Abstract
Introduction
Complex, coronary stenosis remains a technical challenge that may be responsible for in-stent restenosis and vessel thrombosis. Here we investigated the efficacy and safety of excimer laser coronary atherectomy (ELCA) with contrast mix injection for improving vessel wall stent apposition in undilatable, mostly calcified lesions.
Aim
To assess ELCA with contrast mix injection in complex, stented, calcified coronary lesions.
Material and methods
This prospective single-center observational study enrolled 52 consecutive patients (73 lesions), with suboptimal stents implanted in de novo lesions and lesions requiring in-stent restenosis (ISR) due to stent underexpansion using all available means to achieve an optimal result. Patients presenting with ST-segment elevation myocardial infarction were excluded. All patients underwent coronary angiography 6 months after ELCA with intravascular ultrasound or optical coherence tomography study. We used contrast media mixed with saline (25–75%) to supply maximum laser energy output when a standard approach was unsuccessful. Procedural success was defined as relative stent expansion of > 80% minimal stent area (MSA) divided by average reference lumen area.
Results
Procedural success was achieved in all cases. The cross-sectional area measured in treated segment improved significantly from 2.9 (0.72) mm2 to 7.3 (0.79) mm2 after ELCA. The in-hospital device-oriented major adverse cardiac event (DOCE) rate was 9.6%. No vessel perforation occurred during ELCA. After 6 months, the DOCE rate was 13.4%, while the rate of target lesion revascularization (TLR) was 8.2%.
Conclusions
This registry confirms the efficacy and safety of ELCA with contrast mix injection as a possible approach for stent expansion/ISR in failed PCI.
Keywords: angioplasty, contrast-mix, laser, stent, underexpansion
Summary
Currently, transluminal percutaneous coronary angioplasty (PCI) is the main method of invasive treatment of ischemic heart disease. Complex and calcified coronary lesions are responsible for many PCI failures due to restenosis and in-stent thrombosis. As the majority of PCIs are completed with coronary stenting, we investigated the efficacy and safety of excimer laser coronary atherectomy (including with enhanced laser atherectomy force by contrast mix injection) to improve vessel wall stent apposition in undilatable calcified lesions. This approach is also suitable to treat in-stent restenosis due to stent underexpansion. We concluded that this approach was both safe and highly effective.
Introduction
Transluminal percutaneous coronary angioplasty (PCI) with stent implantation is the standard approach to treating coronary artery disease. With the increasing number of treated patients undergoing PCI, a significant number of complex lesions remain difficult to treat using the conventional approach. Heavily calcified or fibrotic and undeletable/balloon uncrossable lesions are a challenging subset for interventional cardiologists. These lesions may be resistant to or untreatable with traditional percutaneous techniques, which restricts therapeutic options in such cases [1–3].
Stent suboptimal expansion is a key factor for serious post-PCI complications such as stent thrombosis and in-stent restenosis (ISR) [3]. It is crucial to optimize lesion preparation before stenting in these cases using cutting balloons and some available atherectomy systems (rotational atherectomy (RA), orbital atherectomy, or excimer laser coronary atherectomy (ELCA)). Unfortunately, these techniques are still infrequently used. The use of RA within an underexpanded stent could be associated with serious complications [4]. The implantation of another stent within an underexpanded stent is not a solution and only worsens the situation.
ELCA helps stent expansion in balloon-resistant lesions [5–7]. In specific settings, ELCA generates an important pulse-wave force that interacts with the vessel tissue outside of the previously implanted stent. This force depends on three important players: contrast media, saline, and blood [8]. Compared to the “flush and bathe” technique, which is currently performed during ELCA-facilitated PCI, contrast mix ELCA PCI generates an important force directed to vessel wall tissue up to 100 bars [7–9].
In patients with underexpanded stents, contrast-mix ELCA can lead to optimized lesion expansion. The acoustic wave disrupts the underlying plaque matrix, supporting the restriction of stent expansion [10]. The use of contrast medium during lasing helps to create larger bubbles with a more powerful shockwave effect outside the stent. This technique ablates the material in the vessel lumen and softens the tissue outside the stent. This approach proved effective without serious complications [10, 11].
Study definitions
In our study we collected consecutive patients with non-well-expanded stents and ISR where the standard ELCA flush and batch technique was ineffective. All patients were stable; no ST-elevation myocardial infarction (STEMI) or non-ST-elevation myocardial infarction (NSTEMI) patients were collected. Stent expansion index was defined according to Uren et al. as the ratio of minimal stent cross-sectional area (CSA) measured in intravascular ultrasound (IVUS) divided by the mean proximal and distal reference lumen areas. Finally, stent underexpansion index was defined as stent expansion below 0.7 (< 70%) [12]. To demonstrate the effectiveness of ELCA for improving stent expansion and the in-stent vessel lumen, we defined procedural success as relative stent expansion of > 80% minimal stent area (MSA) divided by average reference lumen area using IVUS. The change in minimal stent cross-sectional area (CSA; at least 1 mm2) was measured by IVUS (or OCT in selected cases). Coronary angiograms were analyzed using QCA software (QCA Stenosis Analysis, version 1.6.264; GE, USA). Vessel characteristics (MSD, reference vessel diameter, and percent diameter stenosis) were calculated at baseline and after the procedure. IVUS was performed in all patients using an Opticross HD 3.0 F imaging catheter (Boston Scientific, USA). Motorized IVUS pullbacks were performed at 0.5 mm/s. OCT was performed in selected patients with the Dragonfly Duo OPTISTM Imaging Catheter (Abbott, Santa Clara, CA, USA) using an appropriate technique.
IVUS/OCT quantitative analysis was performed by an experienced interventional cardiologist using commercial software (Boston/Abbott; at every 1 mm) within the analyzed stent. The minimal lumen area (MLA) was calculated at the start and end of the procedure. The stent area was measured at the same cross-section of the MLA. Lumen gain was calculated as the difference between the final and initial MLA. Study endpoints were assessed during the procedure: slow-flow or no-reflow, vessel perforation, dissection, and periprocedural myocardial infarction. In-hospital device-oriented major adverse cardiac events (DOCE); the occurrence of STEMI/NSTEMI, target lesion revascularization (TLR), vessel perforation, stroke and death were noted. During follow-up (6 months) IVUS HD or OCT and DOCE were noted. We defined TLR as any repeat PCI of the target lesion or coronary bypass surgery (CABG) for restenosis or other complications of the target lesion. In our study cohort, TLR was performed when angiography with IVUS/OCT during follow-up showed a stenosis ≥ 50%. We used bi-monthly telephone follow-up collect data about patient clinical status. The standardized Academic Research Consortium definitions were applied for myocardial infarction and revascularization [13].
Aim
The aim of our study was to evaluate use of ELCA in a very specific cohort of patients with in-stent restenosis, underexpanded stents and frequently calcified lesions resistant to high-pressure non-compliant balloon inflation.
Material and methods
This single-center study enrolled fifty-two consecutive patients (73 lesions) treated in our hospital during 2018-2021. Patients with STEMI were excluded. All patients presented ISR and non-well expanded stents (stent expansion index below 0.7, with luminal narrowing > 50% diameter in the stented coronary segment using a classical Academic Research Consortium definition [12]. All patients had refused surgical revascularization (CABG) or had been disqualified from CABG. The general study flowchart is presented in Figure 1. We started the ELCA procedure with the standard flush and bath technique (the contrast system was purged with saline first; in the second step saline infusion with 2 ml/s speed was infused during lasing) as the first attempt with the following laser output: 80/80 (fluence [mJ mm2]/rate [Hz]), when ineffective – 30/80 (fluence[mJ mm2]/rate [Hz]). Three ELCA attempts with maximum energy were provided. When, ineffective – especially with severe calcification, > 180º; measured by IVUS or OCT respectively – contrast-mix technique was implemented. In this technique we used contrast medium mixed with saline (from 25–50 up to 75%) and blood to supply maximal laser energy output. All contrast-mix ELCA procedures were performed inside of the stent only. All patients underwent coronary angiography 6 months after ELCA with high-definition intravascular ultrasound (IVUS HD) or optical coherence tomography (OCT) – in the case of use of BVS only. Informed consent was obtained from all patients. This registry was approved by the Lublin Medical University Bioethics Committee.
Figure 1.
General study flowchart
ISR – in-stent restenosis, POBA – plain old balloon angioplasty, NC – non-compliant balloon, IVUS – intravascular ultrasound, OCT – optical coherence tomography, ELCA – excimer laser atherectomy, DES – drug eluting balloon, BVS – bioresorbable vascular scaffolds.
Procedure
All procedures were performed according to the current techniques. All patients received dual-antiplatelet therapy according to the current PCI protocol (acetylsalicylic acid + ticagrelor). Ticagrelor was preferred because the PCI approach using ELCA (photoablation, thermoablation and shockwave force complex) is more aggressive than the standard PCI procedure. During aggressive procedures (such as ELCA or RA) risk of clotting formation is substantially higher.
During the procedures, we used unfractionated heparin 100 UI/kg. In the first attempt, pre-dilatation with a noncompliant balloon prior to ELCA of the target lesion (except for chronic total occlusion lesions) was performed. IVUS HD was performed when balloon underexpansion occurred. The ELCA catheter (0.9-mm X-80; Philips/Spectranetics, NL) was passed over the guide wire, inserted within the stent and advanced slowly toward the underexpanded zone to obtain the best possible result. Several laser passes were applied to achieve the best debulking. The total number of laser pulses count/energy delivered was calculated. At the end of the procedure, the laser catheter was removed, and an additional balloon and stent were used in accordance with standard practice. In 3 patients, the procedure was completed with only plain old balloon angioplasty (POBA) with a drug-eluting balloon (DEB) due to excellent angiographic and IVUS results.
The laser energy (fluency and repetition rate) was used as described in Methods and depended on lesion complexity.
Generally, after the first lasing (flush and bathe technique) with the highest available energy (fluence 80 [mJ/mm2]/rate 80 [Hz]), the subsequent lasing was performed with the lowest fluency and highest repetition rates (i.e., 30 [mJ/mm2] and 80 [Hz] for the 0.9-mm X-80 catheter [Philips/Spectranetics]) to achieve a more powerful shockwave effect outside the stent with minimal risk. Shorter excimer (XeCl) laser pulse duration with lower energy generates more powerful shock waves, which is suitable for treatment of hard lesions [11]. When the result was suboptimal, the next step was ELCA with contrast mix injection. After 3 attempts (flush and bathe technique) starting with 30/80 up to 80/80 (fluence [mJ/mm2]/rate [Hz]) we used contrast medium mixed with saline (from 25–50 up to 75%) and blood to supply maximal laser energy output. The mix volume was injected with 2.0 ml/s speed during lasing. All contrast-mix ELCA procedures were performed inside of the stent only to avoid a possible complication (vessel perforation). In the case of significant vessel tortuosity, we used a guide extension catheter (Guideliner V3; Teleflex, USA) to achieve coaxial laser catheter position and the best photomechanical force propagation without vascular complications. In 4 patients, a bioresorbable vascular stent (BVS) (Desolve; Elixir Medical Corporation) were implanted. These lesions had already been stented with three layers of metal stent (BMS/DES). BVSs were used like an antiproliferative drug carrier rather than a scaffolding platform.
Statistical analysis
For continuous values we used Wilcoxon (for non-parametric distribution) and Student’s t-tests (for parametric, normal distribution). The Shapiro-Wilk test of normality was used. Tests for related couples were performed. All statistical values are expressed as mean (SD). Other qualitative variables are presented as numbers and percentages as mean (SD). We used Statistica software (Stat Soft, Tulsa, OK, USA). All authors read and agreed to the submission of this manuscript.
Results
We studied patients who underwent ELCA procedures between June 2016 and July 2020 with underexpanded stents and ISR. Fifty-two patients with an underexpanded stent in predominantly (93.1%) calcified lesions (n = 73) after unsuccessful high-pressure balloon inflation were included. The mean (SD) age was 71.0 (9.73) years; 32 (61.53%) patients were male. Fourteen (26.97%) patients had diabetes mellitus type 2 (Table I).
Table I.
Patients’ clinical characteristics (n = 52)
| Parameter | Value |
|---|---|
| Age [years] mean (SD) | 71 (9.73) |
| Male gender, n (%) | 32 (61.53) |
| LVEF, mean (SD) | 48 (9.6) |
| Earlier MI, n (%) | 27 (51.9) |
| Earlier CABG, n (%) | 8 (15.3) |
| Diabetes mellitus, n (%) | 14 (26.9) |
| Hypertension, n (%) | 44 (84.6) |
| Dyslipidemia, n (%) | 31 (59.6) |
| Smoker, n (%) | 16 (30.7) |
| Unstable angina, n (%) | 3 (5.7) |
| ISR, n (%) | 52 (100) |
| Non-well-expanded stent, n (%) | 52 (100) |
| Mean time of last PCI* [months] | 8.4 (2.1) |
CABG – coronary artery bypass graft surgery, MI – myocardial infarction, LVEF – left ventricular ejection fraction, ISR – in-stent restenosis
PCI in targeted lesion segment.
Detailed descriptions of coronary target lesions are presented in Table II. The mean (SD) stent size was 3.2(0.6) mm (Table III). Post-dilatation was performed using a mean (SD) 3.5 (0.6) mm non-compliant balloon at mean (SD) 22.9 (4.5) atm. We used only 0.9-mm X80 laser catheters (X-80 Philips/Spectranetics). Mean energy with fluence of 68 (11) mJ/mm2 at a rate of 61 (18) Hz was essential to achieve the best result. Contrast-mix ELCA was used for 61 (83.5%) treated lesions. Before ELCA, IVUS was performed in 66 (86.5%) lesions (Table III).
Table II.
Target lesion specification
| Parameter | Value |
|---|---|
| Target vessel (N = 55) | |
| LAD, n (%) | 15 (31.5) |
| LCX, n (%) | 13 (24.6) |
| RCA, n (%) | 18 (34.2) |
| LM, n (%) | 8 (8.2) |
| SCV, n (%) | 1 (1.3) |
| Lesion types (AHA)* (N = 73) | |
| A1, n (%) | 0 |
| B1, n (%) | 3 (4.1) |
| B2, n (%) | 4 (5.5) |
| C, n (%) | 90.4 (65) |
| Calcification, n (%) | 68 (93.1) |
| Ostial, n (%) | 29 (39.7) |
| CTO, n (%) | 7 (9.58) |
| Tortuous, n (%) | 45 (73.61) |
| ISR, n (%) | 73 (100) |
| DES-ISR, n (%) | 6 (84.7) |
| BMS-ISR, n (%) | 12 (16.1) |
American Heart Association. LAD – left anterior descending artery, LCX – left circumflex artery, RCA – right coronary artery, LM – left main trunk, SVG – saphenous vein graft, ISR – in-stent restenosis, CTO-chronic total occlusion, DES – drug eluting stent, BMS – bare metal stent.
Table III.
Description of procedures
| Parameter | Value | |
|---|---|---|
| Procedure type (N = 73): | ||
| IVUS/OCT before and after procedure | n (%) | 66 (86.5) |
| IVUS/OCT after procedure only | n (%) | 7 (9.58) |
| Max balloon diameter [mm] (pre-ELCA) | Mean (SD) | 3.5 (0.2) |
| Max balloon diameter [mm] (post-ELCA) | Mean (SD) | 3.5 (0.6) |
| Max dilatation pressure [mm] (pre-ELCA) | Mean (SD) | 23.1 (3.8) |
| Max dilatation pressure [mm] (post-ELCA) | Mean (SD) | 22.9 (4.5) |
| ELCA – characteristics (N = 73): | ||
| Catheter 0.9 mm X80 | n (%) | 73 (100) |
| Fluence [mJ/mm2] | Mean (SD) | 68 (11) |
| Rate [Hz] | Mean (SD) | 61 (18) |
| Pulses | Mean (SD) | 8704 (4101) |
| Contrast mix attempt | n (%) | 61 (83.5) |
| Extension catheter | n (%) | 3 (4.1) |
| Post ELCA proceeding (N = 73): | ||
| Stent size | Mean (SD) | 3.2 (0.6) |
| POBA/DEB | n (%) | 4 (5.4) |
| BVS | n (%) | 4 (7.2) |
| DES | n (%) | 65 (89) |
IVUS – intravascular ultrasound, OCT – optical coherence tomography, BVS – bioresorbable vascular scaffolds, POBA – plain old balloon angioplasty, DEB – drug eluting balloon, SD – standard deviation.
For seven lesions (9.58%), it was not possible to cross the IVUS catheter before ELCA. In all cases, noncompliant balloon PCI at high pressure was performed before ELCA. No deaths occurred during any of the procedures. Periprocedural NSTEMI occurred in 4 (7.6%) patients with a peak cTnT elevation of more than 5× the upper reference limit. No STEMI observed. The patient who died during hospitalization had severe left ventricular dysfunction (left ventricular ejection fraction (EF) of 25%) and experienced sudden death 14 days after PCI of the left anterior descending artery. The patient’s death was related to the procedure as we performed a very high-risk procedure in the patient with very low EF. Follow-up coronary angiography performed at 10 days after PCI revealed particularly good results with TIMI3 flow in all coronary arteries. This patient had been waiting for cardioverter-defibrillator implantation briefly during the same hospitalization (as decided by the Heart Team before the PCI procedure). Four patients who underwent POBA only or DEB after the ELCA procedure presented with TLR. Successful contrast-mix ELCA and subsequent stent dilatation were achieved in all cases (Tables IV). During the 6-month follow-up, one death was observed, while 6 cases of TLR occurred (Table IV).
Table IV.
Clinical characteristics following PCI-ELCA procedures (n = 52)
| Variable | Value | |
|---|---|---|
| Hospital STEMI | n (%) | 0 |
| Hospital CABG | n (%) | 0 |
| Hospital Death | n (%) | 1 (1.9) |
| Periprocedural NSTEMI (cTnTx5) | n (%) | 4 (7.6) |
| Dissection | n (%) | 2 (3.8) |
| Perforation | n (%) | 0 |
| ST-elevation alone | n (%) | 0 |
| Bradycardia and ST-alone | n (%) | 0 |
| In-hospital DOCE (n = 52) | n (%) | 5 (9.6) |
| 6-month follow-up after PCI-ELCA: | ||
| DOCE | n (%) | 7 (13.4) |
| TLR* | n (%) | 6 (8.2) |
| Death | n (%) | 1 (1.9) |
| ISR | n (%) | 4 (7.6) |
MI – myocardial infarction, CABG – coronary artery bypass graft surgery, DOCE – device-oriented major adverse cardiac events, TLR – target lesion revascularization, CTnT – cardiac troponin level;
n = 73 (lesions).
Four patients who underwent POBA only or had DEB after the ELCA procedure presented restenosis after 6 months of follow-up. Target vessel visualization quantitative analysis (QCA, IVUS, OCT) is presented in Table V.
Table V.
Target vessel visualization assessment study
| Mode (n) | Variable | Mean | Max. | Min. | SD | P-value |
|---|---|---|---|---|---|---|
| QCA | Pre-ELCA PCI stenosis (%) | 77.98 | 96.00 | 63.00 | 7.69 | < 0.01 |
| Post-ELCA PCI stenosis (%) | 14.08 | 19.00 | 6.00 | 3.47 | ||
| Ref. diameter [mm] pre-ELCA PCI | 3.29 | 4.00 | 2.60 | 0.40 | 0.911 | |
| Ref. diameter [mm] post-ELCA PCI | 3.30 | 4.10 | 2.60 | 0.43 | ||
| Minimal stent diameter [mm] pre-ELCA PCI | 1.42 | 2.60 | 0.60 | 0.42 | < 0.01 | |
| Minimal stent diameter [mm] post-ELCA PCI | 2.92 | 3.90 | 2.10 | 0.47 | ||
| IVUS/OCT* | Pre-ELCA PCI CSA [mm2] | 2.9 | 4.3 | 1.5 | 0.72 | < 0.01 |
| IVUS/OCT** | Post-ELCA PCI CSA [mm2] | 7.30 | 8.9 | 6.2 | 0.79 | |
| IVUS/OCT* | Stent expansion at minimum stent area (MSA/reference lumen area) pre-ELCA PCI (%) | 32.1 | 19.1 | 46.3 | 14.2 | < 0.01 |
| IVUS/OCT** | Stent expansion at minimum stent area (MSA/reference lumen area) post-ELCA PCI (%) | 88.4 | 81.2 | 99.1 | 10.9 | < 0.01 |
IVUS n = 41, OCT n = 4;
IVUS n = 48, OCT n = 4. IVUS – intravascular ultrasound, OCT – optical coherence tomography (only for BVS cases; n = 4), CSA – cross-sectional area, MSA – minimal stent area.
Discussion
ELCA angioplasty with undilatable or calcified coronary lesions has been well documented previously [14, 15]. Some side effects such as coronary dissections were reported at 5–7% when using a standard procedural protocol (saline injection) [4]. However, the occurrence of coronary vessel perforation was exceptionally low (0–1.4%). The use of ELCA to expand an undilatable stent was described only in a few small registries and case reports [4, 10, 14–16]. Noble and Bilodeau described ELCA as a suitable method for balloon refractory lesion treatment in underexpanded stents [15]. The ELLEMENT registry (n = 28) was the first larger case series to evaluate contrast-enhanced ELCA procedures to modify plaques that are stented but high-pressure balloon-resistant [11]. Although contrast-mix laser therapy was reported by Goldberg in 1998, it was not used due to the risk of important dissection or vessel wall perforation [4]. Some reports of successful procedures using rotational atherectomy within underexpanded stents have been published. Some procedural risks were reported, such as burr entrapment, distal embolization of microparticles, and stent strut destruction [3]. A relatively new successful approach with shockwave lithotripsy has also been reported [17, 18]. In very resistant, recurrent cases of ISR (but without calcification) brachytherapy was also discussed recently [19]. Our registry demonstrates the feasibility and efficacy of contrast mix ELCA for facilitating undilatable stent expansion and ISR treatment after failure of the conventional PCI approach (Figures 2, 3, Table V).
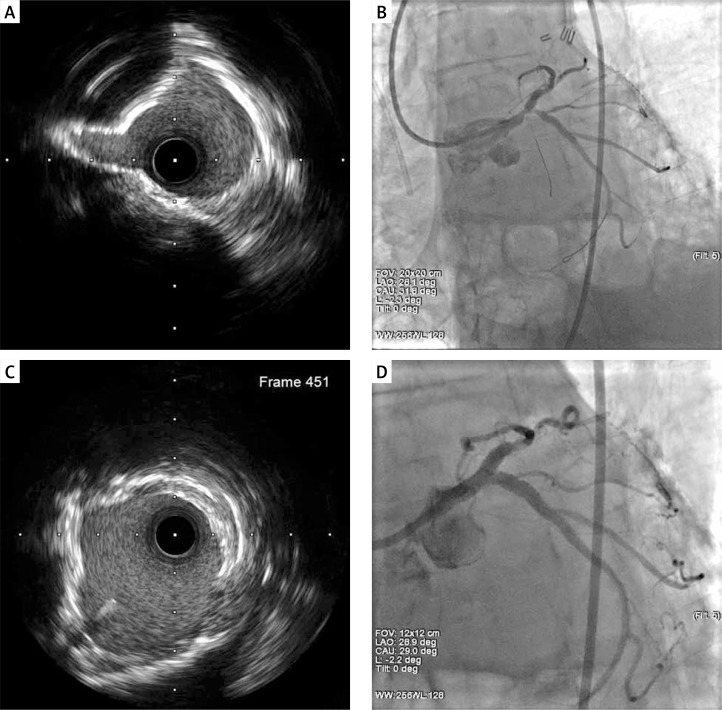
Figure 2.
A, B – Proximal CX. 3 layers non-well-expanding stents/BMS, DES, DES, status after complex PCI LM/Cx/LAD MLA 5.9 mm2. C, D – Final result after 5 passages of ELCA 0.9 mm X80 catheter, 18 000 pulses, fluence 30–80 mJ/mm2, rate 80 Hz. Contrast facilitated lasing. Finalizing non-compliant balloon 24 atm. MLA 10.1 mm2.
Δ 169%
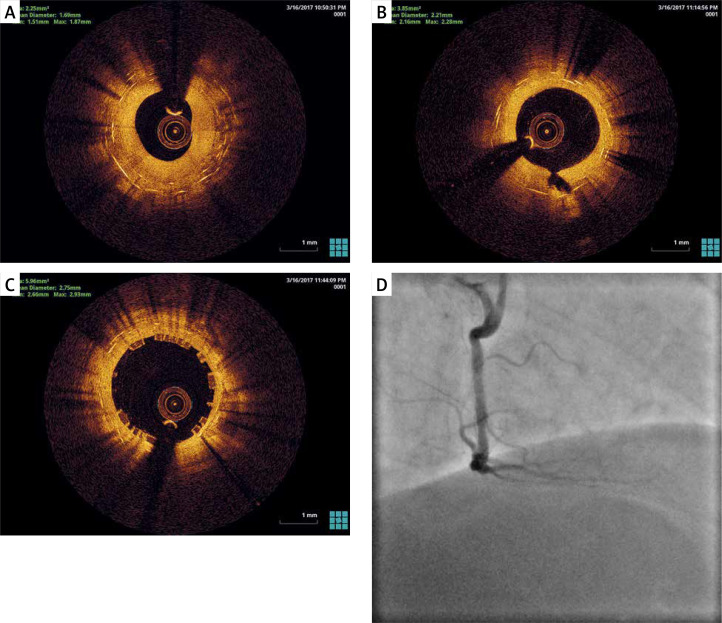
Figure 3.
A – Before ELCA, ISR, 2 DES layers. B – After ELCA, Laser catheter, 0.9 mm X80, fluence 30 mJ/mm2, rate 80 Hz. Contrast facilitated lasing. Finalizing with non-compliant balloon, 3.5 a 22 atm. C – After BVS, Desolve 3.5/ 22 mm. D – Final result
Laser coronary atherectomy with the contrast mix technique inside the stent resulted in our registry with a low incidence of periprocedural complications. The total occurrence of TLR at 6 months was 8.2% (Table IV), which may be acceptable for these very complex and demanding lesions. In the recently published IVL Multicenter European Study TLR was reported at 6% [20]. These results are quite comparable with those of standard PCI with DES, which are currently (depending on stent size, lesion length and morphology) estimated at 6–10% [21].
Laser energy tissue absorption is photochemical, photomechanical, or photothermal in origin. ELCA produces vapor microbubbles [22, 23]. These microbubbles generate shock waves (up to tens of kilobars) [23]. The pulse pressure force causes some beneficial and detrimental effects with ELCA. No pressure microbubbles are generated by laser energy when the laser tip is submerged in saline [8, 11]. Important pulse pressure force is produced during ELCA when the laser tip is surrounded with a concentration of 25% vol/vol blood in saline or as little as 1% vol/vol contrast [8]. The flush and bathe technique was finally introduced to decrease the incidence of laser-induced dissection by this phenomenon [9]. Low fluence (30 mJ/mm2) together with the high repetition rate (80 Hz) achieved during the wash and bath technique allows the safe augmentation of the acoustic/mechanical effect, especially on the vessel wall [7, 9, 11]. This effect is best achieved with a 0.9-mm X80 catheter due to its low profile and high energy output. We recommend keeping the catheter tip coaxially in the vessel lumen to avoid future complications. For this purpose, we used guide wire extension catheter support to achieve the coaxial laser catheter position and prevent potential complications after the lasing of tortuous vessels.
During ELCA with contrast media mix, a low fluence and high repetition rate force close to 100 bars can be generated [7]. This force can safely break down the balloon-resistant plaque beneath the stent. Contrast media mix ELCA should be performed with particular care inside the stent. This approach minimizes the risk of vessel perforation. For the reasons described above, clinicians should use only small laser catheters, such as the 0.9-mm X80. Baumbach et al. reported that microbubbles generated during ELCA in the coronary arteries are three times larger than the diameter of the laser catheter [7]. The use of larger laser catheters (in the coronary arteries) in blood or with contrast mix should be limited to large vessel diameters (> 3.5 mm) to minimize vessel dissection or perforation. This study had a small sample size because of the small number of patients treated at experienced centers. Randomization for ethical problems is unlikely in this case. The safety of this technique (ELCA for ISR) was also confirmed in the recently published retrospective NCDR/PCI registry [24]. However, a large-scale multicenter study with the current PCI approach is necessary.
Conclusions
Good lesion preparation prior to stent implantation using the best available technology is crucial. ELCA with contrast-mix could be used more widely in failed cases by means of the flush and bath technique. This registry confirms the safety of contrast mix ELCA for changing the underlying plaque and improving stent expansion in undilatable lesions.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Mintz GS, Kovach JA, Javier SP, et al. Mechanisms of lumen enlargement after excimer laser coronary angioplasty. An intravascular ultrasound study. Circulation 1995; 92: 3408-14. [DOI] [PubMed] [Google Scholar]
- 2.Herzum M, Cosmeleata R, Maisch B. Managing a complication after direct stenting: removal of a maldeployed stent with rotational atherectomy. Heart 2005; 91: e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sonoda S, Morino Y, Ako J, et al. Impact of Final stent dimensions on long-term results following sirolimus-eluting stent implantation: serial intravascular ultrasound analysis from the Sirius trial. J Am Coll Cardiol 2004; 43: 1959–63. [DOI] [PubMed] [Google Scholar]
- 4.Herzum M, Cosmeleata R, Maisch B. Managing a complication after direct stenting: removal of a maldeployed stent with rotational atherectomy. Heart 2005; 91: e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldberg SL, Colombo A, Akiyama T. Stent under-expansion refractory to balloon dilatation: a novel solution with excimer laser. J Invasive Cardiol 1998; 10: 269-73. [PubMed] [Google Scholar]
- 6.Ahmed WH, Al-Anazi MM, Bittl JA. Excimer laser-facilitated angioplasty for undilatable coronary narrowing’s. Am J Cardiol 1996; 78: 1045-6. [DOI] [PubMed] [Google Scholar]
- 7.Baumbach A, Haase KK, Rose C, et al. Formation of pressure waves during in vitro excimer laser irradiation in whole blood and the effect of dilution with contrast media and saline. Lasers Surg Med 1994; 14: 3-6. [DOI] [PubMed] [Google Scholar]
- 8.Deckelbaum LI, Natarajan MK, Bittl JA, et al. Effect of intracoronary saline infusion on dissection during excimer laser coronary angioplasty: a randomized trial. J Am Coll Cardiol 1995; 26: 1264-9. [DOI] [PubMed] [Google Scholar]
- 9.Viceconte N, Biscione C, Tarsia G, et al. Laser “explosion” technique for treatment of unexpanded coronary stent. Int J Cardiol 2011; 149: 395-7. [DOI] [PubMed] [Google Scholar]
- 10.Berthe L, Sollier A, Peyre P, et al. The generation of laser shock waves in a water-confinement regime with 50 ns and 150 ns XeCI excimer laser pulses. J Phys D Appl Phys 2000; 33: 2142-5. [Google Scholar]
- 11.Latib A, Takagi K, Chiazzola G, et al. Excimer laser lesion modification to expand non-dilatable sTents; the ELLEMENT Registry. Cardiovasc Revasc Med 2014; 15: 8-12. [DOI] [PubMed] [Google Scholar]
- 12.Uren NG, Schwarzacher SP, Metz JA, et al. Predictors, and outcomes of stent thrombosis. Eur Heart J 2002; 23: 124-32. [DOI] [PubMed] [Google Scholar]
- 13.Cutlip DE, Windecker S, Mehran R, et al. Academic Research Consortium . Clinical end points in coronary stent trials: a case for standardized definitions. Circulation 2007; 115: 2344-51. [DOI] [PubMed] [Google Scholar]
- 14.Waciński P, Bilodeau L, Crepeau J, et al. Excimer laser 0.9 mm catheter for calcified uncrossable lesions. Am J Cardiol 2004; 94(6S): 42E. [Google Scholar]
- 15.Noble S, Bilodeau L. High energy excimer laser to treat coronary in-stent restenosis in an underexpanded stent. Catheter Cardiovasc Interv 2008; 71: 803-7. [DOI] [PubMed] [Google Scholar]
- 16.Ben-Dor I, Maluenda G, Pichard AD, et al. The use of excimer laser for complex coronary artery lesions. Cardiovasc Revasc Med 2011; 12: e61-8. [DOI] [PubMed] [Google Scholar]
- 17.Iwańczyk S, Siniawski A, Panowicz M, et al. Successful intravascular lithotripsy for covered stent underexpansion due to severely calcified plaque. Kardiol Pol 2020; 78: 247-8. [DOI] [PubMed] [Google Scholar]
- 18.Wanha W, Tomaniak M, Wanczura P, et al. Intravascular lithotripsy for the treatment of stent underexpansion: the multicenter IVL-DRAGON Registry. J Clin Med 2022; 11: 1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Savage MP, Fishman D. Resistant drug-eluting stent restenosis and resurrection of intracoronary brachytherapy: the lazarus of contemporary coronary intervention. J Soc Cardiovasc Angiography Interv 2023; 2: 100566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Basavarajaiah S, Ielasi A, Raja W, et al. Long-term outcomes following intravascular lithotripsy (IVL) for calcified coronary lesions: a Real-World Multicenter European Study. Catheter Cardiovasc Interv 2023; 1: 250-60. [DOI] [PubMed] [Google Scholar]
- 21.Shlofmitz E, Iantorno M, Waksman R. Restenosis of drug-eluting stents a new classification system based on disease mechanism to guide treatment and state-of-the-art review. Circ Cardiovasc Interv 2019; 12: e007023. [DOI] [PubMed] [Google Scholar]
- 22.Niccoli G, Giubilato S, Conte M, et al. Laser for complex coronary lesions: impact of excimer lasers and technical advancements. Int J Cardiol 2011; 146: 296-9. [DOI] [PubMed] [Google Scholar]
- 23.Oraevsky AA, Jacques SL, Pettit GH, et al. XeCl laser ablation of atherosclerotic aorta: optical properties and energy pathways. Lasers Surg Med 1992; 12: 585-97. [DOI] [PubMed] [Google Scholar]
- 24.Sintek M, Coverstone E, Bach R, et al. Excimer laser coronary angioplasty in coronary lesions: use and safety from the NCDR/CATH PCI Registry. J Circ Cardiovasc Interv 2021; 14: e010061. [DOI] [PubMed] [Google Scholar]