Abstract
Objective
To date, there is no consensus on the surgery strategies of cranioplasty (CP) and ventriculoperitoneal shunt (VPS) placement. This meta‐analysis aimed to investigate the safety of staged and simultaneous operation in patients with comorbid cranial defects with hydrocephalus to inform future surgery protocols.
Methods
A meta‐analysis of PubMed, Ovid, Web of Science, and Cochrane Library databases from the inception dates to February 8, 2023 adherent to PRISMA guidelines was conducted. The pooled analyses were conducted using RevMan 5.3 software. The outcomes included postoperative infection, reoperation, shunt obstruction, hematoma, and subdural effusion.
Results
Of the 956 studies initially retrieved, 10 articles encompassing 515 patients were included. Among the total patients, 193 (37.48%) and 322 (62.52%), respectively, underwent simultaneous and staged surgeries. The finding of pooled analysis indicated that staged surgery was associated with lower rate of subdural effusion (14% in the simultaneous groups vs. 5.4% in the staged groups; OR = 2.39, 95% CI: 1.04–5.49, p = 0.04). However, there were no significant differences in overall infection (OR = 1.92, 95% CI: 0.74–4.97, p = 0.18), central nervous system infection (OR = 1.50, 95% CI: 0.68–3.31, p = 0.31), cranioplasty infection (OR = 1.58, 95% CI: 0.50–5.00, p = 0.44), shunt infection (OR = 1.30, 95% CI: 0.38–4.52, p = 0.67), reoperation (OR = 1.51, 95% CI: 0.38–6.00, p = 0.55), shunt obstruction (OR = 0.73, 95% CI: 0.25–2.16, p = 0.57), epidural hematoma (OR = 2.20, 95% CI: 0.62–7.86, p = 0.22), subdural hematoma (OR = 1.20, 95% CI: 0.10–14.19, p = 0.88), and intracranial hematoma (OR = 1.31, 95% CI: 0.42–4.07, p = 0.64). Moreover, subgroup analysis failed to yield new insights.
Conclusions
Staged surgery is associated with a lower rate of postoperative subdural effusion. However, from the evidence of sensitivity analysis, this result is not stable. Therefore, our conclusion should be viewed with caution, and neurosurgeons in practice should make individualized decisions based on each patient's condition and cerebrospinal fluid tap test.
Keywords: complications, craniectomy, cranioplasty, hydrocephalus, ventriculoperitoneal shunt
Up to now, there is no consensus on the surgery strategies of cranioplasty and ventriculoperitoneal shunt placement in patients with co‐morbid cranial defects with hydrocephalus. Therefore, a meta‐analysis was conducted to investigate this issue. Our cautiously indicated that patients undergo staged cranioplasty and ventriculoperitoneal shunt procedures might benefit from a potentially lower trend in subdural effusion rate.

1. INTRODUCTION
Decompressive craniectomy (DC) is an ancient surgical procedure, dating back to trepanation around 12,000 BC. 1 It is generally believed that the earliest report in modern neurosurgery using DC to treat neurosurgical disorders was Kocher's treatment of patients with elevated intracranial pressure (ICP) after traumatic brain injury (TBI) in 1901. 2 DC is mainly used as a second‐line treatment in patients with drug‐refractory intracranial hypertension caused by various reasons, including TBI, intracerebral hemorrhage (ICH), subarachnoid hemorrhage (SAH), and ischemic stroke (IS), etc. 3 , 4 , 5 , 6 , 7 , 8
The disruption of cerebral blood flow and cerebrospinal fluid (CSF) hydrodynamics after DC puts patients at higher risk of subdural effusion and hydrocephalus, potentially resulting in worse neurological outcomes. 9 , 10 , 11 The sinking of the scalp due to the lack of skeletal support affects the blood supply, neurological function of the cortex, and aesthetics, which may cause sinking skin flap syndrome (SSFS) with a series of neuropsychiatric symptoms. 12 , 13 , 14 Therefore, cranioplasty (CP) is necessary in order to repair the cranial bone defect. In addition to mechanical protection and cosmetology, CP can also effectively improve cerebral blood flow and CSF hydrodynamics, thereby improving cerebral metabolism and promoting neurological function recovery. 15 , 16 , 17 , 18 , 19 , 20 , 21
Due to the changes of CSF dynamics caused by DC, hydrocephalus is an important complication after DC, with an incidence rate of 10%–40%. 22 , 23 , 24 Initial disease can also cause post‐DC hydrocephalus, such as hydrocephalus following TBI or spontaneous SAH. Hydrocephalus is a dilatation of the ventricular system of the brain accompanied by an altered intraventricular pressure, which leads to secondary brain tissue damage and affects neurological recovery. 25 CSF shunt is the standard treatment for hydrocephalus, the most common of which is ventriculoperitoneal shunt (VPS), which has been reported to be used in approximately 5%–15% of patients after DC. 26 , 27
Clinicians have primarily focused on the optimal surgical protocol of CP and VPS placement for post‐DC hydrocephalus patients. However, there is currently no consensus on the staged or simultaneous of CP and VPS placement. In such circumstances, the formulation of the surgical plans will be interfered with by the neurosurgeon's personal preference. This preference has an appropriately subjective character, which is primarily reflected in the surgeon's potential perceived merits or demerits of staged or simultaneous operations. Recent studies suggested that simultaneous surgery had higher rate of postoperative complications, especially infections, compared with staged surgery. 28 , 29 , 30 , 31 However, some neurosurgery experts indicated that simultaneous CP and VPS placement may be reduce surgical procedures, potential risks associated with general anesthesia, length of stay, medical expenses, and several clinical studies also indicated that there were no significant difference in the incidence of postoperative adverse events between staged and simultaneous surgery. 32 , 33 , 34 , 35 , 36 , 37
Given the preceding facts, we conducted a meta‐analysis on the clinical research data published on CP and VPS placement in patients with cranial defect comorbid with hydrocephalus and the aimed to investigate the ideal surgical protocol (staged or simultaneous) by comparing the incidence of postoperative complications, including infection, reoperation, shunt obstruction, hematoma, and subdural effusion. Furthermore, if staged surgery is listed as overwhelming, then the order of CP versus VPS also needs to be clarified. Therefore, subgroup analysis of staged procedures was also put on the agenda.
2. METHODS
2.1. Search strategy
This systemic review and meta‐analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines. 38 The electronic database including PubMed, Ovid, Web of Science, and Cochrane Library were searched from the date of inception to February 8, 2023. A deliberately simple strategy was performed to cast a broad search. 39 For this purpose, the keywords “cranioplasty” and “ventriculoperitoneal shunt” were applied to identify all relevant studies. The literature search was independently conducted by J.Z. and X.Y.D. to ensure the accuracy. Any discrepancy was solved by consultation of an investigator, not involved in the initial procedure. The reference lists were also screened to avoid missing any articles.
2.2. Inclusion and exclusion criteria
The inclusion criteria were as follows: (1) studies enrolling patients with hydrocephalus after DC (that is, comorbid cranial defect with hydrocephalus), (2) studies that involved both simultaneous and staged CP and VPS placement, (3) studies were divided into simultaneous and staged groups, (4) studies that clearly reported the data of adverse events, (5) prospective or retrospective studies. The exclusion criteria were as follows: (1) case reports, reviews, conference abstracts, and editorial letters; (2) studies that included patients who underwent other shunting operations (e.g., lumbar‐peritoneal shunt replacement); (3) studies lacking sufficient data.
2.3. Quality assessment
Quality assessment was independently conducted by two authors (Z.J. and X.Y.D.). The Newcastle‐Ottawa Scale (NOS) was used to evaluate the quality of the finally included studies. The NOS includes three aspects [selection, comparability, and exposure (case–control studies) or outcome (cohort studies)] and a total score of 9 points (0 = worst to 9 = best, ≥7 was defined as high quality). 40
2.4. Data extraction
Two authors (J.Z. and X.Y.D.) reviewed every eligible study and extracted the data including (1) information of the literature (title, author, publication date, etc.); (2) features of the studies (design, inclusion criteria, staging order, sample sizes, etc.); (3) baseline characteristics (demographic characteristics, clinical features, etc.); and (4) data on adverse events, including infection, reoperation, shunt obstruction, hematoma, and subdural effusion. For studies with insufficient information, the reviewers contacted the primary authors, when possible, to acquire and verify the data. In the event of disagreements about the quality of the studies or the data extracted, a third reviewer's opinion was sought.
2.5. Statistical analysis
Data synthesis and analysis was used Review Manager (version 5.3, Cochrane Collaboration). The odds ratios (ORs) with 95% confidence intervals (CIs) was applied as the effect indicator for the dichotomous variables. We calculated 95% CIs performing the Mantel–Haenszel statistical method. I‐square (I 2) statistics and Q tests were applied to evaluate the impact of study heterogeneity on the findings of this meta‐analysis. According to the Cochrane review guidelines, 41 if severe heterogeneity was present at p < 0.1 or I 2 > 50%, the randomized effect models was applied; otherwise, the fixed‐effect models was used. Subgroup analyses were performed according to the staged procedures (CP after VPS, CP after VPS, both, and unknown). Sensitivity analysis was performed by removing single study sequentially. Forest plots were charted for pooled results, and funnel plots were used if no less than five studies were included to assess the publication bias.
3. RESULTS
3.1. Search results
Our search yielded a total of 956 relevant records from various databases (PubMed, Ovid, Web of Science, and Cochrane Library). We excluded 117 duplicate articles, and selected 14 among the remaining 839 papers for full‐text assessment after screening titles and abstracts (Figure 1). The two repeated studies and two studies with non‐target outcome indicators were excluded. Finally, 10 articles met our inclusion criteria and were included in the pooled analysis. 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 These articles provided data on patient recruitment criteria and outcome variables, allowing us to conduct a thorough analysis of the available evidence. In addition, we performed a manual search of the reference lists of the included studies to identify any other potentially relevant articles.
FIGURE 1.
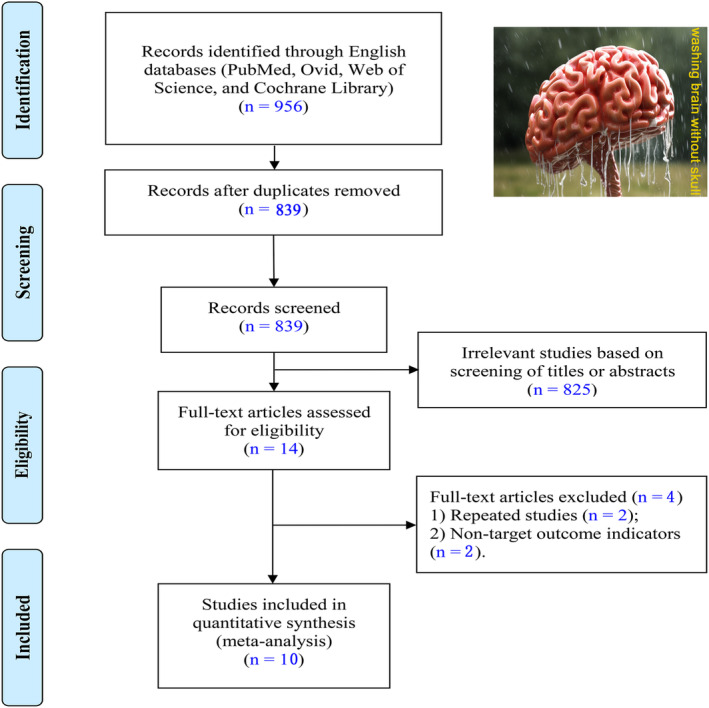
Flow diagram for literature search and screening process.
3.2. Characteristics and quality
The baseline characteristics, quality, patient recruitment criteria, and outcomes of the 10 individual studies are summarized in Tables 1 and 2. All these studies were retrospective study and year of publication ranged from 2014 to 2021. A total of 515 patients underwent simultaneous or staged CP and VPS after DC. Among the total patients, 193 (37.48%) and 322 (62.52%) respectively underwent simultaneous and staged surgeries. Nine articles showed the order of CP and VPS in case of staged groups, and three studies were CP after VPS, two papers were VPS after CP, and four studies had both. The findings of quality evaluation demonstrated that the nine studies were of moderate quality (NOS score 5 or 6) and the one article was of low quality (NOS score 3).
TABLE 1.
Baseline characteristics and quality of included studies.
| Study & Year | Design | Age (year) | Number | Initial presentation | Staging order | Quality |
|---|---|---|---|---|---|---|
| simultaneous, staged | Simultaneous, staged | |||||
| Gill, 2021 | RS | 60.9 ± 15.3, 62.5 ± 12.4 | 18, 63 | TBI, SAH, ICH, IS, AVM | VPS after CP, CP after VPS | ★★★★★★ |
| Ting, 2020 | RS | 57.4 ± 13.7, 51.2 ± 17.5 | 17, 32 | TBI | CP after VPS | ★★★★★★ |
| Zhang, 2021 | RS | 43.0–57.0, 33.0–53.0 | 22, 64 | TBI, SAH, ICH | VPS after CP, CP after VPS | ★★★★★★ |
| Jung, 2015 | RS | 22–74 | 14, 10 | TBI, ICH | VPS after CP | ★★★★★ |
| Lin, 2019 | RS | 57.5 ± 18.0, 52.6 ± 15.6 | 19, 37 | TBI, ICH, SAH, IS, SDH | VPS after CP, CP after VPS | ★★★★★★ |
| Heo, 2014 | RS | 57.3, 55.3 | 32, 19 | TBI, ICH, SAH, IS, BT | ‐ | ★★★★★ |
| Rosinski, 2020 | RS | 54.7 ± 10.1, 45.0 ± 10.6 | 18, 22 | ICH, IS | CP after VPS | ★★★★★ |
| Schuss, 2015 | RS | 52.0 ± 13.0, 53.0 ± 18.0 | 17, 24 | TBI, ICH, SAH, IS | CP after VPS | ★★★★★★ |
| Brelie, 2016 | RS | 42.8 ± 17.89 | 10, 27 | TBI, IS | VPS after CP | ★★★ |
| Meyer, 2017 | RS | 43.0 ± 15.0 | 26, 24 | TBI, ICH, SAH, IS, BT, BA | VPS after CP, CP after VPS | ★★★★★ |
Abbreviations: AVM, arteriovenous malformation; BA, brain abscess; BT, brain tumor; CP, cranioplasty; ICH, intracerebral hemorrhage; IS, ischemic stroke; RS, retrospective study; SAH, subarachnoid hemorrhage; SDH, subdural hemorrhage; TBI, traumatic brain injury; VPS, ventriculoperitoneal shunt.
TABLE 2.
Detailed inclusion and outcomes for each study.
| Study & Year | Inclusion criteria | Outcomes |
|---|---|---|
| Gill, 2021 | Patients underwent CP and VPS in simultaneous or staged operations following DC | Brain abscess, infections, intracranial hemorrhage, pneumocephalus, and neurological functional |
| Ting, 2020 | Patients with TBI who had Glasgow Coma Scale score of <13 on admission and underwent unilateral DC | Infections, subdural hygroma, intracranial hematoma, reoperation, and neurological functional |
| Patients underwent CP and VPS within 6 months after DC | ||
| Zhang, 2021 | Patients developed communicating hydrocephalus after DC and subsequently underwent CP and VPS placement | Infections, shunt malfunction, seizure, intracranial hematoma, subdural hygroma, and paradoxical herniation |
| Patients who were not lost to follow‐up within 3 months | ||
| Jung, 2015 | Patients underwent DC, due to refractory intracranial hypertension after they had suffered a TBI or a vascular lesion | Intracranial hematoma, pseudomembranous colitis, subdural hygroma, infections, shunt malfunction, sunken bone plate |
| All patients underwent early CP (an autologous bone flap, 5 to 8 weeks after DC) | ||
| Programmable shunt valve type (Codman‐Medos programmable VPS, Medos SA, Le Loche) | ||
| Lin, 2019 | Patients >18‐year‐old | Infections, over‐drainage, and reoperation |
| Patients followed up for >3 months | ||
| Patients with non‐malignant brain tumor as the reason for DC | ||
| Heo, 2014 | Patients underwent CP and VPS operations after a DC for refractory intracranial hypertension | Intracranial hematoma, infections, and subdural hygroma |
| The interval between the CP and VPS placement was within 6‐month | ||
| In all CP procedures were used autologous bone | ||
| Rosinski, 2020 | Adult patients who had undergone CP and VPS placement at any time after DC | Intracranial hematoma, reoperation, hospital‐acquired infection, cerebrospinal fluid leak, infections, shunt issues, length of stay |
| Non‐pregnant | ||
| Schuss, 2015 | CP procedures with simultaneous or subsequent VPS placement in patients who previously underwent DC | Intracranial hematoma, infections, and subdural hygroma |
| CP and VPS varied according to the treating neurosurgeon (no time limit) | ||
| Brelie, 2016 | Only patients with cranial vault Reconstruction after DC due to TBI and ischemic/hemorrhagic stroke | Infections, reoperation, subdural empyema, aseptic bone flap necrosis, neurological functional |
| Patients were surgically treated in tertiary care center | ||
| Meyer, 2017 | All adult patients who underwent CP and VPS placement for any indication | Infections, shunt issues |
| Follow‐up >3 months |
Abbreviations: CP, cranioplasty; DC, decompressive craniectomy; TBI, traumatic brain injury; VPS, ventriculoperitoneal shunt.
3.3. Risk of infection
The 10 papers were all included, with a total of 70 infected patients of all participants, including 36 (18.1%) in the simultaneous groups and 34 (10.4%) in the staged groups. 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 We applied OR to estimate the incidence of overall infection in both groups. The heterogeneity test suggested moderate difference between studies (χ2 = 20.25, I 2 = 56%, p = 0.02), so the fixed‐effect model was used. The pooled result revealed that although the incidence of infection was a trend toward greater in the simultaneous group, there were no statistically significant between two groups (OR = 1.92, 95% CI: 0.74–4.97, p = 0.18; Figure 2A).
FIGURE 2.
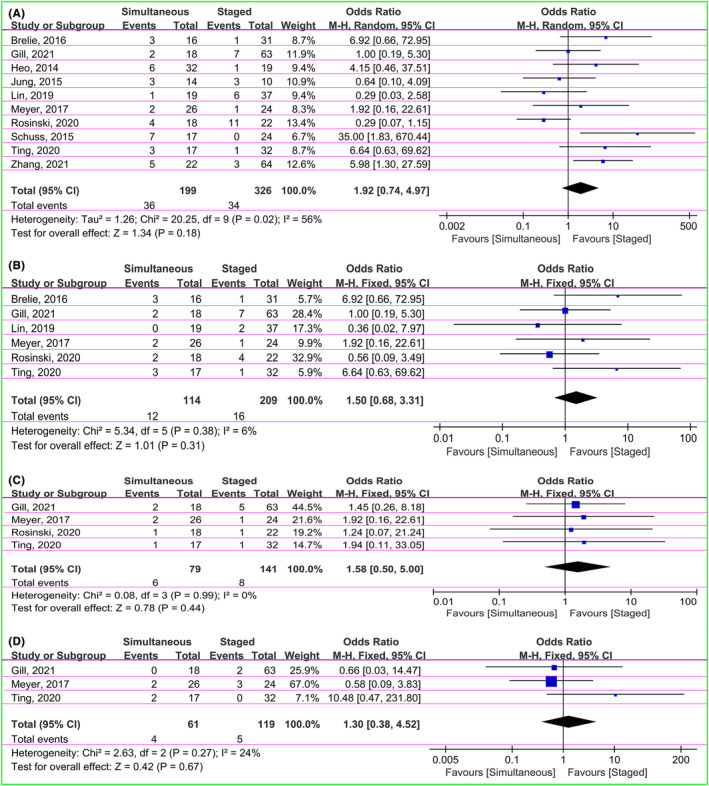
Forest plot showing comparison of overall infection (A), central nervous system infection (B), cranioplasty infection (C), and shunt infection (D) between simultaneous and staged groups.
The group‐level differences of the infection types were performed using OR to estimate the effect sizes. There were no substantial differences between studies (I 2 = 6%, 0%, and 24% on central nervous system infection, cranioplasty infection, and shunt infection, respectively), so the fixed‐effect model were applied. The central nervous system infection was reported in 28 of 323 patients in six articles, including 12 (10.5%) in the simultaneous groups and 16 (7.7%) in the staged groups (OR = 1.50, 95% CI: 0.68–3.31, p = 0.31; Figure 2B). 28 , 32 , 34 , 35 , 36 , 37 The cranioplasty infection was presented in 14 (six in the simultaneous groups and eight in the staged groups) of 220 patients in four studies (OR = 1.58, 95% CI: 0.50–5.00, p = 0.44; Figure 2C). 28 , 32 , 35 , 37 There were three papers showing the data of shunt infection (four in the simultaneous groups and five in the staged groups). The pooled OR was 1.30 (95% CI: 0.38–4.52, p = 0.67; Figure 2D). 28 , 32 , 37 The findings suggested that the simultaneous groups had a trend of higher incidence of all infection types than the staged groups. However, there were no statistically significant difference between two groups.
3.4. Risk of reoperation
A total of 31 patients with reoperation were reported in four articles, including 13 (18.1%) in the simultaneous and 18 (11.7%) in the staged groups. 28 , 32 , 34 , 35 The OR was applied to estimate the incidence of reoperation. There was moderate heterogeneity (χ2 = 7.30, I 2 = 59%, p = 0.06), so the random‐effect model was used. The pooled OR was 1.51 (95% CI: 0.38–6.00, p = 0.55), suggesting statistically no significant in reoperation rate of patients in both groups (Figure 3).
FIGURE 3.
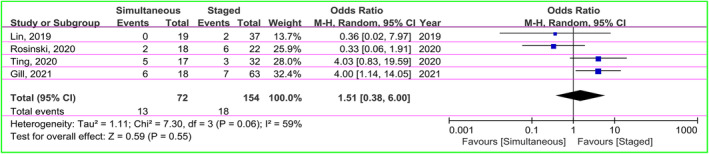
The forest plot displaying a comparison of the risk of reoperation between the two groups.
3.5. Risk of shunt obstruction
There were five studies presenting data of shunt obstruction (n = 5 in the simultaneous groups; n = 9 in the staged groups). 29 , 31 , 33 , 35 , 37 The OR as the effect size was used. There was no heterogeneity between groups (χ2 = 2.22, I 2 = 0%, p = 0.69), so the fixed‐effect model was applied. The pooled result indicated no significant difference in risk of shunt obstruction between groups (OR = 0.73, 95% CI: 0.25–2.16, p = 0.57; Figure 4).
FIGURE 4.
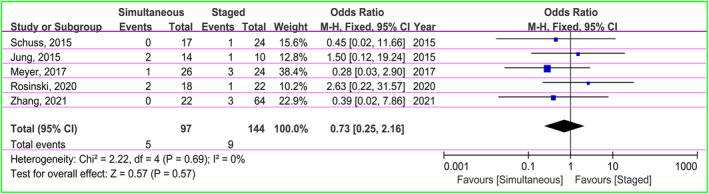
Forest plot of meta‐analysis on the occurrence of shunt obstruction risk in both groups.
3.6. Risk of hematoma
The data of epidural hematoma, subdural hematoma, and intracranial hematoma were included in the pooled analysis. The seven patients in four studies had epidural hematoma, including 4 (4.7%) in the simultaneous groups and 3 (1.9%) in the staged groups. 28 , 29 , 30 , 33 Pooled finding with low heterogeneity (χ2 = 3.18, I 2 = 6%, p = 0.36) using fixed‐effect model did not suggest any statistically significant difference (OR = 2.20, 95% CI: 0.62–7.86, p = 0.22; Figure 5A). There was one article reporting three patients with subdural hematoma, including 2 (6.3%) in the simultaneous and 1 (5.3%) in the staged groups. The pooled OR was 1.20 (95% CI: 0.10–14.19, p = 0.88), indicating no statistically significant difference between two groups (Figure 5B). 30 The five papers showed data of intracranial hematoma (five in the simultaneous and staged groups, respectively). 28 , 30 , 32 , 33 , 35 The result of pooled analysis with no heterogeneity (χ2 = 2.96, I 2 = 0%, p = 0.56) using fixed‐effect model did not demonstrate any statistically significant difference in both groups (OR = 1.31, 95% CI: 0.42–4.07, p = 0.64; Figure 5C).
FIGURE 5.
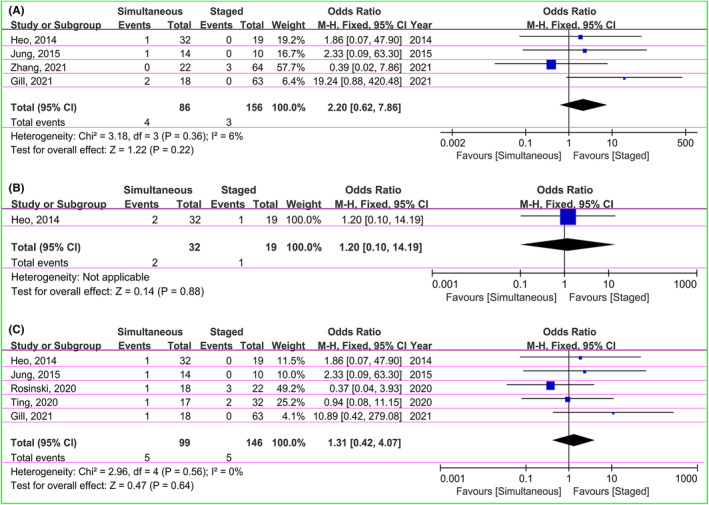
Forest plot indicating comparison of epidural hematoma (A), subdural hematoma (B), and intracranial hematoma (C) in simultaneous and staged groups.
3.7. Risk of subdural effusion
There were 27 patients suffering from subdural effusion in six studies, including 17 (14.0%) in the simultaneous and 10 (5.4%) in the staged groups. 29 , 30 , 31 , 32 , 33 , 34 The OR was used to estimate the incidence of subdural effusion between two groups. The heterogeneity test indicated no difference between studies (χ2 = 2.38, I 2 = 0%, p = 0.79), so the fixed‐effect model was applied. The pooled OR was 2.39 (95% CI: 1.04–5.49, p = 0.04), indicating the incidence of subdural effusion in the staged groups was statistically significantly lower compared with the simultaneous groups (Figure 6).
FIGURE 6.
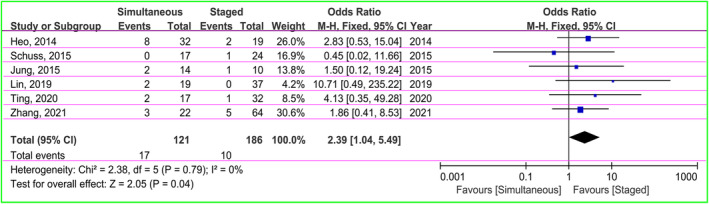
Forest plot of subdural effusion risk between two groups.
3.8. Subgroup analysis
Patients in the staged groups were divided into four subgroups: CP after VPS, VPS after CP, both, and unknown. They were compared with the simultaneous group respectively to explore the influence of the order of CP and VPS on risk of overall infection, central nervous system infection, cranioplasty infection, shunt infection, reoperation, shunt obstruction, epidural hematoma, intracranial hematoma, and subdural effusion. Some trials did not distinguish surgical procedures were defined as “both”. Studies that did not report a specific surgical order were defined as “unknown”. The findings of the subgroup analysis are shown in Table 3. The results demonstrated that the influences of the order of CP and VPS in prognosis are still not known.
TABLE 3.
Subgroup analysis of staged procedures (both, CP after VPS, CP after VPS, and unknown).
| Subgroups | Studies | χ2 | I 2, % | Odds ratio | 95%CI | p value |
|---|---|---|---|---|---|---|
| Overall infection | 10 | 20.25 | 56 | 1.92 | 0.74–4.97 | 0.18 |
| Both | 4 | 5.62 | 47 | 1.51 | 0.41–5.58 | 0.54 |
| CP after VPS | 3 | 11.66 | 83 | 3.32 | 0.16–69.23 | 0.44 |
| VPS after CP | 2 | 2.44 | 59 | 1.87 | 0.18–19.29 | 0.60 |
| Unknown | 1 | ‐ | ‐ | 4.15 | 0.46–37.51 | 0.20 |
| Central nervous system infection | 6 | 5.34 | 6 | 1.50 | 0.68–3.31 | 0.31 |
| Both | 3 | 1.66 | 0 | 1.89 | 0.58–6.22 | 0.29 |
| CP after VPS | 2 | 2.65 | 62 | 1.72 | 0.15–19.19 | 0.66 |
| VPS after CP | 1 | ‐ | ‐ | 6.92 | 0.66–72.95 | 0.11 |
| Unknown | 0 | ‐ | ‐ | ‐ | ‐ | ‐ |
| Cranioplasty infection | 4 | 0.08 | 0 | 1.58 | 0.50–5.00 | 0.44 |
| Both | 2 | 0.03 | 0 | 1.60 | 0.39–6.53 | 0.51 |
| CP after VPS | 2 | 0.05 | 0 | 1.54 | 0.21–11.18 | 0.67 |
| VPS after CP | 0 | ‐ | ‐ | ‐ | ‐ | ‐ |
| Unknown | 0 | ‐ | ‐ | ‐ | ‐ | ‐ |
| Shunt infection | 3 | 2.63 | 24 | 1.30 | 0.38–4.52 | 0.67 |
| Both | 2 | 0.01 | 0 | 0.61 | 0.12–3.04 | 0.54 |
| CP after VPS | 1 | ‐ | ‐ | 10.48 | 0.47–231.80 | 0.14 |
| VPS after CP | 0 | ‐ | ‐ | ‐ | ‐ | ‐ |
| Unknown | 0 | ‐ | ‐ | ‐ | ‐ | ‐ |
| Reoperation | 4 | 1.11 | 59 | 1.51 | 0.38–6.00 | 0.55 |
| Both | 2 | 2.08 | 52 | 1.82 | 0.19–17.41 | 0.60 |
| CP after VPS | 2 | 4.32 | 77 | 1.19 | 0.10–13.77 | 0.89 |
| VPS after CP | 0 | ‐ | ‐ | ‐ | ‐ | ‐ |
| Unknown | 0 | ‐ | ‐ | ‐ | ‐ | ‐ |
| Obstruction | 5 | 2.22 | 0 | 0.73 | 0.25–2.16 | 0.57 |
| Both | 2 | 0.03 | 0 | 0.32 | 0.05–2.07 | 0.23 |
| CP after VPS | 2 | 0.72 | 0 | 1.31 | 0.21–8.04 | 0.77 |
| VPS after CP | 1 | ‐ | ‐ | 1.50 | 0.12–19.24 | 0.76 |
| Unknown | 0 | ‐ | ‐ | ‐ | ‐ | ‐ |
| Epidural hemorrhage | 4 | 3.18 | 6 | 2.20 | 0.62–7.86 | 0.22 |
| Both | 2 | 3.16 | 68 | 2.70 | 0.06–123.98 | 0.61 |
| CP after VPS | 0 | ‐ | ‐ | ‐ | ‐ | ‐ |
| VPS after CP | 1 | ‐ | ‐ | 2.33 | 0.09–63.30 | 0.61 |
| Unknown | 1 | ‐ | ‐ | 1.86 | 0.07–47.90 | 0.71 |
| Intracranial hematoma | 5 | 2.96 | 0 | 1.31 | 0.42–4.07 | 0.64 |
| Both | 1 | ‐ | ‐ | 10.89 | 0.42–279.08 | 0.15 |
| CP after VPS | 2 | 0.28 | 0 | 0.56 | 0.10–3.06 | 0.51 |
| VPS after CP | 1 | ‐ | ‐ | 2.33 | 0.09–63.30 | 0.61 |
| Unknown | 1 | ‐ | ‐ | 1.86 | 0.07–47.90 | 0.71 |
| Subdural effusion | 6 | 2.38 | 0 | 2.39 | 1.04–5.49 | 0.04 |
| Both | 2 | 1.08 | 7 | 2.96 | 0.83–10.56 | 0.09 |
| CP after VPS | 2 | 1.14 | 12 | 1.68 | 0.29–9.85 | 0.57 |
| VPS after CP | 1 | ‐ | ‐ | 1.50 | 0.12–19.24 | 0.76 |
| Unknown | 1 | ‐ | ‐ | 2.83 | 0.53–15.04 | 0.22 |
Note: p < 0.05 is favorable for staging.
Abbreviations: CI, confidence interval; CP, cranioplasty.VPS, ventriculoperitoneal shunt.
3.9. Sensitivity analysis and publication bias
Sensitivity analysis was performed by individually removing each study from overall pooled analysis to evaluate the stability of findings. The results of this meta‐analysis were basically stable. However, individual studies can change the pooled result of subdural effusion. This could be associated with the small sample size. Funnel plots suggested that there was no significant publication bias for overall infection, central nervous system infection, shunt obstruction, intracranial hematoma, and subdural effusion (Figure 7).
FIGURE 7.
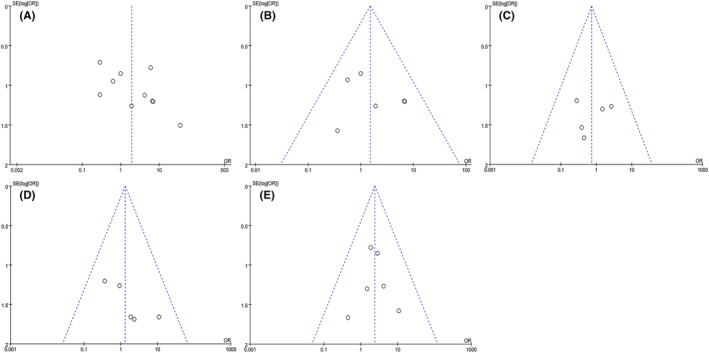
The funnel plots of overall infection (A), central nervous system infection (B), shunt obstruction (C), intracranial hematoma (D), and subdural effusion (E).
4. DISCUSSION
Decompressive craniectomy is a common treatment for patients with refractory intracranial hypertension, and patients who have received DC some require CP and VPS for further treatment. However, due to the lack of guidelines or expert consensus, the order of performing CP and VPS often depends on the neurosurgeon's personal preference. In recent years, several studies have been conducted to evaluate the effect of simultaneous and staged CP and VPS on postoperative complication rates, reporting different perspectives. 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 However, there were characteristics such as small sample size and lack of unified conclusions. Therefore, we performed this meta‐analysis by pooling data from related studies to assess the effect of simultaneous and staged (also including the exploration of the order of staged) CP and VPS on postoperative infection, reoperation, shunt obstruction, hematoma, and subdural effusion.
Infection is one of the most observed complication after CP and VPS. In our meta‐analysis, there was no statistically significant difference in the incidence of postoperative infection. However, there was a higher trend in the simultaneous groups. On the one hand, in the simultaneous groups, longer operative duration and greater operative scope are risk factors for infection. Meyer et al. 37 reported a higher infection rate in the cohort of patients and emphasized the need for more careful aseptic technique when performing multiple simultaneous surgeries. On the other hand, simultaneous surgery could be susceptible to infections caused by the materials used, such as cranial material and shunt devices. 28 In addition, implanting two types of medical materials at the same time also puts patients at a higher risk of infection. This could be related to the microorganisms that colonize the surface of heterogeneous materials can cause recurrent infections. 42 Study by Tsang et al. 43 suggested that CP could theoretically increase the risk of infection in CSF shunts through direct contamination or hematogenous spread. 43 In fact, the two influence and promote each other, other studies have shown that CSF shunt‐related infections can cause post‐CP infections through CSF circulation. 42 , 44 In addition, study from Schuss et al. 31 indicated that poorer general and neurological conditions of patients might predispose neurosurgeons to perform simultaneous surgery to avoid possible complications from secondary surgery, which may also be a contributing factor to higher postoperative infection rates. Some researchers believed that using an antibiotic‐impregnated shunt system during surgery may reduce the incidence of infection. 45 , 46 However, there is an urgent need for large well‐designed randomized controlled trials to clarify the immediate and long‐term effect.
In this meta‐analysis, there were no statistically significant differences in the incidence of reoperation and shunt obstruction after CP and VPS between the simultaneous and staged groups. Several reasons can cause shunt obstruction. 47 Although controversial, neurosurgery generally agrees that pathologically high levels of CSF protein and cells are associated with shunt obstruction. 48 , 49 , 50 In addition, shunt siphoning resulting from overdrainage may cause shunts obstructed by a ventricular tissue protrusion. 51 Causes of reoperation include infection, shunt dysfunction, bone flap resorption, and cosmetic defects, etc. 29 Clinical study demonstrated that the presence of shunts was an independent risk factor for bone flap resorption after CP. 52 Shunt placement reduces dura mater adhesion to the bone flap, ICP fluctuations, and negatively affects cranial repair, thereby contributing to bone flap resorption. 53 , 54 , 55 , 56 , 57 However, Behbahani et al. 58 reported that the presence of shunts had a protective effect on bone flap resorption. Therefore, further studies are needed to determine the relationship between bone flap absorption and the presence of shunts.
In addition, different skull materials may also involve different risks of pressure absorption of skin flaps at ultra‐long‐term, which could occur in patients with both artificial skull and VPS device. This condition is not affected by the surgical plan, but mainly due to the artificial skull itself. It is worth noting that ultra‐long‐term complications are mostly caused by artificial materials with meshes or sharp edges. First, sharp edges are due to the blunting treatment technology of the material edge itself, and second to the deformation of the material itself. Once the material slowly changes from convex to concave due to the multidimensional pressure difference, its edge is forced to curl up, and the scalp is gradually consumed under shear force. Therefore, in the selection of artificial skull, we need to consider the structure of the material itself and its mechanical effects (long‐term material deformation risk). As of now, there are advantages and disadvantages to different artificial skull materials, and further long‐term follow‐up studies on various materials are worthwhile. Therefore, only by fully understanding the material characteristics and combining them with different surgical techniques could reduce the above‐mentioned long‐term complications to some extent. For example, clear understanding of the scalp anatomy, sufficient and thorough skin flap thickness, and clever combination with the mechanical properties of the connecting material.
There was no statistical difference in the incidence of hematoma after CP and VPS between two groups. However, the simultaneous groups was significantly associated with a higher incidence of subdural effusion. Complications such as hematoma and subdural effusion in patients undergoing simultaneous CP and VPS have been reported to be associated with VPS‐induced sunken down effect of the brain. 30 , 33 Heo et al. 30 suggested a higher incidence of hematoma and subdural effusion in the simultaneous groups and indicated that this might be due to the difficulty in regulating VPS pressure in these patients. Therefore, it is particularly concerning to reasonably match the type of shunt. Fixed pressure shunt with limited adjustable pressure range, making precise adjustments on an individual basis difficult. However, programmable shunts can set the appropriate shunt pressure based on the patient's CSF pressure. Of course, its advantage lies in this, it could adjust the pressure in time after the operation and reduce the complications caused by insufficient or excessive shunt.
Furthermore, difficult‐to‐eliminate epidural dead space may increase the risk of hematoma and subdural effusion. 59 Study by Liao and Kao 60 further confirms the above theoretical analysis and demonstrated that temporary clipping of the shunt device could eliminate the dead space between the skull plate and the dura, thereby reducing the risk of subdural effusion and hematoma. Similar results reported from Jung et al, arguing that blocking CSF drainage could avoid the sunken down effect of the brain and prevent the occurrence of hematoma and subdural effusion. 33 , 61 Given this, it is necessary to choose appropriate flow rate and volume of CSF shunt base on programmable shunts. If these complications are not properly avoided, the risk of reoperation may be increased. However, the precise regulation of CSF in time needs to be further explored. In addition, the guidelines for regulation also need to be clarified and refined. Obviously, there is no uniform measure of whether the VPS pressure in these patients is appropriate. Just imagine, the invention of a system that intelligently calculates flow rate based on ideal indicators may have potential value of clinical application. But before that, we need to clarify what is the ideal indicators.
In subgroup analysis, there were no statistical differences in all comparisons. There is no consensus on the sequence of CP and VPS in staged surgery. The traditional clinical experience is usually CP after VPS. But, a one‐size‐fits‐all approach may be arbitrary. Study by Oh et al. 62 confirmed this and their found that the outcomes of VPS after CP tend to be better than CP after shunt operation in patients with large, concave flaccid skull defect. If the VPS is performed first, the patient's ICP will be reduced, resulting in an increase in the difference between atmospheric pressure and ICP, thereby disrupting the normal anatomy of the brain, blood supply, and CSF circulation, which may cause SSFS with symptoms including epilepsy, headaches, dizziness, language deficits, motor deficits, or even paradoxical herniation. 63 , 64 , 65 In contrast, initial CP can significantly improve DC‐induced CSF dynamics dysfunction, thereby partially relieving symptoms of hydrocephalus and potential avoiding the need for subsequent shunting. 66 , 67 But it is not absolute, Morton et al. 68 found that post‐CP was associated with a 9.0% risk of hydrocephalus. In addition, for patients with tense convex cranial defects, VPS must be performed first, and CP should be performed after the bone window is slightly sunken or flattened. 69 However, as with the surgical options, the diagnosis of hydrocephalus in patients with cranial defects is also highly challenging. The cerebrospinal fluid tap test has been regarded as a core tool for the prediction of VPS effectiveness in hydrocephalus patients. Multiple cerebrospinal fluid tap test could be put on the agenda when it is difficult to make a choice through intuitive clinical manifestations. Combined with the above findings, we cautiously believe that staged (also its order) or simultaneous surgery should be selected according to the specific conditions of the patients.
4.1. Limitations
This meta‐analysis has some limitations. The 10 included studies were all single‐center retrospective studies with heterogeneity of study protocols. There may be some relevant articles that were missed, even though we searched major databases and tracked the reference lists of included studies. In addition, there were differences in the CP transplantation materials, the degree of brain bulging, timing of surgery following the initial causative event, and the interval and sequence between CP and VPS in different studies. However, it will not be possible to answer these issues unambiguously, based on data that are difficult to subdivide into subgroups. Thus, the above questions could be the focus of future study. Although 10 studies were included in the pooled analysis, the total sample size was relatively small. Our findings should be analyzed with caution, and neurosurgeons in practice should design individualized treatment plans based on each patient's condition. Prospective studies and randomized controlled trials with strict inclusion criteria are needed in the future to further analyze the pros and cons of simultaneous and staged CP and VPS. Furthermore, neurological functional outcome should also be investigated.
5. CONCLUSIONS
The differential diagnosis cannot be ignored between encephalocele and hydrocephalus. Once the diagnosis of hydrocephalus combined with a cranial defect is confirmed, follow‐up issues will be on the agenda. While these findings were not stable, the authors cautiously indicated that patients with cranial defects and hydrocephalus who undergo staged CP and VPS procedures might benefit from a potentially lower trend in complication rate. However, more studies, ideally using larger, prospective, and multicentre designs are needed to clarify this. Ultimately, neurosurgeons in practice should make individualized decisions based on each patient's condition (also including cerebrospinal fluid tap test), to guide the formulation of staged or imultaneous surgical protocols, even the order of CP and VPS in staged surgeries.
FUNDING INFORMATION
The funding was provided by the National Natural Science Foundation of China (NSFC Grants 82171382, 81870968, and 81901243).
CONFLICT OF INTEREST STATEMENT
The authors report no conflicts of interest in this work.
INFORMED CONSENT
N/A.
ACKNOWLEDGMENTS
We thank “CNS Neuroscience & Therapeutics” for providing such an excellent academic exchange platform.
Zhang J, Deng X, Yuan Q, et al. Staged or simultaneous operations for ventriculoperitoneal shunt and cranioplasty: Evidence from a meta‐analysis . CNS Neurosci Ther. 2023;29:3136‐3149. doi: 10.1111/cns.14347
The first three authors contributed equally to this work
Contributor Information
Zhuoying Du, Email: zdu10@fudan.edu.cn.
Jin Hu, Email: hujin_dana@126.com.
DATA AVAILABILITY STATEMENT
The data that support the findings of our study could be available from contacting the corresponding author.
REFERENCES
- 1. Goodrich JT. How to get in and out of the skull: from tumi to “hammer and chisel” to the Gigli saw and the osteoplastic flap. Neurosurg Focus. 2014;36(4):E6. doi: 10.3171/2014.2.FOCUS13543 [DOI] [PubMed] [Google Scholar]
- 2. Kocher T. Hirnerschütterung, Hirndruck und chirurgische Eingriffe bei Hirnkrankheiten. Alfred Hölder; 1901. [Google Scholar]
- 3. Eberle BM, Schnüriger B, Inaba K, Peter Gruen J, Demetriades D, Belzberg H. Decompressive craniectomy: surgical control of traumatic intracranial hypertension may improve outcome. Injury. 2010;41(9):894‐898. doi: 10.1016/j.injury.2010.02.023 [DOI] [PubMed] [Google Scholar]
- 4. Guresir E, Beck J, Vatter H, et al. Subarachnoid hemorrhage and intracerebral hematoma: incidence, prognostic factors, and outcome. Neurosurgery. 2008;63(6):1088‐1093. doi: 10.1227/01.NEU.0000335170.76722.B9 [DOI] [PubMed] [Google Scholar]
- 5. Guresir E, Raabe A, Setzer M, et al. Decompressive hemicraniectomy in subarachnoid haemorrhage: the influence of infarction, haemorrhage and brain swelling. J Neurol Neurosurg Psychiatry. 2009;80(7):799‐801. doi: 10.1136/jnnp.2008.155630 [DOI] [PubMed] [Google Scholar]
- 6. Münch E, Horn P, Schürer L, Piepgras A, Paul T, Schmiedek P. Management of severe traumatic brain injury by decompressive craniectomy. Neurosurgery. 2000;47(2):315‐322. doi: 10.1097/00006123-200008000-00009 [DOI] [PubMed] [Google Scholar]
- 7. Schwab S, Steiner T, Aschoff A, et al. Early hemicraniectomy in patients with complete middle cerebral artery infarction. Stroke. 1998;29(9):1888‐1893. doi: 10.1161/01.str.29.9.1888 [DOI] [PubMed] [Google Scholar]
- 8. Skoglund TS, Eriksson‐Ritzén C, Jensen C, Rydenhag B. Aspects on decompressive craniectomy in patients with traumatic head injuries. J Neurotrauma. 2006;23(10):1502‐1509. doi: 10.1089/neu.2006.23.1502 [DOI] [PubMed] [Google Scholar]
- 9. Aarabi B, Hesdorffer DC, Simard JM, et al. Comparative study of decompressive craniectomy after mass lesion evacuation in severe head injury. Neurosurgery. 2009;64(5):927‐939. doi: 10.1227/01.NEU.0000341907.30831.D2 [DOI] [PubMed] [Google Scholar]
- 10. Lang JK, Ludwig HC, Mursch K, Zimmerer B, Markakis E. Elevated cerebral perfusion pressure and low colloid osmotic pressure as a risk factor for subdural space‐occupying hygromas? Surg Neurol. 1999;52(6):630‐637. doi: 10.1016/s0090-3019(99)00144-5 [DOI] [PubMed] [Google Scholar]
- 11. De Bonis P, Sturiale CL, Anile C, et al. Decompressive craniectomy, interhemispheric hygroma and hydrocephalus: a timeline of events? Clin Neurol Neurosurg. 2013;115(8):1308‐1312. doi: 10.1016/j.clineuro.2012.12.011 [DOI] [PubMed] [Google Scholar]
- 12. Grant FC, Norcross NC. Repair of cranial defects by cranioplasty. Ann Surg. 1939;110(4):488‐512. doi: 10.1097/00000658-193910000-00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sarov M, Guichard JP, Chibarro S, et al. Sinking skin flap syndrome and paradoxical herniation after hemicraniectomy for malignant hemispheric infarction. Stroke. 2010;41(3):560‐562. doi: 10.1161/STROKEAHA.109.568543 [DOI] [PubMed] [Google Scholar]
- 14. Ashayeri K, Jackson EM, Huang J, et al. Syndrome of the trephined: a systematic review. Neurosurgery. 2016;79(4):525‐534. doi: 10.1227/NEU.0000000000001366 [DOI] [PubMed] [Google Scholar]
- 15. Dujovny M, Aviles A, Agner C, Fernandez P, Charbel FT. Cranioplasty: cosmetic or therapeutic? Surg Neurol. 1997;47(3):238‐241. doi: 10.1016/s0090-3019(96)00013-4 [DOI] [PubMed] [Google Scholar]
- 16. Dujovny M, Agner C, Aviles A. Syndrome of the trephined: theory and facts. Crit Rev Neurosurg. 1999;9(5):271‐278. doi: 10.1007/s003290050143 [DOI] [PubMed] [Google Scholar]
- 17. Dujovny M, Fernandez P, Alperin N, Betz W, Misra M, Mafee M. Post‐cranioplasty cerebrospinal fluid hydrodynamic changes: magnetic resonance imaging quantitative analysis. Neurol Res. 1997;19(3):311‐316. doi: 10.1080/01616412.1997.11740818 [DOI] [PubMed] [Google Scholar]
- 18. Fodstad H, Ekstedt J, Fridén H. CSF hydrodynamic studies before and after cranioplasty. Acta Neurochir Suppl (Wien). 1979;28(2):514‐518. [PubMed] [Google Scholar]
- 19. Fodstad H, Love JA, Ekstedt J, Fridén H, Liliequist B. Effect of cranioplasty on cerebrospinal fluid hydrodynamics in patients with the syndrome of the trephined. Acta Neurochir. 1984;70(1–2):21‐30. doi: 10.1007/BF01406039 [DOI] [PubMed] [Google Scholar]
- 20. Gooch MR, Gin GE, Kenning TJ, German JW. Complications of cranioplasty following decompressive craniectomy: analysis of 62 cases. Neurosurg Focus. 2009;26(6):E9. doi: 10.3171/2009.3.FOCUS0962 [DOI] [PubMed] [Google Scholar]
- 21. Segal DH, Oppenheim JS, Murovic JA. Neurological recovery after cranioplasty. Neurosurgery. 1994;34(4):729‐731. doi: 10.1227/00006123-199404000-00024 [DOI] [PubMed] [Google Scholar]
- 22. Aarabi B, Hesdorffer DC, Ahn ES, Aresco C, Scalea TM, Eisenberg HM. Outcome following decompressive craniectomy for malignant swelling due to severe head injury. J Neurosurg. 2006;104(4):469‐479. doi: 10.3171/jns.2006.104.4.469 [DOI] [PubMed] [Google Scholar]
- 23. Kan P, Amini A, Hansen K, et al. Outcomes after decompressive craniectomy for severe traumatic brain injury in children. J Neurosurg. 2006;105(5 Suppl):337‐342. doi: 10.3171/ped.2006.105.5.337 [DOI] [PubMed] [Google Scholar]
- 24. Yang XF, Wen L, Shen F, et al. Surgical complications secondary to decompressive craniectomy in patients with a head injury: a series of 108 consecutive cases. Acta Neurochir. 2008;150:1241‐1247; discussion 1248. doi: 10.1007/s00701-008-0145-9 [DOI] [PubMed] [Google Scholar]
- 25. Kahle KT, Kulkarni AV, Limbrick DD Jr, Warf BC. Hydrocephalus in children. Lancet. 2016;387(10020):788‐799. doi: 10.1016/S0140-6736(15)60694-8 [DOI] [PubMed] [Google Scholar]
- 26. de Oliveira JG, Beck J, Setzer M, et al. Risk of shunt‐dependent hydrocephalus after occlusion of ruptured intracranial aneurysms by surgical clipping or endovascular coiling: a single‐institution series and meta‐analysis. Neurosurgery. 2007;61(5):924‐933. doi: 10.1227/01.neu.0000303188.72425.24 [DOI] [PubMed] [Google Scholar]
- 27. Sobani ZA, Shamim MS, Zafar SN, et al. Cranioplasty after decompressive craniectomy: an institutional audit and analysis of factors related to complications. Surg Neurol Int. 2011;2:123. doi: 10.4103/2152-7806.85055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gill JH, Choi HH, Lee SH, et al. Comparison of postoperative complications between simultaneous and staged surgery in cranioplasty and ventriculoperitoneal shunt placement after decompressive craniectomy. Korean J Neurotrauma. 2021;17(2):100‐107. doi: 10.13004/kjnt.2021.17.e20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang X, Fang X, Gao A, et al. Safety analysis of simultaneous cranioplasty and ventriculoperitoneal shunt placement. Turk Neurosurg. 2022;32(2):195‐203. doi: 10.5137/1019-5149.JTN.30740-20.2 [DOI] [PubMed] [Google Scholar]
- 30. Heo J, Park SQ, Cho SJ, Chang JC, Park HK. Evaluation of simultaneous cranioplasty and ventriculoperitoneal shunt procedures. J Neurosurg. 2014;121(2):313‐318. doi: 10.3171/2014.2.JNS131480 [DOI] [PubMed] [Google Scholar]
- 31. Schuss P, Borger V, Güresir Á, Vatter H, Güresir E. Cranioplasty and ventriculoperitoneal shunt placement after decompressive craniectomy: staged surgery is associated with fewer postoperative complications. World Neurosurg. 2015;84(4):1051‐1054. doi: 10.1016/j.wneu.2015.05.066 [DOI] [PubMed] [Google Scholar]
- 32. Ting CW, Lu CH, Lan CM, Lee TH, Hsu SW, Su TM. Simultaneous cranioplasty and ventriculoperitoneal shunt placement in patients with traumatic brain injury undergoing unilateral decompressive craniectomy. J Clin Neurosci. 2020;79:45‐50. doi: 10.1016/j.jocn.2020.07.015 [DOI] [PubMed] [Google Scholar]
- 33. Jung YT, Lee SP, Cho JI. An improved one‐stage operation of cranioplasty and ventriculoperitoneal shunt in patient with hydrocephalus and large cranial defect. Korean J Neurotrauma. 2015;11(2):93‐99. doi: 10.13004/kjnt.2015.11.2.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lin HY, Lin KC, Tsai CC, Wan D. Simultaneous or staged operation? Timing of cranioplasty and ventriculoperitoneal shunt after decompressive craniectomy. Formos J Surg. 2019;52(4):122‐126. doi: 10.4103/fjs.fjs_18_19 [DOI] [Google Scholar]
- 35. Rosinski CL, Behbahani M, Geever B, et al. Concurrent versus staged procedures for ventriculoperitoneal shunt and cranioplasty: a 10‐year retrospective comparative analysis of surgical outcomes. World Neurosurg. 2020;143:e648‐e655. doi: 10.1016/j.wneu.2020.08.062 [DOI] [PubMed] [Google Scholar]
- 36. von der Brelie C, Stojanovski I, Meier U, Lemcke J. Open traumatic brain injury is a strong predictor for aseptic bone necrosis after cranioplasty surgery: a retrospective analysis of 219 patients. J Neurol Surg A Cent Eur Neurosurg. 2016;77(1):19‐24. doi: 10.1055/s-0035-1558410 [DOI] [PubMed] [Google Scholar]
- 37. Meyer RM 4th, Morton RP, Abecassis IJ, et al. Risk of complications with simultaneous cranioplasty and placement of ventriculoperitoneal shunt. World Neurosurg. 2017;107:830‐833. doi: 10.1016/j.wneu.2017.08.034 [DOI] [PubMed] [Google Scholar]
- 38. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264‐269. doi: 10.7326/0003-4819-151-4-200908180-00135 [DOI] [PubMed] [Google Scholar]
- 39. Kweh BTS, Rosenfeld JV, Hunn M, Tee JW. Tumor characteristics and surgical outcomes of intracranial subependymomas: a systematic review and meta‐analysis. J Neurosurg. 2022;136(3):736‐748. doi: 10.3171/2021.2.JNS204052 [DOI] [PubMed] [Google Scholar]
- 40. Wells GA, Shea B, O'Connell D, et al. The Newcastle‐Ottawa Scale (NOS) for Assessing the Quality if Nonrandomized Studies in Meta‐Analyses. 2000. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed January 1, 2023
- 41. Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions. 2nd ed. John Wiley & Sons; 2019. [Google Scholar]
- 42. Yang SM, Park HK, Cho SJ, Chang JC, Park SQ, Kim RS. The current analysis of the risk factors for bone graft infection after cranioplasty. Korean J Neurotrauma. 2013;9(2):57‐63. doi: 10.13004/kjnt.2013.9.2.57 [DOI] [Google Scholar]
- 43. Tsang AC, Hui VK, Lui WM, et al. Complications of post‐craniectomy cranioplasty: risk factor analysis and implications for treatment planning. J Clin Neurosci. 2015;22(5):834‐837. doi: 10.1016/j.jocn.2014.11.021 [DOI] [PubMed] [Google Scholar]
- 44. Mustroph CM, Malcolm JG, Rindler RS, et al. Cranioplasty infection and resorption areassociated with the presence of a ventriculoperitoneal shunt: a systematic review and meta‐analysis. World Neurosurg. 2017;103:686‐693. doi: 10.1016/j.wneu.2017.04.066 [DOI] [PubMed] [Google Scholar]
- 45. Albanese A, De Bonis P, Sabatino G, et al. Antibiotic‐impregnated ventriculo‐peritoneal shunts in patients at high risk of infection. Acta Neurochir. 2009;151(10):1259‐1263. doi: 10.1007/s00701-009-0317-2 [DOI] [PubMed] [Google Scholar]
- 46. Govender ST, Nathoo N, van Dellen JR. Evaluation of an antibiotic‐impregnated shunt system for the treatment of hydrocephalus. J Neurosurg. 2003;99(5):831‐839. doi: 10.3171/jns.2003.99.5.0831 [DOI] [PubMed] [Google Scholar]
- 47. Pillai SV. Techniques and nuances in Ventriculoperitoneal shunt surgery. Neurol India. 2021;69(8):471‐475. doi: 10.4103/0028-3886.332261 [DOI] [PubMed] [Google Scholar]
- 48. Fehrenbach MK, Bernhard M, Siekmeyer M, et al. VP‐shunt dysfunction caused by malaria CNS infection. Childs Nerv Syst. 2016;32(4):759‐760. doi: 10.1007/s00381-015-2912-2 [DOI] [PubMed] [Google Scholar]
- 49. Kamat AS, Gretschel A, Vlok AJ, Solomons R. CSF protein concentration associated with ventriculoperitoneal shunt obstruction in tuberculous meningitis. Int J Tuberc Lung Dis. 2018;22(7):788‐792. doi: 10.5588/ijtld.17.0008 [DOI] [PubMed] [Google Scholar]
- 50. Lorber J, Bhat US. Posthaemorrhagic hydrocephalus: diagnosis, differential diagnosis, treatment, and long‐term results. Arch Dis Child. 1974;49(10):751‐762. doi: 10.1136/adc.49.10.751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kraemer MR, Koueik J, Rebsamen S, et al. Overdrainage‐related ependymal bands: a postulated cause of proximal shunt obstruction. J Neurosurg Pediatr. 2018;22(5):567‐577. doi: 10.3171/2018.5.PEDS18111 [DOI] [PubMed] [Google Scholar]
- 52. Mracek J, Hommerova J, Mork J, Richtr P, Priban V. Complications of cranioplasty using a bone flap sterilised by autoclaving following decompressive craniectomy. Acta Neurochir. 2015;157(3):501‐506. doi: 10.1007/s00701-014-2333-0 [DOI] [PubMed] [Google Scholar]
- 53. Bowers CA, Riva‐Cambrin J, Hertzler DA 2nd, et al. Risk factors and rates of bone flap resorption in pediatric patients after decompressive craniectomy for traumatic brain injury. J Neurosurg Pediatr. 2013;11(5):526‐532. doi: 10.3171/2013.1.PEDS12483 [DOI] [PubMed] [Google Scholar]
- 54. Dünisch P, Walter J, Sakr Y, Kalff R, Waschke A, Ewald C. Risk factors of aseptic bone resorption: a study after autologous bone flap reinsertion due to decompressive craniectomy. J Neurosurg. 2013;118(5):1141‐1147. doi: 10.3171/2013.1.JNS12860 [DOI] [PubMed] [Google Scholar]
- 55. Grant GA, Jolley M, Ellenbogen RG, Roberts TS, Gruss JR, Loeser JD. Failure of autologous bone‐assisted cranioplasty following decompressive craniectomy in children and adolescents. J Neurosurg. 2004;100(2):163‐168. doi: 10.3171/ped.2004.100.2.0163 [DOI] [PubMed] [Google Scholar]
- 56. Martin KD, Franz B, Kirsch M, et al. Autologous bone flap cranioplasty following decompressive craniectomy is combined with a high complication rate in pediatric traumatic brain injury patients. Acta Neurochir. 2014;156(4):813‐824. doi: 10.1007/s00701-014-2021-0 [DOI] [PubMed] [Google Scholar]
- 57. Schwarz F, Dünisch P, Walter J, Sakr Y, Kalff R, Ewald C. Cranioplasty after decompressive craniectomy: is there a rationale for an initial artificial bone‐substitute implant? A single‐center experience after 631 procedures. J Neurosurg. 2016;124(3):710‐715. doi: 10.3171/2015.4.JNS159 [DOI] [PubMed] [Google Scholar]
- 58. Behbahani M, Rosenberg DM, Rosinski CL, Chaudhry NS, Nikas D. Cranioplasty in infants less than 24 months of age: a retrospective case review of pitfalls, outcomes, and complications. World Neurosurg. 2019;132:e479‐e486. doi: 10.1016/j.wneu.2019.08.106 [DOI] [PubMed] [Google Scholar]
- 59. Han PY, Kim JH, Kang HI, Kim JS. “Syndrome of the sinking skin‐flap” secondary to the ventriculoperitoneal shunt after craniectomy. J Korean Neurosurg Soc. 2008;43(1):51‐53. doi: 10.3340/jkns.2008.43.1.51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Liao CC, Kao MC. Cranioplasty for patients with severe depressed skull bone defect after cerebrospinal fluid shunting. J Clin Neurosci. 2002;9(5):553‐555. doi: 10.1054/jocn.2002.1116 [DOI] [PubMed] [Google Scholar]
- 61. Zheng WJ, Li LM, Hu ZH, et al. Excessive hemostasis on the scalp increases superficial surgical site infection rate in cranioplasty. World Neurosurg. 2018;120:e811‐e817. doi: 10.1016/j.wneu.2018.08.172 [DOI] [PubMed] [Google Scholar]
- 62. Oh CH, Park CO, Hyun DK, Park HC, Yoon SH. Comparative study of outcomes between shunting after cranioplasty and in cranioplasty after shunting in large concave flaccid cranial defect with hydrocephalus. J Korean Neurosurg Soc. 2008;44(4):211‐216. doi: 10.3340/jkns.2008.44.4.211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Akins PT, Guppy KH. Sinking skin flaps, paradoxical herniation, and external brain tamponade: a review of decompressive craniectomy management. Neurocrit Care. 2008;9(2):269‐276. doi: 10.1007/s12028-007-9033-z [DOI] [PubMed] [Google Scholar]
- 64. Annan M, De Toffol B, Hommet C, et al. Sinking skin flap syndrome (or syndrome of the trephined): a review. Br J Neurosurg. 2015;29(3):314‐318. doi: 10.3109/02688697.2015.1012047 [DOI] [PubMed] [Google Scholar]
- 65. Romero FR, Zanini MA, Ducati LG, Gabarra RC. Sinking skin flap syndrome with delayed dysautonomic syndrome—an atypical presentation. Int J Surg Case Rep. 2013;4(11):1007‐1009. doi: 10.1016/j.ijscr.2013.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Pachatouridis D, Alexiou GA, Zigouris A, et al. Management of hydrocephalus after decompressive craniectomy. Turk Neurosurg. 2014;24(6):855‐858. doi: 10.5137/1019-5149.JTN.8871-13.1 [DOI] [PubMed] [Google Scholar]
- 67. Peraio S, Calcagni ML, Mattoli MV, et al. Decompressive craniectomy and hydrocephalus: proposalof a therapeutic flow chart. J Neurosurg Sci. 2017;61(6):673‐676. doi: 10.23736/S0390-5616.16.03427-X [DOI] [PubMed] [Google Scholar]
- 68. Morton RP, Abecassis IJ, Hanson JF, et al. Timing of cranioplasty: a 10.75‐year single‐center analysis of 754 patients. J Neurosurg. 2018;128(6):1648‐1652. doi: 10.3171/2016.11.JNS161917 [DOI] [PubMed] [Google Scholar]
- 69. Hirschmann D, Kranawetter B, Kirchschlager C, et al. Cranioplasty following ventriculoperitoneal shunting: lessons learned. Acta Neurochir. 2021;163(2):441‐446. doi: 10.1007/s00701-020-04597-y [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of our study could be available from contacting the corresponding author.


