Abstract
People who have dementia with Lewy bodies often have sleep disorders. We used non-wearable devices to record and categorize the sleep patterns of patients with Lewy body dementia. Individual sleep data at a dementia–care unit in Japan were recorded using non-wearables. One week’s worth of data from 18 patients was analyzed. Median metrics for all participants were the following: sleep efficiency, 68% (23-89); sleep duration at night, 6.8 hours (1.6-11.1); times getting out of bed at night, 3.5 (0-13). We identified three types of abnormal sleep: extremely short sleep duration, excessive sleep duration at night, and excessive number of times getting out of bed at night. Sleep disturbances in Lewy body dementia patients are treated using various practices; staff must choose the most effective plan for each patient’s situation. Monitoring patient sleep using non-wearable provides more objective data that can help staff better personalize nursing care.
Keywords: nursing care, non-wearable devices, dementia with Lewy bodies, sleep
Significance Statement
• Quantification of long-term sleep status in DLB patients.
• Development of indicators of medication reconciliation for DLB patients with hypersensitivity to antipsychotic drugs
• Productive discussions with interdisciplinary teams and timely nursing interventions based on reliable data
Introduction
Patients with dementia with Lewy bodies (DLB) tend to have sleep disturbances that are part of the behavioral and psychological symptoms of dementia (BPSD). 1 DLB patients with sleep disturbances present with a variety of symptoms, including nocturnal awakenings, sleep talking, bad dreams, excessive daytime somnolence, confusional arousals, periodic limb movements during sleep, sleep apnea, 2 and circadian rhythm disturbances. Among these, REM-sleep behavior disorder (RBD) is certainly an important sleep disorder in DLB, but the updated diagnostic guidelines, published in 2017, also point out the presence of other symptoms that can severely affect patients’ quality of life, such as sleep-wake rhythm disturbances and excessive daytime sleepiness. These rhythm disturbances are clinically very problematic, and hypersomnia and insomnia increase the burden on caregivers.3-5 However, little evidence exists for these sleep disorders of patients with dementia, and we must rely exclusively on clinical staff reports of their responses to drug prescriptions and of patients’ responses to occupational therapy and recreation during the day. There is a need, therefore, to visualize and accumulate evidence for various sleep disorders that appear in DLB patients.
However, there is a lack of robust evidence for interventions of sleep-wake rhythm disorders and hypersomnia in DLB. Previous studies show that pharmacotherapy is rarely used and reports abound for the use of non-pharmacological therapies. However, the level of evidence for non-pharmacological therapies is not high, and diagnostic guidelines suggest that evidence should be built on a combination of non-pharmacological and pharmacological therapies based on experience with Alzheimer’s disease (AD) and other types of dementia. 5 A Cochrane review updated in 2020 suggests that some drugs may have some effect on sleep disturbances in AD. 6 However, DLB patients are hypersensitive to antipsychotic medications and are at high risk of overreaction. 5 Therefore, it is necessary to carefully control each patient’s condition. Non-pharmacological therapies such as light therapy and occupational therapy have also been suggested, but individual sleep status must be assessed to implement these treatments. 5
With regard to pharmacotherapy, there are two randomized controlled trials that evaluated the efficiency of medications for DLB patients with sleep disorders. These studies used questionnaires to obtain data on sleep patterns, which is a difficult way to obtain correct information (especially about sleep status) from DLB patients because they often have memory loss. Furthermore, because cognitive level varies greatly from patient to patient, measurements cannot uniformly assess the sleep status of all individuals.7,8
Another randomized controlled trial evaluated a non-pharmacological therapy as light therapy, and collected data via a wearable actigraph rather than a questionnaire. 9 This approach had good ecological validity because the patients in the trial were in a nursing home; by contrast, patients in hospitals are more likely to have severe BPSD, making it difficult for them to wear an actigraph for extended periods of time. In addition, it is still difficult to correctly measure the sleep status of DLB patients with actigraphs, as the accuracy varies depending on the degree of Parkinsonism symptoms. 10 Thus, DLB is often accompanied by physical characteristics that make sleep-state measurement difficult. To treat sleep disorders in these patients, it is necessary for medical professionals to understand the patient’s rhythm patterns and actual sleep state, and to seek an individualized solution.
To date, there have been few studies that have monitored the sleep of patients with DLB over a long period of time. One case report described a study in which patients wore a polysomnogram for 3 days to measure specific sleep patterns in DLB, but it was difficult for patients to wear the polysomnogram continuously, which made it challenging to use in routine care. In another study, Ancoli-Israel et al. successfully monitored AD for several weeks using a wristwatch-type wearable actigraph. 11 However, long-term monitoring is considered impractical because the skin becomes weaker in old age, there is a risk that wearing an actigraph may lead to skin problems. Furthermore, if the actigraph has to be removed, such as during bathing, it may be cumbersome to remove it, making long-term monitoring impractical. Recently, there have been studies in which single-lead ECG patches were worn for 3 days by homebound dementia patients to check for the presence of sleep apnea, but the sleep data from those studies were not used for care. 12
Thus, few efforts are being made to use long-term sleep monitoring data for care. In our previous study, we used a non-wearable device to record the sleep disturbance data of AD patients 3 and found that the sleep states of individual patients could be continuously recorded. Importantly, once individual sleep states are ascertained, it is possible to intervene whenever necessary. In this study, sleep states were recorded from individual DLB patients using a non-wearable device to examine whether individual sleep data can be useful for nursing practice. We report three cases: one of a patient with extremely short sleep duration, one with excessive nighttime sleep duration, and one with excessive number of times getting out of bed at night.
Materials and Methods
This study had a prospective design, which was a part of a larger sleep monitoring project that used a non-wearable device called the Nemuri SCAN sensor to record sleep data. This device is commercially available from Paramount Bed Corporation (Tokyo, Japan). The sensor is placed under the mattress and detects small movements to determine whether the subject is asleep or awake. Furthermore, because it is a sheet-type device that is less than 15 mm thick, it does not interfere with the participant’s sleep behavior. The sensing device records sleep duration, time spent awake, time between bedtime and falling asleep, and sleep efficiency. Sleep efficiency is a measure of how efficient each person’s sleep is, and is calculated by dividing the number of minutes of sleep by the number of minutes spent in bed. This device has been validated and used in previous studies.3,13
In this project, after recording the individuals’ sleep data, we used them in nursing practice to identify sleep disturbances and devise treatments. The study period was from 2013 to 2015, and the setting was a 60-bed dementia–care unit for treating severe BPSD at General Hospital A in Osaka, Japan. Many of the ward staff who belong to the dementia–care unit had been trained by dementia specialists and specialized nurses for over 5 years, and therefore had a high degree of specialty in caring for patients with dementia. These staff monitored patients using a comprehensive assessment sheet and were excellent at making their assessments.
The ethics committee of Osaka University approved this research. Written informed consent was obtained from the patients or their families. In cases in which dementia symptoms were highly evident and the decision to participate was difficult, the decision was made and the consent form was signed by the patient’s family or a decision-making agent. The assessment as to whether the patients were able to make their own decisions was made by the patients’ physicians. The study was explained to the patients and we assigned ID numbers so that identification in the data would be anonymous.
Patient Selection and Characteristics
Inclusion criteria were as follows: (i) Diagnosis of probable DLB by psycho-geriatrists using published guidelines for diagnosis and management of DLB 5 ; (ii) a request for inclusion by the patient’s doctors or medical staff because of suspected sleep disturbances; (iii) monitoring for a total of at least 2 weeks, 1 week of which was the first week. As it was expected that there would be unintentional periods of time when data may not be obtained owing to the behavior of the dementia patients, the patients who had been monitored for a total of 2 weeks or more, 1 week of which was the first week, were included in the study. Of the 125 participants, 18 (mean age 78 ± 7.2 years; 8 men) satisfied the inclusion criteria.
Data Collection
We collected medical/nursing data (diagnosis, medical history, disease duration, nursing interventions, and patient conditions from medical records). Before monitoring their sleep, we assessed the degree of dementia in all patients using the Clinical Dementia Rating (CDR) scale, as well as cognitive function using the Mini-Mental State Examination (MMSE). The CDR is a tool for evaluating the severity of dementia and the score is defined by four stages: .5 = Very Mild Dementia, 1 = Mild Dementia, 2 = Moderate Dementia, and 3 = Severe Dementia. 14 Developed in 1975, the MMSE is a 30-point questionnaire (11 questions) used to measure cognitive function. Generally, scores lower than 23 are considered an indication of dementia. 15
How Sleep Data Were Used in Clinical Practice
We collected patient sleep data using the Nemuri SCAN sensor, assessed sleep states and daytime sleepiness, analyzed the data, and gave regular feedback to the staff about the analysis results. Patient-care plans were modified if researchers and staff decided it was appropriate after discussion. If intervention was needed, the staff carried out the new care plan.
At the researcher/staff meetings, we discussed the effects of medication and the need for non-pharmacological therapy. In addition, we discussed how to adjust the rhythm of the patients’ daily lives. We also discussed ways to care for sleep disturbances from various perspectives. When staff observed dementia symptoms such as resistance to nursing care and worsening irritability, or napping owing to the effects of drugs, they asked the attending physician whether the medication should be adjusted. If necessary, the attending physician prescribed a new medication or adjusted the current prescription. The following drugs were prescribed to the patients in this study: cholinesterase inhibitors, atypical antipsychotics, hypnotics, antidepressants, antiepileptic drugs, melatonin receptor agonists, and traditional Japanese Kampo medicine. Non-pharmacological therapy comprised light therapy and occupational therapy; the patients who frequently napped during the day or who tended to fall asleep participated in them. When patients could not remember where their room was or where the toilet was, medical staff assisted them to the appropriate location. When patients were negatively affected by other patients in the same room or by the room environment itself, medical staff moved them to another room. Physical restriction was also used if necessary.
This monitoring-feedback-adjustment cycle continued for more than 2 weeks. After measuring the participant’s sleep for 1 week using the device, the research staff collected the measured data, nursing records, and medical records, and prepared data on the participant’s sleep for that week. The data were then presented to the medical staff in a weekly conference, and both the medical staff and the research staff interpreted the data. On the basis of those interpretations, a nursing plan was formulated (formulated, revised, and added from the second week onward), and then the medical staff implemented the intervention and evaluated it. The above cycle was repeated once a week. In the conference, the medical and research staff evaluated the patient’s sleep status and decided whether to continue the intervention. The end point of the study was as follows: (i) Patient sleep status improved; (ii) patient sleep status did not improve by the end of the study and there was no hope for improvement.
Results
Our analysis of the data collected from the first week of monitoring 18 patients produced the following results. Medians for the recorded sleep parameters: sleep efficiency = 68% (range: 23-89); sleep duration at night = 6.8 hours (1.6-11.1); number of times patients got out of bed at night = 3.5 times (0-13).
The 90th percentile for sleep duration at night was 9.32 hours and two patients with longer sleep durations were considered to have excessive sleep duration at night. The 10th percentile for sleep duration at night was 2.65 hours. Two patients who slept less than that were considered to have extremely short sleep duration. The 90th percentile for times getting out of bed at night was 8.3 times. The two patients whose scores were above this threshold were considered to have gotten out of bed an excessive number of times at night. Among these six patients, we selected three for the case study series because they had sleep disorders that strongly affected their daily lives, and there was concern about prolonged hospitalization. We observed three types of sleep disorders in these three patients. The first, Ms A, regularly experienced extremely short sleep duration; the second, Ms B, regularly experienced excessive sleep duration at night; and the third, Ms C, regularly got out of bed at night an excessive number of times.
Extremely Short Sleep Duration
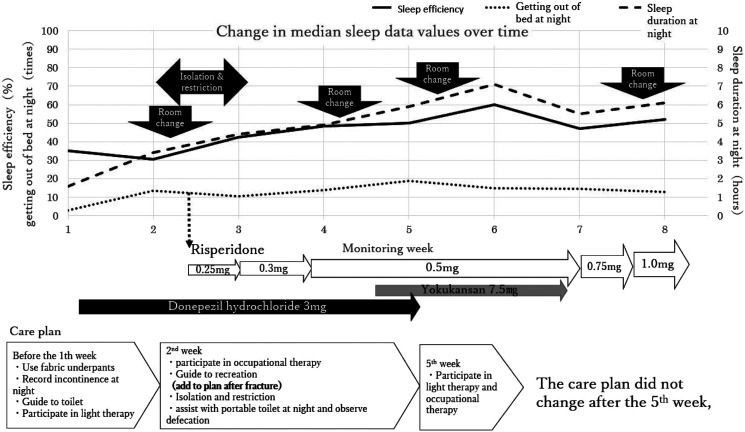
In treating Ms A, we implemented our nursing practice to address extremely short sleep duration. Ms A was aged in her 80s. Five years before the study, she started seeing hallucinations and was hospitalized 2 years before the study began. She presented with delusions and wandering, and was diagnosed as possible DLB, having experienced a progressive decline in cognitive function, repeated visual hallucinations, delusions, and depression. We could not acquire her MMSE score, but her CDR score was 3. When sleep monitoring began, she was prescribed donepezil hydrochloride (3 mg) and magnesium oxide (330 mg). In the CT image of Ms A’s brain, atrophy near the hippocampus was not noticeable, and atrophy was generally minimal, especially when considering that she was in her early 80s. The overview of patient-tailored care for Ms A is shown in Figure 1. In brief, after the first week of monitoring, magnesium was canceled and only donepezil hydrochloride (3 mg) was prescribed for Ms A going forward.
Figure 1.
Patient-tailored care for Ms A.
During the second week of monitoring, magnetic resonance imaging (MRI) revealed a fracture of the right femoral neck. We therefore changed the care plan accordingly. She resisted the restrictions and removed the Nemuri SCAN device. This prevented data collection for the week. Because she strongly resisted restrictions, staff moved her bed to an intensive care room where they could monitor her condition more closely. Isolation and restrictions disrupted her ability to rest because she continued to strongly resist the restrictions. Staff could not implement her care plan because of the fracture, and thus she only participated in light therapy and occupational therapy once. We suspected her delirium was caused by her insomnia, and thus prescribed risperidone (.25 mg).
In the third week, her doctors judged that her sleep state had improved because of the risperidone, and therefore they increased the dosage. During the last half of the third week, they increased the dosage again because she experienced irritability related to misidentification and delirium. At the researcher/staff meetings, one staff member reported that her agitation had improved. However, another said that the intensive care room was not suitable for her. We therefore thought that the room environment might be causing some of her symptoms.
In the fourth week, she was moved to a general room and became less agitated. Her doctor stopped the donepezil hydrochloride and prescribed Yokukan-san (YKS; also written as Yokukansan or Yoku-kan-san in Japanese, which is a traditional Japanese Kampo medicine used to treat BPSD 16 ) because the Japanese insurance system has imposed restrictions on the types of drugs that patients can use, depending on the facility.
In the fifth week, we moved Ms A to another general room because she was bothered by other patients. Although she slept from 9 am to 11 am and from 12 pm to 4 pm (Figure 2) during the fourth week, she did not sleep at these times during the fifth week.
Figure 2.
Sleep scores during the fourth and fifth weeks, recorded by Nemuri SCAN sensor. The circled region in the figure during the fourth week indicates time spent taking a nap.
In the sixth week, her doctors stopped prescribing YKS because long-term care facilities cannot prescribe it. She participated in light therapy and occupational therapy, and a staff member reported that her cognitive function had slightly improved.
During the seventh week, her doctors increased the dosage of risperidone because she became more irritable. She was moved to another general room, again because she had trouble with other patients. This was the final week before she left the hospital.
Excessive Sleep Duration at Night
In treating Ms B, we introduced our nursing practice to address excessive sleep duration at night. Ms B was aged in her 70s. She had been gradually losing her memory for 3 years. She was hospitalized for the purpose of entering the facility, and was diagnosed as possible DLB, having experienced a progressive decline in cognitive function, repeated falls, delusions, and depression. Her MMSE score was 22 and her CDR score was .5. When we began sleep monitoring, she was prescribed brotizolam (.25 mg), escitalopram oxalate (5 mg), and donepezil hydrochloride (5 mg). In the computerized tomography (CT) image of Ms B’s brain, no atrophy near the hippocampus was visible and the medial temporal lobe was preserved.
At the first week’s conference, staff observed that Ms. B basically lived in her own room during the day (Figure 3). The measurement results revealed hypersomnia, but the staff commented that she complained of not sleeping well.
Figure 3.
Patient-tailored care for Ms B.
In the second week, doctors increased her dosage of brotizolam from .25 mg to .5 mg because she complained of insomnia. Additionally, her dosage of escitalopram oxalate was increased from 5 mg to 10 mg because she exhibited decreased motivation. At the staff conference, some felt that it was suggested that she tended to stay in her room most of the time during the first week, but since she spent more time in the common space during the day and before bedtime, her activity during the day might have increased.
Her dosage of brotizolam was decreased from .5 mg to .25 mg to assess changes in sleep. In addition, because Ms B was often silent when she was in the common areas, we considered making a plan that encouraged individual activities rather than group activities.
After the end of the second week, we implemented a plan to increase the amount of her activity during the day. Looking at the results of the sleep index for the third week, her average sleep duration at night was 10.2 hours, her average sleep efficiency was 84%, and she got out of bed an average of two times per night (Figure 4). Ms B’s average sleep efficiency fell from 90% to 84% compared with the second week, but other sleep indicators remained unchanged. Staff observed, moreover, that she could not be seen sleeping in the common areas outside her room.
Figure 4.
Sleep scores for week 1 recorded by the Nemuri SCAN device.
No data were available during the fourth week because of a failure in her Nemuri SCAN device. Although a quantitative evaluation using Nemuri SCAN data was therefore not possible, we ended her sleep monitoring because staff determined that her activity during the day increased and her sleep at night was maintained.
Excessive Number of Times Getting out of Bed at Night
In treating Ms C, we implemented our nursing practice to address getting out of bed an excessive number of times per night. Ms C was aged in her 70s. Three years before the study, she began to have delusions and visited the hospital. However, she then stopped going to the hospital. A year later, her delusions became severe, and she was admitted to the hospital and diagnosed with DLB. Her MMSE score was 20 and her CDR score was 1. At the beginning of sleep monitoring, she was prescribed donepezil hydrochloride (5 mg), magnesium oxide (330 mg), ramelteon (4 mg), and eight other medications. Ms C had progressive decline in cognitive function but did not have BPSD. On the MMSE, her “Delayed recall” score was 2 and her “Serial 7” score was 1. On the MRI and CT images of Ms C’s brain, atrophy near the hippocampus was not very noticeable. Cerebral infarction lesions (white areas) were visible in the CT image.
In the first week of monitoring, Ms C got out of bed nine times during the night. According to Nemuri SCAN data, she tended to sleep all day. At the staff meeting, one staff member reported that she was more delirious from the evening through the night than she was earlier in the day. We discovered that she did not communicate with other patients. Therefore, we intervened to reset her circadian rhythm.
In the second week, her doctors increased her clonazepam dosage. She did not like taking medication or taking a bath. Therefore, the staff wanted to implement an intervention. They tried, but were unable, to discover why she stayed in bed during the daytime. The increased dosage did not affect her sleep state.
In the third week, ramelteon was discontinued and quetiapine was prescribed because her BPSD continued. The staff made a plan in which they encouraged her activity during the daytime; however, her sleep disturbances at night still continued. Comparing the first and third weeks, sleep efficiency increased from 68% to 80%, the number of times getting out of bed at night decreased from nine to four, and sleep duration at night increased from 8.7 hours to 10.6 hours. Our intervention ended when she was moved to another unit.
Discussion
In this study, we measured sleep states in patients with DLB using a non-wearable device and assessed ways in which the measured data could be used in nursing practice. We observed three main types of sleeping problems among the participants: extremely short sleep duration, excessive sleep duration at night, and excessive number of times getting out of bed at night. It is difficult to fully understand a patient’s state of sleep only through observations made by medical staff. Furthermore, in some cases, a participant’s sleep state according to the non-wearable device differed from their actual sleep state. Therefore, a combination of observation and non-wearable device sensing is useful for accurately comprehending patient sleep patterns.
Patients With Extremely Short Sleep Duration
For Ms A, who initially slept very little at night, the primary treatment was pharmacological, although the room environment also influenced her sleep. The measurement results revealed that her napping decreased and sleep duration at night increased by moving her to a different room, thus reducing her interactions with other patients in her room. Thus, patients can affect one another’s sleep. Suzuki and colleagues discuss causes of chronic insomnia in older people, but do not clarify what effects roommates and cohabitants had on DLB patients’ sleep. 17 Although two systematic reviews related to non-pharmacological therapy, including adjusting the sleeping environment, have been published, none have specifically studied the effects of roommates on sleep for those with DLB.18,19 Therefore, this relationship should be examined in greater detail.
Suzuki et al 17 suggest that good sleep hygiene includes regular exercise and meals, the avoidance of stimulants such as caffeine and tobacco, and the creation of a comfortable sleep environment. 17 More specifically, they recommend the following bedroom environment: “Keep the bedroom dark and quiet because noises and dim light can interrupt sleep. Maintain a comfortable bedroom temperature (below 24°C [75 °F]). During the summer, consider using an air conditioner.” In our study, medical staff occasionally moved patients to different rooms in attempts to create an ideal room environment that fit a patient’s character. However, the room temperature, bed layout, and the number of patients per room were not specifically determined. Moreover, switching rooms sometimes worsens a patient’s sleep disorder. In Ms A’s case, device measurements revealed that room changes had some effect on her sleep. We do not think that room change should be the first choice for treating insomnia in older people, but light and noise do influence sleep. Therefore, considering room environment is important.
Excessive Sleep Duration at Night
Our patients suffered from lifestyle-related diseases such as hypertension and diabetes. Because their severity was unclear, the cause of excessive sleeping at night could not be directly determined. However, lifestyle-related diseases are thought to worsen sleep. When sleep quality deteriorates, daytime napping increases as does sleep duration at night.
In Ms B’s case, the monitoring data did not match the patient’s complaint. In addition, another patient who was thought to be asleep according to the staff’s observation was not actually asleep. Patients with dementia may have difficulty in correctly communicating their sleep state to others due to the decline in cognitive function. The number of medical staff is limited, and especially at night, the number decreases further. Therefore, how well medical staff can accurately assess a patient’s condition through observation or patient’s complaint is limited, and in turn, so is the ability to use their assessments to create a nursing practice plan that will actually help the patient. 20 The use of non-wearable devices is thought not only to enable an accurate understanding of patient behavior and sleep state, and to facilitate care that aligns with the actual situation, but also to reduce the daily workload of medical staff.
Excessive Number of Times Getting out of Bed at Night
In our research, getting out of bed means leaving one’s bed for some reason. If someone gets out of bed nine times during the night, as Ms C did in the first week, it means that a caregiver must watch the patient’s behavior throughout the night. In such cases, the additional workload may make it difficult for the caregiver to also carry out their other night care duties. No research has specifically defined “excess number of times” as a numerical value. Therefore, we suggest that monitoring DLB patients’ sleep and trying to discern why they get up and leave their beds so many times at night are crucial.
Importantly, getting out of bed because of nocturnal awakening also increases the risk of falling among patients with dementia, particularly those with DLB, who are at a higher risk of falling than other dementia patients. 21 Thus, we believe that a non-wearable device can be used for early detection of this behavior, which could prevent future falls.
This study has some limitations. There were several patients for whom sufficient data could not be obtained during the survey period. In some cases, even if monitoring was continued, the data could not be measured accurately because the patient did not sleep in bed. In such cases, the staff could provide alternative data from their observation records, but without quantitative data, it is difficult to review the nursing plan in detail. Many of the patients who participated in this study had symptoms of advanced dementia, and often severe BPSD. In addition, clinical characteristics that make patients with DLB susceptible to sleep disorders also make them difficult to treat. Early care that is in accordance with the patient’s condition is thought to lead to improvement in symptoms and shortening of hospitalization. Therefore, it is necessary to accurately understand and respond to patients’ symptoms using non-wearable devices like the one used in the present study.
In the future, the use of devices such as the Nemuri SCAN sensor in clinical settings will be widespread, and it is necessary to consider ways to enable ward staff to analyze measurement data using such devices even when no researchers are present. Furthermore, while the data in this study are several years old, at the time, no studies were available that could understand the condition of DLB patients in such detail and use that information in their care. Therefore, we were able to clarify an efficacious approach for observing the characteristics of DLB patients who have sleep disturbances; this includes using the observations made by ward staff, particularly those who specialize in dementia. It is necessary to develop an easy-to-understand interface that can incorporate detailed data into electronic medical records using Information and Communication Technology similar to the Nemuri SCAN sensor used in the present study, and to design an educational scheme that can fully use such data.
Conclusion
We measured sleep states in patients with DLB using non-wearable devices, analyzed the sleep data and patient information, and used the individual sleep data in nursing practice. The care plans were designed, assessed, and modified over the course of more than 2 weeks. We discovered several kinds of sleep disturbances, such as extremely short sleep duration, excessive sleep duration at night, and getting out of bed many times in the night. Several practices are used to treat patients with DLB, including drug adjustment, environmental control, and light therapy. Using a non-wearable device and thus monitoring DLB patients’ sleep patterns more objectively, nurses cared for patients in concert with medical staff who closely considered individual patient conditions. Medical staff must consider some of the interventions we examined in this research and judge which practices are most effective for their patients.
Acknowledgments
The authors thank the patients and the staff of Asakayama General Hospital. We thank Adam Phillips, PhD, and Anita Harman, PhD, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by JSPS KAKENHI Grant Number 25893129. This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Abbreviations: AD: Alzheimer’s disease; BPSD: Behavioral and Psychological Symptoms of Dementia; CDR: Clinical Dementia Rating scale; CT: Computerized Tomography; DLB: Dementia with Lewy Bodies; MRI: Magnetic Resonance Imaging; MMSE: Mini-Mental State Examination; RBD: REM-sleep behavior disorder; YKS: Yokukan-san; Significance statement.
ORCID iDs
Hideki Kanemoto https://orcid.org/0000-0003-3662-1469
Miyae Yamakawa https://orcid.org/0000-0002-2399-8366
References
- 1.Kryger MH, Dement WC, Roth T. Principles and practice of sleep medicine. 5th ed.; 2010: Elsevier Health Sciences. [Google Scholar]
- 2.Kazui H, Adachi H, Kanemoto H, et al. Effects of donepezil on sleep disturbances in patients with dementia with Lewy bodies: An open-label study with actigraphy. Psychiatr Res. 2017. May 1; 251: 312-318. [DOI] [PubMed] [Google Scholar]
- 3.Higami Y, Yamakawa M, Shigenobu K, Kamide K, Makimoto K. High frequency of getting out of bed in patients with Alzheimer's disease monitored by non-wearable actigraphy. Geriatr Gerontol Int. 2019. Feb 1; 19(2): 130-134. [DOI] [PubMed] [Google Scholar]
- 4.Tabata K, Saijo Y, Morikawa F, et al. Association of premorbid personality with behavioral and psychological symptoms in dementia with Lewy bodies: Comparison with Alzheimer's disease patients. Psychiatry Clin Neurosci. 2017. Jun 1; 71(6): 409-416. [DOI] [PubMed] [Google Scholar]
- 5.McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB consortium. Neurology. 2017; 89: 88-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCleery J, Sharpley AL. Pharmacotherapies for sleep disturbances in dementia. Cochrane Database Syst Rev. 2020. Nov 15; 11: CD009178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edwards K, Royall D, Hershey L, et al. Efficacy and safety of galantamine in patients with dementia with Lewy bodies: A 24-week open-label study. Dement Geriatr Cogn Disord. 2007; 23: 401-405. [DOI] [PubMed] [Google Scholar]
- 8.Litvinenko IV, Odinak MM, Mogil’naya VI, Emelin AY. Efficacy and safety of galantamine (Reminyl) for dementia in patients with Parkinson's disease (an open controlled trial). Zh Nevrol Psikhiatr Im S S Korsakova. 2007; 107: 25-33. [PubMed] [Google Scholar]
- 9.Burns A, Allen H, Tomenson B, Duignan D, Byrne J. Bright light therapy for agitation in dementia: A randomized controlled trial. Int Psychogeriatrics. 2009. Aug; 21(4): 711-721. [DOI] [PubMed] [Google Scholar]
- 10.Maglione JE, Liu L, Neikrug AB, et al. Actigraphy for the assessment of sleep measures in Parkinson's disease. Sleep. 2013. Aug 1; 36(8): 1209-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gehrman P, Marler M, Martin JL, Shochat T, Corey-Bloom J, Ancoli-Israel S. The relationship between dementia severity and rest/activity circadian rhythms. Neuropsychiatr Dis Treat. 2005; 1(2): 155-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu WT, Lin SY, Tsai CY, et al. Comparison of hospital-based and home-based obstructive sleep apnoea severity measurements with a single-lead electrocardiogram patch. Sensors. 2021; 21(23): 8097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kogure T, Shirakawa S, Shimokawa M, Hosokawa Y. Automatic sleep/wake scoring from body motion in bed: Validation of a newly developed sensor placed under a mattress. J Physiol Anthropol. 2011; 30(3): 103-109. [DOI] [PubMed] [Google Scholar]
- 14.Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982; 140(6): 566-572. [DOI] [PubMed] [Google Scholar]
- 15.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975; 12(3): 189-198. [DOI] [PubMed] [Google Scholar]
- 16.Mizoguchi K, Ikarashi Y. Cellular pharmacological effects of the traditional Japanese kampo medicine yokukansan on brain cells. Front Pharmacol. 2017; 8: 655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suzuki K, Miyamoto M, Hirata K. Sleep disorders in the elderly: Diagnosis and management. J Family Med. 2017; 18: 61-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Connors MH, Quinto L, Mckeith I, et al. Non-pharmacological interventions for Lewy body dementia: A systematic review. Psychological Medicine. 2018; 48: 1749-1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morrin H, Fang T, Servant D, Aarsland D, Rajkumar AP. Systematic review of the efficacy of non-pharmacological interventions in people with Lewy body dementia. Int Psychogeriatrics. 2018; 30(3): 395-407. [DOI] [PubMed] [Google Scholar]
- 20.Yamakawa M, Suto S, Shigenobu K, Kunimoto K, Makimoto K. Comparing dementia patients' nighttime objective movement indicators with staff observations. Psychogeriatrics. 2012. Mar; 12(1): 18-26. [DOI] [PubMed] [Google Scholar]
- 21.Allan LM, Ballard CG, Rowan EN, Kenny RA. Incidence and prediction of falls in dementia: A prospective study in older people. PLoS One. 2009; 4(5): e5521. [DOI] [PMC free article] [PubMed] [Google Scholar]