ABSTRACT
The antimicrobial resistance (AMR) in gonorrhea poses global threat of increasing public health concern. In response to this concern, molecular surveillance has been widely utilized to detail the changes in the evolution and distribution of Neisseria gonorrhoeae during AMR transmission. In this study, we performed a comprehensive molecular surveillance of 664 N. gonorrhoeae isolates collected in Shenzhen, one of the cities with the largest mobile population in China, 2019–2020. In 2020, ceftriaxone showed an unprecedented high resistance rate of 24.87%, and 67.83% of the ceftriaxone-resistant (Cro-R) isolates harbored a nonmosaic penA allele. The Cro-R isolates with nonmosaic penA alleles showed a tremendous increasing trend from 0.00% in 2014 to 20.45% in 2020, which proves the need for monitoring nonmosaic penA-related resistance. Importantly, genotyping indicated that multilocus sequence typing ST11231 (35.71%) had a notable rate of ceftriaxone resistance, which might become the focus of future surveillance. Whole-genome sequencing analysis showed that the internationally spreading FC428 clones have circulated in Shenzhen region with typical ceftriaxone resistance (MIC ≥ 0.5 mg/L) maintained. Our surveillance combined with genomic analysis provides current information to update gonorrhea management guidelines and emphasizes that continuous AMR surveillance for N. gonorrhoeae is essential.
IMPORTANCE
We conducted a comprehensive molecular epidemiology analysis for antimicrobial-resistant Neisseria gonorrhoeae in Shenzhen during 2019–2020, which provided important data for personalized treatment and adjustment of monitoring strategy. Briefly, the proportion of ceftriaxone-resistant (Cro-R) isolates reached a stunning prevalence rate of 24.87% in 2020. A typical increment of Cro-R isolates with nonmosaic penA alleles proves the necessity of monitoring nonmosaic AMR mechanism and involving it into developing molecular detection methods. Whole-genome sequencing analysis showed that the international spreading FC428 clone has been circulating in Shenzhen with typical ceftriaxone resistance (MIC ≥ 0.5 mg/L) maintained. In summary, we conducted a comprehensive epidemiology study, providing significant data for therapy management. Our results not only improve the understanding of the distribution and transmission of AMR in N. gonorrhoeae but also provide effective AMR data for improving surveillance strategies in China.
KEYWORDS: molecular surveillance, antimicrobial resistance, multilocus sequence typing, N. gonorrhoeae sequence typing for antimicrobial resistance, whole-genome sequencing
INTRODUCTION
Gonorrhea, an infection caused by Neisseria gonorrhoeae, is a common global sexually transmitted disease, with an estimated 82 million annual cases (https://www.who.int/teams/immunization-vaccines-and-biologicals/diseases/neisseria-gonorrhoeae). Antimicrobial resistance (AMR) has developed for most antimicrobials in the past few decades and has become a growing global public health burden that requires global monitoring and intervention (1). Excessive use and/or misuse of antimicrobials, limitations in surveillance and control of AMR, and lagging updates of guidelines will drive N. gonorrhoeae to acquire and develop AMR (2). N. gonorrhoeae has been included in “priority pathogens” by the WHO since 2017 due to the emergence of multi-drug-resistant isolates and rapid growth of antimicrobial resistance rate (3).
It has been proposed to undertake multiple prospective studies on slow-growing resistance to combat widespread AMR in N. gonorrhoeae; these include pharmacokinetics/pharmacodynamics (PK/PD) analyses to predict which antimicrobial has the highest likelihood of successful treatment of gonorrhea (4). Another mathematical model aims to repurpose “abandoned” antimicrobial through calculating fitness benefit/cost (5). These promising strategies require AMR surveillance data, reinforcing the importance and urgency for comprehensive surveillance. Nevertheless, the actual AMR distribution of N. gonorrhoeae remains vague in most settings because phenotype surveillance produces gaps in AMR surveillance, which is mainly caused by the limited availability of culture methods (6). Moreover, resistant-related isolates with novel penA alleles are constantly emerging (7), and therefore, timely surveillance is the only approach to ensure that local guidelines match the actual mode of AMR in N. gonorrhoeae (6).
Notably, molecular surveillance fills the gaps in interpretability and comparability, as well as significant differences in the methodology from several regions. Genetic analysis was the most popular molecular analysis for tracking AMR in N. gonorrhoeae, including whole-genome sequencing (WGS), multilocus sequence typing (MLST), and N. gonorrhoeae sequence typing for antimicrobial resistance (NG-STAR) methods.
Globally, N. gonorrhoeae MLST ST1901, ST1903, and ST7363 are the most prevalent MLST genotypes among cefixime-resistant and ceftriaxone-resistant (Cro-R) N. gonorrhoeae strains (8, 9). In our previous study, ST7363 was predicted to have the potential to become the next internationally spreading Cro-R sequence type after ST1901 in Shenzhen (10). Another resistance clone of international concern is the FC428 clone (MLST ST1903 and NG-STAR ST233), which has circulated worldwide since it was first reported in Japan (11). Given its typical resistance phenotype, this clone has become the focus of global epidemiological investigations (12).
The rapidly increasing cases of gonorrhea in China place a heavy burden on the country’s healthcare system; in 2021, 127,803 new cases of gonorrhea were reported (http://www.nhc.gov.cn/). However, the incidence of gonorrhea is most likely highly underestimated in China, due to the suboptimal diagnostics, screening, reporting, and epidemiological surveillance performed in many settings. Luo et al. reported an overall prevalence of 0.17% (95% CI, 0.11%–0.28%) for gonorrhea in Shenzhen in 2018 (13), which showed 50% higher than the national rates (1999–2000) reported in 2003 (14). Based on the current situation, high-risk areas should be prioritized while improving the national monitoring capacity. Shenzhen, an economic zone in southern coastal China, has the highest incidence and spread of gonorrhea, given its rapid economic growth, increasing active migrant population, and the permanent residents’ average age is only 32.5 years (15). The special demographic characteristics of highly developed economies make Shenzhen a likely hotspot for gonorrhea, making the region a focus for national surveillance. Therefore, it is vital to monitor AMR in gonorrhea in the Shenzhen region, which would be of great benefit to the national surveillance strategy.
Previously, we performed a comprehensive epidemiological study and explored the correlation between AMR and specific STs using 909 gonococcal isolates in Shenzhen from 2014 to 2018, which provides important data for studies of molecular epidemiology of AMR in N. gonorrhoeae (10). Briefly, the increase in ceftriaxone resistance was observed from 0.00% in 2014 and 2016 to 3.62% in 2018 (10); however, the data are limited. Therefore, in this study, to investigate whether this increment would become a potential threat, we performed a follow-up AMR surveillance of 664 N. gonorrhoeae isolates collected from Shenzhen in 2019–2020 using a genome analysis. Seven AMR alleles in NG-STAR and seven housekeeping alleles in MLST were extracted from the WGS data using bioinformatics method and were used to investigate the evolution and variation of AMR and further identify the distribution of AMR in N. gonorrhoeae. Maximum-likelihood method was used to infer what the phylogenetic tree of 664 gonococcal isolates looks like. Overall, early and regular surveillance of Shenzhen, one of the areas with the highest prevalence of gonorrhea in China, can slow the spread of AMR in N. gonorrhoeae and allow time for the development of novel antimicrobials and vaccines. Notably, this is the first large-scale, WGS-based, follow-up molecular epidemiology study in the Shenzhen region that may be helpful for gonococcal AMR monitoring strategies and updating gonorrhea management guidelines.
RESULTS
A total of 664 N. gonorrhoeae isolates were collected (specifically 270 and 394 isolates in 2019 and 2020, respectively). Isolates were obtained from outpatients with gonorrhea who attended the Shenzhen Center for Chronic Disease Control from 2019 to 2020. No restrictions regarding age, gender, partner, and other behaviors of the patients were considered.
Demographic characteristics and antimicrobial susceptibility
Based on the demographic characteristics, 591 (89.01%) infections occurred in males with a median age of 30 years. Most patients (656, 98.80%) were heterosexually oriented, and 97.59% of the patients indicated having no previous gonorrhea infections. However, the proportion of patients with symptoms of abnormal discharge showed a significant decrease from 98.68% (897/909, 2014–2018) to 82.23% (546/664, 2019–2020) (chi-square test, P = 0.000) (10), indicating a possible increased missed diagnosis rate due to lack of obvious symptoms. The demographic characteristics of the isolates are listed in Table S1.
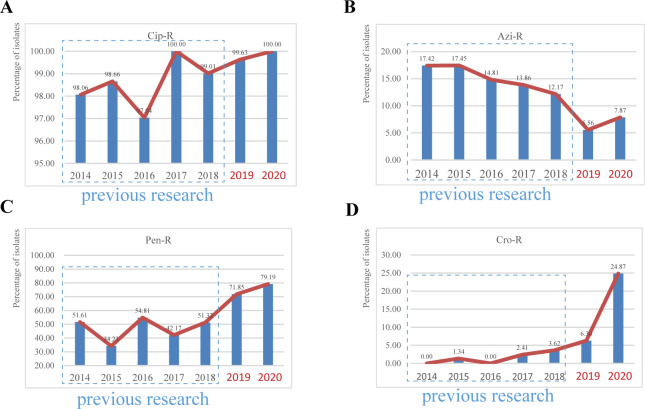
Antimicrobial susceptibility testing was performed for 664 N. gonorrhoeae isolates. All 664 N. gonorrhoeae isolates were susceptible to spectinomycin (Spec-S), and ciprofloxacin-resistant (Cip-R) isolates remained stable and high since 2014 (Fig. 1A) (10). Azithromycin-resistant (Azi-R) isolates showed a decrease from 12.17% in 2018 to 5.56% in 2019, followed by a slight increase to 7.87% in 2020 (Fig. 1B). Penicillin resistance (Pen-R) showed a steady rise (Fig. 1C). The prevalence rate of ceftriaxone resistance increased from 0.00% in 2014 to 6.30% in 2019, reaching a peak of 24.87% in 2020 (Fig. 1D). The rapid increase in ceftriaxone resistance suggests that the effect of ceftriaxone is powerful threated in Shenzhen that requires an urgent novel therapy. In addition, ST2473, ST1143, ST233, and ST506 range for the major types in the isolates possessing high ceftriaxone MIC values (Fig. S1).
Fig 1.
Major antimicrobial susceptibility of N. gonorrhoeae in Shenzhen, China, 2014–2020. Percentages of gonococcal isolates resistant to (A) Cip, (B) Azi, (C) Pen, and (D) Cro. Bars in dashes boxes were data from our previous research in Shenzhen for 2014–2018. Cip-R, resistant to ciprofloxacin; Azi-R, resistant to azithromycin; Pen-R, resistant to penicillin; Cro-R, resistant to ceftriaxone.
Molecular typing with MLST
A total of 73 MLST STs were identified, of which 11 were new STs to the database (Fig. S2A). The most common (top five) STs were ST8123 (92/664, 13.86%), followed by ST7363 (77/664, 11.60%), ST7360 (61/664, 9.19%), ST7365 (53/664, 7.98%), and ST1901 (45/664, 6.78%) (Fig. S2A). Compared to previous surveillance in Shenzhen from 2014 to 2018, among all major STs, the ST8123 (9.79% → 13.86%), ST7363 (9.35% → 11.60%), ST7360 (6.05% → 9.19%), and ST7365 (6.16% → 7.98%) increased slightly. Conversely, ST1901 (8.36% → 6.78%) and ST1600 (5.94% → 3.16%) showed a decreasing trend, suggesting that the fitness cost of the ST1901 and ST1600 clones may become higher for continued propagation in the Shenzhen region. ST1901 is widely associated with Cro-DS worldwide (4, 16), and ST1600 has been frequently reported in China because of general ceftriaxone resistance (MIC ≥ 0.75 mg/L) (12, 17). It is worth noting that ST11231, ST7365, and ST8123 showed a notable rate of Cro-R [35.71% (5/14), 28.30% (15/53), and 13.04% (12/92)] in 2019–2020, whereas these STs showed 100% susceptibility to ceftriaxone in 2014–2018. Besides, Cro-R ration of the predominant ST1901 showed significant increment in this study (5.26% → 15.56%) compared to previous AMR surveillance (10). The predominant ST types from 2019 to 2020 are listed in Table 1.
TABLE 1.
The detailed information of molecular typing of 664 isolates from Shenzhen in 2019–2020
| Year | No. of isolates with mosaic penA types (%) | Major mosaic penA types (no. of isolates) | No. of penA types | Major penA types (no. of isolates) | No. of NG-STAR STs | Major NG-STAR STs (no. of isolates) | No. of MLST STs | Major MLST STs (no. of isolates) |
|---|---|---|---|---|---|---|---|---|
| 2019 (270) |
62(22.96%) | 10.001(26) 34.007(18) 10.003(10) | 32 | 13.001(47) 21.001(34) 10.001(26) 18.001(23) 5.002(22) 34.007(18) 13.002(17) 12.001(13) 10.003(10) | 128 | 495(18) 506(14) 348(11) 2477(9) 1704(7) 501(7) 1102(7) 497(7) | 46 | 7363(40) 7360(35) 8123(25) 1901(24) 7365(17) 9899(14) 1600(11) 7827(10) |
| 2020 (394) |
81(20.56%) | 34.007(31) 10.001(18) 60.001(14) 10.003(8) | 39 | 12.001(54) 18.001(48) 13.001(44) 13.002(40) 34.007(31) 5.002(23) 21.001(22) 10.001(18) 2.002(17) 103.002(15) 60.001(14) 43.002(11) | 202 | 2473(27) 2477(14) 497(13) 1707(12) 2746(8) 1463(7) | 57 | 8123(67) 7363(37) 7365(36) 7360(26) 1901(21) 10314(18) 1903(13) 7822(12) 1583(11) 7367(11) 7827(11) 1600(10) 1928(10) 15251(10) |
| Total (664) |
143(21.54%) | 34.007(49) 10.001(44) 10.003(18) 60.001(15) | 51 | 13.001(91) 18.001(71) 12.001(67) 13.002(57) 21.001(56) 34.007(49) 5.002(45) 10.001(44) | 281 | 2473(33) 2477(23) 497(20) 495(18) 506(17) 1707(16) 348(13) 199(10) 1148(10) 1463(10) | 73 | 8123(92) 7363(77) 7360(61) 7365(53) 1901(45) 10314(27) 7827(21) 7822(21) 1600(21) |
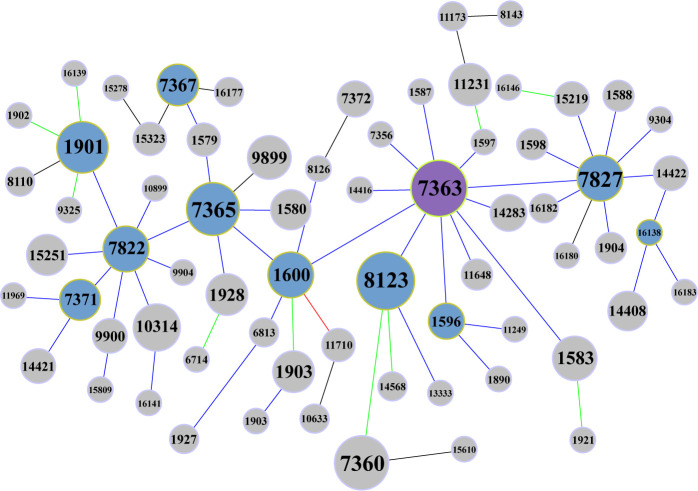
GoeBURST provides the ability to create groups of closely related strains from MLST data, which presumes that a clonal complex (lineage) is founded by a founder genotype, and genetic variants of that founder reflect the progressive accumulation of additional variations over time (18). The goeBURST divided the 73 MLST STs into 6 groups in this study (Table S2). Most isolates (655/664) were found in group 0. The group founder changed into the ESC-resistant-related ST7363 in Shenzhen for 2019 to 2020 from the local prevalent genotype ST7827 in Shenzhen for 2014 to 2018, using the goeBURST algorithm. Through a logistic regression analysis, ST7363 was found to be closely related to increased MIC of ceftriaxone and was predicted to serve as a reservoir for resistance-related STs (10). Fig. 2 shows that ST7827, ST8123, ST7365, and ST1901 are the subgroup founders with a high-frequency occurrence. Compared to previous surveillance (10), the number of subgroup founders typically decreased (17 → 10), which indicated that the genome complex during 2019–2020 showed a lower diversity.
Fig 2.
Group 0 of goeBURST analysis. The frequency of occurrence is represented by the font size of MLST STs and the size of the nodes. Node color: purple, group founder; desaturated blue, subgroup founder; light gray, common node. Link color: black, link drawn without recourse to tiebreak rules; blue, link drawn using tiebreak rule 1 (number of SLVs); green, link drawn using tiebreak rule 2 (number of DLVs); red, link drawn using tiebreak rule 3 (number of TLVs).
Molecular typing with NG-STAR
Typing for AMR using the NG-STAR scheme identified 281 NG-STAR types with 154 new STs (Fig. S2B). The most common (top five) STs were ST2473 (33/664, 4.97%), ST2477 (23/664, 3.46%), ST497 (20/664, 3.01%), ST495 (18/664, 2.71%), and ST506 (17/664, 2.56%) (Fig. S2B). ST2473 and ST497 were also predominant STs in the surveillance database for 2014–2018 (10). Notably, the Cro-R rate of the “old” predominant ST2473 and ST497 significantly increased from 0.00% (0/42) in 2014–2018 to 27.27% (9/33) and 3.13% (1/32) in 2014–2018 to 10% (2/20) in 2019–2020, respectively, which may indicate that these clones rely on the ability to improve MICs to resist strong selection pressure (Fig. 3). Besides, ST495 and ST506 showed a notable Cro-R rate of 16.67% (3/18) and 29.41% (5/17), respectively (Fig. 3). Consequently, consistent with the phenotypic analysis, the AMR rates of most antimicrobials increased by varying degrees during 2019–2020, which may result in additional recombination of AMR alleles produced in N. gonorrhoeae to deal with selective pressure (19). The distribution of different ST types from 2019 to 2020 is listed in Table 1.
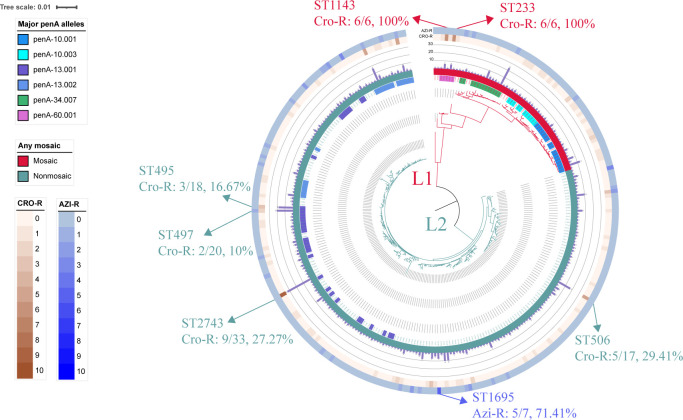
Fig 3.
Phylogenetic tree of 281 NG-STAR STs constructed using MEGA 11. The external color trips that range from inner to outer are as follows: trip 1, mosaic or nonmosaic penA allele, crimson, mosaic penA, cadet blue, nonmosaic penA; trip 2, bar chart showing number of isolates in each ST; trip 3, number of Cro-R isolates in each ST; trip 4, number of Azi-R isolates in each ST. STs indexed in the main text were indicated with arrows outside trips.
The phylogenetic analysis based on the seven AMR determinants showed that all 281 STs were grouped into two main clusters: L1 contains 59 STs (n = 143) that harbor mosaic penA alleles and L2 contains 222 STs (n = 521) that harbor nonmosaic penA alleles, respectively (Fig. 3). The proportion of Cro-R isolates in mosaic clusters (37/143, 25.87%) was significantly higher than that in nonmosaic clusters (78/521, 14.97%) (chi-square test, P = 0.004). The ST1143 and ST233 harboring penA-60.001 allele showed Cro-R rate of 100% (Fig. 3). In addition, the proportion of Azi-R isolates in mosaic clusters (4/143, 2.80%) was significantly lower than that in nonmosaic clusters (41/521,7.87%) (chi-square test, P = 0.037). Notably, the ST1695 showed a high Azi-R rate of 71.41% (5/7) (Fig. 3).
Characterization of AMR determinants and Cro-R isolates
There were 51 penA types among the 664 isolates analyzed, including 16 mosaic (n = 143, 21.54%) and 35 nonmosaic (n = 521, 78.46%) types. The most common mosaic penA allele was penA-34.007 (49/143, 34.27%), followed by penA-10.001 (44/143, 30.77%), penA-10.003 (18/143, 12.59%), and penA-60.001 (15/143, 10.49%). The proportion of isolates harboring mosaic penA alleles increased from 8.39% (13/155) in 2014 to 20.56% (81/394) in 2020 (Table 1). Along with this trend, the rate of Cro-R in the mosaic group increased from 0.00% (0/13) in 2014 to 41.98% (34/81) in 2020. The continuous increasing rate of mosaic penA is likely to facilitate the development of AMR in gonorrhea treatment. In particular, the mosaic penA-60.001 showed rapid growth from 0.60% (1/166) in 2017 to 3.55% (14/394) in 2020, which proves that FC428 is no longer sporadic in China. Additionally, 14.97% (78/521) of the isolates harboring nonmosaic penA showed resistance to ceftriaxone. The most prevalent nonmosaic penA types were penA-13.001 (n = 91) and penA-18.001 (n = 71), of which both clones were the predominant ST in China, suggesting that the AMR of the nonmosaic group mainly occurred in the local common clones (Table 1).
A total of 18 isolates with A311V alteration were identified, 15 of which were in the penA-60.001 allele with 1 in penA-214.001 and 2 in penA-195.001 allele. The latter two were novel mosaics in the database with Cro-R and cefixime-resistant phenotypes, which were reported for the first time in this study.
Phylogenomic analysis
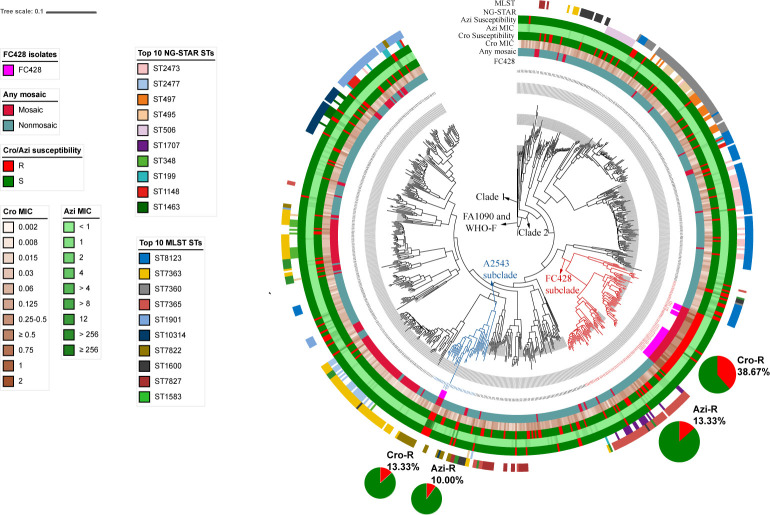
A maximum-likelihood phylogenetic analysis based on the core-genome alignment of 46305 SNP sites was performed. A total of 34 previously reported FC428 isolates and WHO-F were also mapped to FA1090 to construct a single-nucleotide variation phylogenetic tree. According to the ML phylogeny, the 698 gonococcal isolates (664 Shenzhen isolates plus 34 previously reported FC428 isolates) generated two distinct clades, namely, Clade 1 and Clade 2 (Fig. 4). Clade 1 represents that about 0.86% of the strains evolves earlier than Clade 2 that represents the rest of the strains (Fig. 4). Interestingly, in Clade 2, a total of 75 isolates from Shenzhen were observed closely related to 30 of the FC428 isolates and were clustered in the FC428 subclade (indicated in red in Fig. 4). Within the FC428 subclade, isolates from Shenzhen showed a high rate of Cro-R (38.67%, 29/75) as indicated in the pie charts (Fig. 4). Among the 29 Cro-R isolates in this subclade, 44.83% (13/29) and 51.72% (15/29) of the isolates harbored mosaic penA-60.001 and nonmosaic penA-13.002 allele, respectively. Besides, 48.28% (14/29) and 41.38% (12/29) of the Cro-R isolates were MLST ST7365 and ST1903, respectively. Both clones were closely related to ceftriaxone resistance among various surveillance, which was the main reason for the high proportion of ceftriaxone resistance in this clade (10, 20). Besides, in the FC428 subclade, 13.33% (10/75) isolates showed resistance to azithromycin, 60% (6/10) of which were the local predominant MLST ST8123.
Fig 4.
Maximum-likelihood phylogeny of 664 Shenzhen gonococcal isolates, 34 previously reported FC428 clones, and WHO-F. The maximum-likelihood phylogeny was constructed based on 46,305 SNP sites along the whole genome. The external color trips that range from inner to outer are as follows: trip 1, previously reported FC428 clones; trip 2, any mosaic penA alleles; trip 3, ceftriaxone MIC; trip 4, susceptibility categories of ceftriaxone (R, resistant; S, susceptible); trip 5, azithromycin MIC; trip 6, susceptibility categories of azithromycin (R, resistant; S, susceptible); trip 7, predominant NG-STAR STs; trip 8, predominant MLST STs. The percentages of Cro-R and Azi-R in FC428 subclade and A2543 subclade are represented by pie charts.
In addition, 30 Shenzhen isolates were closely clustered in a Cro-R and high-level Azi-R A2543 subclade (A2543, A2735, AT159, and WHO-Q), which showed evidence of circulating in Asia (21) (indicated in blue in Fig. 4). Isolates in this subclade showed a 13.33% and 10.00% rate of ceftriaxone resistance and azithromycin resistance, respectively (Fig. 4). Over 93% of isolates in A2543 subclade harbored a nonmosaic structure, and MLST ST7822 presented the most predominant MLST type. The NG-STAR type distribution showed a high diversity.
DISCUSSION
In this study, all antimicrobials, except for spectinomycin, showed varying levels of resistance since 2019. Ciprofloxacin and penicillin have been removed from the Chinese treatment guidelines for gonorrhea since 2007 (22); however, multidrug resistance was perpetuated at a high level (>70%) due to the high proportion (>50%) of prescription antimicrobials used in outpatient visits (23). Consequently, the repurposing of “old” antimicrobials is not applicable to the current situation in China (24). Fortunately, spectinomycin has been available since 2012 and might be considered as a potential option for the treatment of gonorrhea in Shenzhen. However, there is concern that resistance would be acquired rapidly once spectinomycin is introduced as a first-line monotherapy (2). Therefore, developing new antimicrobials and vaccines remains the most important interventions to curb widespread AMR of N. gonorrhoeae.
As predicted, such a substantial increase was found in the Cro-R group, from 0.00% in 2016 to 24.87% in 2020, which was mainly caused by the widespread and inappropriate use of ceftriaxone therapy (25). Notably, 5% or higher of Cro-R gonococcal isolates have been previously reported in Shanghai and Guangdong in China (26, 27), which indicates an alarming situation in which a period of untreatable gonorrhea may be near. To the best of our knowledge, this is the first report of a high rate of 24.87% ceftriaxone resistance. Besides, Shenzhen has unique demographic characteristics, such as large mobile population and immigrant population, and young permanent residents’ average age (15). This alarming situation highlights the need to enhance AMR monitoring in other countries because the AMR transmission around the world could be connected through travel (6). These studies should be focused on countries where ceftriaxone remains sufficient for most settings, such as Kyrgyzstan in Central Asia, a country near China, attached a perfect rate (100%) of susceptibility to ceftriaxone (28). This information reinforces the importance of the “comprehensive and specific monitoring” strategy, which is aimed at those countries that cannot afford the resources required for comprehensive surveillance, to nevertheless aim to monitor high-risk regions.
Among the 115 Cro-R isolates (115/664, 17.32%, Table 2), the isolates in mosaic group (37/143, 25.87%) showed a significant higher proportion than that in the nonmosaic group (78/521, 14.97%) (chi-square test, P = 0.004). However, an increase in nonmosaic penA-related Cro-R from 2014 (0/142, 0%) to 2020 (64/313, 20.45%) was observed, which strongly challenged the current strategies of molecular surveillance. The mosaic structure is widely recognized as a potential predictive marker for ceftriaxone resistance (29). The trend of increasing resistance rates in the nonmosaic penA group continues to weaken the effectiveness of current strategies and further reduce the efficacy of molecular epidemiology, which has been reported in several research (30, 31). In the Shenzhen region, the penA-13.002 (n = 15) and penA-60.001 (n = 15) showed the highest rate of Cro-R, and penA-13.002 is one of the most common local types in China, whereas it is rare in other countries, thereby decreasing the risk of global transmission (32). In addition, two novel mosaic penA types (penA-195.001, n = 2; penA-214.001, n = 1) were screened in this study and harbored penA A311V mutation, which is the well-known molecular marker to capture ceftriaxone-resistance-associated penA-60.001 (33). In addition, mutations in mtrR, ponA, and porB also provide contributions to resistance to beta-lactams (Table 2). Overall, the highly diverse mechanisms of ceftriaxone resistance pose a threat for the rapid detection of penA-60.001. This difficult situation highlights the importance of rapidly producing molecular targets for emerging resistant isolates based on basic science research.
TABLE 2.
Detailed information of 115 Cro-R isolates in Shenzhen, China, 2019–2020
| Details | |
|---|---|
| No. of isolates | 115 |
| No. of MLST STs | 32 |
| Predominant MLST STs | 7365(15) 1903(12) 8123(12) 1583(8) 1901(8) 7360(7) |
| No. of NG-STAR STs | 74 |
| Predominant NG-STAR STs | 2473(9) 233(6) 1143(6) 1696(5) 1707(5) 506(5) |
| No. of mosaic penA allele types | 10 |
| Predominant mosaic penA allele types | 60.001(15) 10.001(12) 10.003(3) |
| No. of nonmosaic penA allele types | 13 |
| Predominant nonmosaic penA allele types | 13.002(15) 13.001(13) 18.001(13) 12.001(10) |
| Predominant mtrR mutations | −35A Del(85) −35A Del, G45D(10) |
| Predominant porB mutations | G120K, A121D(51) G120K, A121G(32) G120K, A121N(14) |
| Predominant ponA mutations | L421P(113) |
Compared to a previous surveillance in Shenzhen (10), no obvious change was observed in the distribution of the MLST genotype except for ST11231. The rate of ceftriaxone resistance of ST11231 increased from 0.00% (0/14) during 2014–2018 to 35.71% (5/14) during 2019–2020, and alarmingly, 40% (2/5) isolates showed a potential of multi-resistance (ceftriaxone resistance combined with Azi MIC value of 1 mg/L, exactly at the clinical breakpoint for resistance), which suggests that the potential threat of the local clone can evolve into dangerous resistance clones by acquiring new phenotypes within a short time. Another difference is that the cluster from the goeBURST analysis in this study was observed to be lower than that in previous surveillance, which suggests that the diversity of the genome complex tended to be stable during 2019–2020. Conversely, high diversity was observed in the distribution of the NG-STAR genotype. Among the top five STs in a previous surveillance in Shenzhen, only two STs (ST2473 and ST497) were still reported as the predominant epidemic clones (10). Both STs were recognized as ceftriaxone-resistance-related clones in a previous study (10), which may be the main reason for maintaining the stability of the two clones. Therefore, these resistance-associated STs (such as MLST ST11231, NG-STAR ST2473, and NG-STAR ST497) should be the focus of monitoring.
Whole-genome sequences provide a complete explanation of the characteristics of gonococcal isolates compared with those obtained from MLST and NG-STAR. In this study, a total of 644 NG isolates coupled with 34 previously described gonococcal isolates were performed phylogenetic analyses and generated two distinct clades, namely, Clade 1 and Clade 2 (Fig. 4). Specifically, there were 75 isolates clustered in the Cro-R FC428 subclade, which contained the internationally spreading FC428 isolates as well as some isolates from different regions of China (12, 34 – 36) (Fig. 4). In addition, 30 isolates were clustered with four Cro-R and high-level Azi-R isolates (A2543 subclade). Three of the four cases were associated with travel to Asia, suggesting a potential circulation in Asia (37 – 39) (Fig. 4). These results suggest that the FC428 clone was further disseminated locally and may further acquire azithromycin resistance along transmission. Furthermore, 10% of isolates clustered in the A2543 subclade showed azithromycin resistance (Fig. 4). It poses a risk of isolates in this subclade might acquire ceftriaxone resistance combined with high-level azithromycin resistance and threaten the dual-antimicrobial therapy, then bring burden to the management and control of gonorrhea on public health level.
Importantly, the global spread of the Cro-R N. gonorrhoeae FC428 isolates showed a typical increase from 0.60% (1/166) in 2017, first reported in Shenzhen, to 3.55% (14/394) in 2020. Notably, the FC428 was still reported sporadically in 2019 in the Shenzhen region, with explosive growth in just 1 year. Similar to China, the FC428 has been reported as an epidemic clone at 12.28% (14/114) in Vietnam during 2019–2020 (40), which proves that the FC428 has circulated in several countries. Consequently, countries should aim to monitor epidemiologically relevant FC428 clones, which are also the only clones that show 100% relevance to the resistance phenotype.
This study had several limitations. One was the uneven gender rate of samples because males have a higher ratio of outpatient visits. Another limitation was that most samples were collected from the symptomatic population because asymptomatic patients rarely choose to seek hospital care. The interpretability and comparability of our study may be influenced by these limitations, and we will further adjust the sampling strategy for future surveillance.
In conclusion, this study investigated the transmission pattern and evolution of AMR in the Shenzhen region between 2019 and 2020 and further characterized the resistance-associated epidemic clone. Our data provided real-time surveillance data to help match clinical guidelines to the actual patterns of AMR of N. gonorrhoeae. Overall, our findings provide current data on gonococcal AMR and are helpful for the adjustment and improvement of gonorrhea surveillance strategies.
MATERIALS AND METHODS
Antimicrobial susceptibility testing
All isolates perform species identification using culture method, and agar dilution methods were used to assess the isolates’ susceptibility to penicillin, ceftriaxone, cefixime, ciprofloxacin, tetracycline, azithromycin, and spectinomycin (10). The thresholds of resistance were defined in accordance with European Committee on Antimicrobial Susceptibility Testing (EUCAST) (https://www.eucast.org/clinical_breakpoints/): resistance to penicillin (Pen-R): MIC > 1 mg/L; resistance to ceftriaxone (Cro-R), MIC > 0.125 mg/L; resistance to cefixime (Cef-R), MIC > 0.125 mg/L; resistance to ciprofloxacin (Cip-R), MIC > 0.06 mg/L; resistance to tetracycline (Tet-R), MIC > 1 mg/L; resistance to azithromycin (Azi-R), MIC > 1 mg/L; and resistance to spectinomycin (Spec-R), MIC > 64 mg/L.
Genomic DNA extraction and WGS
Genomic DNA from each isolate was extracted and purified using a QIAamp DNA Mini Kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s protocol. DNA libraries were prepared using a Nextera XT DNA Library Preparation Kit (Illumina, San Diego, CA, USA). Libraries were then sequenced using the Illumina NovaSeq 6000 platform, according to the manufacturer’s instructions.
Quality control and phylogenic analysis
Trimmomatic software version 0.39 (http://www.usadellab.org/cms/?page=trimmomatic) was used to filter out the adapter sequence and low-quality bases/reads. A quality assessment of the sequence reads was performed using FastQC version 0.11.9 (https://www.bioinformatics.babraham.ac.uk/projects/fastqc/). After the QC procedures were completed, the clean reads were mapped to the reference strain FA1090 (GenBank accession no. AE004969.1) using BWA MEM (41).
Single-nucleotide polymorphisms (SNPs) were identified using VarScan version 2.4.3 (42) and SAMtools version 1.9 (43). SNPs within repetitive regions and prophages of the FA1090 genome, identified by RepeatMasker (http://repeatmasker.org/) and PHAST (44), were excluded. To avoid the potential effects of homoplasy of drug resistance-associated mutations in the phylogeny tree construction, SNPs located in known genes/regions, including penA, porB, mtrR, and mtrR promoter, were further excluded from the data set, as previously described (45). The SNP set obtained in the previous steps was used to construct the maximum-likelihood phylogeny using FastTree 2.1.10 (46). Sequencing data of all isolates were deposited in Sequence Read Archive (PRJNA560592).
Molecular characterization and genotyping using MLST and NG-STAR
MLST and NG-STAR were performed using gene sequences extracted in silico from the WGS data by mapping clean reads to the reference genome FA1090 (GenBank accession no. AE004969.1) and submitted to the Neisseria MLST (http://www.mlst.net/) and NG-STAR (https://ngstar.canada.ca/welcome/home) websites to determine the respective STs. The allelic profiles for MLST were visualized using PHYLOViZ 2.0 to investigate the evolutionary patterns and explore the founding genotypes (10). A phylogenetic analysis was also performed using the seven AMR determinants of NG-STAR and a neighbor-joining phylogenetic tree with 1,000 bootstrapped replicates using MEGA 11 (10). The iTOL online tool was used for further phylogenetic tree visualization and annotation (10).
Statistical analysis
Statistical analysis was performed using SPSS version 26.0 (Chicago, Illinois) for Windows. The chi-square test was used in this study, and a P-value of < 0.05 was statistically significant.
ACKNOWLEDGMENTS
This study was financially supported by the grants from the CAMS Innovation Fund for Medical Sciences (CIFMS, 2021-I2M-1-038), Nonprofit Central Research Institute Fund of Chinese Academy of Medical Sciences (2019PT310029), and the Fundamental Research Funds for the Central Universities (3332021092).
J.P. and F.W. contributed to the conception and design of the study and acquisition of data. D.W. and Y.L. contributed to the analysis and interpretation of data and writing of the manuscript. C.Z. and Y.Z. help conducted the experiment and assisted in writing the manuscript. All authors read and approved the final version of the manuscript.
The authors declare no conflict of interest.
Contributor Information
Junping Peng, Email: pengjp@hotmail.com.
Feng Wang, Email: biowangfeng@163.com.
Ana-Maria Dragoi, Yale University, New Haven, Connecticut, USA .
ETHICS APPROVAL
This study was approved by the National Center for Sexually Transmitted Disease Control, Nanjing, China (approval number 2014-LS-026) and Shenzhen Center for Chronic Disease Control (approval number SZCCC 2021-008-01-PJ).
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/spectrum.01728-23.
Demographic characteristics, MLST and NG-STAR distribution.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. WHO . 2018. Report on global sexually transmitted infection surveillance 2018
- 2. Unemo M, Shafer WM. 2014. Antimicrobial resistance in Neisseria gonorrhoeae in the 21st century: past, evolution, and future. Clin Microbiol Rev 27:587–613. doi: 10.1128/CMR.00010-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. WHO . 2017. Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. Geneva: [Google Scholar]
- 4. Alonso R, Rodríguez-Achaerandio A, Aguirre-Quiñonero A, Artetxe A, Martínez-Ballesteros I, Rodríguez-Gascón A, Garaizar J, Canut A. 2021. Molecular epidemiology, antimicrobial surveillance, and PK/PD analysis to guide the treatment of Neisseria gonorrhoeae infections. Pharmaceutics 13:1699. doi: 10.3390/pharmaceutics13101699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Whittles LK, White PJ, Didelot X. 2017. Estimating the fitness cost and benefit of cefixime resistance in neisseria gonorrhoeae to inform prescription policy: a modelling study. PLoS Med 14:e1002416. doi: 10.1371/journal.pmed.1002416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Medland NA, Zhang Y, Gunaratnam P, Lewis DA, Donovan B, Whiley DM, Guy RJ, Kaldor JM. 2022. Surveillance systems to monitor antimicrobial resistance in Neisseria gonorrhoeae: a global, systematic review. Euro Surveill 27:2100917. doi: 10.2807/1560-7917.ES.2022.27.18.2100917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xiu L, Yuan Q, Li Y, Zhang C, Tang L, Peng J. 2020. Emergence of ceftriaxone-resistant Neisseria gonorrhoeae strains harbouring a novel mosaic penA gene in China. J Antimicrob Chemother 75:907–910. doi: 10.1093/jac/dkz530 [DOI] [PubMed] [Google Scholar]
- 8. Hanao M, Aoki K, Ishii Y, Shimuta K, Ohnishi M, Tateda K. 2021. Molecular characterization of Neisseria gonorrhoeae isolates collected through a national surveillance programme in Japan, 2013: evidence of the emergence of a ceftriaxone-resistant strain from a ceftriaxone-susceptible lineage. J Antimicrob Chemother 76:1769–1775. doi: 10.1093/jac/dkab104 [DOI] [PubMed] [Google Scholar]
- 9. Carannante A, Ciammaruconi A, Vacca P, Anselmo A, Fillo S, Palozzi AM, Fortunato A, Lista F, Stefanelli P. 2019. Genomic characterization of gonococci from different anatomic sites, Italy, 2007-2014. Microb Drug Resist 25:1316–1324. doi: 10.1089/mdr.2018.0371 [DOI] [PubMed] [Google Scholar]
- 10. Li Y, Li Y, Xiu L, Zeng Y, Zhang C, Sun L, Zhang L, Wang F, Peng J. 2021. Typing of Neisseria Gonorrhoeae isolates in Shenzhen, China from 2014-2018 reveals the shift of Genotypes associated with antimicrobial resistance. Antimicrob Agents Chemother 65:e02311-20. doi: 10.1128/AAC.02311-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Golparian D, Rose L, Lynam A, Mohamed A, Bercot B, Ohnishi M, Crowley B, Unemo M. 2018. Multidrug-resistant Neisseria gonorrhoeae isolate, belonging to the internationally spreading Japanese FC428 clone, with ceftriaxone resistance and intermediate resistance to azithromycin. Euro Surveill 23:1800617. doi: 10.2807/1560-7917.ES.2018.23.47.1800617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yan J, Chen Y, Yang F, Ling X, Jiang S, Zhao F, Yu Y, van der Veen S. 2021. High percentage of the ceftriaxone-resistant Neisseria gonorrhoeae FC428 clone among isolates from a single hospital in Hangzhou, China. J Antimicrob Chemother 76:936–939. doi: 10.1093/jac/dkaa526 [DOI] [PubMed] [Google Scholar]
- 13. Luo ZZ, Li W, Wu QH, Zhang L, Tian LS, Liu LL, Ding Y, Yuan J, Chen ZW, Lan LN, Wu XB, Cai YM, Hong FC, Feng TJ, Zhang M, Chen XS. 2018. Population-based study of chlamydial and gonococcal infections among women in Shenzhen, China: implications for programme planning. PLoS One 13:e0196516. doi: 10.1371/journal.pone.0196516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Parish WL, Laumann EO, Cohen MS, Pan S, Zheng H, Hoffman I, Wang T, Ng KH. 2003. Population-based study of chlamydial infection in China: a hidden epidemic. JAMA 289:1265–1273. doi: 10.1001/jama.289.10.1265 [DOI] [PubMed] [Google Scholar]
- 15. Wang F, Liu JW, Li YZ, Zhang LJ, Huang J, Chen XS, Chen SC, Yin YP. 2020. Surveillance and molecular epidemiology of Neisseria gonorrhoeae isolates in Shenzhen. J Glob Antimicrob Resist 23:269–274. doi: 10.1016/j.jgar.2020.08.013 [DOI] [PubMed] [Google Scholar]
- 16. Cassu-Corsi D, Santos FF, Cayô R, Martins WMBS, Nodari CS, Almeida LGP, Martins RA, Carvalho da Silva RJ, Vasconcelos ATR, Pignatari ACC, Gales AC. 2022. Genomic analyses of ciprofloxacin-resistant Neisseria gonorrhoeae isolates recovered from the largest South American metropolitan area. Genomics 114:110287. doi: 10.1016/j.ygeno.2022.110287 [DOI] [PubMed] [Google Scholar]
- 17. Zhang L, Zhang C, Zeng Y, Li Y, Huang S, Wang F, Peng J. 2021. Emergence and characterization of a ceftriaxone-resistant Neisseria gonorrhoeae FC428 clone evolving moderate-level resistance to azithromycin in Shenzhen, China. Infect Drug Resist 14:4271–4276. doi: 10.2147/IDR.S336212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Francisco AP, Bugalho M, Ramirez M, Carriço JA. 2009. Global optimal eBURST analysis of multilocus typing data using a graphic matroid approach. BMC Bioinformatics 10:152. doi: 10.1186/1471-2105-10-152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lahra MM, Martin I, Demczuk W, Jennison AV, Lee KI, Nakayama SI, Lefebvre B, Longtin J, Ward A, Mulvey MR, Wi T, Ohnishi M, Whiley D. 2018. Cooperative recognition of internationally disseminated ceftriaxone-resistant Neisseria gonorrhoeae strain. Emerg Infect Dis 24:735–740. doi: 10.3201/eid2404.171873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nakayama S, Shimuta K, Furubayashi K, Kawahata T, Unemo M, Ohnishi M. 2016. New ceftriaxone- and multidrug-resistant Neisseria gonorrhoeae strain with a novel mosaic penA gene isolated in Japan. Antimicrob Agents Chemother 60:4339–4341. doi: 10.1128/AAC.00504-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jennison AV, Whiley D, Lahra MM, Graham RM, Cole MJ, Hughes G, Fifer H, Andersson M, Edwards A, Eyre D. 2019. Genetic relatedness of ceftriaxone-resistant and high-level azithromycin resistant Neisseria gonorrhoeae cases. Euro Surveill 24:1900118. doi: 10.2807/1560-7917.ES.2019.24.8.1900118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. W QQ. 2007. Guidelines for diagnosis and treatment of sexual transmitted diseases. Shanghai: Shanghai Science and Technology Press. [Google Scholar]
- 23. Yin YP, Han Y, Dai XQ, Zheng HP, Chen SC, Zhu BY, Yong G, Zhong N, Hu LH, Cao WL, Zheng ZJ, Wang F, Zhi Q, Zhu XY, Chen XS, Broutet N. 2018. Susceptibility of Neisseria gonorrhoeae to azithromycin and ceftriaxone in China: a retrospective study of national surveillance data from 2013 to 2016. PLoS Med 15:e1002499. doi: 10.1371/journal.pmed.1002499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lin EY, Adamson PC, Klausner JD. 2021. Epidemiology, treatments, and vaccine development for antimicrobial-resistant Neisseria gonorrhoeae: current strategies and future directions. Drugs 81:1153–1169. doi: 10.1007/s40265-021-01530-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Han Y, Yin Y, Dai X, Chen S, Yang L, Zhu B, Zhong N, Cao W, Zhang X, Wu Z, Yuan L, Zheng Z, Feng L, Liu J, Chen X. 2020. Widespread use of high-dose ceftriaxone therapy for uncomplicated gonorrhea without reported ceftriaxone treatment failure results from 5 years of multicenter surveillance data in China. Clin Infect Dis 70:99–105. doi: 10.1093/cid/ciz170 [DOI] [PubMed] [Google Scholar]
- 26. Lin X, Qin X, Wu X, Liao Y, Yu Y, Xie Q, Tang S, Guo C, Pei J, Wu Z, Cai C, Wang F, Wu S, Chen H, Liu X, Li M, Cao W, Zheng H. 2022. Markedly increasing antibiotic resistance and dual treatment of Neisseria gonorrhoeae isolates in Guangdong, China, from 2013 to 2020. Antimicrob Agents Chemother 66:e0229421. doi: 10.1128/aac.02294-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dong Y, Yang Y, Wang Y, Martin I, Demczuk W, Gu W. 2020. Shanghai Neisseria gonorrhoeae isolates exhibit resistance to extended-spectrum cephalosporins and clonal distribution. Front Microbiol 11:580399. doi: 10.3389/fmicb.2020.580399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Karymbaeva S, Boiko I, Jacobsson S, Mamaeva G, Ibraeva A, Usupova D, Golparian D, Unemo M. 2021. Antimicrobial resistance and molecular epidemiological typing of Neisseria gonorrhoeae isolates from Kyrgyzstan in central Asia, 2012 and 2017. BMC Infect Dis 21:559. doi: 10.1186/s12879-021-06262-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xiu L, Li Y, Wang F, Zhang C, Li Y, Zeng Y, Yin Y, Peng J. 2020. Multiplex high-resolution melting assay for simultaneous identification of molecular markers associated with extended-spectrum cephalosporins and azithromycin resistance in Neisseria gonorrhoeae. J Mol Diagn 22:1344–1355. doi: 10.1016/j.jmoldx.2020.08.003 [DOI] [PubMed] [Google Scholar]
- 30. de Laat MM, Wind CM, Bruisten SM, Dierdorp M, de Vries HJC, Schim van der Loeff MF, van Dam AP. 2019. Ceftriaxone reduced susceptible Neisseria gonorrhoeae in the Netherlands, 2009 to 2017: from PenA Mosaicism to A501T/V Nonmosaicism. Sex Transm Dis 46:594–601. doi: 10.1097/OLQ.0000000000001031 [DOI] [PubMed] [Google Scholar]
- 31. Abrams AJ, Kirkcaldy RD, Pettus K, Fox JL, Kubin G, Trees DL. 2017. A case of decreased susceptibility to ceftriaxone in Neisseria gonorrhoeae in the absence of a mosaic penicillin-binding protein 2 (penA) allele. Sex Transm Dis 44:492–494. doi: 10.1097/OLQ.0000000000000645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Deng X, Allan-Blitz LT, Klausner JD. 2019. Using the genetic characteristics of Neisseria gonorrhoeae strains with decreased susceptibility to cefixime to develop a molecular assay to predict cefixime susceptibility. Sex Health 16:488–499. doi: 10.1071/SH18227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Xiu L, Zhang C, Li Y, Wang F, Peng J. 2020. High-resolution melting analysis for rapid detection of the internationally spreading ceftriaxone-resistant Neisseria gonorrhoeae FC428 clone. J Antimicrob Chemother 75:106–109. doi: 10.1093/jac/dkz395 [DOI] [PubMed] [Google Scholar]
- 34. Yuan Q, Li Y, Xiu L, Zhang C, Fu Y, Jiang C, Tang L, Peng J. 2019. Identification of multidrug-resistant Neisseria gonorrhoeae isolates with combined resistance to both ceftriaxone and azithromycin, China, 2017-2018. Emerg Microbes Infect 8:1546–1549. doi: 10.1080/22221751.2019.1681242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chen SC, Yuan LF, Zhu XY, van der Veen S, Yin YP. 2020. Sustained transmission of the ceftriaxone-resistant Neisseria gonorrhoeae FC428 clone in China. J Antimicrob Chemother 75:2499–2502. doi: 10.1093/jac/dkaa196 [DOI] [PubMed] [Google Scholar]
- 36. Wang H, Wang Y, Yong G, Li X, Yu L, Ma S, Luo T. 2020. Emergence and genomic characterization of the ceftriaxone-resistant Neisseria gonorrhoeae FC428 clone in Chengdu, China. J Antimicrob Chemother 75:2495–2498. doi: 10.1093/jac/dkaa123 [DOI] [PubMed] [Google Scholar]
- 37. Whiley DM, Jennison A, Pearson J, Lahra MM. 2018. Genetic characterisation of Neisseria gonorrhoeae resistant to both ceftriaxone and azithromycin. Lancet Infect Dis 18:717–718. doi: 10.1016/S1473-3099(18)30340-2 [DOI] [PubMed] [Google Scholar]
- 38. Eyre DW, Sanderson ND, Lord E, Regisford-Reimmer N, Chau K, Barker L, Morgan M, Newnham R, Golparian D, Unemo M, Crook DW, Peto TE, Hughes G, Cole MJ, Fifer H, Edwards A, Andersson MI. 2018. Gonorrhoea treatment failure caused by a Neisseria gonorrhoeae strain with combined Ceftriaxone and high-level azithromycin resistance. Euro Surveill 23:1800323. doi: 10.2807/1560-7917.ES.2018.23.27.1800323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pleininger S, Indra A, Golparian D, Heger F, Schindler S, Jacobsson S, Heidler S, Unemo M. 2022. Extensively drug-resistant (XDR) Neisseria gonorrhoeae causing possible Gonorrhoea treatment failure with ceftriaxone plus azithromycin in Austria, April 2022. Euro Surveill. 27:2200455. doi: 10.2807/1560-7917.ES.2022.27.24.2200455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Trinh TM, Nguyen TT, Le TV, Nguyen TT, Ninh DT, Duong BH, Van Nguyen M, Kesteman T, Pham LT, Rogier van Doorn H. 2022. Neisseria Gonorrhoeae FC428 Subclone Vietnam, 2019-2020. Emerg Infect Dis 28:432–435. doi: 10.3201/eid2802.211788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Li H. 2013. Aligning sequence reads, clone sequences and assembly Contigs with BWA-MEM arXiv preprint arXiv:13033997.
- 42. Koboldt DC, Zhang Q, Larson DE, Shen D, McLellan MD, Lin L, Miller CA, Mardis ER, Ding L, Wilson RK. 2012. Varscan 2: somatic mutation and copy number alteration discovery in cancer by exome sequencing. Genome Res 22:568–576. doi: 10.1101/gr.129684.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R, 1000 Genome Project Data Processing Subgroup . 2009. The sequence alignment/map format and Samtools. Bioinformatics 25:2078–2079. doi: 10.1093/bioinformatics/btp352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhou Y, Liang Y, Lynch KH, Dennis JJ, Wishart DS. 2011. PHAST: a fast phage search tool. Nucleic Acids Res 39:W347–52. doi: 10.1093/nar/gkr485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Peng JP, Yin YP, Chen SC, Yang J, Dai XQ, Zheng HP, Gu WM, Zhu BY, Yong G, Zhong N, Hu LH, Cao WL, Zheng ZJ, Wang F, Zhi Q, Zhang C, Xiu LS, Liu B, Dong J, Sun LL, Zhu YF, Chen XS, Jin Q. 2019. A whole-genome sequencing analysis of Neisseria gonorrhoeae isolates in China: an observational study. EClinicalMedicine 7:47–54. doi: 10.1016/j.eclinm.2019.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Price MN, Dehal PS, Arkin AP. 2010. FastTree 2--approximately maximum-likelihood trees for large alignments. PLoS One 5:e9490. doi: 10.1371/journal.pone.0009490 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Demographic characteristics, MLST and NG-STAR distribution.