ABSTRACT
Carbapenemase-producing Enterobacteriaceae (CPE) are one of the most detrimental species of antibiotic-resistant bacteria globally. Phage therapy has emerged as an effective strategy for the treatment of CPE infections. In western Japan, the rise of Klebsiella pneumoniae strains harboring the pKPI-6 plasmid encoding bla IMP-6 is of increasing concern. To address this challenge, we isolated 29 phages from Japanese sewage, specifically targeting 31 K. pneumoniae strains and one Escherichia coli strain harboring the pKPI-6 plasmid. Electron microscopy analysis revealed that among the 29 isolated phages, 21 (72.4%), 5 (17.2%), and 3 (10.3%) phages belonged to myovirus, siphovirus, and podovirus morphotypes, respectively. Host range analysis showed that 18 Slopekvirus strains within the isolated phages infected 25–26 K. pneumoniae strains, indicating that most of the isolated phages have a broad host range. Notably, K. pneumoniae strain Kp21 was exclusively susceptible to phage øKp_21, whereas Kp22 exhibited susceptibility to over 20 phages. Upon administering a phage cocktail composed of 10 phages, we observed delayed emergence of phage-resistant bacteria in Kp21 but not in Kp22. Intriguingly, phage-resistant Kp21 exhibited heightened sensitivity to other bacteriophages, indicating a “trade-off” for resistance to phage øKp_21. Our proposed phage set has an adequate number of phages to combat the K. pneumoniae strain prevalent in Japan, underscoring the potential of a well-designed phage cocktail in mitigating the occurrence of phage-resistant bacteria.
IMPORTANCE
The emergence of Klebsiella pneumoniae harboring the bla IMP-6 plasmid poses an escalating threat in Japan. In this study, we found 29 newly isolated bacteriophages that infect K. pneumoniae strains carrying the pKPI-6 plasmid from clinical settings in western Japan. Our phages exhibited a broad host range. We applied a phage cocktail treatment composed of 10 phages against two host strains, Kp21 and Kp22, which displayed varying phage susceptibility patterns. Although the phage cocktail delayed the emergence of phage-resistant Kp21, it was unable to hinder the emergence of phage-resistant Kp22. Moreover, the phage-resistant Kp21 became sensitive to other phages that were originally non-infective to the wild-type Kp21 strains. Our study highlights the potential of a well-tailored phage cocktail in reducing the occurrence of phage-resistant bacteria.
KEYWORDS: bacteriophages, Klebsiella pneumoniae, bla IMP-6 , trade-off, phage-resistant bacteria, phage library
INTRODUCTION
Carbapenemase-producing Enterobacteriaceae (CPE) pose a considerable risk in clinical settings worldwide. Among them, Klebsiella pneumoniae, a member of the Enterobacteriaceae family, is a leading cause of nosocomial infections and a major contributor to life-threatening infections caused by multidrug-resistant bacteria globally (1). The bla IMP genes belong to the class B metallo-β-lactamases. bla IMP-1 and bla IMP-6 genes are predominantly identified in CPE isolated in Japan (2, 3), whereas other types of carbapenemases such as NDM, KPC, and OXA-48 are more prevalent in CPE strains isolated from other countries (4). Klebsiella pneumoniae strains carrying the bla IMP-6-encoding pKPI-6 plasmid (5), which is susceptible to imipenem but resistant to meropenem, have become increasingly common in clinical settings in western Japan since their emergence in 2009 (6). These strains are therefore of major concern in clinical settings because of their inappropriate response to commonly used antibiotics.
Recently, bacteriophage therapy has gained considerable attention as an alternative treatment for infections caused by antimicrobial-resistant bacteria (7). Phage therapy targeting Staphylococcus aureus (8) and Mycobacterium tuberculosis (9) has been administered successfully to patients. Furthermore, a recent study has demonstrated the efficacy of phage therapy against CPE in clinical settings (10). Thus, phage therapy is now recognized as a highly reliable strategy to combat nosocomial pathogens.
However, the use of phages against bacteria has led to the emergence of phage-resistant bacteria (11) in vitro (12) and in vivo (13). To address this issue, phage cocktails, which consist of multiple phage types, are often used to prevent the emergence of phage-resistant bacteria. Establishing a phage bank is pertinent for the rapid deployment of phage cocktails in clinical settings (14), especially in emergency cases. As national phage banks are pertinent for the instant management of contingent nosocomial pathogen outbreaks, several countries have constructed public phage banks for the efficient use of phage therapy (14, 15). However, in Japan, there is currently no public phage bank optimized to address the evolving trends in antibiotic-resistant bacteria.
In this study, we isolated and characterized 29 bacteriophages targeting IMP-6-producing K. pneumoniae and Escherichia coli clinical isolates as the first step in establishing a public phage library in Japan. Additionally, we described the mechanisms by which phage cocktails can effectively reduce the emergence of phage-resistant K. pneumoniae strains.
RESULTS
Phage hunting and morphological analysis of newly isolated bacteriophages
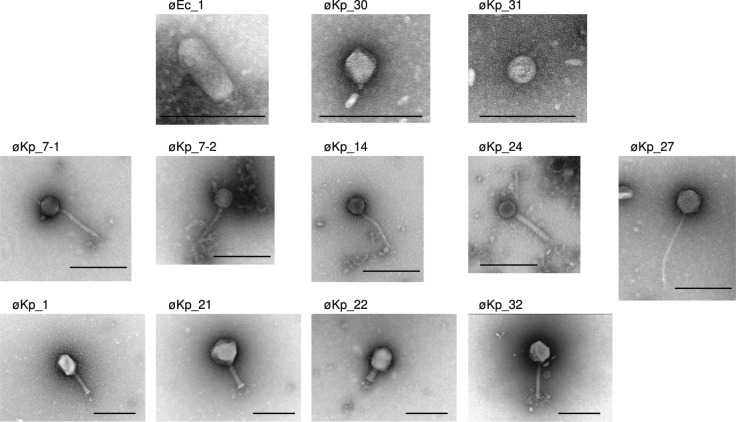
We performed a phage hunting expedition in the sewage system of western Japan, resulting in the isolation of 29 phages targeting 32 strains of K. pneumoniae (Kp1–Kp32) and one strain of E. coli (Ec1), all harboring the pKPI-6 plasmid. It is worth noting that 81.2% (26/32) of the hosts used in this study belonged to sequence type 37, which represents the predominant clone frequently encountered in Japanese clinical settings and commonly associated with the presence of IMP-6 (Table S1) (5). Each phage was assigned a name corresponding to its host number. For instance, the phages øEc_1 and øKp_1 were isolated from E. coli strains Ec1 and K. pneumoniae strain Kp1, respectively, as their corresponding hosts. All phage-corresponding host combinations are listed in Table S2. We did not identify suitable phages capable of infecting K. pneumoniae strains (Kp2, Kp6, Kp25, Kp28, and Kp29). Morphological analysis using electron microscopy revealed that 21 out of 29 (72.4%) isolated phages belonged to the myovirus morphotype, 5 out of 29 (17.2%) belonged to the siphovirus morphotype, and 3 out of 29 (10.3%) belonged to the podovirus morphotype (Fig. 1). All transmission electron microscopy (TEM) images of the myovirus morphotype are shown in Fig. S1 except representative phages (øKp_1, øKp_21, øKp_22, and øKp_32) which are shown in Fig. 1. Notably, based on transmission electron microscopy images, phage øEc_1 was identified as belonging to the podovirus morphotype and C3 morphotype (honeycomb-like) phages (16 – 18), characterized by an elongated head (height, 136.6 nm ± 1.8 nm; width, 61.7 nm ± 3.6 nm; tail, 15.8 nm ± 2.3 nm) (Fig. 1). øEc_1 formed turbid plaques on the E. coli Ec1 strain. øKp_21 was classified as a myovirus morphotype (height, 133.8nm± 3.1 nm; width, 137.1 nm ± 1.1 nm; tail, 109.7 nm ± 1.0 nm) (Fig. 1). Furthermore, øKp_21 possessed a branched tail (tail spike) fiber and formed clear plaques on a lawn of K. pneumoniae Kp21.
Fig 1.
Transmission electron microscopy images of 29 isolated phages. Each sample was negatively stained and magnified at ×50,000. Representative myovirus morphotype phages, øKp_1, øKp_21, øKp_22, and øKp_32, are displayed. All podovirus morphotype phages and siphovirus morphotype phages are shown. The scale bar in each image represents 200 nm.
Bacteriophage classification
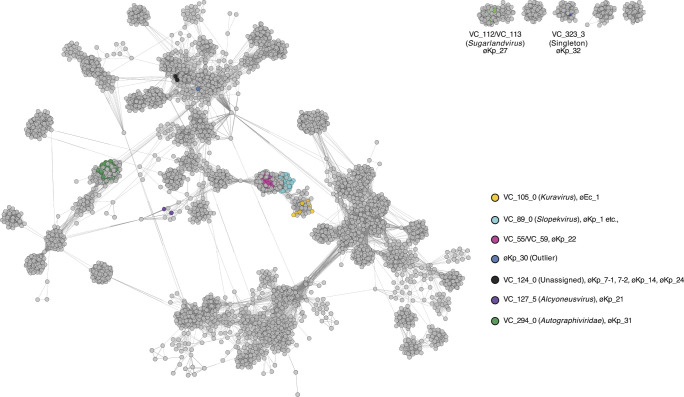
To classify the newly isolated phages, we employed a two-step approach. First, we conducted phage classification using network analysis based on phage-encoded protein ortholog families using vConTACT2 (19). Subsequently, we performed an average nucleotide identity (ANI) analysis. vConTACT2 generated “viral clusters” based on shared protein similarities and proposed taxonomic classifications for the phages of interest. vConTACT2 integrated our isolated phages into nine viral clusters (VC_105_0, VC_89_0, VC_55/VC_59, VC_124_0, VC127_5, VC_294_0, VC_112/VC_113, VC_323_3, and an outlier) (Fig. 2).
Fig 2.
Network images of the newly isolated phages based on shared protein ortholog families. vConTACT2 v0.11.3 was used for clustering each phage. vConTACT2 assigned viral cluster (VC) to each phage. Our phages were divided into nine VCs. Each VC, including our phages, is highlighted in different colors.
Among the three phages exhibiting a podovirus morphotype, øEc_1 and øKp_31 were classified as Kuravirus at the genus level and Autographiviridae at the family level, respectively (Fig. 2). Conversely, øKp_30 could not be classified at either the family or genus level using vConTACT2 (Fig. 2). øEc_1 exhibited nucleotide identities of 97.9% and 95.4% with Escherichia phage MN03 (NC_070990.1) and øEco32, respectively (16) (Fig. S2A), both classified as Kuravirus. øKp_31 displayed nucleotide identities of 95.7% and 93.4% with KPP-5 (20) and T3 phage (NC_003298), respectively. For øKp_30, nucleotide identities of 95.3% and 95.9% were observed with Escherichia phage CUS-3 (21) and vB_EcoP_Kapi1 (22), both classified as Lederbergvirus in the National Center for Biotechnology Information (NCBI) taxonomy database (Fig. S2A). This indicates that øKp_30 belongs to the Lederbergvirus. It is worth noting that Lederbergvirus mainly infects Escherichia coli or Salmonella species, and we did not find any Lederbergvirus infecting K. pneumoniae in the NCBI database. Therefore, this may be the first report of a complete genome of a Lederbergvirus infecting K. pneumoniae. Life cycle analysis in silico suggests that øKp_30 is likely a temperate phage (Table S2), but we have not confirmed whether øKp_30 integrates into the host chromosome.
Among the five phages exhibiting a siphovirus morphotype, øKp_27 was classified as Sugarlandvirus, while the remaining four siphovirus morphotype phages (øKp_7–1, øKp_7–2, øKp_14, and øKp_24) were clustered in the same viral cluster (VC_124_0) and were not classified at the family or genus levels based on vConTACT2 (Fig. 2). These unclassified siphoviruses displayed similar nucleotide identities to each other (Fig. S3) and were highly similar to vB_Kp3 (ON602766) and vB_KleS-HSE3 (23), which are also unclassified phages (Fig. S2B). øKp_27 exhibited nucleotide identities of 95.2% and 95.3% with Sugarland phages (24) and vB_Kpn_IME260 phage (25) (Fig. S2B).
Of the 21 phages exhibiting a myovirus morphotype, øKp_21 is classified as Alcyoneusvirus, whereas øKp_22 and øKp_32 could not be classified at the family or genus levels (genus not assigned) based on vConTACT2. All other myovirus phages are classified under the Slopekvirus genus. The Slopekvirus phages isolated in this study share a remarkably high sequence identity (over 99.8%) and coverage (over 98.7%) with each other, suggesting they are variants of same species. øKp_1, classified as a Slopekvirus, exhibits nucleotide identities of 98.8% and 98.4% with Klebsiella phage PMBT1 (26) and Miro (27) (Fig. S2C), respectively. Among the other myovirus morphotype phages, øKp_21 exhibits 97.6% nucleotide identity with Klebsiella phage Muenster (28). øKp_22 displays 97.9% nucleotide identity with Klebsiella phage KP1 (29), classified as Jiaodavirus (Fig. S2C) in the NCBI taxonomic database. øKp_32 exhibits 95.9% nucleotide identity with Klebsiella phage Miami (30) (Fig. S2C). øKp_21 and øKp_32 possess genome sizes of 353,382 bp (532 CDS) and 251,460 bp (275 CDS), respectively, indicating their classification as jumbo phages (Table S2).
Other genomic information
Recently, phage defense systems and anti-phage defense mechanisms have garnered considerable attention due to their impact on infection efficacy and host range, which are crucial for the successful implementation of phage therapy. Anti-CRISPR (Acr) proteins, which protect phages from the host CRISPR immune system, represent well-characterized anti-defense systems (31). We predicted Acr and Acr-associated regulator (Aca) proteins from our representative phage genomes using AcrFinder (Table S3). The results revealed the formation of genomic clusters of Acr and Aca, consistent with previous reports (32), and majority of phages in our study possess at least one Acr clustered genomic island. Notably, we identified 18 predicted Acr and/or Aca proteins encoded by øKp_27 and 25 by øKp_32. Most of these proteins were found to be less than 200 amino acids long.
To determine the number of encoded tRNAs, we employed tRNAscan-SE (Table S2). Remarkably, øKp_22 and øKp_27 encode 16 and 27 tRNAs, respectively, forming tRNA islands (Fig. S4; Table S4).
Host range determination and analysis of the correlation between plaque size and efficiency of plating
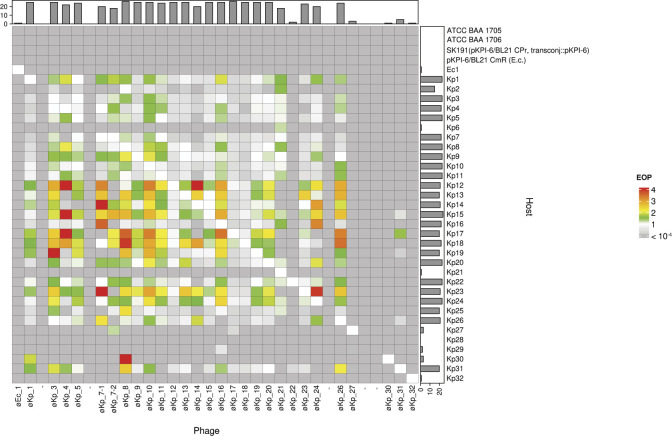
We next determined the host range and its efficiency of plating (EOP) for all phage-host combinations (Fig. 3) (Table S5). We selected two standard strains (ATCC BAA 1705 and ATCC BAA 1706) as the controls for K. pneumoniae. Although no newly isolated phages against Kp2, Kp6, Kp25, Kp28, or Kp29 were isolated, the host range experiment indicated that several isolated phages formed plaques on Kp2, Kp6, Kp25, and Kp29 but not Kp28 (Fig. 3). Phages øKp_8 and øKp_17 exhibited the broadest host range, infecting 26 out of 32 K. pneumoniae host strains. Most phages (øKp_3, øKp_5, øKp_9, øKp_10, øKp_12, øKp_13, øKp_15, øKp_16, øKp_18, øKp_19, øKp_20, and øKp_26) demonstrated plaque formation on 25 host K. pneumoniae strains, indicating that these phages have a broad host range (infecting ≥25 host strains). Conversely, several phages exhibited a narrow host range (infecting ≤4 host strains). For instance, øKp_31 infected Kp15, Kp17, Kp26, and Kp31. øKp_27 (Sugarlandvirus), øKp_30 (Lederbergvirus), and øKp_32 (myovirus) infected only Kp27, Kp30, and Kp32, respectively (Fig. 3). Overall, our phage set encompasses K. pneumoniae commonly encountered in clinical settings and offers an adequate variety of phage types to facilitate the development of a phage cocktail targeting K. pneumoniae in this study.
Fig 3.
A heatmap illustrating the host range of each phage. The X- and Y-axes represent phages and host strains, respectively. Klebsiella pneumoniae ATCC BAA 1705 and ATCC BAA 1706 served as standard strains, whereas Escherichia coli SK191 and BL21 served as control strains. The color in the heatmap represents the EOP. Bar charts on the X- and Y-axes represent the number of infections in each phage and host, respectively.
We observed a positive correlation between EOP values and the formation of large plaques. To investigate this further, we conducted a correlation analysis between EOP values and plaque size for eight representative phages that exhibited distinct host range patterns. Pearson’s correlation coefficients (R values) were 0.75 for øKp_1, 0.86 for øKp_7–1, 0.70 for 7–2, 0.55 for øKp_14, 0.25 for øKp_21, 0.80 for øKp_24, 0.83 for øKp_27, and 0.65 for øKp_31 (Fig. S5). These results demonstrate a positive association between plaque size and EOP. We hypothesized that EOP and plaque size are determined by adsorption efficiency, phage life cycle time, and/or burst size. To assess the factors influencing plaque size in our phages, we conducted adsorption analysis and one-step growth curve experiments on three different phages: myovirus øKp_1 (Slopekvirus), siphovirus øKp_7–1 (genus not assigned), and podovirus øKp_31 (Autographiviridae) (Fig. S6). We selected two hosts with lower and higher EOP values for each phage. The adsorption assay revealed no statistically significant difference between the two hosts for all phages (P = 0.117 for øKp_1, P = 0.351 for øKp_7–1, and P = 0.127 for øKp_31) (Fig. S6). However, the host with a higher EOP exhibited a significantly larger burst size compared to the host with a lower EOP at 38 min for øKp_1, 40 min for øKp_7–1, and 40 min for øKp_31 (P = 0.0229, 0.0493, and 0.00414, respectively). These findings suggest that plaque size for each host is primarily defined by burst size rather than adsorption efficiency.
OD600 kinetics of K. pneumoniae challenged with phages
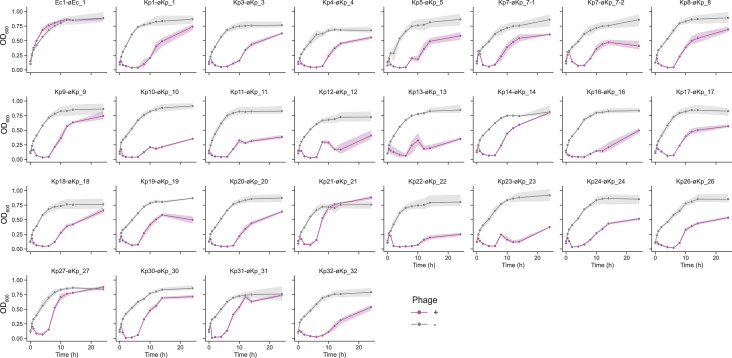
The OD600 kinetics were analyzed for all phage-indicator host combinations. Individual phages were added at a concentration of 108 pfu/mL (multiplicity of infection [MOI)],~10) when the host bacteria entered the exponential phase (OD600 = 0.1). Within 1 h of phage addition, a noticeable decrease in OD600 was observed, while the mixture of Ec1 and øEc_1 did not exhibit a decrease and maintained an OD600 level similar to that of the host strain without any phages (Fig. 4). Although øEc_1 and øKp_30 were predicted to be temperate phages (Table S2), the OD600 of øKp_30 decreased 1 h after its addition, resembling the OD600 kinetics of other virulent phages. Therefore, øKp_30 has the potential to be applied in phage therapy. Furthermore, the OD600 in all phage combinations began to rise again 6–10 h after the addition of each phage. These findings indicate the emergence of phage-resistant bacteria in almost all phage-host combinations.
Fig 4.
OD600 kinetics of indicator bacteria incubated with phages. Bacterial strains were incubated until reaching an OD600 of 0.1, after which each phage was added at a concentration of 109 pfu/mL. OD600 measurements were taken at appropriate time intervals for up to 24 h. All experiments in this section were performed in triplicate. Gray and magenta represent the OD600 without (negative control) and with phages, respectively.
Cocktail analysis of phage-resistant bacteria Kp21 and Kp22
Kp21 exhibited susceptibility only to øKp_21, whereas the Kp22 strain was susceptible to 23 different phages (Fig. 3). Notably, øKp_21 could infect 18 hosts, including Kp21, whereas øKp_22 could only infect Kp22 and Kp31 strains (Fig. 3). Given this contrast, we selected øKp_21 and øKp_22 for the phage cocktail experiment. The phage cocktail comprised 10 phages (øKp_16–26), encompassing eight Slopekviruses, one Alcyoneusvirus, and one siphovirus. In the Kp21 and øKp_21 combination, the OD600 increased after 6 h and eventually reached the level of the negative control after 24 h (Fig. 5A). However, the OD600 did not increase until 14 h after Kp21 was combined with the phage cocktail (Fig. 5A). This finding suggests that the phage cocktail effectively delayed the emergence of phage-resistant Kp21 in the in vitro assay. Conversely, the OD600 of the cocktail against Kp22 exhibited a similar pattern to that of single øKp_22, increasing again after 10 h (Fig. 5C). Thus, the phage cocktail failed to delay the emergence of phage-resistant Kp22. We isolated øKp_21-resistant Kp21 (Kp21r) and øKp_22-resistant Kp22 (Kp22r) using the methodology described in the Materials and Methods section. Although the OD600 kinetics in the mixture of Kp21r and øKp_21 did not decrease and approximated those of Kp21r without any phages, the OD600 was reduced in the Kp21r-phage cocktail combination (Fig. 5B). This result indicates that the Kp21r strain is resistant to phage 21 but susceptible to the phage cocktail. However, the OD600 did not decrease in either the Kp22r-øKp_22 or Kp22r-cocktail combinations, suggesting that the phage-resistant Kp22r is not susceptible to either øKp_22 or the phage cocktail (Fig. 5D).
Fig 5.
Cocktail experiment of Kp21r with Kp22r. Cocktail experiment of Kp21r with Kp22r. OD600 kinetics of Kp21 and Kp21r combined with phage cocktail are shown in panels A and B, respectively. OD600 kinetics of Kp22 and Kp22r combined with phage cocktail are shown in panels C and D, respectively. Phage-resistant Kp21 (Kp21r) and Kp22 (Kp22r) strains were obtained from the culture medium after 24 h of incubation with øKp_21 or øKp_22. The cocktail comprised 10 phages, each at a concentration of 107 pfu/mL. OD600 measurements were taken at appropriate time intervals for up to 24 h. All experiments in this section were performed in triplicate.
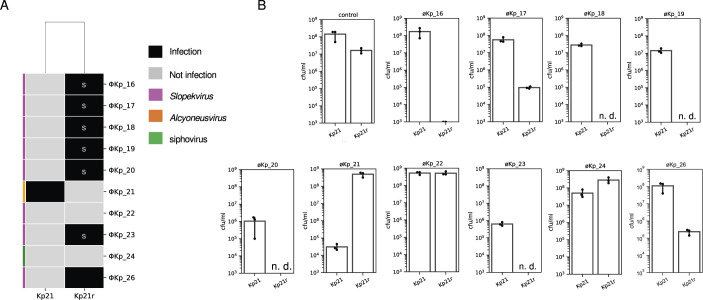
Phage sensitivity shift between Kp21 and Kp21r
To investigate the susceptibility of Kp21r to the phage cocktail, we compared phage plaque formation between Kp21 and Kp21r. According to the host range analysis, no phages formed plaques on Kp21. However, the Kp21r strain exhibited susceptibility to øKp_16, 17, 18, 19, 20, 23, and 26, which belong to the Slopekvirus genus. This finding suggests that Kp21r becomes susceptible to other phages as compensation for its resistance to øKp21 (Fig. 5A). Furthermore, the Kp21r strain showed a sparse background on Luria-Bertani (LB) agar plates containing Slopekvirus strains (Fig. 6A). Importantly, this sparse background was not due to confluent plaque lysis, indicating the presence of a mechanism by which the phage cocktail prevents the emergence of phage-resistant bacteria even without phage infection. To assess the bactericidal efficiency of phages against the Kp21r strain, we measured the colony-forming unit (cfu) values of both Kp21 and Kp21r by individually mixing the phages used in the phage cocktail (Fig. 6B). In the case of øKp_21, the cfu of Kp21 was reduced to 104 cfu/mL, whereas the cfu of Kp21r remained nearly the same as that of the control Kp21r (107 cfu/mL–108 cfu/mL). As for øKp_22 and øKp_24, the cfu per milliliter in Kp21r did not show a considerable decrease compared to that in Kp21, consistent with the observation that these phages are incapable of infecting both Kp21 and Kp21r. However, in other phages, the cfu of the Kp21r strain significantly decreased compared to that of Kp21. Notably, colonies were not detected in combinations of øKp_18, 19, 20, or 23-Kp21r. These results indicate that Kp21r viable cells were effectively eliminated by phages that newly infected Kp21r.
Fig 6.
Analysis of the shift in susceptibility to the phage cocktail in Kp21 and Kp21r. (A) The host range of Kp21 and Kp21r against a phage cocktail comprising 10 phages. “S” denotes a sparse bacterial lawn. (B) Colony-forming units are mentioned under each phage. Kp21 or Kp21r were mixed with individual phages, and 2 h after phage addition, samples were diluted to 10−2 and 10−4 and lawned onto LB plates. “n.d.” indicates that no colonies were detected at the 10−2 dilution.
Characterization of Kp21 and Kp21r
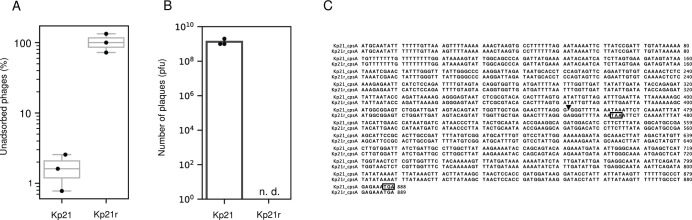
To elucidate the distinctions between the strains Kp21 and Kp21r, we conducted an adsorption assay of øKp_21 for both Kp21 and Kp21r. The assay revealed that the unadsorbed fraction of øKp_21 for the Kp21r strain reached approximately 100% at 5 min after phage addition, whereas for Kp21, this value ranged between 2% and 5% (Fig. 7A). Moreover, no plaques were observed when øKp_21 was added at 1.0 × 109 pfu (EOP <10−9) (Fig. 7B), indicating that øKp_21 loses its ability to adsorb to Kp21r.
Fig 7.
Characterization of the phage-resistant Kp21r strain. (A) Adsorption assay of øKp_21 against Kp21 and Kp21r strains. Kp21 and Kp21r were incubated until reaching an OD600 of 0.5. Subsequently, øKp_21 was added at an MOI of 0.01, and the mixture was incubated at 37°C with shaking at 200 rpm. After 5 min, 200 µL of the mixture was withdrawn and centrifuged at 9,100 × g for 1 min. The number of phages in the supernatant was measured. (B) 1.0 × 109 pfu of øKp_21 were mixed with 100 µL of overnight culture of Kp21 or Kp21r. Then, 5 mL of 0.6% YT (yeast extract and tryptone)-soft agar was added to the host and phage mixture and incubated at 37°C overnight. “n.d.” indicates that no plaques were detected. (C) Nucleotide sequences of cpsA in Kp21 and Kp21r were aligned using ClustalW (https://www.genome.jp/tools-bin/clustalw). The insertion mutation (A) is indicated by an arrow, and the stop codons of cspA in Kp21 and Kp21r are shown by a square.
Subsequently, we detected single nucleotide polymorphisms (SNPs) between Kp21 and Kp21r. Insertion mutations were detected in two genes, thpA (encoding the inner membrane protein) and cpsA (encoding exopolysaccharide synthesis genes), as shown in Table S6. This finding suggests that the øKp_21 phage specifically recognizes the capsular polysaccharide of Kp21. Insertion mutations in cpsA in Kp21r were observed at the 452nd nucleotide position, and a stop codon appeared at the 463rd nucleotide position (Fig. 7C). Consequently, 141 amino acids were truncated at the C-terminus of CpsA in Kp21r (154 amino acids long), compared to the wild-type CpsA amino acid with a length of 295 amino acids. This severe truncation likely impairs the biosynthesis of capsular polysaccharide in Kp21r. Additionally, we isolated eight new øKp21-resistant Kp21 strains (Kp21r-1 to Kp21r-8) and sequenced their draft genomes. SNPs and/or insertions/deletions mutations were identified in genes associated with capsular polysaccharide biosynthesis (Table S7). These findings provide further support for our hypothesis that øKp_21 recognizes Kp21 through its interaction with capsular polysaccharide.
DISCUSSION
Phage therapy is increasingly recognized as an effective strategy for combating antimicrobial-resistant bacteria, especially nosocomial pathogens (8 – 10, 33). A recent study demonstrated the successful suppression of inflammation in a mouse model of inflammatory bowel disease (IBD) through the eradication of K. pneumoniae using a phage cocktail, suggesting its potential therapeutic application for treating IBD in humans (34).
In this study, we isolated and characterized newly isolated bacteriophages targeting antimicrobial-resistant K. pneumoniae strains harboring the bla IMP-6 encoding pKPI-6 plasmid. We found 29 phages from sewage samples in western Japan against both K. pneumoniae and E. coli strains harboring the pKPI-6 plasmid. Genomic sequence analysis revealed that, except for øKp_22, the Slopekvirus members in our study shared similar genome sizes and identities, indicating their classification as variants of the same species (Table S2). Given the concerns about the potential presence of antimicrobial resistance (AMR) and virulence factor (VF) genes in our phages, as these genes can be transferred in clinical settings (35 – 37), we conducted an analysis and found no detection of AMR or VF genes in the isolated phage genomes. Consequently, these phages can be safely applied in clinical settings.
Our host range experiment demonstrated that most of the Slopekvirus phages formed plaques on the 25 tested K. pneumoniae strains. Specific host strains (K. pneumoniae Kp12 to Kp20) exhibited higher EOP (Fig. 3) against most phages, suggesting that several host strains exhibit high susceptibility to newly isolated phages isolated from western Japan. Moreover, host range experiment results indicated a positive correlation between EOP and plaque size for several phages, such as øKp_1, 7, 7–1, 14, 24, 27, and 31. To the best of our knowledge, few reports have described the correlation of these factors (38); however, our adsorption assay and one-step growth experiment suggest that plaque size in each host associated with the burst size and/or phage growth rate (Fig. S6). These findings contribute to the development of newly isolated phages with enhanced virulence against the host bacteria and guide the selection of phage strains for the development of phage cocktails (39, 40).
Our SNP analysis suggested that Kp21r exhibits a deficiency in capsular polysaccharide.
Although no differences in colony morphology between Kp21 and Kp21r were observed on LB agar plates or in cell growth in LB medium, it is well known that capsular polysaccharide provides protection against various stressors, including the host immune system and antimicrobial agents (41). Consequently, Kp21r may be more susceptible to such stressors than Kp21 as a trade-off for its resistance to øKp_21.
The phage cocktail experiment revealed that Kp21r acquired susceptibility to other phages in the phage cocktail. In contrast to the results observed with the Kp21 strain, the addition of Kp22 did not impede the emergence of phage-resistant Kp22 (Kp22r). This divergent outcome in the Kp21 and Kp22 cocktail experiments indicates that the phage cocktail is not an all-round strategy. However, to date, it remains the most reliable strategy to combat bacteriophages. The development of a phage cocktail lacks established universal methods or guidelines, making it challenging to predict the most efficient combination of phages to inhibit the emergence of phage-resistant bacteria. Nevertheless, previous studies have suggested that the mixing of several phages that recognize different host receptors is pertinent to effectively reducing the occurrence of phage-resistant bacteria (42 – 44).
Our experimental findings demonstrate that Kp21r exhibited susceptibility to øKp_16, 17, 18, 19, 20, 23, and 26, whereas Kp21 was exclusively susceptible to øKp_21 (Fig. 6). This outcome suggests that phage-resistant bacteria are more vulnerable to attacks by other phages present in the environment during an evolutionary arms race. Adsorption assays revealed that øKp_21 lacks the ability to adsorb to Kp21r. SNP analysis in Kp21 and Kp21r revealed insertion mutations in at least two genes: cpsA, which encodes a putative capsular biosynthesis protein, and thpA, which encodes a sugar ABC transporter substrate-binding protein. It has been reported that capsular polysaccharides function as the barrier to infect phages (45). In a recent publication, a newly discovered phage that recognizes the capsular polysaccharide of K. pneumoniae was reported (46), suggesting that capsular polysaccharide may be a contributing factor enabling øKp_21 to adsorb to its host, Kp21. We observed that the inclusion of Slopekvirus in the phage cocktail diminished the lawn density of Kp21r on plates. We posit that this phenomenon was caused by lysis mechanisms such as “lysis from without (LO)” or “rapid lysis” (47, 48). Gp5 in T4 phage, which has tail lysozyme, is known to cause LO (49). Gp5 forms a complex with the T4 phage tail, and when the T4 phage adsorbs to their host, Gp5 degrades the peptidoglycan layer. We found that Slopekvirus members, which were used in the phage cocktail in this study, encode a baseplate gene which has a domain of tail lysozyme (Table S8), possessing the capability to degrade peptidoglycan. SNP analysis of Kp21 and Kp21r suggests that Kp21r exhibits deficient capsular polysaccharide, thereby making the phage tail protein encoded in Slopekvirus more effective in degrading Kp21r peptidoglycan and leading to rapid lysis. Some studies have reported that phage-resistant bacteria, due to mutations in genes associated with antibiotic resistance, can become susceptible to antibiotics, and combining phages with antibiotics has been shown to effectively eliminate the target bacteria (50 – 53). Our findings demonstrate that combinations of phages and phage-encoded tail lysozyme efficiently eliminate and/or inhibit the growth of phage-resistant growth (54).
In conclusion, we have successfully isolated and characterized newly isolated phages capable of infecting K. pneumoniae and E. coli harboring the pKPI-6 plasmid, marking a pertinent step toward establishing a public phage bank and advancing phage therapy in Japan. Our phage sets can diminish the threat posed by K. pneumoniae harboring the pKPI-6 plasmid in clinical settings. Furthermore, our phage sets encompass an ample variety of phage types suitable for the development of a phage cocktail. However, the development of a high-throughput method is imperative to efficiently isolate and characterize additional new phages.
MATERIALS AND METHODS
Phage isolation and host information
A total of 32 IMP-6-producing isolates of K. pneumoniae and one IMP-6-producing isolate of E. coli were isolated from clinical settings in western Japan. Additionally, 29 newly isolated phages were collected from sewage in western Japan. To begin, 100 µL of sewage was mixed with an overnight culture of the indicator host, and this mixture was then added to 3 mL of 0.6% YT-soft agar prior to inoculation onto Luria-Bertani (LB) agar plates. The plates were subsequently incubated at 37°C overnight; after which, single-plaque isolation was performed. The plaques were suspended in 1 mL of LB medium and incubated for 2 h. Next, 50 µL of chloroform (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan) was added to each solution, and the mixture was vortexed before being centrifuged at 10,000 × g for 10 min at 4°C. The supernatants were then mixed with individual indicator hosts and incubated at 37°C overnight on LB agar plates. The single-plaque isolation procedure was repeated three times, and the isolated phages were stored at 4°C until further use. Kp21 was renamed from the K. pneumoniae f22 strain.
Phage propagation and purification
Pre-cultured host strains were inoculated into 3 mL of fresh LB medium (diluted 1:100) and incubated at 37°C until reaching an OD600 of 0.5. Subsequently, each phage, originally isolated using the indicated host, was added to the culture and incubated at 37°C with shaking at 200 rpm for 4–6 h. Following the lysis, 50 µL of chloroform (FUJIFILM Wako Pure Chemical Corporation) was added to 1 mL of the phage lysate, vortexed, and then centrifuged at 9,100 × g for 10 min. The resulting supernatants were filtered through a 0.22 µm pore-size membrane (Millipore, MA, USA). Cesium chloride (CsCl) density gradient phage purification was performed as described previously (55, 56) with some modifications. Specifically, 10% polyethylene glycol 6000 (FUJIFILM Wako Pure Chemical Corporation) and 0.5 M NaCl were added to the phage lysates and kept at 4°C for 1.5 h. Following this, the phage lysates were centrifuged at 10,000 × g for 30 min, and the resulting phage pellets were suspended in 1 mL of TM buffer (10 mM Tris-HCl and 5 mM MgCl2 [pH 7.5]). Subsequently, 100 µg/mL of DNase I (Roche, Basel, Switzerland) and RNase I (Thermo Fisher Scientific, MA, USA) were added to the phage solution and incubated at 37°C for 30 min. CsCl (FUJIFILM Wako Pure Chemical Corporation) at three different densities (ρ = 1.3, 1.5, and 1.7) and the phage solution were layered in tubes and ultracentrifuged (Optima MAX-TL; Beckman Coulter, California, USA) at 100,000 × g for 1 h. The resulting phage bands were then collected and dialyzed in SM buffer (25 mM Tris-HCl [pH 7.5], 100 mM NaCl, and 8 mM MgSO4).
Phage propagation and electron microscopic imaging
Copper mesh grids coated with formvar and carbon (Veco grids; Nissin EM, Tokyo, Japan) were glow-discharged and then placed on drops of the phage suspension for 1 min. Subsequently, the grids were rinsed with distilled water and stained with a 2% uranyl acetate solution. The samples were examined using transmission electron microscopy (HT7700; Hitachi Ltd., Tokyo, Japan) at 80 kV.
Host range determination and EOP assay
Each host was incubated at 37°C overnight, and 100 µL of each overnight culture was mixed with 100 µL of each phage suspension. Next, 5 mL of 0.6% YT-soft agar was added to the host-phage mixture and inoculated onto LB agar plates. The plates were then incubated at 37°C overnight, and the number of plaques on each plate was counted. The EOP was calculated using the formula below:
The detection limit for EOP was set at 10−4 pfu. EOP values were measured for all phage-bacteria combinations. Each plaque image was captured using a scan1200 (Interscience, Montpellier, France) for measuring their sizes. The size of each plaque size (mm2) was measured using Fiji (https://fiji.sc) version 2.3.0, with a conversion factor of 11 pixels per millimeter. For very small plaques, the edge of individual plaques was detected using the “find edge” tool in Fiji. Ten plaque areas were measured for each phage-host combination if the number of plaques on the plate exceeded 10.
OD600 kinetics and cocktail experiment
The host colony was pre-cultured in LB medium overnight at 37°C. Subsequently, the pre-cultured bacteria were inoculated (1:100) into fresh LB medium and incubated at 37°C with shaking at 200 rpm until reaching an OD600 of 0.1. Each indicated phage was added to the culture at a concentration of 1.0 × 108 pfu, and the mixed culture was incubated at 37°C with shaking at 200 rpm. The OD600 was measured at appropriate time intervals over a 24-h period. In the phage cocktail experiments, a combination of 10 phages (øKp_16, 17, 18, 19, 20, 21, 22, 23, 24, and 26) was mixed at a concentration of 1.0 × 107 pfu/mL for each individual phage. All experiments in this section were performed in triplicate.
Host and phage genome sequences
All phage genomic DNA was extracted using the Norgen phage DNA isolation kit (Norgen Biotak, Birmingham, UK), following the manufacturer’s instructions. Each phage DNA library was constructed using the QIA seq FX DNA library kit (Qiagen), and sequencing was performed on the Illumina MiSeq platform. Genome assembly was performed using Shovill with default settings. Phage contigs were filtered based on a contig length <200 and coverage of <25. Bacteria and phage strains were annotated using prokka (57) or PGAP (58) version 2021-07-01.build5508. For Nanopore long-read sequencing, we used the Monarch HMW DNA Extraction Kit for Tissue (NEB, MA, USA) following the manufacturer’s instructions.
A long-read library was prepared using the Rapid Barcoding kit (Oxford Nanopore Technologies, Oxford, United Kingdom, catalog number: SQK-RBK004) and sequenced on an R9 flow cell (Oxford Nanopore Technologies, catalog number: FLO-MIN106) using a GridION device (Oxford Nanopore Technologies). Basecalling was performed using Guppy version 5.0.12 in high accuracy mode. The obtained long reads, as well as the MiSeq short reads, which were trimmed using fastp v0.20.1, were assembled using Unicycler v0.4.8 with default parameters. Annotation was conducted using PGAP version 2021-07-01.build5508.
Bioinformatics analysis
For protein prediction in phages, we constructed the phage protein databases from the International Committee on Taxonomy of Viruses (ICTV), which comprise 4,312 genomes and 4,62,579 proteins (https://www.ncbi.nlm.nih.gov/genomes/GenomesGroup.cgi?taxid=28883) (September 2021), using local blastp (59) with an e-value threshold of <2e−20. Protein domains were identified using hmmer (https://www.ebi.ac.uk/Tools/hmmer/) with the Pfam-A 35.0 database, employing an e-value of <1e−10. Phage classification was determined based on the NCBI GenBank and ICTV. The average nucleotide identity was calculated using the average_nucleotide_identity.py program in the pyani packages (60). MUMmer was used to align nucleotide sequences. AMR genes and virulence genes were detected using ABRicate version 1.0.1 (https://github.com/tseemann/abricate) under default settings. The ResFinder database was used to extract AMR genes (61), and the Virulence Factors Database was used to extract virulence genes (62). The packaging mechanism and terminal repeats were analyzed using Phagetermvirome version 4.0.1 (63), and tRNAs were detected using tRNAscan-SE 2.0 (64). Kp21 and Kp21r SNP analysis was performed using SNIPPY (65) with default settings. The anti-defense system in each phage was predicted using AcrFinder (66), employing default settings.Each sequence type and serotype based on the K locus were determined using Kleborate (67). Each phage lifecycle was presumed using PhaTYP (68).
Taxonomic classification
Taxonomy classification was performed using vConTACT2 v0.11.3 with the Prokaryotic Viral RefSeq211-Merged database, under default settings (19). The resulting network was visualized using cytoscape. Each proposed taxonomy was further validated using the ICTV 2022 taxonomy classification. All phage classifications are available in Table S2. ANI was conducted using the average_nucleotide_identity.py program in pyani packages (60).
Phage-resistant Kp21 (Kp21r) and Kp22 (Kp22r) strains
Kp21 and Kp22 were cultured with øKp_21 or øKp_22, respectively, at 37°C. After 24 h of incubation, 1 mL of each culture was centrifuged at 4,400 × g for 10 min. The supernatant was discarded, and the pellets were washed with LB medium. This washing procedure was repeated twice. Subsequently, the pellet was resuspended in saline solution (0.85% NaCl), and the suspension was plated on LB agar and incubated at 37°C overnight. A single colony was selected and incubated overnight at 37°C with shaking at 200 rpm. The glycerol stock of the Kp21r culture was stored at −80°C until further use.
Kp21 and Kp21r adsorption assay
Kp21 and Kp21r were cultured in LB medium and incubated at 37°C until reaching an OD600 of 0.5. Subsequently, 3.0 × 106 pfu of øKp21 was added (MOI = 0.01) and incubated at 37°C with shaking at 200 rpm for 5 min. Next, 20 µL of chloroform was added to 200 µL of the mixture and vortexed. The samples were then centrifuged at 9,100 × g for 1 min, and the supernatant was collected. A 100 µL aliquot of the supernatant was mixed with Kp21, and plaque assays were performed to determine the number of unadsorbed phages. The percentage of unadsorbed phages was calculated using the formula below:
Characterization of switched phage sensitivity between Kp21 and Kp21r
The phage sensitivity of Kp21 and Kp21r was examined. Briefly, 1.0 × 109 pfu of each phage included in the phage cocktail were mixed with 100 µL of overnight Kp21 or Kp21r culture. Subsequently, 5 mL of 0.6% YT-soft agar was added to the host-phage mixture and then poured onto LB agar and incubated at 37°C overnight. The colony count was examined as follows: Kp21 and Kp21r were incubated at 37°C until reaching an OD600 of 0.1. Then, each phage was added at 1.0 × 109 pfu, and the mixture was incubated at 37°C. After 2 h of incubation following phage addition, the mixture was collected and centrifuged at 3,300 × g for 15 min. The supernatant was discarded, and the pellet was suspended in 500 µL of phosphate-buffered saline (0.137 M NaCl, 0.27 mM KCl, 0.1 M Na2HPO4, 18 mM KH2PO4). The suspension was diluted to 10−2 and 10−4, and 100 µL of each dilution was spread onto LB agar.
One-step growth experiment
Host K. pneumoniae was grown overnight at 37°C. The overnight culture was diluted 1:100 in LB medium and incubated until the OD600 reached 0.5. Each phage was added at an MOI of 0.25 or 0.5 against each host strain and incubated in the water bath for 6 min at 37°C for phage adsorption to the host. Then, 100 µL of the culture was withdrawn and centrifuged at 13,000 × g for 2 min, and the supernatant was transferred to 96-well tubes. Additionally, 1 µL of the culture was transferred to 10 mL of fresh LB medium. Samples were withdrawn every 8 min, and 100 µL of each sample was centrifuged at 13,000 × g for 1 min, and the supernatant was transferred to 96-well plates. Tenfold dilutions were spotted on 0.6% LB-soft agar containing each host showing a higher EOP. The burst size was calculated using the formula below:
Statistical analysis
All statistical analyses were conducted using a two-sided Student t test with Python version 3.9.7 and the SciPy Module version 1.4.1. A P-value less than 0.05 was considered statistically significant.
ACKNOWLEDGMENTS
We thank members of the Laboratory of Microbiology at Kawasaki Medical School, including Professor Mineki Saito for their support.
This work was partially supported by JSPS KAKENHI (21K1633 to K.K., 18K08455 and 22K08592 to M.K.) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan; grants (19lm0203008j0003 A058 and 22ym0126811j0001 A255 to M.K.) from the Japan Agency for Medical Research and Development (AMED) and Research and Development Grant Program from Egashira Zaidan to M.K.; and grants (JP21fk0108604j0001, 22fk0108604j0002, 21fk0108132j0002 to M.S.) from Research Program on Emerging and Re-emerging infection Diseases (AMED).
Contributor Information
Mitsuoki Kawano, Email: mkawano@nakamura-u.ac.jp.
Daria Van Tyne, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA .
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/spectrum.04761-22.
Fig. S1 to Fig. S6.
Tables S1 to S8.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Effah CY, Sun T, Liu S, Wu Y. 2020. Klebsiella pneumoniae: an increasing threat to public health. Ann Clin Microbiol Antimicrob 19:1. doi: 10.1186/s12941-019-0343-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yamagishi T, Matsui M, Sekizuka T, Ito H, Fukusumi M, Uehira T, Tsubokura M, Ogawa Y, Miyamoto A, Nakamori S, Tawa A, Yoshimura T, Yoshida H, Hirokawa H, Suzuki S, Matsui T, Shibayama K, Kuroda M, Oishi K. 2020. A prolonged multispecies outbreak of IMP-6 carbapenemase-producing enterobacterales due to horizontal transmission of the IncN plasmid. Sci Rep 10:4139. doi: 10.1038/s41598-020-60659-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Abe R, Akeda Y, Sugawara Y, Takeuchi D, Matsumoto Y, Motooka D, Yamamoto N, Kawahara R, Tomono K, Fujino Y, Hamada S, Langelier C. 2020. Characterization of the plasmidome encoding carbapenemase and mechanisms for dissemination of carbapenem-resistant Enterobacteriaceae. mSystems 5:e00759-20. doi: 10.1128/mSystems.00759-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nordmann P, Naas T, Poirel L. 2011. Global spread of carbapenemase producing Enterobacteriaceae. Emerg Infect Dis 17:1791–1798. doi: 10.3201/eid1710.110655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kayama S, Shigemoto N, Kuwahara R, Oshima K, Hirakawa H, Hisatsune J, Jové T, Nishio H, Yamasaki K, Wada Y, Ueshimo T, Miura T, Sueda T, Onodera M, Yokozaki M, Hattori M, Ohge H, Sugai M. 2015. Complete nucleotide sequence of the IncN plasmid encoding Imp-6 and CTX-M-2 from emerging carbapenem-resistant Enterobacteriaceae in Japan. Antimicrob Agents Chemother 59:1356–1359. doi: 10.1128/AAC.04759-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shigemoto N, Kuwahara R, Kayama S, Shimizu W, Onodera M, Yokozaki M, Hisatsune J, Kato F, Ohge H, Sugai M. 2012. Emergence in Japan of an imipenem-susceptible, meropenem-resistant Klebsiella pneumoniae carrying BLA IMP-6. Diagn Microbiol Infect Dis 72:109–112. doi: 10.1016/j.diagmicrobio.2011.09.019 [DOI] [PubMed] [Google Scholar]
- 7. Cao F, Wang X, Wang L, Li Z, Che J, Wang L, Li X, Cao Z, Zhang J, Jin L, Xu Y. 2015. Evaluation of the efficacy of a bacteriophage in the treatment of pneumonia induced by multidrug resistance Klebsiella pneumoniae in mice. Biomed Res Int 2015:752930. doi: 10.1155/2015/752930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Petrovic Fabijan A, Lin RCY, Ho J, Maddocks S, Ben Zakour NL, Iredell JR, Westmead Bacteriophage Therapy Team, Khalid A, Venturini C, Chard R, Morales S, Sandaradura I, Gilbey T. 2020. Safety of bacteriophage therapy in severe Staphylococcus aureus infection. Nat Microbiol 5:465–472. doi: 10.1038/s41564-019-0634-z [DOI] [PubMed] [Google Scholar]
- 9. Dedrick RM, Guerrero-Bustamante CA, Garlena RA, Russell DA, Ford K, Harris K, Gilmour KC, Soothill J, Jacobs-Sera D, Schooley RT, Hatfull GF, Spencer H. 2019. Engineered bacteriophages for treatment of a patient with a disseminated drug-resistant Mycobacterium abscessus. Nat Med 25:730–733. doi: 10.1038/s41591-019-0437-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Eskenazi A, Lood C, Wubbolts J, Hites M, Balarjishvili N, Leshkasheli L, Askilashvili L, Kvachadze L, van Noort V, Wagemans J, Jayankura M, Chanishvili N, de Boer M, Nibbering P, Kutateladze M, Lavigne R, Merabishvili M, Pirnay J-P. 2022. Combination of pre-adapted bacteriophage therapy and antibiotics for treatment of fracture-related infection due to pandrug-resistant Klebsiella pneumoniae. Nat Commun 13:302. doi: 10.1038/s41467-021-27656-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Oechslin F. 2018. Resistance development to bacteriophages occurring during bacteriophage therapy. Viruses 10:351. doi: 10.3390/v10070351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Oechslin F, Piccardi P, Mancini S, Gabard J, Moreillon P, Entenza JM, Resch G, Que YA. 2017. Synergistic interaction between phage therapy and antibiotics clears Pseudomonas aeruginosa infection in endocarditis and reduces virulence. J Infect Dis 215:703–712. doi: 10.1093/infdis/jiw632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schooley RT, Biswas B, Gill JJ, Hernandez-Morales A, Lancaster J, Lessor L, Barr JJ, Reed SL, Rohwer F, Benler S, Segall AM, Taplitz R, Smith DM, Kerr K, Kumaraswamy M, Nizet V, Lin L, McCauley MD, Strathdee SA, Benson CA, Pope RK, Leroux BM, Picel AC, Mateczun AJ, Cilwa KE, Regeimbal JM, Estrella LA, Wolfe DM, Henry MS, Quinones J, Salka S, Bishop-Lilly KA, Young R, Hamilton T. 2017. Development and use of personalized bacteriophage-based therapeutic cocktails to treat a patient with a disseminated resistant Acinetobacter baumannii infection. Antimicrob Agents Chemother 61. doi: 10.1128/AAC.00954-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yerushalmy O, Khalifa L, Gold N, Rakov C, Alkalay-Oren S, Adler K, Ben-Porat S, Kraitman R, Gronovich N, Shulamit Ginat K, Abdalrhman M, Coppenhagen-Glazer S, Nir-Paz R, Hazan R. 2020. The Israeli Phage Bank (IPB). Antibiotics (Basel) 9:269. doi: 10.3390/antibiotics9050269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nagel T, Musila L, Muthoni M, Nikolich M, Nakavuma JL, Clokie MR. 2022. Phage banks as potential tools to rapidly and cost-effectively manage antimicrobial resistance in the developing world. Curr Opin Virol 53:101208. doi: 10.1016/j.coviro.2022.101208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Savalia D, Westblade LF, Goel M, Florens L, Kemp P, Akulenko N, Pavlova O, Padovan JC, Chait BT, Washburn MP, Ackermann HW, Mushegian A, Gabisonia T, Molineux I, Severinov K. 2008. Genomic and proteomic analysis of PhiEco32, a novel Escherichia coli bacteriophage. J Mol Biol 377:774–789. doi: 10.1016/j.jmb.2007.12.077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mirzaei MK, Eriksson H, Kasuga K, Haggård-Ljungquist E, Nilsson AS. 2014. Genomic, proteomic, morphological, and phylogenetic analyses of vB-EcoP-SU10, a podoviridae phage with C3 morphology. PloS One 9:1–19. doi: 10.1371/journal.pone.0116294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Koonjan S, Cooper CJ, Nilsson AS. 2021. Complete genome sequence of vb_ecop_su7, a podoviridae coliphage with the rare C3 morphotype. Microorganisms 9:1–11. doi: 10.3390/microorganisms9081576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bin Jang H, Bolduc B, Zablocki O, Kuhn JH, Roux S, Adriaenssens EM, Brister JR, Kropinski AM, Krupovic M, Lavigne R, Turner D, Sullivan MB. 2019. Taxonomic assignment of uncultivated prokaryotic virus genomes is enabled by gene-sharing networks. Nat Biotechnol 37:632–639. doi: 10.1038/s41587-019-0100-8 [DOI] [PubMed] [Google Scholar]
- 20. Sofy AR, El-Dougdoug NK, Refaey EE, Dawoud RA, Hmed AA. 2021. Characterization and full genome sequence of novel kpp-5 Lytic Phage against llebsiella pneumoniae responsible for recalcitrant infection. Biomedicines 9:342. doi: 10.3390/biomedicines9040342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. King MR, Vimr RP, Steenbergen SM, Spanjaard L, Plunkett G, Blattner FR, Vimr ER. 2007. Escherichia coli K1-specific bacteriophage CUS-3 distribution and function in phase-variable capsular polysialic acid O acetylation. J Bacteriol 189:6447–6456. doi: 10.1128/JB.00657-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pick K, Ju T, Willing BP, Raivio TL. 2022. Isolation and characterization of a novel temperate Escherichia coli bacteriophage, kapi1, which modifies the O-antigen and contributes to the competitiveness of its host during colonization of the murine gastrointestinal tract. mBio 13:e0208521. doi: 10.1128/mbio.02085-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Peng Q, Fang M, Liu X, Zhang C, Liu Y, Yuan Y. 2020. Isolation and characterization of a novel phage for controlling multidrug-resistant Klebsiella pneumoniae. Microorganisms 8:542. doi: 10.3390/microorganisms8040542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Erickson SG, Lessor L, O’Leary CJ, Gill JJ, Liu M. 2018. Complete genome sequence of Klebsiella pneumoniae siphophage Sugarland. Microbiol Resour Announc 7:e01014-18. doi: 10.1128/MRA.01014-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Xing S, Pan X, Sun Q, Pei G, An X, Mi Z, Huang Y, Zhao B, Tong Y. 2017. Complete genome sequence of a novel multidrug-resistant Klebsiella pneumoniae phage, vB_Kpn_IME260. Genome Announc 5. doi: 10.1128/genomeA.00055-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Koberg S, Brinks E, Fiedler G, Hüsing C, Cho GS, Hoeppner MP, Heller KJ, Neve H, Franz CMAP. 2017. Genome sequence of Klebsiella pneumoniae bacteriophage PMBT1 isolated from raw sewage. Genome Announc 5:4–5. doi: 10.1128/genomeA.00914-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mijalis EM, Lessor LE, Cahill JL, Rasche ES, Kuty Everett GF. 2015. Complete genome sequence of Klebsiella pneumoniae carbapenemase-producing K. pneumoniae myophage Miro. Genome Announc 3:e01137-15. doi: 10.1128/genomeA.01137-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Martin C, Lessor L, Clark J, Le T, Gill JJ, Young R, Liu M. 2021. Complete genome sequence of Klebsiella pneumoniae myophage muenster. Microbiol Resour Announc 10:e01403-20. doi: 10.1128/MRA [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kim Y, Lee SM, Nong LK, Kim J, Kim SB, Kim D. 2022. Characterization of Klebsiella pneumoniae bacteriophages, KP1 and KP12, with deep learning-based structure prediction. Front Microbiol 13:990910. doi: 10.3389/fmicb.2022.990910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mora D, Lessor L, Le T, Clark J, Gill JJ, Liu M. 2021. Complete genome sequence of Klebsiella pneumoniae jumbo phage miami. Microbiol Resour Announc 10:e01404-20. doi: 10.1128/MRA.01404-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Borges AL, Davidson AR, Bondy-Denomy J. 2017. The discovery, mechanisms, and evolutionary impact of anti-CRISPRs. Annu Rev Virol 4:37–59. doi: 10.1146/annurev-virology-101416-041616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shehreen S, Birkholz N, Fineran PC, Brown CM. 2022. Widespread repression of anti-CRISPR production by anti-CRISPR-associated proteins. Nucleic Acids Res 50:8615–8625. doi: 10.1093/nar/gkac674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Terwilliger A, Clark J, Karris M, Hernandez-Santos H, Green S, Aslam S, Maresso A. 2021. Phage therapy related microbial succession associated with successful clinical outcome for a recurrent urinary tract infection. Viruses 13:2049. doi: 10.3390/v13102049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Federici S, Kredo-Russo S, Valdés-Mas R, Kviatcovsky D, Weinstock E, Matiuhin Y, Silberberg Y, Atarashi K, Furuichi M, Oka A, Liu B, Fibelman M, Weiner IN, Khabra E, Cullin N, Ben-Yishai N, Inbar D, Ben-David H, Nicenboim J, Kowalsman N, Lieb W, Kario E, Cohen T, Geffen YF, Zelcbuch L, Cohen A, Rappo U, Gahali-Sass I, Golembo M, Lev V, Dori-Bachash M, Shapiro H, Moresi C, Cuevas-Sierra A, Mohapatra G, Kern L, Zheng D, Nobs SP, Suez J, Stettner N, Harmelin A, Zak N, Puttagunta S, Bassan M, Honda K, Sokol H, Bang C, Franke A, Schramm C, Maharshak N, Sartor RB, Sorek R, Elinav E. 2022. Targeted suppression of human IBD-associated gut microbiota commensals by phage consortia for treatment of intestinal inflammation. Cell 185:2879–2898. doi: 10.1016/j.cell.2022.07.003 [DOI] [PubMed] [Google Scholar]
- 35. Anand T, Bera BC, Vaid RK, Barua S, Riyesh T, Virmani N, Hussain M, Singh RK, Tripathi BN. 2016. Abundance of antibiotic resistance genes in environmental bacteriophages. J Gen Virol 97:3458–3466. doi: 10.1099/jgv.0.000639 [DOI] [PubMed] [Google Scholar]
- 36. Gómez-Gómez C, Blanco-Picazo P, Brown-Jaque M, Quirós P, Rodríguez-Rubio L, Cerdà-Cuellar M, Muniesa M. 2019. Infectious phage particles packaging antibiotic resistance genes found in meat products and chicken feces. Sci Rep 9:13281. doi: 10.1038/s41598-019-49898-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kohei K, Mitsuoki K, Motoyuki S, Mariana C. 2021. Distribution of antimicrobial resistance and virulence genes within the prophage-associated regions in nosocomial pathogens. mSphere:e00452-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Haines MEK, Hodges FE, Nale JY, Mahony J, van Sinderen D, Kaczorowska J, Alrashid B, Akter M, Brown N, Sauvageau D, Sicheritz-Pontén T, Thanki AM, Millard AD, Galyov EE, Clokie MRJ. 2021. Analysis of selection methods to develop novel phage therapy cocktails against antimicrobial resistant clinical isolates of bacteria. Front Microbiol 12:613529. doi: 10.3389/fmicb.2021.613529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Molina F, Simancas A, Ramírez M, Tabla R, Roa I, Rebollo JE. 2021. A new pipeline for designing phage cocktails based on phage-bacteria infection networks. Front Microbiol 12:564532. doi: 10.3389/fmicb.2021.564532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Molina Felipe, Menor-Flores M, Fernández L, Vega-Rodríguez MA, García P. 2022. Systematic analysis of putative phage – phage interactions on minimum – sized phage cocktails. Sci Rep 12:2458. doi: 10.1038/s41598-022-06422-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Paczosa MK, Mecsas J. 2016. Klebsiella pneumoniae: going on the offense with a strong defense. Microbiol Mol Biol Rev 80:629–661. doi: 10.1128/MMBR.00078-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tanji Y, Shimada T, Yoichi M, Miyanaga K, Hori K, Unno H. 2004. Toward rational control of Escherichia coli O157:H7 by a phage cocktail. Appl Microbiol Biotechnol 64:270–274. doi: 10.1007/s00253-003-1438-9 [DOI] [PubMed] [Google Scholar]
- 43. Hesse S, Rajaure M, Wall E, Johnson J, Bliskovsky V, Gottesman S, Adhya S. 2020. Phage resistance in multidrug-resistant Klebsiella pneumoniae ST258 evolves via diverse mutations that culminate in impaired adsorption. mBio 11:1–14. doi: 10.1128/mBio.02530-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gordillo Altamirano FL, Barr JJ. 2021. Unlocking the next generation of phage therapy: the key is in the receptors. Curr Opin Biotechnol 68:115–123. doi: 10.1016/j.copbio.2020.10.002 [DOI] [PubMed] [Google Scholar]
- 45. Olszak T, Shneider MM, Latka A, Maciejewska B, Browning C, Sycheva LV, Cornelissen A, Danis-Wlodarczyk K, Senchenkova SN, Shashkov AS, Gula G, Arabski M, Wasik S, Miroshnikov KA, Lavigne R, Leiman PG, Knirel YA, Drulis-Kawa Z. 2017. The O-specific polysaccharide lyase from the phage LKA1 tailspike reduces Pseudomonas virulence. Sci Rep 7:16302. doi: 10.1038/s41598-017-16411-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hao G, Shu R, Ding L, Chen X, Miao Y, Wu J, Zhou H, Wang H. 2021. Bacteriophage SRD2021 recognizing capsular polysaccharide shows therapeutic potential in serotype K47 Klebsiella pneumoniae infections. Antibiotics (Basel) 10:894. doi: 10.3390/antibiotics10080894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chegini Z, Khoshbayan A, Taati Moghadam M, Farahani I, Jazireian P, Shariati A. 2020. Bacteriophage therapy against Pseudomonas aeruginosa biofilms: a review. Ann Clin Microbiol Antimicrob 19:45. doi: 10.1186/s12941-020-00389-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Liao Y-T, Zhang Y, Salvador A, Harden LA, Wu VCH. 2022. Characterization of a T4-like bacteriophage vB_EcoM-Sa45lw as a potential biocontrol agent for shiga toxin-producing Escherichia coli O45 contaminated on Mung bean seeds. Microbiol Spectr 10:e0222021. doi: 10.1128/spectrum.02220-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ye N, Nemoto N. 2004. Processing of the tail lysozyme (gp5) of bacteriophage T4. J Bacteriol 186:6335–6339. doi: 10.1128/JB.186.18.6335-6339.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Burmeister AR, Fortier A, Roush C, Lessing AJ, Bender RG, Barahman R, Grant R, Chan BK, Turner PE. 2020. Pleiotropy complicates a trade-off between phage resistance and antibiotic resistance. Proc Natl Acad Sci U S A 117:11207–11216. doi: 10.1073/pnas.1919888117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gurney J, Pradier L, Griffin JS, Gougat-Barbera C, Chan BK, Turner PE, Kaltz O, Hochberg ME. 2020. Phage steering of antibiotic-resistance evolution in the bacterial pathogen, Pseudomonas aeruginosa Evol Med Public Health 2020:148–157. doi: 10.1093/emph/eoaa026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Liu CG, Green SI, Min L, Clark JR, Salazar KC, Terwilliger AL, Kaplan HB, Trautner BW, Ramig RF, Maresso AW. 2020. Phage-antibiotic synergy is driven by a unique combination of antibacterial mechanism of action and stoichiometry. mBio. doi: 10.1101/2020.02.27.967034 [DOI] [PMC free article] [PubMed]
- 53. Nakamura K, Fujiki J, Nakamura T, Furusawa T, Gondaira S, Usui M, Higuchi H, Tamura Y, Iwano H. 2021. Fluctuating bacteriophage-induced galU deficiency region is involved in trade-off effects on the phage and fluoroquinolone sensitivity in Pseudomonas aeruginosa. Virus Res 306:198596. doi: 10.1016/j.virusres.2021.198596 [DOI] [PubMed] [Google Scholar]
- 54. Roach DR, Donovan DM. 2015. Antimicrobial bacteriophage-derived proteins and therapeutic applications. Bacteriophage 5:e1062590. doi: 10.1080/21597081.2015.1062590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Uchiyama J, Rashel M, Maeda Y, Takemura I, Sugihara S, Akechi K, Muraoka A, Wakiguchi H, Matsuzaki S. 2008. Isolation and characterization of a novel Enterococcus faecalis bacteriophage Φef24C as a therapeutic candidate. FEMS Microbiol Lett 278:200–206. doi: 10.1111/j.1574-6968.2007.00996.x [DOI] [PubMed] [Google Scholar]
- 56. Kitamura N, Sasabe E, Matsuzaki S, Daibata M, Yamamoto T. 2020. Characterization of two newly isolated Staphylococcus aureus bacteriophages from Japan belonging to the genus silviavirus. Arch Virol 165:2355–2359. doi: 10.1007/s00705-020-04749-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Seemann T. 2014. Prokka: rapid prokaryotic genome annotation. Bioinformatics 30:2068–2069. doi: 10.1093/bioinformatics/btu153 [DOI] [PubMed] [Google Scholar]
- 58. Zaslavsky L, Ciufo S, Fedorov B, Tatusova T. 2016. Clustering analysis of proteins from microbial genomes at multiple levels of resolution. BMC Bioinformatics 17 Suppl 8:276. doi: 10.1186/s12859-016-1112-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. J Mol Biol 215:403–410. doi: 10.1016/S0022-2836(05)80360-2 [DOI] [PubMed] [Google Scholar]
- 60. Pritchard L, Glover RH, Humphris S, Elphinstone JG, Toth IK. 2016. Genomics and taxonomy in diagnostics for food security: soft-rotting enterobacterial plant pathogens. Anal Methods 8:12–24. doi: 10.1039/C5AY02550H [DOI] [Google Scholar]
- 61. Zankari E, Hasman H, Cosentino S, Vestergaard M, Rasmussen S, Lund O, Aarestrup FM, Larsen MV. 2012. Identification of acquired antimicrobial resistance genes. J Antimicrob Chemother 67:2640–2644. doi: 10.1093/jac/dks261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Chen L, Zheng D, Liu B, Yang J, Jin Q. 2016. VFDB 2016: hierarchical and refined dataset for big data analysis – 10 years on. Nucleic Acids Res 44:D694–D697. doi: 10.1093/nar/gkv1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Garneau JR, Legrand V, Marbouty M, Press MO, Vik DR, Fortier L-C, Sullivan MB, Bikard D, Monot M. 2021. High-throughput identification of viral termini and packaging mechanisms in virome datasets using PhageTermVirome. Sci Rep 11:1–9. doi: 10.1038/s41598-021-97867-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Chan PP, Lin BY, Mak AJ, Lowe TM. 2021. TRNAscan-SE 2.0: improved detection and functional classification of transfer RNA genes. Nucleic Acids Res 49:9077–9096. doi: 10.1093/nar/gkab688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Page AJ, Taylor B, Delaney AJ, Soares J, Seemann T, Keane JA, Harris SR. 2016. SNP-sites: rapid efficient extraction of SNPs from multi-FASTA alignments. Microb Genom 2:e000056. doi: 10.1099/mgen.0.000056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Yi H, Huang L, Yang B, Gomez J, Zhang H, Yin Y. 2020. Acrfinder: genome mining anti-CRISPR operons in prokaryotes and their viruses. Nucleic Acids Res 48:W358–W365. doi: 10.1093/nar/gkaa351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lam MMC, Wick RR, Watts SC, Cerdeira LT, Wyres KL, Holt KE. 2021. A genomic surveillance framework and genotyping tool for Klebsiella pneumoniae and its related species complex. Nat Commun 12:4188. doi: 10.1038/s41467-021-24448-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Shang J, Tang X, Sun Y. 2023. PhaTYP: predicting the lifestyle for bacteriophages using BERT. Brief Bioinform 24:24. doi: 10.1093/bib/bbac487 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 to Fig. S6.
Tables S1 to S8.