Abstract
Background:
Evobrutinib is an oral, central nervous system (CNS)-penetrant and highly selective covalent Bruton’s tyrosine kinase inhibitor under clinical development for patients with relapsing multiple sclerosis (RMS).
Objective:
To investigate the effect of evobrutinib on immune responses in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccinated patients with RMS from a Phase II trial (NCT02975349).
Methods:
A post hoc analysis of patients with RMS who received evobrutinib 75 mg twice daily and SARS-CoV-2 vaccines during the open-label extension (n = 45) was conducted. Immunoglobulin (Ig)G anti-S1/S2-specific SARS-CoV-2 antibodies were measured using an indirect chemiluminescence immunoassay.
Results:
In the vaccinated subgroup, mean/minimum evobrutinib exposure pre-vaccination was 105.2/88.7 weeks. In total, 43 of 45 patients developed/increased S1/S2 IgG antibody levels post-vaccination; one patient’s antibody response remained negative post-vaccination and the other had antibody levels above the upper limit of detection, both pre- and post-vaccination. Most patients (n = 36/45), regardless of pre-vaccination serostatus, had a 10–100-fold increase of antibody levels pre- to post-vaccination. Antibody levels post-booster were higher versus post-vaccination.
Conclusion:
These results suggest evobrutinib, an investigational drug with therapeutic potential for patients with RMS, acts as an immunomodulator, that is, it inhibits aberrant immune cell responses in patients with RMS, while responsiveness to foreign de novo and recall antigens is maintained.
Keywords: Evobrutinib, Bruton’s tyrosine kinase, COVID-19, SARS-CoV-2, vaccines, multiple sclerosis
Introduction
Multiple sclerosis (MS) is an inflammatory, progressive neurodegenerative immune-mediated disease of the central nervous system (CNS). 1 Despite the effectiveness of currently available MS treatments at reducing relapses,2–6 there is an ongoing unmet need for treatments that can effectively target immune cells without sustained depletion and/or immunosuppression. Some disease-modifying therapies (DMTs), including sphingosine-1-phosphate receptor (S1PR) modulators and anti-CD20 monoclonal antibodies, are associated with immunosuppression, including an increased risk of infections,7–10 attenuated vaccine responses to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)11–14 and increased severity of coronavirus disease 2019 (COVID-19). 15 Therefore, immunomodulators, a class of therapeutic agents that modulates dysregulated immune systems back to a more tolerogenic state and improve the ability to distinguish self from foreign antigens, have long been pursued.
Bruton’s tyrosine kinase (BTK), one of the Tec family of non-receptor tyrosine kinases, is expressed in B cells, and innate cells such as macrophages and microglia. 16 BTK propagates signals from multiple receptors including B-cell, Fc, toll-like and chemokine receptors. 16 BTK contains a kinase domain plus four additional domains that together contribute to multiple functions critical for intracellular signalling. 17 Mouse models indicate that autoreactive B cells are more dependent on BTK signalling than normal B cells, 18 and higher BTK expression levels lower the threshold for activation of hyperresponsive B cells. 19 These data indicate BTK activity acts to modulate immune function rather than as a simple ‘on/off’ switch of immune cell function.
In patients with MS, elevated levels of phospho-BTK have been detected in peripheral blood B-cell subsets and in microglia in CNS lesions, suggesting that activated BTK signalling plays a role in MS-relevant inflammatory immune responses.20,21 Therefore, modulation of BTK signalling in the periphery and CNS may reduce both peripherally- driven and CNS-compartmentalised inflammation associated with relapses, brain tissue loss and disability progression, while still maintaining normally protective immune system responsiveness to foreign antigens.
Evobrutinib is an oral, CNS-penetrant, highly selective covalent BTK inhibitor.22,23 Evobrutinib can decrease the activation, migration, proliferation and cytokine release of B cells, inhibit proinflammatory microglia/macrophage differentiation and change the polarisation of microglia/macrophages to a neuroprotective phenotype.21,24–26 In a Phase II trial (NCT02975349) in patients with relapsing MS (RMS), evobrutinib 75 mg once daily (QD) and twice daily (BID) showed treatment benefits on T1 gadolinium-positive lesions versus placebo (week 24; presumably via an effect on peripheral B cells)27,28 and on slowly expanding lesions (suggesting an effect on chronic active lesions, possibly through action on CNS-derived microglia). 29 The reduction in T1 gadolinium-positive lesions, an indicator of relapse biology, was consistent with the numerical reductions in annualised relapse rate (ARR) seen with evobrutinib 75 mg QD and BID at week 48 versus placebo/evobrutinib 25 mg QD. Currently, the impact of evobrutinib on vaccine responses in patients with RMS has not been investigated. However, a preliminary, post hoc analysis of a Phase II trial (NCT02975336) has indicated that evobrutinib-treated patients with systemic lupus erythematosus (75 mg QD and 50 mg BID versus placebo) can mount a recall humoral response to seasonal influenza vaccination. 30
To better understand how evobrutinib may be modulating immune cell function, the effect on de novo and recall immune responses in SARS-CoV-2 vaccinated patients with RMS was investigated.
Patients and methods
Trial design
The analyses described here included data from the Phase II trial of evobrutinib in RMS (NCT02975349; https://clinicaltrials.gov/ct2/show/NCT02975349), which has been described previously. 27
Briefly, the double-blind, randomised controlled trial with a parallel, open-label, dimethyl fumarate (DMF) reference group in patients with RMS, comprised a 48-week double-blind period (DBP) followed by an open-label extension (OLE; see Supplemental eFigure 1). Patients were randomly assigned 1:1:1:1:1 to oral evobrutinib (25 mg QD, 75 mg QD, or 75 mg BID); placebo (switched to evobrutinib 25 mg QD after week 24), or open-label DMF (120 mg BID for 7 days, then 240 mg BID thereafter). 27 After 48 weeks, all patients were eligible to enter the OLE and were switched to evobrutinib 75 mg QD. Following a protocol amendment, all patients in the OLE switched to evobrutinib 75 mg BID after a mean (±SD) of 49.8 (±6.17) weeks. This amendment was informed by an additional review of the DBP data. An added benefit of the BID versus QD dose across several endpoints (MRI, ARR and additional exposure–response analyses) 31 was observed during the review of the efficacy and safety data from the primary analysis at 24 weeks and the blinded extension analysis at 48 weeks.
The trial was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonisation Good Clinical Practice Guidelines. Written informed consent was obtained from each patient before any trial-related activities were performed.
Vaccinated subgroup
A post hoc analysis was performed on samples identified retrospectively in a subgroup of patients who were vaccinated during the OLE. This vaccinated subgroup included those patients who had received both evobrutinib 75 mg BID and a complete SARS-CoV-2 vaccination cycle (messenger RNA (mRNA) or non-mRNA vaccines) with or without boosters (mRNA) during the OLE. A complete vaccination cycle was defined as per the specific dosing recommendations for each vaccine (i.e., two doses except for the Janssen (Johnson & Johnson) vaccine that only required one). The pre-dose plasma sample was defined as the latest available sample prior to the first vaccine dose. As a result of the OLE visit schedule, the post-dose and post-booster samples were taken from the first visit ⩾ 28 days and ⩾ 7 days after the last vaccine/booster dose, respectively.
In addition to assessing the SARS-CoV-2 vaccination response, safety was assessed by the nature, severity and occurrence of treatment-emergent adverse events (TEAEs).
SARS-CoV-2 anti-Spike 1 and 2 antibody assay
To investigate the SARS-CoV-2 vaccination response, the DiaSorin LIAISON SARS-CoV-2 antibody test kit, a chemiluminescence immunoassay, was used to quantitatively assess the combined levels of SARS-CoV-2 anti-Spike 1 (S1) and S2 antibodies (details are described in the Supplemental Material).
The lower limit of quantification was 3.8 arbitrary units (AU)/mL. Seronegativity was defined as < 15.0 AU/mL and seropositivity as ⩾ 15.0 AU/mL. 32 The clinical reporting range for the assay was 3.8–2900.0 AU/mL.
Results
Patient population
A total of 267 patients were randomised into the Phase II trial. Of the 213 patients who then entered the OLE, 160 (75.1%) completed ⩾ 192 weeks of treatment. Of these, 45 patients who received both evobrutinib 75 mg BID and a complete SARS-CoV-2 vaccination cycle during the OLE were included in this post hoc vaccinated subgroup analysis. The baseline characteristics of the full patient cohort and the vaccinated subgroup are summarised in Table 1. Prior to any SARS-CoV-2 vaccination, 6 out of 45 patients reported a COVID-19 infection-related TEAE. These patients were seropositive (⩾ 15.0 AU/mL) at the pre-vaccination sample.
Table 1.
Baseline characteristics.
| Vaccinated subgroup (n = 45) a |
Treatment received during the DBP (full trial cohort) | |||||
|---|---|---|---|---|---|---|
| Placebo + evobrutinib 25 mg QD n = 39 (100%) |
Evobrutinib 25 mg QD n = 39 (100%) |
Evobrutinib 75 mg QD n = 42 (100%) |
Evobrutinib 75 mg BID n = 44 (100%) |
DMF 240 mg BID n = 49 (100%) |
||
| Female, n (%) | 31 (68.9) | 28 (71.8) | 22 (56.4) | 29 (69.0) | 30 (68.2) | 36 (73.5) |
| Age, years (mean (± SD)) | 46.0 (±9.6) | 42.0 (±11.1) | 42.7 (±9.6) | 43.5 (±9.9) | 44.1 (±11.3) | 42.4 (±11.7) |
| Time since MS onset, years (median (min; max)) | – | 7.5 (0.1;39.4) | 8.4 (1.6;26.4) | 13.2 (0.4;23.3) | 11.2 (0.2;39.4) | 7.3 (3.9;14.5) |
| Type of vaccine, n (%) | ||||||
| mRNA | 37 (82.2) | – | – | – | – | – |
| Non-mRNA | 8 (17.8) | – | – | – | – | – |
| Received booster, n (%) | 14 (31.1) | – | – | – | – | – |
| Serostatus, n (%) | ||||||
| Seropositive | 13 (28.9) | – | – | – | – | – |
| Seronegative | 32 (71.1) | – | – | – | – | – |
Evobrutinib 75 mg BID exposure pre-dose: mean (±SD): 105.2 (±7.9) weeks; median (min;max): 104.4 (88.7;132.4) weeks.
BID: twice daily; DMF: dimethyl fumarate; DBP: double-blind period; mRNA: messenger ribonucleic acid; MS: multiple sclerosis; QD: once daily; SD: standard deviation; %: proportion of patients.
In the vaccinated subgroup (n = 45), most patients received an mRNA SARS-CoV-2 vaccine (n = 37, 82.2%), with eight patients (17.8%) receiving a non-mRNA vaccine. Fourteen patients (31.1%) also received an mRNA booster. The mean (±SD) evobrutinib 75 mg BID exposure time during the OLE pre-vaccination was 105.2 (±7.9) weeks (minimum 88.7 weeks). Prior to vaccination, 32 patients (71.1%) were seronegative (< 15.0 AU/mL) and 13 patients (28.9%) were seropositive (⩾ 15.0 AU/mL). The mean (±SD) time between the last vaccine dose and post-dose sample was 10.3 (±3.4) weeks (median (min;max) 10.6 (4.9;19.1) weeks). The mean (±SD) time between the booster dose and post-booster sample was 6.4 (±3.6) weeks (median (min;max) 7.6 (1.6;12.3) weeks).
Immune response to SARS-CoV-2 vaccines
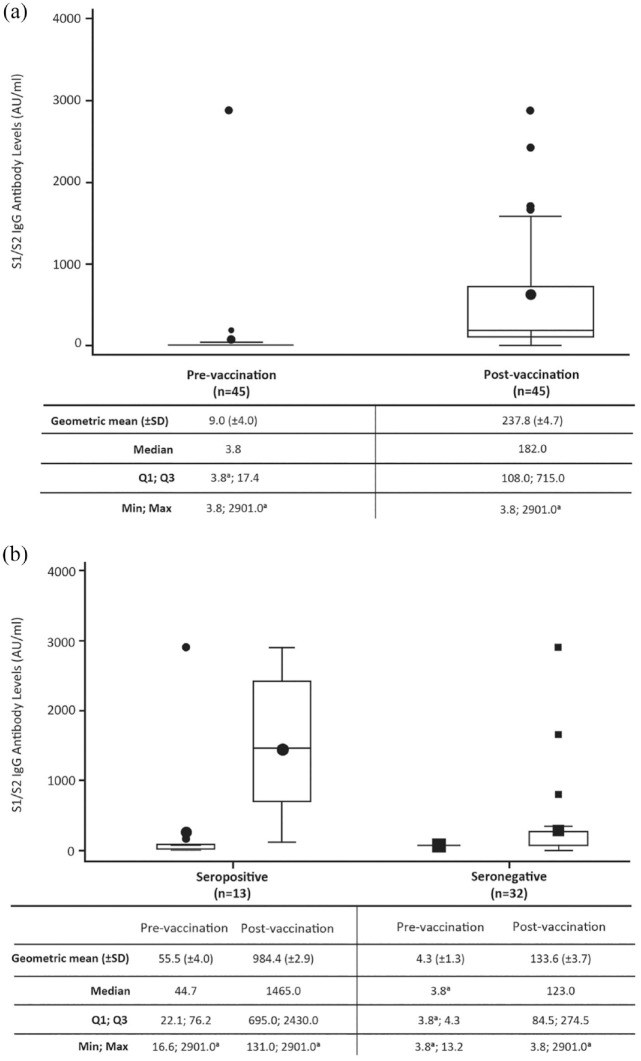
Of the 45 evobrutinib-treated patients, 43 developed/increased S1/S2 IgG antibody levels post-vaccination. Overall, geometric mean (±SD) S1/S2 IgG antibody levels were 9.0 (±4.0) AU/mL (median 3.8) before the first vaccination and increased to 237.8 (±4.7) AU/mL (median 182.0) after a complete vaccination cycle (see Figure 1(a)). S1/S2 IgG antibodies post-vaccination were higher in patients who were seropositive pre-vaccination (n = 13; geometric mean (±SD) 984.4 (±2.9) AU/mL) versus those who were seronegative (n = 32; 133.6 (±3.7) AU/mL); see Figure 1(b)). Two patients did not have detectable changes in S1/S2 IgG antibody levels after vaccination. One patient was seronegative both pre- and post-vaccination. The other patient had antibody levels > 2900 AU/mL (the upper limit of detection for the assay) both pre- and post-vaccination with no reported COVID-19 infection.
Figure 1.
(a) Pre- and post-vaccination S1/S2 IgG antibody levels and (b) by serostatus.
Patients were treated with evobrutinib 75 mg BID.
a2900 AU/mL is the upper limit of reporting of the assay and 3.8 AU/mL is the lower limit of reporting of the assay.
AU: arbitrary unit; BID: twice daily; IgG: immunoglobulin G; SD: standard deviation.
The majority of patients (n = 36/45) had a 10–100-fold induction of S1/S2 IgG antibody levels from pre-vaccination to post-vaccination; the geometric mean (±SD) fold change of S1/S2 IgG antibody levels for all patients was 26.4 (±3.4) AU/mL. The fold changes in the pre-vaccination seropositive patients were lower versus the pre-vaccination seronegative patients since the IgG antibody levels were already increased pre-vaccination in seropositive patients (see Table 2).
Table 2.
S1/S2 IgG antibody levels from pre-vaccination to post-vaccination.
| Seropositive at pre-vaccination n = 13 (100%) |
Seronegative at pre-vaccination n = 32 (100%) |
All patients n = 45 (100%) |
|
|---|---|---|---|
| Pre-vaccination, AU/mL | |||
| Geometric mean (±SD) | 55.5 (±4.0) | 4.3 (±1.3) | 9.0 (±4.0) |
| Median | 44.7 | 3.8 | 3.8 |
| Post-vaccination, AU/mL | |||
| Geometric mean (±SD) | 984.4 (±2.9) | 133.6 (±3.7) | 237.8 (±4.7) |
| Median | 1465.0 | 123.0 | 182.0 |
| Fold changes to post-vaccination a , n (%) | |||
| ⩽ 1X | 1 (7.7) | 1 (3.1) | 2 (4.4) |
| > 1–10X | 3 (23.1) | 2 (6.3) | 5 (11.1) |
| > 10–30X | 4 (30.8) | 13 (40.6) | 17 (37.8) |
| > 30–60X | 3 (23.1) | 8 (25.0) | 11 (24.4) |
| > 60–100X | 2 (15.4) | 6 (18.8) | 8 (17.8) |
| > 100X | 0 | 2 (6.3) | 2 (4.4) |
| Geometric mean fold change (±SD) | 17.7 (±3.33) | 31.0 (±3.40) | 26.4 (±3.43) |
| 95% CI | [8.57; 36.70] | [19.91; 48.15] | [18.20; 38.16] |
| Median fold change | 18.3 | 30.3 | 28.4 |
| Q1; Q3 | 8.3; 34.0 | 21.7; 56.2 | 18.3; 49.3 |
| Min; Max | 1.0; 90.5 | 1.0; 557.9 | 1.0; 557.9 |
Patients were treated with evobrutinib 75 mg BID.
Fold changes are the ratio between the post-dose and pre-dose IgG antibody levels.
AU: arbitrary unit; BID: twice daily; CI: confidence interval; IgG: immunoglobulin G; SD: standard deviation; %: proportion of patients.
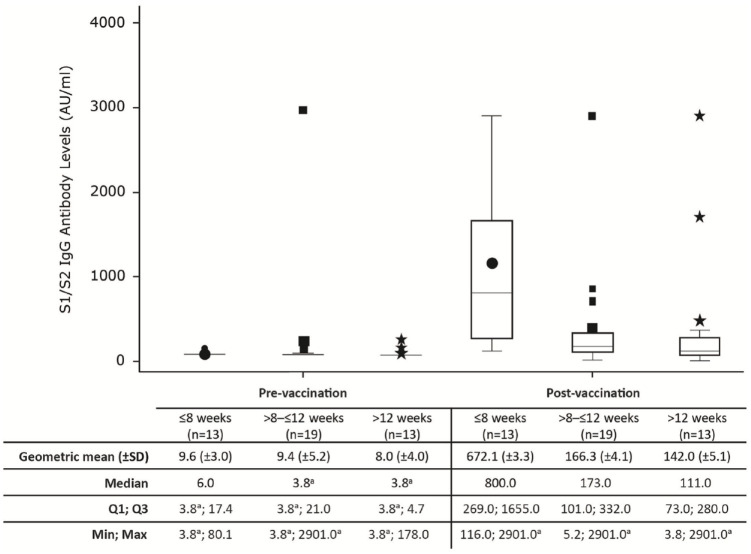
As this was a post hoc analysis, the time between the last vaccine dose to the post-dose antibody assessment ranged from 4.9 to 19.1weeks. For the majority of patients, the time between the last vaccine dose and antibody assessment was > 8 weeks (n = 32/45, 71.1%). With increasing time between the last vaccine dose and antibody assessment, a lower S1/S2 IgG antibody level was observed (geometric mean (±SD) ⩽ 8 weeks: 672.1 (±3.3); >8–⩽12 weeks: 166.3 (±4.1); >12 weeks: 142.0 (±5.1) see Figure 2). It should be noted that prior to vaccination, the antibody levels were similar across these time period categories (⩽ 8 weeks: 9.6 (±3.0); > 8–⩽ 12 weeks: 9.4 (±5.2); > 12 weeks: 8.0 (±4.0)).
Figure 2.
Persistence of S1/S2 IgG antibody levels over time.
Patients were treated with evobrutinib 75 mg BID.
a2900 AU/mL is the upper limit of reporting of the assay and 3.8 AU/mL is the lower limit of reporting of the assay.
AU: arbitrary unit; BID: twice daily; IgG: immunoglobulin G; SD: standard deviation.
Immune response to SARS-CoV-2 booster vaccines
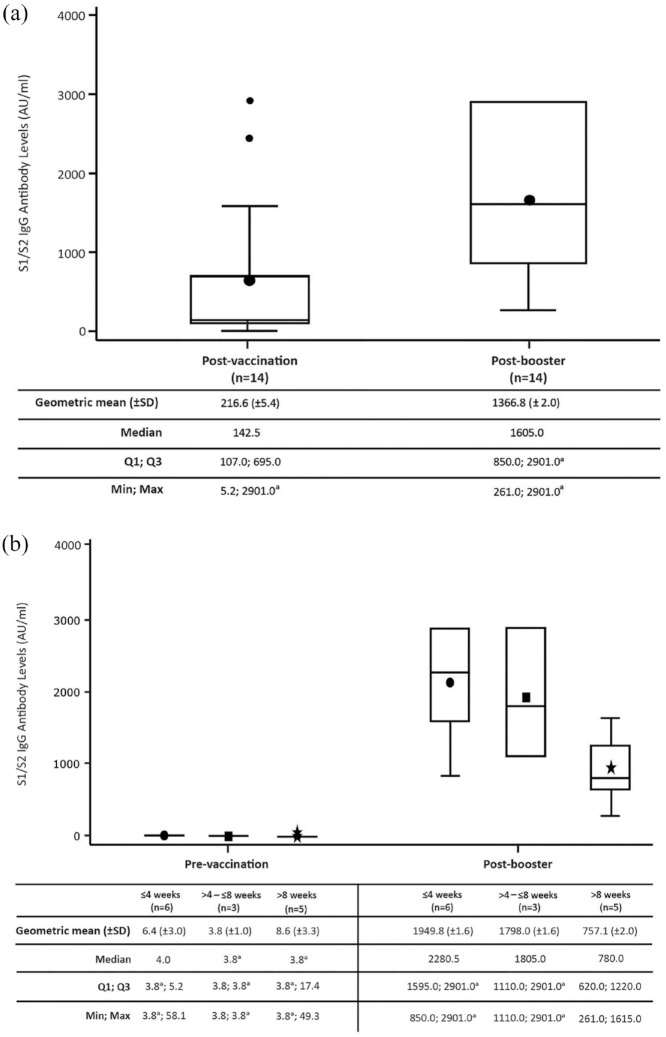
S1/S2 IgG antibody levels increased in 12 of the 14 evobrutinib-treated patients who received a booster when compared with levels generated following the initial vaccination cycle (post-booster: geometric mean (±SD) 1366.8 (±2.0) AU/mL; median 1605.0; post-vaccination: geometric mean (±SD) 216.6 (±5.4) AU/mL; median 142.5; see Figure 3(a)). S1/S2 IgG antibody levels were similar for seropositive (n = 3; geometric mean (±SD) 1464.8 (±1.2) AU/mL) and seronegative (n = 11; 1341.3 (±2.2) AU/mL) patients who had received a booster, albeit the small number of patients limits this comparison. Two patients did not have a substantial or detectable change in S1/S2 IgG antibody levels after the booster. One patient had S1/S2 IgG antibody levels of 1575.0 and 1615.0 AU/mL post-vaccination and post-booster, respectively. The other patient had levels ⩾ 2900 AU/mL (the upper limit of detection for the assay) at both time points.
Figure 3.
(a) Post-booster S1/S2 IgG antibody levels and (b) persistence of S1/S2 IgG antibody levels post-booster over time.
Patients were treated with evobrutinib 75 mg BID.
a2900 AU/mL is the upper limit of reporting of the assay and 3.8 AU/mL is the lower limit of reporting of the assay.
AU: arbitrary unit; BID: twice daily; IgG: immunoglobulin G; SD: standard deviation.
The time between the booster dose and the post-booster sample ranged from 1.6 to 12.3 weeks. For over half of patients who received a booster, the time between the booster vaccine dose and antibody assessment was > 4 weeks (n = 8/14, 57.1%). With increasing time between the booster dose and antibody assessment, a lower S1/S2 IgG antibody level was observed (geometric mean (±SD) ⩽ 4 weeks: 1949.8 (±1.6 AU/mL); > 4–⩽ 8 weeks: 1798.0 (±1.6) AU/mL; > 8 weeks: 757.1 (±2.0) AU/mL; see Figure 3(b)).
Overall, no substantial differences in S1/S2 IgG antibody levels were observed with the mRNA versus non-mRNA vaccines post-vaccination or post-booster (see Table 3). However, due to the small sample size, no strong conclusions can be made.
Table 3.
S1/S2 IgG antibody levels by mRNA and non-mRNA vaccines.
| Pre-vaccination | Post-vaccination | Post-booster | ||||
|---|---|---|---|---|---|---|
| mRNA (n = 37) |
non-mRNA (n = 8) |
mRNA (n = 37) |
non-mRNA (n = 8) |
mRNA (n = 12) |
non-mRNA (n = 2) |
|
| Geometric mean (±SD) | 8.5 (±4.3) | 12.1 (±3.2) | 247.7 (±4.5) | 197.1 (±6.2) | 1402.4 (±2.1) | 1171.6 (±1.6) |
| Median | 3.8 | 12.1 | 173.0 | 184.0 | 1627.5 | 1232.5 |
| Q1; Q3 | 3.8; 13.2 | 4.1; 31.1 | 108.0; 715.0 | 116.0; 972.0 | 945.0; 2901.0 | 850.0; 1615.0 |
| Min; Max | 3.8; 2901.0 | 3.8; 76.2 | 3.8; 2901.0 | 5.2; 1685.0 | 261.0; 2901.0 | 850.0; 1615.0 |
IgG: immunoglobulin G; mRNA: messenger ribonucleic acid; SD: standard deviation.
Safety in the vaccinated subgroup
Overall, during the vaccination period (time from the first vaccine dose until 42 days after the last dose) no new safety signals were identified, and patients had a similar local and systemic TEAE profile following vaccination as the general population (data not reported). Eight patients reported 17 vaccine-related TEAEs, which were all listed as adverse reactions specific to the vaccines (immunisation reaction, vaccination site pain, vaccination site bruising and vaccination complication (further reported as fatigue)). No clinically meaningful differences among the TEAEs reported after the first, second or booster vaccine doses were observed.
Discussion
Overall, our data indicate that almost all patients (96%) receiving evobrutinib treatment for at least 192 weeks were able to mount immune responses to SARS-CoV-2 vaccines that were in line with published reports of neutralising antibody levels in untreated patients with MS or healthy controls.13,33,34 In a Phase II trial involving patients with RMS, evobrutinib has been shown previously to lower MRI lesions, reflecting a decreased relapse biology.27,28 The results shown here suggest evobrutinib may act as an immune modulator, such that pathogenic CNS autoimmunity is inhibited, while allowing generation of immunoreactivity to foreign epitopes, including novel antigens.
Currently, most published data on the roles of BTK in normal immune cell function are derived from knockout mice 19 and the consequences of genetic mutations in humans. 35 Such data provide insights into BTK involvement in the earliest stages of immune system development, but do not clarify its roles in the mature immune system. In adult humans, knowledge is largely limited to studies of less selective BTK inhibitors used in oncology,36–38 and important gaps remain in our understanding of the impact of BTK inhibition on the mature immune system in non-neoplastic contexts such as autoimmune disease. Our results are the first to report the impact of long-term BTK inhibition on vaccine responses in adults with RMS.
Case series and registry studies have reported that anti-CD20-treated patients with MS are at higher risk of more severe COVID-19 disease compared with those taking other DMTs.15,39,40 There is evidence that MS DMTs that work through B-cell depletion (anti-CD20 monoclonal antibodies) or lymphocyte sequestration (S1PR modulators) adversely affect humoral responses to SARS-CoV-2 vaccination in people with MS.12–14 To date, most of the available data on BTK inhibitors and SARS-CoV-2 vaccine responses are derived from their use in chronic lymphocytic leukaemia (CLL). In patients with CLL, the BTK inhibitors ibrutinib and acalabrutinib are associated with a blunted immune response to recombinant zoster, hepatitis and SARS-CoV-2 vaccines.36–38,41–44 However, the BTK inhibitors used in these studies were less selective than evobrutinib.22,23 It is also important to note that significant immune dysfunction, resulting in an increased infection risk, is a key feature of CLL and reduced responses to vaccination among non-BTK inhibitor-treated patients have been observed. 45 Data herein support that humoral responses to SARS-CoV-2 vaccinations are not adversely affected by evobrutinib. In addition, the safety profile observed in this study was similar to the general population in terms of vaccine-related adverse events. Taken together with previous safety data from the OLE of the Phase II trial indicating that, for most evobrutinib-treated patients, CD19+ B-cell counts (96 weeks) and Ig levels (120 weeks) remained in the normal range, 28 these results are reassuring. There is currently no standard definition or assay for assessing the humoral response to SARS-CoV-2 vaccines and a wide range of assays have been used to investigate these responses in patients with MS, making direct comparisons between our data and the literature challenging. To date, we are aware of only one publication using the same assay as ours. 13 Brill et al. 13 evaluated serologic SARS-CoV-2 vaccine responses in untreated patients with MS and healthy controls and showed that the mean S1/S2 IgG antibody levels generated in these participants were comparable to our study (283 and 288 AU/mL, respectively). Prior work has shown that humoral responses wane over time in healthy volunteers after SARS-CoV-2 mRNA vaccination46,47 and the post-dose samples in Brill et al. 13 were evaluated 2–4 weeks after the last vaccine dose, as compared with 5–19 weeks after the last vaccination in our study. Indeed, we observed a waning of responses beyond the 8-week post-vaccination time point. Overall, our results appear in line with the published report of humoral responses generated using the same antibody assay in healthy persons and untreated MS patients.
Our study has limitations. The analysis of vaccine responses in samples obtained from the Phase II OLE trial was not pre-specified and relatively small numbers of patient samples over a range of time points post-vaccination were included. In addition, a comparator treatment group was not available. Information from a larger patient population and a comparator group is anticipated from the ongoing Phase III trials of evobrutinib. Since most patients in the current OLE received mRNA vaccinations, with a limited number receiving other types of SARS-CoV-2 vaccine types, we are not powered to analyse immune response by individual vaccine type. 12 Our study assessed only humoral and not cellular immunity and, finally, we used only IgG as the measure of vaccine response and while this is the standard approach, IgA and IgM which are also considered important in vaccine response measures, were not assessed here.42,48
Conclusion
The increases in antibody levels generated after COVID-19 immunisation in both seropositive and seronegative patients suggest that evobrutinib treatment does not substantially inhibit serologic responses to both de novo and recall antigens.
Supplemental Material
Supplemental material, sj-docx-1-msj-10.1177_13524585231192460 for Antibody response to SARS-CoV-2 vaccines in patients with relapsing multiple sclerosis treated with evobrutinib: A Bruton’s tyrosine kinase inhibitor by Amit Bar-Or, Anne H Cross, Anthony L Cunningham, Yann Hyvert, Andrea Seitzinger, Hans Gühring, Elise E Drouin, Nektaria Alexandri, Davorka Tomic and Xavier Montalban in Multiple Sclerosis Journal
Acknowledgments
The authors thank the patients and their families, as well as the investigators, coinvestigators and the trial teams at each of the participating centres; Merck Healthcare KGaA, Darmstadt, Germany was involved in the trial design, collection, analysis and interpretation of the data, and the development of this manuscript. Medical writing and editorial support were provided by Francesca Hemingway of Bioscript Group Ltd, Macclesfield, UK and supported by Merck Healthcare KGaA, Darmstadt, Germany (CrossRef Funder ID: 10.13039/100009945).
Footnotes
Data Availability Statement: Data are available upon reasonable request. Any requests for data by qualified scientific and medical researchers for legitimate research purposes will be subject to Merck Healthcare KGaA, Darmstadt, Germany’s Data Sharing Policy. All requests should be submitted in writing to Merck Healthcare KGaA, Darmstadt, Germany’s data sharing portal https://www.merckgroup.com/en/research/our-approach-to-research-and-development/healthcare/clinical-trials/commitment-responsible-data-sharing.html. When Merck Healthcare KGaA, Darmstadt, Germany has a co-research, co-development, or co-marketing or co-promotion agreement, or when the product has been out-licenced, the responsibility for disclosure might be dependent on the agreement between parties. Under these circumstances, Merck Healthcare KGaA, Darmstadt, Germany will endeavour to gain agreement to share data in response to requests.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: A Bar-Or holds the Melissa and Paul Anderson Chair. He has participated as a speaker in meetings sponsored by and received consulting fees from Accure, Atara Biotherapeutics, Biogen, BMS/Celgene/Receptos, GlaxoSmithKline, Gossamer, Janssen/Actelion, Medimmune, Merck Healthcare KGaA, Darmstadt, Germany, EMD Serono Research & Development Institute, Inc., Billerica, MA, USA, an affiliate of Merck KGaA, Novartis, Roche/Genentech and Sanofi-Genzyme. He has received grant support to the University of Pennsylvania from Biogen Idec, Merck Healthcare KGaA, Darmstadt, Germany, EMD Serono Research & Development Institute, Inc., Billerica, MA, USA, an affiliate of Merck KGaA, Novartis and Roche/Genentech.
AH Cross has received consulting fees, research support and honoraria from Biogen, Bristol-Myers Squibb, EMD Serono Research & Development Institute, Inc., Billerica, MA, USA, an affiliate of Merck KGaA, Merck Healthcare KGaA, Darmstadt, Germany, Genentech, Roche, Horizon, Janssen (subsidiary of Johnson & Johnson), Novartis, OCTAVE, TG Therapeutics, Academic CME, and WebMD; serves on the scientific advisory boards for ASCLEPIOS I/II for Novartis, and EVOLUTION I/II for EMD Serono Research & Development Institute, Inc., Billerica, MA, USA, an affiliate of Merck KGaA; has received grants from the United States Department of Defense; is President of the Board of Governors of the Consortium of Multiple Sclerosis Centers; and is a member of the advisory board of the International Progressive MS Alliance.
AL Cunningham has received consultant fees to his institution from GSK, Seqirus and EMD Serono Research & Development Institute, Inc., Billerica, MA, USA, an affiliate of Merck KGaA.
Y Hyvert, A Seitzinger, N Alexandri and H Gühring are employees of Merck Healthcare KGaA, Darmstadt, Germany.
EE Drouin is an employee of EMD Serono Research & Development Institute, Inc., Billerica, MA, USA, an affiliate of Merck KGaA
D Tomic is an employee of Ares Trading SA, Eysins, Switzerland, an affiliate of Merck KGaA, Darmstadt, Germany, and received stock or an ownership interest from Novartis.
X Montalban has received speaking honoraria and travel expenses for participation in scientific meetings, has been a steering committee member of clinical trials or participated in advisory boards of clinical trials in the past years with Abbvie, Actelion, Alexion, Bayer, Biogen, Bristol-Myers Squibb/Celgene, EMD Serono Research & Development Institute, Inc., Billerica, MA, USA, an affiliate of Merck KGaA, Darmstadt, Germany, Genzyme, F. Hoffmann-La Roche Ltd., Immunic, Janssen Pharmaceuticals, Medday, Merck KGaA, Darmstadt, Germany, Mylan, Nervgen, Novartis, Sandoz, Sanofi-Genzyme, Teva Pharmaceutical, TG Therapeutics, Excemed, MSIF and NMSS.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This trial was funded by Merck Healthcare KGaA, Darmstadt, Germany (CrossRef Funder ID: 10.13039/100009945).
ORCID iDs: Anne H Cross  https://orcid.org/0000-0003-0829-7569
https://orcid.org/0000-0003-0829-7569
Anthony L Cunningham  https://orcid.org/0000-0002-6744-5667
https://orcid.org/0000-0002-6744-5667
Supplemental Material: Supplemental material for this article is available online.
Contributor Information
Amit Bar-Or, Center for Neuroinflammation and Experimental Therapeutics, Department of Neurology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA.
Anne H Cross, Department of Neurology, Center for Neuroimmunology and Neuroinfectious Diseases, Washington University School of Medicine, St. Louis, MO, USA.
Anthony L Cunningham, Centre for Virus Research, The Westmead Institute for Medical Research, The University of Sydney, Westmead, NSW, Australia.
Yann Hyvert, Merck Healthcare KGaA, Darmstadt, Germany.
Andrea Seitzinger, Merck Healthcare KGaA, Darmstadt, Germany.
Hans Gühring, Merck Healthcare KGaA, Darmstadt, Germany.
Elise E Drouin, EMD Serono Research & Development Institute, Inc., Billerica, MA, USA, an affiliate of Merck KGaA.
Nektaria Alexandri, Merck Healthcare KGaA, Darmstadt, Germany.
Davorka Tomic, Ares Trading SA, Eysins, Switzerland, an affiliate of Merck KGaA.
Xavier Montalban, Department of Neurology-Neuroimmunology, Centre d’Esclerosi Múltiple de Catalunya (Cemcat), Hospital Universitari Vall d’Hebron, Barcelona, Spain.
References
- 1. Filippi M, Bar-Or A, Piehl F, et al. Multiple sclerosis. Nat Rev Dis Prim 2018; 4: 43. [DOI] [PubMed] [Google Scholar]
- 2. Ayzenberg I, Hoepner R, Kleiter I. Fingolimod for multiple sclerosis and emerging indications: Appropriate patient selection, safety precautions, and special considerations. Ther Clin Risk Manag 2016; 12: 261–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bar-Or A, Grove RA, Austin DJ, et al. Subcutaneous ofatumumab in patients with relapsing-remitting multiple sclerosis: The MIRROR study. Neurology 2018; 90: e1805–e1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Havrdova E, Cohen JA, Horakova D, et al. Understanding the positive benefit: risk profile of alemtuzumab in relapsing multiple sclerosis: Perspectives from the Alemtuzumab Clinical Development Program. Ther Clin Risk Manag 2017; 13: 1423–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mulero P, Midaglia L, Montalban X. Ocrelizumab: A new milestone in multiple sclerosis therapy. Ther Adv Neurol Disord 2018; 11: 8773025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Singer BA. The role of natalizumab in the treatment of multiple sclerosis: Benefits and risks. Ther Adv Neurol Disord 2017; 10: 327–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tallantyre EC, Whittam DH, Jolles S, et al. Secondary antibody deficiency: A complication of anti-CD20 therapy for neuroinflammation. J Neurol 2018; 265: 1115–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Derfuss T, Weber MS, Hughes R, et al. Serum immunoglobulin levels and risk of serious infections in the pivotal Phase III trials of ocrelizumab in multiple sclerosis and their open-label extensions. Multi Scler 2019; 25(Suppl. 2): 20–21. [Google Scholar]
- 9. Jalkh G, Abi Nahed R, Macaron G, et al. Safety of newer disease modifying therapies in multiple sclerosis. Vaccines 2020; 9: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Buonomo AR, Zappulo E, Viceconte G, et al. Risk of opportunistic infections in patients treated with alemtuzumab for multiple sclerosis. Exp Opin Drug Saf 2018; 17: 709–717. [DOI] [PubMed] [Google Scholar]
- 11. Capuano R, Bisecco A, Conte M, et al. Six-month humoral response to mRNA SARS-CoV-2 vaccination in patients with multiple sclerosis treated with ocrelizumab and fingolimod. Mult Scler Relat Disord 2022; 60: 103724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sormani MP, Inglese M, Schiavetti I, et al. Effect of SARS-CoV-2 mRNA vaccination in MS patients treated with disease modifying therapies. eBioMedicine 2021; 72: 103581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brill L, Rechtman A, Zveik O, et al. Humoral and T-cell response to SARS-CoV-2 vaccination in patients with multiple sclerosis treated with ocrelizumab. JAMA Neurol 2021; 78: 1510–1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pitzalis M, Idda ML, Lodde V, et al. Effect of different disease-modifying therapies on humoral response to BNT162b2 vaccine in sardinian multiple sclerosis patients. Front Immunol 2021; 12: 781843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Simpson-Yap S, De Brouwer E, Kalincik T, et al. Associations of disease-modifying therapies with COVID-19 severity in multiple sclerosis. Neurology 2021; 97: e1870–e1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Garg N, Padron EJ, Rammohan KW, et al. Bruton’s tyrosine kinase inhibitors: The next frontier of B-cell-targeted therapies for cancer, autoimmune disorders, and multiple sclerosis. J Clin Med 2022; 11: 6139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mohamed AJ, Yu L, Bäckesjö CM, et al. Bruton’s tyrosine kinase (Btk): Function, regulation, and transformation with special emphasis on the PH domain. Immunol Rev 2009; 228: 58–73. [DOI] [PubMed] [Google Scholar]
- 18. Kil LP, de Bruijn MJ, van Nimwegen M, et al. Btk levels set the threshold for B-cell activation and negative selection of autoreactive B cells in mice. Blood 2012; 119: 3744–3756. [DOI] [PubMed] [Google Scholar]
- 19. Whyburn LR, Halcomb KE, Contreras CM, et al. Reduced dosage of Bruton’s tyrosine kinase uncouples B cell hyperresponsiveness from autoimmunity in lyn–/– mice. J Immunol 2003; 171: 1850–1858. [DOI] [PubMed] [Google Scholar]
- 20. Gruber R, Dufault M, Chretien N, et al. Decoding Bruton’s tyrosine kinase signalling in neuroinflammation. Mult Scler 2020; 26(Suppl. 3): 270. [Google Scholar]
- 21. Rijvers L, van Langelaar J, Bogers L, et al. Human T-bet+ B cell development is associated with BTK activity and suppressed by evobrutinib. JCI Insight 2022; 7: e160909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Haselmayer P, Camps M, Liu-Bujalski L, et al. Efficacy and pharmacodynamic modeling of the BTK inhibitor evobrutinib in autoimmune disease models. J Immunol 2019; 202: 2888–2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Caldwell RD, Qiu H, Askew BC, et al. Discovery of evobrutinib: An oral, potent, and highly selective, covalent Bruton’s tyrosine kinase (BTK) inhibitor for the treatment of immunological diseases. J Med Chem 2019; 62: 7643–7655. [DOI] [PubMed] [Google Scholar]
- 24. Torke S, Pretzsch R, Häusler D, et al. Inhibition of Bruton’s tyrosine kinase interferes with pathogenic B-cell development in inflammatory CNS demyelinating disease. Acta Neuropathol 2020; 140: 535–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Alankus Y, Grenningloh R, Haselmayer P, et al. BTK inhibition prevents inflammatory macrophage differentiation: A potential role in MS. Mult Scler 2018; 24(Suppl. 2): 264. [Google Scholar]
- 26. Geladaris A, Torke S, Weber M, et al. Targeting BTK in chronic CNS autoimmunity inhibits activation of microglia. Mult Scler 2021; 27(Suppl. 2): 790–791.32749910 [Google Scholar]
- 27. Montalban X, Arnold DL, Weber MS, et al. Placebo-controlled trial of an oral BTK inhibitor in multiple sclerosis. N Engl J Med 2019; 380: 2406–2417. [DOI] [PubMed] [Google Scholar]
- 28. Montalban X, Wolinsky J, Arnold DL, et al. Safety and efficacy of evobrutinib, a Bruton’s tyrosine kinase inhibitor in relapsing multiple sclerosis over 2.5 years of the open-label extension to a Phase II trial. Neurology 2022; 98(Suppl. 18): 2812. [Google Scholar]
- 29. Arnold D, Elliott C, Montalban X, et al. Effects of evobrutinib, a Bruton’s tyrosine kinase inhibitor, on slowly expanding lesions: An emerging imaging marker of chronic tissue loss in multiple sclerosis. Mult Scler 2021; 27(Suppl. 2): 69. [Google Scholar]
- 30. Cross AH, Wallace D, Cunningham AL, et al. Immune response following vaccination against seasonal influenza in patients treated with the BTK inhibitor evobrutinib. Eur J Neurol 2022; 29(Suppl. 1): 348. [Google Scholar]
- 31. Papasouliotis O, Mitchell D, Girard P, et al. Determination of a clinically effective evobrutinib dose: Exposure-response analyses of a Phase II relapsing multiple sclerosis study. Clin Transl Sci 2022; 15: 2888–2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. U.S. Food and Drug Administration. LIAISON® SARS-CoV-2 S1/S2 IgG, https://www.fda.gov/media/137359/download (accessed 5 July 2023).
- 33. Xiong X, Qu K, Ciazynska KA, et al. A thermostable, closed SARS-CoV-2 spike protein trimer. Nat Struct Mol Biol 2020; 27: 934–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med 2021; 27: 1205–1211. [DOI] [PubMed] [Google Scholar]
- 35. Crofford LJ, Nyhoff LE, Sheehan JH, et al. The role of Bruton’s tyrosine kinase in autoimmunity and implications for therapy. Expert Rev Clin Immunol 2016; 12: 763–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pleyer C, Laing KJ, Ali MA, et al. BTK inhibitors impair humoral and cellular responses to recombinant zoster vaccine in CLL. Blood Adv 2022; 6: 1732–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pleyer C, Ali MA, Cohen JI, et al. Effect of Bruton tyrosine kinase inhibitor on efficacy of adjuvanted recombinant hepatitis B and zoster vaccines. Blood 2021; 137: 185–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Parry H, McIlroy G, Bruton R, et al. Impaired neutralisation of SARS-CoV-2 delta variant in vaccinated patients with B cell chronic lymphocytic leukaemia. J Hematol Oncol 2022; 15: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Louapre C, Collongues N, Stankoff B, et al. Clinical characteristics and outcomes in patients with coronavirus disease 2019 and multiple sclerosis. JAMA Neurol 2020; 77: 1079–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sormani MP, De Rossi N, Schiavetti I, et al. Disease-modifying therapies and coronavirus disease 2019 severity in multiple sclerosis. Ann Neurol 2021; 89: 780–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Muchtar E, Koehler AB, Johnson MJ, et al. Humoral and cellular immune responses to recombinant herpes zoster vaccine in patients with chronic lymphocytic leukemia and monoclonal B cell lymphocytosis. Am J Hematol 2022; 97: 90–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shen Y, Freeman JA, Holland J, et al. COVID-19 vaccine failure in chronic lymphocytic leukaemia and monoclonal B-lymphocytosis; humoural and cellular immunity. Br J Haematol 2022; 197: 41–51. [DOI] [PubMed] [Google Scholar]
- 43. Benjamini O, Rokach L, Itchaki G, et al. Safety and efficacy of the BNT162b mRNA COVID-19 vaccine in patients with chronic lymphocytic leukemia. Haematologica 2022; 107: 625–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Herishanu Y, Avivi I, Aharon A, et al. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with chronic lymphocytic leukemia. Blood 2021; 137: 3165–3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Langerbeins P, Eichhorst B. Immune dysfunction in patients with chronic lymphocytic leukemia and challenges during COVID-19 pandemic. Acta Haematol 2021; 144: 508–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Naaber P, Tserel L, Kangro K, et al. Dynamics of antibody response to BNT162b2 vaccine after six months: A longitudinal prospective study. Lancet Reg Health Eur 2021; 10: 100208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Favresse J, Bayart JL, Mullier F, et al. Antibody titres decline 3-month post-vaccination with BNT162b2. Emerg Microbes Infect 2021; 10: 1495–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Shen Y, Freeman JA, Holland J, et al. Multiple COVID-19 vaccine doses in CLL and MBL improve immune responses with progressive and high seroconversion. Blood 2022; 140: 2709–2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-msj-10.1177_13524585231192460 for Antibody response to SARS-CoV-2 vaccines in patients with relapsing multiple sclerosis treated with evobrutinib: A Bruton’s tyrosine kinase inhibitor by Amit Bar-Or, Anne H Cross, Anthony L Cunningham, Yann Hyvert, Andrea Seitzinger, Hans Gühring, Elise E Drouin, Nektaria Alexandri, Davorka Tomic and Xavier Montalban in Multiple Sclerosis Journal