Abstract
We evaluated the effect of an option B-plus Enhanced Adherence Package (BEAP), on early ART uptake in a randomized controlled trial. HIV-positive, ART naïve pregnant women in Lusaka, Zambia, were randomized to receive BEAP (phone calls/home visits, additional counseling, male partner engagement and missed-visit follow-up) versus standard of care (SOC). The primary outcome was initiating and remaining on ART at 30 days. Analysis was by intention to treat (ITT) using logistic regression. Additional per protocol analysis was done. We enrolled 454 women; 229 randomized to BEAP and 225 to SOC. Within 30 days of eligibility, 445 (98.2%) initiated ART. In ITT analysis, 82.5% BEAP versus 80.4% SOC participants reached primary outcome (crude relative risk [RR] 1.03; 95% confidence interval [CI] 0.91–1.16; Wald test statistic = 0.44; p-value = 0.66). In per protocol analysis, (92 participants (40.2%) excluded), 91.9% BEAP versus 80.4% SOC participants reached primary outcome (crude RR 1.14; 95% CI 1.02–1.29; Wald test statistic = 2.23; p-value = 0.03). Early ART initiation in pregnancy was nearly universal but there was early drop out suggesting need for additional adherence support.
Keywords: HIV seropositivity, Pregnant women, Antiretroviral therapy, Option B + uptake, Enhanced adherence
Introduction
Test and treat, for HIV infection, is now recommended to optimize health and reduce transmission [1]. In pregnancy, this is important as mother to child transmission is directly related to duration of antiretroviral therapy (ART) [2–4]. Test and treat for pregnant women (Option B +) was originally implemented in Malawi and provided an early example of feasibility and effectiveness [5, 6]. Although test and treat has been expanded to other HIV-positive populations, pregnant women remain a group with unique challenges. During the early period of implementation in Malawi, early attrition was noted to be 20 to 30% [7]. Strategies to optimize uptake of, adherence to, and retention on ART in pregnant women are needed.
In Zambia, Option B + was introduced in 2013 [8]. During early scale-up, issues similar to what was reported in Malawi emerged [9, 10]. Based on formative research with patients, community members, health workers, and other stakeholders, we developed an intervention called B + Enhanced Adherence Package (BEAP) to improve uptake of Option B +. We hypothesized that, when delivered immediately after Option B + eligibility, BEAP would increase ART uptake and augment early adherence. We evaluated the BEAP intervention in a randomized controlled trial.
Methods
Trial Design
This was a two-arm parallel individual-randomized controlled trial of HIV-positive pregnant women attending antenatal care. Participants were randomized to the intervention (BEAP) or the standard of care (SOC) in a ratio of 1:1. Field data collection, data entry and statistical analysis were not masked. Eligibility criteria did not change after trial commencement.
The University of Zambia Biomedical Research Ethics Committee (Lusaka, Zambia) and the University of North Carolina at Chapel Hill Institutional Review Board (Chapel Hill, NC, USA) approved the study. The study was reviewed in accordance with the Centers for Disease Control and Prevention (CDC) human research protection procedures and was determined to be research, but CDC investigators did not interact with human subjects or have access to identifiable data or specimens for research purposes. The trial was registered at ClinicalTrials.gov (trials number NCT02459678).
Participants
We sensitized and recruited HIV-positive pregnant women at their first antenatal visit, after receipt of an HIV positive result or within 7 days. Women become eligible for Option B + on the day of an HIV-positive diagnosis. Inclusion criteria were age 18 years and above, pregnancy and HIV-1/2-positivity. Exclusion criteria were previous ART initiation for own health or having taken ART for more than 7 days in the current pregnancy, intrauterine fetal demise, significant mental illness, and not intending to receive further care at the same facility. Women provided written informed consent. The study was conducted in three public sector clinics in Lusaka, Zambia between March 2017 and May 2018. The study sites were selected based on their large patient volumes and study space availability.
Study Procedures
We enrolled participants into the study after they had been enrolled into routine antenatal care at the Maternal and Child Health (MCH) clinic. Participants were counseled regarding PMTCT and recommended to start ART that same day unless there was a clinical indication to delay. We collected demographic and obstetrical information for each participant, and blood for standard tests including CD4. ART was dispensed to willing participants. Where antenatal appointments coincided with study visits, we conducted both at the study site. We followed up the women using the national ANC guidelines and the HIV care follow-up schedule [8]. We encouraged women who declined same day ART to return the following week for further counseling. The initial dispensation of antiretroviral drugs was for 14 days, followed by another 14-day refill; thereafter, monthly until the third month, then three-monthly. For this primary outcomes’ analysis, we report ART uptake and adherence by 30-days post study enrolment. Adherence was based on the assumption that the women possessed medication based on the last dispensed amounts as per pharmacy records.
BEAP Intervention
We developed the BEAP intervention based on multiple sources including in-depth interviews and focus groups with HIV-positive pregnant women and male partners during the early Option B + era in Zambia, review of the medical literature, and consultation with key PMTCT implementers, including from the Ministry of Health (MoH). These data sources suggested that in Zambia, interventions to increase option B + uptake should focus on correcting medication beliefs in conflict with PMTCT, addressing stigma, and encouraging support from male partners. Rather than target one specific barrier, we brought together a combination of elements that could be feasibly delivered in the community. These were: (a) Staffing—the BEAP team was a male and female pair of community workers, on a monthly stipend, who were members of the local community, trained in HIV counseling and testing, and had knowledge of facility-based HIV care procedures, (b) Timing—a BEAP counselor attempted to contact participants within 3 days of assignment to the BEAP arm in order to introduce themselves and discuss the services they could provide, (c) Optional services made available to the pregnant woman and her partner, which included:
One-on-one counseling regarding HIV/AIDS and maternal and child health delivered at a location chosen by the participant;
Home couples counseling and HIV testing;
Home male partner HIV testing; and
Male partner referral and linkage to ART (if male partner was HIV-positive)
Appointment reminders by phone call and/or SMS.
The BEAP intervention was designed to increase initiation and early adherence and retention on ART; however, the number, type, and intensity of services provided were at the choice of the pregnant woman and/or her partner. They were informed of what services were available and were free to choose all or any service combination. The BEAP team provided services to the intervention group within 7 days of study enrollment, and followed-up missed appointments during pregnancy for 3 months or until delivery if this occurred before 3 months. If a participant in the intervention arm was one day late for a visit, the respective BEAP team was informed to make an immediate follow-up phone call to confirm the missed visit, understand the reasons, and provide social support. If the 2-week visit was missed, the BEAP team was allowed to dispense a 3-day pack of ART to the woman at home or in the community to reduce treatment interruptions.
The BEAP team generally worked Monday-Fridays, during the day and only worked weekends on as needed basis. We closely monitored intervention delivery for feasibility, uptake, and fidelity to protocols. At every in-person or phone contact with clients, the BEAP counselors were required to complete a brief form documenting the topics discussed. Adherence to the intervention at 30 days was based on the intervention activities documented for each participant from the date of enrollment to 30 days post-enrollment. At 1 month after enrollment, we conducted a brief survey with participants in the BEAP arm to assess its acceptability.
Participants in the SOC arm received all their routine PMTCT services at the facility without any additional counseling and home follow-up services. In the SOC, the counseling strategy is based on group health education on pregnancy, birth preparedness, HIV, PMTCT and couple counseling for those who come with their partners. One-on-one counseling is only done during post-test HIV counseling.
Outcomes
The primary outcome was the proportion of women who initiated ART by and remained on ART at 30 days after Option B + eligibility. This composite outcome acknowledged that some women may not be ready to start same-day but take up ART soon after, while other women may accept the pills and then disengage from care, as noted in Malawi [7]. Both initiation and being on ART at 30 days were based on pharmacy records.
Sample Size Calculation
Programmatic data from the study setting estimated that the primary outcome occurred in 60% of newly diagnosed HIVpositive pregnant women in routine care. We anticipated that the BEAP intervention would increase the primary outcome to 75%. The primary analysis was to compare these proportions between the two groups using z-test. Therefore, a total of 432 (216 per arm) participants were required to give a 90% power at 2-sided 5% level of significance. We then adjusted for 5% to account for lost-to-follow-up, giving 454 participants (227 per arm) to be enrolled in this study. The estimation was done in Stata 15 MP (StataCorp, College Station, TX, USA).
Randomization: Sequence Generation and Implementation
In order to compare the effect of the BEAP intervention versus the current Ministry of Health-endorsed SOC counseling strategy, participants were randomized in a 1:1 ratio to either the BEAP intervention or the SOC arm. To decrease the disparity between the 2 arms, we used computer-generated randomization with blocks of 2, 4, and 6. Sealed opaque envelopes containing the randomization assignment or list were created by the trial statistician (SB) at the Centre for Infectious Diseases Research in Zambia before study activation. The randomization list was generated using a computerized random number generator in Stata 15 MP (StataCorp, College Station, TX, USA). After signing the consent form, the participant was assigned a study ID, and in the presence of the participant the study nurse or research assistant opened the corresponding envelope revealing the assignment.
Statistical Methods
We used mean and proportions to summarize individual and facility-level characteristics as appropriate. We presented baseline characteristics by randomization group to check for baseline imbalances after randomization. We assessed the effect of the intervention for our primary outcomes of interest through intent-to-treat analyses. We used generalized linear model (GLM) to estimate the effect of the intervention on the proportion of HIV-positive pregnant women who initiated and were on ART within 30 days post-Option B + eligibility date. We obtained crude relative risks (RRs) as our effect measure using log-link binomial-family specification in the GLM. Robust standard errors were calculated to allow for intra-site correlation. We also estimated risk difference (RD), using identity-link binomial-family specification, between intervention and SOC arms as well as the number needed to treat (NNT) in order to prevent one early loss from care. We planned a similar analysis adjusting for age, education, baseline CD4 count, disclosure of HIV status to a male partner at/before enrollment, initiation of ART at/before enrollment, and baseline characteristics determined to be imbalanced, if any. Where there was no convergence in the GLM specifications for the adjusted estimates, we used margins to derive RR and RD after logistic regression estimation. A per-protocol analysis was also performed where we dropped from analysis participants in the BEAP arm who did not receive BEAP services and repeated the analysis using similar model. Statistical significance was set at p < 0.05. We also performed subgroup analysis on parity (i.e. first-time mothers vs. all others), age (aged 18–24 vs. 25 +), baseline HIV disclosure status (disclosure to partner vs no disclosure), and baseline CD4 (< 350 cells/μl vs ≥ 350 cells/μl). All analyses were performed in Stata 15 MP (Stata-Corp, College Station, Texas, USA).
Results
Between March 02, 2017 and December 18, 2017, 717 women were screened. Of those, 215 were eligible but not interested in participation, 48 were ineligible, and 454 enrolled (68% of eligible). During randomization, 229 were assigned to BEAP and 225 to the SOC (Fig. 1). The median age was 26.9 years (IQR 23.6–31.8), median gestational age was 22.0 weeks (IQR 18–25), and 418 (92.1%) indicated having started ART within 7 days of enrolment. The prevalence of initiating ART on the same day as HIV diagnosis among study participants was 74.7%. There were no significant differences by study arm in the prevalence of initiating ART on the same day as HIV diagnosis (Chi-squared test statistic = 0.74; p = 0.39), initiation of ART at/before enrollment (Chi-squared test statistic = 1.5; p = 0.22), or other baseline characteristics (Table 1). Prior to the primary outcome, one participant who had initiated ART withdrew consent and was excluded from all further study activities.
Fig. 1.
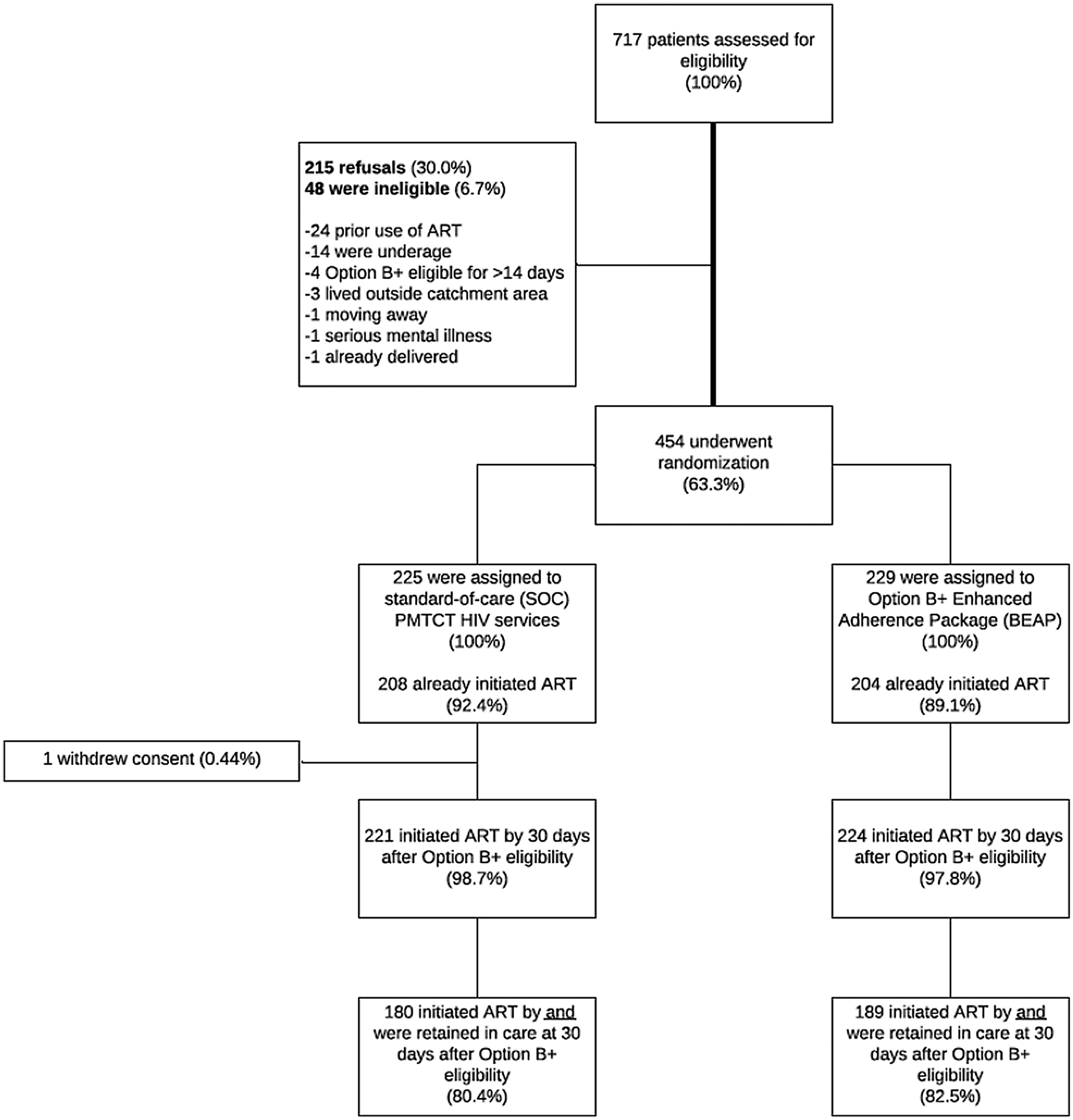
For each group, the numbers of participants who were randomly assigned, received intended treatment, and were analysed for the primary outcome in intent-to-treat (ITT) analysis
Table 1.
Baseline descriptive characteristics of enrolled participants (N = 454)
| Characteristic at baseline | Study arm | ||
|---|---|---|---|
| BEAP (n = 229) | Standard-of-care (n = 225) | p-value | |
| Age, years | 26.3 (23.3–31.7) | 27.5 (24.0–32.0) | .16 |
| Gestational age, weeks | 22 (18–25) | 23 (19–26) | 0.09 |
| Number of pregnancies | 3 (2–4) | 3 (2–4) | 0.23 |
| Number of live births | 2 (1–3) | 2 (1–3) | 0.37 |
| Marital status | 0.48 | ||
| Cohabitating/married | 200 (87.3) | 199 (88.4) | |
| Single/widowed/divorced | 29 (12.7) | 26 (11.6) | |
| Educational level | 0.28 | ||
| None | 15 (6.6) | 11 (4.9) | |
| Nursery/primary | 69 (30.1) | 87 (38.7) | |
| ≥ Secondary | 145 (63.3) | 127 (56.4) | |
| Initiated ART on same day as diagnosisa | 167 (72.9) | 172 (76.8) | 0.30 |
| Initiated ART at/before enrollment | 204 (89.1) | 208 (92.4) | 0.32 |
| CD4 countb | 287 (195.5–392.5) | 317 (201–426) | 0.20 |
| CD4 count b | 0.14 | ||
| < 350 cells/mm3 | 113 | 99 | |
| ≥ 350 cells/mm3 | 63 | 76 | |
| Clinic | 1.00 | ||
| Kanyama | 76 (33.2) | 75 (33.3) | |
| Chawama | 77 (33.6) | 75 (33.3) | |
| Matero | 76 (33.2) | 75 (33.3) | |
| Disclosure of HIV status to male partner at/before enrollment | 84 (36.7) | 73 (32.4) | 0.22 |
| Participant aware of male partner’s HIV status | 77 (33.6) | 64 (28.4) | 0.28 |
| Of those aware, whose partner is positive | 39 (50.6) | 40 (62.5) | 0.25 |
Data are in n (%) or Median (IQR) with Chi-Squared and Wilcoxon Rank-Sum p-values, respectively
1 missing value (0.22%)
103 missing values (23%)
After enrollment and within 30 days of Option B + eligibility, an additional 34 participants initiated ART, bringing the total to 445 (98.2%). The number who met the primary outcome (initiated and on ART at 30 days) was 369 (81.5%) as 76 women who initiated were not in possession of medication as per pharmacy records at 30 days. Among women assigned to the intervention arm who provided information on specific BEAP services they were interested in receiving (160 of 229 randomized to BEAP), services desired included: one-on-one counseling for 143 (89.4%), appointment reminders for 132 (82.5%), male partner outreach for 50 (31.3%), home couple counseling in 30 (18.8%), home partner testing in 20 (12.5%), and male partner referrals for 11 (6.9%) of the women. Per documentation, 136 of 229 (59.4%) participants in the BEAP arm received BEAP services (defined as receiving at least one of the services offered by the BEAP team) prior to primary outcome at 30 days. BEAP counselors reported 2 main challenges to delivery of the intervention: lack of or incorrect phone number and/or home address, which undermined their ability to reach clients, and lack of privacy in densely populated areas, which made it hard to provide confidential services. Relative to the number of women indicating interest in each specific BEAP service, services received included: one-on-one counseling for 128 (89.5%), appointment reminders for 92 (69.7%), male partner outreach for 17 (34.0%), home couple counseling in 3 (9.7%), home partner testing in 2 (10.0%), and male partner referrals for 4 (36.4%) of the women. All the services received were less than the number that the women had indicated a desire for.
Effect of the BEAP Intervention on ART Initiation and Remaining on ART at 30 Days (ITT)
Compared to the 180 (80.4%) patients in the SOC arm, 189 (82.5%) randomly assigned to BEAP initiated and were on ART at 30 days, representing a crude relative risk (RR) of 1.03 (95% CI 0.91, 1.16; Wald test statistic = 0.44; p = 0.66) and a risk difference (RD) of 2.18 percentage points (95% CI − 7.73, 12.09; Wald test statistic = 0.43; p = 0.67) in our ITT analysis (Table 2). Similarly, in adjusted analyses those randomly assigned to BEAP had 1.03 times the risk of ART initiation and remaining on ART compared with patients in the SOC arm (95% CI 0.92, 1.17; Wald test statistic = 0.52; p = 0.59), representing an adjusted risk difference of 2.70 percentage points (95% CI: − 7.32, 12.73; Wald test statistic = 0.51; p = 0.60). The number of patients needed to provide BEAP in order to prevent one early loss from care was 46 in unadjusted analyses and 37 in adjusted analyses. Subgroup analyses assessing for potential effect modification by parity, age, baseline HIV partner disclosure status, and baseline CD4 count revealed no significant results in unadjusted and adjusted analyses.
Table 2.
Relative risk of initiating and adhering to ART at 30 days post-B+ eligibility date for HIV-positive pregnant women enrolled in enhanced adherence package (BEAP) compared to those + in standard-of-care, intent-to-treat and per-protocol analyses, Lusaka, Zambia, 2017
| Analysis | n (%) | Crude relative risk (95% Cl) | Wald test statistic (p-value) | Adjusted Relative riska (95% Cl) | Wald test statistic (p-value) | Crude risk difference (95% Cl) | Wald test statistic (p-value) | Adjusted risk differencea (95% Cl) | Wald test statistic (p-value) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Intention to treat (N = 453) | ||||||||||
| SOC | 224 | 180 (80.4) | Reference 1.03 | Reference 1.03 | Reference 2.18 | Reference 2.70 | ||||
| BEAP | 229 | 189 (82.5) | (0.91, 1.16) | 0.44 (0.66) | (0.92, 1.17) | 0.52 (0.59) | (− 7.73, 12.09) | 0.43 (0.67) | (− 7.32, 12.73) | 0.51 (0.60) |
| Per Protocol (N = 360)b | ||||||||||
| SOC | 224 | 180 (80.4) | Reference 1.14 | Reference 1.15 | Reference 11.55 | Reference 12.26 | ||||
| BEAP | 136 | 125 (91.9) | (1.02, 1.29) | 2.23 (0.03) | (1.04, 1.28) | 2.67 (0.008) | (6.53, 22.46) | 2.08 (0.04) | (2.43, 22.09) | 2.45 (0.01) |
Adjusted models included age, education, baseline CD4 count, disclosure of HIV status to a male partner at/before enrollment, and initiation of ART at/before enrollment
93 participants (20.5%) were excluded from the per protocol analysis
Per Protocol Analysis
In a per protocol analysis, we repeated our analysis after excluding, from the BEAP arm, the 92 participants who did not receive any BEAP services, as well as 1 participant that was included despite failure to meet enrolment eligibility. Among the 136 in BEAP who received early services, 125 (91.9%) had initiated and were on ART at 30 days, compared with 80.4% in SOC arm (crude RR of 1.14; 95% CI 1.02, 1.29; Wald test statistic = 2.23; p = 0.03; and a 11.55 percentage point RD; Wald test statistic = 2.08; p = 0.04) (Table 2).
The association between BEAP and ART initiation and being on ART at 30 days remained statistically significant after adjustment for potential confounders (adjusted RR of 1.15; 95% CI 1.04, 1.28; Wald test statistic = 2.67; p = 0.008; and a 12.26 percentage point RD; Wald test statistic = 2.45; p = 0.01). No significant variation was found in the effect of BEAP on the primary outcome in subgroup analyses, and the number needed to be provided with BEAP in order to prevent one early loss from care was 9 in unadjusted analyses and 8 in adjusted analyses.
Acceptability of the BEAP
We obtained responses to the BEAP acceptability survey from 136 of the 229 women in the BEAP arm (response rate = 59.4%). Among these women, 119 (87.5%) stated that being visited at home by the BEAP team was either very acceptable (N = 75; 55.1.0%) or acceptable (N = 44; 32.4%). Feedback on the acceptability of receiving phone calls from the BEAP team was available for 134 women; 117 of whom (87.3%) stated that receiving a phone call from the BEAP team was either very acceptable (N = 75; 56.0%) or acceptable (N = 42; 31.3%).
Discussion
ART initiation within 30 days of eligibility among HIV-positive pregnant women at three urban sites in Zambia was nearly universal; however, like other reports, early signs of disengagement from ART were seen [7, 11, 12]. Slightly higher number of women who were randomised to the BEAP intervention were more likely to initiate and adhere to ART at 30 days compared to those on standard of care. Though the increase in ART initiation and adherence was not statistically significant, our findings highlight high initial option B + program uptake in Zambia, suggesting that interventions like BEAP could help to optimize uptake.
The BEAP intervention was developed in response to data from Malawi, Zambia, and other sites, that showed that many PMTCT clients may not be ‘ready’ for same day lifelong ART. Among eligible women randomized to the intervention, 55.9% received an initial visit for the BEAP intervention, which included home/community counseling to reinforce the benefits of ART among those who initiated and quell persistent myths around ART use in pregnancy, rapid follow-up to women late for their first follow-up, and/or support with partner disclosure. Including only the 55.9% in the per protocol analysis showed a statistically significant ART uptake compared to standard of care. However, the per protocol analysis might have overestimated the effect of BEAP possibly because the about 44% of the women randomised to BEAP but did not receive BEAP might be those who were not ‘ready’ to initiate and adhere to ART at 30 days. While none of these services are innovative by themselves, this was the first time a package intervention was evaluated on option B + outcomes in a rigorous trial in Zambia. These data build on a growing number of PMTCT interventions to optimize the outcomes of the Option B + policy [13–17]. Unlike most, we focused entirely on initial uptake and adherence. Our trial also demonstrates the importance of careful measurement of intervention fidelity to facilitate per protocol analyses.
Our study has several limitations. First, only one-third of 18 + year-old women who presented for Option B + at the study facilities enrolled in the study, which may have led to enrollment of a selected group more likely to take up ART. This would have led us to conclude that B + uptake was higher than reality but would not have undermined our interpretation of the marginal effect of the intervention in real-world setting. However, we enrolled 76% of women who were screened for study participation. Also, inclusion of adolescent pregnant women would have strengthened these data as programmatic data indicated that adolescents represent approximately 20% of women receiving PMTCT services. Challenges encountered in the community such as lack of privacy and inability to locate participants due to wrong addresses and/or phone numbers affected full implementation of the intervention. Although both home visits and phone calls were reported to be either very acceptable or acceptable by the BEAP participants, we received a response rate on acceptability survey from only 59.4% of the women in the BEAP arm. It is possible that women who did not find the intervention acceptable may have opted out of the survey. Another limitation was our inability to exclude selftransfers to other facilities. Some women classified as having stopped ART may have been on it at another facility. Silent transfer to other health facilities is a common phenomenon and has been reported in other settings [18–20]. While the proportion achieving the primary outcome overall may be inaccurate, we do not believe this would have altered our evaluation of the BEAP intervention. Finally, these data may not be generalizable to settings outside Lusaka.
Although these are clinical trial data, we believe these results provide real world insights that are valuable in other settings. The study team was embedded in the public clinics and we conducted study activities during regular PMTCT and ART visits. Therefore, our participants likely reflected HIV-positive pregnant women not on ART in Zambia and other similar countries, and our study outcomes would be representative of non-study conditions. While the BEAP was resource intensive, standards provided to all women reflected what is provided in the facility and these data can inform program management. Finally, qualitative research, not presented in this paper, suggested that barriers to B + uptake and retention were similar between Zambia and other African settings [21, 22]. Therefore, an intervention such as BEAP would likely be feasible and useful in other settings. In a study in Nigeria using a combination intervention that included a community component, ART initiation and retention were significantly improved in a PMTCT program that was option B-based [23]. In that study, retention was assessed until 12 weeks postpartum. Poor early retention, however, has been identified as a barrier to timely viral load suppression [24], an important factor in prevention of mother to child transmission of HIV [4]. Our study was focused on ART initiation, in the first month of ART after antenatal booking, and adherence. It would have been helpful to assess the intervention effect for a longer period. A study in Malawi found that 47% of women in option B + who were lost to followup never came back for a followup visit after the first ARV dispensation [25]. We believe that improving early adherence would impact on longer-term adherence which is critical for pregnancy outcomes. Other interventions to improve ART uptake and/or retention have included conditional cash transfers, use of lay cadres, integration of PMTCT into routine care and continuous quality improvement [26–29]. Whereas different interventions have been effective, combination interventions have been found more useful [28].
Conclusion
Our findings showed modest improvement in ART uptake among option B + women using a multicomponent community-based intervention (BEAP). Since BEAP is relatively resource-intensive, it may be utilized for women identified as most in need of additional support rather than the general population. These are some of the first option B + data to be published highlighting high overall initial uptake in the Zambian context. Further research on the effects of such an intervention on long-term outcomes, as well as its cost effectiveness, is needed.
Acknowledgements
This research has been supported by the President’s Emergency Plan for AIDS Relief (PEPFAR) through the United States Centers for Disease Control and Prevention (CDC) under the terms of cooperative agreement number U01GH000530. Additional investigator support was provided by the National Institutes of Health (K24 AI120796). The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the funding agencies. The authors acknowledge the B-Plus study team for their work in participant recruitment and follow-up; and all the HIV infected pregnant women who participated in the study.
Footnotes
This trial was registered at ClinicalTrials.gov (trials number NCT02459678) on May 14, 2015.
References
- 1.WHO. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Recommendations for a public health approacg. 2nd ed. Geneva: WHO; 2016. [PubMed] [Google Scholar]
- 2.Chagomerana MB, Miller WC, Tang JH, Hoffman IF, Mthiko BC, Phulusa J, et al. Optimizing prevention of HIV mother to child transmission: duration of antiretroviral therapy and viral suppression at delivery among pregnant Malawian women. PLoS ONE. 2018;13(4):e0195033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Myer L, Phillips TK, McIntyre JA, Hsiao NY, Petro G, Zerbe A, et al. HIV viraemia and mother-to-child transmission risk after antiretroviral therapy initiation in pregnancy in Cape Town. S Afr HIV Med. 2017;18(2):80–8. [DOI] [PubMed] [Google Scholar]
- 4.Mandelbrot L, Tubiana R, Le Chenadec J, Dollfus C, Faye A, Pannier E, et al. No perinatal HIV-1 transmission from women with effective antiretroviral therapy starting before conception. Clin Infect Dis. 2015;61(11):1715–25. [DOI] [PubMed] [Google Scholar]
- 5.van Lettow M, Bedell R, Mayuni I, Mateyu G, Landes M, Chan AK, et al. Towards elimination of mother-to-child transmission of HIV: performance of different models of care for initiating lifelong antiretroviral therapy for pregnant women in Malawi (Option B+). J Int AIDS Soc. 2014;17(1):18994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalua T, Barr BAT, Van Oosterhout JJ, Mbori-Ngacha D, Schouten EJ, Gupta S, et al. Lessons learned from option B+ in the evolution toward “test and start” from Malawi, Cameroon, and the United Republic of Tanzania. J Acquir Immune Defic Syndr. (1999). 2017;75(Suppl 1):S43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tenthani LHA, Tweya H, et al. Retention in care under universal antiretroviral therapy for HIV-infected pregnant and breastfeeding women (‘Option B+’) in Malawi. AIDS. 2014;28(4):589–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ministry of Health Z. Zambia Consolidated Guidelines for Treatment and Prevention of HIV Infection. Lusaka: Ministry of Health Z; 2014. [Google Scholar]
- 9.Tweya H, Gugsa S, Hosseinipour M, Speight C, Ng’ambi W, Bokosi M, et al. Understanding factors, outcomes and reasons for loss to follow-up among women in O ption B+ PMTCT programme in L ilongwe, M alawi. Trop Med Int Health. 2014;19(11):1360–6. [DOI] [PubMed] [Google Scholar]
- 10.Haas AD, Tenthani L, Msukwa MT, Tal K, Jahn A, Gadabu OJ, et al. Retention in care during the first 3 years of antiretroviral therapy for women in Malawi’s option B+ programme: an observational cohort study. Lancet HIV. 2016;3(4):e175–e182182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dionne-Odom J, Massaro C, Jogerst KM, Li Z, Deschamps M-M, Destine CJ, et al. Retention in care among HIV-infected pregnant women in haiti with PMTCT option B. AIDS Res Treat. 2016. 10.1155/2016/6284290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chan AK, Kanike E, Bedell R, Mayuni I, Manyera R, Mlotha W, et al. Same day HIV diagnosis and antiretroviral therapy initiation affects retention in Option B+ prevention of mother-to-child transmission services at antenatal care in Zomba District, Malawi. J Int AIDS Soc. 2016;19(1):20672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colvin CJ, Konopka S, Chalker JC, Jonas E, Albertini J, Amzel A, et al. A systematic review of health system barriers and enablers for antiretroviral therapy (ART) for HIV-infected pregnant and postpartum women. PLoS ONE. 2014;9(10):e108150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geldsetzer P, Yapa HMN, Vaikath M, Ogbuoji O, Fox MP, Essajee SM, et al. A systematic review of interventions to improve post-partum retention of women in PMTCT and ART care. J Int AIDS Soc. 2016;19(1):20679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nance N, Pendo P, Masanja J, Ngilangwa DP, Webb K, Noronha R, et al. Short-term effectiveness of a community health worker intervention for HIV-infected pregnant women in Tanzania to improve treatment adherence and retention in care: A clusterrandomized trial. PLoS ONE. 2017;12(8):e0181919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rollins NC, Essajee SM, Bellare N, Doherty M, Hirnschall GO. Improving retention in care among pregnant women and mothers living with HIV: lessons from INSPIRE and implications for future WHO guidance and monitoring. J Acquir Immune Defic Syndr. (1999). 2017;75(2):S111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herce ME, Mtande T, Chimbwandira F, Mofolo I, Chingondole CK, Rosenberg NE, et al. Supporting option B+ scale up and strengthening the prevention of mother-to-child transmission cascade in central Malawi: results from a serial cross-sectional study. BMC Infect Dis. 2015;15(1):328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geng EH, Odeny TA, Lyamuya R, Nakiwogga-Muwanga A, Diero L, Bwana M, et al. Retention in care and patient-reported reasons for undocumented transfer or stopping care among HIV-infected patients on antiretroviral therapy in Eastern Africa: application of a sampling-based approach. Clin Infect Dis. 2015;62(7):935–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Domercant JW, Puttkammer N, Young P, Yuhas K, François K, Grand Pierre R, et al. Attrition from antiretroviral treatment services among pregnant and non-pregnant patients following adoption of Option B+ in Haiti. Global Health Action. 2017;10(1):1330915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zürcher K, Mooser A, Anderegg N, Tymejczyk O, Couvillon MJ, Nash D, et al. Outcomes of HIV-positive patients lost to followup in African treatment programmes. Tropical Med Int Health. 2017;22(4):375–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buregyeya E, Naigino R, Mukose A, Makumbi F, Esiru G, Arinaitwe J, et al. Facilitators and barriers to uptake and adherence to lifelong antiretroviral therapy among HIV infected pregnant women in Uganda: a qualitative study. BMC Pregnancy Child-birth. 2017;17(1):94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hodgson I, Plummer ML, Konopka SN, Colvin CJ, Jonas E, Albertini J, et al. A systematic review of individual and contextual factors affecting ART initiation, adherence, and retention for HIV-infected pregnant and postpartum women. PLoS ONE. 2014;9(11):e111421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aliyu MH, Blevins M, Audet CM, Kalish M, Gebi UI, Onwujekwe O, et al. Integrated prevention of mother-to-child HIV transmission services, antiretroviral therapy initiation, and maternal and infant retention in care in rural north-central Nigeria: a cluster-randomised controlled trial. Lancet HIV. 2016;3(5):e202–e211211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mugavero MJ, Amico KR, Westfall AO, Crane HM, Zinski A, Willig JH, et al. Early retention in HIV care and viral load suppression: implications for a test and treat approach to HIV prevention. J Acquir Immune Defic Syndr. (1999). 2012;59(1):86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mitiku I, Arefayne M, Mesfin Y, Gizaw M. Factors associated with loss to follow-up among women in Option B+ PMTCT programme in northeast Ethiopia: a retrospective cohort study. J Int AIDS Soc. 2016;19(1):20662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yotebieng M, Thirumurthy H, Moracco KE, Kawende B, Chalachala JL, Wenzi LK, et al. Conditional cash transfers and uptake of and retention in prevention of mother-to-child HIV transmission care: a randomised controlled trial. Lancet HIV. 2016;3(2):e85–e93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DiCarlo A, Fayorsey R, Syengo M, Chege D, Sirengo M, Reidy W, et al. Lay health worker experiences administering a multi-level combination intervention to improve PMTCT retention. BMC Health Serv Res. 2018;18(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vrazo AC, Firth J, Amzel A, Sedillo R, Ryan J, Phelps BR. Interventions to significantly improve service uptake and retention of HIV-positive pregnant women and HIV-exposed infants along the prevention of mother-to-child transmission continuum of care: systematic review. Trop Med Int Health. 2018;23(2):136–48. [DOI] [PubMed] [Google Scholar]
- 29.Fayorsey RN, Wang C, Chege D, Reidy W, Syengo M, Owino SO, et al. Effectiveness of a Lay Counselor-led combination intervention for retention of mothers and infants in HIV Care: a randomized trial in Kenya. J Acquir Immune Defic Syndr. (1999). 2019;80(1):56. [DOI] [PMC free article] [PubMed] [Google Scholar]


