@ERSpublications
Dysanapsis – an anthropometric mismatch between airway tree calibre and lung size that is common in the general population – is strongly associated with all-cause mortality and increases susceptibility to tobacco smoking-related diseases https://bit.ly/42oDe8J
To the Editor:
The human airway tree is a vital conduit for gas exchange and our first line of defence against noxious aerosols such as tobacco smoke: a leading cause of death worldwide [1].
Dysanapsis refers to an anthropometric mismatch of airway tree calibre that was first inferred from inter-individual differences in maximum expiratory airflow among healthy adults [2] and later confirmed by direct measurement using computed tomography (CT) in community-based samples [3]. Variation in airway tree calibre is evident by early adulthood [4], extends to the peripheral airways (a major site of tobacco smoke-associated pathobiology) [5] and is associated with greater aerosol deposition in computational models [6].
We tested the hypothesis that CT-assessed dysanapsis is associated with all-cause and cause-specific mortality from major diseases attributed to tobacco smoking.
The Multi-Ethnic Study of Atherosclerosis (MESA) is a multi-site community-based sample of 6814 non-Hispanic white, black, Hispanic and Chinese participants aged 45–84 years old in 2000–2002 [7]. Exclusion criteria included clinical cardiovascular disease and impediments to long-term follow-up. The MESA Lung and MESA Air substudies measured airway and lung dimensions on CT and outdoor residential air pollutant concentrations. Institutional review board approval was obtained, and participants provided written informed consent.
Airway and lung measurements were performed on baseline cardiac CT images acquired at full inspiration on cardiac-gated multidetector and electron-beam scanners according to standardised protocol [8].
The primary index of dysanapsis was calculated as the mean of airway lumen diameters (in cm) divided by the cube-root of total lung volume (in cm3) estimated from cardiac CT (airway-to-lung ratiocardiacCT) [9].
A secondary index of dysanapsis was the mean of airway lumen diameters in mm (airway tree calibre) without a lung volume term.
The primary outcome was time-to-death from any cause ascertained via the National Death Index of the US National Vital Statistics System, a centralised database of death records compiled from state vital statistics offices [10]. Cause-specific death from atherosclerotic cardiovascular disease (ASCVD), lung cancer and COPD were defined by the International Classification of Diseases code listed as the primary cause of death.
Time-at-risk was the interval between the day of baseline study assessment and day of death or, if no death, the last follow-up assessment, or 31 December 2018 if a follow-up assessment occurred on or after 1 January 2019.
Age, sex, race/ethnicity, health insurance status, educational attainment, and physician diagnosis of asthma were assessed by questionnaire items. Height and weight were measured following standardised protocols. Principal components of genetic ancestry were derived from genome-wide data from the Affymetrix 6.0 chip [11].
Cigarette, pipe and cigar smoking status (current/former/never) and cigarette pack-years, pipe-years and cigar-years were assessed by questionnaire items and confirmed by urine cotinine. Second-hand smoke exposure was assessed by self-report as living or working with someone who smokes regularly indoors and by the average number of hours of exposure per week.
Residential outdoor particulate matter with aerodynamic diameter <2.5 μm (PM2.5), oxides of nitrogen (NOx), and ozone (O3) concentrations were estimated over 1 year of the baseline enrolment period using validated spatiotemporal models based on government regulatory monitors and spatially dense supplemental data [12].
The percentage of emphysema-like lung was quantified as the percent lung volume below −950 Hounsfield units [13].
The associations of airway-to-lung ratiocardiacCT and airway tree calibre with mortality (all-cause and cause-specific) were assessed by fitting Poisson and proportional hazard regression models to estimate mortality rate differences and hazard ratios (SAS v9.4). Estimates were tabulated by quartile of airway-to-lung ratiocardiacCT.
Poisson regression models used log of person-time as the offset and adjusted for study entry age, age2, sex, height, body mass index (BMI), BMI2, race/ethnicity, principal components of genetic ancestry, study site, educational attainment, health insurance status, cigarette smoking status, pack-years, pack-years2, cigar smoking status, cigar-years, pipe smoking status, pipe-years, second-hand smoke exposure status and hours per week of exposure, hours per week of exposure2, asthma diagnosis, residential outdoor PM2.5, NOx and O3 concentrations, the spatial location and number of airways measured and CT voxel size. Proportional hazard models were adjusted for the same covariables. Interactions with cigarette smoking status, sex, age tertile, and race/ethnicity were assessed with product terms. Secondary analyses included 1) unadjusted models and 2) additional adjustment for percentage of emphysema-like lung (log-transformed). An exploratory analysis of participants surviving to and completing the first MESA exam with spirometry (July 2005) additionally adjusted for forced expiratory volume in 1 s (FEV1) to forced vital capacity (FVC) ratio, FEV1 and FVC.
Two-sided p-values<0.05 were considered statistically significant.
Among 6814 participants enrolled in MESA, 6678 (98.1%) were included. Excluded participants were generally similar to those included, except they were more likely to smoke and report lower educational attainment.
Included participants had a mean±SD age of 62±10 years, 52.9% were female, and race/ethnicity proportions were 38.5% non-Hispanic white, 27.6% non-Hispanic black, 21.9% Hispanic and 12.0% Chinese. The mean±SD airway-to-lung ratiocardiacCT was 0.024±0.007 and participants with smaller airway-to-lung ratiocardiacCT were more likely to report non-Hispanic white race/ethnicity, lower educational attainment, current smoking status, and physician diagnosis of asthma. There were 1635 deaths over 96 877 person-years, including 232 deaths from ASCVD, 128 from lung cancer and 59 from COPD. From smallest-to-largest quartile of airway-to-lung ratiocardiacCT, there were 515, 425, 372 and 323 deaths, respectively.
Smaller airway-to-lung ratiocardiacCT was associated with significantly higher 10-year all-cause mortality (adjusted death rate difference comparing lowest versus highest of airway-to-lung ratiocardiacCTquartile 60.1 excess deaths per 10 000 person-years (95% CI 43.5–76.7; p<0.0001); adjusted hazard ratio (aHR) 2.38 (95% CI 1.88–3.00; p<0.0001)) (figure 1a). This association was also evident among never-smoking participants (35.8 excess deaths per 10 000 person-years (95% CI 15.6–56.0); aHR 2.05 (95% CI 1.43–2.93; p<0.0001)) and among ever-smoking participants (94.3 excess deaths per 10 000 person-years (95% CI 67.2–121.4); aHR 2.97 (95% CI 2.15–4.09; p<0.0001)).
FIGURE 1.
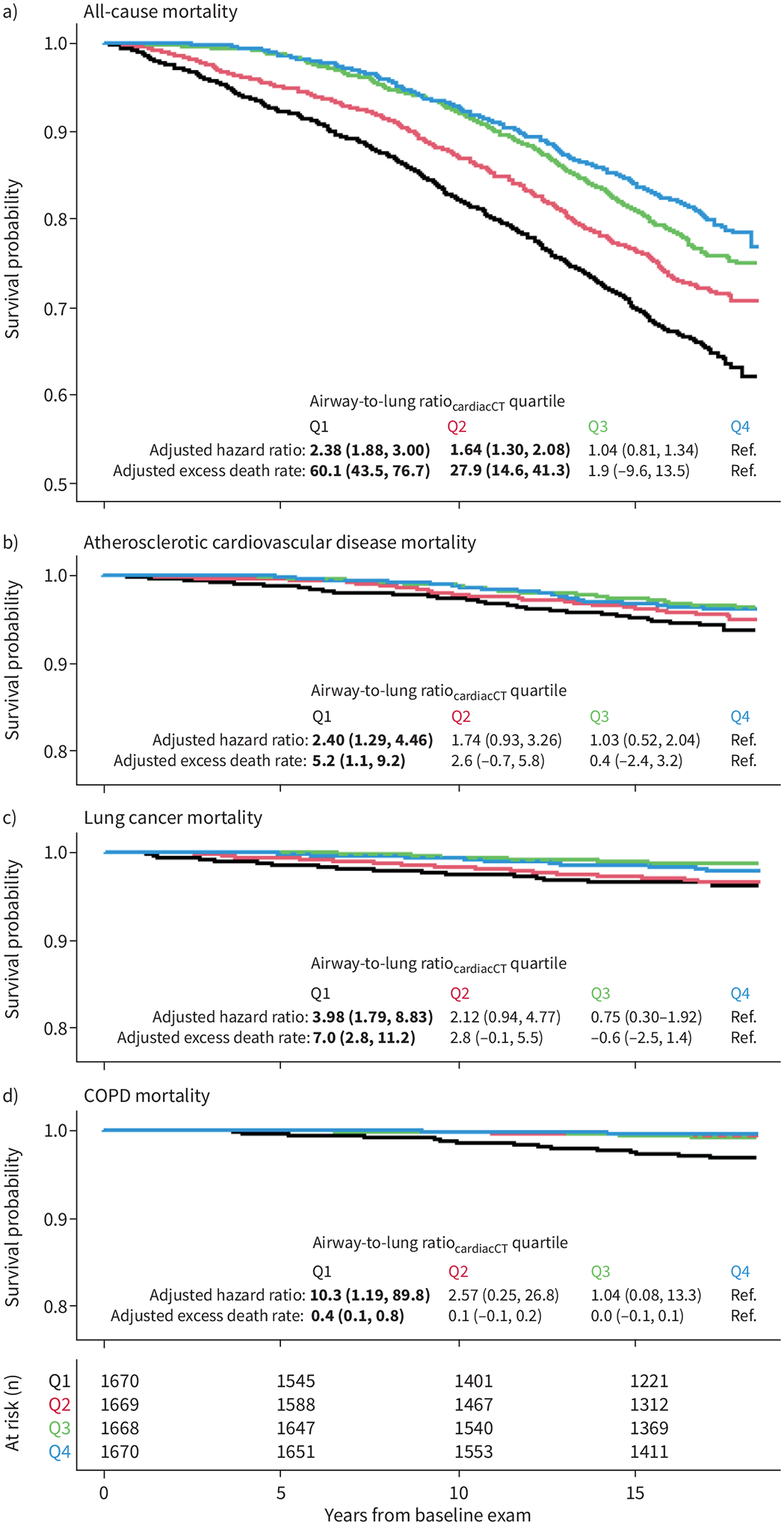
Association of computed tomography (CT)-assessed dysanapsis with mortality. Kaplan–Meier survival curves for a) all-cause mortality and b–d) cause-specific mortality by quartile of airway-to-lung ratiocardiacCT (calculated as the mean of airway lumen diameters (in cm) divided by the cube-root of total lung volume (in cm3) estimated from cardiac CT; black: quartile 1, smallest; red: quartile 2; green: quartile 3; blue: quartile 4, largest). Likelihood ratio test p<0.001 for each panel. The adjusted 10-year hazard ratios and excess death rates per 10 000 person-years were calculated using proportional hazard and Poisson regression models, respectively, adjusting for baseline age, age2, sex, height, body mass index (BMI), BMI2, race/ethnicity, principal components of genetic ancestry, health insurance status, educational attainment, study site, baseline cigarette smoking status, pack-years, pack-years2, cigar smoking status, cigar-years, pipe smoking status, pipe-years, environmental tobacco smoke exposure status, environmental tobacco smoke exposure (hours per week), environmental tobacco smoke exposure (hours per week)2, 1-year residential outdoor air pollutant concentrations (particulate matter with aerodynamic diameter <2.5 μm, oxides of nitrogen and ozone), physician diagnosis of asthma, spatial location of airways proportion sampled from core versus peel, left versus right, and upper versus mid versus lower lung zones and number of airways measured and voxel size. Cause-specific death was defined by the primary (underlying) International Classification of Diseases code listed as underlying cause of death (atherosclerotic cardiovascular disease: I21–25, I63, I65–66, I69.3, F01, I70–72, I74–75; lung cancer: C33–34; COPD: J41–44).
Smaller airway-to-lung ratiocardiacCT was associated with higher 10-year ASCVD mortality (aHR 2.40 (95% CI 1.29–4.46; p=0.006)), lung cancer mortality (aHR 3.98 (95% CI 1.79–8.83; p<0.001)) and COPD mortality (aHR 10.33 (95% CI 1.19–89.80; p=0.034)) (figure 1b–d).
All-cause and cause-specific mortality results were consistent when airway-to-lung ratiocardiacCT was replaced with airway tree calibre.
Models unadjusted and additionally adjusted for percentage of emphysema-like lung yielded consistent associations. The adjusted mean differences in mortality were similar over 18.5 years of follow-up whereas the hazard ratio decreased in magnitude with increasing follow-up duration but remained statistically significant.
There was statistical evidence that airway-to-lung ratiocardiacCT augmented cigarette smoking-associated all-cause mortality (p-interaction=0.028) over the 18.5-year follow-up interval, but this was not statistically significant over the 10-year follow-up interval (p-interaction=0.087). There was no evidence of interactions by sex (p-interaction=0.870), age tertile (p-interaction=0.090) or race/ethnicity (p-interaction=0.870).
Among 3791 participants surviving to and completing spirometry at MESA Exam 4 (743 subsequent deaths over 43 333 person-years), the aHR for all-cause mortality was 1.47 (p=0.057) when comparing smallest to largest airway tree calibre quartile. This association was partially attenuated with additional adjustment for FEV1/FVC (aHR 1.45; p=0.070), FEV1 (aHR 1.28; p=0.221) and FVC (aHR 1.34; p=0.157).
In a multi-ethnic community-based sample of older adults, quantitative assessment of dysanapsis was associated with all-cause mortality and cause-specific mortality from ASCVD, lung cancer and COPD. These associations were independent of demographics, body size and potential confounders including tobacco exposures, and were also evident within never-smoking and ever-smoking strata. These findings suggest that dysanapsis represents an important host factor associated with mortality. Moreover, whereas the original dysanapsis definition referred to mismatch between airway tree calibre and lung size (i.e. smaller or larger), the current findings provide evidence that smaller airway tree calibre relative to lung size in particular is associated with adverse health outcomes.
Studies of older adults with heavy tobacco smoke exposure and COPD have demonstrated associations between various measures of airway lumen calibre and mortality [14, 15] but few, to our knowledge, have examined this relationship in the general population or among non-smoking individuals [11]. The present study builds upon prior observations by showing that CT-assessed dysanapsis is associated with all-cause mortality among never- and ever-smoking persons, and with cause-specific mortality from ASCVD and lung cancer, in addition to COPD. These new findings suggest that the excess mortality associated with airway tree structure is not due exclusively to tobacco smoking-mediated airway remodelling, and may instead represent a developmental trait that increases susceptibility to death from both pulmonary and non-pulmonary conditions.
Mechanisms linking dysanapsis to mortality were not assessed in this study, but three hypotheses are considered in light of present and prior observations. First, dysanapsis may increase susceptibility to noxious aerosols (e.g. tobacco smoke, air pollutants) via altered lung deposition/clearance (i.e. higher dose delivered [6]) or via an intrinsically impaired capacity for airway homeostasis (e.g. an impaired epithelial response to injury). Consistent with this hypothesis, we note that CT-assessed dysanapsis was 1) associated with higher mortality rates for ASCVD, lung cancer and COPD, the top three causes of death attributed to tobacco smoke and air pollution [1]; and 2) appeared to augment cigarette smoking-associated all-cause mortality, though this only reached statistical significance in the 18.5-year follow-up and merits replication. Second, dysanapsis may reflect an aberrant early life morphogenic programme that impacts other organ systems, which in turn increase mortality through mechanisms unrelated to airway pathophysiology. In support of this hypothesis, we note that biological pathways governing airway tree morphogenesis overlap with other (branched) organ systems (e.g. renal, vascular) [16]. Finally, airway-to-lung ratiocardiacCT and airway tree calibre may simply represent a better biomarker of cumulative tobacco smoke exposure than self-report. We think such residual confounding is unlikely because mortality associations were independent of tobacco smoke exposures and CT-assessed emphysema, and were evident among non-smoking participants.
This study has limitations. First, airway lumens were quantified using cardiac-gated CT images, which may be less precise than full-lung CT. We note, however, that cardiac gating reduces cardiac-related motion artifacts and that reproducibility of the airway measures was excellent [8]. Second, airways were not sampled at standard anatomic locations, which may introduce selection bias in the presence of conditions associated with peripheral airway loss (e.g. smoking-related COPD, ageing). We note, however, that 1) the dysanapsis–mortality association was unchanged with adjustment for spatial location and number of airways sampled, 2) dysanapsis–mortality associations were evident within age strata and among never-smoking participants and, critically, 3) biased sampling of more central airways (i.e. larger lumen airways) among older participants or participants with smoking-related COPD, if present, would tend to underestimate the association between dysanapsis and mortality. Third, while several established and potential confounders were accounted for, unmeasured or imprecisely measured factors may have contributed to residual confounding. Fourth, the exploratory analysis adjusting for spirometry acquired at a later examination was likely underpowered. Nevertheless, we hypothesise that the dysanapsis–mortality association is at least partly mediated by dysanapsis-associated lung function impairment [3] and an adequately powered mediation analysis is warranted. Fifth, cause-specific mortality defined by administrative coding may be misclassified. We note, however, that this outcome was secondary to all-cause mortality.
In a multi-ethnic community-based sample of older adults, lower airway-to-lung ratiocardiacCT was associated with higher all-cause mortality and cause-specific mortality from ASCVD, lung cancer and COPD. These observations suggest that dysanapsis is associated with increased risk of premature death from both pulmonary and non-pulmonary conditions.
Acknowledgement:
Thanks to James A. Hanley for his statistical guidance at the study design stage and his constructive feedback on the manuscript. The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Conflict of interest:
C. Sack reports grants from NIEHS K23ES030725-04, outside the submitted work. E.A. Hoffman reports support for the present manuscript from NIH; E.A. Hoffman also reports that he is founder and shareholder of VIDA Diagnostics, and an unpaid member of Photon Counting CT advisory board for Siemens Healthineers, outside the submitted work. N.B. Allen reports support for the present manuscript from NIH/NHLBI; N.B. Allen also reports grants from NIH/NHLBI, outside the submitted work. J. Guo reports support for the present manuscript from National Institutes of Health; J. Guo also reports being a shareholder of VIDA Diagnostics, outside the submitted work. E.D. Michos reports advisory board participation with AstraZeneca, Bayer, Boehringer Ingelheim, Esperion, Novartis, Novo Nordisk and Pfizer, outside the submitted work. S.J. Shea reports support for the present manuscript from the National Heart, Lung, and Blood Institute. R.G. Barr reports support for the present manuscript from NIH, COPD Foundation and Foundation for the NIH; R.G. Barr also reports grants from American Lung Association, and advisory board participation with COPD Foundation, outside the submitted work. B.M. Smith reports support for the present manuscript from NIH, Canadian Lung Association, CIHR and Quebec Health Research Fund. All other authors have no potential conflicts of interest to disclose.
Support statement:
This research was supported by R01-HL130506, R01-HL077612, R01-093081, R01-HL155816, R01-HL121270, K23ES030725, K25ES034064, contracts 75N92020D00001, HHSN268201500003I, N01-HC-95159, 75N92020D00005, N01-HC-95160, 75N92020D00002, N01-HC-95161, 75N92020D00003, N01-HC-95162, 75N92020D00006, N01-HC-95163, 75N92020D00004, N01-HC-95164, 75N92020D00007, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute, by grants UL1-TR-000040, UL1-TR-001079 and UL1-TR-001420 from the National Center for Advancing Translational Sciences (NCATS) and by the American Lung Association. M. Vameghestahbanati was supported by Vanier Canada Graduate Scholarship. The information contained herein was derived in part from data provided by the Bureau of Vital Statistics, New York City Department of Health, and Mental Hygiene. This publication was developed under STAR research assistance agreements, numbers RD831697 (MESA Air) and RD-83830001 (MESA Air Next Stage), awarded by the US Environmental Protection Agency. It has not been formally reviewed by the EPA. The views expressed in this document are solely those of the authors and the EPA does not endorse any products or commercial services mentioned in this publication. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.GBD 2019 Risk Factors Collaborators. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020; 396: 1223–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Green M, Mead J, Turner JM. Variability of maximum expiratory flow-volume curves. J Appl Physiol 1974; 37: 67–74. [DOI] [PubMed] [Google Scholar]
- 3.Smith BM, Kirby M, Hoffman EA, et al. Association of dysanapsis with chronic obstructive pulmonary disease among older adults. JAMA 2020; 323: 2268–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vameghestahbanati M, Hiura GT, Barr RG, et al. CT-assessed dysanapsis and airflow obstruction in early and mid adulthood. Chest 2022; 161: 389–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vameghestahbanati M, Kirby M, Tanabe N, et al. Central airway tree dysanapsis extends to the peripheral airways. Am J Respir Crit Care Med 2021; 203: 378–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang X, Li F, Rajaraman PK, et al. A computed tomography imaging-based subject-specific whole-lung deposition model. Eur J Pharm Sci 2022; 177: 106272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bild DE, Bluemke DA, Burke GL, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol 2002; 156: 871–881. [DOI] [PubMed] [Google Scholar]
- 8.Donohue KM, Hoffman EA, Baumhauer H, et al. Cigarette smoking and airway wall thickness on CT scan in a multi-ethnic cohort: the MESA lung study. Respir Med 2012; 106: 1655–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wysoczanski A, Angelini ED, Sun Y, et al. Multi-view CNN for total lung volume inference on cardiac computed tomography. IEEE International Symposium on Biomedical Imaging 2023; 2023: 04061834. [Google Scholar]
- 10.National Center for Health Statistics. National Death Index. www.cdc.gov/nchs/ndi/about.htm
- 11.Oelsner EC, Smith BM, Hoffman EA, et al. Prognostic significance of large airway dimensions on computed tomography in the general population. The multi-ethnic study of atherosclerosis (MESA) lung study. Ann Am Thorac Soc 2018; 15: 718–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keller JP, Olives C, Kim SY, et al. A unified spatiotemporal modeling approach for predicting concentrations of multiple air pollutants in the multi-ethnic study of atherosclerosis and air pollution. Environ Health Perspect 2015; 123: 301–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang M, Aaron CP, Madrigano J, et al. Association between long-term exposure to ambient air pollution and change in quantitatively assessed emphysema and lung function. JAMA 2019; 322: 546–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bodduluri S, Kizhakke Puliyakote A, Nakhmani A, et al. Computed tomography-based airway surface area-to-volume ratio for phenotyping airway remodeling in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2021; 203: 185–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhatt SP, Terry NL, Nath H, et al. Association between expiratory central airway collapse and respiratory outcomes among smokers. JAMA 2016; 315: 498–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Affolter M, Zeller R, Caussinus E. Tissue remodelling through branching morphogenesis. Nat Rev Mol Cell Biol 2009; 10: 831–842. [DOI] [PubMed] [Google Scholar]


