ABSTRACT
Klebsiella pneumoniae is a well-known human nosocomial pathogen with an arsenal of virulence factors, including capsular polysaccharides (CPS), fimbriae, flagella, and lipopolysaccharides (LPS). Our previous study found that alcohol acted as an essential virulence factor for high-alcohol-producing K. pneumoniae (HiAlc Kpn). Integration host factor (IHF) is a nucleoid-associated protein that functions as a global virulence regulator in Escherichia coli. However, the regulatory role of IHF in K. pneumoniae remains unknown. In the present study, we found that deletion of ihfA or ihfB resulted in a slight defect in bacterial growth, a severe absence of biofilm formation and cytotoxicity, and a significant reduction in alcohol production. RNA sequencing differential gene expression analysis showed that compared with the wild-type control, the expression of many virulence factor genes was downregulated in ΔihfA and ΔihfB strains, such as those related to CPS (rcsA, galF, wzi, and iscR), LPS (rfbABCD), type I and type III fimbriae (fim and mrk operon), cellulose (bcs operon), iron transporter (feoABC, fhuA, fhuF, tonB, exbB, and exbD), quorum sensing (lsr operon and sdiA), type II secretion system (T2SS) and type VI secretion system (T6SS) (tssG, hcp, and gspE). Of these virulence factors, CPS, LPS, fimbriae, and cellulose are involved in biofilm formation. In addition, IHF could affect the alcohol production by regulating genes related to glucose intake (ptsG), pyruvate formate-lyase, alcohol dehydrogenase, and the tricarboxylic acid (TCA) cycle. Our data provided new insights into the importance of IHF in regulating the virulence of HiAlc Kpn.
IMPORTANCE
Klebsiella pneumoniae is a well-known human nosocomial pathogen that causes various infectious diseases, including urinary tract infections, hospital-acquired pneumonia, bacteremia, and liver abscesses. Our previous studies demonstrated that HiAlc Kpn mediated the development of nonalcoholic fatty liver disease by producing excess endogenous alcohol in vivo. However, the regulators regulating the expression of genes related to metabolism, biofilm formation, and virulence of HiAlc Kpn remain unclear. In this study, the regulator IHF was found to positively regulate biofilm formation and many virulence factors including CPS, LPS, type I and type III fimbriae, cellulose, iron transporter, AI-2 quorum sensing, T2SS, and T6SS in HiAlc Kpn. Furthermore, IHF positively regulated alcohol production in HiAlc Kpn. Our results suggested that IHF could be a potential drug target for treating various infectious diseases caused by K. pneumoniae. Hence, the regulation of different virulence factors by IHF in K. pneumoniae requires further investigation.
KEYWORDS: HiAlc Kpn , IHF, biofilm, virulence, regulate
INTRODUCTION
Klebsiella pneumoniae is a Gram-negative human nosocomial pathogen responsible for various infections, including pneumonia, bacteremia, and liver abscesses (1 – 3). The bacterium comprises a variety of virulence factors, including capsule, lipopolysaccharides (LPS), type I and type III fimbriae, type VI secretion system (T6SS), quorum-sensing (QS) system, and siderophores, many of which are involved in biofilm formation (1, 4). Previously, we found that high-alcohol-producing K. pneumoniae (HiAlc Kpn), which was present in the intestines of 60% of patients with nonalcoholic fatty liver disease (NAFLD), was a primary causative agent of NAFLD (2). This indicated that alcohol production was also an important virulence factor for K. pneumoniae.
Integration host factor (IHF) belongs to nucleoid-associated proteins and was first identified as a λ-phage accessory protein involved in the site-specific recombination process (5, 6). IHF is a heterodimeric protein encoded by ihfA and ihfB which binds to sequence-specific DNA and mediates its bending, thereby facilitating the assembly of nucleoprotein structures and regulating DNA replication, recombination, repair, and other processes (7). As a global regulator, IHF plays an essential role in bacterial response to environmental stresses and pathogenesis by controlling the expression of numerous genes (8, 9). In Escherichia coli, IHF contributes to the formation of capsule and type I fimbriae and helps stabilize pathogenicity islands that play an important role in bacterial colonization (10 – 12). Moreover, IHF can promote the development of E. coli persisters by decreasing energy-generating components, resulting in higher levels of antibiotic tolerance (13). In Salmonella enterica, ihf deletion mediates a deficiency in biofilm formation by reducing curli fimbriae, cellulose, and pellicle production (14). In Vibrio cholerae, IHF positively regulates two main virulence genes, tcpA and ctx, which encode for the synthesis of toxin co-regulated fimbriae and cholera toxin (15). However, the role of IHF in K.pneumoniae is still unknown.
In this study, we found that deletion of ihfA or ihfB in HiAlc Kpn severely impaired biofilm formation and virulence and significantly reduced alcohol production. IHF positively regulated the expression of a number of virulence factors, such as capsular polysaccharides (CPS), LPS, cellulose, iron transporter, QS, type II secretion system (T2SS), T6SS, and type I and type III fimbriae. Downregulated expression of these virulence factors can explain the impaired biofilm formation in HiAlc Kpn. Furthermore, IHF affected the alcohol production of HiAlc Kpn by regulating genes related to glucose intake, the tricarboxylic acid (TCA) cycle, and fermentation. By elucidating the global regulatory role of IHF in bacterial virulence, we demonstrated that IHF could be a potential target for treating various diseases caused by K. pneumoniae.
MATERIALS AND METHODS
Bacterial strains, plasmids, and primers
The wild-type strain used in this study was high-alcohol-producing Klebsiella pneumoniae (W14), obtained from a patient with auto-brewery syndrome combined with NAFLD (2). Methods by Link et al. (16) and Pan et al. (17) were applied to obtain the gene deletion and complementation strains. All the strains used for culture and phenotypic analysis were grown in Luria-Bertani (LB) broth (5-g/L yeast extract, 10-g/L sodium chloride, and 10-g/L tryptone) in a shaking incubator (180–200 rpm) at 37°C. All the bacterial strains, plasmids, and primers used in this study are listed in Table S1.
Bacterial growth curves
Growth curves of W14, ΔihfA, ΔihfB, ΔihfA/ihfA, and ΔihfB/ihfB strains were derived after subculturing in LB broth overnight. Briefly, the overnight cultures of strains were diluted 1:100 into 20 mL of fresh LB broth and grown by shaking at 180–200 rpm at 37°C. Optical density (OD)600 measurements were performed per hour to determine the cell density. Three independent cultures were used for each assay.
Measurement of alcohol concentration
LB cultured strains were incubated overnight, and the supernatant was taken by centrifugation at 12,000 rpm to measure alcohol concentration. The level of alcohol in all strains was determined by headspace gas chromatography (Agilent 6850) with flame ionization detection (Headspace) in both aerobic and anaerobic conditions.
Biofilm formation analysis
W14, ΔihfA, ΔihfB, ΔihfA/ihfA, and ΔihfB/ihfB strains were grown overnight, diluted to OD600 of 0.025 in LB broth, and incubated 200 µL in each well of a 96-well plate for 24 h. Subsequently, the liquid was poured from the medium, followed by washing with phosphate-buffered saline (PBS) and drying at 65°C for 15 min. Each well was stained with 1% crystal violet and washed twice with 200 µL of bleaching solution (malcohol:glacial acetic acid:H2O (vol/vol/vol) =4:1:5) per well. The medium was incubated at room temperature with gentle shaking. Finally, the biofilm quantification with crystal violet was measured at a wavelength of 590 nm.
Observation of biofilm production by CLSM
To better analyze the effect of IHF on biofilm formation, confocal laser scanning microscope (CLSM) was used to analyze biofilm production. The cultured strains were incubated overnight and diluted at a ratio of 1:100 with fresh LB. A total of 1 mL of the diluted solution was added to a 15-mm glass bottom cell culture dish and incubated at 37°C for 48 h to form biofilm at the bottom. The culture dish was washed thrice with PBS buffer and fixed with 2.5% glutaraldehyde for 1.5 h at 4°C. After washing twice with PBS buffer, 400 µL of 50-µg/mL fluorescein isothiocyanate-conjugated concanavalin A (FITC-conA) solution was added to stain the extracellular polysaccharides at 4°C for 1 h. After washing with PBS again, the bacteria in the biofilm were observed by fluorescent staining with 10-µg/mL propidium iodide (PI) solution at 4°C for 15 min. The resulting solution was then poured and dried at room temperature. A CLSM system was used to digitize all confocal images. Fluorescent intensity, biofilm production, bacterial density, and other analyses were analyzed using ImageJ.
Cytotoxicity assay
The cytotoxicity of K. pneumoniae was measured by a cell-lifting assay with subtle adjustments (18). A549 cells (1 × 105) were inoculated into each well in a 24-well plate and cultured in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal calf serum with 5% CO2 at 37°C for 24 h. A549 cells were infected with K. pneumoniae at a multiplicity of infection of 100 for 10 h. After that, the medium in each well was aspirated, and the remaining cells were washed with PBS twice and stained with 0.025% crystal violet (200 µL) at 37°C for 15 min. The crystal violet was discarded, and the plate was washed with 1-mL phosphate-buffered saline twice. Next, 200-µL 95% alcohol was added into each well and incubated with gentle shaking for 30 min at room temperature to dissolve the dye. Alcohol solution was used to measure absorbance at a wavelength of 490 nm.
RNA sequencing and differential expression analysis
RNA sequencing was performed using Gene Denovo Biotechnology Co., Ltd (Guangzhou, China). Briefly, total RNA was extracted from late-exponentially growing period of W14, ΔihfA, and ΔihfB using Trizol reagent kit (Invitrogen, Carlsbad, CA, USA), and genomic DNA was removed using recombinant DNase I. After total RNA was extracted, prokaryotic mRNA was enriched by removing rRNA by Ribo-Zero Magnetic Kit (Epicentre, Madison, WI, USA). Then, the enriched mRNA was reverse-transcribed into cDNA using NEBNext Ultra RNA Library Prep Kit for Illumina (NEB #7530; New England Biolabs, Ipswich, MA, USA). The resulting cDNA library was sequenced using Illumina Novaseq6000 by Gene Denovo Biotechnology Co. RNA differential expression analysis between two groups was performed by DESeq2 software (19). Kyoto Encyclopedia of Genes and Genomes (KEGG) and Gene Ontology (GO) enrichment analysis were conducted using KOBAS version 2.06 and Goatools. Bioinformatics analysis was performed using Omicsmart, a real-time interactive online platform for data analysis (http://www.omicsmart.com).
RNA extraction, reverse transcription, and qRT-PCR
The bacterial suspension to be tested was first centrifuged at 12,000 rpm, and the obtained bacterial precipitate was resuspended with 1-mL trizol. Then, 0.2-mL trichloromethane was added, and the solution was vortexed for 15 s and centrifuged at 12,000 rpm for 10 min. Equal amounts of the aqueous phase were mixed lightly with isopropanol, and total RNA was extracted using the RNeasy Mini kit (Tiangen Biotech, Beijing, China). Approximately 0.5–1.0 μg of RNA was used for reverse transcription, and cDNA was synthesized by PrimeScript Reverse Transcriptase (TaKaRa, Dalian, China). The quantitative real-time PCR (qRT-PCR) experiment was performed using 20 µL of a mix including the SYBR Premix Ex TaqTM II (TaKaRa), cDNA templates, and specific forward and reverse primers. The experiment was performed using the CFX Connect Real-Time system (Bio-Rad, USA). rpoB, a gene encoding the DNA-directed RNA polymerase subunit β, was used as an internal reference.
Statistical analysis
Each assay used three biological replicates to ensure accuracy. Data were statistically analyzed by GraphPad Prism (version 5.0, USA) and expressed as mean ± standard deviations. Statistically significant differences were determined by Student’s t-test and *P < 0.05, **P < 0.01, and ***P < 0.001 all reflected statistical significance.
RESULTS
IHF slightly influenced in vitro growth in HiAlc Kpn
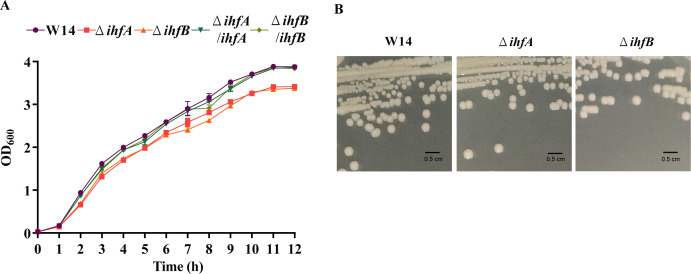
IHF typically comprises two subunits, IHFɑ and IHFβ, encoded by ihfA and ihfB, respectively (20, 21). To characterize the function of IHF in the pathogenicity of HiAlc Kpn, we generated two mutants: ΔihfA and ΔihfB. Compared with wild-type strain W14, the growth curves of ΔihfA and ΔihfB in LB broth demonstrated a slightly slower growth, and compensation of ihfA and ihfB restored bacterial growth (Fig. 1A). However, no difference in growth was observed among wild-type strain W14, ΔihfA, and ΔihfB mutant strains on the solid plates (Fig. 1B). These results indicated that the deletion of IHF slightly affected the growth of HiAlc Kpn.
FIG 1.
Deletion of ihfA or ihfB slightly influenced the growth of HiAlc Kpn in LB broth. (A) The growth curves of W14, ΔihfA, ΔihfB, ΔihfA/ihfA, and ΔihfB/ihfB were measured by optical density (OD600) per hour over a period of 12 h. (B) Typical colony images of W14, ΔihfA, and ΔihfB.
IHF affected biofilm formation and cytotoxicity in HiAlc Kpn
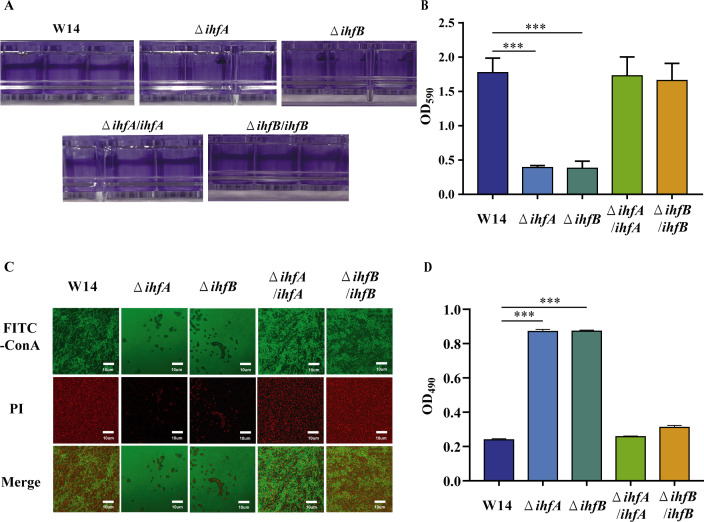
To examine whether IHF affected biofilm formation in HiAlc Kpn, we performed Crystal violet (CV) staining and CLSM. Deletion of ihfA or ihfB significantly impaired the ability of biofilm formation in HiAlc Kpn. Compensation with ihfA or ihfB restored biofilm formation to the level of wild-type strain W14 (Fig. 2A through C). Meanwhile, we tested bacterial cytotoxicity by measuring their ability to detach A549 cells from the culture plates. Compared to wild-type strain W14, ihfA and ihfB deletion exhibited decreased cytotoxicity. Complementation of ihfA or ihfB restored the bacterial cytotoxicity (Fig. 2D). Our results demonstrated that IHF affected the biofilm formation and bacterial virulence, similar to the effects observed in Salmonella enterica and E. coli (10, 21, 22).
FIG 2.
Deletion of ihfA or ihfB decreased the biofilm formation and virulence in HiAlc Kpn. (A) Images of biofilm formed by W14, ΔihfA, ΔihfB, ΔihfA/ihfA, and ΔihfB/ihfB were stained with 1% CV and adhered on plastic centrifuge tubes. (B) Biofilm was stained with 1% CV. The extracted color was dissolved with 33% bleaching solution and measured at OD590. ***P < 0.001, compared to wild-type strain W14 by Student’s t-test. (C) CLSM of biofilm formation in W14, ΔihfA, ΔihfB, ΔihfA/ihfA, and ΔihfB/ihfB was observed after incubation for 48 h. (D) Cytotoxicity of W14, ΔihfA, ΔihfB, ΔihfA/ihfA, and ΔihfB/ihfB. A549 cells were infected with the indicated strains at a MOI of 100. After 10 h of infection and 15-min crystal violet staining, cells attached to the plate were measured at OD490. ***P < 0.001, compared to wild-type strain W14 by Student’s t-test.
IHF contributed to alcohol production in HiAlc Kpn
Our previous study found that HiAlc Kpn could produce large amounts of endogenous alcohol, which led to NAFLD, indicating that alcohol might be a critical pathogenic virulence factor in HiAlc Kpn (2). As shown in Fig. 3, alcohol production significantly decreased in ΔihfA and ΔihfB under both aerobic and anaerobic conditions, while compensation with ihfA and ihfB restored alcohol production. These results showed that IHF was required for alcohol production in HiAlc Kpn.
Fig 3.
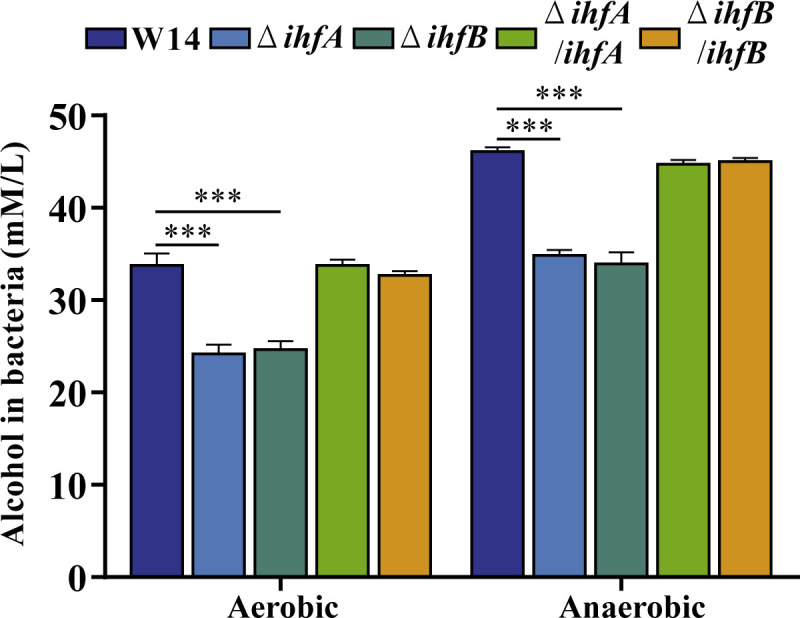
Deletion of ihfA or ihfB decreased the alcohol production in HiAlc Kpn. The alcohol-producing ability of W14, ΔihfA, ΔihfB, ΔihfA/ihfA, and ΔihfB/ihfB was measured in aerobic and anaerobic conditions. ***P < 0.001, compared to wild-type strain W14 by Student’s t-test.
Profiling gene expression of ΔihfA and ΔihfB
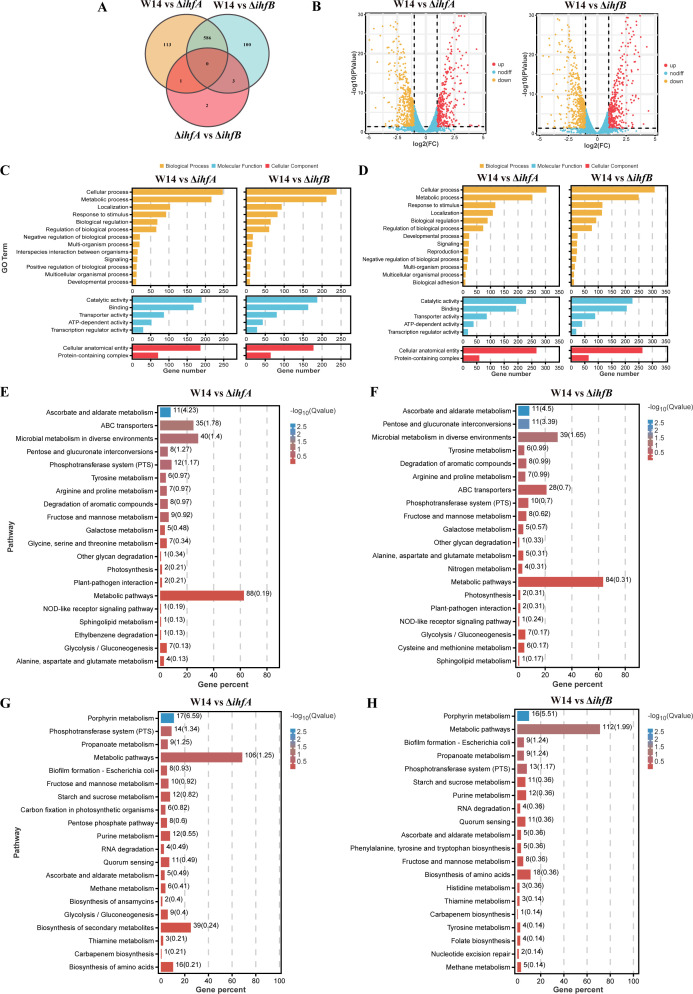
To understand the mechanism of IHF-mediated regulation of bacterial biofilm and virulence, we examined the gene expression profiles of the late-exponentially growing W14, ΔihfA, and ΔihfB. Differentially expressed genes (DEGs) with P < 0.05 and |log2 (fold change)| of >1 were identified by comparing the RNA-sequencing (RNA-seq) data with that of wild-type strain W14. Compared with wild-type strain W14, the number of DEGs in ΔihfA and ΔihfB strains was 700 (320 upregulated and 380 downregulated genes) and 689 (315 upregulated and 374 downregulated genes), respectively. The number of DEGs between ΔihfA and ΔihfB was only six (five upregulated and one downregulated genes), which suggested that ihfA and ihfB might play similar regulatory roles in HiAlc Kpn (Fig. 4A and B). The DEGs of ΔihfA and ΔihfB strains were analyzed using GO enrichment. A total of 20 significantly enriched GO terms from three categories (biological process, molecular function, and cellular component) are shown in Fig. 4C and D. Most enriched DEGs among GO terms corresponded to cellular process, metabolic process, catalytic activity, binding, and cellular anatomical entity. Based on the KEGG pathway analysis, the upregulated genes were enriched in metabolic pathways, microbial metabolism in diverse environments, and ABC transporters pathways (Fig. 4E and F). Notably, the downregulated DEGs in ΔihfA and ΔihfB strains relative to wild-type strain W14 were enriched in the biofilm information, quorum-sensing pathways, suggesting that IHF can play an important role in biofilms and virulence of K. pneumoniae (Fig. 4G and H).
FIG 4.
Profiling gene expression of ΔihfA and ΔihfB. Analysis of total RNA sequencing. (A) The Venn diagram shows the overlapped DEGs numbers of W14 and ΔihfA, W14 and ΔihfB, ΔihfA and ΔihfB. (B) Volcano plot of DEGs between W14 and ΔihfA or ΔihfB. GO enrichment analysis of upregulated (C) and downregulated (D) DEGs in ΔihfA and ΔihfB. KEGG pathway enrichment analysis of upregulated DEGs in ΔihfA (E) and ΔihfB (F). KEGG pathway enrichment analysis of downregulated DEGs in ΔihfA (G) and ΔihfB (H).
IHF-regulated genes related to bacterial biofilm and virulence
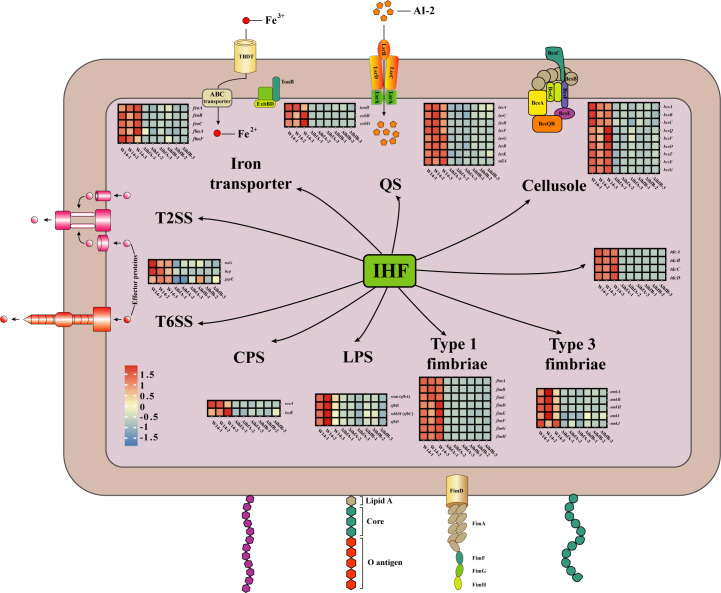
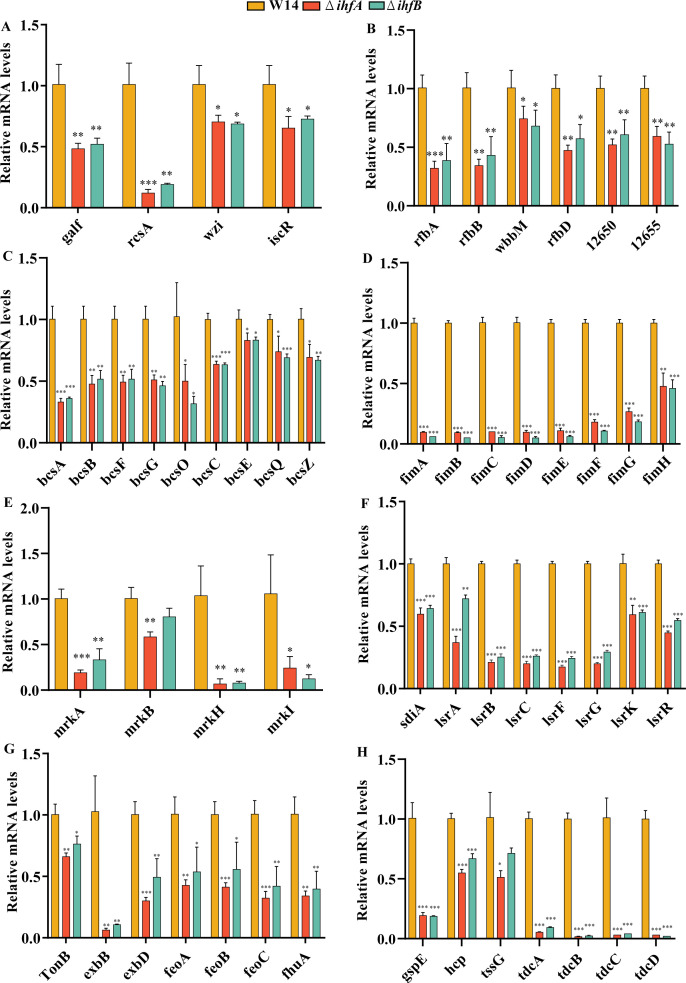
CPS are closely related to biofilm formation and bacterial virulence (23). RcsA plays an important role in Rcs phosphorelay system, which regulates CPS and biofilm formation in K. pneumoniae (24). When RcsA is inhibited, CPS expression is significantly decreased (25). The genes galF and wzi are involved in capsule formation, coding for UDP-glucose pyrophosphorylase and an outer membrane protein, respectively (26). RcsA participates in the CRP-cAMP-mediated regulation of galF transcription and influences CPS biosynthesis in K. pneumoniae. IscR, an Fe-S cluster-containing transcription factor, plays a key role in CPS synthesis and iron acquisition in K. pneumoniae (4, 27). RNA-seq and qRT-PCR results indicated that the expression of rcsA, galF, wzi, and iscR was significantly lower in ΔihfA and ΔihfB and showed that IHF could positively regulate the synthesis of CPS in HiAlc Kpn (Fig. 5 and 6A).
FIG 5.
Regulatory network of IHF in HiAlc Kpn. The regulatory network of IHF in HiAlc Kpn. IHF positively regulates the synthesis of CPS, LPS, cellulose, type I and type III fimbriae, T2SS, T6SS, iron transporter and QS by IHF. Gene expression measured by RNA sequencing is shown by heat map.
FIG 6.
Deletion of ihfA or ihfB decreased the expressions of biofilm and virulence-related genes. The relative mRNA levels of W14, ΔihfA, and ΔihfB in CPS (A), LPS (B), cellulose (C), type I fimbriae (D), type III fimbriae (E), QS system (F), iron acquisition (G), and other virulence-related genes (H) were calculated by qRT-PCR. *P < 0.05, **P < 0.01, ***P < 0.001, compared to wild-type strain W14 by Student’s t-test.
LPS, cellulose, type I fimbriae, and type III fimbriae are also involved in K. pneumoniae biofilm formation. wzm (rfbA) and wbbM (rfbC) belong to the rfb gene cluster, encoding enzymes for the biosynthesis of O-antigen in K. pneumoniae (4, 28). Cellulose is an important component of bacterial biofilm. Bacterial cellulose synthesis and translocation are regulated by the inner membrane-associated bcsABZC and bcsEFG operons in E. coli and Salmonella (29, 30). bcsA, bcsB, and bcsC encode a cellulose synthase enzyme, c-di-GMP-binding protein, and cellulose oxidoreductase enzyme, respectively (30). Type I and type III fimbriae are important virulence factors affecting biofilm formation, cell attachment, and pathogenicity in K. pneumoniae, which are encoded by fim and mrk operons, respectively (31, 32). RNA-seq differential gene expression analysis and qRT-PCR showed that the expression of genes related to LPS (rfbABCD) (Fig. 6B), cellulose (bcsABCZ, bcsEFG, bcsO, and bcsQ) (Fig. 6C), and type I and type III fimbriae (fimABCDEFGH, mrkAB, and mrkHIJ) (Fig. 6D and E) was significantly downregulated compared to that in wild-type control (Fig. 5).
LuxS/AI-2 QS system can detect cell density and affect the virulence of K. pneumoniae (33, 34). AI-2 is produced by the enzyme LuxS and consumed intracellularly by the Lsr system, which consists of the ATP-binding cassette transporter LsrACDB, AI-2 modifier protein LsrFG, AI-2 uptake repressor LsrR, and AI-2 phosphorylation kinase LsrK (35). SdiA, a LuxR-type regulator, negatively regulates fimbriae expression, biofilm synthesis, and AI-2 production in K. pneumoniae (34). In this study, the expression of lsrACB, lsrFG, lsrRK, and sdiA was significantly downregulated in ihfA and ihfB deletion strains (Fig. 5 and 6F).
Iron acquisition is vital for bacterial survival and growth. Its absorption depends on a specific outer membrane transporter, in which TonB-dependent transporters (TBDTs) can efficiently bind to the iron-containing substrates. After binding to the substrates, the conformation of TBDTs changes, followed by interaction with TonB via the N-terminal conserved region to obtain energy to complete the transport (36). After passing through the outer membrane, the iron-containing substrate binds to a periplasmic cavity-binding protein. It then enters the cytoplasm via the Feo transport system (including FeoABC) on the inner membrane. The Feo system is crucial for the colonization and virulence of pathogens (37). FhuA is a multifunctional protein acting as a transmembrane receptor in the outer membrane that recognizes iron carriers and enters the periplasm in a TonB-dependent manner (38). FhuF is a member of the iron carrier reductase (FSR) subfamily (39). It participates in the removal of iron carriers (40). Our results showed that iron transport-related gene expression (feoABC, fhuA, fhuF, tonB, exbB, and exbD) was significantly decreased in ihfA and ihfB deletion strains (Fig. 5 and 6G).
Secretion systems, from type I secretion system to T6SS, are regulated by IHF in Dickeya zeae (41). In HiAlc Kpn, we found that the expression of genes related to T2SS and T6SS (tssG, hcp, and gspE) was significantly decreased in ΔihfA and ΔihfB mutant strains (Fig. 5 and 6H). Type II secretion system is a sophisticated multiprotein machinery essential for bacterial pathogenicity (42, 43). GspE is an ATPase belonging to the type II/type IV secretion family. The energy for T2SS is generated by GspE-mediated ATP hydrolysis to transport folded proteins outside the cell through the GspD outer membrane channel (44). T6SS is a contact-dependent protein secretion apparatus involved in multiple processes related to bacterial virulence. Bacteria use T6SS to compete with the host and to coordinate the invasion process (45 – 47). T6SS needle consists of an Hcp hexamer ring, and TssG is a protein that helps to localize the needle in the donor cell (48).
The operon tdcABCDEFG, which consists of the regulatory gene tdcA and the structural gene tdcBCDEFG, is involved in the transport and metabolism of L-threonine and L-serine during anaerobic growth in E. coli (49). Research indicated that Tdc affects the intestinal colonization of K. pneumoniae (50). Our data showed that the expression of tdcABCD was significantly downregulated in both ΔihfA and ΔihfB (Fig. 5 and 6H).
IHF-regulated genes related to the TCA cycle and fermentation
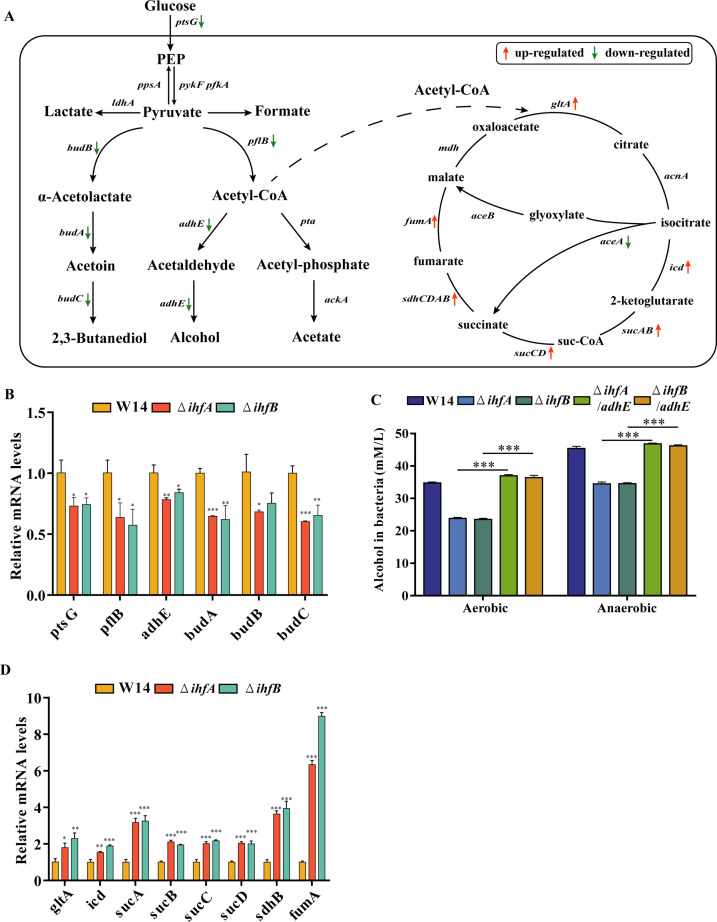
HiAlc Kpn can mediate the induction of NAFLD by producing excess endogenous alcohol using glucose as the main carbon source. EIICBglc, a major glucose transporter, is encoded by ptsG. Glucose that enters the cells is converted to pyruvate via glycolysis (51). The generation of acetyl-coenzyme A (acetyl-CoA) from pyruvate is mediated by pyruvate dehydrogenase complex and pyruvate formate-lyase (PFL) in two main ways. Acetyl-CoA can enter the TCA cycle or be catalyzed by alcohol dehydrogenase (adhE) to convert acetaldehyde to produce alcohol (52 – 55) (Fig. 7A). In K. pneumoniae, pyruvate formate-lyase (pflB) and adhE encodes PFL and the main alcohol dehydrogenase, respectively. qRT-PCR results showed that ptsG, pflB, and adhE expression decreased significantly in ΔihfA and ΔihfB under high glucose condition (Fig. 7B). Overexpression of adhE significantly increased alcohol production in the ΔihfA and ΔihfB mutant strains (Fig. 7C). Meanwhile, the expression of genes related to the TCA cycle (gltA, icd, sucABCD, sdhB, and fumA) increased significantly (Fig. 7D). These results indicated that IHF was required for glucose intake and regulated the expression of TCA and fermentation-related genes, thereby affecting the production of alcohol.
FIG 7.
IHF-regulated genes related to the TCA cycle and fermentation. (A) Metabolic pathways and related genes of HiAlc Kpn. The picture shows up- and downregulated genes in ΔihfA and ΔihfB compared with wild-type strain W14. (B) The relative mRNA levels of fermentation-related genes in W14, ΔihfA, and ΔihfB were calculated by qRT-PCR. (C) The alcohol-producing ability of W14, ΔihfA, ΔihfB, ΔihfA/adhE, and ΔihfB/adhE was measured in aerobic and anaerobic conditions. (D) The relative mRNA levels of TCA cycle-related genes in W14, ΔihfA, and ΔihfB were calculated by qRT-PCR. *P < 0.05; **P < 0.01, ***P < 0.001, compared to wild-type strain W14 by Student’s t-test.
DISCUSSION
Our previous study found that HiAlc Kpn is associated with nearly 60% of individuals with NAFLD in a Chinese cohort (2). The large amount of endogenous alcohol produced by intestinal bacteria can lead to hepatic steatosis, mitochondrial dysfunction, and inflammatory response, and the long-term accumulation of these pathological changes can predispose an individual to the development of NAFLD (2). Furthermore, the overgrowth of LPS-producing pathogenic strains with high endotoxin activity including K. pneumoniae is related to the onset of NAFLD (56, 57). LPS acts as a pathogen-associated molecular pattern that binds to toll-like recepter 4 and triggers an essential inflammatory cascade during the progression of NAFLD (57, 58). Therefore, both high-alcohol and high-endotoxin activities of bacteria are important for the development of NAFLD. This study demonstrated that IHF can positively regulate alcohol production and LPS in HiAlc Kpn. Therefore, IHF may be a potential drug target for treating NAFLD caused by HiAlc Kpn.
As a regulator, IHF binds to DNA by recognizing consensus sequences 5′‐WATCAANNNNTTR‐3′ (where W = A or T, N = any base, and R = A or G) in E. coli (59, 60). The consensus sequence was used to predict potential sites for binding IHF to DNA in HiAlc Kpn W14 using FIMO version 5.5.1 (61). Among the genes of HiAlc Kpn regulated by IHF at the transcriptional level, IHF-binding sites were found in the promoter regions of genes fimA, mrkH, and tdcA. However, the precise IHF-binding sites in K. pneumoniae still need to be further identified, which will deepen the understanding of the regulatory mechanism of IHF.
The function of IHF has been explored in several bacterial species. In E.coli, the expression of type I pili, group 2 capsule, and tdc operon and the colonization of the urinary tract can be affected by IHF (10, 12, 62, 63). In S. enterica, IHF positively regulated the biofilm formation by affecting the curli fimbriae expression, cellulose production, and pellicle formation (10, 12). In Vibrio fluvialis, IHF bound to the promoter of T6SS major cluster to regulate the expression and secretion of Hcp (64). Consistent with the previous research, our results indicated that in K. pneumoniae, IHF positively regulated genes related to cellulose synthesis (bcs operon), CPS (rcsA, galF, wzi, and iscR), type I and type III fimbriae (fim and mrk operons), T2SS and T6SS (gspE, hcp, and tssG), LPS (rfbABCD), and tdc operon. These results indicated that the regulatory function of IHF is relatively conserved among different strains.
It is worth noting that IHF positively regulates lsrACDBFG operon and negatively regulates lsrRK in Aggregatibacter actinomycetemcomitans, which differs from the findings presented here (65). Our RNA-seq differential gene expression analysis and qRT-PCR demonstrated that both lsrABCFG and lsrRK were positively regulated by IHF (Fig. 5 and 6F). The function of lsrRK operon and its regulation by IHF are worth investigating. Furthermore, we found that IHF was a positive regulator of iron transporters including TBDTs, Feo transport system, and ExbB-ExbD-TonB protein complex, which has not yet been reported. In summary, IHF acts as a global regulator affecting a wide range of virulence factors. There may still be unknown virulence factors regulated by IHF, and further mechanisms should be explored.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank Editage (www.editage.cn) for English language editing.
This work was supported by grants from the National Natural Science Foundation for Key Programs of China Grants (82130065) and National Natural Science Foundation of China (32200159 and 82272352).
J.Y., Z.F., and T.F. designed the experiments. Z.F., T.F., Z.L., B.D., X.C., R.Z., Y.F., and H.Z. performed the experiments. The other authors analyzed the results. Z.F. and Z.L. wrote the manuscript. J.Y. and Z.F. revised the manuscript. All authors reviewed the manuscript.
Contributor Information
Jing Yuan, Email: yuanjing6216@163.com.
Fei Chen, Beijing Institute of Genomics, Chinese Academy of Sciences, Beijing, China .
DATA AVAILABILITY
The raw sequence data of RNA sequencing in this study have been deposited in the Genome Sequence Archive in National Genomics Data Center, Beijing Institute of Genomics (China National Center for Bioinformation), Chinese Academy of Sciences, under accession number CRA010192.
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/spectrum.01170-23.
Bacterial strains, plasmids, and primers used in this study.
An accounting of the reviewer comments and feedback.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Wang G, Zhao G, Chao X, Xie L, Wang H. 2020. The characteristic of virulence, biofilm and antibiotic resistance of Klebsiella pneumoniae. Int J Environ Res Public Health 17:6278. doi: 10.3390/ijerph17176278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yuan J, Chen C, Cui J, Lu J, Yan C, Wei X, Zhao X, Li N, Li S, Xue G, Cheng W, Li B, Li H, Lin W, Tian C, Zhao J, Han J, An D, Zhang Q, Wei H, Zheng M, Ma X, Li W, Chen X, Zhang Z, Zeng H, Ying S, Wu J, Yang R, Liu D. 2019. Fatty liver disease caused by high-alcohol-producing Klebsiella pneumoniae. Cell Metab 30:1172. doi: 10.1016/j.cmet.2019.11.006 [DOI] [PubMed] [Google Scholar]
- 3. Federici S, Kredo-Russo S, Valdés-Mas R, Kviatcovsky D, Weinstock E, Matiuhin Y, Silberberg Y, Atarashi K, Furuichi M, Oka A, Liu B, Fibelman M, Weiner IN, Khabra E, Cullin N, Ben-Yishai N, Inbar D, Ben-David H, Nicenboim J, Kowalsman N, Lieb W, Kario E, Cohen T, Geffen YF, Zelcbuch L, Cohen A, Rappo U, Gahali-Sass I, Golembo M, Lev V, Dori-Bachash M, Shapiro H, Moresi C, Cuevas-Sierra A, Mohapatra G, Kern L, Zheng D, Nobs SP, Suez J, Stettner N, Harmelin A, Zak N, Puttagunta S, Bassan M, Honda K, Sokol H, Bang C, Franke A, Schramm C, Maharshak N, Sartor RB, Sorek R, Elinav E. 2022. Targeted suppression of human ibd-associated gut microbiota commensals by phage consortia for treatment of intestinal inflammation. Cell 185:2879–2898. doi: 10.1016/j.cell.2022.07.003 [DOI] [PubMed] [Google Scholar]
- 4. Vuotto C, Longo F, Pascolini C, Donelli G, Balice MP, Libori MF, Tiracchia V, Salvia A, Varaldo PE. 2017. Biofilm formation and antibiotic resistance in Klebsiella pneumoniae urinary strains. J Appl Microbiol 123:1003–1018. doi: 10.1111/jam.13533 [DOI] [PubMed] [Google Scholar]
- 5. Dillon SC, Dorman CJ. 2010. Bacterial nucleoid-associated proteins, nucleoid structure and gene expression. Nat Rev Microbiol 8:185–195. doi: 10.1038/nrmicro2261 [DOI] [PubMed] [Google Scholar]
- 6. Miller HI, Friedman DI. 1980. An E. coli gene product required for lambda site-specific recombination. Cell 20:711–719. doi: 10.1016/0092-8674(80)90317-7 [DOI] [PubMed] [Google Scholar]
- 7. Reverchon S, Meyer S, Forquet R, Hommais F, Muskhelishvili G, Nasser W. 2021. The nucleoid-associated protein ihf acts as a 'transcriptional domainin' protein coordinating the bacterial virulence traits with global transcription. Nucleic Acids Res 49:776–790. doi: 10.1093/nar/gkaa1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gama-Castro S, Jiménez-Jacinto V, Peralta-Gil M, Santos-Zavaleta A, Peñaloza-Spinola MI, Contreras-Moreira B, Segura-Salazar J, Muñiz-Rascado L, Martínez-Flores I, Salgado H, Bonavides-Martínez C, Abreu-Goodger C, Rodríguez-Penagos C, Miranda-Ríos J, Morett E, Merino E, Huerta AM, Treviño-Quintanilla L, Collado-Vides J. 2008. RegulonDB (version 6.0): gene regulation model of Escherichia coli K-12 beyond transcription, active (experimental) annotated promoters and textpresso navigation. Nucleic Acids Res 36:D120–4. doi: 10.1093/nar/gkm994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dame RT. 2005. The role of nucleoid-associated proteins in the organization and compaction of bacterial chromatin. Mol Microbiol 56:858–870. doi: 10.1111/j.1365-2958.2005.04598.x [DOI] [PubMed] [Google Scholar]
- 10. Chittò M, Berger M, Berger P, Klotz L, Dröge P, Dobrindt U. 2020. IHF stabilizes pathogenicity island I of uropathogenic Escherichia coli strain 536 by attenuating integrase I promoter activity. Sci Rep 10:9397. doi: 10.1038/s41598-020-66215-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Justice SS, Li B, Downey JS, Dabdoub SM, Brockson ME, Probst GD, Ray WC, Goodman SD. 2012. Aberrant community architecture and attenuated persistence of uropathogenic Escherichia coli in the absence of individual IHF subunits. PLoS One 7:e48349. doi: 10.1371/journal.pone.0048349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rowe S, Hodson N, Griffiths G, Roberts IS. 2000. Regulation of the Escherichia coli K5 capsule gene cluster: evidence for the roles of H-NS, BipA, and integration host factor in regulation of group 2 capsule gene clusters in pathogenic E. coli. J Bacteriol 182:2741–2745. doi: 10.1128/JB.182.10.2741-2745.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nicolau SE, Lewis K. 2022. The role of integration host factor in Escherichia coli persister formation. mBio 13:e0342021. doi: 10.1128/mbio.03420-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Leite B, Werle CH, Carmo CP do, Nóbrega DB, Milanez GP, Culler HF, Sircili MP, Alvarez-Martinez CE, Brocchi M. 2017. Integration host factor is important for biofilm formation by Salmonella enterica Enteritidis. Pathog Dis 75. doi: 10.1093/femspd/ftx074 [DOI] [PubMed] [Google Scholar]
- 15. Stonehouse E, Kovacikova G, Taylor RK, Skorupski K. 2008. Integration host factor positively regulates virulence gene expression in Vibrio cholerae. J Bacteriol 190:4736–4748. doi: 10.1128/JB.00089-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Link AJ, Phillips D, Church GM. 1997. Methods for generating precise deletions and insertions in the genome of wild-type Escherichia coli: application to open reading frame characterization. J Bacteriol 179:6228–6237. doi: 10.1128/jb.179.20.6228-6237.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pan YJ, Fang HC, Yang HC, Lin TL, Hsieh PF, Tsai FC, Keynan Y, Wang JT. 2008. Capsular polysaccharide synthesis regions in Klebsiella pneumoniae serotype K57 and a new capsular serotype. J Clin Microbiol 46:2231–2240. doi: 10.1128/JCM.01716-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wu W, Jin S. 2005. PtrB of Pseudomonas aeruginosa suppresses the type III secretion system under the stress of DNA damage. J Bacteriol 187:6058–6068. doi: 10.1128/JB.187.17.6058-6068.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Love MI, Huber W, Anders S. 2014. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15. doi: 10.1186/s13059-014-0550-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zulianello L, de la Gorgue de Rosny E, van Ulsen P, van de Putte P, Goosen N. 1994. The HimA and HimD subunits of integration host factor can specifically bind to DNA as homodimers. EMBO J 13:1534–1540. doi: 10.1002/j.1460-2075.1994.tb06415.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mangan MW, Lucchini S, Danino V, Cróinín TO, Hinton JCD, Dorman CJ. 2006. The integration host factor (IHF) integrates stationary-phase and virulence gene expression in Salmonella enterica serovar Typhimurium. Mol Microbiol 59:1831–1847. doi: 10.1111/j.1365-2958.2006.05062.x [DOI] [PubMed] [Google Scholar]
- 22. Li M, Rosenshine I, Tung SL, Wang XH, Friedberg D, Hew CL, Leung KY. 2004. Comparative proteomic analysis of extracellular proteins of enterohemorrhagic and enteropathogenic Escherichia coli strains and their ihf and ler mutants. Appl Environ Microbiol 70:5274–5282. doi: 10.1128/AEM.70.9.5274-5282.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Walker KA, Miller VL. 2020. The intersection of capsule gene expression, hypermucoviscosity and hypervirulence in Klebsiella pneumoniae. Curr Opin Microbiol 54:95–102. doi: 10.1016/j.mib.2020.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Su K, Zhou X, Luo M, Xu X, Liu P, Li X, Xue J, Chen S, Xu W, Li Y, Qiu J. 2018. Genome-wide identification of genes regulated by RcsA, RcsB, and RcsAB phosphorelay regulators in Klebsiella pneumoniae NTUH-K2044. Microb Pathog 123:36–41. doi: 10.1016/j.micpath.2018.06.036 [DOI] [PubMed] [Google Scholar]
- 25. Ebel W, Trempy JE. 1999. Escherichia coli RcsA, a positive activator of colanic acid capsular polysaccharide synthesis, functions to activate its own expression. J Bacteriol 181:577–584. doi: 10.1128/JB.181.2.577-584.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ares MA, Sansabas A, Rodríguez-Valverde D, Siqueiros-Cendón T, Rascón-Cruz Q, Rosales-Reyes R, Jarillo-Quijada MD, Alcántar-Curiel MD, Cedillo ML, Torres J, Girón JA, De la Cruz MA. 2019. The interaction of Klebsiella pneumoniae with lipid rafts-associated cholesterol increases macrophage-mediated phagocytosis due to down regulation of the capsule polysaccharide. Front Cell Infect Microbiol 9:255. doi: 10.3389/fcimb.2019.00255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wu CC, Wang CK, Chen YC, Lin TH, Jinn TR, Lin CT. 2014. IscR regulation of capsular polysaccharide biosynthesis and iron-acquisition systems in Klebsiella pneumoniae CG43. PLoS One 9:e107812. doi: 10.1371/journal.pone.0107812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Balestrino D, Ghigo J-M, Charbonnel N, Haagensen JAJ, Forestier C. 2008. The characterization of functions involved in the establishment and maturation of Klebsiella pneumoniae in vitro biofilm reveals dual roles for surface exopolysaccharides. Environ Microbiol 10:685–701. doi: 10.1111/j.1462-2920.2007.01491.x [DOI] [PubMed] [Google Scholar]
- 29. Ross P, Mayer R, Benziman M. 1991. Cellulose biosynthesis and function in bacteria. Microbiol Rev 55:35–58. doi: 10.1128/mr.55.1.35-58.1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zogaj X, Nimtz M, Rohde M, Bokranz W, Römling U. 2001. The multicellular morphotypes of Salmonella Typhimurium and Escherichia coli produce cellulose as the second component of the extracellular matrix. Mol Microbiol 39:1452–1463. doi: 10.1046/j.1365-2958.2001.02337.x [DOI] [PubMed] [Google Scholar]
- 31. Stahlhut SG, Struve C, Krogfelt KA, Reisner A. 2012. Biofilm formation of Klebsiella pneumoniae on urethral catheters requires either type 1 or type 3 fimbriae. FEMS Immunol Med Microbiol 65:350–359. doi: 10.1111/j.1574-695X.2012.00965.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Murphy CN, Clegg S. 2012. Klebsiella pneumoniae and type 3 fimbriae: nosocomial infection, regulation and biofilm formation. Future Microbiol 7:991–1002. doi: 10.2217/fmb.12.74 [DOI] [PubMed] [Google Scholar]
- 33. Chen L, Wilksch JJ, Liu H, Zhang X, Torres VVL, Bi W, Mandela E, Cao J, Li J, Lithgow T, Zhou T. 2020. Investigation of LuxS-mediated quorum sensing in Klebsiella pneumoniae. J Med Microbiol 69:402–413. doi: 10.1099/jmm.0.001148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pacheco T, Gomes AÉI, Siqueira NMG, Assoni L, Darrieux M, Venter H, Ferraz LFC. 2021. SdiA, a quorum-sensing regulator, suppresses fimbriae expression, biofilm formation, and quorum-sensing signaling molecules production in Klebsiella pneumoniae. Front Microbiol 12:597735. doi: 10.3389/fmicb.2021.597735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Xue T, Zhao L, Sun H, Zhou X, Sun B. 2009. LsrR-binding site recognition and regulatory characteristics in Escherichia coli AI-2 quorum sensing. Cell Res 19:1258–1268. doi: 10.1038/cr.2009.91 [DOI] [PubMed] [Google Scholar]
- 36. Ferguson AD, Deisenhofer J. 2004. Metal import through microbial membranes. Cell 116:15–24. doi: 10.1016/s0092-8674(03)01030-4 [DOI] [PubMed] [Google Scholar]
- 37. Hsueh KL, Yu LK, Hsieh YC, Hsiao YY, Chen CJ. 2023. FeoC from Klebsiella pneumoniae uses its iron sulfur cluster to regulate the GTPase activity of the ferrous iron channel. Biochim Biophys Acta Proteins Proteom 1871:140855. doi: 10.1016/j.bbapap.2022.140855 [DOI] [PubMed] [Google Scholar]
- 38. Wang Y, Chen X, Hu Y, Zhu G, White AP, Köster W. 2018. Evolution and sequence diversity of FhuA in Salmonella and Escherichia. Infect Immun 86:1128 doi: 10.1128/IAI.00573-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Trindade IB, Hernandez G, Lebègue E, Barrière F, Cordeiro T, Piccioli M, Louro RO. 2021. Conjuring up a ghost: structural and functional characterization of FhuF, a ferric siderophore reductase from E. coli. J Biol Inorg Chem 26:313–326. doi: 10.1007/s00775-021-01854-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Matzanke BF, Anemüller S, Schünemann V, Trautwein AX, Hantke K. 2004. FhuF, part of a siderophore-reductase system. Biochemistry 43:1386–1392. doi: 10.1021/bi0357661 [DOI] [PubMed] [Google Scholar]
- 41. Chen S, Hu M, Hu A, Xue Y, Wang S, Liu F, Li C, Zhou X, Zhou J. 2022. The integration host factor regulates multiple virulence pathways in bacterial pathogen Dickeya zeae MS2. Mol Plant Pathol 23:1487–1507. doi: 10.1111/mpp.13244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Korotkov KV, Sandkvist M, Hol WGJ. 2012. The type II secretion system: biogenesis, molecular architecture and mechanism. Nat Rev Microbiol 10:336–351. doi: 10.1038/nrmicro2762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Michel-Souzy S, Douzi B, Cadoret F, Raynaud C, Quinton L, Ball G, Voulhoux R. 2018. Direct interactions between the secreted effector and the T2SS components GspL and GspM reveal a new effector-sensing step during type 2 secretion. J Biol Chem 293:19441–19450. doi: 10.1074/jbc.RA117.001127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nivaskumar M, Francetic O. 2014. Type II secretion system: a magic beanstalk or a protein escalator. Biochim Biophys Acta 1843:1568–1577. doi: 10.1016/j.bbamcr.2013.12.020 [DOI] [PubMed] [Google Scholar]
- 45. Boyer F, Fichant G, Berthod J, Vandenbrouck Y, Attree I. 2009. Dissecting the bacterial type VI secretion system by a genome wide in silico analysis: what can be learned from available microbial genomic resources? BMC Genomics 10:104. doi: 10.1186/1471-2164-10-104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Silverman JM, Brunet YR, Cascales E, Mougous JD. 2012. Structure and regulation of the type VI secretion system. Annu Rev Microbiol 66:453–472. doi: 10.1146/annurev-micro-121809-151619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yu KW, Xue P, Fu Y, Yang L. 2021. T6SS mediated stress responses for bacterial environmental survival and host adaptation. Int J Mol Sci 22:478. doi: 10.3390/ijms22020478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Park Y-J, Lacourse KD, Cambillau C, DiMaio F, Mougous JD, Veesler D. 2018. Structure of the type VI secretion system TssK-TssF-TssG baseplate subcomplex revealed by cryo-electron microscopy. Nat Commun 9:5385. doi: 10.1038/s41467-018-07796-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ganduri YL, Sadda SR, Datta MW, Jambukeswaran RK, Datta P. 1993. TdcA, a transcriptional activator of the tdcABC operon of Escherichia coli, is a member of the LysR family of proteins. Mol Gen Genet 240:395–402. doi: 10.1007/BF00280391 [DOI] [PubMed] [Google Scholar]
- 50. Hsieh PF, Lin HH, Lin TL, Wang JT. 2010. CadC regulates cad and tdc operons in response to gastrointestinal stresses and enhances intestinal colonization of Klebsiella pneumoniae. J Infect Dis 202:52–64. doi: 10.1086/653079 [DOI] [PubMed] [Google Scholar]
- 51. Bobrovskyy M, Vanderpool CK. 2014. The small RNA SgrS: roles in metabolism and pathogenesis of enteric bacteria. Front Cell Infect Microbiol 4:61. doi: 10.3389/fcimb.2014.00061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kedryna T, Gumińska M, Marchut E. 1983. The inhibitory effect of L-cysteine and its derivatives on glycolysis in Ehrlich ascites tumour cells. Biochim Biophys Acta 763:64–71. doi: 10.1016/0167-4889(83)90026-5 [DOI] [PubMed] [Google Scholar]
- 53. Lu Q, Zhao Y, Gao X, Wu J, Zhou H, Tang P, Wei Q, Wei Z. 2018. Effect of tricarboxylic acid cycle regulator on carbon retention and organic component transformation during food waste composting. Bioresour Technol 256:128–136. doi: 10.1016/j.biortech.2018.01.142 [DOI] [PubMed] [Google Scholar]
- 54. Dong R, Liang Y, He S, Cui Y, Shi C, He Y, Shi X. 2022. DsrA modulates central carbon metabolism and redox balance by directly repressing PflB expression in Salmonella Typhimurium. Microbiol Spectr 10:e0152221. doi: 10.1128/spectrum.01522-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Leonardo MR, Cunningham PR, Clark DP. 1993. Anaerobic regulation of the adhE gene, encoding the fermentative alcohol dehydrogenase of Escherichia coli. J Bacteriol 175:870–878. doi: 10.1128/jb.175.3.870-878.1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Carpino G, Del Ben M, Pastori D, Carnevale R, Baratta F, Overi D, Francis H, Cardinale V, Onori P, Safarikia S, Cammisotto V, Alvaro D, Svegliati-Baroni G, Angelico F, Gaudio E, Violi F. 2020. Increased liver localization of lipopolysaccharides in human and experimental NAFLD. Hepatology 72:470–485. doi: 10.1002/hep.31056 [DOI] [PubMed] [Google Scholar]
- 57. Fei N, Bruneau A, Zhang X, Wang R, Wang J, Rabot S, Gérard P, Zhao L. 2020. Endotoxin producers overgrowing in human gut microbiota as the causative agents for nonalcoholic fatty liver disease. mBio 11:e03263-19. doi: 10.1128/mBio.03263-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Guo J, Friedman SL. 2010. Toll-like receptor 4 signaling in liver injury and hepatic fibrogenesis. Fibrogenesis Tissue Repair 3:21. doi: 10.1186/1755-1536-3-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Craig NL, Nash HA. 1984. E. coli integration host factor binds to specific sites in DNA. Cell 39:707–716. doi: 10.1016/0092-8674(84)90478-1 [DOI] [PubMed] [Google Scholar]
- 60. Yang CC, Nash HA. 1989. The interaction of E. coli IHF protein with its specific binding sites. Cell 57:869–880. doi: 10.1016/0092-8674(89)90801-5 [DOI] [PubMed] [Google Scholar]
- 61. Grant CE, Bailey TL, Noble WS. 2011. FIMO: scanning for occurrences of a given motif. Bioinformatics 27:1017–1018. doi: 10.1093/bioinformatics/btr064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Corcoran CP, Dorman CJ. 2009. DNA relaxation-dependent phase biasing of the fim genetic switch in Escherichia coli depends on the interplay of H-NS, IHF and LRP. Mol Microbiol 74:1071–1082. doi: 10.1111/j.1365-2958.2009.06919.x [DOI] [PubMed] [Google Scholar]
- 63. Wu YF, Datta P. 1992. Integration host factor is required for positive regulation of the tdc operon of Escherichia coli. J Bacteriol 174:233–240. doi: 10.1128/jb.174.1.233-240.1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Pan J, Zhao M, Huang Y, Li J, Liu X, Ren Z, Kan B, Liang W. 2018. Integration host factor modulates the expression and function of T6SS2 in Vibrio fluvialis. Front Microbiol 9. doi: 10.3389/fmicb.2018.00962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Torres-Escobar A, Juárez-Rodríguez MD, Demuth DR. 2014. Differential transcriptional regulation of aggregatibacter actinomycetemcomitans lsrACDBFG and lsrRK operons by integration host factor protein. J Bacteriol 196:1597–1607. doi: 10.1128/JB.00006-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Bacterial strains, plasmids, and primers used in this study.
An accounting of the reviewer comments and feedback.
Data Availability Statement
The raw sequence data of RNA sequencing in this study have been deposited in the Genome Sequence Archive in National Genomics Data Center, Beijing Institute of Genomics (China National Center for Bioinformation), Chinese Academy of Sciences, under accession number CRA010192.