ABSTRACT
NorA, an extensively studied efflux pump in Staphylococcus aureus, has been connected to fluoroquinolone, antiseptic, and disinfection resistance. Several studies have also emphasized how efflux pumps, including NorA, function as the first line of defense of S. aureus against antibiotics. In this study, we have screened some chemically synthesized indole derivatives for their activity as efflux pump inhibitors (EPIs). The derivative SMJ-5 was found to be a potent NorA efflux pump inhibitor among the screened indole derivatives, owing to increased ethidium bromide and norfloxacin accumulation in norA over-expressing S. aureus. The combination of SMJ-5 and ciprofloxacin demonstrated the eradication of S. aureus biofilm and prolonged the post-antibiotic effect more than ciprofloxacin alone. SMJ-5 was able to inhibit staphyloxanthin virulence. In in vitro time-kill trials and in vivo efficacy investigations, the combination enhanced the bactericidal activity of ciprofloxacin against S. aureus. Additionally, reverse transcription PCR results revealed that SMJ-5 also inhibits the NorA efflux pump indirectly at the transcriptional level.
IMPORTANCE
The NorA efflux pump is the most effective resistance mechanism in S. aureus. The clinical importance of NorA efflux pumps is demonstrated by the expression of pump genes in S. aureus strains in response to fluoroquinolones and biocides. Along with the repercussions of decreased fluoroquinolone sensitivity, increasing expression of efflux pump genes by their substrate necessitates the importance of efflux pump inhibitors. Reserpine and verapamil are clinically used to treat ailments and have proven NorA inhibitors, but, unfortunately, the concentration needed for these drugs to inhibit the pump is not safe in clinical settings. In the current study, we have screened some indole derivatives, and among them, SMJ-5 was reported to potentiate norfloxacin and ciprofloxacin at their sub-inhibitory concentration by inhibiting the norA gene transcriptionally. Here we highlight the promising points of this study, which could serve as a model to design a therapeutic EPI candidate against norA over-expressing S. aureus.
KEYWORDS: Staphylococcus aureus, major facilitator superfamily (MFS), norA efflux pump, efflux pump inhibitors (EPIs), multidrug resistance (MDR)
INTRODUCTION
Multidrug resistance (MDR) is one of the primary causes of death worldwide and continues to be a severe challenge in treating infectious diseases (1). The advent and fast spread of multidrug-resistant bacteria pose a serious threat to global healthcare. S. aureus, an opportunistic pathogen, can cause many complications with mild to life-threatening outcomes. S. aureus can also form biofilms, leading to higher antibiotic tolerance (2). Fluoroquinolones have a broad spectrum of activity against a range of bacterial species, including methicillin-resistant Staphylococcus aureus (MRSA) (3). However, the clinical relevance of these antibiotics was somewhat debilitated due to their abrupt evolution in resistance (4). Around 25% of S. aureus isolates in Europe are resistant to fluoroquinolones, which comprise about 90% of MRSA isolates (5). Also, studies have proved that resistance against fluoroquinolones is related to a mutation in GrlA and GrlB proteins of topoisomerase IV and GyrA and GyrB proteins of DNA gyrase (6).
Among the various factors that cause resistance, one of the significant factors is the efflux pumps, which transport structurally distinct, noxious compounds such as antibiotics outside the cell; therefore, these efflux pumps reduce their intracellular concentration and lead to multidrug resistance (7, 8). The chromosomally encoded NorA is a 388-amino acid efflux pump with 12 transmembrane helices and a molecular weight of 42.2 kDa (9). The NorA efflux pump has a wide range of substrates, including fluoroquinolones, dyes, antiseptics, and quaternary ammonium compounds (10). NorA efflux pump uses proton-motive force to expel these compounds out of the cell.
Moreover, efflux pumps have been linked to virulence and biofilm formation. Efflux pumps enhance biofilm formation by excreting toxic compounds and signaling molecules while influencing surface adhesion (10, 11). Efflux pump inhibitors (EPIs) have been observed to potentiate the existing antibiotics, overcoming the resistance in bacteria (10). S. aureus strains were unable to form biofilms, and the expression of the norA efflux pump genes was reduced by toluidine blue O-mediated photodynamic treatment (12). Nilotinib was reported as a NorA efflux pump inhibitor. It significantly reduced the growth of S. aureus biofilms, indicating that this substance interacted with the NorA efflux pump and decreased activity (10). The anti-fungal ketoconazole inhibits the NorA efflux pump and biofilm development in S. aureus (13). Considering the role of efflux pumps in antibiotic resistance, biofilm formation, and virulence, we found that there have been various efforts to discover and develop efflux pump inhibitors. Still, none has been approved due to their toxicity. With the barren antibiotic pipeline in today’s era, there is a dire need to adopt new strategies to revitalize existing antibiotics using antibiotic potentiators.
This study observed the efflux pump inhibitory effect and the ciprofloxacin potentiation efficacy of indole derivatives in S. aureus by inhibiting the NorA efflux pump. Our findings shed light on the efflux pump inhibitory effect of indole derivatives, which helps to reduce the pre-formed S. aureus biofilm. This study also demonstrates the efficacy of the most potent efflux pump inhibitor, SMJ-5, in in vivo murine infection models.
RESULTS
Effect of indole-based derivatives on the MIC of antibiotics and its structure-activity relationship
We have synthesized eight derivatives of the indole scaffold (Table S1). The 1H NMR and 13C NMR spectra of 2-(2′-aminophenyl) indole [RP2] (Fig. S1) and mass spectra, 1H NMR, and 13C NMR spectra of its indole derivatives are mentioned in the supplemental data (Fig. S2). The antibiotic boosting activity of indole-based derivatives was quantified by observing the reduction in minimum inhibitory concentrations (MICs) of antibiotics. The MIC value of the antibiotics ciprofloxacin and norfloxacin was reduced when coupled with indole-based derivatives at sub-inhibitory concentrations (1/4×, 1/8×, and 1/16× MIC), where no intrinsic anti-bacterial activity was observed. SMJ-3, SMJ-5, SMJ-9, and SMJ-10 demonstrated a momentous fall in ciprofloxacin and norfloxacin MICs with a fold change of 32, 16, 32, 16, and 16, 32, 8, and 16, respectively, in S. aureus SA-1199B (norA-over-expressed strain). The fractional inhibitory concentration index (FICI) value for SMJ-3, SMJ-5, SMJ-9, and SMJ-10 was ≤0.5, indicating synergy with ciprofloxacin and norfloxacin (Table 1). However, a minor or no change in modulation was observed in the MIC value for S. aureus K-1758 (norA deletion strain), and a FICI value of >0.5 to <4.0 was observed, which indicates indifference (Table S2). Hence, synergy observed with S. aureus SA-1199B norA over-expressing strain may be due to indole derivatives behaving as NorA efflux pump inhibitors.
TABLE 1.
Checkerboard synergy assay of indole derivatives against S. aureus SA-1199B (norA-over-expressed strain) a
|
Modulation factor = fold change in MIC of antibiotic in the presence of the indole derivatives.
Also, the indole derivatives, when coupled with erythromycin in a strain expressing MsrA (S. aureus RN-4220), showed no synergism but was indicative of indifference as the FICI observed was >0.5 and <4.0 (Table S3). The indole derivatives were also coupled with NorB substrate moxifloxacin (14), and a checkerboard synergy assay was performed with the over-expressing NorA strain (S. aureus SA-1199B). Since moxifloxacin is not the substrate of NorA efflux pumps, no synergy was observed (Table S4a) (15). The development of combination therapy will be greatly aided by the use of currently prescribed drugs and NorA inhibitors. Also, the MIC spectrum of fluoroquinolones (ciprofloxacin, norfloxacin, levofloxacin and moxifloxacin) and tetracycline is shown on S. aureus SA-1199B (norA over-expressing), SA-1199 (norA wild-type), and K-1758 (norA deletion) strains (Table S4b) to show the susceptibility profile of these strains toward fluoroquinolones. These findings collectively imply that NorA is a target of the derivatives used in the investigation.
According to the structure-activity relationship (SAR), hydrogen, a weak electron-donating group attached to the Schiff’s base (at the R1 position), reduces the EPI activity in the case of SMJ-6 and SMJ-8, whereas the derivatives (SMJ-1, SMJ-3, SMJ-5, SMJ-7, SMJ-9, and SMJ-10) having methyl substituent at R1 position, being a strong electron-donating group than hydrogen, attached to the Schiff’s base increased the EPI activity. Introducing electron-withdrawing substituents at the R 2 position of the phenyl ring attached to the Schiff base of the indole scaffold resulted in reduced activity (SMJ-6, SMJ-7, and SMJ-8). SMJ-8 containing –CF3 at the R 2 position, which is highly electron-withdrawing, loses its activity ultimately compared to –F and –NO2 substituents at the R 2 position of SMJ-6 and SMJ-7, respectively, which is weakly active. The SMJ-1-bearing methyl (–CH3) group at the R 2 position, being the weakest electron donating group among -OH, -dihydroxyl, -SCH3, and NH2, present in SMJ-3, SMJ-5, SMJ-9, and SMJ-10, respectively, has a minor activity. However, strong electron-donor groups at the R 2 position increase the EPI activity. Hence, the SAR revealed that strong electron-donating groups at the R1 and R 2 positions are the critical feature of bioactivity (SMJ-3, SMJ-5, SMJ-9, and SMJ-10) (Fig. S3).
Effect of SMJ-5 on clinical strains
To check the efficacy of the combination SMJ-5 (1/4× MIC) and ciprofloxacin in a clinical setting, we studied the synergy of the combination in 17 clinical isolates. Moreover, SMJ-5, along with ciprofloxacin, displayed an activity ranging from 2- to 64-fold modulation. According to previous studies, a decrease in the MIC by at least fourfold compared to their initial concentration in the presence of EPI indicated efflux inhibition (16). Here, we observed a ≥4-fold reduction in 14 strains out of 17, and the combination brings 12 strains out of 17 below the clinical resistance breakpoint, emphasizing the combination can be of high applicability in clinical practice (Table S5) (17, 18). The combination of SMJ-5 and ciprofloxacin exhibits ≥8-fold modulation on five clinical strains. The expression of the norA gene in S. aureus clinical strains was confirmed through reverse transcription PCR (RT-PCR) analysis, and all the strains showed higher norA expression than the deletion strain, S. aureus K-1758. S. aureus American Type Culture Collection (ATCC) 29213 was used as a reference sample strain because it has a basal level of NorA efflux pump expression (19). Relative gene expression was determined using the power 2−ΔΔCT technique. The housekeeping gene 16s rRNA was used as an endogenous control to normalize the expression levels of target genes (Fig. S4).
Also, to verify that the result is not due to any other factor than the NorA efflux pump, we assessed the combinatorial action of SMJ-5 with erythromycin and moxifloxacin. Additionally, no discernible decrease in MIC was found with these antibiotics (Table S6).
Uptake of EtBr and norfloxacin by S. aureus SA-1199B cells in the presence of indole derivatives
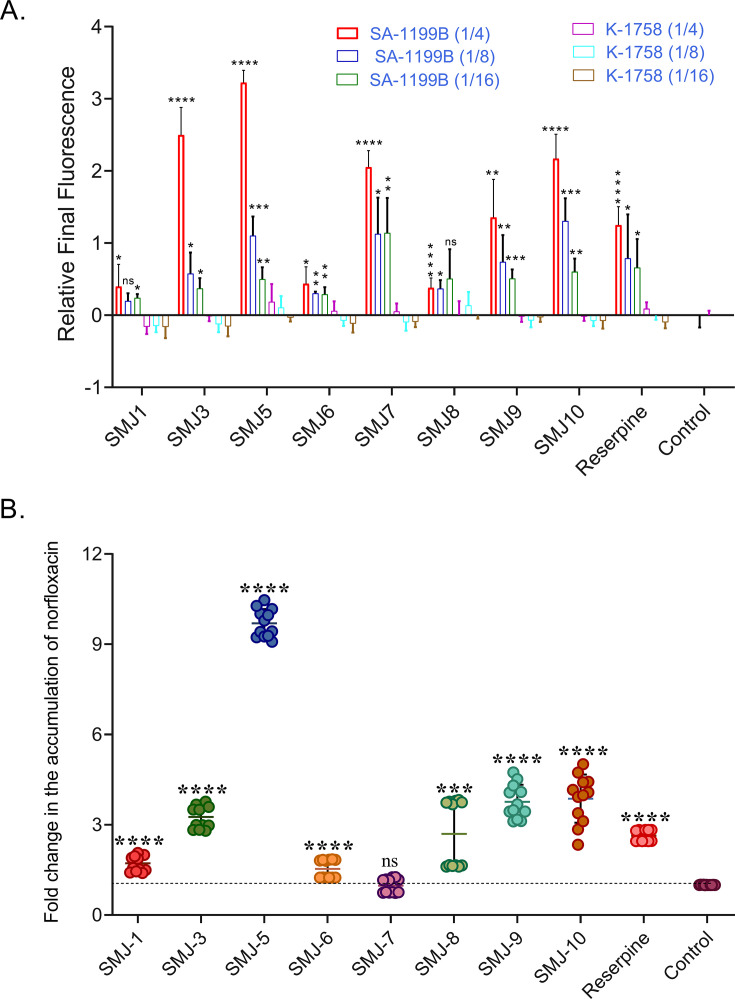
To corroborate the evidence that indole derivatives inhibit the NorA efflux pump, we further monitored the ethidium bromide (EtBr) and norfloxacin uptake, which are fluorescent NorA efflux pump substrates that bind to the major groove of DNA and DNA gyrase, respectively. Inhibition of the NorA pump by indole derivatives was evident by an increased fluorescence of EtBr at 600 nm in the presence of indole derivatives relative to the fluorescence in the absence of indole derivatives, indicating EtBr accumulation. Among the indole derivatives, SMJ-3 and SMJ-5 displayed significantly enhanced EtBr accumulation, even higher than the positive control reserpine. The relative final fluorescence (RFF) values of SMJ-3 and SMJ-5 at 1/4× MIC were found to be 2.49 ± 0.38 and 3.22 ± 0.17, respectively (Fig. 1A). In contrast, the RFF for positive control reserpine was 1.24 ± 0.25 at 1/4× MIC after 20-min incubation. In the presence of reserpine, EtBr fluorescence was slightly increased in the S. aureus K-1758 strain. Still, the negative RFF value for indole derivatives suggested that the treated cells had negligible or no EtBr accumulation than the control cells, suggesting that the NorA efflux pump could be a target for indole derivatives.
FIG 1.
(A) EtBr accumulation assay, RFF value of indole derivatives at 1/4×, 1/8×, and 1/16× MIC in the presence of EtBr on S. aureus SA-1199B (norA over-expression) and K-1758 (norA knockout) strain. (B) Norfloxacin accumulation assay on S. aureus SA-1199B strain. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. P value calculated using 95% class interval. Both experiments were done in biological duplicates, and this is the representation of one, comprising three and six technical replicates, respectively; panel A corresponds to average ± SD, and panel B is the representation of individual readings ± SD. ns, non-significant; SD, standard deviation.
The norfloxacin accumulation assay validated the NorA efflux pump inhibition and NorA efflux pump substrate accumulation. Norfloxacin (16 µg/mL) with the sub-inhibitory concentration of indole derivatives demonstrated a substantial increase in norfloxacin accumulation. The fold change in norfloxacin accumulation was lower than that of reserpine for SMJ-1, SMJ-6, and SMJ-7, slightly higher for SMJ-3, SMJ-9, and SMJ-10, and significantly higher for SMJ-5. SMJ-5 accumulated norfloxacin 8.69- and 7.06-folds more elevated than the control (without EPI) and reserpine (positive control), respectively, indicating that it is a potent efflux pump inhibitor (Fig. 1B).
Rate of extrusion of EtBr in the presence of indole derivatives
We observed EtBr extrusion over time in S. aureus SA-1199B in the presence and absence of indole derivatives. It was found to be significantly reduced in the presence of indole derivatives. These EtBr extrusion results are consistent with the EtBr accumulation assay, and it was determined that SMJ-3, SMJ-5, SMJ-9, and SMJ-10 have a high potential to inhibit efflux pumps. The addition of glucose was observed to lower the efflux pump inhibitory activity of the indole derivatives and reserpine, re-energizing them to promote active efflux (Fig. 2).
FIG 2.
EtBr efflux inhibition assay of indole derivatives at the sub-inhibitory concentration (1/4× MIC) in EtBr presence at 4 µg/mL concentration in the presence and absence of glucose. Reserpine was used as the positive control. The results correspond to the mean of two biological repeats performed in triplicates.
SMJ-5 does not entail membrane depolarization and permeabilization
To track the phenomenon of membrane depolarization, we used the membrane potential-sensitive dye DiSC3(5) to quantify changes in the electrical potential gradient in intact bacteria. Valinomycin, used as a positive control, caused massive fluorescence leakage, demonstrating its membrane-depolarizing property (20). In contrast, no such increase in fluorescence was observed in the case of indole derivatives at MIC and 1/2× MIC concentrations (Fig. 3A). The membrane permeabilization was also assessed in the presence of the indole derivatives using propidium iodide (PI). An increase in fluorescence was observed in positive control paenibacillin (21) and SMJ-8. In contrast, no such increase appeared in the case of other indole derivatives, as PI could not enter the viable cell and bind to the target (Fig. 3B). The results confirmed that indole derivatives do not permeabilize the membrane.
FIG 3.
(A) Membrane depolarization was detected using fluorescence of DiSC3(5) showing high fluorescence of positive control valinomycin; drug-free control was used as the negative control. (B) Membrane permeability was measured using nucleic acid binding dye propidium iodide; paenibacillin was used as the positive control, and drug-free control was used as the negative control. (C) ATP luciferin-luciferase Bioluminescence detection assay; CCCP, the positive control, quenches ATP compared to control. (D) Mammalian Ca2+ channel assay on human embryonic kidney cells. All the experiments represent biological duplicates performed in technical triplicates, and results correspond to average ± SD. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001. P value calculated using 95% class interval. CCCP, carbonyl cyanide m-chlorophenyl hydrazone.
Effect of indole derivatives on the electron transport chain by luciferase luminescence assay
Membrane dysfunction disturbs the respiratory chain functions, resulting in reduced ATP levels. The S. aureus SA-1199B isolate was incubated with indole derivatives for 5 h, and the effect of indole derivatives on the intracellular ATP level was evaluated. The ATP levels remained constant compared to the drug-free control, indicating no change in transmembrane potential, ruling out the possibility of ATP depletion as the cause of efflux inhibition. In contrast, a significant reduction in the intracellular ATP was observed in the case of carbonyl cyanide m-chlorophenyl hydrazone (CCCP) (positive control), which is a potent mitochondrial uncoupling agent that increases the proton permeability across the mitochondrial inner membranes (22) (Fig. 3C).
Effect of indole derivatives on mammalian Ca2+ channels
We were cognizant that many bacterial efflux inhibitors limit mammalian Ca2+ channel activity in clinical settings, such as verapamil, inhibits host Ca2+ channels, eliciting human neurotoxicity (23). The mammalian Ca2+ channel blocking was observed using the Fluo-4 Direct calcium channel assay kit on human embryonic kidney (HEK) 293T cells; the Ca2+ channel activity was not affected by indole derivatives. However, the positive control, verapamil, prevented the accumulation of Ca2+ ions in the cytoplasm. None of the indole derivatives reduced Ca2+ channel activity, overcoming the problem that has hampered the development of efflux pump inhibitors (Fig. 3D).
Lack of toxicity of indole derivatives toward eukaryotic cells
To develop a preliminary idea of the toxicity of indole derivatives, we determined the hemolytic and mammalian cell viability assays. Hemolytic activity was evaluated by measuring the release of hemoglobin from rabbit erythrocytes as a function of concentration. The indole derivatives SMJ-5, SMJ-6, SMJ-7, SMJ-9, and SMJ-10 demonstrated a low hemolytic activity of less than 20% at a significantly higher concentration than their working concentration, whereas SMJ-1, SMJ-3, and SMJ-8 had greater than 20% hemolysis. The resulting dose-response for SMJ-5 was 15% at 500-µg/mL concentration (relatively higher than the effective concentration) (Fig. 4A). Mammalian cell viability assays were conducted on human peripheral blood mononuclear cells (PBMC) and human embryonic kidney cells (HEK 293T) using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT assay at two varying concentrations of 500 and 250 µg/mL. In PBMCs, high cell viability of 59.87 ± 0.21 and 98.31 ± 1.59 for SMJ-5 was observed at 500- and 250-µg/mL concentrations, respectively. In contrast, on HEK 293T cells, SMJ-5 displayed cell viability of 48.35 ± 4.32 and 79.56 ± 2.66 at 500- and 250-µg/mL concentrations, respectively (Fig. 4B).
FIG 4.
(A) Hemolysis assay on 4% red blood cell (RBC) at 64-, 128-, 250-, and 500-µg/mL concentrations. (B) Cytotoxicity assay on human peripheral blood mononuclear cells and human embryonic kidney cells (HEK 293T) at 500- and 250-µg/mL concentrations. (C) Spectrometric quantification of staphyloxanthin intermediates, namely, 4,4-diapophytoene (286 nm), 4,4-diaponeurosporene (435 nm), 4,4-diaponeurosporenic acid (455 nm), and staphyloxanthin (462 nm) extracted from control and indole derivatives (1/4 × MIC) treated ATCC 29,213 cells. Results were considered significant (*) when P < 0.05 and highly significant (**) when P < 0.01 and (***) P < 0.001. P values were calculated using 95% class interval. Panels A through C represent two biological readings performed in triplicates. (D) Schematic representation of the staphyloxanthin biosynthesis pathway. The figure demonstrates SMJ-5 inhibits dehydrosqualene synthase (CrtM) while miconazole inhibits 4,4-diapophytoene desaturase (CrtN). CrtM is an enzyme that catalyzes the condensation of two farnesyl diphosphates to produce 4,4-diapophytoene, further converted to 4,4-diaponeurosporene by CrtN.
Effect of indole derivatives on virulence factors
Staphyloxanthin is a yellow-colored carotenoid and a known virulence factor that promotes resistance to reactive oxygen species and the host immune system found in S. aureus (24). Staphyloxanthin and its metabolic intermediates were quantified in the presence and absence of sub-inhibitory concentration of indole derivatives. Indole derivatives were previously reported to inhibit staphyloxanthin formation, and S. aureus ATCC 29213 is the standard strain reported to produce staphyloxanthin (24). Therefore, out of curiosity, we also checked the staphyloxanthin inhibiting property of indole derivatives apart from its efflux pump inhibiting property. Hence, we studied the activity of our indole derivatives; we evaluated their activity on all intermediates involved. We found indole derivatives showed a significant reduction in spectrometric measurements of staphyloxanthin biosynthesis pathway metabolic intermediates including 4,4-diaponeurosporene, 4,4-diaponeurosporenic acid, and staphyloxanthin, except 4,4-diapophytoene. SMJ-5, SMJ-7, SMJ-9, and SMJ-10 interfered with the condensation of 4,4′-diapophytoene to 4,4′-diaponeurosporene, indicating these derivatives inhibit CrtN. While the positive control miconazole (25) inhibits staphyloxanthin formation by inhibiting dehydrosqualene synthase (CrtM) (Fig. 4C and D). CrtM catalyzes the condensation of two farnesyl diphosphates to create 4,4-diapophytoene and is the first enzyme in S. aureus biosynthetic route for staphyloxanthin, while CrtN catalyzes the formation of 4,4-diaponeurosporene from 4,4-diapophytoene (25). Controlling the carotenoid pigment’s biosynthesis pathway would be very beneficial since it would reduce the persistence of S. aureus infections in individuals and make the pathogen more vulnerable to the host immune response.
Efflux pumps as the target to inhibit biofilm formation and eradicate pre-formed biofilms
Efflux pumps are highly active in bacterial biofilms, making them an excellent target for developing anti-biofilm strategies (26). Encouraged by the potentiation of ciprofloxacin by SMJ-5, we evaluated the effect of the SMJ-5 and ciprofloxacin combination on the S. aureus SA-1199B biofilm. The minimum biofilm inhibitory concentration that inhibits ≥50% of the biofilm (MBIC50) and minimum biofilm eradication concentration that eradicates ≥50% of the biofilm (MBEC50) concentrations of ciprofloxacin were determined. Ciprofloxacin had an MBIC50 and MBEC50 of 8 and 16 µg/mL, respectively. The MBIC50 and MBEC50 concentrations of ciprofloxacin, in combination with 1/4× MIC of SMJ-5, demonstrated significant inhibition in biofilm formation and eradication of pre-formed biofilm (16-fold) (Table S7a).
Furthermore, biofilm viability was determined calorimetrically by measuring the reduction in insoluble formazan produced by metabolically active biofilm cells from MTT. SMJ-5 at its sub-inhibitory concentration (1/4× MIC) combined with ciprofloxacin (1 µg/mL) reduced biofilm viability by eightfold, comparable to the reduction seen with ciprofloxacin at 8 µg/mL (Table S7a). Two-fold increase was observed in biofilm inhibition and biofilm eradication capability of ciprofloxacin in presence of SMJ-5 on S. aureus SA-1199 strain (Table S7b).
Confocal microscopy for visualization of biofilms
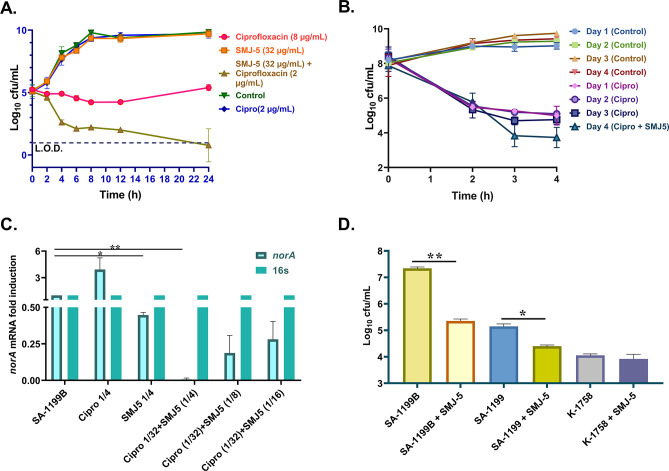
The effect of SMJ-5 and ciprofloxacin alone and in combination was assessed using microscopic examination of the S. aureus SA-1199B biofilm. Ciprofloxacin alone at 4 µg/mL concentration (1/2× MIC) could not eradicate mature pre-formed biofilm. SMJ-5 (32 µg/mL) combined with ciprofloxacin (1 µg/mL) eradicated the pre-formed biofilm to the same extent as MBEC50 of ciprofloxacin alone at 16 µg/mL. Ciprofloxacin’s (1 µg/mL) biofilm eradication efficiency was increased by 16-fold in the presence of SMJ-5. The quantitative data were observed to follow the microscopic study (Fig. 5).
FIG 5.
Confocal microscopic images of biofilm disruption in the presence of ciprofloxacin and SMJ-5 alone and in combination after 24 h of treatment on poly-L-lysine-coated glass coverslips. Confocal microscopy of biofilm in the sequence control, ciprofloxacin (4 µg/mL), SMJ-5 (32 µg/mL), ciprofloxacin (16 µg/mL), and ciprofloxacin (1 µg/mL) + SMJ-5 (32 µg/mL). Scale 50 µm. The image is the best representation of the biological triplicates.
Enhanced post-antibiotic life of ciprofloxacin in the presence of SMJ-3, 5, 9, and 10
From the above experiments, we evaluated SMJ-3, SMJ-5, SMJ-9, and SMJ-10 to be the best hits, showing low in vitro general toxicity in mammalian cells and efficiently eradicating pre-formed biofilm. A post antibiotic effect (PAE) of 0.9 h was observed in an exponential-phase culture of S. aureus SA-1199B subjected to 1× MIC of ciprofloxacin alone, while an enhanced PAE of 1.4, 4.8, 1.9, and 3.05 h was observed in combination with SMJ-3, SMJ-5, SMJ-9, and SMJ-10. The combination showed an increase of 0.5, 3.9, 1.0, and 2.15 h compared to when ciprofloxacin was tested alone at 1× MIC. For ciprofloxacin alone and combined with SMJ-3, SMJ-5, SMJ-9, and SMJ-10, an extension of 0.4, 0, 2.0, 0.9, and 1.4 h was seen at 1/2× MIC. Ciprofloxacin did not exhibit any PAE at 1/4× MIC, and among the combinations, SMJ-3, SMJ-5, SMJ-9, and SMJ-10 displayed a PAE of 0, 1.2, 0.8, and 0.9 h. The PAE results demonstrate an extended post-antibiotic life of SMJ-5 compared to SMJ-3, SMJ-9, and SMJ-10 (Fig. 6). Considering the data above, we discovered SMJ-5 as the most potent hit and chose SMJ-5 for further research.
FIG 6.
Post-antibiotic effect of ciprofloxacin at 1×, 1/2×, and 1/4× MIC alone and in combination with SMJ-3, SMJ-5, SMJ-9, and SMJ-10 at 1×, 1/2×, and 1/4× MIC by turbiditory method on S. aureus SA-1199B strain in H; the results are the representation of two biological repeats performed in triplicates ± SD.
Mutation frequency analysis of SMJ-5 to check the development of its resistant mutants
A compound’s ability to limit or minimize the development of resistant mutants is represented by its mutation prevention concentration (MPC). The occurrence of mutants anticipates the number of survivors divided by CFU plated. Ciprofloxacin alone displayed an MPC at 16× MIC. In comparison, SMJ-5 at 32 µg/mL (1/4× MIC) reduced the MPC of ciprofloxacin by 64-folds in the S. aureus SA-1199B strain, indicating that SMJ-5 holds a clinical relevance in the era of emerging resistance (Table 2).
TABLE 2.
Mutation prevention concentration of ciprofloxacin alone at various concentrations (0.125 to 16× MIC) and in combination with SMJ-5 at 32 µg/mL on S. aureus SA-1199B a ,b
| Ciprofloxacin (concentration) | 0.125× MIC | 0.25× MIC | 0.5× MIC | 1× MIC | 2× MIC | 4× MIC | 8× MIC | 16× MIC |
|---|---|---|---|---|---|---|---|---|
| Ciprofloxacin (CFU) | UC | UC | UC | UC | UC | 6.01 × 10−11 ± 1.11 × 10−11 | 0.4 × 10−10
±3.11 × 10−12 |
<10−10 |
| Ciprofloxacin + SMJ-5 (32 µg/mL) (CFU) | 4.01 × 10−8
±1.32 × 10−8 |
<10−10 | <10−10 | <10−10 | <10−10 | <10−10 | < 0−10 | <10−10 |
Ciprofloxacin (MIC) = 8 µg/mL.
UC, uncountable.
Effect of indole-based inhibitor SMJ-5 on the time-kill kinetics of ciprofloxacin
The time-kill kinetic study establishes the rate at which a compound kills a micro-organism as a function of survival recorded at various exposure time points. Ciprofloxacin was used in this assay at inhibitory (8 µg/mL) and sub-inhibitory (2 µg/mL) concentrations alone and combined with SMJ-5. Ciprofloxacin and SMJ-5 showed comparable growth to the untreated control cells at sub-inhibitory concentrations. Ciprofloxacin alone at 1× MIC (8 µg/mL) displayed a reduction of 2.3 log10 at a 12-h time point but later began to regrow, whereas ciprofloxacin (2 µg/mL) in combination with SMJ-5 (32 µg/mL) reduced CFU by 3.4 log10 at the 12-h time point and 4.6 log10 at the 24-h time point (Fig. 7A).
FIG 7.
(A) Time-kill kinetic assay, the combination of ciprofloxacin (2 µg/mL) and SMJ-5 (32 µg/mL), demonstrated a bactericidal effect as compared to ciprofloxacin alone against S. aureus SA-1199B strain. (B) Persister-kill kinetics, ciprofloxacin at 20× MIC, forms persisters hereditarily for 3 days on S. aureus ATCC 29213; on the fourth day, combination of ciprofloxacin at 20× MIC and SMJ-5 at 1/4× MIC lowers the persister count by 2 log10 CFU/mL. (C) RT-PCR of S.aureus SA-1199B norA in untreated, ciprofloxacin (1/4× MIC), SMJ-5 (1/4× MIC), and ciprofloxacin (1/32 MIC) + SMJ-5 (1/4× MIC), ciprofloxacin (1/32× MIC) + SMJ-5 (1/8× MIC) and ciprofloxacin (1/32× MIC) + SMJ-5 (1/16× MIC). (D) Macrophage invasion assay on S. aureus SA-1199B (norA over-expressing), SA-1199 (norA wild type), and K-1758 (norA knockout) strain in the presence of SMJ-5 at sub-inhibitory concentration; the results in B correspond to the mean of triplicate readings ± SD. Experiments A and D are representations of three biological repeats, and experiments B and C are the representations of two biological repeats. Results were considered significant (*) when P < 0.05 and highly significant (**) when P < 0.01 . P value calculated using 95% class interval.
Effect of SMJ-5 on persister heritability
As per the literature, the persisters develop as an epigenetic variant of their wild-type strain; their survival increase does not transfer to the next generation (19, 27). A typical biphasic killing of the cells was observed when an overnight culture of S. aureus ATCC 29213 was treated with 20× MIC of ciprofloxacin, leading to a fraction of persisters. The persisters survived every cycle of repeated growth and treatment. The cycle was repeated two times. The results in each cycle were identical to those of the original population, demonstrating that persistence is not heritable under the conditions depicted for the experiment. The persisters did not reduce even after two more cycles. Eradication of stationary-phase persister cells was achieved when they were subjected to ciprofloxacin and SMJ-5 combination treatment. The persister biphasic time-kill kinetic assay revealed the excellent anti-persister potential of the combination, demonstrating a 2 log10 reduction compared to ciprofloxacin alone (Fig. 7B).
SMJ-5 has a transcriptional inhibitory impact on norA induction
The expression of the norA mRNA was done following a previous study (28). The expression of norA mRNA in vehicle-treated bacteria was adjusted to a onefold induction, while ciprofloxacin (1/4× MIC) significantly boosted norA expression compared to the vehicle. The expression of norA was considerably reduced in the S. aureus SA-1199B strain following incubation with SMJ-5 (1/4× MIC), but the addition of ciprofloxacin increased the expression of norA. Further, we observed that SMJ-5 (1/4× MIC) in combination with ciprofloxacin (1/32× MIC) significantly reduced the ciprofloxacin-induced expression of norA at the mRNA level, aiding in the treatment of norA-over-expressed S. aureus SA-1199B (Fig. 7C). However, as the concentration of SMJ-5 reduced to 1/8× MIC and 1/16× MIC, the norA expression increased, but it was still less than ciprofloxacin-induced expression. A similar pattern was observed with norA wild type expressing S. aureus SA-1199 strain (Fig. S5A). The results depict that SMJ-5 also inhibits the NorA efflux pump indirectly at the transcriptional level. Further, we wanted to know the effect of SMJ-5 on mgrA as mgrA is the master regulator of norA (29). Moreover, we found that SMJ-5 downregulated mgrA expression in a concentration-dependent manner at 1/4× MIC (32 µg/mL), 1/8× MIC (16 µg/mL), and 1/16× MIC (8 µg/mL) (Fig. S5B).
Role of SMJ-5 to reduce macrophage invasion of NorA over-expressing S. aureus
The S. aureus strains SA-1199B (NorA over-expressing strain), SA-1199 (wild-type strain), and K-1758 (NorA knockout strain) were assessed for the intracellular invasion of the macrophage THP1 cells (105 cells/well) in the presence or absence of SMJ-5 (32 µg/mL). We found that S. aureus SA-1199B had nearly 1.97 log10 higher intracellular invasions than the wild-type SA-1199 strain, while S. aureus K-1758 had no significant invasion. SMJ-5 (32 µg/mL) did not affect S. aureus K-1758 penetration. In contrast, the presence of SMJ-5 (32 µg/mL) reduced the invasion of S. aureus SA-1199B to 2.32 log10 compared to a 1.02 log10 reduction in the wild-type SA-1199 (Fig. 7D).
Acute toxicity in animal model
Unwanted effects that cause biochemical lesions and functional impairments in organs can change a person’s overall functioning or the functioning of specific organs (30, 31). The positive in vitro toxicity results on mammalian cell lines prompted us to investigate SMJ-5’s in vivo acute toxicity to understand the compound’s therapeutic potential better. SMJ-5 was administered sub-cutaneously in two stages: stage 1 consisted of three groups and stage 2 consisted of two groups at a dose of 50 mg/kg (stage 1, group 1), 100 mg/kg (stage 1, group 2), and 250 mg/kg (stage 1, group 3) and in stage 2 and at a dose of 500 mg/kg (stage 2, group 1), and 1000 mg/kg (stage 2, group 2) (Table S8). The experiment was performed in triplicates. None of the animals died during the administration of SMJ-5, and no major change in body weight or blood glucose levels was observed after 7 days of administration. The preliminary acute toxicity study states that SMJ-5 is safe at ≥1,000 mg/kg, which can be further studied for better accuracy. The behavioral pattern of treated mice was the same as that of the placebo group. The mice were sacrificed for histopathological examination, and blood was collected through the retro-orbital route for biochemical tests. Histopathological analyses displayed similar results to the control group’s (Fig. 8A). The renal function test of treated mice resulted in normal calcium, blood urea, serum creatinine, and uric acid level in the body, similar to that of the control group. Liver function test demonstrated that the treated mice’s bilirubin (total); bilirubin, direct (conjugated); bilirubin, indirect (unconjugated); serum glutamic-oxaloacetic transaminase [S.G.O.T. (ALT)] , serum glutamic-pyruvic transaminase [S.G.P.T. (ALT)], total protein, albumin, globulin, A:G ratio, and total cholesterol were all within normal limits (Table S9).
FIG 8.
(A) Histopathological analysis of six major organs of BALB/c mice after sub-cutaneous administration of 1,000- and 500-mg/kg SMJ-5 and control, respectively (n = 3); and ciprofloxacin-SMJ-5 combination is efficacious in two mouse models of infection. (B) Neutropenic mouse thigh infection model: single sub-cutaneous dose treatment (10-mg/kg ciprofloxacin and 10-mg/kg SMJ-5 alone and in combination, six mice per group, 4 h after infection). CFU was determined 24 h post-infection for drug-treated mice; for controls, CFU was calculated 4 and 24 h post-infection. (C) Mouse peritonitis survival model: three sub-cutaneous dose treatments (10-mg/kg ciprofloxacin and 10-mg/kg SMJ-5 alone and in combination, 10 mice per group; after 1, 4, and 6 h post-infection). *P < 0.05, **P < 0.01, ****P < 0.0001. P values were calculated using 95% class interval.
Murine in vivo thigh infection model
The high efficacy of the combination prompted us to evaluate the effect of SMJ-5 and ciprofloxacin combination on the soft-tissue infection model in mice. Using a neutropenic mouse thigh infection model, we assessed the in vivo effectiveness of the SMJ-5 and ciprofloxacin combination. Mice were given 1.865 × 106 CFU of S. aureus SA-1199B infection intramuscularly. After 4 h, the first control group was sacrificed to check if the infection was established successfully, then the right thigh of the mice was homogenized and plated. The CFU count in the control group sacrificed at 4 h was found to be approximately 1.44 × 106. Ciprofloxacin (10 mg/kg) and SMJ-5 (10 mg/kg) alone, administered 4 h after infection, did not lower the bacterial counts 20 h after treatment. However, the combination of SMJ-5 and ciprofloxacin administered 4 h after infection proved very efficacious, demonstrating a reduction of approximately 0.8 and 3.191 log10 compared to 4 and 24 h of untreated control (Fig. 8B).
Effect of the combination on the mouse peritonitis model
A mouse septicemia model was used to perform an animal efficacy study. To initiate septicemia, mice were infected intraperitoneally with 500 µL of 2 × 109 CFU/mL of S. aureus SA-1199B, a dose that leads to ≥90% mortality. SMJ-5 (10 mg/kg) and ciprofloxacin (10 mg/kg) alone and in combination were injected three times in a single day at 1, 3, and 5 h after infection. SMJ-5-treated mice did not survive the infection after 5 d, whereas 30% of ciprofloxacin-treated mice survived, and the group treated with the combination of SMJ-5 and ciprofloxacin cured 80% of the animals, that is, the survival increased to 50% in the combination compared to ciprofloxacin alone (Fig. 8C).
DISCUSSION
S. aureus is an opportunistic, commensal bacterium that colonizes about one-third of the entire population asymptomatically (32) and successfully establishes infections using efflux pumps, genetic composition, and virulence factors (33). Efflux pumps have majorly contributed to the development of antibiotic resistance. In S. aureus, the NorA efflux pump is linked to fluoroquinolone resistance (10, 34, 35). NorA efflux pumps of the major facilitator superfamily play an essential role in innate and acquired antibiotic resistance in Gram-positive bacteria by extruding a myriad of anti-microbial metabolites (36). Previous studies have demonstrated that the lack of efflux pumps plays an essential role in attenuating the bacterial biofilm and virulence of bacteria and successfully treating drug-resistant infections (37).
An EPI 2-(2-aminophenyl) indole was reported by our group that was isolated from the terrestrial bacterial isolate, Streptomyces sp. IMTB 2501 (34). Using the scaffold as the rational, we successfully synthesized eight indole derivatives to improve their efficacy and activity profile. In the current study, we evaluated the efflux pump inhibitory potential of indole derivatives to enable better therapeutic intervention. This study demonstrates the remarkable efficacy of indole derivative SMJ-5 in potentiating fluoroquinolones in norA over-expressing S. aureus. To ensure that indole derivatives cause no bacterial death, the synergy with ciprofloxacin and norfloxacin is observed at sub-inhibitory concentrations of indole derivatives. In the presence of a potentiator, a minimum fourfold decrease in the MICs from their initial values was regarded as evidence of efflux inhibition (38). In the norA over-expressing strain, SMJ-5 synergized with ciprofloxacin and norfloxacin, resulting in lower MICs than the clinical resistance breakpoints of >4 µg/mL of ciprofloxacin and ≥16 µg/mL of norfloxacin (17, 18). In addition, SMJ-5 with ciprofloxacin showed synergy in 20 clinical strains of S. aureus. SMJ-3, SMJ-5, SMJ-9, and SMJ-10 show impressive EPI activity in the EtBr accumulation and inhibition assays, which is the gold standard for evaluating EPI functionality (34). The target of our EPIs, the NorA efflux pump, was partially validated by observing a more significant norfloxacin accumulation in the presence of indole derivatives compared to the control.
A chemical entity must complete a stringent checklist to be an EPI. The compound must not be anti-microbial; an anti-bacterial compound eventually results in the selection of resistant mutants, limiting its worth as an EPI. A true EPI should not hinder general metabolic processes limiting resistance development. Carbonyl cyanide m-chlorophenyl hydrazone is arguably the most well-known laboratory EPI (22). However, it is unlikely that such compounds could turn into medications because they can also be toxic. Thus, the clinical usage of efflux pump inhibitors should not impart toxicity on their own (39). Therefore, to rule out the possibility of any other effect on the bacterial membrane by the indole derivatives, we evaluated the effect of indole derivatives on general metabolic functions of bacterial cells, such as membrane depolarization, membrane permeability, ATP depletion, or antagonizing effects on the activation of eukaryotic Ca2+ channels as a corollary. Compounds disturbing the membrane can lead to resistance development in bacteria and identification of false EPIs, such as verapamil and CCCP (22). Among the four major hits, only SMJ-5, SMJ-9, and SMJ-10 do not disturb these metabolic functions and demonstrate the solidity of indole derivatives as true NorA efflux pump inhibitors.
Secondly, a true EPI must have excellent pharmacological characteristics such as low toxicity, high therapeutic and safety indices, and a favorable absorption, distribution, metabolism, excretion, and toxicity) profile (22). The known EPIs, despite being strong inhibitors, could not proceed to clinical trials due to their unavoidable toxicities. Reserpine, a plant alkaloid, is a general inhibitor of gram-positive efflux pumps, but its clinical utility is limited because later, it was found to be neurotoxic (40). In Gram-positive Staphylococci and Gram-negative Campylobacter spp., an EPI epigallocatechin gallate has been shown to increase the potency of tetracycline, erythromycin, and ciprofloxacin. However, due to toxicity concerns, no additional in vivo or pre-clinical trials were conducted (22). However, here, we found SMJ-5 to be negligibly hemolytic and displayed the highest viability in mammalian PBMC and HEK cell lines and was identified as a safe candidate for future investigation.
Efflux pumps are also known to contribute to biofilm development, and EPIs, such as thioridazine, boeravinone B, and nilotinib, are reported to reduce biofilm formation (10, 26, 41). As a corollary, we investigated the efficacy of indole derivatives in inhibiting biofilm formation and eradicating pre-formed biofilms. In crystal violet and MTT biofilm disruption assays, SMJ-3, SMJ-5, SMJ-7, SMJ-9, and SMJ-10 at sub-inhibitory concentrations successfully increased the efficacy of ciprofloxacin to eradicate the pre-formed S. aureus biofilm. We also used confocal microscopy to confirm SMJ-5’s involvement in biofilm eradication after being influenced by its biofilm eradication capabilities.
Hence, considering in vitro toxicity data, hemolysis data, and biofilm eradication capability results, we evaluated the post-antibiotic effect of ciprofloxacin in the presence of indole derivatives. An antibiotic agent produces PAE, per a prior study, when it results in an instantaneous growth delay of at least 0.5 h on a bacterial culture (42). SMJ-5 demonstrated a significant 3.9-h prolonged life of ciprofloxacin that may aid in maintaining wide dosage intervals. Given these findings, we decided to pursue SMJ-5 for further research.
The bacterial resistance to almost all antibiotics has led to the current state of anti-microbial resistance. To prevent the emergence of resistance to them, it is possible to use ideal EPIs in combination with them to have better therapeutic efficacy. In particular, it has been demonstrated that the non-anti-bacterial adjuvant that blocks the resistance mechanism of that antibiotic can reduce the development of resistance (43). Here we found a significantly high reduction of 64-fold in MPC of ciprofloxacin in the presence of SMJ-5 compared to that of ciprofloxacin alone during mutation frequency analysis demonstrating concurrent therapy to minimize resistance development. A reduction of 2 log10 CFU/mL in time-kill kinetics is referred to as synergy relative to its most active counterpart (here, ciprofloxacin). Moreover, it must be ≥2 log10 CFU/mL below the initial inoculum (44). The CFU decrease in combination was 2.3 log10 lower than ciprofloxacin alone at the 12-h time that establishes synergy. Anti-microbial persisters appear to be formed through multifactorial pathways involving efflux pumps, protein synthesis inhibition, and impaired cell development (19). As a result, we surmised that our EPIs might reduce the number of persister cells. Compared to ciprofloxacin alone, the combined therapy showed a 1 log10 reduction in persister cells, proving its efficacy. Indole derivatives are known to inhibit staphyloxanthin and its intermediates (24), which was validated when we found inhibition of staphyloxanthin and its intermediates by SMJ-5 at its sub-inhibitory concentration. Although S. aureus is not typically thought of as an intracellular pathogen, it might have a chance to survive and spread disease if it were to occupy an intracellular niche (32) momentarily. In this regard, our investigations supported the role of NorA overproduction in S. aureus intracellular invasion in macrophage THP-1 cell lines. Additionally, SMJ-5’s activity as an EPI in preventing NorA-overproducing S. aureus invasion was validated. The THP-1 macrophage invasion of norA-over-expressed S. aureus was observed to reduce by 2.32 log10 in the presence of SMJ-5, whereas no macrophage invasion was observed in the case of norA deletion S. aureus. Additionally, RT-PCR demonstrated that SMJ-5 dramatically decreased norA’s expression at the mRNA level after ciprofloxacin induction, indicating indirect inhibition of the NorA efflux pump by SMJ-5. Moreover, as it is known that norA is regulated by its master regulator mgrA (29), therefore we hypothesized SMJ-5 would also downregulate the mgrA gene. This was then proven by RT-PCR analysis that SMJ-5 downregulates mgrA expression. The outstanding in vitro results prompted us to assess the in vivo efficacy of the combination.
The in vivo effectiveness study was then conducted, and we found a significant reduction in CFU compared to the control. Also, the combination showed a 50% higher survival rate than ciprofloxacin alone in a mouse peritonitis model. In vivo, an acute toxicity study displayed that the safety of SMJ-5 is ≥1,000 mg/kg. No significant histopathological changes were observed.
Our findings conclude that SMJ-5 is a new compound of bacterial EPI that should be investigated further for its clinical therapeutic potential in conjunction with fluoroquinolones as a therapeutic drug.
MATERIALS AND METHODS
General chemistry
All chemicals and reagents used for the synthesis were procured from Sigma Aldrich (St. Louis, MO, USA), Alfa-aesar (Johnson Matthey Company, Ward Hill, MA, USA), and SDFineChem Ltd (India). Silica gel (#100–200) was used for column chromatography (CC), and thin layer chromatography (TLC) plates were purchased from Merck (Germany). The organic solvents used for CC were obtained from Finar and Rankem (India). 1H and 13C NMR spectra were recorded on BrukerAvance DPX 400 spectrometer (Brukers, Germany) at 400 and 100 MHz, and 1H spectra were also recorded for a few compounds on JNM-ECA 500 series NMR JEOL spectrometer (JEOL, Japan) at 500 MHz, respectively. NMR spectra were recorded in CDCl3, MeOD, and/or DMSO-d 6 at room temperature. Chemical shifts values (δ) were expressed in parts per million (ppm) relative to internal standard tetramethylsilane. Coupling constants (J) are given in hertz. Data are reported in the following order: chemical shift, multiplicity (s = singlet, d = doublet, t = triplet, dd = doublet of doublets, dt = doublet of triplets, m = multiplet, br = broad), and combinations of these coupling constants and integration. The mass spectra were recorded on a Thermo LTQ-XL spectrometer and Agilent 6200 series TOF/6500 series Q-TOF 10.1.
General procedure for the synthesis of 2-(2′-aminophenyl) indole [RP2] derivatives
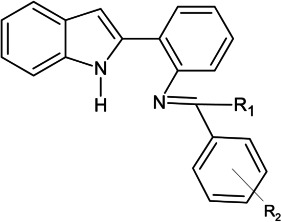
2-(2′-Aminophenyl)indole [4] was synthesized as per the method reported by Agarwal et al. (45). In brief, to a round-bottom flask containing phenyl hydrazine [2] (11.09 mmol) in absolute ethanol, o-aminoacetophenone [1] (7.3 mmol) was added with the addition of 5–10 drops of glacial acetic acid. The reaction mixture was refluxed for 4 h. After cooling, the precipitates (ppt’s) of phenylhydrazone [3] were washed with ethanol to give a solid orange color. Final cyclization was done by pouring phenylhydrazone [3] portionwise to the heated solution of polyphosphoric acid and stirred vigorously for 30 min at 80°C. The resulting mixture was cooled to room temperature and poured over crushed ice containing sodium hydroxide pellets. The solid ppt’s formed were washed with water and dried to afford crude product. Final purification was done using column chromatography over silica gel (ethyl acetate/n-hexane: 1.5/8.5) to give the desired product [4] (Fig. 9).
FIG 9.
Protocol for the synthesis of 2-(2′-aminophenyl) indole [RP2] derivatives.
The subsequent step included synthesizing mono-2-(2-arylideneaminophenyl)indole derivatives [6] under reflux settings using various aromatic aldehydes/acetophenones [5] (46). The substituted aromatic aldehyde/acetophenone [5] (2.88 mmol) was introduced dropwise to a round-bottom flask containing 2-(2′-aminophenyl) indole [4] (2.88 mmol) in absolute ethanol. To catalyze the reaction, a few drops of glacial acetic acid were added. The reaction was maintained under reflux conditions for 8–12 h until the completion of the reaction. Completion of reaction was monitored by TLC until the starting material was consumed. After cooling, the mixture was extracted with ethyl acetate and washed with brine. The combined organic layer was dried over anhydrous sodium sulfate, and the solvent was evaporated under a vacuum. The resultant combination was purified using n-hexane-ethyl acetate gradient (n-hexane: 100% to n-hexane/ethyl acetate: 50/50) on a silica gel column chromatography to give the pure final product (Table S1).
RP2
2-(2′-Aminophenyl) indole; white crystals; yield 74%; 1H NMR (500 MHz, CDCl3) δ ppm 8.45 (br s, 1 h), 7.63 (d, 1H, J = 7.5 Hz), 7.39 (t, 2 h, J = 7.5 Hz),7.21–7.12 (3H, m), 6.86 (m, 1 h), 6.81 (d, 1 h, J = 7.5 Hz),6.72 (m, 1 h), 3.15 (br s, 2 h); Electrospray ionization-mass spectrometry (ESI-MS) m/z calculated for C14H13N2 [M + H]+209.10, found 209.08.
SMJ-1
(E)−2-(1H-indol-2-yl)-N-(1-(p-tolyl)ethylidene)aniline; tan color solid; yield 82%; 1H NMR (400 MHz, DMSO-d 6) δ ppm: 11.39 (s, 1 h), 7.46 (d, J = 7.5 Hz, 1 h), 7.35 (t, J = 8.7 Hz, 3 h), 7.25 (d, J = 7.8 Hz, 1 h), 7.02 (m, 3H), 6.94 (m, J = 8.9 Hz, 1 h), 6.87 (t, J = 15.0 Hz, 1 h),6.66 (m, 1 h), 6.54 (m, 2 h), 2.21 (s, 3 h), 2.05 (s, 3 h); 13C NMR (100 MHz, DMSO-d 6) δ ppm: 19.0, 20.9, 30.0, 56.5, 58.5, 111.6, 113.2, 113.2, 113.7, 116.1, 119.3, 119.5, 121.3, 121.3, 125.7, 126.1, 128.7, 128.8, 131.1, 135.6, 137.7, 144.2, 147.3; ESI-HRMS m/z; calcd for C23H21N2[M + H] +325.1705; found: 325.1704.
SMJ-3
(E)−4-(1-((2-(1H-indol-2-yl)phenyl)imino)ethyl)phenol; white color solid; yield 76%; 1H NMR (400 MHz, DMSO-d 6) δ ppm: 11.35 (s, 1 h), 9.25 (s, 1 h), 7.45 (d, J = 7.6 Hz, 1 h), 7.31 (m, 3 h), 7.16 (d, J = 8 Hz, 1 h), 7.0 (t, J = 7.6 Hz, 1 h), 6.93 (t, J = 8 Hz, 1 h), 6. 84 (t, J = 15.2 Hz, 1 h), 6.63 (m, 3 h), 6.54 (t, J = 8 Hz, 1 h), 6.47 (s, 1 h), 2. 0 (s, 3 h);13C NMR (100 MHz, DMSO-d 6) δ ppm: 30.5, 46.6, 58.2, 111.5, 113.2, 113.6, 114.8, 115.6, 115.9, 119.3, 119.4, 121.3, 121.4, 125.7, 127.5, 128.6, 130.9, 131.2, 137.7, 140.7, 144.2, 156.1; Electrospray ionization-high resolution mass spectrometry (ESI-HRMS) m/z; calcd for C22H19N2O [M + H] +327.1497; found: 327.1494.
SMJ-5
(E)−5-(1-((2-(1H-indol-2-yl)phenyl)imino)ethyl)benzene-1,3-diol; blonde color; yield 91%; 1H NMR (400 MHz, DMSO-d 6) δ ppm: 11.34 (s, 1 h), 9.01 (s, 2 h), 7.46 (d, J = 6.7 Hz, 1 h), 7.34 (d, J = 8.0 Hz, 1 h), 7.29 (d, J = 7.9 Hz, 1 h), 7.04 (t, J = 7.2 Hz, 1 h), 6.94 (dd, J = 1.3, 15.3 Hz, 1 h), 6.89 (m, 1 h), 6.66 (d, J = 7.7 Hz, 1 h), 6.55 (t, J = 7.4 Hz, 1 h), 6.47 (s, 1 h), 6.37 (d, J = 2.1 Hz, 2 h), 6.01 (t, J = 2.0 Hz, 1 h), 1.98 (m, 3 h); 13C NMR (100 MHz, DMSO-d 6)δ ppm: 170.3, 157.6, 151.2, 143.7, 137.2, 130.5, 128.1, 125.2, 120.8, 120.7, 118.9, 118.9, 115.3, 113.0, 104.3, 100.43, 58.1, 48.4, 29.4, 28.9, 20.7, 17.2, 17.1; D2O exchange confirmed the presence of NH proton; ESI-HRMS m/z; calcd forC22H18N2O2[M + H] +343.1447; found: 343.1446.
SMJ-6
(E)-N-(4-fluorobenzylidene)-2-(1H-indol-2-yl)aniline; white color solid; yield 54%; 1H NMR (400 MHz, DMSO-d 6) δ ppm: 12.9 (s, 1 h), 8.60 (d, J = 1.0 Hz, 1 h), 8.58 (d, J = 1.2 Hz, 1 h), 8.15 (d, J = 0.8 Hz, 1 h), 7.90 (m, 3 h), 7.76 (m, 4 h), 7.54 (d, J = 8.0 Hz, 1 h), 7.47 (m, 4H) 7.17 (m, 1 h); 13C NMR (100 MHz, DMSO d 6) δ ppm: 163.7, 154.3, 144.9, 141.0, 139.0, 137.1, 131.1, 131.0, 129.3, 128.5, 125.6, 125.4, 121.9, 121.6, 120.9, 120.3, 116.2, 115.4, 115.2, 111.9, 111.8.; ESI-HRMS m/z; calcd for C21H14FN2 +313.1141; found: 313.1141.
SMJ-7
(E)−2-(1H-indol-2-yl)-N-(1-(4-nitrophenyl)ethylidene)aniline; orange color solid; yield 81%; 1H NMR (400 MHz, DMSO-d 6) δ ppm: 11.54 (s, 1 h), 8.13 (d, J = 8 Hz, 2 h), 7.73 (d, J = 8 Hz, 2 h), 7.5 (d, J = 4 Hz, 1H), 7.36 (t, J = 8 Hz, 2 h), 7.06 (t, J = 7.2 Hz,1H), 6.98 (m, 1H),6.92 (t, J = 8 Hz, 1 h), 6.84 (s, 1H),6.7 (d, J = 8 Hz, 1 h), 6.6(t, J = 7.2 Hz, 1 h), 2.14 (s, 3H); 13C NMR (100 MHz, DMSO-d 6) δ ppm: δ156.8, 145.9, 143.2, 137.3, 130.9, 128.5, 126.9,126.9, 124.9, 123.2,123.2, 121.2, 119.4, 118.6, 116.2, 113.2, 112.9, 111.3, 111.2, 58.4, 29.1; ESI-HRMS m/z; calcd for C22H17N3O2[M + H]+356.1399; found: 356.1393.
SMJ-8
(E)−2-(1H-indol-2-yl)-N-(4-trifluoromethyl)benzylidene)aniline; off-white solid; yield 77%; 1H NMR (400 MHz, DMSO-d 6) δ ppm: 12.97 (s, 1 h), 8.6 (d, J = 8 Hz, 1H), 8.15 (d, J = 8 Hz, 1 h), 8.08 (m, 2 h), 8.0 (m, 2 h), 7.82–7.7 (m, 3 h), 7.4 (m, 2 h), 7.18 (t, J = 8 Hz, 1 h); 13C NMR (100 MHz, DMSO-d 6) δ ppm:154.2, 145.3, 145.2, 141.6, 139.6, 130.3, 129.9, 129.8, 129.6, 129.2, 126.5, 126.2, 126.0, 125.9, 125.8, 123.5, 122.5, 121.7, 121.3, 120.9, 161.8, 112.5, 112.2; ESI-HRMS m/z; calcd for C22H14F3N2 +, 363.1109; found: 365.1109.
SMJ-9
(E)−2-(1H-indol-2-yl)-N-(1-(4-(methylthio)phenyl)ethylidene)aniline; cream color solid; yield-82%; 1H NMR (400 MHz, DMSO-d 6) δ ppm:11.40(s, 1 h) 7.47 (d, J = 6.7 Hz, 1 h), 7.41 (d, J = 8.5 Hz, 2 h), 7.35 (d, J = 8.0 Hz, 1 h), 7.28 (d, J = 8.0 Hz, 1 h), 7.14 (d, J = 8.5 Hz, 2 h), 7.03 (t, J = 7.2 Hz, 1 h), 6.95 (m, 1 h), 6.89 (t, J = 7.5 Hz, 1 h), 6.67 (d, J = 7.7 Hz, 1 h), 6.57 (m, 2 h), 2.4 (s, 3 h), 2.1 (s, 3 h); 13C NMR (100 MHz, DMSO-d 6) δ ppm: 147.1, 144.1, 137.7, 136.1, 131.2, 128.7, 126.8, 126.8, 126.1, 126.0, 125.6, 121.5, 121.4, 119.5, 119.2, 116.1, 113.7, 113.26, 112.9, 111.6, 58.4, 29.8, 15.3; ESI-HRMS m/z; calcd for C23H21N2S [M + H]+ 357.1424; found: 357.1424.
SMJ-10
E)-N-(1-(4-aminophenyl)ethylidene)−2-(1H-indol-2-yl)aniline; cream color solid; yield 58%; 1H NMR (400 MHz, DMSO-d 6) δ ppm:11.29 (s, 1 h), 7.45 (d, J = 7.1 Hz, 1 h), 7.32 (d, J = 8.0 Hz, 1 h), 7.15 (m, 3 h), 7.00 (t, J = 7.3 Hz, 1 h), 6.92 (m, 1 h), 6.83 (m, 1 h), 6.63 (d, J = 7.8 Hz, 1 h), 6.53 (t, J = 7.3 Hz, 1 h), 6.45 (d, J = 8.5 Hz, 2 h),6.35 (s, 1 h), 4.89 (s, 2 h), 1.96 (s, 3 h; 13C NMR (100 MHz, DMSO-d 6) δ ppm: 128.5, 127.1, 121.3, 121.2, 119.4, 119.2, 115.7, 113.5, 113.1, 111.5, 30.0; ESI-HRMS m/z; calcd for C22H20N3[M + H]+ 326.1657; found: 326.1659.
Bacterial strains, cell lines, and growth medium
The S. aureus SA-1199B with A116E GrlA substitution, over-expressing the NorA MDR efflux pump, SA-1199 (norA wild-type), SA-K-1758 (NCTC 8325–4 norA deletion mutant), ATCC 33591, ATCC 29213 and 20 clinical isolates of S. aureus from GMCH were used in the present study. Human peripheral blood mononuclear cells (Himedia), THP-1 human monocytic cell line, and HEK-293 cells were used for ex vivo experiments. For the generation of macrophages, THP-1 cells were induced by phorbol 12-myristate 13-acetate at 100 ng/mL and incubated for 24 h.
All bacterial media and supplements included cation-adjusted BBL Mueller-Hinton II Broth (CA-MHB), Mueller-Hinton agar (MHA), and tryptic soya broth (TSB). Dulbecco's Modified Eagle Medium (DMEM) (ThermoFisher) and Fetal bovine serum (FBS) (Thermo Fisher) were used for cell culture experiments.
MIC determination and synergy assay
The minimum inhibitory concentration was determined using broth microdilution according to the CLSI standards (47, 48). The experiment was conducted in 96-well round-bottom plates with a 200-µL total reaction volume. Twofold serial dilutions of the drugs [ciprofloxacin (256–2 µg/mL) and norfloxacin (256–2 µg/mL)] were prepared. The OD600nm of the bacteria grown to the mid-exponential phase was adjusted to 0.25–0.3, and it was diluted such that each well finally contained 5 × 105 CFU/mL, and the plates were incubated at 37°C for 18 h.
The synergistic efficacy of indole derivatives with norfloxacin (Sigma Aldrich) and ciprofloxacin (Sigma Aldrich) was identified by checkerboard assay (47, 48). The MIC of antibiotics and the indole derivatives were determined by varying the concentration of the indole derivatives and the drugs’ varying concentrations. The fractional inhibitory concentration index was calculated to identify the synergistic efficacy of indole derivatives using the following equation:
FICI = MIC (antibiotic in the presence of EPI) / MIC (antibiotic alone) + MIC (EPI in the presence of antibiotic) / MIC (EPI alone).
The FICI values of ≤0.5, >0.5–4, and >4 were considered to be synergistic, indifferent, and antagonistic, respectively (10).
EtBr accumulation assay
As previously described (10, 35), fluorometric measurement of EtBr (Sigma Aldrich) absorption from S. aureus SA-1199B and SA-K-1758 was performed. Briefly, in uptake buffer (7-mM KCl, 110-mM NaCl, 0.4-mM Na2HPO4, 50-mM NH4Cl, 52-mM Tris base, and 0.2% glucose, adjusted to pH 7.5 with HCl), bacterial suspensions grown to mid-exponential phase in Mueller-Hinton broth (MHB) were diluted to an OD600nm of 0.3. The bacterial suspensions were treated with 4-µg/mL EtBr in the presence of 1/4×, 1/8×, and 1/16× MIC of indole derivatives and reserpine (Sigma Aldrich), a recognized efflux pump inhibitor. In a Synergy microplate reader (BioTek, USA), the fluorescence gain was recorded after 20 min at λexcitation/λemission of 530 and 600 nm. The equation calculated the relative final fluorescence:
RFF = (RFtreated – RFuntreated) / RFuntreated,
where RFtreated represents the fluorescence in the presence of indole derivatives or reserpine at the last time point (20 min), and RFuntreated signifies the fluorescence in the control well (without EPI) at the last time point (20 min) (without EPI and indole derivatives).
When the activity index was greater than 0, the EtBr accumulated more in the presence of the EPI than in the absence of the EPI (untreated cells were considered 0). Negative RFF indicated that less EtBr was accumulated than in the untreated control. RFF greater than 1 in the presence of efflux inhibitors revealed increased EtBr accumulation in the cells (49).
Measurement of norfloxacin accumulation
The norfloxacin accumulation in the norA-over-expressed strain S. aureus SA-1199B was measured by fluorescence assay (38, 50). The culture was grown to the mid-log phase. The bacterial suspensions of OD600nm of 0.7 were treated with 16-µg/mL (1/4× MIC) norfloxacin, 1/4× MIC of indole derivatives and reserpine, and were incubated on ice for 5 min. The cells were resuspended in 1-mL glycine buffer (glycine = 0.1 M, HCl = 0.02 M, pH 3) after rinsing twice with phosphate buffer (Na2HPO4·7H2O = 0.0754M, NaH2PO4·H2O = 0.0246 M, pH 7.4). The samples were centrifuged after incubating for 20 min at room temperature, and 1:10 dilution of the supernatant was used to record the fluorescence at λexcitation and λemission of 281 and 440 nm, respectively.
Efflux inhibition study using ethidium bromide
The impact of indole derivatives on EtBr efflux in S. aureus SA-1199B was studied using fluorometry (10, 35). With shaking, S. aureus SA-1199B was grown to an OD600nm of 0.7 at 37°C. The bacterial suspension was centrifuged, washed thrice, and resuspended in PBS (pH 7.4) (Lonza). Furthermore, in sub-inhibitory concentrations of indole derivatives, the bacterial suspensions were treated with 4-µg/mL (1/4 × MIC) EtBr. Allowing the highest amount of EtBr to accumulate, the suspensions were incubated for 60 min at 25°C. The suspensions were centrifuged to stop the reaction and remove excess EtBr at 4°C. The cells were then resuspended in ice-cold PBS, and fluorescence was measured at 530/600 nm at 37°C for 45 min at 9-min intervals. The experiment was carried out in a Synergy microplate reader.
Membrane potentiation assay
S. aureus SA-1199B was grown at 37°C to mid-exponential phase. The cells were centrifuged, washed thrice at 4000 × g for 10 min, and resuspended in 50-mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffer and 0.1% glucose to achieve an OD600 of 0.1. DiSC3(5) (Sigma Aldrich) dye at 1-µM concentration and 300-mM KCl (to countervail the outer and cytoplasmic K+ concentrations) in HEPES buffer was added to the cells and incubated in the dark at room temperature for 10 min for the probe fluorescence to stabilize (51 – 54). The indole derivatives were added at their MIC to the cells, and valinomycin (Sigma Aldrich) (MIC = 16 µg/mL) was used as the positive control. Bacterial OD600nm was then normalized for all the treatment groups and control. Then, the fluorescence leakage was measured immediately for 54 min at an interval of 9 min at a λexcitation/λemission of 622/670 nm.
Membrane permeability assay
The membrane permeabilization assay measured the fluorescence of propidium iodide penetrating the membrane according to the Invitrogen Propidium Iodide kit (Thermo Fisher Scientific, Inc.) protocol with certain modifications. In CA-MHB, S. aureus SA-1199B was incubated and allowed to grow up to the mid-exponential stage with an OD600nm of 0.3. The 500-µL bacterial suspension was treated with indole derivatives and paenibacillin at 1× MIC for 30 min. After treatment, bacterial cell OD600nm was normalized, and then the culture was washed thrice with normal saline (0.85% NaCl) and suspended in PBS. The suspensions were treated with 30-µM PI and incubated at room temperature for 15–20 min. Any unbound PI was eliminated by centrifuging the suspensions at 13,000 × g for 5 min and then suspending in 500-µL PBS. Suspensions of 100 µL were dispensed in a 96-well plate, and fluorescence was measured at a λexcitation/ λemission of 490 and 635 nm, respectively, after 30 min.
Determination of intracellular ATP levels
An ATP determination kit was used to measure the intracellular ATP levels of bacteria according to the manufacturer’s protocol (Invitrogen, Life Technologies, USA). S. aureus SA-1199B bacterial suspension of 500 µL was treated with indole derivatives separately at a sub-inhibitory concentration of 1/4× MIC and CCCP (Sigma Aldrich) at 0.5 µg/mL in MHB for 4 h at 37°C and 200 rpm. After treatment, bacterial cell OD600nm was normalized, and then the cells were centrifuged and resuspended in 100 µL of PBS after incubation. Finally, the cells were sonicated for 15 min, followed by 10 min of alternate heat and cold shock for lyses of the cells. The supernatant was used to quantify total ATP represented as relative luminescence (34).
Mammalian Ca2+ channel blocking assay
According to the manufacturer’s instructions, the Fluo-4 Direct Ca2+ channel test kit was used to examine the effect of SMJ-5 on human calcium channels (Life Technologies, Carlsbad, CA, USA) (23). HEK-293T cells (5 × 104) were treated with Probenecid (5 mM) and Fluo-4 dye for 1 h, and the amount of fluorescence was measured. Then the cells were treated with indole derivatives at 1/4× MIC, DMSO (vehicle control), and verapamil at 50 µg/mL. Carbachol (50 µg/mL), the Ca2+ channel stimulator, was added after 2 min, and the fluorescence was recorded at 494/516 nm using a microplate reader.
Hemolysis assay
Fresh rabbit blood erythrocytes were used in the hemolysis assay (55). Blood was collected in a heparin tube and then centrifuged and rinsed with PBS thrice. PBS was used to resuspend the RBCs at the final concentration of 4% (vol/vol). In 180 µL of freshly prepared 4% RBC, 20 µL of indole derivatives at a final concentration of 64, 128, 250, and 500 µg/mL, 0.1% Triton-X (positive control), DMSO (vehicle control) was added. DMSO was subtracted from the readings. The suspension was incubated for 1 h at 37°C before centrifuging for 5 min at 4°C at 1000 × g. The supernatant (100 µL) was shifted to a 96-well flat-bottom plate, and absorbance was measured at 570 nm (BioTek, US).
Ac = (Absorbance of control – Absorbance of blank)
Mammalian cytotoxicity
The cytotoxicity of indole derivatives was investigated on human peripheral blood mononuclear cells (PBMC) and Human Embryonic Kidney 293T cells (HEK) (55). Approximately 20,000 PBMC and 7000 HEK cells were seeded separately per well in separate plates, and they were incubated in a CO2 incubator with 5% CO2 at 37°C for 24 h. Indole derivatives (250 and 500 µg/mL) were added and incubated at 37°C in a CO2 incubator. After 24 h of incubation, MTT (500 µg/mL) was added to the wells after discarding the medium. The formazan crystals were solubilized with a 100-µL stopping solution containing 40% (vol/vol) dimethylformamide (DMF) in 2% (vol/vol) glacial acetic acid and 16% (wt/vol) sodium dodecyl sulfate (SDS) at 4.7 pH. The absorbance was assessed at 570 nm (BioTek, USA).
The given equation was used to calculate the percent viability:
% Cell viability = At/ Ac;
At = (absorbance of test compound – absorbance of blank);
Ac = (absorbance of control – absorbance of blank)
Crystal violet biofilm inhibition
For biofilm formation, S. aureus SA-1199B was grown overnight in the Soyabean Casein Digest Medium (Tryptone Soya Broth) (HIMEDIA) with 2.5% glucose. The culture was then diluted to 1 × 109 CFU/mL. Microtiter plates containing indole derivatives and ciprofloxacin combination as detailed for the synergy studies. The wells were washed with PBS twice after 48-h incubation at 37°C to remove the planktonic cells, followed by fixation with methanol for 15 min and air-drying at 37°C in an inverted position for 10 min. The cells in the dried wells were stained with 0.05% (wt/vol) crystal violet (CV) and incubated for 10 min at room temperature. The cells were rinsed in PBS until the control wells holding media only were colorless. 200 µL of ethanol: acetone (80:20) mixture was added to the wells and incubated at room temperature for 10 min. A Synergy H.T. multi-mode microplate reader measured absorbance at 570 nm (BioTek) (56). Compared to the control wells, a reduction of ≥50% absorbance was defined as Minimal biofilm inhibitory concentration (MBIC50).
Crystal violet biofilm eradication
The first step was to generate biofilms, S. aureus SA-1199B was grown overnight in the Soyabean Casein Digest Medium (Tryptone Soya Broth) (HIMEDIA) with 2.5% glucose. The culture was then diluted (1 × 109 CFU/mL). The plates were incubated for 48 h. Indole derivatives and ciprofloxacin combinations, as detailed for the synergy studies, were introduced simultaneously to eradicate the pre-formed biofilm at the maturation stage (48-h biofilms) with 200 µL of TSB. Untreated biofilms were employed as control. The plates were incubated at 37°C for 24. The non-adherent cells were removed from each well the next day, and the adhering biofilm was washed twice with PBS, followed by fixation with methanol for 15 min and air-drying at 37°C in an inverted position for 10 min. CV was used to assess the biofilm, and biofilm eradication was estimated as specified. The cells in the dried wells were stained with 0.05% (wt/vol) CV and incubated for 10 min at room temperature. The cells were rinsed in PBS until the control wells holding the medium were colorless. Ethanol:acetone (80:20) mixture of 200 µL was added to the wells and incubated at room temperature for 10 min. A Synergy H.T. multi-mode microplate reader measured absorbance at 570 nm (BioTek) (56). Compared to the control wells, a ≥50% absorbance reduction was defined as minimal biofilm eradication concentration (MBEC50).
Identifying viable cell populations in biofilms
We also used the MTT test to count the number of live cells in the biofilm. The experiment was carried out exactly as stated before, but instead of a CV, MTT (1-mg/mL final concentration) was employed. MTT was added after cell washing, and plates were incubated at 37°C for 4 h. The MTT was solubilized in a solubilizing solution. The formazan crystals were solubilized with a 100-µL stopping solution containing 40% (vol/vol) DMF in 2% (vol/vol) glacial acetic acid and 16% (wt/vol) SDS at 4.7 pH. The absorbance was assessed at 570 nm (BioTek, USA). The findings are shown as MBEC50 of live adhering bacteria in the biofilm compared to the control (untreated biofilm) (57).
Confocal microscopy
SYTO9 dye (Invitrogen, Life Technologies) was used to stain nucleic acid of the cells in biofilms and visualized using confocal laser scanning microscopy (CLSM). CLSM was used to confirm the impact of ciprofloxacin (4 and 16 µg/mL), SMJ-5 (32 µg/mL), and a combination of ciprofloxacin (1 µg/mL), and SMJ-5 (32 µg/mL) was used at MBEC50 to visualize the effect. S. aureus SA-1199B biofilm was grown on sterile 18-mm glass coverslips coated with poly-L-lysine placed in a 12-well polystyrene plate (Falcon) (58). Ciprofloxacin was utilized at 4 and 16 µg/mL; SMJ-5 was used at 32 µg/mL; and a combination of ciprofloxacin at 1 µg/mL and SMJ-5 at 32 µg/mL was used at MBEC50 to visualize the effect. The plates were incubated at 37°C for 24 h. The plates were washed using normal saline to remove planktonic cells. SYTO9 was diluted 1000 times in PBS, poured into the biofilm wells, and left at room temperature for 20–30 min. After incubation, tfhree more saline washes were given, and on an oil immersion lens (×40), images were visualized using Nikon A1 plus Ti confocal microscope with a Nikon A1R scan head. Images were captured using NIS elements software.
Staphyloxanthin extraction and spectrometric measurement of its biosynthetic intermediates
In CA-MHB, S. aureus ATCC 29213 was incubated and grown overnight. The culture was then diluted 1,000×, and 5-mL culture was treated with indole derivatives at a sub-inhibitory concentration (1/4× MIC) for 48 h. The treated and untreated bacterial cells’ optical densities (OD) were then normalized and centrifuged at 8,000 rpm for 10 min to extract carotenoid pigments. PBS was used to rinse the cell pellets before they were suspended in 500 µL of methanol. The tubes were shaken for 5 h at 400 rpm and 55°C while covered in aluminum foil. The extracted pigments were then separated from the supernatant by centrifuging the tubes. Using a plate reader and various wavelengths, the staphyloxanthin intermediates present in the collected supernatant was quantified. The wavelengths used for the absorbance measurements were 286 nm for 4,4′-diapophytoene, 435 nm for 4,4′-diaponeurosporene, 455 nm for 4,4′-diaponeurosporenic acid, and 462 nm for staphyloxanthin (59). As a negative control, methanol was employed.
Post-antibiotic assay
In CA-MHB, S. aureus SA-1199B was grown to the mid-log phase. The culture was then adjusted to an OD600nm of 0.25. Further, the bacterial culture was diluted a hundred times, and 1-mL bacterial culture was treated with ciprofloxacin at 1×, 1/2×, and 1/4× MIC alone and in combination with SMJ-3, SMJ-5, SMJ-9, and SMJ-10 (1/4× MIC) at 37°C for 2 h. After that, bacterial suspension OD600nm was normalized for all the treatment groups and control. Then, the bacterial suspensions were centrifuged for 10 min at 1200 × g and washed thrice in new media to eliminate the drug’s carry-over effect. The untreated bacterial suspension was included as the control. A 300-µL bacterial suspension was added to the wells of a transparent bottom 96-well flat-bottom plate in triplicate and incubated at 37°C for 18 h. PAE was determined using the formula PAE = T 50 − C 50, where T 50 and C 50 refer to the time taken by the treated and untreated cultures to reach a 50% OD of the final absorbance achieved by the control, respectively (60).
MPC
S. aureus SA-1199B (1010 CFU) bacterial suspensions were spread on MHA plates with varying concentrations of ciprofloxacin alone and in conjunction with SMJ-5 at 32 µg/L and were incubated at 37°C. After 48 h, the colonies were enumerated, and the mutation frequency was estimated by dividing the number of survivors by the number of CFU plated. The MPC was considered to be the concentration at which no colony appeared (61).
Time-kill kinetics
The S. aureus SA-1199B was grown to the mid-exponential phase, and a suspension (OD600nm ~0.3) was prepared. The initial inoculum of the S. aureus SA-1199B was challenged with ciprofloxacin at 8 and 2 µg/mL separately. Likewise, SMJ-5 was tested at its sub-inhibitory concentration of 32 µg/mL both in the presence and absence of 2 µg/mL of ciprofloxacin. At different time points (0, 2, 4, 6, 8, 12, and 24 h), the culture was assessed for the bacterial count and expressed as CFU per milliliter (62).
Persisters assay
S. aureus ATCC 29213 was grown for 24 h to investigate the antibiotic-induced persister cell production. These stationary-phase cells were treated with 10 µg/mL (20× MIC) of ciprofloxacin for 3 h. Aliquots were withdrawn at intervals of 60 min for 3 h to determine the number of viable bacteria that survived ciprofloxacin exposure. DMSO was used as vehicle control. The viable colonies were determined after 24 h of incubation and represented as log10 CFU/mL. After incubation, the remaining cells were pelleted, washed, and allowed to flow in a fresh medium overnight. And the process was repeated for 3 days to check the heritability of persister formation. On the fourth day, the cells were treated with SMJ-5 (32 µg/mL) and ciprofloxacin (10 µg/mL), and aliquots were withdrawn every 60 min for 3 h to determine the viable count (63).
Extraction of total RNA and semi-quantitative RT-PCR
S. aureus SA-1199B was grown overnight. The culture was diluted 1,000× and then treated with SMJ-5 (32 µg/mL), ciprofloxacin (2 µg/mL), and SMJ-5 (32 µg/mL) + ciprofloxacin (0.25 µg/mL) for 16 h. The culture was centrifuged at 10,000 × g and treated with 5 M guanidine thiocyanate for 5 min, then again centrifuged, pelleted, and stored at −80°C until RNA isolation. The pellet was then washed with Diethyl pyrocarbonate (DEPC) water and resuspended in 1-mL TRIzol reagent (Thermo Fisher Scientific, Inc.), and then lysis was performed using a bead beater for 30 s at an interval of 3–5 min thrice at a speed of 6 rpm. The suspension was placed on ice instantly after every cycle. Total RNA was purified from the bacterial suspension using the Qiagen RNeasy Mini kit following the manufacturer’s protocol.
The reverse transcription PCR was conducted using the SuperScript III Platinum One-Step qRT-PCR Kit (Thermo Fisher Scientific, Inc.) to synthesis cDNA following the manufacturer’s protocol. The following were the PCR thermocycling parameters: 50°C for 14.02 min, 95°C for 2 min, 40 cycles of 95°C for 15 s, 60°C for 30 s, and a melt curve of 95°C for 15 s, and 60°C for 1 min. Relative gene expression was determined using the power 2−ΔΔCT technique. The housekeeping gene 16s rRNA was used as an endogenous control to normalize the expression levels of target genes. Each sample was analyzed in duplicate, and all samples were examined parallel for their expression. The fold induction was calculated using the average replicate values. In norA gene expression analysis in clinical strains, the S. aureus ATCC 29213 strain was used as a reference sample strain. The primer sequences for the norA (28) and 16s rRNA genes are available in the supplemental materials (Table S10).
Macrophage invasion assay
The THP-1 macrophage cell line was used to study S. aureus intracellular invasion in the presence of SMJ-5 (35). S. aureus SA-1199B, SA-1199, and K-1758 (106 CFU/well) were employed to infect macrophages (105 cells/well with or without SMJ-5 (32 µg/mL) and were incubated for 2 h. After washing out the extracellular bacteria three times with PBS, the cells were treated for 30 min with gentamicin (50 µg/mL). The cells were treated with 0.1% saponin for a short interval, and the viability of intracellular bacteria was calculated by plating on MHA.
Acute toxicity, blood-biochemical, and histopathological studies in animal model
This experiment was performed in two stages (30). The outcome from each stage determined the next step (i.e., whether to halt or continue to the next level). Stage 1 consisted of three groups, and stage 2 consisted of two groups; each group contained three mice each. SMJ-5 was administered sub-cutaneously at a dose of 50 mg/kg (stage 1, group 1), 100 mg/kg (stage 1, group 2), and 250 mg/kg (stage 1, group 3) and in stage 2 and at a dose of 500 mg/kg (stage 2, group 1) and 1,000 mg/kg (stage 2; group 2) (Table S8). Animals were observed every 2 h after administration for 12 h and then every 12 h for a period of 7 days. Mortality and behavioral toxicity signs were recorded.
Mice were given an intraperitoneal injection of ketamine (90 mg/kg) and xylazine (10 mg/kg) (64) at the end of 7 days. After that, a blood drop was collected from the tail vein of the anesthetized mouse to check the blood glucose level. Blood (400–500 µL) was collected through the retro-orbital route from mice.
The blood in MCT was left undisturbed for 30 min at room temperature and then centrifuged for 10 min at 1,000 × g. After that, the serum was collected and refrigerated at −80°C. The mice were dissected, and six organs, namely, the lung, heart, brain, liver, kidney, and spleen, were removed and stored in 10% neutral buffered formalin. The blood-biochemical and histopathological studies were outsourced from a private medicos center.
In vivo thigh infection model
Female BALB/c mice received two intraperitoneal doses of 150 and 100 mg/kg of cyclophosphamide for 4 days to introduce neutropenia. The mice were after that injected intramuscularly with 50 µL of S. aureus SA-1199B 3.2 × 107 CFU/mL). Four hours post-infection, a single sub-cutaneous dose of 10 mg/kg of SMJ-5 and 10 mg/kg of ciprofloxacin (Sigma Aldrich) alone and in combination was administered in mice. The fourth group was administered 50 mg/kg of ciprofloxacin alone to check if the combination was better than the high-dose ciprofloxacin. One group of infected mice was euthanized pre-treatment by cervical dislocation after 4 h of infection. The right thighs were removed and homogenized, and CFUs were determined. The other four groups of mice were euthanized by cervical dislocation 20 h after injecting the administered dose of SMJ-5 and ciprofloxacin. All four groups’ CFU was enumerated from the right thigh (65).
Mouse peritonitis model
The experiment was carried out as stated previously, with a few changes (66). The mice were given 500 µL of 2 × 109 CFU/mL S. aureus SA-1199B suspension prepared in 5% mucin intraperitoneally. All groups (n = 10) were sub-cutaneously administered at 1, 3, and 5 h after infection with 10 mg/kg of SMJ-5 and 10 mg/kg of ciprofloxacin alone and in combination. The survival condition and weight variations of mice were monitored for 7 days. The mice that survived until the seventh day were euthanized by cervical dislocation.
Statistical analyses
Most trials used biological replicates, and consistent outcomes were attained each time. The information is shown as mean and standard deviation. Different mean t-tests (two-tailed) were employed to find group differences. P values of 0.05 (*) were regarded as statistically significant, and 0.01 (**), 0.001 (***), and 0.0001 (***) were considered highly significant.
ACKNOWLEDGMENTS
We thank the director of Council of Scientific and Industrial Research (CSIR)-Institute of Microbial Technology for providing the necessary facilities and infrastructure to complete this work. N.C. expresses gratitude to the Indian CSIR for the senior research scholarship. We are grateful for the norA efflux pump over-expression and deletion strains provided by Dr. Inshad Ali Khan of Central University of Rajasthan in India. The clinical isolates utilized in this investigation were provided as a generous gift by Professor Varsha Gupta of Government Medical College and Hospital, Chandigarh, India. We appreciate Mr. Deepak Bhatt’s technical support at the Confocal Microscopy facility. We also acknowledge Dr. Rajneesh Dadwal for his insightful comments on the manuscript. We acknowledge Dr. Kusum Joshi from Chandigarh’s Medicos Centre for histopathological evaluations. The authors gratefully thank the Department of Biotechnology, India, which supported this project (IMT/GAP/2019/04893-GAP0193).
The present work was supported by GAP/2019/04893-GAP0193.
H.N., S.J., N.C., and R.T. designed the project. N.C., R.T., and N.M. performed the microbiological experiments. N.C. and N.M. conducted animal studies. R.K., G.K., and S.J. performed the chemical synthesis. Manuscript writing: original draft preparation by N.C.; writing—review and editing by N.C. and H.N. All of the authors read and approved the final version of the manuscript.
The authors declare no competing financial interest.
AFTER EPUB
[This article was published on 27 September 2023 without the supplemental material. The supplemental material was included in the current version, posted on 17 October 2023.]
Contributor Information
Sanjay Jachak, Email: sjachak11@gmail.com.
Hemraj Nandanwar, Email: hemraj@imtech.res.in.
Brian Conlon, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, USA .
ETHICS APPROVAL
This study was carried out in compliance with the recommendations of the Committee for the Purpose of Control and Supervision of Experiments on Animals. The protocol (IAEC/20/04) was approved by the CSIR-Institute of Microbial Technology’s Institutional Animal Ethics Committee in Chandigarh, India. Randomization and blinding were not regarded as necessary for infection animal models. During the histopathology investigation, the observer was unaware of the control and treatment groups.
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/spectrum.04876-22.
Tables S.1 to S.10 and Fig. S.1 to S.5.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Nikaido H. 2009. Multidrug resistance in bacteria. Annu Rev Biochem 78:119–146. doi: 10.1146/annurev.biochem.78.082907.145923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tong SYC, Davis JS, Eichenberger E, Holland TL, Fowler VG. 2015. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev 28:603–661. doi: 10.1128/CMR.00134-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wolfson JS, Hooper DC. 1985. The fluoroquinolones: structures, mechanisms of action and resistance, and spectra of activity in vitro. Antimicrob Agents Chemother 28:581–586. doi: 10.1128/AAC.28.4.581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hooper DC. 1999. Mechanisms of fluoroquinolone resistance. Drug Resist Updat 2:38–55. doi: 10.1054/drup.1998.0068 [DOI] [PubMed] [Google Scholar]
- 5. Gagliotti C, Balode A, Baquero F, Degener J, Grundmann H, Gür D, Jarlier V, Kahlmeter G, Monen J, Monnet DL, Rossolini GM, Suetens C, Weist K, Heuer O, EARS-Net Participants (Disease Specific Contact Points for AMR) . 2011. Escherichia coli and Staphylococcus aureus: bad news and good news from the European antimicrobial resistance surveillance network (EARS-net, formerly EARSS), 2002 to 2009. Euro Surveill 16:19819. doi: 10.2807/ese.16.11.19819-en [DOI] [PubMed] [Google Scholar]
- 6. Tanaka M, Wang T, Onodera Y, Uchida Y, Sato K. 2000. Mechanism of quinolone resistance in Staphylococcus aureus. J Infect Chemother 6:131–139. doi: 10.1007/s101560070010 [DOI] [PubMed] [Google Scholar]
- 7. Piddock LJV. 2006. Multidrug-resistance efflux pumps? Not just for resistance. Nat Rev Microbiol 4:629–636. doi: 10.1038/nrmicro1464 [DOI] [PubMed] [Google Scholar]
- 8. De Rossi E, Aínsa JA, Riccardi G. 2006. Role of mycobacterial efflux transporters in drug resistance: an unresolved question. FEMS Microbiol Rev 30:36–52. doi: 10.1111/j.1574-6976.2005.00002.x [DOI] [PubMed] [Google Scholar]
- 9. McAleese F, Petersen P, Ruzin A, Dunman PM, Murphy E, Projan SJ, Bradford PA. 2005. A novel MATE family efflux pump contributes to the reduced susceptibility of laboratory-derived Staphylococcus aureus mutants to tigecycline . Antimicrob Agents Chemother 49:1865–1871. doi: 10.1128/AAC.49.5.1865-1871.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zimmermann S, Klinger-Strobel M, Bohnert JA, Wendler S, Rödel J, Pletz MW, Löffler B, Tuchscherr L. 2019. Clinically approved drugs inhibit the Staphylococcus aureus multidrug NorA efflux pump and reduce biofilm formation. Front Microbiol 10:2762. doi: 10.3389/fmicb.2019.02762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lopes LAA, Dos Santos Rodrigues JB, Magnani M, de Souza EL, de Siqueira-Júnior JP. 2017. Inhibitory effects of flavonoids on biofilm formation by Staphylococcus aureus that overexpresses efflux protein genes. Microb Pathog 107:193–197. doi: 10.1016/j.micpath.2017.03.033 [DOI] [PubMed] [Google Scholar]
- 12. Yu Y, Zhao Y, He Y, Pang J, Yang Z, Zheng M, Yin RJP, Therapy P. 2022. Inhibition of efflux pump encoding genes and biofilm formation by sub-lethal photodynamic therapy in methicillin susceptible and resistant Staphylococcus aureus. Photodiagnosis Photodyn Ther 39:102900. doi: 10.1016/j.pdpdt.2022.102900 [DOI] [PubMed] [Google Scholar]
- 13. Abd El-Baky RM, Sandle T, John J, Abuo-Rahma G-D, Hetta HF. 2019. A novel mechanism of action of ketoconazole: inhibition of the NorA efflux pump system and biofilm formation in multidrug-resistant Staphylococcus aureus. Infect Drug Resist 12:1703–1718. doi: 10.2147/IDR.S201124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Truong-Bolduc QC, Bolduc GR, Okumura R, Celino B, Bevis J, Liao CH, Hooper DC. 2011. Implication of the NorB efflux pump in the adaptation of Staphylococcus aureus to growth at acid pH and in resistance to moxifloxacin. Antimicrob Agents Chemother 55:3214–3219. doi: 10.1128/AAC.00289-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ince D, Zhang X, Hooper DC. 2003. Activity of and resistance to moxifloxacin in Staphylococcus aureus. Antimicrob Agents Chemother 47:1410–1415. doi: 10.1128/AAC.47.4.1410-1415.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lee MD, Galazzo JL, Staley AL, Lee JC, Warren MS, Fuernkranz H, Chamberland S, Lomovskaya O, Miller GH. 2001. Microbial fermentation-derived inhibitors of efflux-pump-mediated drug resistance. Farmaco 56:81–85. doi: 10.1016/s0014-827x(01)01002-3 [DOI] [PubMed] [Google Scholar]
- 17. Humphries R, Bobenchik AM, Hindler JA, Schuetz AN. 2021. Overview of changes to the clinical and laboratory standards Institute Performance standards for antimicrobial susceptibility testing, M100, 31st edition. J Clin Microbiol 59:e0021321. doi: 10.1128/JCM.00213-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Patel JB. 2017. Performance standards for antimicrobial susceptibility testing. Clinical and laboratory standards institute. [Google Scholar]
- 19. Martini CL, Coronado AZ, Melo MCN, Gobbi CN, Lopez ÚS, de Mattos MC, Amorim TT, Botelho AMN, Vasconcelos ATR, Almeida LGP, Planet PJ, Zingali RB, Figueiredo AMS, Ferreira-Carvalho BT. 2021. Cellular growth arrest and efflux pumps are associated with antibiotic persisters in Streptococcus pyogenes induced in biofilm-like environments. Front Microbiol 12:716628. doi: 10.3389/fmicb.2021.716628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Uribe EG. 1973. ATP synthesis driven by a K+-valinomycin-induced charge imbalance across chloroplast grana membranes. FEBS Lett. 36:143–147. doi: 10.1016/0014-5793(73)80356-4 [DOI] [PubMed] [Google Scholar]
- 21. Jangra M, Kaur M, Nandanwar H. 2019. In-vitro studies on a natural lantibiotic, paenibacillin: a new-generation antibacterial drug candidate to overcome multi-drug resistance. Int J Antimicrob Agents 53:838–843. doi: 10.1016/j.ijantimicag.2019.03.020 [DOI] [PubMed] [Google Scholar]
- 22. Sharma A, Gupta VK, Pathania R. 2019. Efflux pump inhibitors for bacterial pathogens: from bench to bedside. Indian J Med Res 149:129–145. doi: 10.4103/ijmr.IJMR_2079_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Blanchard C, Barnett P, Perlmutter J, Dunman PM. 2014. Identification of Acinetobacter baumannii serum-associated antibiotic efflux pump inhibitors. Antimicrob Agents Chemother 58:6360–6370. doi: 10.1128/AAC.03535-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee J-H, Cho HS, Kim Y, Kim J-A, Banskota S, Cho MH, Lee J. 2013. Indole and 7-benzyloxyindole attenuate the virulence of Staphylococcus aureus. Appl Microbiol Biotechnol 97:4543–4552. doi: 10.1007/s00253-012-4674-z [DOI] [PubMed] [Google Scholar]
- 25. Elmesseri RA, Saleh SE, Elsherif HM, Yahia IS, Aboshanab KM. 2022. Staphyloxanthin as a potential novel target for deciphering promising anti-Staphylococcus aureus agents. Antibiotics (Basel) 11:298. doi: 10.3390/antibiotics11030298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kvist M, Hancock V, Klemm P. 2008. Inactivation of efflux pumps abolishes bacterial biofilm formation. Appl Environ Microbiol 74:7376–7382. doi: 10.1128/AEM.01310-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Brauner A, Fridman O, Gefen O, Balaban NQ. 2016. Distinguishing between resistance, tolerance and persistence to antibiotic treatment. Nat Rev Microbiol 14:320–330. doi: 10.1038/nrmicro.2016.34 [DOI] [PubMed] [Google Scholar]
- 28. Yu H, Wang Y, Wang X, Guo J, Wang H, Zhang H, Du F. 2019. Jatrorrhizine suppresses the antimicrobial resistance of methicillin-resistant Staphylococcus aureus. Exp Ther Med 18:3715–3722. doi: 10.3892/etm.2019.8034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Juárez-Verdayes MA, Parra-Ortega B, Hernández-Rodríguez C, Betanzos-Cabrera G, Rodríguez-Martínez S, Cancino-Diaz ME, Cancino-Diaz JC. 2012. Identification and expression of nor efflux family genes in Staphylococcus epidermidis that act against gatifloxacin. Microb Pathog 52:318–325. doi: 10.1016/j.micpath.2012.03.001 [DOI] [PubMed] [Google Scholar]
- 30. Chinedu E, Arome D, Ameh FS. 2013. A new method for determining acute toxicity in animal models. Toxicol Int 20:224–226. doi: 10.4103/0971-6580.121674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Walum E. 1998. Acute oral toxicity. Environ Health Perspect 106:497–503. doi: 10.1289/ehp.98106497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wertheim HFL, Vos MC, Ott A, van Belkum A, Voss A, Kluytmans JAJW, van Keulen PHJ, Vandenbroucke-Grauls CMJE, Meester MHM, Verbrugh HA. 2004. Risk and outcome of nosocomial Staphylococcus aureus bacteraemia in nasal carriers versus non-carriers. Lancet 364:703–705. doi: 10.1016/S0140-6736(04)16897-9 [DOI] [PubMed] [Google Scholar]
- 33. Pérez VKC, Costa GM da, Guimarães AS, Heinemann MB, Lage AP, Dorneles EMS. 2020. Relationship between virulence factors and antimicrobial resistance in Staphylococcus aureus from bovine mastitis. J Glob Antimicrob Resist 22:792–802. doi: 10.1016/j.jgar.2020.06.010 [DOI] [PubMed] [Google Scholar]
- 34. Tambat R, Jangra M, Mahey N, Chandal N, Kaur M, Chaudhary S, Verma DK, Thakur KG, Raje M, Jachak S, Khatri N, Nandanwar H. 2019. Microbe-derived Indole metabolite demonstrates potent multidrug efflux pump inhibition in Staphylococcus aureus Front Microbiol 10:2153. doi: 10.3389/fmicb.2019.02153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kalia NP, Mahajan P, Mehra R, Nargotra A, Sharma JP, Koul S, Khan IA. 2012. Capsaicin, a novel inhibitor of the NorA efflux pump, reduces the intracellular invasion of Staphylococcus aureus. J Antimicrob Chemother 67:2401–2408. doi: 10.1093/jac/dks232 [DOI] [PubMed] [Google Scholar]
- 36. Poole K. 2007. Efflux pumps as antimicrobial resistance mechanisms. Ann Med 39:162–176. doi: 10.1080/07853890701195262 [DOI] [PubMed] [Google Scholar]
- 37. Li XZ, Nikaido H. 2009. Efflux-mediated drug resistance in bacteria: an update. Drugs 69:1555–1623. doi: 10.2165/11317030-000000000-00000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mahey N, Tambat R, Chandal N, Verma DK, Thakur KG, Nandanwar H. 2021. Repurposing approved drugs as fluoroquinolone potentiators to overcome efflux pump resistance in Staphylococcus aureus. Microbiol Spectr 9:e0095121. doi: 10.1128/Spectrum.00951-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Li X-Z, Plésiat P, Nikaido H. 2015. The challenge of efflux-mediated antibiotic resistance in gram-negative bacteria. Clin Microbiol Rev 28:337–418. doi: 10.1128/CMR.00117-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Neyfakh AA, Bidnenko VE, Chen LB. 1991. Efflux-mediated multidrug resistance in bacillus subtilis: similarities and dissimilarities with the mammalian system. Proc Natl Acad Sci U S A 88:4781–4785. doi: 10.1073/pnas.88.11.4781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Singh S, Kalia NP, Joshi P, Kumar A, Sharma PR, Kumar A, Bharate SB, Khan IA. 2017. Boeravinone B, A novel dual inhibitor of NorA bacterial efflux pump of Staphylococcus aureus and human P-glycoprotein, reduces the biofilm formation and intracellular invasion of bacteria. Front Microbiol 8:1868. doi: 10.3389/fmicb.2017.01868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Craig WA. 1991. The postantibiotic effect. Clinical Microbiology Newsletter 13:121–124. doi: 10.1016/0196-4399(91)90030-Y [DOI] [Google Scholar]
- 43. Fleeman RM, Debevec G, Antonen K, Adams JL, Santos RG, Welmaker GS, Houghten RA, Giulianotti MA, Shaw LN. 2018. Identification of a novel polyamine scaffold with potent efflux pump inhibition activity toward multi-drug resistant bacterial pathogens. Front Microbiol 9:1301. doi: 10.3389/fmicb.2018.01301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Belley A, Neesham-Grenon E, Arhin FF, McKay GA, Parr TR, Moeck G. 2008. Assessment by time-kill methodology of the synergistic effects of oritavancin in combination with other antimicrobial agents against Staphylococcus aureus. Antimicrob Agents Chemother 52:3820–3822. doi: 10.1128/AAC.00361-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Agarwal PK, Sawant D, Sharma S, Kundu B. 2009. New route to the synthesis of the isocryptolepine alkaloid and its related skeletons using a modified pictet–spengler reaction. Eur J Org Chem 2009:292–303. doi: 10.1002/ejoc.200800929 [DOI] [Google Scholar]
- 46. Rohini R, Reddy PM, Shanker K, Kanthaiah K, Ravinder V, Hu A. 2011. Synthesis of mono, bis-2-(2-arylideneaminophenyl) indole azomethines as potential antimicrobial agents. Arch Pharm Res 34:1077–1084. doi: 10.1007/s12272-011-0705-z [DOI] [PubMed] [Google Scholar]
- 47. Hemaiswarya S, Kruthiventi AK, Doble M. 2008. Synergism between natural products and antibiotics against infectious diseases. Phytomedicine 15:639–652. doi: 10.1016/j.phymed.2008.06.008 [DOI] [PubMed] [Google Scholar]
- 48. Abbey TC, Deak E. 2019. What’s new from the CLSI subcommittee on antimicrobial susceptibility testing M100. Clin Microbiol Newsl 41:203–209. doi: 10.1016/j.clinmicnews.2019.11.002 [DOI] [Google Scholar]
- 49. Machado D, Fernandes L, Costa SS, Cannalire R, Manfroni G, Tabarrini O, Couto I, Sabatini S, Viveiros M. 2017. Mode of action of the 2-phenylquinoline efflux inhibitor PQQ4R against Escherichia coli. PeerJ 5:e3168. doi: 10.7717/peerj.3168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Grimsey EM, Fais C, Marshall RL, Ricci V, Ciusa ML, Stone JW, Ivens A, Malloci G, Ruggerone P, Vargiu AV, Piddock LJV, Davies JE. 2020. Chlorpromazine and amitriptyline are substrates and inhibitors of the AcrB multidrug efflux pump. mBio 11:mBio doi: 10.1128/mBio.00465-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wu M, Maier E, Benz R, Hancock RE. 1999. Mechanism of interaction of different classes of cationic antimicrobial peptides with planar bilayers and with the cytoplasmic membrane of Escherichia coli. Biochemistry 38:7235–7242. doi: 10.1021/bi9826299 [DOI] [PubMed] [Google Scholar]
- 52. Morin N, Lanneluc I, Connil N, Cottenceau M, Pons AM, Sablé S. 2011. Mechanism of bactericidal activity of microcin L in Escherichia coli and Salmonella enterica. Antimicrob Agents Chemother 55:997–1007. doi: 10.1128/AAC.01217-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Cheng M, Huang JX, Ramu S, Butler MS, Cooper MA. 2014. Ramoplanin at bactericidal concentrations induces bacterial membrane depolarization in Staphylococcus aureus. Antimicrob Agents Chemother 58:6819–6827. doi: 10.1128/AAC.00061-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. French S, Farha M, Ellis MJ, Sameer Z, Côté J-P, Cotroneo N, Lister T, Rubio A, Brown ED. 2020. Potentiation of antibiotics against gram-negative bacteria by polymyxin B analogue SPR741 from unique perturbation of the outer membrane. ACS Infect Dis 6:1405–1412. doi: 10.1021/acsinfecdis.9b00159 [DOI] [PubMed] [Google Scholar]
- 55. Tambat R, Mahey N, Chandal N, Verma DK, Jangra M, Thakur KG, Nandanwar H. 2022. A microbe-derived efflux pump inhibitor of the resistance-nodulation-cell division protein restores antibiotic susceptibility in Escherichia coli and Pseudomonas aeruginosa ACS Infect Dis 8:255–270. doi: 10.1021/acsinfecdis.1c00281 [DOI] [PubMed] [Google Scholar]
- 56. Singh S, Kalia NP, Joshi P, Kumar A, Sharma PR, Kumar A, Bharate SB, Khan IA. 2017. Boeravinone B, A novel dual inhibitor of NorA bacterial efflux pump of Staphylococcus aureus and human P-glycoprotein, reduces the biofilm formation and intracellular invasion of bacteria. Front Microbiol 8:1868. doi: 10.3389/fmicb.2017.01868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Trafny EA, Lewandowski R, Zawistowska-Marciniak I, Stępińska M. 2013. Use of MTT assay for determination of the biofilm formation capacity of microorganisms in metalworking fluids. World J Microbiol Biotechnol 29:1635–1643. doi: 10.1007/s11274-013-1326-0 [DOI] [PubMed] [Google Scholar]
- 58. Yang H, Wang M, Yu J, Wei H. 2015. Aspartate inhibits Staphylococcus aureus biofilm formation. FEMS Microbiol Lett 362:fnv025. doi: 10.1093/femsle/fnv025 [DOI] [PubMed] [Google Scholar]
- 59. Leejae S, Hasap L, Voravuthikunchai SP. 2013. Inhibition of staphyloxanthin biosynthesis in Staphylococcus aureus by rhodomyrtone, a novel antibiotic candidate. J Med Microbiol 62:421–428. doi: 10.1099/jmm.0.047316-0 [DOI] [PubMed] [Google Scholar]
- 60. Rázquin-Olazarán I, Shahrour H, Martínez-de-Tejada G. 2020. A synthetic peptide sensitizes multi-drug resistant Pseudomonas aeruginosa to antibiotics for more than two hours and permeabilizes its envelope for twenty hours. J Biomed Sci 27:85. doi: 10.1186/s12929-020-00678-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Drugeon HB, Juvin ME, Bryskier A. 1999. Relative potential for selection of fluoroquinolone-resistant Streptococcus pneumoniae strains by levofloxacin: comparison with ciprofloxacin, sparfloxacin and ofloxacin. J Antimicrob Chemother 43 Suppl C:55–59. doi: 10.1093/jac/43.suppl_3.55 [DOI] [PubMed] [Google Scholar]
- 62. Tambat R, Jangra M, Mahey N, Chandal N, Kaur M, Chaudhary S, Verma DK, Thakur KG, Raje M, Jachak S, Khatri N, Nandanwar H. 2019. Microbe-derived indole metabolite demonstrates potent multidrug efflux pump inhibition in Staphylococcus aureus. Front Microbiol 10:2153. doi: 10.3389/fmicb.2019.02153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lechner S, Lewis K, Bertram R. 2012. Staphylococcus aureus persisters tolerant to bactericidal antibiotics. Microb Physiol 22:235–244. doi: 10.1159/000342449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Levin-Arama M, Abraham L, Waner T, Harmelin A, Steinberg DM, Lahav T, Harlev M. 2016. Subcutaneous compared with intraperitoneal KetamineXylazine for anesthesia of mice. J Am Assoc Lab Anim Sci 55:794–800. [PMC free article] [PubMed] [Google Scholar]
- 65. Ling LL, Schneider T, Peoples AJ, Spoering AL, Engels I, Conlon BP, Mueller A, Schäberle TF, Hughes DE, Epstein S, Jones M, Lazarides L, Steadman VA, Cohen DR, Felix CR, Fetterman KA, Millett WP, Nitti AG, Zullo AM, Chen C, Lewis K. 2015. A new antibiotic kills pathogens without detectable resistance. Nature 520:455–459. doi: 10.1038/nature14303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zhou L, She P, Tan F, Li S, Zeng X, Chen L, Luo Z, Wu Y. 2020. Repurposing antispasmodic agent otilonium bromide for treatment of Staphylococcus aureus infections. Front Microbiol 11:1720. doi: 10.3389/fmicb.2020.01720 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S.1 to S.10 and Fig. S.1 to S.5.