Abstract
The melanocortin-4 receptor agonist setmelanotide is now recommended for the treatment of genetic obesity due to proopiomelanocortin (POMC), proprotein convertase subtilisin/kexin type 1 (PCSK1), or leptin receptor (LEPR) deficiency in patients aged 6 years and older. Here, we describe the clinical benefit of setmelanotide administration in a 5-year-old child with severe hyperphagia and obesity due to POMC deficiency. Daily administration of 0.5 mg setmelanotide for 12 months resulted in significant weight loss of −30 kg from baseline (−36% of weight loss) and improvements in hyperphagia and metabolic status. No major side effects were observed, except for hyperpigmentation and transient spontaneous erections. Interestingly, the clinical improvement of the child was associated with a remarkable improvement in the quality of life of the parents, along with a decrease in their emotional scores. This observation supports the early use of setmelanotide in young children with melanocortin pathway variants, in order to limit the adverse consequences of early and extreme weight gain, and to improve the quality of life of patients and of their relatives.
Keywords: monogenic obesity, proopiomelanocortin, MC4R, quality of life
Introduction
The leptin/melanocortin pathway plays an undisputed role in the hypothalamic control of food intake. Its interruption due to a genetic defect is associated with early and severe obesity, with major hyperphagia in the first weeks of life, and with endocrine deficits and/or neurodevelopmental disorders [1]. These genetic obesities are a heavy burden for children and for their families, with deterioration in their quality of life. Until recently, and apart from the use of recombinant leptin in congenital leptin deficiency, no therapeutic option was available for patients with genetic variants downstream of the leptin receptor. Treatment was based on the use of conventional clinical approaches to obesity management and associated symptoms, with limited effectiveness on weight and hyperphagia [2, 3]. This ended with the development of the melanocortin-4 receptor (MC4R) agonist setmelanotide, which showed a beneficial effect not only on weight loss, reduction of hyperphagia, and metabolic complications, but also on the improvement of the quality of life of patients older than 12 years after 52 weeks of treatment [4, 5]. Sustained beneficial effect and safety was recently reported after 7 years of setmelanotide administration in the first 2 treated adults with proopiomelanocortin (POMC) deficiency [6]. The MC4R agonist setmelanotide is now recommended for the treatment of genetic obesity and control of hunger due to POMC, proprotein convertase subtilisin/kexin type 1 (PCSK1), or leptin receptor (LEPR) deficiency in patients 6 years and older. However, data are still lacking regarding tolerance, efficacy, and impact on patient and caregiver quality of life among young children under 6. We describe here the clinical effect of 12 months of setmelanotide administration on a 5-year-old POMC-deficient child and the impact of this treatment on his parents’ quality of life.
Case Presentation
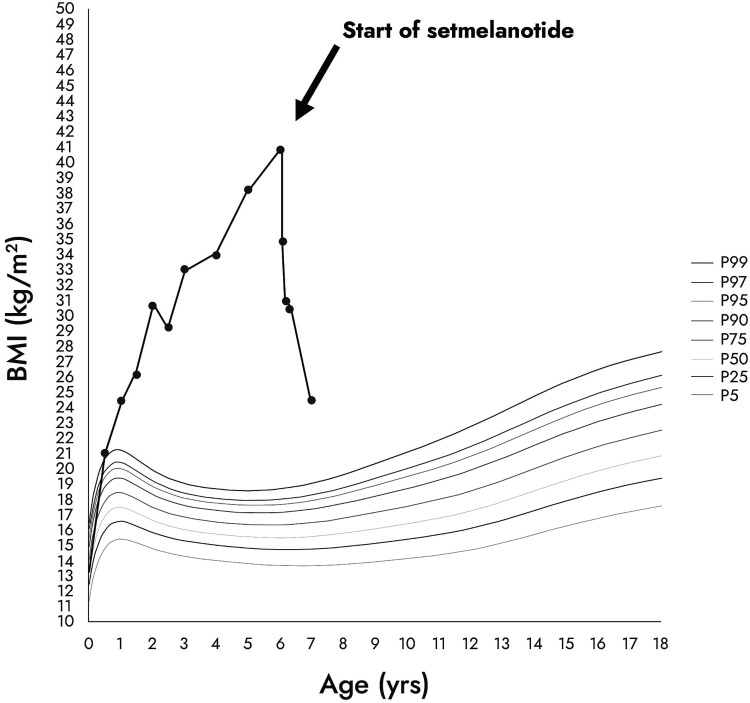
This boy was born after a regular pregnancy from consanguineous parents of Turkish origin with birth parameters in the normal range (weight 3.295 kg; height 50 cm; head circumference 36.5 cm). At birth, the boy was rapidly admitted in the local neonatology department for bilateral pneumothorax with a favorable evolution after 1 week. During his stay, several instances of asymptomatic hypoglycemia were noticed, and he received maltodextrin supplementation. He rapidly developed cholestasis and the diagnosis of adrenocorticotrophin hormone (ACTH) deficiency was made at day 5 (cortisol < 3 nmol/L [<8.3 μg/L] [range 110-520 nmol/L]; ACTH < 1 pmol/L [< 5 pg/mL]). No other hypothalamic-pituitary deficiency or anatomical lesion were found as a newborn or later. Treatment by hydrocortisone was started at day 6 with the usual dose of 20 mg/m2/day. During the first year of life, he developed a severe obesity phenotype (body mass index [BMI] > 97 percentile at the age of 6 months) without adiposity rebound (Fig. 1). The parents described severe hyperphagia and necessity to feed their child every 2 hours after the age of 6 months. Skin pigmentation was normal without the red hair phenotype frequently found in POMC-deficient patients.
Figure 1.
Evolution of the patient's BMI before and after initiation of setmelanotide. The different curves represent the BMI percentile curves from the 5th percentile (P5) to the 99th percentile (P99).
Diagnostic Assessment
Due to the persistent association between ACTH deficiency and early-onset severe obesity with uncontrolled hyperphagia, the diagnosis of POMC deficiency was suspected at the age of 3. Direct sequencing of the POMC gene led to the identification of a homozygous variant c.251G>A in POMC, responsible for a premature stop codon predicting high pathogenic impact. The 2 parents, who did not suffer from severe obesity, were heterozygous carriers of this POMC variant. The child developed severe neurodevelopmental delay illustrated by delayed walking at 28 months of age and absence of speech with less than 20 words at the age of 5, with the need for adapted schooling and speech therapy. Neurogenetic explorations were negative (methylation of chromosome 15 for Prader-Willi syndrome, chromosomal analysis on DNA chip), as was cerebral magnetic resonance imaging, suggesting that this neurodevelopmental delay might be related to cerebral sequalae of undiagnosed initial neonatal hypoglycemia at birth.
Treatment
Despite intensive control of food intake and physical activity by the caregivers, this POMC-deficient patient developed a severe obesity phenotype (weight = 82 kg; height = 142 cm; BMI = 40.7 kg/m2 and BMI Z score +18 SDs at 5 years) with voracious hyperphagia that was assessed by clinical interview of the parents and the use of validated questionnaires (eg, Child Eating Behavior Questionnaire [CEBQ] and the Dykens questionnaire). Metabolic explorations showed increased fasting insulinemia (20.4 μU/mL [141.7 pmol/L] [normal < 5 μU/mL]), elevated liver enzymes (aspartate aminotransferase/alanine aminotransferase 41/37 IU/L [normal < 35 IU/L]), and elevated triglycerides (3.8 mmol/L [normal range, 0.4-1.3]). Given the extreme severity of the disease and the life-threatening condition, we obtained an authorization from the French Health Authorities to use setmelanotide on a compassionate basis in this patient. He received daily treatment at the minimal dose of 0.5 mg through subcutaneous injections from October 2021 until now (12 months of setmelanotide therapy). During the entire period of treatment, comprehensive multidisciplinary management such as intensive control of food intake and physical activity were continued as additional specialized approaches as speech therapy and psychomotor skill training, which have been started early in childhood.
Outcome and Follow-up
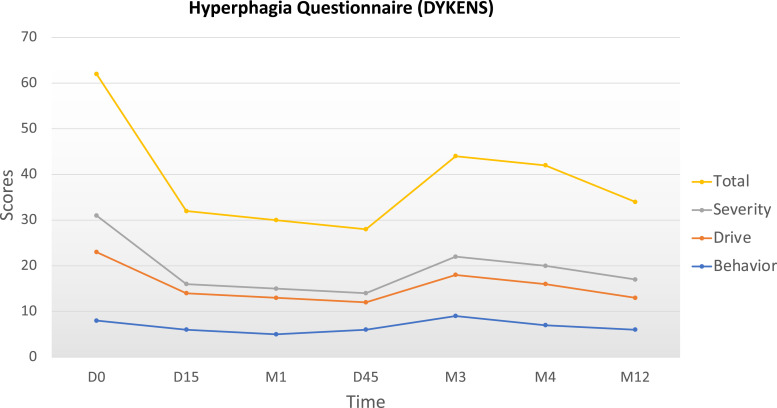
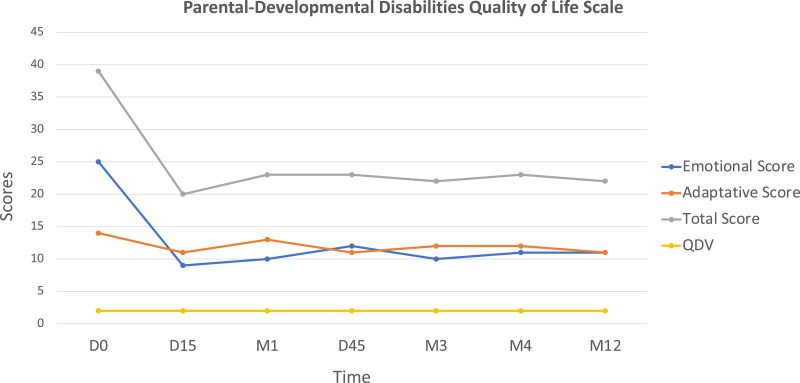
Setmelanotide administration led to rapid weight loss (−4.3 kg) with decreased hyperphagia after the first 15 days of daily injection (Fig. 2). After 4 months of treatment, the child had lost 16.5 kg (−20% from baseline). After 12 months, he had lost a total of 30 kg (−36% weight loss from baseline), resulting in a weight of 52 kg with BMI at 25.4 kg/m2 and BMI Z score + 6 SD (−12 SD after 1 year on setmelanotide) at the age of 6 years and 10 months (Fig. 1). The parents reported unprecedented improvement of the child behavior due to much lower preoccupation for food, substantiated by the assessment of hyperphagia using the validated CEBQ and the Dykens questionnaire. After 15 days, the Dykens hyperphagia score dropped from 31 to 16 (−48%), and the CEBQ total score dropped from 104 to 85. The Dykens score improvement was mostly due to decreased scores in hyperphagic drive (15 to 8) and severity (8 to 2) (Fig. 2). The child showed a 20% reduction in enjoyment of food, emotional overeating, and food responsiveness. After 12 months of setmelanotide administration with 0.5 mg per day, these ameliorations remained stable. Setmelanotide administration also led to a rapid 30% improvement of lipid values, liver enzymes, and fasting insulin. No major adverse side effects were described except for transient erections reported once or twice per day only during the first month of treatment and for previously reported increased skin pigmentation that persisted with time [7]. Interestingly, we also assessed the impact of their child's disease on parents’ quality of life and emotional status using the parental-developmental disabilities quality of life scale [8]. During the setmelanotide treatment period, parents reported a positive impact with a rapid decrease in emotional score (−60% after 15 days that persisted after 12 months) (Fig. 3).
Figure 2.
Evolution of the Dykens questionnaire scores for hyperphagia on setmelanotide. The total score of hyperphagia and the drive score (evaluating the food obsession) rapidly dropped after 15 days on setmelanotide (−48% and −47% respectively) with a persistent effect after 12 months on the minimal dose of setmelanotide (0.5 mg per day). Abbreviations: D, day; M, month.
Figure 3.
Evolution of the score for quality of life of the parents during their child's treatment with setmelanotide. We used the parental-developmental disabilities quality of life scale since the child had neurodevelopmental delay. On setmelanotide, the total score rapidly decreased mainly due to the rapid decrease of the emotional score showing the simultaneous beneficial impact on relatives. Abbreviations: D, day; M, month.
Discussion
Phase 2 and 3 setmelanotide trials included mainly patients older than 12 years, with few children aged 6 to 12. For the approved monogenic obesity indications, only 4 POMC-, 1 PCSK1-, and 1 LEPR-deficient patients of age between 6 and 12 have been treated in clinical trials [4]. For the approved syndromic obesity indication, 8 patients with Bardet-Biedl syndrome aged 6 to 12 were included in the pivotal trial. Until now, no data was available on the tolerance, efficacy, and impact on caregivers of setmelanotide in children under 6 years old with genetically impaired melanocortin pathway. This case report of a 5-year-old boy with POMC deficiency describes dramatic and sustainable (12 months) amelioration of weight, food seeking, and general behavior following 0.5 mg/day setmelanotide administration. This observation shows that early use of setmelanotide in children with POMC deficiency is efficient and safe and supports early therapy. Moreover, the child's rapid response to treatment was associated with a major positive impact for his parents’ quality of life. This effect on relatives helps reduce the major burden on caregivers secondary to uncontrolled hyperphagia and to severe early-onset obesity gain [9, 10], reducing the associated stigma and improving their quality of life.
In conclusion, our observation paves the way for the administration of this new MC4R agonist, setmelanotide, in patients below 6 years of age diagnosed with genetic variants of the melanocortin pathway. Further studies in higher numbers of patients are warranted.
Learning Points
Early-onset severe obesity without adiposity rebound associated to additional phenotypes as unusual hyperphagia, neurodevelopmental, and/or endocrine disorders requires genetic testing.
In case of POMC deficiency diagnosed in a child younger than 6 years, treatment with setmelanotide can be proposed and is well tolerated.
The burden on caregivers associated with childhood early-onset obesity needs to be evaluated and improved in order to limit patient and family stigma.
Acknowledgments
We thank the French National Drug Safety Agency (ANSM) for providing authorization regarding the drug use in a compassion protocol and Rhythm Pharmaceuticals for giving access to setmelanotide.
Abbreviations
- BMI
body mass index
- CEBQ
Child Eating Behavior Questionnaire
- LEPR
leptin receptor
- PCSK1
proprotein convertase subtilisin/kexin type 1
- POMC
proopiomelanocortin
Contributor Information
Beatrice Dubern, Sorbonne Université, Trousseau Hospital, Assistance Publique-Hôpitaux de Paris, 75012 Paris, France; Sorbonne Université, Inserm, Nutrition and Obesities, Systemic Approaches Research Group, NutriOmics, 75013 Paris, France.
Alexandre Lourdelle, Pediatric Endocrinology, American Memorial Hospital, CHU Reims, 51100 Reims, France.
Karine Clément, Sorbonne Université, Inserm, Nutrition and Obesities, Systemic Approaches Research Group, NutriOmics, 75013 Paris, France; Nutrition Department, Assistance Publique-Hôpitaux de Paris, 75013 Paris, France.
Contributors
All authors made individual contributions to authorship. B.D. and A.L. were involved in care management of this patient and manuscript writing. K.C. was involved in the manuscript writing. All authors reviewed and approved the final draft.
Funding
No public or commercial funding
Disclosures
B.D. is a consultant for Novo Nordisk and primary investigator for Rhythm Pharmaceuticals; A.L. has none declared; K.C. is primary investigator for Rhythm Pharmaceuticals trials, consultant for Danone Research, and received a research grant from Confo Therapeutics.
Informed Patient Consent for Publication
Signed informed consent obtained directly from the patient's relatives or guardians.
Data Availability Statement
Original data generated and analyzed for this case report are included in this published article.
References
- 1.Dubern B, Mosbah H, Pigeyre M, Clément K, Poitou C. Rare genetic causes of obesity: diagnosis and management in clinical care. Ann Endocrinol. 2022;83(1):63‐72. [DOI] [PubMed] [Google Scholar]
- 2.Poitou C, Puder L, Dubern B, et al. . Long-term outcomes of bariatric surgery in patients with bi-allelic mutations in the POMC, LEPR, and MC4R genes. Surg Obes Relat Dis. 2021;17(8):1449‐1456. [DOI] [PubMed] [Google Scholar]
- 3.Kühnen P, Biebermann H, Wiegand S. Pharmacotherapy in childhood obesity. Horm Res Paediatr. 2022;95(2):177‐192. [DOI] [PubMed] [Google Scholar]
- 4.Clément K, van den Akker E, Argente J, et al. . Efficacy and safety of setmelanotide, an MC4R agonist, in individuals with severe obesity due to LEPR or POMC deficiency: single-arm, open-label, multicentre, phase 3 trials. Lancet Diabetes Endocrinol. 2020;8(12):960‐970. [DOI] [PubMed] [Google Scholar]
- 5.Kühnen P, Wabitsch M, von Schnurbein J, et al. . Quality of life outcomes in two phase 3 trials of setmelanotide in patients with obesity due to LEPR or POMC deficiency. Orphanet J Rare Dis. 2022;17(1):38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kühnen P, Clément K. Long-term MC4R agonist treatment in POMC-deficient patients. N Engl J Med. 2022;387(9):852‐854. [DOI] [PubMed] [Google Scholar]
- 7.Kanti V, Puder L, Jahnke I, et al. . A melanocortin-4 receptor agonist induces skin and hair pigmentation in patients with monogenic mutations in the leptin-melanocortin pathway. Skin Pharmacol Physiol. 2021;34(6):307‐316. [DOI] [PubMed] [Google Scholar]
- 8.Vernhet C, Michelon C, Dellapiazza F, et al. . Perceptions of parents of the impact of autism spectrum disorder on their quality of life and correlates: comparison between mothers and fathers. Qual Life Res. 2022;31(5):1499‐1508. [DOI] [PubMed] [Google Scholar]
- 9.Kleinendorst L, van Haelst MM, van den Akker ELT. Young girl with severe early-onset obesity and hyperphagia. BMJ Case Rep. 2017;2017:bcr-2017-221067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wabitsch M, Fehnel S, Mallya UG, et al. . Understanding the patient experience of hunger and improved quality of life with setmelanotide treatment in POMC and LEPR deficiencies. Adv Ther. 2022;39(4):1772‐1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Original data generated and analyzed for this case report are included in this published article.