ABSTRACT
Chronic Chagasic cardiomyopathy develops years after infection in 20–40% of patients, but disease progression is poorly understood. Here, we assessed Trypanosoma cruzi parasite dynamics and pathogenesis over a 2.5-year period in naturally infected rhesus macaques. Individuals with better control of parasitemia were infected with a greater diversity of parasite strains compared to those with increasing parasitemia over time. Also, the in vivo parasite multiplication rate decreased with increasing parasite diversity, suggesting competition among strains or a stronger immune response in multiple infections. Significant differences in electrocardiographic (ECG) profiles were observed in Chagasic macaques compared to uninfected controls, suggesting early conduction defects, and changes in ECG patterns over time were observed only in macaques with increasing parasitemia and lower parasite diversity. Disease progression was also associated with plasma fibronectin degradation, which may serve as a biomarker. These data provide a novel framework for the understanding of Chagas disease pathogenesis, with parasite diversity shaping disease progression.
IMPORTANCE
Chagas disease progression remains poorly understood, and patients at increased risk of developing severe cardiac disease cannot be distinguished from those who may remain asymptomatic. Monitoring of Trypanosoma cruzi strain dynamics and pathogenesis over 2–3 years in naturally infected macaques shows that increasing parasite diversity in hosts is detrimental to parasite multiplication and Chagasic cardiomyopathy disease progression. This provides a novel framework for the understanding of Chagas disease pathogenesis.
KEYWORDS: strain interactions, host-parasite relationship, intracellular parasites, competition, pathogenesis
INTRODUCTION
Chagas disease is a neglected tropical disease caused by Trypanosoma cruzi parasites, with a disease burden of 0.55 million disability-adjusted life years lost, 10,600 annual deaths, and an estimated $7.2 billion in annual economic losses (1). Its main clinical manifestation is chronic Chagasic cardiomyopathy, which develops many years after the initial infection in 20–40% of patients, while digestive manifestations such as mega-esophagous or megacolon may be observed in about 10% of patients (2). Despite the severe morbidity associated with disease progression, Chagas disease remains often underdiagnosed and has substandard access to care for patients.
A major issue is that disease progression is still poorly understood, and patients at an increased risk of developing severe disease cannot be distinguished from those who may remain in the asymptomatic chronic stage, making any prognosis uncertain. Pathogenesis has been associated with immune exhaustion, allowing parasite persistence in host tissues (3, 4), providing the rationale to target the parasite through drugs or therapeutic vaccines (2). However, the efficacy of benznidazole treatment is variable, and while it can consistently decrease parasite burden, it can also delay the appearance of new electrocardiographic (ECG) abnormalities and the progression to more severe Kuschnir group of cardiac disease in patients treated early (5, 6), but it fails to stop or delay the progression of fibrotic heart disease in symptomatic patients (7). However, disease progression and response to treatment are difficult to monitor, due in part to the need for long-term follow-up and the lack of precise indicators. For example, seronegativization, which may be considered the gold standard for treatment response, takes many years after treatment to be observed (8, 9).
Several alternative biomarkers of disease progression and response to treatment have been proposed. For example, in Chagasic mice, serum levels of transforming growth factor β, platelet-derived growth factor, and connective tissue growth factor correlate well with cardiac fibrosis and cardiac dysfunction (10). Plasma fibronectin degradation has also been proposed as a biomarker of disease progression and response to treatment in both patients and mouse models (11 – 14). Nonetheless, the prognosis value of these biomarkers remains to be investigated.
Adding to this complexity, parasite genetic diversity has also been suggested to play a role in Chagas disease pathogenesis. Indeed, T. cruzi parasites are genetically very diverse, and the species is currently divided into seven major lineages or discrete typing units (DTUs) named TcI to TcVI and TcBat (15). While clear differences in infectivity, virulence, or drug susceptibility can be observed among parasite strains in vitro or in animal models (16 – 20), marked associations of parasite DTUs with clinical manifestations in patients have been difficult to establish (21 – 25). In addition, the use of genotyping methods of increased sensitivity is providing growing evidence that a large proportion of infections in both triatomine vectors (26 – 28) and mammalian hosts (29 – 33) are in fact infections with multiple parasite strains or DTUs. For example, mixed infections with TcI and non-TcI parasites were detected in 20.6% (7/34) of pregnant women in Mexico, 38.1% (8/21) in Honduras, 43.7% (6/16) in Argentina (30), and in 33% (2/6) of US patients from Texas (34). Another study found that 47% (8/17) of Chagasic patients from Yucatan, Mexico, were infected with different mixtures of TcI, TcII, TcV, and TcVI parasites (31). The implications of such multiple infections on disease progression and clinical manifestations are unclear, but interactions among strains are likely to occur, which will modulate selection pressures on both hosts and parasite strains (35 – 38). For example, experimental co-infection with two strains of Trypanosoma brucei in mice resulted in the suppression of individual strains and increased host survival (39). Accordingly, experimental co-infections with T. cruzi in mice can also result in differences in infection progression (40 – 42), suggesting interactions among strains, but these favor either the host or the parasite, depending on specific strain combinations, making generalization of these results difficult. Theoretical approaches have helped conceptualize these possible outcomes, such as a more severe disease caused by multiple pathogen strains when their “super-infection” is able to better evade or overcome the immune system. Conversely, less severe disease may occur if competition among strains or cross-immunity leads to reduced pathogen growth. However, there is a lack of empirical data to assess the relevance of these potential situations and outcomes (35), particularly in the context of natural infections with T. cruzi.
Therefore, our objective was to assess T. cruzi parasite strain dynamics and disease progression in a cohort of naturally infected rhesus macaques, which closely mimic human infection (43). There is indeed extensive zoonotic circulation of T. cruzi in the southern USA (44), with occasional spill-over infections in captive macaques (45) with T. cruzi strains that are similar to those circulating in local Triatoma sanguisuga vectors and mammalian hosts in Louisiana (26, 29, 32, 33, 46, 47). We measured parasite dynamics and clinical disease progression over a 2.5-year period in macaques that had been infected for 1–6 years to shed light for the first time on a unique interplay between multiple parasite strains and clinical profile during the chronic phase and provide a new framework for understanding Chagas disease progression.
RESULTS
Parasite burden in a cohort of naturally infected rhesus macaques
We followed a cohort of rhesus macaques with a natural T. cruzi infection. These were initially screened as seropositive for T. cruzi during routine health examinations of a breeding colony, and infection was confirmed through additional serological testing (Stat-Pak rapid test) and PCR. Their average age was 10.7 ± 0.7 years old (range four to 19 years), and they had been infected for 4.1 ± 0.3 years at the start of the follow-up (range one to 6 years) (Table S1). The cohort included five macaques of Chinese ancestry and 27 of Indian ancestry. Some animals, but not all, presented a transient loss in body weight around the time seroconversion was detected, which may reflect the acute phase of T. cruzi infection (Fig. 1).
Fig 1.
Body weight history. Body weight history of representative Chagasic macaques. Vertical arrows indicate the time when positive T. cruzi serology was first detected. * indicate pregnancies, which may explain some body weight increases. Note some transient body weight loss detected in some animals around the time of infection.
Quantification of T. cruzi parasitemia by qPCR indicated an average parasite burden of 8.1 ± 1.5 parasite eq./mL of blood that was overall stable over the 24–30 months of follow-up (Fig. 2A). However, analysis of the changes over time in parasite burden in individual macaques revealed two major patterns, with some animals presenting a slow decrease in parasitemia over time, suggesting some control of the infection, which were referred to as “controllers” (Fig. 2B), while others presented a gradual increase in parasitemia, suggestive of disease progression, which were referred to as “progressors” (Fig. 2C). Interestingly, macaques with progressive disease tended to have had more pronounced body weight loss at the time of infection compared with those with better control of the infection, suggesting a more severe acute phase, although this did not reach statistical significance (Fig. 2D, P = 0.076).
Fig 2.
Blood parasite burden. Blood parasite burden was measured by qPCR over time. (A) Time course of parasitemia during 24–30 months of follow-up in Chagasic rhesus macaques. (B) Time course of parasitemia in individual macaques with increasing parasitemia (“progressors”). (C) Time course of parasitemia in individual macaques with decreasing parasitemia (“controllers”). Symbols and lines are color-coded for each macaque, with their ID indicated in the graph in panels B and C. (D) Body weight change at the time of infection in progressor and controller macaques. Body weight loss tended to be more pronounced in progressor macaques, although this did not reach statistical significance (P = 0.076).
Parasite genotyping and strain dynamics
We next genotyped the blood parasites in a subset of 11 macaques using next-generation sequencing of the mini-exon gene, followed by phylogenetic analysis of the sequences. A total of 304 mini-exon sequences were obtained, corresponding to 2–12 unique haplotypes per animal and time point (GenBank accession numbers ON907885–ON908188). Globally, sequences from the cohort clustered with reference sequences belonging to TcI, TcII, TcIV, TcV, and TcVI DTUs (Fig. 3A; Fig. S1), although TcI predominated followed by TcIV, and TcII, TcV, and TcVI were less frequent. As noted before (29), some sequence similarity was observed between mini-exon sequences from these macaques with parasite sequences identified in other mammalian hosts and triatomine vectors from the region, in agreement with these infections being the product of spillover events from local zoonotic transmission cycles. Only one monkey was infected with a single DTU at the start of follow-up (TcI), and all others were infected with mixtures of TcI, TcIV, TcII, TcV, and TcVI parasites in variable proportions, and up to five DTUs could be detected in a single animal (Fig. 3B). Because of the very low prevalence/incidence of infection in these hosts (45), sequential infections with different T. cruzi parasite DTUs are extremely unlikely, and the most likely explanation for these multiple infections is simultaneous co-infections with multiple parasite strains. Indeed, triatomine vectors from the region have often been found co-infected with multiple parasite strains/DTUs (26).
Fig 3.
T. cruzi parasite diversity in naturally infected macaques. (A) Phylogenetic tree of mini-exon sequences from naturally infected macaques, indicating the presence of TcI, TcII, TcIV, TcV, and TcVI parasite DTUs. Parasite DTUs are color-coded as indicated. * indicates sequences from reference strains from each DTU. Bootstrap support of different clades is indicated for each DTU. (B) Comparison of parasite DTUs’ respective proportions at the start of follow-up in progressor and controller macaques. Parasite DTUs are color-coded as indicated. (C) Comparison of the proportion of infections with different numbers of DTUs in progressor and controller macaques. Most progressor macaques were infected with 1–2 parasite DTUs, while most controller macaques were infected with 4–5 DTUs, χ2 = 16.07, P = 0.003. (D) Comparison of the number of unique mini-exon sequence haplotypes in progressor and controller macaques. Controller macaques presented infections with a significantly larger number of T. cruzi haplotypes than progressor macaques, Student’s t = 2.48, P = 0.016. (E) Linear regression between blood parasite burden and the number of infecting T. cruzi mini-exon haplotypes. Parasite burden significantly decreased with increasing number of haplotypes, R 2 = 0.18, P = 0.002.
Comparison of parasite assemblages between progressor and controller macaques revealed significant differences. Indeed, macaques with a better control of T. cruzi parasitemia were predominantly infected with 4–5 parasite DTUs, while those with progressive parasite burden were predominantly infected with only 1–2 parasite DTUs (Fig. 3C, χ2 = 16.07, P = 0.003). We also assessed the diversity of mini-exon sequence haplotypes detected and accordingly found that macaques with a better control of T. cruzi burden presented a significantly higher number of parasite haplotypes compared to those with a progressive parasite burden (Fig. 3D, t = 2.48, P = 0.016). Furthermore, there was a significant negative correlation between blood parasite burden and haplotype number, with a decreasing burden as the haplotype number increased (Fig. 3E, R 2 = 0.18, P = 0.002). A low sensitivity of our genotyping approach to detect rare haplotypes/DTUs in samples with lower parasite burden could not account for these observations, as this would have resulted in a decrease in haplotypes/DTUs with decreasing parasite burden. Rather, these data suggested that natural infections with a higher diversity of T. cruzi strains/DTUs were better controlled by hosts compared to infections with a lower parasite diversity.
To further evaluate parasite strain interactions and dynamics, we then analyzed potential changes in parasite assemblages over time. Again, two major patterns were observed. In macaques infected with a limited number of parasite DTUs (mostly TcI and TcIV), the relative proportions of each DTU showed limited variation over the 24–30 months of follow-up, with most of these animals corresponding to progressors (Fig. 4A). On the other hand, infections with a higher number of parasite DTUs often resulted in larger changes in the relative proportion of parasite DTUs over time, leading to sequential changes in the predominant DTU, although most, if not all, DTUs persisted over time (Fig. 4B). Thus, different parasite strain dynamics seemed to be occurring in these two groups of macaques, with progressors having rather stable and well-established infections caused by a limited diversity of parasites that were able to persist, while infections with a higher diversity of parasite strains/DTUs led to a limited parasite growth and regular changes in the predominant DTU persisting in these controller hosts.
Fig 4.
Intra-host T. cruzi parasite strain dynamics over time. Changes in T. cruzi parasite DTU assemblage were measured over 20–30 months of follow-up and are shown for individual monkeys: (A) controllers and (B) progressors. Parasite DTUs are color-coded as indicated.
These observations suggested that T. cruzi strain interactions may be occurring in infections with a higher parasite diversity, resulting in a lower proliferation of the parasites. To assess this point, we calculated DTU-specific parasite multiplication rates in the hosts and found significant differences in the in vivo multiplication rate of T. cruzi according to the DTU (Fig. 5A, ANOVA, F = 4.0, P = 0.004). Indeed, TcI parasites presented the highest multiplication rate, followed by TcIV, and TcII, TcV, and TcVI presented the lowest multiplication rates in chronically infected macaques. Such differences may help explain the relative abundance of each DTU in this host population. However, parasite proliferation was also affected by parasite diversity as we detected a significant negative correlation between the multiplication rate and the number of parasite haplotypes present (Fig. 5B, R 2 = 0.067, P = 0.003), with the parasite multiplication rate decreasing when an increasing number of haplotypes were present. These observations strongly supported the hypothesis that increasing parasite diversity in hosts was detrimental to parasite multiplication, possibly due to competition for resources or a stronger immune control through cross-immunity.
Fig 5.
In vivo T. cruzi multiplication rate. T. cruzi parasite multiplication rate was measured in vivo in naturally infected macaques and compared according to parasite DTU (A). Multiplication rate varied significantly according to parasite DTU, color-coded as indicated, ANOVA, F = 4.0, P = 0.004. (B) Linear regression of parasite multiplication rate as a function of parasite diversity. Multiplication rate significantly decreased with infections with increasing numbers of parasite haplotypes, R 2 = 0.067, P = 0.003.
Because parasite strain assemblages seemed to be key for T. cruzi control, we also investigated the potential association of specific parasite sequence haplotypes with parasite burden. For this, we performed principal component analysis (PCA) of the mini-exon sequences from each DTU among progressor and controller macaques (Fig. 6). We detected significant differences in sequence haplotypes between progressor and controller macaques only for TcI parasites (Fig. 6A, PERMANOVA, P = 0.049), but not for any of the other DTUs (Fig. 6B through E, P > 0.05). Thus, genetic differences among strains within each DTU may play a lesser role than DTU composition in determining parasite burden and disease progression. However, it should be noted that T. cruzi genetic diversity within DTUs may have been limited in this cohort of hosts from the same habitat.
Fig 6.
Genetic diversity of T. cruzi within DTUs. PCA of mini-exon haplotype sequences was performed for each DTU to compare parasite diversity between progressor and controller macaques. Significant differences in parasite haplotypes between groups were observed for TcI strains (PERMANOVA, P = 0.049), but not for TcII, TcIV, TcV, or TcVI parasites (PERMANOVA, P > 0.05).
Electrocardiographic assessment of cardiac function
Next, we also evaluated cardiac function in this cohort of Chagasic macaques, as well as in age- and sex-matched uninfected control animals, through electrocardiographic recordings. Measurement of P wave, PR, QT, QRS, and RR intervals indicated that there were no significant differences in individual ECG parameters between Chagasic and uninfected macaques. However, integrated analysis of these parameters through linear discriminant analysis (LDA) showed that ECGs from Chagasic macaques differed from those from uninfected controls (PERMANOVA, P = 0.007), and 75.6% of individuals could be correctly reclassified between Chagasic and controls based on their ECG profiles (Table S2). These data indicated that while major arrhythmias were not detected, Chagasic macaques presented small conduction defects suggestive of the onset of cardiac alterations. Interestingly, one Chagasic macaque presented major cardiomegaly after only one year of infection, associated with severe kyphosis and generalized muscle atrophy, suggestive of a rapid and advanced clinical disease in this animal (Fig. 7A and B).
Fig 7.
Cardiomegaly and ECG alterations in Chagasic macaques. Thoracic X-ray image of a Chagasic (A) and uninfected age-matched macaque (B) showing pronounced cardiomegaly in the Chagasic animal. (C) LDA of ECG parameters of uninfected, and progressor and controller macaques, indicating significant differences in their ECG profiles, PERMANOVA, P = 0.036. Up to 63.6% of animals could be correctly classified into their respective groups by the LDA. (D) LDA of ECG parameters of progressor macaques at two time points about 6–15 months apart, indicating significant changes in ECG profiles over time, PERMANOVA, P = 0.015. Up to 73.0% of animals could be correctly classified into their respective groups by the LDA. (E) LDA analysis of ECG parameters of controller macaques at two time points about 6–15 months apart, indicating no significant changes in ECG profiles over time, PERMANOVA, P = 0.23, although up to 64.7% of animals could be correctly classified in their respective groups by the LDA.
Further analysis of ECG parameters based on the blood parasitemia of Chagasic macaques (progressor vs controller) also revealed significant differences in ECG profiles between these two groups, which differed from uninfected controls (Fig. 7C, PERMANOVA, P = 0.036), and 63.6% of animals could be correctly reclassified by the LDA. A subset of animals had two ECG recordings available taken 8–15 months apart during our follow-up, allowing us to examine potential changes over time. Again, no significant changes were detected among single ECG parameters. However, the LDA indicated that ECGs from macaques classified as progressors based on parasitemia presented significant differences over time and with uninfected controls (Fig. 7D, PERMANOVA, P = 0.015), while ECGs from controller macaques remained unchanged over time (Fig. 7E, PERMANOVA, P = 0.23). Together, these observations indicate that macaques with increasing parasitemia present more rapidly progressing ECG alterations, while those with more controlled parasitemia have no or more limited progression of the ECG alterations over the follow-up period.
Biomarker of disease progression
To expand on our assessment of disease progression in these macaques, we then measured plasma levels of fibronectin, as this protein has been proposed as a biomarker for Chagas disease. Indeed, the levels of degraded fibronectin have been found to be increased in Chagasic patients as well as in mouse models (11 – 13), as it is a proposed substrate for cruzipain, a parasite cysteine protease (48). Western blot analysis of macaque plasma proteins indicated that Chagasic macaques indeed presented a reduced amount of high-molecular-weight intact fibronectin, together with a higher amount of multiple degradation fragments of lower molecular weight, compared with uninfected animals (Fig. 8A). In particular, a 37-kDa degradation fragment was observed, which may correspond to a comparable low-molecular-weight fragment identified in Chagasic patients and experimentally infected mice (11 – 13). Further quantitative analysis of fibronectin degradation and its changes over time indicated that individual levels were rather stable over the 24–30 months of follow-up and that macaques controlling their blood parasite burden had a plasma fibronectin level similar to that of uninfected animals, while those with progressive parasite burden presented a significantly elevated fibronectin degradation (Fig. 8B and C, t = 4.5, P < 0.0001). Finally, fibronectin degradation was significantly correlated with parasite haplotype number, and infections with a lower number of haplotypes were associated with a higher level of fibronectin degradation (Fig. 8D, R 2 = 0.23, F = 15.64, P = 0.0002). Fibronectin degradation also significantly increased with increasing blood parasite burden (Fig. 8E, R 2 = 0.07, F = 4.03, P = 0.049). These observations suggested that fibronectin degradation may be associated with disease severity and may be a valuable prognostic biomarker of disease progression.
Fig 8.
Plasma fibronectin degradation as a biomarker of disease progression. Plasma fibronectin degradation was measured by western blot (A). High-molecular-weight fibronectin (FN) is partially degraded in Chagasic macaques compared to uninfected controls, which results in an increase in lower-molecular-weight fragments, including a fragment of about 37 kDA (dFN). Plasma transferrin (Transf) was used as the loading control. Densitometric quantification of fibronectin degradation (B) indicated levels comparable to uninfected macaques in controller Chagasic macaques and significantly increased levels in progressor Chagasic macaques (t = 4.5, P < 0.0001). (C) Measurement of fibronectin degradation over time indicated very stable levels in both controller and progressor macaques over 20–30 months of follow-up. Color-coded lines and symbols indicate individual macaques, whose ID is indicated. (D) Fibronectin degradation was negatively correlated with parasite haplotype number, R 2 = 0.23, F = 15.64, P = 0.0002, and (E) positively correlated with blood parasite burden, R 2 = 0.07, F = 4.03, P = 0.049).
Integrative modeling of disease progression
Finally, we built a statistical model to identify key variables allowing us to predict Chagas disease progression in our cohort of naturally infected macaques, based on the progression in blood parasite burden (progressors vs controllers). The best model provided a rather good fit of the data (R 2 = 0.55, P = 0.004) and included the duration of the infection, fibronectin degradation level, QRS duration, and parasite haplotype number as variables (Table 1). Another model provided a somewhat better fit (R 2 = 0.63, P = 0.004), although AIC indicated that it was slightly worse than the best model (AIC = 28.7 vs 26.6), and this model included the same variables plus the body weight change at the time of infection (Table 1). These models strongly suggested that changes in blood parasite burden over time were associated with the duration of infection, parasite genetic diversity, and changes in ECG profiles, and that fibronectin degradation may serve as a valuable biomarker of disease progression. Body weight loss at the time of infection, which may reflect the severity of the acute phase, may also be an important predictor of disease development at later stages.
TABLE 1.
Logistic models for blood parasitemia changes over time a
| Model #1 | X2 | P | R 2 | AIC |
|---|---|---|---|---|
| 15.22 | 0.0043* | 0.55 | 26.6 |
* indicates statistically significant P values (<0.05).
DISCUSSION
Chagas disease progression during the chronic phase remains poorly understood, and naturally infected macaques offer an unprecedented opportunity to investigate this process. Thus, we evaluated for the first time intra-host T. cruzi strain dynamics in association with parasitological and clinical indicators of disease progression in rhesus macaques chronically infected for 1–6 years.
Blood parasite burden is a key parasitological indicator, and it is the primary outcome to assess disease progression and response to treatment in clinical trials of drug therapies against Chagas disease (7, 49 – 51). We found that at the population level, blood parasite burden was low but rather stable over time in our untreated macaques, which is similar to previous observations from placebo-treated human patients in the chronic phase. However, we also detected important individual variations in changes in parasitemia over time, suggestive of differences in parasite control, with some animals presenting a gradual increase in parasitemia while others presented a decrease in parasitemia over time. We then assessed possible explanations for these differences in parasitological profiles between the two groups.
We found that differences in blood parasite burden profiles were in large part associated with parasite genetic diversity, more specifically with the parasite strain assemblage infecting each host, as well as associated with more rapid changes in ECG alterations. Indeed, we first confirmed the circulation of a large diversity of DTU, expanding previous work (29). While the use of the mini-exon as a single marker for genotyping T. cruzi has limitations, particularly the inability to assess the exact number of parasite strains within DTUs (31, 52, 53), it allowed a reliable follow-up of parasite dynamics over time. Future studies based on additional markers or the isolation of parasites may provide more detailed data on infecting strains, but have limitations in terms of sensitivity and can be biased toward strains growing better in vitro (31, 54). Thus, our data shed some new light on T. cruzi strain interactions in such multiple infections. Indeed, a major finding of our study was that infections with an increased diversity of parasite strains/DTUs were associated with a decreasing parasitemia and a lower in vivo multiplication rate of the parasite compared to infections with a more limited parasite diversity. Accordingly, these observations suggest that in macaques, infections with mixtures of T. cruzi strains/DTUs do not benefit the parasite by allowing a severe “super-infection” to develop, but rather favor the host by allowing for a better control of the parasite. This reduced parasite proliferation may be due to multiple non-exclusive mechanisms (35, 55). For example, it may involve (1) direct interference, in which strains produce substances to inhibit each other; (ii) resource competition, such as for nutrients or host infection sites (tissue/cell specificity of strains) that are used and no longer available for other strains and limit their growth, which is an indirect form of competition; or (iii) a stronger immune response that controls parasite infection through cross-reactivity among strains. In this case, strains with higher similarity suffer from increased immune pressure, and more divergent strains may be favored by a reduced antigenic overlap allowing for some immune evasion (35, 38, 56). In this respect, it is worth noting that, in spite of the detrimental interactions involving multiple strains, parasite diversity is largely maintained over time in these chronically infected macaques, although at low levels of parasitemia. Also, as cardiac disease progression appears slower when a high parasite diversity is present, it is likely that inflammatory responses leading to myocarditis are decreased. Thus, our overall observations provide the first evidence that T. cruzi proliferation and cardiac disease progression may be limited as the diversity of infecting strains/DTUs increases, and further studies should help disentangle how these possible mechanisms may be involved. Nonetheless, it should be noted that a limitation of our study is that we could only assess parasite diversity in the blood, and different patterns of strain/DTU diversity and their interactions may occur in other tissues.
Previous studies of experimental infections in mice with a mixture of T. cruzi strains have mostly focused on the comparison of combinations of pairs of strains vs each strain alone (40 – 42), and to our knowledge, mixtures of more than two strains have not been evaluated in experimental infection models. The experimental co-infection with two strains of Trypanosoma brucei in mice similarly resulted in the suppression of individual strains and increased host survival, indicative of strain competition in the host (39). Natural infections with Plasmodium vivax are composed of mixtures of closely related parasites, and mutation and selection processes could be identified over time in patients, indicative of parasite evolution in the host (57).
We also detected differences in in vivo multiplication rates among T. cruzi DTUs. Multiplication rate is an important determinant of the virulence and fitness of pathogens in hosts (58), and the observed differences among DTUs may help explain, at least in part, the relative proportions/abundance of the respective T. cruzi DTUs in macaques. Other host species from the region, such as cats, dogs, or rodents (32, 33, 46), seem to harbor somewhat different proportions of T. cruzi DTUs, and further studies of strain dynamics in these hosts should help better characterize parasite traits, which may vary according to host species.
We also observed a limited role of specific parasite genotype/haplotype on disease progression compared to the effect of strain mixtures. This appears contrary to many observations of individual strain biological variability in vitro and in vivo (16 – 20). However, within DTUs, parasite diversity was overall low in our rather homogenous cohort of parasites and hosts from the same habitat. Comparison with a broader diversity of strains from other geographic regions may be needed to assess this point as the geographic differentiation of T. cruzi strains is also likely contributing to biological differences (52, 59).
Interestingly, our data on body weight loss at the time of T. cruzi infection, although rather crude, also suggest that this may be a very early indicator of the long-term outcome of infections during the chronic phase. However, as the acute phase is rarely identified in patients, this may be difficult to confirm. On the other hand, plasma fibronectin degradation is emerging as a valuable biomarker of disease progression as proposed before (11 – 14). Indeed, we found that it was specifically increased in macaques with increasing parasite burden and progressive alterations in ECG recordings. The development of novel assays for an easier and more quantitative measurement of fibronectin degradation fragments than western blot would thus be helpful for its further evaluation. Also, measurements of cruzipain protease levels may provide important information as it may mediate fibronectin degradation (48).
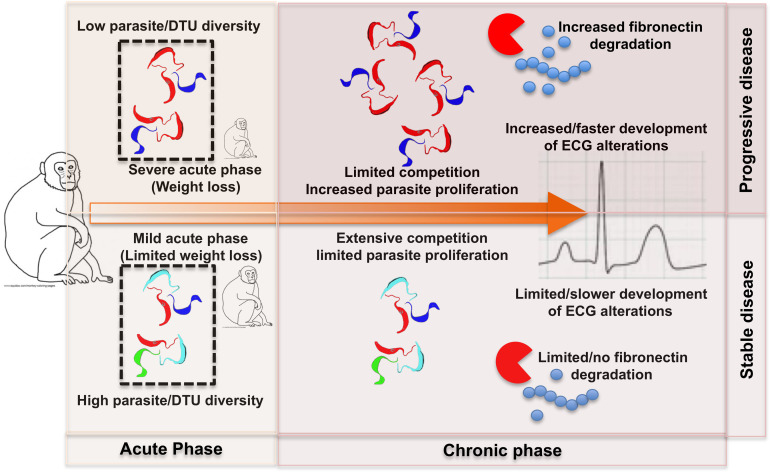
Finally, our study provides a general framework for a better understanding of the relationship between parasitological and clinical disease progression in naturally infected Chagasic macaques. Indeed, our data suggest that disease progression in the chronic phase is in large part determined by the onset of infection according to parasite diversity (Fig. 9). Thus, on the one hand, infection with a limited diversity of parasite strains/DTUs would result in a more severe acute phase and a favorable environment for their multiplication and long-term persistence in the host at increasing levels. This would be associated with more rapidly progressing ECG alterations, which may eventually lead to severe arrhythmias and cardiac dysfunction. Also, increased levels of plasma fibronectin may serve as a biomarker of this situation. On the other hand, infection with a higher diversity of parasite strains/DTUs would result in a milder acute phase, with limited parasite proliferation, and lead to lower levels of persisting parasites. This would be associated with no or very slow development of ECG alterations, which may not progress toward severe cardiac dysfunction. Also, normal levels of plasma fibronectin may serve as a biomarker of this outcome. While additional factors, such as host intrinsic factors (60, 61), are likely to also affect disease progression, our simple framework allows for further evaluating the mechanisms of pathogenesis in Chagasic hosts.
Fig 9.
A framework for disease progression in Chagasic macaques. We propose that disease progression in the chronic phase is in large part determined by the onset of infection according to T. cruzi parasite diversity. See the text for additional explanations.
Indeed, this framework may help explain differences in clinical profiles and disease progression in Chagasic patients, and warrants further studies aimed at better assessing T. cruzi parasite diversity in patient cohorts associated with their clinical profile (31, 34). For example, a recently reported acute case from Belize was found to be co-infected with a mixture of TcIV, TcII, and TcV parasites and effectively treated with nifurtimox (62), and treatment effectiveness may have benefited from the multiplicity of parasite strains interacting. Also, preclinical models of T. cruzi experimental infection rely mostly, if not exclusively, on infections with single parasite strains, which accordingly would be the most stringent models for testing the efficacy of drugs or vaccines.
In conclusion, our integrative evaluation of parasitological and clinical aspects of T. cruzi infection in naturally infected rhesus macaques, with details of the intra-host parasite DTU dynamics over prolonged time, reveals unique interconnections between parasite genetic diversity and disease progression. In particular, we provide evidence of the multiplicity of infection in the host as a major driver of disease progression. It would be of key interest to further assess the potential mechanisms underlying the interactions among multiple strains, and such empirical data in natural infections are critical to complement other more theoretical approaches, given the paucity of experimental data. Fibronectin degradation is also emerging as a valuable biomarker in Chagas disease, which may eventually lead to a better prognosis for patients. Finally, further testing and refining the proposed framework for Chagas disease progression in Chagasic patients is warranted to reach a better understanding of pathogenesis and ultimately improve patient care and treatment.
MATERIALS AND METHODS
Animals
Rhesus macaques (Macaca mulatta) of Indian and Chinese ancestry were housed in the breeding colony of the Tulane National Primate Research Center (TNPRC) in species-appropriate social groups in outdoor enclosures in accordance with the Animal Welfare Act, the Guide for the Care and Use of Laboratory Animals, and other federal statutes and regulations. Both Indian-origin and Chinese-origin macaques are used in research, and they differ in appearance and genetic background. Differences in susceptibility to infections have been described between the subspecies for some pathogens (63), but no information is available regarding susceptibility to T. cruzi infection. Outdoor enclosures are constructed of wire mesh walls and a roof with natural ground cover and a variety of perches, shelters, and swings. Water is available ad libitum, and a standard, commercially formulated nonhuman primate diet is provided daily and supplemented with fresh fruit, vegetables, and/or other forage materials several times a week. In addition to daily observations, all macaques receive routine examinations and preventative medicine treatments (vaccines, anthelmintics) during semiannual health assessments. Blood samples are also collected for colony surveillance and diagnostic purposes. All breeding and management protocols are reviewed and approved by the Tulane University Institutional Animal Care and Use Committee. Animals were identified as seropositive for T. cruzi parasites (Table S1) during routine colony surveillance. Infections occurred naturally upon contact with infected Triatoma sanguisuga, the main vector in Louisiana (26), which may occasionally be present in outdoor enclosures. Additional blood samples were collected in subsequent routine examinations to allow for T. cruzi parasite monitoring, but unfortunately, no historic samples from when animals seroconverted were available to assess initial parasite burden or genotypes. Between two and five blood samples per animal were thus collected over a 2.5-year period of follow-up, although for a few animals, only one sample was available. Because sampling only occurred during routine care, it is biased for females as these were followed more closely than males in the breeding colony (Table S1). When possible, electrocardiographic recordings were performed to assess cardiac function, yielding between one and four ECG recordings per animal over the study period. Age- and sex-matched, healthy, uninfected animals were used to obtain control blood samples and ECG recordings for comparison. All procedures were performed on sedated animals. The study protocol was approved by the Tulane Institutional Animal Care and Use Committee.
Trypanosoma cruzi infection diagnostic
Trypanosoma cruzi infection was initially diagnosed through a Multiplexed Fluorometric Immunoassay (MFIA, Charles River Laboratories), which simultaneously tests for multiple pathogens and is routinely performed as part of health monitoring of the TNPRC breeding colony. Infection was then confirmed using Stat-Pak rapid immunochromatographic test (Chembio, Medford, NY), two diagnostic PCR reactions targeting nuclear satellite DNA (Sat. DNA PCR, TcZ1/TcZ2 primers) (64), and parasite kinetoplast DNA (kDNA PCR, Tc121/Tc122 primers) (65). Body weight, medical histories, and X-ray images were obtained from health records from routine care of the animals. Changes in body weight at the time of infection were obtained by calculating the difference in weight at the time of seroconversion and the previous measurement.
Blood parasite burden
Blood parasite burden was measured using a validated and standardized duplex qPCR method based on Taq-Man probes, targeting T. cruzi SatDNA and RNAse P gene as an internal amplification control (50). Because blood parasite burden in chronically infected hosts may be close to or even below the detection limit (66, 67), targeting a multi-copy sequence allows increased sensitivity. This assay is used in clinical trials evaluating treatment effectiveness in Chagasic patients and has been extensively validated (68). While some possible differences in sensitivity among parasite DTUs have been noted (for TcId and TcIe, not detected in these macaques, and for TcIV, but not for the other DTUs), the limited number of parasite strains tested does not allow for any generalization or correction/adjustment of the measured parasite burden (68). Briefly, DNA was extracted from 200 µL of blood with the Qiagen DNEasy Blood and Tissue Kit as per the manufacturer’s instructions, and amplifications were performed with 5 µL of DNA in a final volume of 20 µL. Uracil-DNA Glycosylase (Thermo Fisher Scientific) was added to the reaction mix, as a carry-over contamination control, and TaqMan RNase P Control Reagents Kit (Applied Biosystems) was used. Cycling conditions will be as follows: a first step of 2 min at 50°C and a second step of 10 min at 95°C, followed by 40 cycles at 95°C for 15 s and 58°C for 1 min. All samples were analyzed in duplicate. The qPCR results were converted to parasitic load (parasite equivalents/mL of blood) using a standard calibration curve, and data were log-transformed for statistical analysis.
T. cruzi parasite genotyping
Parasite genotyping was performed using a multiplex PCR targeting the mini-exon sequence, which gives PCR products of different sizes according to the DTU (69), and TrypME3 and TcCH primers, which amplify a larger fragment of 500 bp of this marker from all DTUs (53), as in previous studies (32). Briefly, amplicons were pooled for each sample and sequenced on a MiSeq (Illumina) platform. Reads were competitively mapped to mini-exon reference sequences from each DTU (70), and sequence variants were identified with the FreeBayes SNP/variant tool (71). Phylogenetic trees based on maximum likelihood were built using FastTree (72) to assess haplotype clustering with reference sequences from all DTUs. The number of reads of each unique haplotype and for parasite DTUs was used to calculate their relative proportions, and only those representing at least 1% of the reads were included in the analysis. Mini-exon sequences were deposited in the GenBank database under accession numbers ON907885–ON908188.
Absolute parasite burden for each DTU was calculated by multiplying their relative proportions with the corresponding total parasite burden of each sample measured by qPCR at each time point. From these data, DTU-specific parasite multiplication rates were measured by calculating absolute changes in parasite number from each DTU between the time points of our follow-up and expressed as parasite equivalents/mL of blood/month. Data were further log-transformed for statistical analysis.
Mini-exon sequence haplotype diversity was further assessed by principal component analysis. Because of the large sequence difference among DTUs, separate analyses were performed with sequences from each DTU. The statistical significance of differences among groups of sequences was analyzed by permutation ANOVA (PERMANOVA) based on 10,000 permutations and Jukes-Cantor distances among sequences. Analyses were performed in PAST 4.0 (73).
Electrocardiographic recording
Six lead electrocardiographic recordings were performed on sedated macaques to assess cardiac function using a DRE TrueVET ECG-1 Single Channel recorder at a speed of 25 mm/s and 40 mm/mV. Most animals were assessed once, but a subset had repeated ECG recordings at 8- to 12-month intervals to assess potential changes over time. For all recordings, the duration of the P wave, PR, RR, QRS, QT, and QTc intervals was measured (an average of 10 beats for each animal), and individual parameters were compared between groups using the Student’s t-test. For multivariate analysis, ECG parameters were integrated into the LDA (74), and groups were compared according to the first and second LDA axes. One-way PERMANOVA was used to assess the statistical significance of differences among groups with 10,000 permutations based on Manhattan distances. The confusion matrix of the LDA was also used to evaluate the accuracy of the reclassification of individual macaques among groups based on the similarities/differences in their ECG patterns.
Measurement of plasma fibronectin
Fibronectin and its degradation fragments were identified in plasma samples by western blot with a commercial antibody (Rabbit anti-fibronectin; Sigma-Aldrich) and quantified by densitometric analysis as before (14). Plasma samples were stored with protease inhibitors (protease inhibitor cocktail: 10 mM Iodoacetamide; 1 mM N-Methylmaleimide; 1 mM Benzamidine; 1 mM EDTA; 1 mM Sodium Orthovanadate; 1 mM PMSF; 50 mM Tris Base [all from Sigma]), and 20 µg of proteins in Laemmli buffer was separated on a 12% polyacrylamide gel and transferred onto nitrocellulose membranes. Fibronectin and its degradation products were detected with a 1:800 dilution of polyclonal antibody (Rabbit anti-fibronectin; Sigma-Aldrich) followed by a peroxidase-labeled secondary antibody [Goat anti-Rabbit IgG (H + L) HRP Antibody, EMD Millipore Corp.] at a 1:10,000 dilution. Plasma transferrin was used as the sample loading control after detecting fibronectin. The nitrocellulose membranes were stripped in a stripping solution of 100 mM 2-mercaptoethanol, 2% (wt/vol) SDS, 62.5 mM Tris-HCl, pH 6.7, at 50°C for 30 min and reprobed with an anti-transferrin rabbit polyclonal antibody (Proteintech) at a dilution of 1:1,000, followed by the secondary antibody [Goat anti-Rabbit IgG (H + L) HRP Antibody, EMD Millipore Corp., 1:10,000 dilution]. Blots were scanned on a Bio-Rad ChemiDoc Imaging System for densitometric analysis, and image analysis was performed using ImageJ. Fibronectin levels were normalized to transferrin and expressed as the ratio of degradation fragments/intact fibronectin (in arbitrary units). Data were log-transformed for statistical analysis.
Statistical modeling
We used logistic regression to model profiles of blood parasite burden as a function of parasite diversity, duration of infection, ECG alterations, and other clinical variables. Several models were built based on different sets of variables, and we used Akaike information criteria for model comparison and selection of the best model(s). Analyses were performed in SAS JMP 9.0.
ACKNOWLEDGMENTS
This work was supported by the National Center for Research Resources and the Office of Research Infrastructure Programs (ORIP) of the NIH through grant P51 OD011104 to the Tulane National Primate Research Center and grant R21AI175523 from the National Institute of Allergy and Infectious Diseases to E.D.
We also thank the Division of Veterinary Medicine for expert animal care.
E. Dumonteil and C. Herrera conceived of the study, designed the research, and analyzed the data. H. Desale, W. Tu, N. Hernández-Cuevas, M. Shroyer, and K. Goff performed the experiments. P.A. Marx supervised all animal work. E. Dumonteil drafted the manuscript. All authors revised the manuscript.
The authors have declared that no conflict of interest exists.
Contributor Information
Eric Dumonteil, Email: edumonte@tulane.edu.
Denis Sereno, Institut de Recherche pour le Développement, Montpellier, France .
DATA AVAILABILITY
DNA sequence data that support the findings of this study have been deposited in GenBank with the accession numbers ON907885–ON908188. Additional data are available within the paper and its supplementary information files or from the corresponding author upon reasonable request.
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/spectrum.04236-22.
Supplemental figure and tables.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Lee BY, Bacon KM, Bottazzi ME, Hotez PJ. 2013. Global economic burden of Chagas disease: a computational simulation model. Lancet Infect Dis 13:342–348. doi: 10.1016/S1473-3099(13)70002-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rassi A, Marin JA, Rassi A. 2017. Chronic Chagas cardiomyopathy: a review of the main pathogenic mechanisms and the efficacy of aetiological treatment following the BENznidazole Evaluation for Interrupting Trypanosomiasis (BENEFIT) trial. Mem Inst Oswaldo Cruz 112:224–235. doi: 10.1590/0074-02760160334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tarleton RL. 2001. Parasite persistence in the aetiology of Chagas disease. Int J Parasitol 31:550–554. doi: 10.1016/s0020-7519(01)00158-8 [DOI] [PubMed] [Google Scholar]
- 4. Natale MA, Minning T, Albareda MC, Castro Eiro MD, Álvarez MG, Lococo B, Cesar G, Bertocchi G, Elias MJ, Caputo MB, Tarleton RL, Laucella SA, Almeida IC. 2021. Immune exhaustion in chronic Chagas disease: pro-inflammatory and immunomodulatory action of IL-27 in vitro. PLoS Negl Trop Dis 15:e0009473. doi: 10.1371/journal.pntd.0009473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Viotti R, Vigliano C, Lococo B, Bertocchi G, Petti M, Alvarez MG, Postan M, Armenti A. 2006. Long-term cardiac outcomes of treating chronic Chagas disease with benznidazole versus no treatment: a nonrandomized trial. Ann Intern Med 144:724–734. doi: 10.7326/0003-4819-144-10-200605160-00006 [DOI] [PubMed] [Google Scholar]
- 6. Hasslocher-Moreno AM, Saraiva RM, Sangenis LHC, Xavier SS, de Sousa AS, Costa AR, de Holanda MT, Veloso HH, Mendes F, Costa FAC, Boia MN, Brasil P, Carneiro FM, da Silva GMS, Mediano MFF. 2021. Benznidazole decreases the risk of chronic Chagas disease progression and cardiovascular events: a long-term follow up study. EClinicalMedicine 31:100694. doi: 10.1016/j.eclinm.2020.100694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Morillo CA, Marin-Neto JA, Avezum A, Sosa-Estani S, Rassi A, Rosas F, Villena E, Quiroz R, Bonilla R, Britto C, Guhl F, Velazquez E, Bonilla L, Meeks B, Rao-Melacini P, Pogue J, Mattos A, Lazdins J, Rassi A, Connolly SJ, Yusuf S. 2015. Randomized trial of benznidazole for chronic Chagas' cardiomyopathy. N Engl J Med 373:1295–1306. doi: 10.1056/NEJMoa1507574 [DOI] [PubMed] [Google Scholar]
- 8. Viotti R, Vigliano C, Alvarez MG, Lococo B, Petti M, Bertocchi G, Armenti A, De Rissio AM, Cooley G, Tarleton R, Laucella S. 2011. Impact of aetiological treatment on conventional and multiplex serology in chronic Chagas disease. PLoS Negl Trop Dis 5:e1314. doi: 10.1371/journal.pntd.0001314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Solari A, Ortíz S, Soto A, Arancibia C, Campillay R, Contreras M, Salinas P, Rojas A, Schenone H. 2001. Treatment of Trypanosoma cruzi-infected children with nifurtimox: a 3 year follow-up by PCR. J Antimicrob Chemother 48:515–519. doi: 10.1093/jac/48.4.515 [DOI] [PubMed] [Google Scholar]
- 10. Hoffman KA, Reynolds C, Bottazzi ME, Hotez P, Jones K. 2019. Improved biomarker and imaging analysis for characterizing progressive cardiac fibrosis in a mouse model of chronic Chagasic cardiomyopathy. J Am Heart Assoc 8:e013365. doi: 10.1161/JAHA.119.013365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Santamaria C, Chatelain E, Jackson Y, Miao Q, Ward BJ, Chappuis F, Ndao M. 2014. Serum biomarkers predictive of cure in Chagas disease patients after nifurtimox treatment. BMC Infect Dis 14:302. doi: 10.1186/1471-2334-14-302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ruiz-Lancheros E, Rasoolizadeh A, Chatelain E, Garcia-Bournissen F, Moroni S, Moscatelli G, Altcheh J, Ndao M. 2018. Validation of apolipoprotein A-1 and fibronectin fragments as markers of Parasitological cure for congenital Chagas disease in children treated with benznidazole. Open Forum Infect Dis 5:fy236. doi: 10.1093/ofid/ofy236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ndao M, Spithill TW, Caffrey R, Li H, Podust VN, Perichon R, Santamaria C, Ache A, Duncan M, Powell MR, Ward BJ. 2010. Identification of novel diagnostic serum biomarkers for Chagas' disease in asymptomatic subjects by mass spectrometric profiling. J Clin Microbiol 48:1139–1149. doi: 10.1128/JCM.02207-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hernández-Cuevas NA, Marín-Cervera A, Garcia-Polanco S, Martínez-Vega P, Rosado-Vallado M, Dumonteil E. 2021. Fibronectin degradation as biomarker for Trypanosoma cruzi infection and treatment monitoring in mice. Parasitology 148:1067–1073. doi: 10.1017/S0031182021000809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zingales B, Andrade SG, Briones MRS, Campbell DA, Chiari E, Fernandes O, Guhl F, Lages-Silva E, Macedo AM, Machado CR, Miles MA, Romanha AJ, Sturm NR, Tibayrenc M, Schijman AG, Second Satellite Meeting . 2009. A new consensus for Trypanosoma cruzi intraspecific nomenclature: second revision meeting recommends TcI to TcVI. Mem Inst Oswaldo Cruz 104:1051–1054. doi: 10.1590/s0074-02762009000700021 [DOI] [PubMed] [Google Scholar]
- 16. Magalhães-Santos IF, Souza MM, Lima CSC, Andrade SG. 2004. Infection of Calomys callosus (Rodentia Cricetidae) with strains of different Trypanosoma cruzi biodemes: pathogenicity, histotropism, and fibrosis induction. Mem Inst Oswaldo Cruz 99:407–413. doi: 10.1590/s0074-02762004000400011 [DOI] [PubMed] [Google Scholar]
- 17. Martínez-Díaz RA, Escario JA, Nogal-Ruiz JJ, Gómez-Barrio A. 2001. Biological characterization of Trypanosoma cruzi strains. Mem Inst Oswaldo Cruz 96:53–59. doi: 10.1590/s0074-02762001000100006 [DOI] [PubMed] [Google Scholar]
- 18. Murta SM, Gazzinelli RT, Brener Z, Romanha AJ. 1998. Molecular characterization of susceptible and naturally resistant strains of Trypanosoma cruzi to benznidazole and nifurtimox. Mol Biochem Parasitol 93:203–214. doi: 10.1016/s0166-6851(98)00037-1 [DOI] [PubMed] [Google Scholar]
- 19. Veloso VM, Carneiro CM, Toledo MJ, Lana M, Chiari E, Tafuri WL, Bahia MT. 2001. Variation in susceptibility to benznidazole in isolates derived from Trypanosoma cruzi parental strains. Mem Inst Oswaldo Cruz 96:1005–1011. doi: 10.1590/s0074-02762001000700021 [DOI] [PubMed] [Google Scholar]
- 20. Herreros-Cabello A, Callejas-Hernández F, Fresno M, Gironès N. 2019. Comparative proteomic analysis of trypomastigotes from Trypanosoma cruzi strains with different pathogenicity. Infect Genet Evol 76:104041. doi: 10.1016/j.meegid.2019.104041 [DOI] [PubMed] [Google Scholar]
- 21. Gomes ML, Macedo AM, Pena SD, Chiari E. 1998. Genetic relationships between Trypanosoma cruzi strains isolated from chronic chagasic patients in southern Brazil as revealed by RAPD and SSR-PCR analysis. Acta Trop 69:99–109. doi: 10.1016/s0001-706x(97)00122-8 [DOI] [PubMed] [Google Scholar]
- 22. Andrade SG, Magalhães JB. 1997. Biodemes and zymodemes of Trypanosoma cruzi strains: correlations with clinical data and experimental pathology. Rev Soc Bras Med Trop 30:27–35. doi: 10.1590/s0037-86821997000100006 [DOI] [PubMed] [Google Scholar]
- 23. Santana RAG, Magalhães LKC, Magalhães LKC, Prestes SR, Maciel MG, da Silva GAV, Monteiro WM, de Brito FR, de Aguiar Raposo Câmara Coelho LI, Barbosa-Ferreira JM, Guerra JAO, Silveira H, das Graças Vale Barbosa M. 2014. Trypanosoma cruzi strain TcI is associated with chronic Chagas disease in the Brazilian Amazon. Parasit Vectors 7:267. doi: 10.1186/1756-3305-7-267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Brenière SF, Carrasco R, Revollo S, Aparicio G, Desjeux P, Tibayrenc M. 1989. Chagas' disease in Bolivia: clinical and epidemiological features and zymodeme variability of Trypanosoma cruzi strains isolated from patients. Am J Trop Med Hyg 41:521–529. doi: 10.4269/ajtmh.1989.41.521 [DOI] [PubMed] [Google Scholar]
- 25. Zingales B. 2018. Trypanosoma cruzi genetic diversity: something new for something known about Chagas disease manifestations, serodiagnosis and drug sensitivity. Acta Trop 184:38–52. doi: 10.1016/j.actatropica.2017.09.017 [DOI] [PubMed] [Google Scholar]
- 26. Dumonteil E, Pronovost H, Bierman EF, Sanford A, Majeau A, Moore R, Herrera C. 2020. Interactions among Triatoma sanguisuga blood feeding sources, gut microbiota and Trypanosoma cruzi diversity in southern Louisiana. Mol Ecol 29:3747–3761. doi: 10.1111/mec.15582 [DOI] [PubMed] [Google Scholar]
- 27. Murillo-Solano C, López-Domínguez J, Gongora R, Rojas-Gulloso A, Usme-Ciro J, Perdomo-Balaguera E, Herrera C, Parra-Henao G, Dumonteil E. 2021. Diversity and interactions among triatomine bugs, their blood feeding sources, gut microbiota and Trypanosoma cruzi in the Sierra Nevada de Santa Marta in Colombia. Sci Rep 11:12306. doi: 10.1038/s41598-021-91783-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Polonio R, López-Domínguez J, Herrera C, Dumonteil E. 2021. Molecular ecology of Triatoma dimidiata in southern Belize reveals risk for human infection and the local differentiation of Trypanosoma cruzi parasites. Int J Infect Dis 108:320–329. doi: 10.1016/j.ijid.2021.05.083 [DOI] [PubMed] [Google Scholar]
- 29. Herrera C, Majeau A, Didier P, Falkenstein KP, Dumonteil E. 2019. Trypanosoma cruzi diversity in naturally infected non-human primates in Louisiana assessed by deep sequencing of the mini-exon gene. Trans R Soc Trop Med Hyg 113:281–286. doi: 10.1093/trstmh/try119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Herrera C, Truyens C, Dumonteil E, Alger J, Sosa-Estani S, Cafferata ML, Gibbons L, Ciganda A, Matute ML, Zuniga C, Carlier Y, Buekens P. 2019. Phylogenetic analysis of Trypanosoma cruzi from pregnant women and newborns from Argentina, Honduras and Mexico suggests an association of parasite haplotypes with congenital transmission of the parasite. J Mol Diagn 21:1095–1105. doi: 10.1016/j.jmoldx.2019.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Villanueva-Lizama L, Teh-Poot C, Majeau A, Herrera C, Dumonteil E. 2019. Molecular genotyping of Trypanosoma cruzi by next-generation sequencing of the mini-exon gene reveals infections with multiple parasite DTUs in Chagasic patients from Yucatan, Mexico. J Infect Dis 219:1980–1988. doi: 10.1093/infdis/jiz047 [DOI] [PubMed] [Google Scholar]
- 32. Dumonteil E, Elmayan A, Majeau A, Tu W, Duhon B, Marx P, Wolfson W, Balsamo G, Herrera C. 2020. Genetic diversity of Trypanosoma cruzi parasites infecting dogs in southern Louisiana sheds light on parasite transmission cycles and serological diagnostic performance. PLoS Negl Trop Dis 14:e0008932. doi: 10.1371/journal.pntd.0008932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dumonteil E, Desale H, Tu W, Duhon B, Wolfson W, Balsamo G, Herrera C. 2021. Shelter cats host infections with multiple Trypanosoma cruzi discrete typing units in southern Louisiana. Vet Res 52:53. doi: 10.1186/s13567-021-00923-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Garcia MN, Burroughs H, Gorchakov R, Gunter SM, Dumonteil E, Murray KO, Herrera CP. 2017. Molecular identification and genotyping of Trypanosoma cruzi DNA in autochthonous Chagas disease patients from Texas, USA. Infect Genet Evol 49:151–156. doi: 10.1016/j.meegid.2017.01.016 [DOI] [PubMed] [Google Scholar]
- 35. Balmer O, Tanner M. 2011. Prevalence and implications of multiple-strain infections. Lancet Infect Dis 11:868–878. doi: 10.1016/S1473-3099(11)70241-9 [DOI] [PubMed] [Google Scholar]
- 36. Susi H, Barrès B, Vale PF, Laine A-L. 2015. Co-infection alters population dynamics of infectious disease. Nat Commun 6:5975. doi: 10.1038/ncomms6975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kinnula H, Mappes J, Sundberg L-R. 2017. Coinfection outcome in an opportunistic pathogen depends on the inter-strain interactions. BMC Evol Biol 17:77. doi: 10.1186/s12862-017-0922-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Seppälä O, Jokela J. 2016. Do coinfections maintain genetic variation in parasites? Trends Parasitol 32:930–938. doi: 10.1016/j.pt.2016.08.010 [DOI] [PubMed] [Google Scholar]
- 39. Balmer O, Stearns SC, Schötzau A, Brun R. 2009. Intraspecific competition between co-infecting parasite strains enhances host survival in African trypanosomes. Ecology 90:3367–3378. doi: 10.1890/08-2291.1 [DOI] [PubMed] [Google Scholar]
- 40. Ragone PG, Pérez Brandán C, Monje Rumi M, Tomasini N, Lauthier JJ, Cimino RO, Uncos A, Ramos F, Alberti D’Amato AM, Basombrío MA, Diosque P. 2015. Experimental evidence of biological interactions among different isolates of Trypanosoma cruzi from the Chaco Region. PLoS One 10:e0119866. doi: 10.1371/journal.pone.0119866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Martins HR, Toledo MJO, Veloso VM, Carneiro CM, Machado-Coelho GLL, Tafuri WL, Bahia MT, Valadares HM, Macedo AM, Lana M. 2006. Trypanosoma cruzi: impact of dual-clone infections on parasite biological properties in BALB/c mice. Exp Parasitol 112:237–246. doi: 10.1016/j.exppara.2005.11.006 [DOI] [PubMed] [Google Scholar]
- 42. Rodrigues CM, Valadares HMS, Francisco AF, Arantes JM, Campos CF, Teixeira-Carvalho A, Martins-Filho OA, Araujo MSS, Arantes RME, Chiari E, Franco GR, Machado CR, Pena SDJ, Faria AMC, Macedo AM. 2010. Coinfection with different Trypanosoma cruzi strains interferes with the host immune response to infection. PLoS Negl Trop Dis 4:e846. doi: 10.1371/journal.pntd.0000846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Padilla AM, Yao PY, Landry TJ, Cooley GM, Mahaney SM, Ribeiro I, VandeBerg JL, Tarleton RL. 2021. High variation in immune responses and parasite phenotypes in naturally acquired Trypanosoma cruzi infection in a captive non-human primate breeding colony in Texas, USA. PLoS Negl Trop Dis 15:e0009141. doi: 10.1371/journal.pntd.0009141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Curtis-Robles R, Hamer SA, Lane S, Levy MZ, Hamer GL. 2018. Bionomics and spatial distribution of triatomine vectors of Trypanosoma cruzi in Texas and other Southern States, USA. Am J Trop Med Hyg 98:113–121. doi: 10.4269/ajtmh.17-0526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dorn PL, Daigle ME, Combe CL, Tate AH, Stevens L, Phillippi-Falkenstein KM. 2012. Low prevalence of Chagas parasite infection in a nonhuman primate colony in Louisiana. J Am Assoc Lab Anim Sci 51:443–447. [PMC free article] [PubMed] [Google Scholar]
- 46. Pronovost H, Peterson AC, Chavez BG, Blum MJ, Dumonteil E, Herrera CP. 2020. Deep sequencing reveals multiclonality and new discrete typing units of Trypanosoma cruzi in rodents from the southern United States. J Microbiol Immunol Infect 53:622–633. doi: 10.1016/j.jmii.2018.12.004 [DOI] [PubMed] [Google Scholar]
- 47. Majeau A, Cloherty E, Anderson AN, Straif-Bourgeois SC, Dumonteil E, Herrera C. 2023. Genetic diversity of Trypanosoma cruzi infecting raccoons (Procyon lotor) in 2 metropolitan areas of southern Louisiana: implications for parasite transmission networks. Parasitology 150:1–8. doi: 10.1017/S0031182023000070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Maeda FY, Cortez C, Izidoro MA, Juliano L, Yoshida N. 2014. Fibronectin-degrading activity of Trypanosoma cruzi cysteine proteinase plays a role in host cell invasion. Infect Immun 82:5166–5174. doi: 10.1128/IAI.02022-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Torrico F, Gascón J, Barreira F, Blum B, Almeida IC, Alonso-Vega C, Barboza T, Bilbe G, Correia E, Garcia W, Ortiz L, Parrado R, Ramirez JC, Ribeiro I, Strub-Wourgaft N, Vaillant M, Sosa-Estani S, BENDITA study group . 2021. New regimens of benznidazole monotherapy and in combination with fosravuconazole for treatment of Chagas disease (BENDITA): a phase 2, double-blind, randomised trial. Lancet Infect Dis 21:1129–1140. doi: 10.1016/S1473-3099(20)30844-6 [DOI] [PubMed] [Google Scholar]
- 50. Cafferata ML, Toscani MA, Althabe F, Belizán JM, Bergel E, Berrueta M, Capparelli EV, Ciganda Á, Danesi E, Dumonteil E, Gibbons L, Gulayin PE, Herrera C, Momper JD, Rossi S, Shaffer JG, Schijman AG, Sosa-Estani S, Stella CB, Klein K, Buekens P. 2020. Short-course benznidazole treatment to reduce Trypanosoma cruzi parasitic load in women of reproductive age (BETTY): a non-inferiority randomized controlled trial study protocol. Reprod Health 17:128. doi: 10.1186/s12978-020-00972-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Torrico F, Gascon J, Ortiz L, Alonso-Vega C, Pinazo M-J, Schijman A, Almeida IC, Alves F, Strub-Wourgaft N, Ribeiro I, E1224 Study Group . 2018. Treatment of adult chronic indeterminate Chagas disease with benznidazole and three E1224 dosing regimens: a proof-of-concept, randomised, placebo-controlled trial. Lancet Infect Dis 18:419–430. doi: 10.1016/S1473-3099(17)30538-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Majeau A, Murphy L, Herrera C, Dumonteil E. 2021. Assessing Trypanosoma cruzi parasite diversity through comparative genomics: implications for disease epidemiology and diagnostics. Pathogens 10:212. doi: 10.3390/pathogens10020212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Majeau A, Herrera C, Dumonteil E. 2019. An improved approach to Trypanosoma cruzi molecular genotyping by next-generation sequencing of the mini-exon gene. Methods Mol Biol 1955:47–60. doi: 10.1007/978-1-4939-9148-8_4 [DOI] [PubMed] [Google Scholar]
- 54. Sá ARN, Kimoto KY, Steindel M, Grisard EC, Gomes ML. 2018. Limit of detection of PCR/RFLP analysis of cytochrome oxidase II for the identification of genetic groups of Trypanosoma cruzi and Trypanosoma rangeli in biological material from vertebrate hosts. Parasitol Res 117:2403–2410. doi: 10.1007/s00436-018-5928-1 [DOI] [PubMed] [Google Scholar]
- 55. Bashey F. 2015. Within-host competitive interactions as a mechanism for the maintenance of parasite diversity. Philos Trans R Soc Lond B Biol Sci 370:20140301. doi: 10.1098/rstb.2014.0301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bhattacharyya S, Gesteland PH, Korgenski K, Bjørnstad ON, Adler FR. 2015. Cross-immunity between strains explains the dynamical pattern of paramyxoviruses. Proc Natl Acad Sci U S A 112:13396–13400. doi: 10.1073/pnas.1516698112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Dia A, Jett C, Trevino SG, Chu CS, Sriprawat K, Anderson TJC, Nosten F, Cheeseman IH. 2021. Single-genome sequencing reveals within-host evolution of human malaria parasites. Cell Host Microbe 29:1496–1506. doi: 10.1016/j.chom.2021.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Stewart LB, Diaz-Ingelmo O, Claessens A, Abugri J, Pearson RD, Goncalves S, Drury E, Kwiatkowski DP, Awandare GA, Conway DJ. 2020. Intrinsic multiplication rate variation and plasticity of human blood stage malaria parasites. Commun Biol 3:624. doi: 10.1038/s42003-020-01349-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Schwabl P, Maiguashca Sánchez J, Costales JA, Ocaña-Mayorga S, Segovia M, Carrasco HJ, Hernández C, Ramírez JD, Lewis MD, Grijalva MJ, Llewellyn MS, Sirugo G. 2020. Culture-free genome-wide locus sequence typing (GLST) provides new perspectives on Trypanosoma cruzi dispersal and infection complexity. PLoS Genet 16:e1009170. doi: 10.1371/journal.pgen.1009170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Frade AF, Pissetti CW, Ianni BM, Saba B, Lin-Wang HT, Nogueira LG, de Melo Borges A, Buck P, Dias F, Baron M, Ferreira LRP, Schmidt A, Marin-Neto JA, Hirata M, Sampaio M, Fragata A, Pereira AC, Donadi E, Kalil J, Rodrigues V, Cunha-Neto E, Chevillard C. 2013. Genetic susceptibility to Chagas disease cardiomyopathy: involvement of several genes of the innate immunity and chemokine-dependent migration pathways. BMC Infect Dis 13:587. doi: 10.1186/1471-2334-13-587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Casares-Marfil D, Strauss M, Bosch-Nicolau P, Lo Presti MS, Molina I, Chevillard C, Cunha-Neto E, Sabino E, Ribeiro ALP, González CI, Martín J, Acosta-Herrera M. 2021. A genome-wide association study identifies novel susceptibility Loci in chronic Chagas cardiomyopathy. Clin Infect Dis 73:672–679. doi: 10.1093/cid/ciab090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Murray KO, Saldaña MA, Gunter SM, Manzanero R, Zielinski-Gutierrez E, Herrera C, Thompson JM, Maliga A, Bautista K, Lino A, Hawes E, Ronca SE, Morey F, Fuentes RC, Lopez B, Dumonteil E, Morazan GH. 2022. Diagnosis of acute Chagas disease in a Belizean child with evidence of a multi-clonal Trypanosoma cruzi infection. Am J Trop Med Hyg 107:992–995. doi: 10.4269/ajtmh.22-0338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zhou Y, Bao R, Haigwood NL, Persidsky Y, Ho W. 2013. SIV infection of rhesus macaques of Chinese origin: a suitable model for HIV infection in humans. Retrovirology 10:89. doi: 10.1186/1742-4690-10-89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Moser DR, Kirchhoff LV, Donelson JE. 1989. Detection of Trypanosoma cruzi by DNA amplification using the polymerase chain reaction. J Clin Microbiol 27:1477–1482. doi: 10.1128/jcm.27.7.1477-1482.1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sturm NR, Degrave W, Morel C, Simpson L. 1989. Sensitive detection and schizodeme classification of Trypanosoma cruzi cells by amplification of kinetoplast minicircle DNA sequences: use in diagnosis of Chagas' disease. Mol Biochem Parasitol 33:205–214. doi: 10.1016/0166-6851(89)90082-0 [DOI] [PubMed] [Google Scholar]
- 66. Abad-Franch F. 2022. Trypanosoma cruzi parasitemia in chronic Chagas disease: insights from hierarchical modeling. PLoS Negl Trop Dis 16:e0010612. doi: 10.1371/journal.pntd.0010612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. De Salazar PM, Sosa-Estani S, Salvador F, Sulleiro E, Sánchez-Montalvá A, Ribeiro I, Molina I, Buckee CO. 2022. Human Trypanosoma cruzi chronic infection leads to individual level steady-state parasitemia: implications for drug-trial optimization in Chagas disease. PLoS Negl Trop Dis 16:e0010828. doi: 10.1371/journal.pntd.0010828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ramírez JC, Cura CI, da Cruz Moreira O, Lages-Silva E, Juiz N, Velázquez E, Ramírez JD, Alberti A, Pavia P, Flores-Chávez MD, Muñoz-Calderón A, Pérez-Morales D, Santalla J, Marcos da Matta Guedes P, Peneau J, Marcet P, Padilla C, Cruz-Robles D, Valencia E, Crisante GE, Greif G, Zulantay I, Costales JA, Alvarez-Martínez M, Martínez NE, Villarroel R, Villarroel S, Sánchez Z, Bisio M, Parrado R, Maria da Cunha Galvão L, Jácome da Câmara AC, Espinoza B, Alarcón de Noya B, Puerta C, Riarte A, Diosque P, Sosa-Estani S, Guhl F, Ribeiro I, Aznar C, Britto C, Yadón ZE, Schijman AG. 2015. Analytical validation of quantitative real-time PCR methods for quantification of Trypanosoma cruzi DNA in blood samples from Chagas disease patients. J Mol Diagn 17:605–615. doi: 10.1016/j.jmoldx.2015.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Souto RP, Fernandes O, Macedo AM, Campbell DA, Zingales B. 1996. DNA markers define two major phylogenetic lineages of Trypanosoma cruzi. Mol Biochem Parasitol 83:141–152. doi: 10.1016/s0166-6851(96)02755-7 [DOI] [PubMed] [Google Scholar]
- 70. Villanueva-Lizama L, Teh-Poot C, Majeau A, Herrera C, Dumonteil E. 2019. Molecular genotyping of Trypanosoma cruzi by next-generation sequencing of the mini-exon gene reveals infections with multiple parasite discrete typing units in chagasic patients from Yucatan, Mexico. J Infect Dis 219:1980–1988. doi: 10.1093/infdis/jiz047 [DOI] [PubMed] [Google Scholar]
- 71. Garrison E, Marth G. 2012. Haplotype-based variant detection from short-read sequencing. arXiv Preprint
- 72. Price MN, Dehal PS, Arkin AP. 2010. FastTree 2--approximately maximum-likelihood trees for large alignments. PLoS One 5:e9490. doi: 10.1371/journal.pone.0009490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Hammer Ø, Harper DAT, Ryan PD. 2001. PAST: paleontological statistics software package for education and data analysis. Palaeontologia Electronica 4:9pp. [Google Scholar]
- 74. Kornreich F, Selvester RH, Montague TJ, Rautaharju PM, Saetre HA, Ahmad J. 1992. Discriminant analysis of the standard 12-lead ECG for diagnosing non-Q wave myocardial infarction. J Electrocardiol 24 Suppl:163–172. doi: 10.1016/s0022-0736(10)80039-1 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental figure and tables.
Data Availability Statement
DNA sequence data that support the findings of this study have been deposited in GenBank with the accession numbers ON907885–ON908188. Additional data are available within the paper and its supplementary information files or from the corresponding author upon reasonable request.