [PubMed] [Google Scholar]
- [57].Gruver AL, Sempowski GD. Cytokines, leptin, and stress-induced thymic atrophy. J Leukoc Biol 2008;84(4):915–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Billard MJ, Gruver AL, Sempowski GD. Acute endotoxin-induced thymic atrophy is characterized by intrathymic inflammatory and wound healing responses. PloS One 2011;6(3):e17940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Di Naro E, Cromi A, Ghezzi F, Raio L, Uccella S, D’Addario V, et al. Fetal thymic involution: a sonographic marker of the fetal inflammatory response syndrome. Am J Obstet Gynecol 2006;194(1):153–9. [DOI] [PubMed] [Google Scholar]
- [60].Sciaky-Tamir Y, Hershkovitz R, Mazor M, Shelef I, Erez O. The use of imaging technology in the assessment of the fetal inflammatory response syndrome-imaging of the fetal thymus. Prenat Diagn 2015;35(5):413–9. [DOI] [PubMed] [Google Scholar]
- [61].Kuban JD, Allred EN, Leviton A. Thymus involution and cerebral white matter damage in extremely low gestational age neonates. Biol Neonate 2006;90(4): 252–7. [DOI] [PubMed] [Google Scholar]
- [62].Kunzmann S, Glogger K, Been JV, Kallapur SG, Nitsos I, Moss TJ, et al. Thymic changes after chorioamnionitis induced by intraamniotic lipopolysaccharide in fetal sheep. Am J Obstet Gynecol 2010;202(5). 476.e1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Kuypers E, Wolfs TG, Collins JJ, Jellema RK, Newnham JP, Kemp MW, et al. Intraamniotic lipopolysaccharide exposure changes cell populations and structure of the ovine fetal thymus. Reprod Sci 2013;20(8):946–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Tarcic N, Ovadia H, Weiss DW, Weidenfeld J. Restraint stress-induced thymic involution and cell apoptosis are dependent on endogenous glucocorticoids. J Neuroimmunol 1998;82(1):40–6. [DOI] [PubMed] [Google Scholar]
- [65].Sempowski GD, Rhein ME, Scearce RM, Haynes BF. Leukemia inhibitory factor is a mediator of Escherichia coli lipopolysaccharide-induced acute thymic atrophy. Eur J Immunol 2002;32(11):3066–70. [DOI] [PubMed] [Google Scholar]
- [66].Démoulins T, Abdallah A, Kettaf N, Baron ML, Gerarduzzi C, Gauchat D, et al. Reversible blockade of thymic output: an inherent part of TLR ligand-mediated immune response. J Immunol 2008;181(10):6757–69. [DOI] [PubMed] [Google Scholar]
- [67].Anz D, Thaler R, Stephan N, Waibler Z, Trauscheid MJ, Scholz C, et al. Activation of melanoma differentiation-associated gene 5 causes rapid involution of the thymus. J Immunol 2009;182(10):6044–50. [DOI] [PubMed] [Google Scholar]
- [68].Melville JM, Bischof RJ, Meeusen EN, Westover AJ, Moss TJ. Changes in fetal thymic immune cell populations in a sheep model of intrauterine inflammation. Reprod Sci 2012;19(7):740–7. [DOI] [PubMed] [Google Scholar]
- [69].Luciano AA, Yu H, Jackson LW, Wolfe LA, Bernstein HB. Preterm labor and chorioamnionitis are associated with neonatal T cell activation. PloS One 2011;6 (2):e16698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Brüning T, Daiminger A, Enders G. Diagnostic value of CD45RO expression on circulating T lymphocytes of fetuses and newborn infants with pre-, peri- or early post-natal infections. Clin Exp Immunol 1997;107(2):306–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Peoples JD, Cheung S, Nesin M, Lin H, Tatad AM, Hoang D, et al. Neonatal cord blood subsets and cytokine response to bacterial antigens. Am J Perinatol 2009; 26(9):647–57. [DOI] [PubMed] [Google Scholar]
- [72].Matsuda Y, Kato H, Imanishi K, Mitani M, Ohta H, Uchiyama T. T cell activation in abnormal perinatal events. Microbiol Immunol 2010;54(1):38–45. [DOI] [PubMed] [Google Scholar]
- [73].Kallapur SG, Presicce P, Senthamaraikannan P, Alvarez M, Tarantal AF, Miller LM, et al. Intra-amniotic IL-1β induces fetal inflammation in rhesus monkeys and alters the regulatory T cell/IL-17 balance. J Immunol 2013;191(3): 1102–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Lewis SM, Williams A, Eisenbarth SC. Structure and function of the immune system in the spleen. Science immunology 2019;4(33). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Bronte V, Pittet MJ. The spleen in local and systemic regulation of immunity. Immunity 2013;39(5):806–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Toti P, De Felice C, Occhini R, Schuerfeld K, Stumpo M, Epistolato MC, et al. Spleen depletion in neonatal sepsis and chorioamnionitis. Am J Clin Pathol 2004; 122(5):765–71. [DOI] [PubMed] [Google Scholar]
- [77].Musilova I, Kacerovsky M, Andrys C, Kostal M, Slaba K, Jacobsson B. The fetal splenic vein flow pattern and fetal inflammatory response in the preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med 2014;27(8):770–4. [DOI] [PubMed] [Google Scholar]
- [78].Musilova I, Spacek R, Stranik J, Jacobsson B, Kacerovsky M. Fetal portal system flowmetry and intra-amniotic inflammation in preterm prelabor rupture of membranes. Fetal Diagn Ther 2019:1–10. [DOI] [PubMed] [Google Scholar]
- [79].Musilova I, Kacerovsky M, Hornychova H, Kostal M, Jacobsson B. Pulsation of the fetal splenic vein–a potential ultrasound marker of histological chorioamnionitis and funisitis in women with preterm prelabor rupture of membranes. Acta Obstet Gynecol Scand 2012;91(9):1119–23. [DOI] [PubMed] [Google Scholar]
- [80].Kuypers E, Willems MG, Jellema RK, Kemp MW, Newnham JP, Delhaas T, et al. Responses of the spleen to intraamniotic lipopolysaccharide exposure in fetal sheep. Pediatr Res 2015;77(1–1):29–35. [DOI] [PubMed] [Google Scholar]
- [81].Yoon BH, Romero R, Jun JK, Maymon E, Gomez R, Mazor M, et al. An increase in fetal plasma cortisol but not dehydroepiandrosterone sulfate is followed by the onset of preterm labor in patients with preterm premature rupture of the membranes. Am J Obstet Gynecol 1998;179(5):1107–14. [DOI] [PubMed] [Google Scholar]
- [82].Seckl JR, Cleasby M, Nyirenda MJ. Glucocorticoids, 11beta-hydroxysteroid dehydrogenase, and fetal programming. Kidney Int 2000;57(4):1412–7. [DOI] [PubMed] [Google Scholar]
- [83].Nathanielsz PW, Berghorn KA, Derks JB, Giussani DA, Docherty C, Unno N, et al. Life before birth: effects of cortisol on future cardiovascular and metabolic function. Acta Paediatr 2003;92(7):766–72. [PubMed] [Google Scholar]
- [84].Seckl JR, Meaney MJ. Glucocorticoid programming. Ann N Y Acad Sci 2004; 1032:63–84. [DOI] [PubMed] [Google Scholar]
- [85].Moritz KM, Boon WM, Wintour EM. Glucocorticoid programming of adult disease. Cell Tissue Res 2005;322(1):81–8. [DOI] [PubMed] [Google Scholar]
- [86].Seckl JR, Holmes MC. Mechanisms of disease: glucocorticoids, their placental metabolism and fetal ‘programming’ of adult pathophysiology. Nat Clin Pract Endocrinol Metabol 2007;3(6):479–88. [DOI] [PubMed] [Google Scholar]
- [87].Williams FL, Visser TJ, Hume R. Transient hypothyroxinaemia in preterm infants. Early Hum Dev 2006;82(12):797–802. [DOI] [PubMed] [Google Scholar]
- [88].De Felice C, Bagnoli F, Toti P, Musaro MA, Peruzzi L, Paffetti P, et al. Transient hypothyroxinemia of prematurity and histological chorioamnionitis. J Perinat Med 2005;33(6):514–8. [DOI] [PubMed] [Google Scholar]
- [89].Reuss ML, Paneth N, Pinto-Martin JA, Lorenz JM, Susser M. The relation of transient hypothyroxinemia in preterm infants to neurologic development at two years of age. N Engl J Med 1996;334(13):821–7. [DOI] [PubMed] [Google Scholar]
- [90].Luo B, Yu Z, Li Y. Thyroid hormone disorders and sepsis. Bio Med Mater Eng 2017;28(s1). S237-s41. [DOI] [PubMed] [Google Scholar]
- [91].Kim JG, Shin H, Kim W, Lim TH, Jang B, Cho Y, et al. The value of decreased thyroid hormone for predicting mortality in adult septic patients: a systematic review and meta-analysis. Sci Rep 2018;8(1):14137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Stephens JD, Birnholz JC. Noninvasive verification of fetal respiratory movements in normal pregnancy. J Am Med Assoc 1978;240(1):35–6. [PubMed] [Google Scholar]
- [93].Stephens JD, Birnholz JC. Verification of human fetal breathing with phased array ultrasound imaging. J Clin Ultrasound 1978;6(2):100–2. [DOI] [PubMed] [Google Scholar]
- [94].Isaacson G, Birnholz JC. Human fetal upper respiratory tract function as revealed by ultrasonography. Ann Otol Rhinol Laryngol 1991;100(9 Pt 1):743–7. [DOI] [PubMed] [Google Scholar]
- [95].Kalache KD, Chaoui R, Bollmann R. Doppler assessment of tracheal and nasal fluid flow during fetal breathing movements: preliminary observations. Ultrasound Obstet Gynecol 1997;9(4):257–61. [DOI] [PubMed] [Google Scholar]
- [96].Burgess AM, Hutchins GM. Inflammation of the lungs, umbilical cord and placenta associated with meconium passage in utero. Review of 123 autopsied cases. Pathol Res Pract 1996;192(11):1121–8. [DOI] [PubMed] [Google Scholar]
- [97].Mortensen E, Kearney MS. Meconium aspiration in the midtrimester fetus: an autopsy study. Pediatr Dev Pathol 2009;12(6):438–42. [DOI] [PubMed] [Google Scholar]
- [98].Cassell GH, Waites KB, Crouse DT, Rudd PT, Canupp KC, Stagno S, et al. Association of Ureaplasma urealyticum infection of the lower respiratory tract with chronic lung disease and death in very-low-birth-weight infants. Lancet 1988;2(8605):240–5. [DOI] [PubMed] [Google Scholar]
- [99].Vintzileos AM, Campbell WA, Nochimson DJ, Connolly ME, Fuenfer MM, Hoehn GJ. The fetal biophysical profile in patients with premature rupture of the membranes–an early predictor of fetal infection. Am J Obstet Gynecol 1985;152 (5):510–6. [DOI] [PubMed] [Google Scholar]
- [100].Vintzileos AM, Campbell WA, Nochimson DJ, Weinbaum PJ. Fetal breathing as a predictor of infection in premature rupture of the membranes. Obstet Gynecol 1986;67(6):813–7. [DOI] [PubMed] [Google Scholar]
- [101].Goldstein I, Romero R, Merrill S, Wan M, O’Connor TZ, Mazor M, et al. Fetal body and breathing movements as predictors of intraamniotic infection in preterm premature rupture of membranes. Am J Obstet Gynecol 1988;159(2):363–8. [DOI] [PubMed] [Google Scholar]
- [102].Sherer DM, Spong CY, Salafia CM. Fetal breathing movements within 24 hours of delivery in prematurity are related to histologic and clinical evidence of amnionitis. Am J Perinatol 1997;14(6):337–40. [DOI] [PubMed] [Google Scholar]
- [103].Speer CP. Inflammatory mechanisms in neonatal chronic lung disease. Eur J Pediatr 1999;158(Suppl 1):S18–22. [DOI] [PubMed] [Google Scholar]
- [104].Speer CP. New insights into the pathogenesis of pulmonary inflammation in preterm infants. Biol Neonate 2001;79(3–4):205–9. [DOI] [PubMed] [Google Scholar]
- [105].Thebaud B, Goss KN, Laughon M, Whitsett JA, Abman SH, Steinhorn RH, et al. Bronchopulmonary dysplasia. Nature reviews Disease primers 2019;5(1):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Watterberg KL, Demers LM, Scott SM, Murphy S. Chorioamnionitis and early lung inflammation in infants in whom bronchopulmonary dysplasia develops. Pediatrics 1996;97(2):210–5. [PubMed] [Google Scholar]
- [107].Villamor-Martinez E, Alvarez-Fuente M, Ghazi AMT, Degraeuwe P, Zimmermann LJI, Kramer BW, et al. Association of chorioamnionitis with bronchopulmonary dysplasia among preterm infants: a systematic review, meta-analysis, and meta-regression. JAMA network open 2019;2(11):e1914611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Ghezzi F, Gomez R, Romero R, Yoon BH, Edwin SS, David C, et al. Elevated interleukin-8 concentrations in amniotic fluid of mothers whose neonates subsequently develop bronchopulmonary dysplasia. Eur J Obstet Gynecol Reprod Biol 1998;78(1):5–10. [DOI] [PubMed] [Google Scholar]
- [109].Yoon BH, Romero R, Jun JK, Park KH, Park JD, Ghezzi F, et al. Amniotic fluid cytokines (interleukin-6, tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-8) and the risk for the development of bronchopulmonary dysplasia. Am J Obstet Gynecol 1997;177(4):825–30. [DOI] [PubMed] [Google Scholar]
- [110].Yoon BH, Romero R, Kim KS, Park JS, Ki SH, Kim BI, et al. A systemic fetal inflammatory response and the development of bronchopulmonary dysplasia. Am J Obstet Gynecol 1999;181(4):773–9. [DOI] [PubMed] [Google Scholar]
- [111].Jobe AH, Newnham JP, Willet KE, Sly P, Ervin MG, Bachurski C, et al. Effects of antenatal endotoxin and glucocorticoids on the lungs of preterm lambs. Am J Obstet Gynecol 2000;182(2):401–8. [DOI] [PubMed] [Google Scholar]
- [112].Kallapur SG, Willet KE, Jobe AH, Ikegami M, Bachurski CJ. Intra-amniotic endotoxin: chorioamnionitis precedes lung maturation in preterm lambs. Am J Physiol Lung Cell Mol Physiol 2001;280(3):L527–36. [DOI] [PubMed] [Google Scholar]
- [113].Moss TJ, Nitsos I, Ikegami M, Jobe AH, Newnham JP. Experimental intrauterine Ureaplasma infection in sheep. Am J Obstet Gynecol 2005;192(4):1179–86. [DOI] [PubMed] [Google Scholar]
- [114].Bry K, Lappalainen U, Hallman M. Intraamniotic interleukin-1 accelerates surfactant protein synthesis in fetal rabbits and improves lung stability after premature birth. J Clin Invest 1997;99(12):2992–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Willet KE, Jobe AH, Ikegami M, Newnham J, Brennan S, Sly PD. Antenatal endotoxin and glucocorticoid effects on lung morphometry in preterm lambs. Pediatr Res 2000;48(6):782–8. [DOI] [PubMed] [Google Scholar]
- [116].Bachurski CJ, Ross GF, Ikegami M, Kramer BW, Jobe AH. Intra-amniotic endotoxin increases pulmonary surfactant proteins and induces SP-B processing in fetal sheep. Am J Physiol Lung Cell Mol Physiol 2001;280(2):L279–85. [DOI] [PubMed] [Google Scholar]
- [117].Newnham JP, Moss TJ, Padbury JF, Willet KE, Ikegami M, Ervin MG, et al. The interactive effects of endotoxin with prenatal glucocorticoids on short-term lung function in sheep. Am J Obstet Gynecol 2001;185(1):190–7. [DOI] [PubMed] [Google Scholar]
- [118].Moss TJ, Nitsos I, Kramer BW, Ikegami M, Newnham JP, Jobe AH. Intra-amniotic endotoxin induces lung maturation by direct effects on the developing respiratory tract in preterm sheep. Am J Obstet Gynecol 2002;187(4):1059–65. [DOI] [PubMed] [Google Scholar]
- [119].Kallapur SG, Jobe AH, Ikegami M, Bachurski CJ. Increased IP-10 and MIG expression after intra-amniotic endotoxin in preterm lamb lung. Am J Respir Crit Care Med 2003;167(5):779–86. [DOI] [PubMed] [Google Scholar]
- [120].Kallapur SG, Bachurski CJ, Le Cras TD, Joshi SN, Ikegami M, Jobe AH. Vascular changes after intra-amniotic endotoxin in preterm lamb lungs. Am J Physiol Lung Cell Mol Physiol 2004;287(6):L1178–85. [DOI] [PubMed] [Google Scholar]
- [121].Kallapur SG, Moss TJ, Ikegami M, Jasman RL, Newnham JP, Jobe AH. Recruited inflammatory cells mediate endotoxin-induced lung maturation in preterm fetal lambs. Am J Respir Crit Care Med 2005;172(10):1315–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Kramer BW, Joshi SN, Moss TJ, Newnham JP, Sindelar R, Jobe AH, et al. Endotoxin-induced maturation of monocytes in preterm fetal sheep lung. Am J Physiol Lung Cell Mol Physiol 2007;293(2):L345–53. [DOI] [PubMed] [Google Scholar]
- [123].Kallapur SG, Nitsos I, Moss TJ, Polglase GR, Pillow JJ, Cheah FC, et al. IL-1 mediates pulmonary and systemic inflammatory responses to chorioamnionitis induced by lipopolysaccharide. Am J Respir Crit Care Med 2009;179(10):955–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Shah TA, Hillman NH, Nitsos I, Polglase GR, Pillow JJ, Newnham JP, et al. Pulmonary and systemic expression of monocyte chemotactic proteins in preterm sheep fetuses exposed to lipopolysaccharide-induced chorioamnionitis. Pediatr Res 2010;68(3):210–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Polglase GR, Hooper SB, Gill AW, Allison BJ, Crossley KJ, Moss TJ, et al. Intrauterine inflammation causes pulmonary hypertension and cardiovascular sequelae in preterm lambs. J Appl Physiol 2010;108(6):1757–65. Bethesda, Md : 1985. [DOI] [PubMed] [Google Scholar]
- [126].Kramer BW, Kallapur SG, Moss TJ, Nitsos I, Polglase GP, Newnham JP, et al. Modulation of fetal inflammatory response on exposure to lipopolysaccharide by chorioamnion, lung, or gut in sheep. Am J Obstet Gynecol 2010;202(1). 77.e1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Collins JJ, Kuypers E, Nitsos I, Jane Pillow J, Polglase GR, Kemp MW, et al. LPS-induced chorioamnionitis and antenatal corticosteroids modulate Shh signaling in the ovine fetal lung. Am J Physiol Lung Cell Mol Physiol 2012;303(9):L778–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Kemp MW, Kannan PS, Saito M, Newnham JP, Cox T, Jobe AH, et al. Selective exposure of the fetal lung and skin/amnion (but not gastro-intestinal tract) to LPS elicits acute systemic inflammation in fetal sheep. PloS One 2013;8(5):e63355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Moss TJ, Knox CL, Kallapur SG, Nitsos I, Theodoropoulos C, Newnham JP, et al. Experimental amniotic fluid infection in sheep: effects of Ureaplasma parvum serovars 3 and 6 on preterm or term fetal sheep. Am J Obstet Gynecol 2008;198 (1):122.e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Moss TJ, Nitsos I, Knox CL, Polglase GR, Kallapur SG, Ikegami M, et al. Ureaplasma colonization of amniotic fluid and efficacy of antenatal corticosteroids for preterm lung maturation in sheep. Am J Obstet Gynecol 2009; 200(1). 96.e1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Novy MJ, Duffy L, Axthelm MK, Sadowsky DW, Witkin SS, Gravett MG, et al. Ureaplasma parvum or Mycoplasma hominis as sole pathogens cause chorioamnionitis, preterm delivery, and fetal pneumonia in rhesus macaques. Reprod Sci 2009;16(1):56–70. [DOI] [PubMed] [Google Scholar]
- [132].Collins JJ, Kallapur SG, Knox CL, Nitsos I, Polglase GR, Pillow JJ, et al. Inflammation in fetal sheep from intra-amniotic injection of Ureaplasma parvum. Am J Physiol Lung Cell Mol Physiol 2010;299(6):L852–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Ikegami M, Moss TJ, Kallapur SG, Mulrooney N, Kramer BW, Nitsos I, et al. Minimal lung and systemic responses to TNF-alpha in preterm sheep. Am J Physiol Lung Cell Mol Physiol 2003;285(1):L121–9. [DOI] [PubMed] [Google Scholar]
- [134].Kallapur SG, Presicce P, Rueda CM, Jobe AH, Chougnet CA. Fetal immune response to chorioamnionitis. Semin Reprod Med 2014;32(1):56–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Jj C In: Bland RDCJ, editor. Pathology of chronic lung disease of early infancy. Marcel Dekker; 2000. [Google Scholar]
- [136].Parker MM, Shelhamer JH, Bacharach SL, Green MV, Natanson C, Frederick TM, et al. Profound but reversible myocardial depression in patients with septic shock. Ann Intern Med 1984;100(4):483–90. [DOI] [PubMed] [Google Scholar]
- [137].Parker MM, McCarthy KE, Ognibene FP, Parrillo JE. Right ventricular dysfunction and dilatation, similar to left ventricular changes, characterize the cardiac depression of septic shock in humans. Chest 1990;97(1):126–31. [DOI] [PubMed] [Google Scholar]
- [138].Vincent JL, Gris P, Coffernils M, Leon M, Pinsky M, Reuse C, et al. Myocardial depression characterizes the fatal course of septic shock. Surgery 1992;111(6): 660–7. [PubMed] [Google Scholar]
- [139].Kumar A, Haery C, Parrillo JE. Myocardial dysfunction in septic shock. Crit Care Clin 2000;16(2):251–87. [DOI] [PubMed] [Google Scholar]
- [140].Rudiger A, Singer M. Mechanisms of sepsis-induced cardiac dysfunction. Crit Care Med 2007;35(6):1599–608. [DOI] [PubMed] [Google Scholar]
- [141].Court O, Kumar A, Parrillo JE, Kumar A. Clinical review: myocardial depression in sepsis and septic shock. Crit Care 2002;6(6):500–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Romero R, Espinoza J, Gonçalves LF, Gomez R, Medina L, Silva M, et al. Fetal cardiac dysfunction in preterm premature rupture of membranes. J Matern Fetal Neonatal Med 2004;16(3):146–57. [DOI] [PubMed] [Google Scholar]
- [143].Di Naro E, Cromi A, Ghezzi F, Giocolano A, Caringella A, Loverro G. Myocardial dysfunction in fetuses exposed to intraamniotic infection: new insights from tissue Doppler and strain imaging. Am J Obstet Gynecol 2010;203(5). 459.e1–7. [DOI] [PubMed] [Google Scholar]
- [144].Yanowitz TD, Jordan JA, Gilmour CH, Towbin R, Bowen A, Roberts JM, et al. Hemodynamic disturbances in premature infants born after chorioamnionitis: association with cord blood cytokine concentrations. Pediatr Res 2002;51(3): 310–6. [DOI] [PubMed] [Google Scholar]
- [145].Vandenbroucke L, Doyen M, Le Lous M, Beuchee A, Loget P, Carrault G, et al. Chorioamnionitis following preterm premature rupture of membranes and fetal heart rate variability. PloS One 2017;12(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Rounioja S, Räsänen J, Glumoff V, Ojaniemi M, Mäkikallio K, Hallman M. Intra-amniotic lipopolysaccharide leads to fetal cardiac dysfunction. A mouse model for fetal inflammatory response. Cardiovasc Res 2003;60(1):156–64. [DOI] [PubMed] [Google Scholar]
- [147].Rounioja S, Räsänen J, Ojaniemi M, Glumoff V, Autio-Harmainen H, Hallman M. Mechanism of acute fetal cardiovascular depression after maternal inflammatory challenge in mouse. Am J Pathol 2005;166(6):1585–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Tare M, Bensley JG, Moss TJ, Lingwood BE, Kim MY, Barton SK, et al. Exposure to intrauterine inflammation leads to impaired function and altered structure in the preterm heart of fetal sheep. Clin Sci (Lond) 2014;127(9):559–69. [DOI] [PubMed] [Google Scholar]
- [149].Durosier LD, Herry CL, Cortes M, Cao M, Burns P, Desrochers A, et al. Does heart rate variability reflect the systemic inflammatory response in a fetal sheep model of lipopolysaccharide-induced sepsis? Physiol Meas 2015;36(10):2089–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Stock SJ, Patey O, Thilaganathan B, White S, Furfaro LL, Payne MS, et al. Intrauterine Candida albicans infection causes systemic fetal candidiasis with progressive cardiac dysfunction in a sheep model of early pregnancy. Reprod Sci 2017;24(1):77–84. [DOI] [PubMed] [Google Scholar]
- [151].Mitchell T, MacDonald JW, Srinouanpranchanh S, Bammler TK, Merillat S, Boldenow E, et al. Evidence of cardiac involvement in the fetal inflammatory response syndrome: disruption of gene networks programming cardiac development in nonhuman primates. Am J Obstet Gynecol 2018;218(4). 438.e1-. e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Carr H, Cnattingius S, Granath F, Ludvigsson JF, Edstedt Bonamy AK. Preterm birth and risk of heart failure up to early adulthood. J Am Coll Cardiol 2017;69 (21):2634–42. [DOI] [PubMed] [Google Scholar]
- [153].Bonamy AK, Martin H, Jörneskog G, Norman M. Lower skin capillary density, normal endothelial function and higher blood pressure in children born preterm. J Intern Med 2007;262(6):635–42. [DOI] [PubMed] [Google Scholar]
- [154].Bensley JG, Stacy VK, De Matteo R, Harding R, Black MJ. Cardiac remodelling as a result of pre-term birth: implications for future cardiovascular disease. Eur Heart J 2010;31(16):2058–66. [DOI] [PubMed] [Google Scholar]
- [155].Lewandowski AJ, Bradlow WM, Augustine D, Davis EF, Francis J, Singhal A, et al. Right ventricular systolic dysfunction in young adults born preterm. Circulation 2013;128(7):713–20. [DOI] [PubMed] [Google Scholar]
- [156].Lewandowski AJ, Augustine D, Lamata P, Davis EF, Lazdam M, Francis J, et al. Preterm heart in adult life: cardiovascular magnetic resonance reveals distinct differences in left ventricular mass, geometry, and function. Circulation 2013;127 (2):197–206. [DOI] [PubMed] [Google Scholar]
- [157].Been JV, Lievense S, Zimmermann LJ, Kramer BW, Wolfs TG. Chorioamnionitis as a risk factor for necrotizing enterocolitis: a systematic review and meta-analysis. J Pediatr 2013;162(2). 236–42.e2. [DOI] [PubMed] [Google Scholar]
- [158].Neu J, Mshvildadze M, Mai V. A roadmap for understanding and preventing necrotizing enterocolitis. Curr Gastroenterol Rep 2008;10(5):450–7. [DOI] [PubMed] [Google Scholar]
- [159].Wolfs TG, Buurman WA, Zoer B, Moonen RM, Derikx JP, Thuijls G, et al. Endotoxin induced chorioamnionitis prevents intestinal development during gestation in fetal sheep. PloS One 2009;4(6):e5837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Wolfs TG, Kallapur SG, Polglase GR, Pillow JJ, Nitsos I, Newnham JP, et al. IL-1alpha mediated chorioamnionitis induces depletion of FoxP3+ cells and ileal inflammation in the ovine fetal gut. PloS One 2011;6(3):e18355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Fricke EM, Elgin TG, Gong H, Reese J, Gibson-Corley KN, Weiss RM, et al. Lipopolysaccharide-induced maternal inflammation induces direct placental injury without alteration in placental blood flow and induces a secondary fetal intestinal injury that persists into adulthood. Am J Reprod Immunol 2018;79(5): e12816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Elgin TG, Fricke EM, Gong H, Reese J, Mills DA, Kalantera KM, et al. Fetal exposure to maternal inflammation interrupts murine intestinal development and increases susceptibility to neonatal intestinal injury. Dis Model Mech 2019;12 (10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Yan X, Managlia E, Tan XD, De Plaen IG. Prenatal inflammation impairs intestinal microvascular development through a TNF-dependent mechanism and predisposes newborn mice to necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol 2019;317(1). G57-g66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Lee J, Romero R, Lee KA, Kim EN, Korzeniewski SJ, Chaemsaithong P, et al. Meconium aspiration syndrome: a role for fetal systemic inflammation. Am J Obstet Gynecol 2016;214(3). 366.e1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Romero R, Hanaoka S, Mazor M, Athanassiadis AP, Callahan R, Hsu YC, et al. Meconium-stained amniotic fluid: a risk factor for microbial invasion of the amniotic cavity. Am J Obstet Gynecol 1991;164(3):859–62. [DOI] [PubMed] [Google Scholar]
- [166].Mazor M, Furman B, Wiznitzer A, Shoham-Vardi I, Cohen J, Ghezzi F. Maternal and perinatal outcome of patients with preterm labor and meconium-stained amniotic fluid. Obstet Gynecol 1995;86(5):830–3. [DOI] [PubMed] [Google Scholar]
- [167].Romero R, Yoon BH, Chaemsaithong P, Cortez J, Park CW, Gonzalez R, et al. Bacteria and endotoxin in meconium-stained amniotic fluid at term: could intra-amniotic infection cause meconium passage? J Matern Fetal Neonatal Med 2014; 27(8):775–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Hsieh TT, Hsieh CC, Hung TH, Chiang CH, Yang FP, Pao CC. Differential expression of interleukin-1 beta and interleukin-6 in human fetal serum and meconium-stained amniotic fluid. J Reprod Immunol 1998;37(2):155–61. [DOI] [PubMed] [Google Scholar]
- [169].Yamada T, Minakami H, Matsubara S, Yatsuda T, Kohmura Y, Sato I. Meconium-stained amniotic fluid exhibits chemotactic activity for polymorphonuclear leukocytes in vitro. J Reprod Immunol 2000;46(1):21–30. [DOI] [PubMed] [Google Scholar]
- [170].Okazaki K, Kondo M, Kato M, Kakinuma R, Nishida A, Noda M, et al. Serum cytokine and chemokine profiles in neonates with meconium aspiration syndrome. Pediatrics 2008;121(4):e748–53. [DOI] [PubMed] [Google Scholar]
- [171].Hofer N, Jank K, Strenger V, Pansy J, Resch B. Inflammatory indices in meconium aspiration syndrome. Pediatr Pulmonol 2016;51(6):601–6. [DOI] [PubMed] [Google Scholar]
- [172].Yokoi K, Iwata O, Kobayashi S, Muramatsu K, Goto H. Influence of foetal inflammation on the development of meconium aspiration syndrome in term neonates with meconium-stained amniotic fluid. PeerJ 2019;7:e7049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Cope E, Dilly S. Kupffer cell numbers during human development. Clin Exp Immunol 1990;81(3):485–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Kutteh WH, Rainey WE, Carr BR. Regulation of interleukin-6 production in human fetal Kupffer cells. Scand J Immunol 1991;33(5):607–13. [DOI] [PubMed] [Google Scholar]
- [175].Kutteh WH, Rainey WE, Beutler B, Carr BR. Tumor necrosis factor-alpha and interleukin-1 beta production by human fetal Kupffer cells. Am J Obstet Gynecol 1991;165(1):112–20. [DOI] [PubMed] [Google Scholar]
- [176].Stallmach T, Karolyi L. Augmentation of fetal granulopoiesis with chorioamnionitis during the second trimester of gestation. Hum Pathol 1994;25 (3):244–7. [DOI] [PubMed] [Google Scholar]
- [177].Pfisterer C, Faber R, Horn LC. Chorioamnionitis-induced changes of fetal extramedullar hematopoiesis in the second trimester of gestation. Is diagnosis from fetal autopsy possible? Virchows Arch 2005;446(2):150–6. [DOI] [PubMed] [Google Scholar]
- [178].Miranda RN, Omurtag K, Castellani WJ, De las Casas LE, Quintanilla NM, Kaabipour E. Myelopoiesis in the liver of stillborns with evidence of intrauterine infection. Arch Pathol Lab Med 2006;130(12):1786–91. [DOI] [PubMed] [Google Scholar]
- [179].Bieghs V, Vlassaks E, Custers A, van Gorp PJ, Gijbels MJ, Bast A, et al. Chorioamnionitis induced hepatic inflammation and disturbed lipid metabolism in fetal sheep. Pediatr Res 2010;68(6):466–72. [DOI] [PubMed] [Google Scholar]
- [180].Vlassaks E, Gavilanes AW, Bieghs V, Reinartz A, Gassler N, Van Gorp PJ, et al. Antenatal exposure to chorioamnionitis affects lipid metabolism in 7-week-old sheep. J Dev Orig Health Dis 2012;3(2):103–10. [DOI] [PubMed] [Google Scholar]
- [181].Carroll SG, Papaioannou S, Nicolaides KH. Assessment of fetal activity and amniotic fluid volume in the prediction of intrauterine infection in preterm prelabor amniorrhexis. Am J Obstet Gynecol 1995;172(5):1427–35. [DOI] [PubMed] [Google Scholar]
- [182].Yoon BH, Kim YA, Romero R, Kim JC, Park KH, Kim MH, et al. Association of oligohydramnios in women with preterm premature rupture of membranes with an inflammatory response in fetal, amniotic, and maternal compartments. Am J Obstet Gynecol 1999;181(4):784–8. [DOI] [PubMed] [Google Scholar]
- [183].Lee SE, Romero R, Lee SM, Yoon BH. Amniotic fluid volume in intra-amniotic inflammation with and without culture-proven amniotic fluid infection in preterm premature rupture of membranes. J Perinat Med 2010;38(1):39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Rangel-Frausto MS, Pittet D, Costigan M, Hwang T, Davis CS, Wenzel RP. The natural history of the systemic inflammatory response syndrome (SIRS). A prospective study. J Am Med Assoc 1995;273(2):117–23. [PubMed] [Google Scholar]
- [185].Schlichtig R, Kramer DJ, Pinsky MR. Flow redistribution during progressive hemorrhage is a determinant of critical O2 delivery. J Appl Physiol 1991;70(1): 169–78. Bethesda, Md : 1985. [DOI] [PubMed] [Google Scholar]
- [186].Kato R, Pinsky MR. Personalizing blood pressure management in septic shock. Ann Intensive Care 2015;5(1):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Azpurua H, Dulay AT, Buhimschi IA, Bahtiyar MO, Funai E, Abdel-Razeq SS, et al. Fetal renal artery impedance as assessed by Doppler ultrasound in pregnancies complicated by intraamniotic inflammation and preterm birth. Am J Obstet Gynecol 2009;200(2):203 e1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Hudalla H, Karenberg K, Kuon RJ, Poschl J, Tschada R, Frommhold D. LPS-induced maternal inflammation promotes fetal leukocyte recruitment and prenatal organ infiltration in mice. Pediatr Res 2018;84(5):757–64. [DOI] [PubMed] [Google Scholar]
- [189].Galinsky R, Moss TJ, Gubhaju L, Hooper SB, Black MJ, Polglase GR. Effect of intra-amniotic lipopolysaccharide on nephron number in preterm fetal sheep. Am J Physiol Ren Physiol 2011;301(2):F280–5. [DOI] [PubMed] [Google Scholar]
- [190].Brenner BM, Garcia DL, Anderson S. Glomeruli and blood pressure. Less of one, more the other? Am J Hypertens 1988;1(4 Pt 1):335–47. [DOI] [PubMed] [Google Scholar]
- [191].Mendez N, Torres-Farfan C, Salazar E, Bascur P, Bastidas C, Vergara K, et al. Fetal programming of renal dysfunction and high blood pressure by chronodisruption. Front Endocrinol 2019;10:362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Murphy DJ, Sellers S, MacKenzie IZ, Yudkin PL, Johnson AM. Case-control study of antenatal and intrapartum risk factors for cerebral palsy in very preterm singleton babies. Lancet 1995;346(8988):1449–54. [DOI] [PubMed] [Google Scholar]
- [193].Yoon BH, Kim CJ, Romero R, Jun JK, Park KH, Choi ST, et al. Experimentally induced intrauterine infection causes fetal brain white matter lesions in rabbits. Am J Obstet Gynecol 1997;177(4):797–802. [DOI] [PubMed] [Google Scholar]
- [194].Yoon BH, Jun JK, Romero R, Park KH, Gomez R, Choi JH, et al. Amniotic fluid inflammatory cytokines (interleukin-6, interleukin-1beta, and tumor necrosis factor-alpha), neonatal brain white matter lesions, and cerebral palsy. Am J Obstet Gynecol 1997;177(1):19–26. [DOI] [PubMed] [Google Scholar]
- [195].Grether JK, Nelson KB. Maternal infection and cerebral palsy in infants of normal birth weight. J Am Med Assoc 1997;278(3):207–11. [PubMed] [Google Scholar]
- [196].Nelson KB, Dambrosia JM, Grether JK, Phillips TM. Neonatal cytokines and coagulation factors in children with cerebral palsy. Ann Neurol 1998;44(4): 665–75. [DOI] [PubMed] [Google Scholar]
- [197].Al-Haddad BJS, Oler E, Armistead B, Elsayed NA, Weinberger DR, Bernier R, et al. The fetal origins of mental illness. Am J Obstet Gynecol 2019;221(6):549–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Eastman NJ, Deleon M. The etiology of cerebral palsy. Am J Obstet Gynecol 1955; 69(5):950–61. [DOI] [PubMed] [Google Scholar]
- [199].Leviton A, Gilles FH. Custering of the morphological components of perinatal telencephalic leucoencephalopathy. J Neurol Neurosurg Psychiatry 1971;34(5): 642–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Gilles FH, Leviton A, Kerr CS. Endotoxin leucoencephalopathy in the telencephalon of the newborn kitten. J Neurol Sci 1976;27(2):183–91. [DOI] [PubMed] [Google Scholar]
- [201].Gilles FH, Leviton A. Neonatal white matter damage and the fetal inflammatory response. Semin Fetal Neonatal Med 2020:101111. [DOI] [PubMed] [Google Scholar]
- [202].Nelson KB. The epidemiology of FIRS in term and late preterm births. Semin Fetal Neonatal Med 2020:101141. [DOI] [PubMed] [Google Scholar]
- [203].Wiswell TE. Evaluation for the etiology of neonatal encephalopathy and the diagnosis of FIRS. Seminars in Fetal and Neonatal Medicine. 2020. [DOI] [PubMed] [Google Scholar]
- [204].Yap V, Perlman JM. Mechanisms of brain injury in newborn infants associated with the fetal inflammatory response syndrome. Semin Fetal Neonatal Med 2020. Apr 9:101110. [DOI] [PubMed] [Google Scholar]
- [205].Scher MS. Neurologic outcome after fetal inflammatory response syndrome: trimester-specific considerations. Semin Fetal Neonatal Med 2020. [DOI] [PubMed] [Google Scholar]
- [206].Nelson KB, Grether JK. Causes of cerebral palsy. Curr Opin Pediatr 1999;11(6): 487–91. [DOI] [PubMed] [Google Scholar]
- [207].Nelson KB, Willoughby RE. Infection, inflammation and the risk of cerebral palsy. Curr Opin Neurol 2000;13(2):133–9. [DOI] [PubMed] [Google Scholar]
- [208].Nelson KB, Blair E. Prenatal factors in cerebral palsy. N Engl J Med 2015;373(23): 2288–9. [DOI] [PubMed] [Google Scholar]
- [209].Garite TJ, Freeman RK, Linzey EM, Braly P. The use of amniocentesis in patients with premature rupture of membranes. Obstet Gynecol 1979;54(2):226–30. [PubMed] [Google Scholar]
- [210].Bobitt JR, Hayslip CC, Damato JD. Amniotic fluid infection as determined by transabdominal amniocentesis in patients with intact membranes in premature labor. Am J Obstet Gynecol 1981;140(8):947–52. [DOI] [PubMed] [Google Scholar]
- [211].Zlatnik FJ, Cruikshank DP, Petzold CR, Galask RP. Amniocentesis in the identification of inapparent infection in preterm patients with premature rupture of the membranes. J Reprod Med 1984;29(9):656–60. [PubMed] [Google Scholar]
- [212].Broekhuizen FF, Gilman M, Hamilton PR. Amniocentesis for gram stain and culture in preterm premature rupture of the membranes. Obstet Gynecol 1985;66 (3):316–21. [PubMed] [Google Scholar]
- [213].Romero R, Sirtori M, Oyarzun E, Avila C, Mazor M, Callahan R, et al. Infection and labor. V. Prevalence, microbiology, and clinical significance of intraamniotic infection in women with preterm labor and intact membranes. Am J Obstet Gynecol 1989;161(3):817–24. [DOI] [PubMed] [Google Scholar]
- [214].DiGiulio DB, Romero R, Kusanovic JP, Gomez R, Kim CJ, Seok KS, et al. Prevalence and diversity of microbes in the amniotic fluid, the fetal inflammatory response, and pregnancy outcome in women with preterm pre-labor rupture of membranes. Am J Reprod Immunol 2010;64(1):38–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Romero R, Miranda J, Chaiworapongsa T, Chaemsaithong P, Gotsch F, Dong Z, et al. A novel molecular microbiologic technique for the rapid diagnosis of microbial invasion of the amniotic cavity and intra-amniotic infection in preterm labor with intact membranes. Am J Reprod Immunol 2014;71(4):330–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Romero R, Miranda J, Kusanovic JP, Chaiworapongsa T, Chaemsaithong P, Martinez A, et al. Clinical chorioamnionitis at term I: microbiology of the amniotic cavity using cultivation and molecular techniques. J Perinat Med 2015; 43(1):19–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Yoon BH, Romero R, Shim JY, Shim SS, Kim CJ, Jun JK. C-reactive protein in umbilical cord blood: a simple and widely available clinical method to assess the risk of amniotic fluid infection and funisitis. J Matern Fetal Neonatal Med 2003; 14(2):85–90. [DOI] [PubMed] [Google Scholar]
- [218].Kim CJ, Romero R, Chaemsaithong P, Chaiyasit N, Yoon BH, Kim YM. Acute chorioamnionitis and funisitis: definition, pathologic features, and clinical significance. Am J Obstet Gynecol 2015;213(4 Suppl):S29–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Gilles FH, Murphy SF. Perinatal telencephalic leucoencephalopathy. J Neurol Neurosurg Psychiatr 1969;32(5):404–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Leviton A, Gilles FH. An epidemiologic study of perinatal telencephalic leucoencephalopathy in an autopsy population. J Neurol Sci 1973;18(1):53–66. [DOI] [PubMed] [Google Scholar]
- [221].Dammann O, Leviton A. Infection remote from the brain, neonatal white matter damage, and cerebral palsy in the preterm infant. Semin Pediatr Neurol 1998;5 (3):190–201. [DOI] [PubMed] [Google Scholar]
- [222].Debillon T, Gras-Leguen C, Vérielle V, Winer N, Caillon J, Rozé JC, et al. Intrauterine infection induces programmed cell death in rabbit periventricular white matter. Pediatr Res 2000;47(6):736–42. [DOI] [PubMed] [Google Scholar]
- [223].Duncan JR, Cock ML, Scheerlinck JP, Westcott KT, McLean C, Harding R, et al. White matter injury after repeated endotoxin exposure in the preterm ovine fetus. Pediatr Res 2002;52(6):941–9. [DOI] [PubMed] [Google Scholar]
- [224].Hagberg H, Peebles D, Mallard C. Models of white matter injury: comparison of infectious, hypoxic-ischemic, and excitotoxic insults. Ment Retard Dev Disabil Res Rev 2002;8(1):30–8. [DOI] [PubMed] [Google Scholar]
- [225].Bell MJ, Hallenbeck JM. Effects of intrauterine inflammation on developing rat brain. J Neurosci Res 2002;70(4):570–9. [DOI] [PubMed] [Google Scholar]
- [226].Dammann O, Kuban KC, Leviton A. Perinatal infection, fetal inflammatory response, white matter damage, and cognitive limitations in children born preterm. Ment Retard Dev Disabil Res Rev 2002;8(1):46–50. [DOI] [PubMed] [Google Scholar]
- [227].Debillon T, Gras-Leguen C, Leroy S, Caillon J, Rozé JC, Gressens P. Patterns of cerebral inflammatory response in a rabbit model of intrauterine infection-mediated brain lesion. Brain Res Dev Brain Res 2003;145(1):39–48. [DOI] [PubMed] [Google Scholar]
- [228].Mallard C, Welin AK, Peebles D, Hagberg H, Kjellmer I. White matter injury following systemic endotoxemia or asphyxia in the fetal sheep. Neurochem Res 2003;28(2):215–23. [DOI] [PubMed] [Google Scholar]
- [229].Dammann O, Leviton A. Inflammation, brain damage and visual dysfunction in preterm infants. Semin Fetal Neonatal Med 2006;11(5):363–8. [DOI] [PubMed] [Google Scholar]
- [230].Gilles FH, Leviton A, Dooling EC. The developing human brain: growth and epidemiologic neuropathology. Elsevier Science; 2013. [Google Scholar]
- [231].Korzeniewski SJ, Romero R, Cortez J, Pappas A, Schwartz AG, Kim CJ, et al. A “multi-hit” model of neonatal white matter injury: cumulative contributions of chronic placental inflammation, acute fetal inflammation and postnatal inflammatory events. J Perinat Med 2014;42(6):731–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [232].Lu HY, Zhang Q, Wang QX, Lu JY. Contribution of histologic chorioamnionitis and fetal inflammatory response syndrome to increased risk of brain injury in infants with preterm premature rupture of membranes. Pediatr Neurol 2016;61. 94–8.e1. [DOI] [PubMed] [Google Scholar]
- [233].Gussenhoven R, Westerlaken RJJ, Ophelders D, Jobe AH, Kemp MW, Kallapur SG, et al. Chorioamnionitis, neuroinflammation, and injury: timing is key in the preterm ovine fetus. J Neuroinflammation 2018;15(1):113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [234].Stojanovska V, Atik A, Nitsos I, Skiold B, Barton SK, Zahra VA, et al. Effects of intrauterine inflammation on cortical gray matter of near-term lambs. Front Pediatr 2018;6:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [235].Disdier C, Awa F, Chen X, Dhillon SK, Galinsky R, Davidson JO, et al. Lipopolysaccharide-induced changes in the neurovascular unit in the preterm fetal sheep brain. J Neuroinflammation 2020;17(1):167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [236].Zhang Z, Narayan S, Su L, Al-Alawyat H, Liu J, Kannan S. Cerebellar injury and impaired function in a rabbit model of maternal inflammation induced neonatal brain injury. Neurobiol Learn Mem 2019;165:106901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [237].Galinsky R, Dhillon SK, Dean JM, Davidson JO, Lear CA, Wassink G, et al. Tumor necrosis factor inhibition attenuates white matter gliosis after systemic inflammation in preterm fetal sheep. J Neuroinflammation 2020;17(1):92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [238].Gilles FH, Averill DR Jr, Kerr CS. Neonatal endotoxin encephalopathy. Ann Neurol 1977;2(1):49–56. [DOI] [PubMed] [Google Scholar]
- [239].Grafe MR. The correlation of prenatal brain damage with placental pathology. J Neuropathol Exp Neurol 1994;53(4):407–15. [DOI] [PubMed] [Google Scholar]
- [240].Roland EH, Magee JF, Rodriguez E, Lupton BA, Hill A. Placental abnormalities: insights into pathogenesis of cystic periventricular leukomalacia. Ann Neurol 1996;40:3213. [Google Scholar]
- [241].Verma U, Tejani N, Klein S, Reale MR, Beneck D, Figueroa R, et al. Obstetric antecedents of intraventricular hemorrhage and periventricular leukomalacia in the low-birth-weight neonate. Am J Obstet Gynecol 1997;176(2):275–81. [DOI] [PubMed] [Google Scholar]
- [242].O’Shea TM, Klinepeter KL, Dillard RG. Prenatal events and the risk of cerebral palsy in very low birth weight infants. Am J Epidemiol 1998;147(4):362–9. [DOI] [PubMed] [Google Scholar]
- [243].O’Shea TM, Klinepeter KL, Meis PJ, Dillard RG. Intrauterine infection and the risk of cerebral palsy in very low-birthweight infants. Paediatr Perinat Epidemiol 1998;12(1):72–83. [PubMed] [Google Scholar]
- [244].Redline RW, Wilson-Costello D, Borawski E, Fanaroff AA, Hack M. Placental lesions associated with neurologic impairment and cerebral palsy in very low-birth-weight infants. Arch Pathol Lab Med 1998;122(12):1091–8. [PubMed] [Google Scholar]
- [245].Alexander JM, Gilstrap LC, Cox SM, McIntire DM, Leveno KJ. Clinical chorioamnionitis and the prognosis for very low birth weight infants. Obstet Gynecol 1998;91(5 Pt 1):725–9. [DOI] [PubMed] [Google Scholar]
- [246].Bejar R, Wozniak P, Allard M, Benirschke K, Vaucher Y, Coen R, et al. Antenatal origin of neurologic damage in newborn infants. I. Preterm infants. Am J Obstet Gynecol 1988;159(2):357–63. [DOI] [PubMed] [Google Scholar]
- [247].Leviton A, Paneth N, Reuss ML, Susser M, Allred EN, Dammann O, et al. Maternal infection, fetal inflammatory response, and brain damage in very low birth weight infants. Developmental Epidemiology Network Investigators. Pediatr Res 1999;46 (5):566–75. [DOI] [PubMed] [Google Scholar]
- [248].Ng EAE, Rose T. The association of clinical and histologic chorioamnionitis (CA) with cystic periventricular leukomalacia (cPVL) and cerebral palsy (CP) in preterm infants. Pediatr Res 2000:318A. [Google Scholar]
- [249].Matsuda Y, Kouno S, Hiroyama Y, Kuraya K, Kamitomo M, Ibara S, et al. Intrauterine infection, magnesium sulfate exposure and cerebral palsy in infants born between 26 and 30 weeks of gestation. Eur J Obstet Gynecol Reprod Biol 2000;91(2):159–64. [DOI] [PubMed] [Google Scholar]
- [250].Wu YW, Colford JM Jr. Chorioamnionitis as a risk factor for cerebral palsy: a meta-analysis. J Am Med Assoc 2000;284(11):1417–24. [DOI] [PubMed] [Google Scholar]
- [251].Wu YW. Systematic review of chorioamnionitis and cerebral palsy. Ment Retard Dev Disabil Res Rev 2002;8(1):25–9. [DOI] [PubMed] [Google Scholar]
- [252].Nelson KB. The epidemiology of cerebral palsy in term infants. Ment Retard Dev Disabil Res Rev 2002;8(3):146–50. [DOI] [PubMed] [Google Scholar]
- [253].Jacobsson B, Hagberg G, Hagberg B, Ladfors L, Niklasson A, Hagberg H. Cerebral palsy in preterm infants: a population-based case-control study of antenatal and intrapartal risk factors. Acta Paediatr 2002;91(8):946–51. [DOI] [PubMed] [Google Scholar]
- [254].Grether JK, Nelson KB, Walsh E, Willoughby RE, Redline RW. Intrauterine exposure to infection and risk of cerebral palsy in very preterm infants. Arch Pediatr Adolesc Med 2003;157(1):26–32. [DOI] [PubMed] [Google Scholar]
- [255].Nelson KB. Can we prevent cerebral palsy? N Engl J Med 2003;349(18):1765–9. [DOI] [PubMed] [Google Scholar]
- [256].Yoon BH, Park CW, Chaiworapongsa T. Intrauterine infection and the development of cerebral palsy. BJOG 2003;110(Suppl 20):124–7. [DOI] [PubMed] [Google Scholar]
- [257].Wharton KN, Pinar H, Stonestreet BS, Tucker R, McLean KR, Wallach M, et al. Severe umbilical cord inflammation-a predictor of periventricular leukomalacia in very low birth weight infants. Early Hum Dev 2004;77(1–2):77–87. [DOI] [PubMed] [Google Scholar]
- [258].Redline RW. Severe fetal placental vascular lesions in term infants with neurologic impairment. Am J Obstet Gynecol 2005;192(2):452–7. [DOI] [PubMed] [Google Scholar]
- [259].Mehta R, Nanjundaswamy S, Shen-Schwarz S, Petrova A. Neonatal morbidity and placental pathology. Indian J Pediatr 2006;73(1):25–8. [DOI] [PubMed] [Google Scholar]
- [260].Gilles FH, Nelson MD. The developing human brain: growth and adversities. Wiley; 2012. [Google Scholar]
- [261].Dammann O, Leviton A. Intermittent or sustained systemic inflammation and the preterm brain. Pediatr Res 2014;75(3):376–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [262].Yanni D, Korzeniewski SJ, Allred EN, Fichorova RN, O’Shea TM, Kuban K, et al. Both antenatal and postnatal inflammation contribute information about the risk of brain damage in extremely preterm newborns. Pediatr Res 2017;82(4):691–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [263].Debillon T, Gras-Leguen C, Verielle V, Winer N, Caillon J, Roze JC, et al. Intrauterine infection induces programmed cell death in rabbit periventricular white matter. Pediatr Res 2000;47(6):736–42. [DOI] [PubMed] [Google Scholar]
- [264].Debillon T, Gras-Leguen C, Leroy S, Caillon J, Roze JC, Gressens P. Patterns of cerebral inflammatory response in a rabbit model of intrauterine infection-mediated brain lesion. Brain Res Dev Brain Res 2003;145(1):39–48. [DOI] [PubMed] [Google Scholar]
- [265].Saadani-Makki F, Kannan S, Lu X, Janisse J, Dawe E, Edwin S, et al. Intrauterine administration of endotoxin leads to motor deficits in a rabbit model: a link between prenatal infection and cerebral palsy. Am J Obstet Gynecol 2008;199(6). 651.e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [266].Burd I, Bentz AI, Chai J, Gonzalez J, Monnerie H, Le Roux PD, et al. Inflammation-induced preterm birth alters neuronal morphology in the mouse fetal brain. J Neurosci Res 2010;88(9):1872–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [267].Saadani-Makki F, Kannan S, Makki M, Muzik O, Janisse J, Romero R, et al. Intrauterine endotoxin administration leads to white matter diffusivity changes in newborn rabbits. J Child Neurol 2009;24(9):1179–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [268].Kannan S, Dai H, Navath RS, Balakrishnan B, Jyoti A, Janisse J, et al. Dendrimer-based postnatal therapy for neuroinflammation and cerebral palsy in a rabbit model. Sci Transl Med 2012;4(130):130ra46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [269].Beloosesky R, Gayle DA, Amidi F, Nunez SE, Babu J, Desai M, et al. N-acetyl-cysteine suppresses amniotic fluid and placenta inflammatory cytokine responses to lipopolysaccharide in rats. Am J Obstet Gynecol 2006;194(1):268–73. [DOI] [PubMed] [Google Scholar]
- [270].Beloosesky R, Gayle DA, Ross MG. Maternal N-acetylcysteine suppresses fetal inflammatory cytokine responses to maternal lipopolysaccharide. Am J Obstet Gynecol 2006;195(4):1053–7. [DOI] [PubMed] [Google Scholar]
- [271].Wang X, Svedin P, Nie C, Lapatto R, Zhu C, Gustavsson M, et al. N-acetylcysteine reduces lipopolysaccharide-sensitized hypoxic-ischemic brain injury. Ann Neurol 2007;61(3):263–71. [DOI] [PubMed] [Google Scholar]
- [272].Castillo-Melendez M, Yawno T, Jenkin G, Miller SL. Stem cell therapy to protect and repair the developing brain: a review of mechanisms of action of cord blood and amnion epithelial derived cells. Front Neurosci 2013;7:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [273].Faulkner SD, Ruff CA, Fehlings MG. The potential for stem cells in cerebral palsy–piecing together the puzzle. Semin Pediatr Neurol 2013;20(2):146–53. [DOI] [PubMed] [Google Scholar]
- [274].Ruff CA, Faulkner SD, Fehlings MG. The potential for stem cell therapies to have an impact on cerebral palsy: opportunities and limitations. Dev Med Child Neurol 2013;55(8):689–97. [DOI] [PubMed] [Google Scholar]
- [275].Li J, McDonald CA, Fahey MC, Jenkin G, Miller SL. Could cord blood cell therapy reduce preterm brain injury? Front Neurol 2014;5:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [276].Gonzales-Portillo GS, Reyes S, Aguirre D, Pabon MM, Borlongan CV. Stem cell therapy for neonatal hypoxic-ischemic encephalopathy. Front Neurol 2014;5:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [277].Chicha L, Smith T, Guzman R. Stem cells for brain repair in neonatal hypoxia-ischemia. Childs Nerv Syst 2014;30(1):37–46. [DOI] [PubMed] [Google Scholar]
- [278].Sharabi H, Khatib N, Ginsberg Y, Weiner Z, Ross MG, Tamar BK, et al. Therapeutic N-Acetyl-Cysteine (nac) following initiation of maternal inflammation attenuates long-term offspring cerebral injury, as evident in magnetic resonance imaging (MRI). Neuroscience 2019;403:118–24. [DOI] [PubMed] [Google Scholar]
- [279].Thompson DK, Thai D, Kelly CE, Leemans A, Tournier JD, Kean MJ, et al. Alterations in the optic radiations of very preterm children-Perinatal predictors and relationships with visual outcomes. NeuroImage Clinical 2014;4:145–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [280].Hellgren KM, Tornqvist K, Jakobsson PG, Lundgren P, Carlsson B, Kallén K, et al. Ophthalmologic outcome of extremely preterm infants at 6.5 Years of age: extremely preterm infants in Sweden study (EXPRESS). JAMA Ophthalmol 2016; 134(5):555–62. [DOI] [PubMed] [Google Scholar]
- [281].Darlow BA, Elder MJ, Kimber B, Martin J, Horwood LJ. Vision in former very low birthweight young adults with and without retinopathy of prematurity compared with term born controls: the NZ 1986 VLBW follow-up study. Br J Ophthalmol 2018;102(8):1041–6. [DOI] [PubMed] [Google Scholar]
- [282].Morken TS, Dammann O, Skranes J, Austeng D. Retinopathy of prematurity, visual and neurodevelopmental outcome, and imaging of the central nervous system. Semin Perinatol 2019;43(6):381–9. [DOI] [PubMed] [Google Scholar]
- [283].Lindqvist S, Vik T, Indredavik MS, Brubakk AM. Visual acuity, contrast sensitivity, peripheral vision and refraction in low birthweight teenagers. Acta Ophthalmol Scand 2007;85(2):157–64. [DOI] [PubMed] [Google Scholar]
- [284].Allred EN, Capone A Jr, Fraioli A, Dammann O, Droste P, Duker J, et al. Retinopathy of prematurity and brain damage in the very preterm newborn. J AAPOS 2014;18(3):241–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [285].Ogunyemi D, Murillo M, Jackson U, Hunter N, Alperson B. The relationship between placental histopathology findings and perinatal outcome in preterm infants. J Matern Fetal Neonatal Med 2003;13(2):102–9. [DOI] [PubMed] [Google Scholar]
- [286].Polam S, Koons A, Anwar M, Shen-Schwarz S, Hegyi T. Effect of chorioamnionitis on neurodevelopmental outcome in preterm infants. Arch Pediatr Adolesc Med 2005;159(11):1032–5. [DOI] [PubMed] [Google Scholar]
- [287].Dammann O, Brinkhaus MJ, Bartels DB, Dordelmann M, Dressler F, Kerk J, et al. Immaturity, perinatal inflammation, and retinopathy of prematurity: a multi-hit hypothesis. Early Hum Dev 2009;85(5):325–9. [DOI] [PubMed] [Google Scholar]
- [288].Moscuzza F, Belcari F, Nardini V, Bartoli A, Domenici C, Cuttano A, et al. Correlation between placental histopathology and fetal/neonatal outcome: chorioamnionitis and funisitis are associated to intraventricular haemorrage and retinopathy of prematurity in preterm newborns. Gynecol Endocrinol 2011;27(5): 319–23. [DOI] [PubMed] [Google Scholar]
- [289].Sato M, Nishimaki S, Yokota S, Seki K, Horiguchi H, An H, et al. Severity of chorioamnionitis and neonatal outcome. J Obstet Gynaecol Res 2011;37(10): 1313–9. [DOI] [PubMed] [Google Scholar]
- [290].Woo SJ, Park KH, Jung HJ, Kim S, Choe G, Ahn J, et al. Effects of maternal and placental inflammation on retinopathy of prematurity. Graefes Arch Clin Exp Ophthalmol 2012;250(6):915–23. [DOI] [PubMed] [Google Scholar]
- [291].Villamor-Martinez E, Cavallaro G, Raffaeli G, Mohammed Rahim OMM, Gulden S, Ghazi AMT, et al. Chorioamnionitis as a risk factor for retinopathy of prematurity: an updated systematic review and meta-analysis. PloS One 2018;13(10): e0205838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [292].Sood BG, Madan A, Saha S, Schendel D, Thorsen P, Skogstrand K, et al. Perinatal systemic inflammatory response syndrome and retinopathy of prematurity. Pediatr Res 2010;67(4):394–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [293].Yu H, Yuan L, Zou Y, Peng L, Wang Y, Li T, et al. Serum concentrations of cytokines in infants with retinopathy of prematurity. APMIS 2014;122(9):818–23. [DOI] [PubMed] [Google Scholar]
- [294].Wang X, Tang K, Chen L, Cheng S, Xu H. Association between sepsis and retinopathy of prematurity: a systematic review and meta-analysis. BMJ open 2019;9(5):e025440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [295].Cristobal R, Oghalai JS. Hearing loss in children with very low birth weight: current review of epidemiology and pathophysiology. Arch Dis Child Fetal Neonatal Ed 2008;93(6):F462–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [296].Zimmerman E, Lahav A. Ototoxicity in preterm infants: effects of genetics, aminoglycosides, and loud environmental noise. J Perinatol 2013;33(1):3–8. [DOI] [PubMed] [Google Scholar]
- [297].Leung JC, Cifra CL, Agthe AG, Sun CC, Viscardi RM. Antenatal factors modulate hearing screen failure risk in preterm infants. Arch Dis Child Fetal Neonatal Ed 2016;101(1):F56–61. [DOI] [PubMed] [Google Scholar]
- [298].Jackson W, Taylor G, Selewski D, Smith PB, Tolleson-Rinehart S, Laughon MM. Association between furosemide in premature infants and sensorineural hearing loss and nephrocalcinosis: a systematic review. Maternal health, neonatology and perinatology 2018;4:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [299].Aschoff L Die Otitis media neonatorum. Z Ohr 1897;31:295. [Google Scholar]
- [300].de Sa DJ. Infection and amniotic aspiration of middle ear in stillbirths and neonatal deaths. Arch Dis Child 1973;48(11):872–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [301].Ermocilla R, Cassady G, Ceballos R. Otitis media in the pathogenesis of neonatal meningitis with group B beta-hemolytic streptococcus. Pediatrics 1974;54(5): 643–4. [PubMed] [Google Scholar]
- [302].Matarazzo L, Nider S, Battelino S, Muzzi E, Orzan E, Ventura A, et al. Meningitis as a consequence of otitis media in a child referred from the newborn hearing screening programme: a missed opportunity. J Paediatr Child Health 2018;54(7): 810–2. [DOI] [PubMed] [Google Scholar]
- [303].Takumida M, Anniko M. Localization of endotoxin in the inner ear following inoculation into the middle ear. Acta Otolaryngol 2004;124(7):772–7. [DOI] [PubMed] [Google Scholar]
- [304].Shim YJ, Choi BY, Park KH, Lee H, Jung YM, Kim YM. Inflammatory and immune proteins in umbilical cord blood: association with hearing screening test failure in preterm neonates. Mediat Inflamm 2018;2018:4209359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [305].Kim YM, Romero R, Chaiworapongsa T, Espinoza J, Mor G, Kim CJ. Dermatitis as a component of the fetal inflammatory response syndrome is associated with activation of Toll-like receptors in epidermal keratinocytes. Histopathology 2006; 49(5):506–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [306].Kemp MW, Saito M, Nitsos I, Jobe AH, Kallapur SG, Newnham JP. Exposure to in utero lipopolysaccharide induces inflammation in the fetal ovine skin. Reprod Sci 2011;18(1):88–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [307].Kemp MW, Saito M, Kallapur SG, Jobe AH, Keelan JA, Li S, et al. Inflammation of the fetal ovine skin following in utero exposure to Ureaplasma parvum. Reprod Sci 2011;18(11):1128–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [308].Gaines Das RE, Poole S. The international standard for interleukin-6. Evaluation in an international collaborative study. J Immunol Methods 1993;160(2):147–53. [DOI] [PubMed] [Google Scholar]
- [309].Meister B, Herold M, Mayr A, Widschwendter M, Maurer H, Heim K, et al. Interleukin-3, interleukin-6, granulocyte-macrophage colony-stimulating factor and erythropoietin cord blood levels of preterm and term neonates. Eur J Pediatr 1993;152(7):569–73. [DOI] [PubMed] [Google Scholar]
- [310].Mestan K, Yu Y, Thorsen P, Skogstrand K, Matoba N, Liu X, et al. Cord blood biomarkers of the fetal inflammatory response. J Matern Fetal Neonatal Med 2009;22(5):379–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [311].Malamitsi-Puchner A, Protonotariou E, Boutsikou T, Makrakis E, Sarandakou A, Creatsas G. The influence of the mode of delivery on circulating cytokine concentrations in the perinatal period. Early Hum Dev 2005;81(4):387–92. [DOI] [PubMed] [Google Scholar]
- [312].Duncombe G, Veldhuizen RA, Gratton RJ, Han VK, Richardson BS. IL-6 and TNFalpha across the umbilical circulation in term pregnancies: relationship with labour events. Early Hum Dev 2010;86(2):113–7. [DOI] [PubMed] [Google Scholar]
- [313].Chan CJ, Summers KL, Chan NG, Hardy DB, Richardson BS. Cytokines in umbilical cord blood and the impact of labor events in low-risk term pregnancies. Early Hum Dev 2013;89(12):1005–10. [DOI] [PubMed] [Google Scholar]
- [314].Barug D, Goorden S, Herruer M, Muller M, Brohet R, de Winter P. Reference values for interleukin-6 and interleukin-8 in cord blood of healthy term neonates and their association with stress-related perinatal factors. PloS One 2014;9(12): e114109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [315].Trevino-Garza C, Villarreal-Martinez L, Estrada-Zuniga CM, Leal-Trevino M, Rodriguez-Balderrama I, Nieto-Sanjuanero A, et al. IL-6 and TNF-alpha levels in umbilical cord blood of healthy term newborns in relation to mode of delivery. J Obstet Gynaecol 2016;36(6):719–21. [DOI] [PubMed] [Google Scholar]
- [316].Ebenebe CU, Boiger A, Perez A, Mathies FL, Hecher K, Singer D. Interleukin-6 elevation in healthy neonates. J Perinatol 2020;40(2):294–8. [DOI] [PubMed] [Google Scholar]
- [317].Howman RA, Charles AK, Jacques A, Doherty DA, Simmer K, Strunk T, et al. Inflammatory and haematological markers in the maternal, umbilical cord and infant circulation in histological chorioamnionitis. PloS One 2012;7(12):e51836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [318].Dollner H, Vatten L, Linnebo I, Zanussi GF, Laerdal A, Austgulen R. Inflammatory mediators in umbilical plasma from neonates who develop early-onset sepsis. Biol Neonate 2001;80(1):41–7. [DOI] [PubMed] [Google Scholar]
- [319].Cobo T, Kacerovsky M, Andrys C, Drahosova M, Musilova I, Hornychova H, et al. Umbilical cord blood IL-6 as predictor of early-onset neonatal sepsis in women with preterm prelabour rupture of membranes. PloS One 2013;8(7):e69341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [320].Su H, Chang SS, Han CM, Wu KY, Li MC, Huang CY, et al. Inflammatory markers in cord blood or maternal serum for early detection of neonatal sepsis-a systemic review and meta-analysis. J Perinatol 2014;34(4):268–74. [DOI] [PubMed] [Google Scholar]
- [321].Vaisbuch E, Romero R, Gomez R, Kusanovic JP, Mazaki-Tovi S, Chaiworapongsa T, et al. An elevated fetal interleukin-6 concentration can be observed in fetuses with anemia due to Rh alloimmunization: implications for the understanding of the fetal inflammatory response syndrome. J Matern Fetal Neonatal Med 2011;24(3):391–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [322].Takahashi N, Nagamatsu T, Fujii T, Takahashi K, Tsuchida Y, Fujio K, et al. Extremely high levels of multiple cytokines in the cord blood of neonates born to mothers with systemic autoimmune diseases. Cytokine 2020;127:154926. [DOI] [PubMed] [Google Scholar]
- [323].Yoon BH, Romero R, Park JS, Kim M, Oh SY, Kim CJ, et al. The relationship among inflammatory lesions of the umbilical cord (funisitis), umbilical cord plasma interleukin 6 concentration, amniotic fluid infection, and neonatal sepsis. Am J Obstet Gynecol 2000;183(5):1124–9. [DOI] [PubMed] [Google Scholar]
- [324].Kim CJ, Yoon BH, Park SS, Kim MH, Chi JG. Acute funisitis of preterm but not term placentas is associated with severe fetal inflammatory response. Hum Pathol 2001;32(6):623–9. [DOI] [PubMed] [Google Scholar]
- [325].Pacora P, Chaiworapongsa T, Maymon E, Kim YM, Gomez R, Yoon BH, et al. Funisitis and chorionic vasculitis: the histological counterpart of the fetal inflammatory response syndrome. J Matern Fetal Neonatal Med 2002;11(1): 18–25. [DOI] [PubMed] [Google Scholar]
- [326].Redline RW, Heller D, Keating S, Kingdom J. Placental diagnostic criteria and clinical correlation–a workshop report. Placenta 2005;26(Suppl A):S114–7. [DOI] [PubMed] [Google Scholar]
- [327].Oh JW, Park CW, Moon KC, Park JS, Jun JK. The relationship among the progression of inflammation in umbilical cord, fetal inflammatory response, early-onset neonatal sepsis, and chorioamnionitis. PloS One 2019;14(11): e0225328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [328].Kim CJ, Yoon BH, Kim M, Park JO, Cho SY, Chi JG. Histo-topographic distribution of acute inflammation of the human umbilical cord. Pathol Int 2001;51(11): 861–5. [DOI] [PubMed] [Google Scholar]
- [329].Ferrara JL. Cytokine dysregulation as a mechanism of graft versus host disease. Curr Opin Immunol 1993;5(5):794–9. [DOI] [PubMed] [Google Scholar]
- [330].Chatenoud L, Ferran C, Reuter A, Legendre C, Gevaert Y, Kreis H, et al. Systemic reaction to the anti-T-cell monoclonal antibody OKT3 in relation to serum levels of tumor necrosis factor and interferon-gamma [corrected]. N Engl J Med 1989; 320(21):1420–1. [DOI] [PubMed] [Google Scholar]
- [331].Chatenoud L, Ferran C, Legendre C, Thouard I, Merite S, Reuter A, et al. In vivo cell activation following OKT3 administration. Systemic cytokine release and modulation by corticosteroids. Transplantation 1990;49(4):697–702. [DOI] [PubMed] [Google Scholar]
- [332].Sinha P, Matthay MA, Calfee CS. Is a “cytokine storm” relevant to COVID-19? JAMA internal medicine 2020. June 30. 10.1001/jamainternmed.2020.3313. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- [333].Madsen-Bouterse SA, Romero R, Tarca AL, Kusanovic JP, Espinoza J, Kim CJ, et al. The transcriptome of the fetal inflammatory response syndrome. Am J Reprod Immunol 2010;63(1):73–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [334].Gersell DJ, Phillips NJ, Beckerman K. Chronic chorioamnionitis: a clinicopathologic study of 17 cases. Int J Gynecol Pathol 1991;10(3):217–29. [PubMed] [Google Scholar]
- [335].Jacques SM, Qureshi F. Chronic chorioamnionitis: a clinicopathologic and immunohistochemical study. Hum Pathol 1998;29(12):1457–61. [DOI] [PubMed] [Google Scholar]
- [336].Kim JS, Romero R, Kim MR, Kim YM, Friel L, Espinoza J, et al. Involvement of Hofbauer cells and maternal T cells in villitis of unknown aetiology. Histopathology 2008;52(4):457–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [337].Kim MJ, Romero R, Kim CJ, Tarca AL, Chhauy S, LaJeunesse C, et al. Villitis of unknown etiology is associated with a distinct pattern of chemokine up-regulation in the feto-maternal and placental compartments: implications for conjoint maternal allograft rejection and maternal anti-fetal graft-versus-host disease. J Immunol 2009;182(6):3919–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [338].Edmondson N, Bocking A, Machin G, Rizek R, Watson C, Keating S. The prevalence of chronic deciduitis in cases of preterm labor without clinical chorioamnionitis. Pediatr Dev Pathol 2009;12(1):16–21. [DOI] [PubMed] [Google Scholar]
- [339].Kim CJ, Romero R, Kusanovic JP, Yoo W, Dong Z, Topping V, et al. The frequency, clinical significance, and pathological features of chronic chorioamnionitis: a lesion associated with spontaneous preterm birth. Mod Pathol 2010;23(7): 1000–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [340].Lee J, Romero R, Chaiworapongsa T, Dong Z, Tarca AL, Xu Y, et al. Characterization of the fetal blood transcriptome and proteome in maternal anti-fetal rejection: evidence of a distinct and novel type of human fetal systemic inflammatory response. Am J Reprod Immunol 2013;70(4):265–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [341].Kim CJ, Romero R, Chaemsaithong P, Kim JS. Chronic inflammation of the placenta: definition, classification, pathogenesis, and clinical significance. Am J Obstet Gynecol 2015;213(4 Supplement):S53–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [342].Yoon BH, Romero R, Park JY, Oh KJ, Lee J, Conde-Agudelo A, et al. Antibiotic administration can eradicate intra-amniotic infection or intra-amniotic inflammation in a subset of patients with preterm labor and intact membranes. Am J Obstet Gynecol 2019;221(2). 142.e1-.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [343].Oh KJ, Romero R, Park JY, Lee J, Conde-Agudelo A, Hong JS, et al. Evidence that antibiotic administration is effective in the treatment of a subset of patients with intra-amniotic infection/inflammation presenting with cervical insufficiency. Am J Obstet Gynecol 2019;221(2). 140.e1-.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [344].Hassan S, Romero R, Hendler I, Gomez R, Khalek N, Espinoza J, et al. A sonographic short cervix as the only clinical manifestation of intra-amniotic infection. J Perinat Med 2006;34(1):13–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [345].Lee J, Romero R, Kim SM, Chaemsaithong P, Yoon BH. A new antibiotic regimen treats and prevents intra-amniotic inflammation/infection in patients with preterm PROM. J Matern Fetal Neonatal Med 2016;29(17):2727–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [346].Lee J, Romero R, Kim SM, Chaemsaithong P, Park CW, Park JS, et al. A new anti-microbial combination prolongs the latency period, reduces acute histologic chorioamnionitis as well as funisitis, and improves neonatal outcomes in preterm PROM. J Matern Fetal Neonatal Med 2016;29(5):707–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [347].Kacerovsky M, Romero R, Stepan M, Stranik J, Maly J, Pliskova L, et al. Antibiotic administration reduces the rate of intraamniotic inflammation in preterm prelabor rupture of the membranes. Am J Obstet Gynecol 2020;223(1). 114.e1-.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [348].Guaraldi G, Meschiari M, Cozzi-Lepri A, Milic J, Tonelli R, Menozzi M, et al. Tocilizumab in patients with severe COVID-19: a retrospective cohort study. The Lancet Rheumatology 2020;2(8):e474–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [349].Zhang C, Wu Z, Li JW, Zhao H, Wang GQ. Cytokine release syndrome in severe COVID-19: interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int J Antimicrob Agents 2020;55(5):105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [350].Liu B, Li M, Zhou Z, Guan X, Xiang Y. Can we use interleukin-6 (IL-6) blockade for coronavirus disease 2019 (COVID-19)-induced cytokine release syndrome (CRS)? J Autoimmun 2020:102452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [351].Adam Monteagudo L, Boothby A, Gertner E. Continuous intravenous anakinra infusion to calm the cytokine storm in macrophage activation syndrome. ACR open rheumatology 2020;2(5):276–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [352].Cavalli G, De Luca G, Campochiaro C, Della-Torre E, Ripa M, Canetti D, et al. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. The Lancet Rheumatology 2020;2(6):e325–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [353].Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. J Am Med Assoc 2020. Apr 13. 10.1001/jama.2020.6019. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- [354].Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol 2020;20(6):363–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [355].Xiong Y, Wintermark P. Therapeutic interventions for fetal inflammatory response syndrome (FIRS). Semin Fetal Neonatal Med 2020:101112. [DOI] [PubMed] [Google Scholar]
- [356].Schüller SS, Kramer BW, Villamor E, Spittler A, Berger A, Levy O. Immunomodulation to prevent or treat neonatal sepsis: past, present, and future. Front Pediatr 2018;6:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [357].Wakabayashi A, Sawada K, Nakayama M, Toda A, Kimoto A, Mabuchi S, et al. Targeting interleukin-6 receptor inhibits preterm delivery induced by inflammation. Mol Hum Reprod 2013;19(11):718–26. [DOI] [PubMed] [Google Scholar]
- [358].Nadeau-Vallée M, Chin PY, Belarbi L, Brien M, Pundir S, Berryer MH, et al. Antenatal suppression of IL-1 protects against inflammation-induced fetal injury and improves neonatal and developmental outcomes in mice. J Immunol 2017; 198(5):2047–62. [DOI] [PubMed] [Google Scholar]
- [359].Gravett MG, Adams KM, Sadowsky DW, Grosvenor AR, Witkin SS, Axthelm MK, et al. Immunomodulators plus antibiotics delay preterm delivery after experimental intraamniotic infection in a nonhuman primate model. Am J Obstet Gynecol 2007;197(5). 518.e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [360].Motomura K, Romero R, Xu Y, Theis KR, Galaz J, Winters AD, et al. Intra-amniotic infection with Ureaplasma parvum causes preterm birth and neonatal mortality that are prevented by treatment with clarithromycin. mBio 2020;11(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [361].Lei J, Firdaus W, Rosenzweig JM, Alrebh S, Bakhshwin A, Borbiev T, et al. Murine model: maternal administration of stem cells for prevention of prematurity. Am J Obstet Gynecol 2015;212(5). 639.e1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [362].Murohara T, Ikeda H, Duan J, Shintani S, Sasaki K, Eguchi H, et al. Transplanted cord blood-derived endothelial precursor cells augment postnatal neovascularization. J Clin Invest 2000;105(11):1527–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [363].Mahmood A, Lu D, Chopp M. Intravenous administration of marrow stromal cells (MSCs) increases the expression of growth factors in rat brain after traumatic brain injury. J Neurotrauma 2004;21(1):33–9. [DOI] [PubMed] [Google Scholar]
- [364].van Velthoven CT, Kavelaars A, van Bel F, Heijnen CJ. Mesenchymal stem cell treatment after neonatal hypoxic-ischemic brain injury improves behavioral outcome and induces neuronal and oligodendrocyte regeneration. Brain Behav Immun 2010;24(3):387–93. [DOI] [PubMed] [Google Scholar]
- [365].Lee YH, Choi KV, Moon JH, Jun HJ, Kang HR, Oh SI, et al. Safety and feasibility of countering neurological impairment by intravenous administration of autologous cord blood in cerebral palsy. J Transl Med 2012;10:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [366].Feng M, Lu A, Gao H, Qian C, Zhang J, Lin T, et al. Safety of allogeneic umbilical cord blood stem cells therapy in patients with severe cerebral palsy: a retrospective study. Stem Cell Int 2015;2015:325652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [367].Novak I, Walker K, Hunt RW, Wallace EM, Fahey M, Badawi N. Concise review: stem cell interventions for people with cerebral palsy: systematic review with meta-analysis. Stem cells translational medicine 2016;5(8):1014–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [368].Paton MCB, McDonald CA, Allison BJ, Fahey MC, Jenkin G, Miller SL. Perinatal brain injury as a consequence of preterm birth and intrauterine inflammation: designing targeted stem cell therapies. Front Neurosci 2017;11:200. [DOI] [PMC free article] [PubMed] [Google Scholar]