Abstract
Cibacron blue F3GA (CB) was found to reduce the MIC of oxacillin for methicillin-resistant Staphylococcus aureus (MRSA). This effect was not observed with methicillin-susceptible S. aureus. CB alters the resistance level of MRSA through a factor(s) other than mecA-related products, major autolysins, or femAB products. The exact target(s) of CB in causing the effect is unknown.
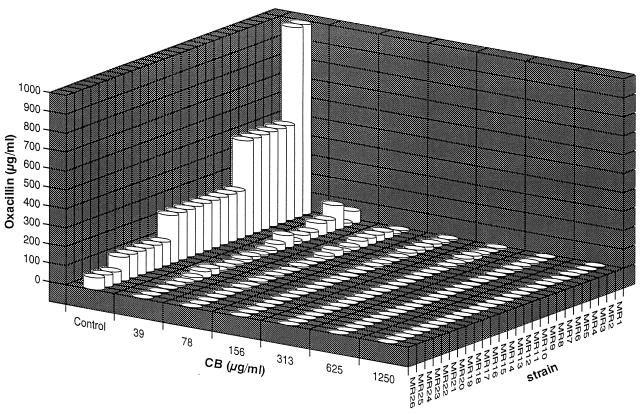
Cibacron blue F3GA (CB) is a triazinyl dye widely used as the affinity ligand for dye-ligand chromatography. CB is structurally similar to naturally occurring heterocycles, such as nucleoside phosphate, NAD+, coenzyme A, and folic acid (1–3, 8). It has been demonstrated that CB specifically binds to nucleotide binding sites of kinases and dehydrogenases and that some of the enzyme activities are inhibited by CB (1, 4, 6, 7). The aim of this study was to investigate the effect of CB on the in vitro susceptibility of methicillin-resistant Staphylococcus aureus (MRSA) to oxacillin. CB used in this study was from Sigma Chemical Co., St. Louis, Mo. (C 9534) and was formerly called reactive blue 2 (R 4502); it has an A-ring orthosulfonic acid (9). CB purified by reversed-phase high-pressure liquid chromatography according to the procedure described by Hanggi and Carr (9) behaved similarly to unpurified CB, and consequently CB was used without purification in this study. MICs were determined by a microdilution method (13), and population analysis was carried out as described elsewhere (12). CB alone was not inhibitory to staphylococcal strains when used at up to 2,500 μg/ml in the experiments. The effect of CB on in vitro susceptibility to oxacillin was evaluated with 28 MRSA and 10 methicillin-susceptible S. aureus (MSSA) strains. For all MRSA strains, the MIC of oxacillin was significantly reduced in the presence of CB concentrations of 39 μg/ml or higher (Fig. 1). Highly resistant MRSA strains appeared to be less susceptible to the sensitizing effect of CB, but 78 to 156 μg of CB per ml markedly reduced the MICs of oxacillin for those strains. We therefore employed 100 μg of CB per ml in further studies, unless otherwise noted. On the other hand, MICs of oxacillin for MSSA did not change at all in the presence of CB (data not shown). A population analysis of 28 MRSA strains and 2 MSSA strains was carried out in the presence and absence of CB. In most cases, the population curve of homogeneously or heterogeneously oxacillin-resistant MRSA shifted to the left in the presence of CB. On the other hand, population curves of MSSA did not change at all in the presence of CB and confirmed the results of MIC analysis. CB alone also had no effect on the population curve. This sensitizing effect of CB was observed only when β-lactam was used with CB (Table 1). Various triazinyl dyes related to CB were tested for an effect on the sensitivity of MRSA to oxacillin and were found to have a very weak effect on a limited number of strains (data not shown).
FIG. 1.
Effect of CB on the susceptibilities of MRSA isolates to oxacillin. The column height indicates the MIC of oxacillin.
TABLE 1.
MICs of various antibiotics for MRSA in the presence and absence of CB
| MRSA strain | MICa (μg/ml) of:
|
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FOM
|
CS
|
VCM
|
BC
|
CP
|
PCG
|
DMPPC
|
MPIPC
|
CEZ
|
CCL
|
|||||||||||
| − | + | − | + | − | + | − | + | − | + | − | + | − | + | − | + | − | + | − | + | |
| MR1 | 512 | 512 | 128 | 128 | 2 | 2 | 64 | 128 | 8 | 8 | 64 | 4 | >512 | 4 | >512 | 16 | 512 | 2 | 512 | 32 |
| MR6 | 256 | >512 | 128 | 64 | 4 | 4 | 64 | 128 | 8 | 4 | 32 | 8 | >512 | 8 | 512 | 64 | 512 | 32 | >512 | >512 |
| MR8 | 32 | 32 | 64 | 64 | 2 | 2 | 64 | 128 | 32 | 32 | 16 | 4 | 256 | 4 | 512 | 8 | 256 | 1 | 512 | 16 |
| MR14 | 512 | >512 | 128 | 64 | 4 | 1 | 64 | 128 | 8 | 8 | 16 | 16 | 64 | 8 | 256 | 2 | 128 | <0.5 | 512 | 4 |
| MR15 | 512 | 512 | 64 | 128 | 1 | 1 | 64 | 128 | 8 | 8 | 64 | 4 | 512 | 4 | 256 | 4 | 128 | 2 | 256 | 4 |
| MR20 | 64 | 64 | 32 | 32 | 1 | 1 | 32 | 64 | 8 | 8 | 32 | 4 | >512 | 4 | 128 | 8 | 256 | 2 | 512 | 32 |
| MR24 | 16 | 16 | 64 | 32 | 1 | 1 | 32 | 32 | 16 | 16 | 32 | <0.5 | 32 | 2 | 64 | 1 | 64 | <0.5 | 256 | 16 |
| MR26 | 16 | 16 | 64 | 32 | 4 | 1 | 16 | 32 | 16 | 16 | 64 | 8 | 32 | 2 | 64 | 2 | 128 | <0.5 | 512 | 2 |
MICs were tested in the absence (−) and presence (+) of CB. FOM, fosfomycin; CS, cycloserine; VCM, vancomycin; BC, bacitracin; CP, chloramphenicol; PCG, benzylpenicillin; DMPPC, methicillin; MPIPC, oxacillin; CEZ, cefazolin; CCL, cefaclor.
We further assessed the effect of CB on the bactericidal activity of oxacillin with S. aureus MR15. When CB was added to exponentially growing MR15, the cells grew in clusters as described previously (20). Measurement of CFU of the culture after brief sonication to disperse clusters revealed that CB did not affect the growth of MR15. Oxacillin at a concentration of 16 μg/ml also did not significantly affect the growth of strain MR15. On the other hand, coincubation of CB with oxacillin (16 μg/ml) completely inhibited growth but did not reduce the number of CFU. These results suggest that the sensitization effect of CB on MRSA cells is bacteriostatic.
We studied the effect of CB on the synthesis of penicillin-binding proteins (PBPs) in strain MR6-2 (β-lactamase free) (13). S. aureus cell membranes were prepared from cells grown in the presence or absence of CB. The binding of β-lactam antibiotics to PBPs was investigated with 14C-labeled benzylpenicillin (10 to 30 Ci/mmol) (Amersham International, Bucks, United Kingdom) (5). The amounts of PBP2′ and PBP2 were not affected by the presence of CB in the culture. Next, the effect of CB on the binding of 14C-labeled benzylpenicillin to PBPs was investigated. The binding study revealed that the kinetics also were not affected by CB.
CB has been shown to inhibit bacteriolytic enzyme activities of S. aureus (16, 19). To determine whether bacteriolytic enzymes are involved in the sensitizing effect of CB, we studied the effect of CB on the resistance levels of Lyt− mutants, which virtually lack major autolysins of S. aureus (the atl gene products), and of their parent, MRSA MR6 (13). Population analysis of two Lyt− mutants, Lyt-2 and Lyt-5, derived from MR6 indicated that the Lyt− phenotype had no effect on the levels of resistance of these strains to oxacillin. Moreover, the mutants were as sensitive as the parent strains to the effect of CB on susceptibility to oxacillin. The mecI-mecR element and penicillinase plasmids were shown to affect the resistance level of MRSA (11, 14, 22). We studied the effect of CB on the resistance level of a prototype MRSA strain, N315, and its isogenic derivatives N315P (penicillinase negative) and N315-IR74 (mecI mecR::tet) (14). The population curve was shifted to the left irrespective of the status of the mecI-mecR element and the penicillinase plasmid. We studied whether CB could affect the structure of peptidoglycan as previously observed in studies demonstrating the lower sensitivity of femAB mutants to lysostaphin compared to that of the wild type (10, 15). We determined the susceptibilities of several MRSA strains grown in the presence or absence of CB to various bacteriolytic enzymes with different bond specificities, including 62-kDa N-acetylmuramyl-l-alanine amidase (18), 51-kDa endo-β-N-acetylglucosaminidase (17), and lysostaphin, by zymography (12). Regardless of whether the cells were grown in the presence or absence of CB, the minimum bacteriolytic doses of N-acetylmuramyl-l-alanine amidase, N-acetylglucosaminidase, and lysostaphin for the strains were identical.
In conclusion, our results suggested that CB alters the resistance level of MRSA through a factor(s) other than mecA-related products, major autolysins, or femAB products. Although the exact target(s) of CB in causing this sensitizing effect is not clear, it is likely that the target is involved in a critical metabolic pathway, given that PBP2′ is the only functional PBP. Further studies of the sensitization effect of CB may help to elucidate the molecular mechanism of methicillin resistance in S. aureus.
Acknowledgments
We thank Keiichi Hiramatsu, Juntendo University, for bacterial strains. We also thank Tadashi Oshida, Tanabe Seiyaku Co., Ltd., for helpful discussions and Masao Kuwabara, Hiroshima Prefectural Hospital, for encouragement.
This investigation was supported in part by a grant from the Scientific Research Funds (07557115 and 084557481) of the Ministry of Education, Science and Culture, Japan (1996 and 1997).
REFERENCES
- 1.Ashton A R, Polya G M. The specific interaction of cibacron and related dyes with cyclic nucleotide phosphodiesterase and lactate dehydrogenase. Biochem J. 1978;175:501–506. doi: 10.1042/bj1750501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baird J K, Sherwood R F, Carr R J G, Atkinson A. Enzyme purification by substrate elution chromatography from procion dye-polysaccharide matrices. FEBS Lett. 1976;70:61–66. doi: 10.1016/0014-5793(76)80726-0. [DOI] [PubMed] [Google Scholar]
- 3.Beissner R S, Rudolph F B. Interaction of cibacron blue 3G-A and related dyes with nucleotide-requiring enzymes. Arch Biochem Biophys. 1978;189:76–80. doi: 10.1016/0003-9861(78)90115-7. [DOI] [PubMed] [Google Scholar]
- 4.Biellmann J-F, Samama J-P, Bränden C I, Eklund H. X-ray studies of the binding of cibacron blue F3GA to liver alcohol dehydrogenase. Eur J Biochem. 1979;102:107–110. doi: 10.1111/j.1432-1033.1979.tb06268.x. [DOI] [PubMed] [Google Scholar]
- 5.Brown D F, Reynolds P E. Intrinsic resistance to β-lactam antibiotics in Staphylococcus aureus. FEBS Lett. 1980;122:275–278. doi: 10.1016/0014-5793(80)80455-8. [DOI] [PubMed] [Google Scholar]
- 6.Clonis Y D, Goldfinch M J, Lowe C R. The interaction of yeast hexokinase with procion green H-4G. Biochem J. 1981;197:203–211. doi: 10.1042/bj1970203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clonis Y D, Lowe C R. Triazine dyes, a new class of affinity labels for nucleotide-dependent enzymes. Biochem J. 1980;191:247–251. doi: 10.1042/bj1910247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edwards R A, Woody R W. Spectroscopic studies of cibacron blue and congo red bound to dehydrogenases and kinases, evaluation of dyes as probes of the dinucleotide fold. Biochemistry. 1979;18:5197–5204. doi: 10.1021/bi00590a026. [DOI] [PubMed] [Google Scholar]
- 9.Hanggi D, Carr P. Analytical evaluation of the purity of commercial preparations of cibacron blue F3GA and related dyes. Anal Biochem. 1985;149:91–104. doi: 10.1016/0003-2697(85)90480-4. [DOI] [PubMed] [Google Scholar]
- 10.Henze U, Sidow T, Wecke J, Labischinski H, Berger-Bächi B. Influence of femB on methicillin resistance and peptidoglycan metabolism in Staphylococcus aureus. J Bacteriol. 1993;175:1612–1620. doi: 10.1128/jb.175.6.1612-1620.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hiramatsu K, Suzuki E, Takayama H, Katayama Y, Yokota T. Role of penicillinase plasmids in the stability of the mecA gene in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1990;34:600–604. doi: 10.1128/aac.34.4.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Komatsuzawa H, Sugai M, Shirai C, Suzuki J, Hiramatsu K, Suginaka H. Triton X-100 alters the resistance level of methicillin-resistant Staphylococcus aureus to oxacillin. FEMS Microbiol Lett. 1995;134:209–212. doi: 10.1111/j.1574-6968.1995.tb07939.x. [DOI] [PubMed] [Google Scholar]
- 13.Komatsuzawa H, Suzuki J, Sugai M, Miyake Y, Suginaka H. The effect of Triton X-100 on the in-vitro susceptibility of methicillin-resistant Staphylococcus aureus to oxacillin. J Antimicrob Chemother. 1994;34:885–897. doi: 10.1093/jac/34.6.885. [DOI] [PubMed] [Google Scholar]
- 14.Kuwahara-Arai K, Kondo N, Hori S, Tateda-Suzuki E, Hiramatsu K. Suppression of methicillin resistance in a mecA-containing pre-methicillin-resistant Staphylococcus aureus strain is caused by the mecI-mediated repression of PBP 2′ production. Antimicrob Agents Chemother. 1996;40:2680–2685. doi: 10.1128/aac.40.12.2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maidhof H, Reinicke B, Blümel P, Berger-Bächi B, Labischinski H. femA, which encodes a factor essential for expression of methicillin resistance, affects glycine content of peptidoglycan in methicillin-resistant and methicillin-susceptible Staphylococcus aureus strains. J Bacteriol. 1991;173:3507–3513. doi: 10.1128/jb.173.11.3507-3513.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sugai M, Akiyama T, Komatsuzawa H, Miyake Y, Suginaka H. Characterization of sodium dodecyl sulfate-stable Staphylococcus aureus bacteriolytic enzymes by polyacrylamide gel electrophoresis. J Bacteriol. 1990;172:6494–6498. doi: 10.1128/jb.172.11.6494-6498.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sugai M, Koike H, Hong Y-M, Miyake Y, Nogami R, Suginaka H. Purification of a 51 kDa endo-β-N-acetylglucosaminidase from Staphylococcus aureus. FEMS Microbiol Lett. 1989;61:267–272. doi: 10.1016/0378-1097(89)90209-7. [DOI] [PubMed] [Google Scholar]
- 18.Sugai M, Komatsuzawa H, Akiyama T, Hong Y-M, Oshida T, Miyake Y, Yamaguchi T, Suginaka H. Identification of endo-β-N-acetylglucosaminidase and N-acetylmuramyl-l-alanine amidase as cluster-dispersing enzymes in Staphylococcus aureus. J Bacteriol. 1995;177:1491–1496. doi: 10.1128/jb.177.6.1491-1496.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sugai M, Ooku K, Akiyama T, Inoue S, Iseda S, Miyake Y, Suginaka H. Suppression of penicillin-induced lysis of Staphylococcus aureus by cibacron blue 3G-A. FEMS Microbiol Lett. 1991;80:151–154. doi: 10.1016/0378-1097(91)90586-y. [DOI] [PubMed] [Google Scholar]
- 20.Sugai M, Ooku K, Takata T, Miyake Y, Suginaka H. A triazine dye, cibacron blue 3G-A induces Staphylococcus aureus to form giant clusters. FEMS Microbiol Lett. 1990;67:175–178. doi: 10.1016/0378-1097(90)90190-2. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki E, Kuwahara-Arai K, Richardson J F, Hiramatsu K. Distribution of mec regulator genes in methicillin-resistant Staphylococcus clinical strains. Antimicrob Agents Chemother. 1993;37:1219–1226. doi: 10.1128/aac.37.6.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tesch W, Ryffel C, Strässle A, Kayser F H, Berger-Bächi B. Evidence of a novel staphylococcal mec-encoded element (mecR) controlling expression of penicillin-binding protein 2′. Antimicrob Agents Chemother. 1990;34:1703–1706. doi: 10.1128/aac.34.9.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]