Abstract
Background:
A majority of women with multiple sclerosis (MS) are diagnosed prior to menopause, yet their experiences during this transition are not well characterized.
Objectives:
To explore associations between mental health, sleep, and other quality of life metrics, and vasomotor symptoms (VMSs) in ambulatory, menopausal women with MS.
Methods:
A secondary analysis was performed of baseline data from two trials enrolling ambulatory peri/postmenopausal women with MS: NCT02710214 (N = 24, bothersome VMS) and NCT04002934 (ongoing, N = 35, myelin repair). Measures analyzed were 36-Item Short-Form Survey (SF-36) (primary scale: general mental health), subjective sleep quality (Pittsburg Sleep Quality Index), VMS (daily diary, interference), mood (Center for Epidemiologist Studies—Depression Scale (CES-D)), walking impairment (timed 25-foot walk (T25FW)), and global disability (Expanded Disability Status Scale (EDSS)).
Results:
Participants’ characteristics (N = 59) were: mean age 51.8 years (SD = 3.4), mean disease duration 11.3 years (SD = 7.6), median EDSS 3.0 (IQR = 2.0–4.0). Mental health was associated with better sleep quality (rho = −0.41, p = 0.019) and better mood (rho = −0.75, p < 0.001), but not with EDSS or T25FW (rho < 0.20, p > 0.10). Worse sleep quality also correlated with more frequent VMS (rho = 0.41, p = 0.02) and VMS interference (rho = 0.59, p < 0.001).
Conclusions:
Findings suggest that optimizing sleep quality, mood, and hot flash quantity/interference could substantially improve mental health in menopausal women with MS—and highlight an important care gap in this population.
Keywords: Sleep, menopause, multiple sclerosis, hot flash, vasomotor symptoms
Introduction
Multiple sclerosis (MS) is a neuroinflammatory demyelinating disorder that affects three times as many females as males. As MS typically is diagnosed between 20 and 40 years of age, a majority of these patients will experience menopause after MS onset. 1 The remarkable advances in disease-modifying therapies (DMTs) over the past two decades have meant that an increasing number of patients are approaching midlife with less cumulative disability than in prior decades. Yet, much is unknown about the quality of life (QOL) and mental health of postmenopausal women with MS—who are typically excluded from randomized clinical trials for new DMTs. 2
Menopause is a normal physiological transition with significant hormonal changes, including fluctuations in estrogen levels before its postmenopausal decline. 3 In women with MS, qualitative studies have suggested that menopausal vasomotor symptoms (VMSs) can exacerbate existing MS symptoms, potentially through an Uhthoff’s-like phenomenon. Furthermore, “overlap symptoms” common to both MS and the menopausal transition, such as worsening mood, fatigue, and bladder control, could cumulatively worsen symptoms in MS.4–7 Finally, in the general population, the perimenopausal period is associated with worse sleep quality, including sleep onset and sleep maintenance. Menopause could therefore also exacerbate MS symptoms through worsening in sleep quality, which disproportionately affects people with MS.8–11 Because of this, it was hypothesized that poor sleep quality and worse VMSs would impact general mental health in this patient population.
To better characterize these relationships, the current study aimed to evaluate contributors to mental health in cis-women at midlife, and particularly to understand how sleep and VMS may influence mental health. To accomplish this, a secondary analysis was performed using baseline data from two clinical trial cohorts, encompassing 59 peri/postmenopausal women at midlife. Trial 1 was enriched for patients experiencing bothersome VMSs.
Participants and methods
Study setting
Secondary analyses were performed using data collected at the baseline visit of two clinical trials conducted in the UCSF Center for MS and Neuroinflammation.
Trial 1
The first trial (ClinicalTrials.gov Identifier: NCT04002934) studied the impact of a hormonal therapy (bazedoxifene + conjugated estrogens, Duavee®) on menopausal symptoms in women with MS. In this trial, 24 ambulatory peri/postmenopausal women with MS and bothersome menopause symptoms were randomized 1:1 to receive either treatment or placebo for 2 months. 12 Key inclusion criteria included females aged 40–62 years, MS by 2010 International Panel Criteria, EDSS 0.0–6.0 (inclusive), amenorrhea for at least 6 months or in early postmenopause (last menstrual period within 2 years), and no change in MS DMT in prior 6 months, or 2 months if changing to anti-CD20 monoclonal antibody therapies (rituximab or ocrelizumab) because of shorter therapeutic lag. Exclusion criteria included: body mass index > 50 kg/m2, uncontrolled co-morbidities (e.g. hypertension, thyroid), substance abuse, moderate or severe depression, relapses or steroid treatment in prior month, or menopausal hormone therapy use within 2 months of enrollment. Known sleep disorders were not excluded.
Trial 2
The second clinical trial (ongoing, ClinicalTrials.gov Identifier: NCT02710214) evaluated the effect of a selective estrogen receptor modulator (bazedoxifene), on remyelination in peri/ postmenopausal women with MS. In this delayed start trial, participants are randomized 1:1 to receive either treatment or placebo for the first 3 months of the study, then treatment for all participants in the last 3 months. Key inclusion criteria include: females aged 45–65 years (or 40+ and postmenopausal), MS by 2010 International Panel Criteria, EDSS 0.0–6.0 (inclusive), and no change or planned change in MS DMT for 6 months. Exclusion criteria include: MS disease duration > 25 years, uncontrolled co-morbidities (e.g. hypertension, thyroid), substance abuse, and moderate or severe depression.
Variables collected
For both trials, at baseline, patient-reported outcomes (PROs) and neurological outcomes were collected from all participants (Table 1).
Table 1.
Demographic and clinical characteristics of 59 peri/postmenopausal women with MS included in the analyses.
| Characteristic | Trial 1 (n = 24) | Trial 2 (n = 35) | Total (n = 59) | Comparison p-value (t-test or χ2) |
|---|---|---|---|---|
| Age, mean (SD) | 51.2 (3.6) | 52.2 (3.3) | 51.8 (3.4) | p = 0.27 |
| Median (IQR) | 51.0 (4.25) | 51.0 (4.0) | 51.0 (4.5) | |
| Disease duration, mean (SD) | 11.0 (8.5) | 11.5 (6.9) | 11.3 (7.6) | p = 0.83 |
| Median (IQR) | 10.0 (12.5) | 9.0 (11.0) | 10.0 (12.0) | |
| Disease type, n (%) | ||||
| RRMS | 21 (87.5%) | 35 (100%) | 56 (94.9%) | χ2 = 4.61, p = 0.10 |
| SPMS | 2 (8.3%) | 0 (0%) | 2 (3.3%) | |
| PPMS | 1 (4.2%) | 0 (0%) | 1 (1.7%) | |
| Race/ethnicity n (%) | ||||
| White, non-Hispanic | 21 (87.5%) | 33 (94.3%) | 54 (91.5%) | χ2 = 1.47, p = 0.48 |
| Hispanic or Latinx | 0 (0%) | 2 (5.7%) | 2 (3.4%) | |
| Black or African American | 2 (8.3%) | 0 (0%) | 2 (3.4%) | |
| Native Hawaiian or Other Pacific Islander | 1 (4.2%) | 0 (0%) | 1 (1.7%) | |
| Years of education, mean (SD) | 16.2 (1.0) | 17.0 (2.7) | 16.7 (2.2) | p = 0.15 |
| Patient-reported quality of life SF-36 (score range: 0–100), mean (SD) | ||||
| Physical functioning | 68.3 (27.3) | 76.0 (24.2) | 73.1 (25.7) | p = 0.29 |
| Role physical | 46.4 (43.2) | 48.6 (40.9) | 47.8 (41.8) | p = 0.86 |
| Bodily pain | 62.4 (23.5) | 71.6 (22.9) | 68.2 (23.5) | p = 0.16 |
| General health perceptions | 50.0 (22.2) | 59.7 (16.3) | 56.1 (19.3) | p = 0.07 |
| Vitality | 38.6 (25.3) | 54.3 (21.7) | 48.4 (24.4) | p = 0.02 |
| Social functioning | 62.5 (30.7) | 78.9 (22.3) | 72.6 (27.1) | p = 0.03 |
| Role emotional | 71.4 (40.2) | 48.6 (43.2) | 57.1 (43.5) | p = 0.06 |
| General mental health | 76.2 (13.2) | 74.2 (19.2) | 74.9 (17.2) | p = 0.04 |
| Physical composite score | 39.1 (11.4) | 44.9 (9.2) | 42.7 (10.5) | p = 0.04 |
| Mental composite score | 48.1 (8.9) | 46.5 (11.4) | 47.1 (10.5) | p = 0.59 |
| Functional measures | ||||
| EDSS total score, median (IQR) | 3.0 (2.5, 4.5) | 2.5 (2.0, 3.5) | 3.0 (2.0, 4.0) | χ2 = 11.66, p = 0.39 |
| T25FW, mean (SD) | 5.2 (1.3) | 4.5 (0.8) | 4.7 (1.0) | p = 0.02 |
| Bowel/bladder FS, median (IQR) | 1.0 (0.0, 2.0) | 1.0 (0.0, 1.0) | 1.0 (0.0, 2.0) | χ2 = 2.78, p = 0.43 |
| SDMT, # correct (SD) | 43.3 (10.4) | 48.2 (7.9) | 46.2 (9.3) | p = 0.05 |
| LNS, mean (SD) | 10.0 (1.8) | – | 10.0 (1.8) | – |
| CVLT-2 total correct, mean (df) | – | 58.2 (13.0) | 58.2 (13.0) | – |
| BVMT | – | 27.0 (5.7) | 27.0 (5.7) | – |
| Menopausal symptoms | ||||
| HF (within 24 hours), mean (SD), (min. – max.) |
5.0 (4.7)
(0.1–17.6) |
1.5 (2.4)
(0.0–7.8) |
3.0 (3.9)
(0.0–17.6) |
p = 0.001 |
| HFRDIS (score range: 0–100), mean (SD) | 24.6 (21.3) | – | 24.6 (21.3) | – |
| Sleep symptoms | ||||
| PSQI (score range: 0 – 3) (mean, SD) | ||||
| Global score (score range: 0 – 21) | 8.1 (2.6) | 7.5 (3.1) | 7.8 (2.8) | p = 0.55 |
| Subjective sleep quality | 1.2 (0.7) | 1.3 (0.8) | 1.2 (0.7) | p = 0.64 |
| Sleep latency | 1.6 (0.9) | 0.9 (0.7) | 1.3 (0.9) | p = 0.02 |
| Sleep duration | 0.8 (0.6) | 0.7 (0.9) | 0.7 (0.7) | p = 0.95 |
| Sleep efficiency | 0.7 (0.9) | 1.1 (1.1) | 0.8 (1.0) | p = 0.23 |
| Sleep disturbance | 1.8 (0.5) | 1.8 (0.5) | 1.8 (0.5) | p = 0.79 |
| Use of sleep medication | 0.9 (1.3) | 1.0 (1.2) | 1.0 (1.2) | p = 0.91 |
| Daytime dysfunction | 1.2 (0.7) | 0.9 (0.7) | 1.0 (0.7) | p = 0.11 |
| ISI (score range: 0–28) (mean, SD) | 10.2 (5.9) | – | 10.2 (5.9) | – |
| Mood | ||||
| CES-D (score range: 0 – 60) (mean, SD) | – | 16.0 (7.2) | 16.0 (7.2) | – |
| DMT, n (%) | ||||
| First-line self-injectables | 4 (16.7%) | 7 (20.0%) | 11 (18.6%) | χ2 = 7.01, p = 0.07 |
| Orals | 8 (33.3%) | 8 (22.9%) | 16 (27.1%) | |
| Monoclonal antibodies | 6 (25%) | 18 (51.4%) | 24 (40.7%) | |
| None | 6 (25%) | 2 (5.7%) | 8 (13.6) | |
Metrics bolded indicate the primary variables analyzed.
Abbreviations: DMT = disease-modifying therapy, EDSS = Expanded Disability Status Scale, HF = hot flash, HFRDIS = Hot Flash-Related Daily Interference Scale, IQR = interquartile range, ISI = insomnia severity index, LNS = letter–number sequencing, MSQOL-54 = multiple sclerosis quality of life 54, SD = standard deviation, SDMT = symbol digit modalities test, T25FW = timed 25-foot walk.
Patient-reported outcomes
All PROs were collected via REDCap, except for hot flash diaries that were collected on paper forms.
Quality of life
Medical Outcomes Study 36-Item Short-Form Survey 13 (SF-36, Trials 1 and 2). This instrument assesses eight health concepts that contribute to two composite scores. The eight health concepts surveyed are: physical functioning, role limitations due to physical health, bodily pain, general health perception, vitality/energy, social functioning, role limitations due to emotional problems, and general mental health. The two composite scores are a physical composite score and mental composite score (MCS). Each health concept is scored individually from 0 (worse) to 100 (better). The general mental health scores were the primary focus of the analyses.
Hot flashes (VMSs). Daily hot flash diary 14 (Trials 1 and 2)
These were collected twice daily (Trial 1) and once daily (Trial 2). The average number of hot flashes per day in the first 2 weeks of the study was included in analyses.
Hot Flash-Related Daily Interference Scale (HFRDIS; Trial 1 only)
This weekly survey 15 utilizes a 10-point rating system to quantify the impact of hot flashes on daily activities and QOL. This measure was collected at the baseline of the study before intervention.
Sleep. Pittsburgh Sleep Quality Index 16 (PSQI, Trials 1 and 2)
This evaluates sleep quality and disturbance over the past month, and is scored into seven subscores (subjective sleep quality, sleep latency, sleep duration, sleep efficiency, sleep disturbance, use of sleep medication, and daytime dysfunction) and one global score, in which higher scores indicate worse sleep. The global PSQI score was used for the present analyses.
Insomnia Severity Index 17 (ISI, Trial 1)
The ISI is a seven-item survey used to assess the severity of insomnia symptoms using daytime and night-time features. Higher scores suggest more severe insomnia symptoms.
Mood. Center for Epidemiologist Studies—Depression Scale 18 (CES-D, Trial 2)
This 20-item scale tests for symptoms of depression. Scores range from 0 to 60, with cutoffs as follows: no/mild (0–16), moderate (17–23), and severe (24 and above).
Objective neurological evaluations (Trials 1 and 2)
Expanded Disability Status Scale (EDSS)
This is a standard disability evaluation test for MS and was completed by a trained neurostatus-certified neurologist; 19 from this, both the total score, and the bowel and bladder functional system (B/B FS) score were extracted.
Timed 25-foot walk (T25FW)
This quantitative test of walking speed is one of the three components of the Multiple Sclerosis Functional Composite (MSFC). 20
Cognitive evaluations. Symbol digit modalities test (SDMT; Trials 1 and 2)
This sensitive and specific test assesses processing speed and working memory, which are typically affected domains in patients with MS who are cognitively impaired.21,22 A higher score on the SDMT measure indicates better cognitive performance.
Letter–number sequencing (LNS; Trial 1)
This subtest of the Wechsler Memory Scale is used to assess verbal working memory. 23 The recall score of the LNS is used in our analyses. A higher score on LNS indicates better cognitive performance.
California verbal learning test—second edition (CVLT-2; Trial 2)
This is a measure of episodic verbal learning and memory. 24 Participants completed five trials and the total correct was included in analysis. A higher score on the CVLT-2 measure indicates better cognitive performance.
Brief visuospatial memory test—revised (BVMT-R; Trial 2)
This is a test of visuospatial learning and memory. 25 Three trials of the BVMT were administered and scores were summed for a total score, used in the analysis. A higher score on the BVMT-R measure indicates better cognitive performance.
Ethical statement
The trials for which these baseline data were collected were approved under the UCSF Institutional Review Board, #15-18004 and #18024511. These trials were listed on clinicaltrials.gov as NCT02710214 and NCT04002934, respectively. Written informed consent was obtained from each participant in each study.
Statistical analyses
In the current study, relationships between general mental health, sleep, VMSs, and MS-related function were evaluated. Demographic and clinical characteristics were compared between each trial via independent t-tests or chi-square tests. To identify important associations, a heat map of the clinical characteristics and PROs collected was generated. Spearman’s correlations were used to examine any significant associations between these variables, focusing on general mental health, PSQI, and HFRDIS. A mediation analysis was also performed to assess whether VMS had direct and/or indirect effects on mental health via sleep. A linear regression model was used for this analysis with significance determined by bootstrapping. As such, the following specific questions were asked: (1) Which variables had the strongest associations with general mental health in this patient cohort? (2) Which variables were correlates of poor sleep quality?
Significance was set at an α = 0.05 for all analyses. The SciPy v1.9.1, Statsmodels v0.13.5, and NumPy v1.21.1 statistical packages were used. 26
Results
Overall, this cohort of 59 cis-women had mean age 51.8 years (SD = 3.4). Participants were well educated (mean years of education: 16.7 years), and 91.5% were non-Hispanic White. At the trials’ baseline visit, no participants were taking hormonal therapy for their VMS. Only two participants were current smokers. Given that VMS was an inclusion criterion for Trial 1, participants in this trial had a greater mean number of 24-hour hot flashes (5.0 (4.7) vs. 1.5 (2.4), p = 0.001) (Table 1).
Overall, the analysis cohort had moderate ambulatory disability, with median EDSS of 3.0 (IQR = 2.0–4.0) and mean T25FW 4.7 seconds (SD = 1.0). MS clinical characteristics were similar across both trials, and mean disease duration was 11.3 years (SD = 7.6); notably, more participants in the more recent trial were using monoclonal antibodies as their DMT. CES-D was available for the Trial 2 participants, and mean score was 16.0 (SD = 7.2), corresponding with the cutoff for mild depression.
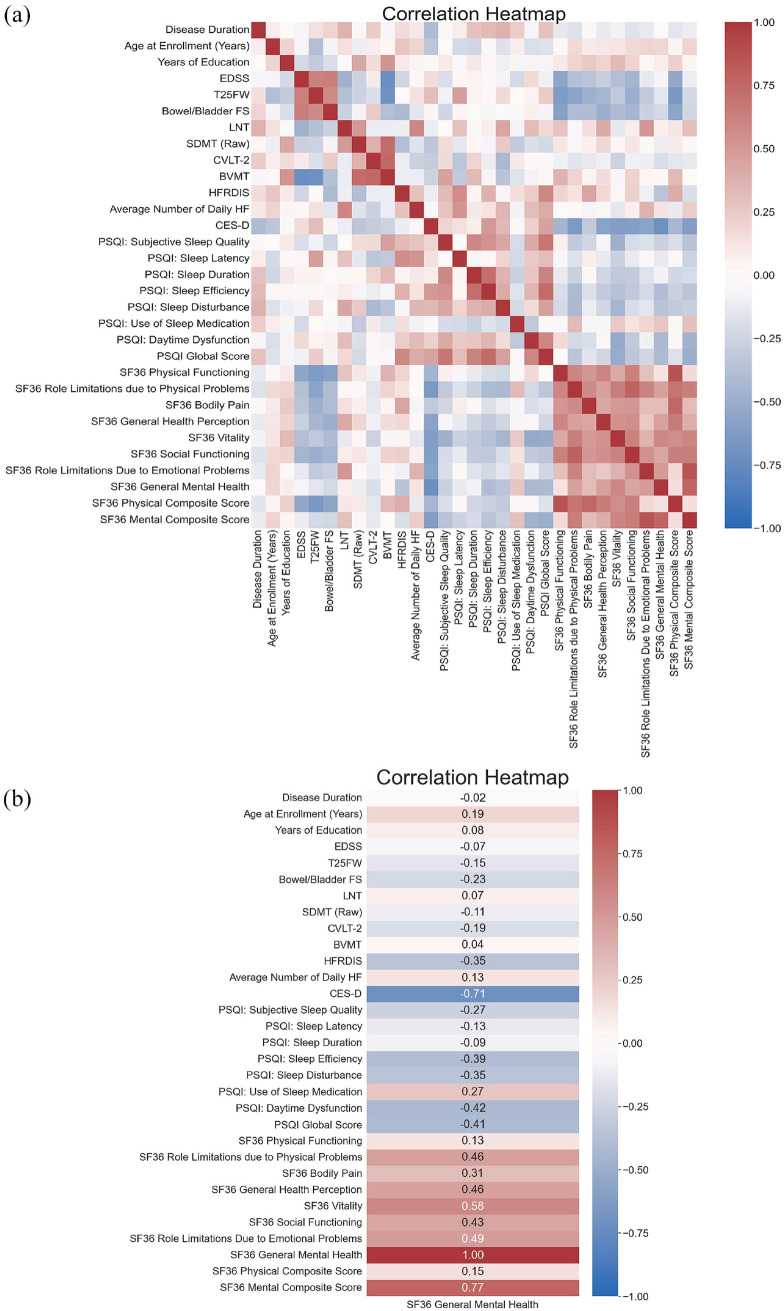
To understand bivariate associations between participants’ clinical characteristics and PROs, a heatmap of Spearman’s correlations was created (Figure 1). As expected, this heatmap revealed moderate to strong correlations among the various measures of QOL (SF-36 general mental health, vitality, physical functioning), of physical disability (EDSS, T25FW, and B/B FS), of cognition (LNS, SDMT, CVLT-II, and BVMT), and measures of sleep and VMS (PSQI, ISI, Daily HF, and HFRDIS).
Figure 1.
Spearman’s correlations across a series of clinical characteristics demonstrate relationships between sleep, hot flashes, and quality of life, suggesting sleep and VMS may be predictors of well-being in women with MS at midlife. (a) Correlation heatmap for all measures. (b) Measures associated with SF-36 general mental health.
SF-36 general mental health was examined as our primary indicator of well-being (Figure 2). This measure showed moderate correlations with SF-36 vitality (rho = 0.59, p < 0.001). However, general mental health showed no correlation with patient-reported physical functioning (rho = 0.13, p = 0.34); nor it was correlated with EDSS (rho = −0.07, p = 0.61) or the T25FW (rho = −0.14, p = 0.32), markers of physical disability in MS. General mental health also showed no correlations with any cognitive assessment (all rho < 0.20, all p > 0.05). In contrast, as hypothesized, general mental health moderately correlated with better sleep as reflected by lower PSQI scores (rho = −0.41, p = 0.019). In particular, the PSQI subscore for daytime dysfunction (challenges staying awake while driving, having enthusiasm to get things done, etc.) was correlated with general mental health (rho = −0.42, p = 0.017). In Trial 2, where information on mood was available, SF-36 general mental health showed strong correlations with CES-D (rho = −0.75, p = 0.0006). Similar correlations were found when considering SF-36 MCS (Figure 1).
Figure 2.
Consideration of vasomotor symptoms and sleep quality at midlife leads to a more comprehensive understanding of quality of life in menopausal women with MS.
This cohort of participants were overall “poor sleepers” with an average PSQI score of 7.8 (SD = 2.8). To ensure that PSQI was a reliable measure of sleep, PSQI values were correlated with insomnia severity (ISI) in the Trial 1 participants who completed this measure. Worse sleep on PSQI was indeed strongly correlated with insomnia on ISI (rho = 0.83, p < 0.0001). Furthermore, PSQI was associated not only with SF-36 general mental health but also with SF-36 vitality score (rho = −0.52, p = 0.002).
Finally, worse sleep on PSQI showed moderate associations with VMS, both with average daily number of hot flashes (rho = 0.40, p = 0.02) and with the HFRDIS (rho = 0.59, p = 0.006), indicating that both the number and impact of VMS correlated with poor sleep. In a mediation analysis, global PSQI scores showed a mediating effect between average daily number of hot flashes and general mental health (indirect effect: −0.7755, 95% CI: (–1.99, −0.19)). However, there was no direct effect between the average daily number of hot flashes and general mental health (direct effect: 0.4155 (95% CI: (−0.6980, 1.5000)). Increased number of daily hot flashes was also moderately correlated with LNS scores (rho = 0.60, p = 0.004).
Stability analyses: distinction by trial
Data were separated by trial to ensure that one trial was not driving effects seen in aggregated analysis. The heatmaps for these analyses are presented in Supplemental Figure 1.
Similar to the grouped analyses, SF-36 general mental health again showed modest correlations with SF-36 vitality (Trial 1: rho = 0.42, p = 0.06; Trial 2: rho = 0.72, p < 0.001) and modest correlations with better PSQI (Trial 1: rho = −0.44, p = 0.10; Trial 2: rho = −0.42, p = 0.12), with similar correlation coefficients despite more limited statistical power.
Again, SF-36 general mental health did not show any significant associations with markers of physical disability or cognition in either trial (all rho < 0.25, all p > 0.10), except for a weak correlation with bowel/bladder FS (rho = −0.33, p = 0.06) in Trial 2 only. Full results are presented in Supplemental Figure 1(a) and (b).
Focusing on PSQI, it was again also associated with SF-36 vitality score in both trials (Trial 1: rho = −0.49, p = 0.046; Trial 2; rho = −0.68, p = 0.005). In Trial 1, which was enriched for participants with bothersome VMS, worse sleep by PSQI showed moderate associations with VMS, both with average daily number of hot flashes (rho = 0.44, p = 0.05) and with the HFRDIS (rho = 0.59, p = 0.006), again indicating that both the number and impact of VMS were correlated with poor sleep. In Trial 2, while not significant, PSQI was moderately correlated with mood, as well (CES-D, rho = 0.46, p = 0.18). CES-D was also significantly correlated with SF-36 vitality (rho = −0.62, p = 0.008).
Discussion
This study of women with MS at midlife uncovers significant associations between mental health and sleep, menopausal VMS, mood, and QOL. Few studies to date have focused on the experience of women with MS during the menopausal transition, or even included them in DMT efficacy clinical trials. 2 In the general population, SF-36 scores have not been shown to be specifically associated with menopausal status or hormone therapy use. 27 Although causation cannot be determined from the current cross-section 31 data, our findings highlight potential pathways between sleep quality, VMS frequency/interference, and mental well-being that represent unmet clinical care and research gaps in this growing population.
As sleep disturbances are linked to impaired cognitive function, mood disorders, physical disability, and limitations in activities of daily living, which may further decrease QOL, it is critical to understand how sleep, menopause, and MS interact. Poor sleep quality may arise from the presence of sleep disorders, such as insomnia, obstructive sleep apnea (OSA), and restless leg syndrome (RLS), which are also more common in both MS and menopause.5,28,29 In an analysis from the Nurses’ Health Study, OSA, sleepiness, and insomnia were found to be more prevalent in patients with MS than in the broader cohort. 9 In this longitudinal cohort study, these sleep disorders differentially moderated or mediated the relationship between MS and perceived cognition. Additional studies focused on objective cognitive testing have also identified associations between sleep disorders and cognitive impairment in MS;8,30 however, the impact of menopause on sleep disturbances in MS, and how these conditions could interact influence mental health in midlife women, have not been studied to date. These data suggest that VMS could impact mental health through sleep disturbances, offering new insights into potential treatment targets that require further investigation.
Similarities across the two trial studies used in these analyses include targeting women with MS at midlife, similar inclusion and exclusion criteria, and similar PROs of sleep and VMS. While the total sample size was modest (N = 59), the fact that the main study findings were significant in two cohorts with some key differences in enrollment criteria (namely, VSM bother), may enhance their generalizability. The current study focused on a demographic that is often neglected in MS clinical trials (i.e. menopausal women), but other enrollment biases characteristic of MS trials persisted, namely, the participants were mostly of moderate disability and predominantly White, and a broader demographic of patients should be studied to increase generalizability. Moreover, neuroimaging evaluations of key brain regions involved in sleep regulation, such as the hypothalamus or brainstem, could provide added mechanistic insight into our clinical findings. Furthermore, this was a cross-section 32 study, and a longitudinal study could further deconstruct the effects of aging and menopause in this patient population. Notably, better SF-36 physical functioning was previously associated with menopausal hormone therapy use in a pre-DMT era Nurses’ Health Study cohort, but causation would not be established. 31 Consequently, it was not clear whether women in better physical function were more likely to receive standard of care general health care including at that time hormone therapy, or whether the hormone therapy contributed to preserved physical functioning.
Historically, around the age of 45 or 50, MS progression advances, with a shift from relapsing phases to more progressive phases of the disease.32,33 Moreover, it has been shown that sex differences in MS disease course and progression are reduced after age 50, with increased similarity in disease progression between males and females,34,35 including in individuals with MS onset after the age of 50. This coincides chronologically with the age of menopause, and it is not clear if effects from MS, age, or hormonal changes influence this progression independently or synergistically.36–39 It is not yet clear whether this is due to neurological disability progression or because the EDSS scores may also reflect non-neurological measures of aging and impairment. 40 The increasing use of highly effective DMTs in recent years appears to be leading to overall lower disability burden in individuals with MS; however, disability measures, such as the EDSS used in DMT clinical trials generally favor “visible” ambulatory impairment, with less attention given to “invisible” symptoms, such as pain, fatigue, and cognitive impairment. In our clinical experience, menopausal women with MS often report poor mental health,3,40 making statements, such as “things are falling apart,” “something has to give,” and commonly asking of their symptomatic worsening, “Is it my menopause, my MS, or both?.” The current findings suggest a significant clinical opportunity in the management of menopausal women with MS whose inflammatory activity is overall well-controlled, to improve their QOL. Beyond optimization of gait, balance, and physical activity, increased attention to optimizing sleep and reducing VMS to support improved energy and mental health is sorely needed.
Supplemental Material
Supplemental material, sj-docx-1-msj-10.1177_13524585231197056 for Well-being at midlife: Correlates of mental health in ambulatory menopausal women with multiple sclerosis by Denisse Morales-Rodriguez, Annika Anderson, Alyssa Nylander, Stephanie Hsu, Jessica Singh, Will Rowles, Christine M Walsh, Tiffany J Braley and Riley Bove in Multiple Sclerosis Journal
Supplemental material, sj-jpg-2-msj-10.1177_13524585231197056 for Well-being at midlife: Correlates of mental health in ambulatory menopausal women with multiple sclerosis by Denisse Morales-Rodriguez, Annika Anderson, Alyssa Nylander, Stephanie Hsu, Jessica Singh, Will Rowles, Christine M Walsh, Tiffany J Braley and Riley Bove in Multiple Sclerosis Journal
Supplemental material, sj-jpg-3-msj-10.1177_13524585231197056 for Well-being at midlife: Correlates of mental health in ambulatory menopausal women with multiple sclerosis by Denisse Morales-Rodriguez, Annika Anderson, Alyssa Nylander, Stephanie Hsu, Jessica Singh, Will Rowles, Christine M Walsh, Tiffany J Braley and Riley Bove in Multiple Sclerosis Journal
Acknowledgments
The authors thank the study participants.
Footnotes
Data Availability Statement: The participants of this study did not give written consent for their data to be shared publicly, so due to the sensitive nature of the research supporting data are not available.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: D.M., A.A., A.N., S.H., J.S., W.R., CW., and T.B. have nothing to disclose. R.B. is the recipient of a National Multiple Sclerosis Harry Weaver Award. She has received research support from the National Multiple Sclerosis Society, the National Institutes of Health, and the Department of Defense. She has also received research support from Biogen, Novartis, and Roche Genentech. She has received personal compensation for consulting from Alexion, EMD Serono, Horizon, Jansen, and TG Therapeutics.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Multiple Sclerosis Society pilot grant (PR-1412-02135 awarded to Dr R.B.), the National Multiple Sclerosis Society Harry Weaver Neuroscience Award, and the Sherak Foundation.
ORCID iDs: Denisse Morales-Rodriguez  https://orcid.org/0000-0002-3272-7023
https://orcid.org/0000-0002-3272-7023
Alyssa Nylander  https://orcid.org/0000-0002-1976-3847
https://orcid.org/0000-0002-1976-3847
Stephanie Hsu  https://orcid.org/0009-0006-7031-3696
https://orcid.org/0009-0006-7031-3696
Tiffany J Braley  https://orcid.org/0000-0003-0680-7828
https://orcid.org/0000-0003-0680-7828
Riley Bove  https://orcid.org/0000-0002-2034-8800
https://orcid.org/0000-0002-2034-8800
Supplemental Material: Supplemental material for this article is available online.
Contributor Information
Denisse Morales-Rodriguez, UCSF Weill Institute for Neurosciences, Department of Neurology, University of California San Francisco, San Francisco, CA, USA.
Annika Anderson, UCSF Weill Institute for Neurosciences, Department of Neurology, University of California San Francisco, San Francisco, CA, USA.
Alyssa Nylander, UCSF Weill Institute for Neurosciences, Department of Neurology, University of California San Francisco, San Francisco, CA, USA.
Stephanie Hsu, UCSF Weill Institute for Neurosciences, Department of Neurology, University of California San Francisco, San Francisco, CA, USA.
Jessica Singh, UCSF Weill Institute for Neurosciences, Department of Neurology, University of California San Francisco, San Francisco, CA, USA.
Will Rowles, UCSF Weill Institute for Neurosciences, Department of Neurology, University of California San Francisco, San Francisco, CA, USA.
Christine M Walsh, UCSF Weill Institute for Neurosciences, Department of Neurology, University of California San Francisco, San Francisco, CA, USA.
Tiffany J Braley, Division of Multiple Sclerosis and Clinical Neuroimmunology, Department of Neurology, University of Michigan, Ann Arbor, MI, USA.
Riley Bove, UCSF Weill Institute for Neurosciences, Department of Neurology, University of California San Francisco, San Francisco, CA, USA.
References
- 1. Bove R, Chitnis T. The role of gender and sex hormones in determining the onset and outcome of multiple sclerosis. Mult Scler 2014; 20(5): 520–526. [DOI] [PubMed] [Google Scholar]
- 2. Houtchens MK, Bove R. A case for gender-based approach to multiple sclerosis therapeutics. Front Neuroendocrinol 2018; 50: 123–134. [DOI] [PubMed] [Google Scholar]
- 3. Harlow SD, Gass M, Hall JE, et al. Executive summary of the Stages of Reproductive Aging Workshop + 10: Addressing the unfinished agenda of staging reproductive aging. J Clin Endocrinol Metab 2012; 97(4): 1159–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bove R, Vaughan T, Chitnis T, et al. Women’s experiences of menopause in an online MS cohort: A case series. Mult Scler Relat Disord 2016; 9: 56–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bove R, Healy BC, Secor E, et al. Patients report worse MS symptoms after menopause: Findings from an online cohort. Mult Scler Relat Disord 2015; 4(1): 18–24. [DOI] [PubMed] [Google Scholar]
- 6. Lee J, Han Y, Cho HH, et al. Sleep disorders and menopause. J Menopausal Med 2019; 25(2): 83–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tandon VR, Sharma S, Mahajan A, et al. Menopause and sleep disorders. J Midlife Health 2022; 13(1): 26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Braley TJ, Chervin RD. A practical approach to the diagnosis and management of sleep disorders in patients with multiple sclerosis. Ther Adv Neurol Disord 2015; 8(6): 294–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Braley TJ, Kratz AL, Kaplish N, et al. Sleep and cognitive function in multiple sclerosis. Sleep 2016; 39(8): 1525–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Braley TJ, Shieu MM, Zaheed AB, et al. Pathways between multiple sclerosis, sleep disorders, and cognitive function: Longitudinal findings from The Nurses’ Health Study. Mult Scler 2023; 29(3): 436–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Whibley D, Goldstein C, Kratz AL, et al. A multidimensional approach to sleep health in multiple sclerosis. Mult Scler Relat Disord 2021; 56: 103271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bove R, Anderson A, Rowles W, et al. A hormonal therapy for menopausal women with MS: A phase Ib/IIa randomized controlled trial. Mult Scler Relat Disord 2022; 61: 103747. [DOI] [PubMed] [Google Scholar]
- 13. Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992; 30(6): 473–483. [PubMed] [Google Scholar]
- 14. Joffe H, Guthrie KA, LaCroix AZ, et al. Low-dose estradiol and the serotonin-norepinephrine reuptake inhibitor venlafaxine for vasomotor symptoms: A randomized clinical trial. JAMA Intern Med 2014; 174(7): 1058–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Carpenter JS. The Hot Flash Related Daily Interference Scale: A tool for assessing the impact of hot flashes on quality of life following breast cancer. J Pain Symptom Manage 2001; 22(6): 979–989. [DOI] [PubMed] [Google Scholar]
- 16. Buysse DJ, Reynolds CF, 3rd, Monk TH, et al. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res 1989; 28(2): 193–213. [DOI] [PubMed] [Google Scholar]
- 17. Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med 2001; 2(4): 297–307. [DOI] [PubMed] [Google Scholar]
- 18. Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Meas 1977; 1(3): 385–401. [Google Scholar]
- 19. Kappos L, D’Souza M, Lechner-Scott J, et al. On the origin of Neurostatus. Mult Scler Relat Disord 2015; 4(3): 182–185. [DOI] [PubMed] [Google Scholar]
- 20. Fischer JS, Rudick RA, Cutter GR, et al. The Multiple Sclerosis Functional Composite Measure (MSFC): An integrated approach to MS clinical outcome assessment. National MS Society Clinical Outcomes Assessment Task Force. Mult Scler 1999; 5(4): 244–250. [DOI] [PubMed] [Google Scholar]
- 21. Benedict RHB, DeLuca J, Phillips G, et al. Validity of the symbol digit modalities test as a cognition performance outcome measure for multiple sclerosis. Mult Scler 2017; 23(5): 721–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Parmenter BA, Weinstock-Guttman B, Garg N, et al. Screening for cognitive impairment in multiple sclerosis using the Symbol digit Modalities Test. Mult Scler 2007; 13(1): 52–57. [DOI] [PubMed] [Google Scholar]
- 23. Wechsler D. WAIS-IV: Wechsler Adult Intelligence Scale. 4th ed. San Antonio, TX: Psychological Corporation, 2008. [Google Scholar]
- 24. Delis DC, Kramer JH, Kaplan E, et al. California verbal learning test–Second edition, 10.1037/t15072-000 [DOI]
- 25. Tam JW, Schmitter—Edgecombe M. The role of processing speed in the Brief Visuospatial Memory Test—Revised. Clin Neuropsychol 2013; 27(6): 962–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Seabold S, Josef P. Statsmodels: Econometric and| statistical modeling with python. In: Proceedings of the 9th Python in science conference, 2010, https://conference.scipy.org/proceedings/scipy2010/pdfs/seabold.pdf
- 27. O’Dea I, Hunter MS, Anjos S. Life satisfaction and health-related quality of life (SF-36) of middle-aged men and women. Climacteric 1999; 2(2): 131–140. [DOI] [PubMed] [Google Scholar]
- 28. Huo N, Smith CY, Gazzuola Rocca L, et al. Association of premenopausal bilateral oophorectomy with restless legs syndrome. JAMA Netw Open 2021; 4(2): e2036058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schaedel Z, Holloway D, Bruce D, et al. Management of sleep disorders in the menopausal transition. Post Reprod Health 2021; 27(4): 209–214. [DOI] [PubMed] [Google Scholar]
- 30. Valentine TR, Kratz AL, Kaplish N, et al. Sleep-disordered breathing and neurocognitive function in multiple sclerosis: Differential associations across cognitive domains. Mult Scler 2023; 29(7): 832–845. [DOI] [PubMed] [Google Scholar]
- 31. Bove R, White CC, Fitzgerald KC, et al. Hormone therapy use and physical quality of life in postmenopausal women with multiple sclerosis. Neurology 2016; 87: 1457–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cree BAC, Hollenbach JA, Bove R, et al. Silent progression in disease activity-free relapsing multiple sclerosis. Ann Neurol 2019; 85(5): 653–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cree BAC, Arnold DL, Chataway J, et al. Secondary progressive multiple sclerosis: New insights. Neurology 2021; 97(8): 378–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Baroncini D, Annovazzi PO, De Rossi N, et al. Impact of natural menopause on multiple sclerosis: A multicentre study. J Neurol Neurosurg Psychiatry 2019; 90(11): 1201–1206. [DOI] [PubMed] [Google Scholar]
- 35. Ladeira F, Salavisa M, Caetano A, et al. The influence of menopause in multiple sclerosis course: A longitudinal cohort study. Eur Neurol 2018; 80(3–4): 223–227. [DOI] [PubMed] [Google Scholar]
- 36. Bove RM, Healy B, Augustine A, et al. Effect of gender on late-onset multiple sclerosis. Mult Scler 2012; 18(10): 1472–1479. [DOI] [PubMed] [Google Scholar]
- 37. Bove R, Healy BC, Musallam A, et al. Exploration of changes in disability after menopause in a longitudinal multiple sclerosis cohort. Mult Scler 2016; 22(7): 935–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Otero-Romero S, Midaglia L, Carbonell-Mirabent P, et al. Menopause does not modify disability trajectories in a longitudinal cohort of women with clinically isolated syndrome and multiple sclerosis followed from disease onset. Eur J Neurol 2022; 29(4): 1075–1081. [DOI] [PubMed] [Google Scholar]
- 39. Lynch S, Baker S, Nashatizadeh M, et al. Disability measurement in Multiple Sclerosis patients 55 years and older: What is the Expanded Disability Status Scale really telling clinicians? Mult Scler Relat Disord 2021; 49: 102724. [DOI] [PubMed] [Google Scholar]
- 40. Bove R, Okai A, Houtchens M, et al. Effects of menopause in women with multiple sclerosis: An evidence-based review. Front Neurol 2021; 12: 554375. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-msj-10.1177_13524585231197056 for Well-being at midlife: Correlates of mental health in ambulatory menopausal women with multiple sclerosis by Denisse Morales-Rodriguez, Annika Anderson, Alyssa Nylander, Stephanie Hsu, Jessica Singh, Will Rowles, Christine M Walsh, Tiffany J Braley and Riley Bove in Multiple Sclerosis Journal
Supplemental material, sj-jpg-2-msj-10.1177_13524585231197056 for Well-being at midlife: Correlates of mental health in ambulatory menopausal women with multiple sclerosis by Denisse Morales-Rodriguez, Annika Anderson, Alyssa Nylander, Stephanie Hsu, Jessica Singh, Will Rowles, Christine M Walsh, Tiffany J Braley and Riley Bove in Multiple Sclerosis Journal
Supplemental material, sj-jpg-3-msj-10.1177_13524585231197056 for Well-being at midlife: Correlates of mental health in ambulatory menopausal women with multiple sclerosis by Denisse Morales-Rodriguez, Annika Anderson, Alyssa Nylander, Stephanie Hsu, Jessica Singh, Will Rowles, Christine M Walsh, Tiffany J Braley and Riley Bove in Multiple Sclerosis Journal