Abstract
The ketone bodies, especially the β-hydroxybutyrate, had been shown to modulate the function of the central nervous system and prevent the pathological progression of Alzheimer’s disease (AD). However, little is known about the role of acetoacetate in the AD brain. Thus, we intraventricularly injected acetoacetate into familial AD mice (APPSWE) for 14 days and monitored their memory and biochemical changes. During the behavior test, acetoacetate at 100 mg/kg led to significant improvement in both Y-maze and novel object recognition tests (NORTs) (both P < .05), indicating ameliorating spatial and recognition memory, respectively. Biomedical tests revealed two mechanisms were involved. Firstly, acetoacetate inhibited the GPR43-pERK pathway, which led to apparent inhibition in tumor necrosis factor-α and Interleukin-6 expression in the hippocampus in a concentration-dependent manner. Secondarily, acetoacetate stimulated the expression of hippocampal brain-derived neurotrophic factor (BDNF). We concluded that acetoacetate could ameliorate AD symptoms and exhibited promising features as a therapeutic for AD.
Keywords: Alzheimer’s disease, acetoacetate, GPR43, neuroinflammation memory, brain-derived neurotrophic factor
Introduction
Neurodegenerative diseases, such as Alzheimer’s disease (AD), have become the one major contributor to cognition impairment and dramatically affect life quality. 1 AD is characterized by pathological accumulation of amyloid-beta (Aβ) protein and the formation of intracellular neurofibrillary tangles, which initiates a cascade of neuroinflammation and leads to neuronal death and a decline in memory and learning behaviors. 2 Since AD is currently considered to be uncurable, addressing modifiable risk factors to prevent AD remains the most promising strategy. In this regard, increasing evidence suggests that daily diet could play a significant role in preventing and slowing AD’s pathological changes. 1
Emerging evidence suggested that the AD brain had impaired glucose metabolism. 3 Previous studies demonstrated that cerebral glucose metabolism is reduced by 20-40% in AD 4 , especially in the hippocampus regions. 5 Although fat is the optimal alternative to glucose, the brain cannot utilize fatty acids. Ketone bodies feature water-soluble and lower molecular weight, which enable them to efficiently cross the blood-brain barrier. 6 Based on these results, literature had shown that ketone has become the major alternative energy substrate for neurons in the AD brain.7-9 Interestingly, emerging data indicates ketones are not only merely nutritional molecules but have potentially positive effects on AD as well. Previously, the β-HB has been shown to improve AD symptoms and prevent AD’s pathological progression.8,10,11 Acetoacetate is the central ketone body as β-HB is generated from acetoacetate by the β-HB dehydrogenase. 12 Additionally, ketones are transported into cells at different rates, with the uptake of acetoacetate being twice that of β-HB at a given arterial concentration. 13 However these features, little is known about the role of acetoacetate on the pathological progression of AD.
GPR43, also known as free fatty acid receptor 2 (FFAR2), is a dual-coupling G protein-coupled receptor (GPCR) that binds with both the pertussis toxin-sensitive Gq and Gi/o proteins. 14 Previously, the GPR43 is identified as a cognate receptor for short-chain fatty acids (SCFA). 15 The SCFAs are microbial metabolites in the intestine, which elicit a plethora of effects on systemic health, including modulating the microbiota-gut-brain axis 16 and the inflammation process. 17 Thus, as the receptor of SCFAs, the GPR43 has been suggested to be also responsible for the diverse regulatory and therapeutic effects across a spectrum of diseases. GPR43 is abundantly expressed in immune cells 15 and modulates inflammatory response. 18 Since inflammation has been strongly implicated in the pathogenesis of AD, 19 the GPR43 has become an appealing pharmaceutical target with therapeutic applications for AD. Recently, GPR43 has been identified as a specific receptor for acetoacetate. 20 Additionally, GPR43 expression has been newly discovered in the brain, especially in the hippocampus. 21 However, basic unanswered questions persist about the involvement of GPR43, as well as its ligand acetoacetate, in preventing the pathological progression of AD.
In the current study, we explore the effects and underlying mechanisms of exogenous acetoacetate on mice with memory disorders. By using the intra-ventricular injection method, we tested the hypothesis that elevation of acetoacetate in the central nervous system (CNS) could improve the memory of AD mice.
Materials and Methods
The research protocol was approved by the Research and Ethics Committee of Shanghai Cancer Center affiliated to Shanghai Fu-Dan University School of Medicine (SHCan.20190903-021). The animal experiment procedures were approved by the Animal Care Committee (IACUC) of Shanghai Cancer Center affiliated to Shanghai Fu-Dan University School of Medicine, which was in accordance with animal care guidelines.
Animal Housing and Sample Collection
A total of 48 male familial AD mice (APPSWE - Model 2789) (9-10 months, 25-30g) were purchased from Taconic Biosciences (La Jolla, USA). They were evenly divided into 6 groups with 8 mice in each group. Animals were singly housed in an animal care facility under room temperature at 12/12 hours of light/dark cycles. Animals were allowed ad libitum access to laboratory chow and water throughout the study.
On the day of sample collection, after euthanized with sodium pentobarbital (120 mg/kg, intraperitoneally [i.p.]), mice were sacrificed with neck dislocation. Bilateral hippocampi were collected and suspended in lysis buffer (50 mM tris pH 7.5 and 1% triton in 10 mL Milli-Q) supplemented with cOmpleteTM Mini Protease Inhibitor Cocktail Tablets (11836153001, Roche, Indianapolis, IN, US). This was followed by homogenizing the samples in the Bullet Blender 5E Pro homogenizer (Next Advance, Troy, NY, US) for 3 min using .5 mm stainless steel beads. Then, after 20 min centrifuged at 14,000 g for 10 min at 4°C, the supernatant and sediment were separately collected for further usage.
Intracerebroventricular Cannulation and Acetoacetate Administration
Cannulae were implanted intracerebroventricularly as previously described. 22 Mice were anesthetized with sodium pentobarbital (120 mg/kg, intraperitoneally [i.p.]) and mounted in a stereotaxic instrument with the incisor bar. Then a guide cannula (2.5 mm length, 26 gauge; Plastics One, Roanoke, VA) was implanted in the right lateral ventricle using the following coordinates from the atlas of Franklin and Paxinos. 23 The cannulas were fixed to the skull using three 1.6 mm stainless steel screws and dental cement. After 4 days of recovery, ethyl acetoacetate (molecular weight 130.14 g/mol, Cas#20412-62-8, Sigma- Sigma-Aldrich, St. Louis, MO, US) solution was freshly prepared in MilliQ water at 2.3 M. A serial dilution using MilliQ water was performed to achieve acetoacetate solution at .23, .023, .0023 and .0023 M. The acetoacetate solution was intraventricularly infused via guide cannulas at 1 µL/min for 5 min twice a day for 14 days. Thus, the final acetoacetate application dosage was 0, .01, .1, 1, 10, and 100 mg/kg for testing groups I to VI, respectively.
Behavioral Tests
All behavioral tests were conducted between 10 AM and 3 PM. Y-maze and novel object recognition tests were utilized to evaluate the spatial and recognition memory.
Y-maze
The Y-maze was used to assess mice memory and conducted as previously described. 24 A classical Y-maze apparatus was used, which consisted of three similar grey acrylic arms (10 * 30 * 17 cm) placed at 120° with respect to each other. During the training session, one arm was blocked off (novel arm); mice were placed facing the end of one of the other two accessible arms (start arm) and allowed to explore the environment for 30 mins for three consecutive days. When the test started, both arena’s arms (start arm & novel arm) were accessible for exploring in a 30 mins period. The ANY-mazeTM video tracking system (Stoelting Co., Wood Dale, US) was used to record the time that mice spend in either novel arms or start arms. The equation to compare the time spent on different arms was as follows:
Novel Object Recognition Tests
The NORT was performed as previously presented. 25 In short, one mouse was placed in an empty grey Perspex square arena (60 × 60 × 35 cm). During the training session, one mouse was placed in the middle of the arena and allowed to explore the environment in the absence of objects for 30 mins for seven consecutive days. On the test day, after the mouse was put in the arena for 30 mins, two identical LEGO® Bricks 3002 (1.6 × 2.4 x 1.1 cm) were placed at opposing corners of the arena. The testing mouse was allowed to explore the pieces for 5 minutes. The percentage of time spent exploring the two identical pieces in the open-field box was considered the baseline. Then, the mouse was taken out of the arena for 10 mins and one LEGO® Brick was replaced with LEGO® Plate 3031 (3.2 × 3.2 × 0.5 cm). The observation started when the mouse was put back into the arena and we calculated how much time the trained mouse spent at each object in another 5 mins session. The relative time spent on exploring the novel object was calculated as follows:
Acetoacetate and its Downstream Pathway Signaling Testing
The Acetoacetate Assay was performed according to the manufacturer’s instructions as previously presented. 26 The Acetoacetate Assay Kit (Abcam Inc., Boston, MA, USA) was used to detect the final acetoacetate concentration in the hippocampus tissue. The samples were treated as described in the previous paragraph and then used for the assay.
Western Blot
The Western blot procedures were conducted according to previously reported with minor modifications. 27 After being euthanized with sodium pentobarbital (120 mg/kg, intraperitoneally), mice were sacrificed with neck dislocation and the hippocampi were obtained and lysed in 4 °C lysis buffer (ab156035, Abcam Inc., Boston, MA, USA). The Bovine Serum Albumin assay was used to determine the total protein concentration of each sample, which was then normalized to the same level. A total of 40 μg of protein was resolved by SDS gel electrophoresis and blotted onto a nitrocellulose membrane. The membrane was then incubated with anti-GPR43 (ab124272) or anti-pERK (ab201015, Abcam Inc., Boston, MA, US) at 1:500 overnight at 4°C. On the second day, the membrane was washed with 4 °C TBS buffer and incubated with horseradish peroxidase (HRP)-conjugated anti-goat IgG antibody (ab6721, Abcam Inc., Boston, MA, USA) at 1:1000 concentration. The band intensity was measured, and the relative expression ratios of β-actin values were calculated.
cAMP Assay
The cAMP concentration was determined by using the cAMP ELISA kit (ab65355) (Abcam, Waltham, US) according to the manufacturer’s instructions. Briefly, hippocampi tissue was homogenized in the 1 mL .1 M HCl buffer by the PowerLyzer 24 Homogenizer (QIAGEN, Germantown, US) following the manufacturer’s recommended settings. After homogenizing, samples were immediately centrifuged at 14,000 g for 10 min at 4°C. The supernatant was collected and kept on ice for immediate use. Then, 50 µl of the sample was added to the Protein G coated 96-well plate followed by 10 μL of the reconstituted cAMP antibody. After 1 hour of incubation at room temperature with gentle agitation, 10 µL of cAMP-HRP was added to each well and was incubated for another 1 hour at room temperature. When the incubation was over, each well was washed with 200 µl 1X Assay Buffer 3 times. Finally, 100 µL of HRP Developer was incubated with each sample for 1 hour at room temperature, which was ceased by adding 100 µL of 1M HCl into each well. Finally, the ELISA plate was read at OD450 nm.
Statistical Analyses
All data were analyzed by using the SPSS Statistics 21 (IBM®, Endicott, US) while the graphs were generated by the GraphPad Prism 8.1 (Graphpad®, San Diego, US). All data were presented in the table and text as mean ± stand deviation. To compare one variable change between two and three groups, the student’s t-test and one-way ANOVA with Tukey’s multiple comparison test were conducted, respectively. To compare two variables across multiple conditions, we used the two-way ANOVA with Tukey’s multiple comparison test A P-value ≤ .05 was used as the significance cutoff value for the entire study.
Patient and Public Involvement
No patient was involved.
Results
AD Mice Demonstrated Impaired Spatial and Recognition Memory
We first used Y-maze to study the spatial memory of AD mice before and after 14 days of acetoacetate application (Figure 1A). A two-way ANOVA revealed that there existed a statistically significant interaction between acetoacetate injection dosage and treatment period [F (5, 84) = 2.435, P = .041]. Simple main effects analysis showed that 14-days treatment could significantly improve the spatial memory [F (1, 84) = 30.96, P < 0001] while the acetoacetate application did not [F (5, 84) = 1.949, P = .094]. Before acetoacetate application, the control group spend an RT of 31.3 ± 11.4% in the novel arms, which was no different from that of the treatment groups. However, after acetoacetate application, the treatment group exhibited gradually increased RT spent in the novel arm. Post hoc analysis demonstrated that the mice exhibited dramatically increased RT in the novel arms when acetoacetate reached 100 mg/kg, as compared to that of the control (P = .039).
Figure 1.
The effects of acetoacetate on spatial and recognition memory of Alzheimer’s disease (AD) mice as reflected in the Y-maze (A) and novel object recognition tests (NORTs) (B). Before acetoacetate application, all groups exhibited similar memory impairment. After 14 days application, although acetoacetate at low concentration did not improve AD mice’s behavior as compared to that of the control group, acetoacetate at 100 mg/kg significantly improve AD mice’s ratio of time spent in the Y-maze novel arm and exploring the novel object, indicating ameliorated spatial and recognition memory, respectively.
Next, we studied the effect of acetoacetate in the NORT setting. Like the results of the Y-maze, a statistically significant interaction was observed between acetoacetate injection dosage and injection time [F (5, 84) = 3.015, P = .015]. Simple main effects analysis demonstrated that treatment time had a dominant effect on recognition memory [F (1, 84) = 5.774, P = .019]. However, the effect of acetoacetate on recognition memory remained insignificant [F (5, 84) = 1.426, P = .224]. While all groups showed similar RT before acetoacetate treatment, mice exhibited increased RT, which was positively correlated to their acetoacetate concentration (Figure 1C). Post hoc analysis demonstrated that the RT of the 100 mg/kg acetoacetate group became significantly improved as compared to that of the control (P = .008).
Acetoacetate Deposition Increased in the Hippocampus After Acetoacetate Application
To understand the mechanism of this memory improvement, we next studied biochemical changes inside the brain of AD mice (Figure 2). The assay indicated that acetoacetate application at 10 mg/kg had significantly elevated acetoacetate concentration in the hippocampi as compared to the control group (P = .002). Further increased acetoacetate concentration to 100 mg/kg further elevated acetoacetate concentration in the hippocampi (P < .001).
Figure 2.
The hippocampal acetoacetate concentration changes after external acetoacetate application. The Alzheimer’s disease (AD) mice were applied with different concentrations of acetoacetate intraventricularly for 14 days. The hippocampi were then retrieved and tested for acetoacetate deposition. The assay results indicated that acetoacetate application at 10and 100 mg/kg could significantly elevate acetoacetate concentration in the hippocampi as compared to the control group and low acetoacetate group (both P < .05).
Acetoacetate Treatment Decreased Inflammatory Factor Expression via GPR43-pERK Pathway Inhibition
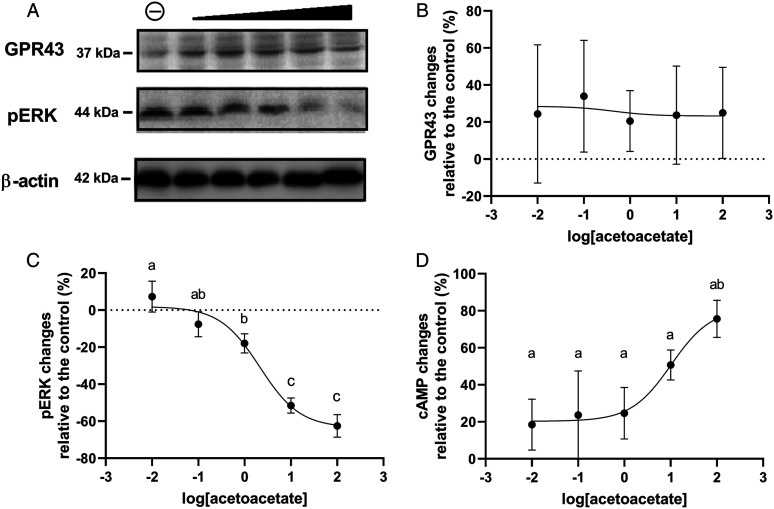
The G-protein-coupled receptor 43 (GPR43) is a receptor for acetoacetate.14,20 Thus, we further test the signal changes of the acetoacetate-GPR43-pERK pathway in the hippocampi. Unlike that of the acetoacetate changes in the hippocampi, the GPR43 expression level was not affected by acetoacetate application, as there was no difference between the control and treatment groups (Figure 3A and B, P = .36). On the contrary, the pERK level was inhibited in a concentration-dependent pattern (Figure 3A and C) and the 50% inhibitory concentration (IC50) of acetoacetate was 18.67 mg/kg (95% CI: 11.27 to 31.49 mg/kg, R2 = .94). Meanwhile, the cAMP, which is another substrate of the GPR43, demonstrated an expression increase in a dose-response manner after acetoacetate application. The 50% excitatory concentration (EC50) of acetoacetate was 10.28 mg/kg (95% CI: 3.6 to 30.02 mg/kg, R2 = .7).
Figure 3.
The hippocampal GPR43 and pERK expression level changes after intra-ventricular acetoacetate application at different concentrations. The hippocampi were then retrieved from Alzheimer’s disease (AD) mice and Western-blot was used to test the signal changes of the acetoacetate-GPR43-pERK pathway. The GPR43 expression level was not affected by acetoacetate application, even at the highest concentration (A & B, P > .05). On the contrary, the pERK level was inhibited in an acetoacetate-dose-dependent manner (A & C) and the 50% inhibitory concentration (IC50) of acetoacetate was 18.67 mg/kg (95% CI: 11.27 to 31.49 mg/kg, R2 = .94). Meanwhile, the cAMP also demonstrated a dose-response manner after acetoacetate application (D) and the 50% excitatory concentration (EC50) of acetoacetate was 10.28 mg/kg (95% CI: 3.6 to 30.02 mg/kg, R2 = .7).
As the two major downstream products of pERK, both IL-6 and TNF-α exhibited apparently inhibited expression in concentration-dependent patterns (Figure 4A and B), and the IC50 of acetoacetate was 2.35 (95%CI: 1.52 to 3.67, R2 = .95) and 1.36 mg/kg (95% CI: .85 to 2.22 mg/kg, R2 = .94) for IL-6 and TNF-α, respectively.
Figure 4.
The hippocampal TNF-α and IL-6 levels after intraventricular acetoacetate application at different concentrations. As the two major downstream products of the acetoacetate-GPR43-pERK pathway, both IL-6 (A) and TNF-α (B) exhibited apparently decreased levels in acetoacetate-concentration dependent patterns, the 50% inhibitory concentration (IC50) of acetoacetate was 2.35 (95% CI: 1.52 to 3.67, R2 = .95) and 1.36 mg/kg (95% CI: .85 to 2.22 mg/kg, R2 = .94) for IL-6 and TNF-α, respectively.
Acetoacetate treatment led to increased brain-derived neurotrophic factor (BDNF)
A previous study indicated that β-HB could promote BDNF expression in the CNS. 9 Since we had observed improved AD mice memory after acetoacetate application, we thus hypothesized that acetoacetate could also increase BDNF expression in the hippocampus. Interestingly, BDNF was elevated by the acetoacetate in a dose-dependent pattern (Figure 5). The EC50 of acetoacetate was 318.4 mg/kg (95%CI: 88.71 to 731.9 mg/kg, R2 = .73).
Figure 5.
The hippocampal brain-derived neurotrophic factor (BDNF) levels after intra-ventricular acetoacetate application at different concentrations. The hippocampal BDNF exhibited an acetoacetate-dose-dependent pattern. The 50% excitatory concentration (EC50) acetoacetate for BDNF was 318.4 mg/kg (95% CI: 88.71 to 731.9 mg/kg, R2 = .73).
Discussion
Although the an increased understanding of AD’s neurobiology and pathogenesis, only a few symptomatic treatments are currently available for AD. According to the Lancet Commission on Dementia Prevention, Intervention, and Care, one-third of dementia cases may be preventable through diet. 1 Thus, applications of ketone bodies, especially the β-HB, showed merit in the previous studies.8,10,11 However, little is known about the role of acetoacetate in modulating CNS function and preventing AD pathological progression. In the current study, we intraventricularly applied acetoacetate to AD mice and studied both behavior and hippocampi biochemical changes. Our results indicate that AD mice, after acetoacetate application, showed significantly improved spatial and recognition memory. This was because intraventricular acetoacetate application could significantly increase the acetoacetate in the hippocampus, which inhibited the neural inflammation process as well as promoted neurotrophic factors in the hippocampus.
It is well known that the ketone body is an alternative energy source for the brain, heart, and skeletal muscles in mammals during states of energy deficit. Recently, the ketone bodies have also been gradually identified as a key factor for preventing AD progression. Among all ketones, the β-HB has received the most thorough studies. For example, β-HB is dramatically decreased in the blood sample of AD patients 8 and oral application of β-HB could improve their results on cognitive tests.8,10,11 Additionally, β-HB has been shown to modify disease in mouse models of AD by unknown mechanisms.28,29 However, the effect of acetoacetate in the AD brain has been barely studied. In the current study, we intraventricularly infused acetoacetate from .01 to 100 mg/kg in AD mice. To our best knowledge, this was the highest acetoacetate dosage that has ever been used via the intraventricular pathway. Previously, Chikahisa et al. 30 reported the intraventricular application of acetoacetate at 6.7 mg/kg, which was the highest intraventricular dosage reported so far. Meanwhile, among other infusion pathways, the highest acetoacetate dosages reported were 1240 mg/kg 31 and 600 mg/kg 32 via the subcutaneous and intraperitoneal pathways, respectively. We believe the intraventricular pathway is superior to other application methods. It was documented that the transfer of ketone bodies across cell membranes (including those of neurons) was enabled by monocarboxylic acid transporters (MCTs). According to previous studies, the central β-HB concentration was only about 26% of β-HB plasma concentration.33,34 Since the kinetics of MCT remain largely unknown for acetoacetate, we believe the intraventricular infusion can provide us with the least interfered evidence of acetoacetate’s effect in the AD brain.
We observed that AD mice demonstrated significantly improved spatial and recognition memory after acetoacetate application at 100 mg/kg, which matched the literature. For example, either acetoacetate injection 32 or ketogenic diets 35 could improve the learning and memory abilities of AD mice in the lab setting. This finding was also supported by several recent RCT studies that enrolled human subjects.36-39 Additionally, we demonstrated that this memory improvement was accompanied by an accumulation of acetoacetate in the hippocampus region. Within only a handful of literature, cerebral acetoacetate concentration was shown to vary from 20 to 30 nmol/g in brain tissue,26,40 which agreed with our study. As is well known, the hippocampus is closely associated with memory, cognitive function, and neurogenesis in the normal brain, and the AD brain shows the degeneration of the hippocampus along with the deposition of tau and Aβ protein. 41 Among the limited literature, Massieu et al. 42 demonstrated that acetoacetate could protect neurons against glutamate neurotoxicity in the hippocampus both in vivo and in vitro after glycolysis inhibitor application. Massieu proposed that acetoacetate served as an alternative energy substrate during glycolysis inhibition. Thus, the accumulation of acetoacetate in the hippocampi, as revealed in the current study, agreed with Massieu’s findings, because the hippocampi neurons in the AD brain could be craving for energy supply and thus deposit as much acetoacetate as possible. Additionally, we elucidated that other than being an energy substrate, acetoacetate could also protect neurons via promoting BDNF expression and inhibiting neuroinflammation in the hippocampus. Our result indicates that acetoacetate could be as beneficial as the β-HB for proper brain function in AD patients.
So far, two orphan GPCRs have been identified as the specific receptor for SCFAs: the GPR43 and GPR41.15,43 Interestingly, although sharing a high sequence identity, GPR43 and GPR41 are coupled with different downstream signals. While the GPR41 couples exclusively through the Gi/o family, the GPR43 couples either the Gi/o and Gq families, which could not only modulate the phosphorylation of ERK and adenylate cyclase (cAMP), but also the intracellular calcium levels. 14 Additionally, there is a plethora of literature suggesting that SCFAs, after binding to GPCRs, have multi-dimensional roles in regulating various physiological and pathologic processes.16,17,44 Together, the biochemical and physiological features make GPR43 a promising drug target for a series of diseases. The β-HB and acetoacetate are the two major ketone bodies, which were long considered mere by-products of fatty acid breakdown that increase during fasting and other situations of glucose shortage. 45 Interestingly, in addition to functioning as an energy source, β-HB acts as a key signaling molecule via GPR41 counteracting AD pathology.46,47 On the contrary, little is known about the effect of acetoacetate on the pathological progress of AD. Recently, Miyamoto et al. 20 identifies that acetoacetate is also a ligand for GPR43. Since the role of GPR43 in inflammatory bowel disease has been extensively investigated, the coupling of acetoacetate and GPR43 in the AD brain becomes extremely intriguing. In the current study, we first observed that acetoacetate accumulated in the hippocampi after intraventricular application. Previously, literature had demonstrated the role of ketones in the hippocampus. For example, Bough et al. 48 demonstrated that ketogenic diets could direct affect hippocampus electrophysiology and achieved the anti-convulsant and anti-epileptogenic effect in vivo. Ruskin et al. 49 showed that ketones in vitro could increase intracellular ATP in CA3 pyramidal neurons of the hippocampus. Although suggested to modulate neurological function via the gut-brain axis, ketones are shown to accumulate in the hippocampus to practice their therapeutic role. Next, we observed that acetoacetate-GPR43 coupling led to inhibition of pERK and its two substrates TNF-α and IL-6. Neuroinflammation has been strongly implicated in the pathogenesis of AD. 19 Thus, decreasing inflammatory mediators’ production in the hippocampus could inhibit the cascade of neuroinflammation, which could be one possible mechanism of memory improvement in AD mice after acetoacetate application. Our result also agreed with previous studies, which demonstrated that another ketone body, β-HB, could regulate the innate immune response in the CNS and efficiently protect neurons. 50 Together, we believe that acetoacetate, like the β-HB, plays a unique role in slowing the AD process.
BDNF is a key protein that affects cognition and memory.51,52 In many neurodegenerative diseases, BDNF signaling is reduced significantly. 53 Previous studies showed that intermittent fasting 54 and ketogenic diet 55 have neuroprotection effects, mainly via increasing BDNF levels in the CNS. This therapy was shown clinically effective and has now been utilized by clinicians to control seizures. 54 Additionally, the β-HB has been shown to promote BDNF expression in the CNS. Potential mechanisms included acetylating histone deposition at the Bdnf promoter, 9 modulating redox balance and transcription factors, 56 and activating the NF-kB signaling pathway. 57 Based on this information, ketone diets were developed for humans and shown to increase plasma BDNF 58 However, whether acetoacetate acquires a similar physiological function in the AD brain remains unknown. Thus, we monitored the BDNF protein expression in the hippocampus after acetoacetate infusion. Surprisingly, we demonstrated a prominently elevated BDNF in an acetoacetate-dosage-dependent manner. To the best of our knowledge, this is the very first study to elucidate the role of acetoacetate on BDNF expression. This finding provides an interesting fact that acetoacetate obtained a multifaceted effect to alleviate the pathological process of the AD brain.
We note some limitations arising from the current study. First, although a significantly increased acetoacetate concentration was observed in the hippocampi, the distribution of acetoacetate remains largely unknown. Since the subdivisions of the hippocampus, including dentate gyrus and components of cornu ammonis, were proposed to be affected differently during AD, carbon-11-labeled acetoacetate will be used in the future study. Second, although we demonstrated that acetoacetate could upregulate BDNF in the hippocampus, the exact mechanism remains inconclusive. As several potential mechanisms have been proposed for β-HB on BDNF regulation,9,56,57 we will focus on the mechanism study of acetoacetate-induced BDNF elevation in the future. Moreover, we did not study the pharmacokinetics of acetoacetate during the injection period in AD mice. Since the ketone bodies become the main energy substrate for the brain under AD status, we will use microdialysis to understand the real-time acetoacetate concentration changes in our future study. Also, the current study only tested the effect of acetoacetate on short-term memory but did not investigate long-term memory. We will include other behavioral tests, including the Morris water maze, in future studies to answer this issue.
In conclusion, our study indicated that acetoacetate present a therapeutic effect on the AD pathophysiological process, which could alleviate AD symptoms. Two potential mechanisms were enrolled, including inhibiting the inflammation process and promoting neuron survival via promoting BDNF expression in the hippocampus.
Abbreviations
- Aβ protein
amyloid-beta protein
- AD
Alzheimer’s disease
- ANOVA
Analysis of Variance
- BDNF
brain-derived neurotrophic factor
- β-HB
β-hydroxybutyrate
- CNS
central nerve system
- EC50
50% excitatory concentration
- FFAR2
free fatty acid receptor 2
- GPR43
G-protein-coupled receptor 43
- IACUC
Institutional Animal Care Committee
- IC50
50% inhibitory concentration
- IL-6
Interleukin-6
- MCT
monocarboxylic acid transporter
- pERK
phosphorylated extracellular signal-regulated kinase
- RT
relative time
- SCFA
short-chain fatty acids
- TNF-α
tumor necrosis factor-α
Footnotes
Author Contributions: All authors contributed to the study’s conception and design. Prof. Xiao-Jun Wu performed material preparation, data collection, and analysis. Prof. Qin-Qin Shu, Bin Wang, Lan Dong, and Bin Hao wrote the first draft of the manuscript. All authors read and approved the final manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical Approval: The research protocol was approved by the Research and Ethics Committee of Shanghai Cancer Center affiliated to Shanghai Fu-Dan University School of Medicine (SHCan.20190903-021). The animal experiment procedures were approved by the Animal Care Committee (IACUC) of Shanghai Cancer Center affiliated to Shanghai Fu-Dan University School of Medicine, which was in accordance with animal care guidelines.
Data Availability: All data are available upon reasonable request.
Significance Statement: Little is known about the role of acetoacetate on the AD brain. We identified that acetoacetate could improve AD symptoms and exhibited features as potential therapeutics for AD.
Disclaimer: We want to express our sincere acknowledgment to all the personnel helping us to complete the current study. We also want to give our deepest appreciation to the animals who had sacrificed their lives for the current study.
ORCID iD
References
- 1.Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet. 2017;390(10113):2673-2734. doi: 10.1016/s0140-6736(17)31363-6 [DOI] [PubMed] [Google Scholar]
- 2.DeTure MA, Dickson DW. The neuropathological diagnosis of alzheimer’s disease. Mol Neurodegener. 2019;14(1):32. doi: 10.1186/s13024-019-0333-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calsolaro V, Edison P. Alterations in glucose metabolism in alzheimer’s disease. Recent Pat Endocr Metab Immune Drug Discov. 2016;10(1):31-39. doi: 10.2174/1872214810666160615102809 [DOI] [PubMed] [Google Scholar]
- 4.Hoyer S. Oxidative energy metabolism in alzheimer brain. Studies in early-onset and late-onset cases. Mol Chem Neuropathol. 1992;16(3):207-224. doi: 10.1007/bf03159971 [DOI] [PubMed] [Google Scholar]
- 5.Griffith CM, Macklin LN, Cai Y, et al. Impaired glucose tolerance and reduced plasma insulin precede decreased AKT phosphorylation and GLUT3 translocation in the hippocampus of old 3xTg-AD mice. J Alzheim Dis. 2019;68(2):809-837. doi: 10.3233/jad-180707 [DOI] [PubMed] [Google Scholar]
- 6.Pollay M, Stevens FA. Starvation-induced changes in transport of ketone bodies across the blood-brain barrier. J Neurosci Res. 1980;5(2):163-172. doi: 10.1002/jnr.490050208 [DOI] [PubMed] [Google Scholar]
- 7.Shippy DC, Wilhelm C, Viharkumar PA, Raife TJ, Ulland TK. β-Hydroxybutyrate inhibits inflammasome activation to attenuate alzheimer’s disease pathology. J Neuroinflammation. 2020;17(1):280. doi: 10.1186/s12974-020-01948-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reger MA, Henderson ST, Hale C, et al. Effects of beta-hydroxybutyrate on cognition in memory-impaired adults. Neurobiology of aging. 2004;25(3):311-314. doi: 10.1016/s0197-4580(03)00087-3 [DOI] [PubMed] [Google Scholar]
- 9.Sleiman SF, Henry J, Al-Haddad R, et al. Exercise promotes the expression of brain derived neurotrophic factor (BDNF) through the action of the ketone body β-hydroxybutyrate. Elife. 2016. 5:e15092. doi: 10.7554/eLife.15092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ota M, Matsuo J, Ishida I, et al. Effects of a medium-chain triglyceride-based ketogenic formula on cognitive function in patients with mild-to-moderate Alzheimer's disease. Neurosci Lett. 2019;690:232-236. doi: 10.1016/j.neulet.2018.10.048 [DOI] [PubMed] [Google Scholar]
- 11.Henderson ST, Vogel JL, Barr LJ, Garvin F, Jones JJ, Costantini LC. Study of the ketogenic agent AC-1202 in mild to moderate Alzheimer’s disease: A randomized, double-blind, placebo-controlled, multicenter trial. Nutrition & metabolism. 2009;6:31. doi: 10.1186/1743-7075-6-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Veech RL. The therapeutic implications of ketone bodies: The effects of ketone bodies in pathological conditions: Ketosis, ketogenic diet, redox states, insulin resistance, and mitochondrial metabolism. Prostaglandins Leukot Essent Fat Acids. 2004;70(3):309-319. doi: 10.1016/j.plefa.2003.09.007 [DOI] [PubMed] [Google Scholar]
- 13.Hawkins RA. Uptake of ketone bodies by rat brain in vivo. Biochem J. 1971;121(1):17p. doi: 10.1042/bj1210017pa [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Le Poul E, Loison C, Struyf S, et al. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J Biol Chem. 2003;278(28):25481-25489. doi: 10.1074/jbc.M301403200 [DOI] [PubMed] [Google Scholar]
- 15.Brown AJ, Goldsworthy SM, Barnes AA, et al. The orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem. 2003;278(13):11312-11319. doi: 10.1074/jbc.M211609200 [DOI] [PubMed] [Google Scholar]
- 16.Dalile B, Van Oudenhove L, Vervliet B, Verbeke K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat Rev Gastroenterol Hepatol. 2019;16(8):461-478. doi: 10.1038/s41575-019-0157-3 [DOI] [PubMed] [Google Scholar]
- 17.Sivaprakasam S, Prasad PD, Singh N. Benefits of short-chain fatty acids and their receptors in inflammation and carcinogenesis. Pharmacology & therapeutics. 2016;164:144-151. doi: 10.1016/j.pharmthera.2016.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maslowski KM, Vieira AT, Ng A, et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461(7268):1282-1286. doi: 10.1038/nature08530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heneka MT, Carson MJ, El Khoury J, et al. Neuroinflammation in alzheimer’s disease. Lancet Neurol. 2015;14(4):388-405. doi: 10.1016/s1474-4422(15)70016-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miyamoto J, Ohue-Kitano R, Mukouyama H, et al. Ketone body receptor GPR43 regulates lipid metabolism under ketogenic conditions. Proc Natl Acad Sci USA. 2019;116(47):23813-23821. doi: 10.1073/pnas.1912573116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamamoto I, Kawasumi K, Ohkusu-Tsukada K, Arai T. Molecular characterization of free fatty acid receptors FFAR2 and FFAR3 in the domestic cat. Veterinary medicine and science. 2021;7(1):77-85. doi: 10.1002/vms3.356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chikahisa S, Fujiki N, Kitaoka K, Shimizu N, Séi H. Central AMPK contributes to sleep homeostasis in mice. Neuropharmacology. 2009;57(4):369-374. doi: 10.1016/j.neuropharm.2009.07.015 [DOI] [PubMed] [Google Scholar]
- 23.Franklin K, Paxinos G. The Mouse Brain in Stereotaxic Coodinates. Academic Press; 2008. [Google Scholar]
- 24.Chesworth R, Downey L, Logge W, Killcross S, Karl T. Cognition in female transmembrane domain neuregulin 1 mutant mice. Behav Brain Res. 2012;226(1):218-223. doi: 10.1016/j.bbr.2011.09.019 [DOI] [PubMed] [Google Scholar]
- 25.Dere E, Huston JP, De Souza Silva MA. The pharmacology, neuroanatomy and neurogenetics of one-trial object recognition in rodents. Neurosci Biobehav Rev. 2007;31(5):673-704. doi: 10.1016/j.neubiorev.2007.01.005 [DOI] [PubMed] [Google Scholar]
- 26.Liśkiewicz D, Liśkiewicz A, Nowacka-Chmielewska MM, et al. Differential response of hippocampal and cerebrocortical autophagy and ketone body metabolism to the ketogenic diet. Front Cell Neurosci. 2021;15:733607. doi: 10.3389/fncel.2021.733607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abcam. Western blot protocol. Abcam. https://www.abcam.cn/protocols/general-western-blot-protocol (2020). [Google Scholar]
- 28.Kashiwaya Y, Bergman C, Lee JH, et al. A ketone ester diet exhibits anxiolytic and cognition-sparing properties, and lessens amyloid and tau pathologies in a mouse model of alzheimer’s disease. Neurobiol Aging. 2013;34(6):1530-1539. doi: 10.1016/j.neurobiolaging.2012.11.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pawlosky RJ, Kemper MF, Kashiwaya Y, King MT, Mattson MP, Veech RL. Effects of a dietary ketone ester on hippocampal glycolytic and tricarboxylic acid cycle intermediates and amino acids in a 3xTgAD mouse model of Alzheimer's disease. J Neurochem. 2017;141(2):195-207. doi: 10.1111/jnc.13958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chikahisa S, Shimizu N, Shiuchi T, Séi H. Ketone body metabolism and sleep homeostasis in mice. Neuropharmacology. 2014;79:399-404. doi: 10.1016/j.neuropharm.2013.12.009 [DOI] [PubMed] [Google Scholar]
- 31.Langhans W, Wiesenreiter F, Scharrer E. Different effects of subcutaneous D, L-3-hydroxybutyrate and acetoacetate injections on food intake in rats. Physiol Behav. 1983;31(4):483-486. doi: 10.1016/0031-9384(83)90070-7 [DOI] [PubMed] [Google Scholar]
- 32.Yin J, Nielsen M, Li S, Shi J. Ketones improves apolipoprotein E4-related memory deficiency via sirtuin 3. Aging. 2019;11(13):4579-4586. doi: 10.18632/aging.102070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pan JW, Rothman TL, Behar KL, Stein DT, Hetherington HP. Human brain beta-hydroxybutyrate and lactate increase in fasting-induced ketosis. J Cerebr Blood Flow Metab. 2000;20(10):1502-1507. doi: 10.1097/00004647-200010000-00012 [DOI] [PubMed] [Google Scholar]
- 34.Pan JW, Telang FW, Lee JH, et al. Measurement of beta-hydroxybutyrate in acute hyperketonemia in human brain. J Neurochem. 2001;79(3):539-544. doi: 10.1046/j.1471-4159.2001.00575.x [DOI] [PubMed] [Google Scholar]
- 35.Newman JC, Covarrubias AJ, Zhao M, et al. Ketogenic diet reduces midlife mortality and improves memory in aging mice. Cell Metabol. 2017;26(3):547-557. doi: 10.1016/j.cmet.2017.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fortier M, Castellano CA, St-Pierre V, et al. A ketogenic drink improves cognition in mild cognitive impairment: Results of a 6-month RCT. Alzheimers Dement. 2021;17(3):543-552. doi: 10.1002/alz.12206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fortier M, Castellano CA, Croteau E, et al. A ketogenic drink improves brain energy and some measures of cognition in mild cognitive impairment. Alzheimers Dement. 2019;15(5):625-634. doi: 10.1016/j.jalz.2018.12.017 [DOI] [PubMed] [Google Scholar]
- 38.Roy M, Fortier M, Rheault F, et al. A ketogenic supplement improves white matter energy supply and processing speed in mild cognitive impairment. Alzheimers Dement. 2021;7(1):e12217. doi: 10.1002/trc2.12217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Myette-Côté E, St-Pierre V, Beaulieu S, et al. The effect of a 6-month ketogenic medium-chain triglyceride supplement on plasma cardiometabolic and inflammatory markers in mild cognitive impairment. Prostaglandins Leukot Essent Fat Acids. 2021;169:102236. doi: 10.1016/j.plefa.2020.102236 [DOI] [PubMed] [Google Scholar]
- 40.Faria MH, Muniz LR, Vasconcelos PR. Ketone bodies metabolism during ischemic and reperfusion brain injuries following bilateral occlusion of common carotid arteries in rats. Acta Cir Bras. 2007;22(2):125-129. doi: 10.1590/s0102-86502007000200009 [DOI] [PubMed] [Google Scholar]
- 41.Hu K, Li Y, Yu H, Hu Y. CTBP1 confers protection for hippocampal and cortical neurons in rat models of alzheimer’s disease. Neuroimmunomodulation. 2019;26(3):139-152. doi: 10.1159/000500942 [DOI] [PubMed] [Google Scholar]
- 42.Massieu L, Haces ML, Montiel T, Hernández-Fonseca K. Acetoacetate protects hippocampal neurons against glutamate-mediated neuronal damage during glycolysis inhibition. Neuroscience. 2003;120(2):365-378. doi: 10.1016/s0306-4522(03)00266-5 [DOI] [PubMed] [Google Scholar]
- 43.Stoddart LA, Smith NJ, Jenkins L, Brown AJ, Milligan G. Conserved polar residues in transmembrane domains V, VI, and VII of free fatty acid receptor 2 and free fatty acid receptor 3 are required for the binding and function of short chain fatty acids. J Biol Chem. 2008;21283(47):32913-32924. doi: 10.1074/jbc.M805601200 [DOI] [PubMed] [Google Scholar]
- 44.Delzenne NM, Neyrinck AM, Bäckhed F, Cani PD. Targeting gut microbiota in obesity: effects of prebiotics and probiotics. Nat Rev Endocrinol. 2011;7(11):639-646. doi: 10.1038/nrendo.2011.126 [DOI] [PubMed] [Google Scholar]
- 45.Rui L. Energy metabolism in the liver. Compr Physiol. 2014;4(1):177-197. doi: 10.1002/cphy.c130024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Puchalska P, Crawford PA. Multi-dimensional roles of ketone bodies in fuel metabolism, signaling, and therapeutics. Cell Metabol. 2017;25(2):262-284. doi: 10.1016/j.cmet.2016.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Altayyar M, Nasser JA, Thomopoulos D, Bruneau M, Jr. The implication of physiological ketosis on the cognitive brain: A narrative review. Nutrients. 2022;25(3):14. doi: 10.3390/nu14030513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bough KJ, Schwartzkroin PA, Rho JM. Calorie restriction and ketogenic diet diminish neuronal excitability in rat dentate gyrus in vivo. Epilepsia. 2003;44(6):752-760. doi: 10.1046/j.1528-1157.2003.55502.x [DOI] [PubMed] [Google Scholar]
- 49.Masino SA, Li T, Theofilas P, et al. A ketogenic diet suppresses seizures in mice through adenosine A receptors. The Journal of clinical investigation. 2011;121(7):2679-2683. doi: 10.1172/jci57813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Youm YH, Nguyen KY, Grant RW, et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nature Med. 2015;21(3):263-269. doi: 10.1038/nm.3804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Balasubramanian P, Sirivelu MP, Weiss KA, et al. Differential effects of inhalation exposure to PM2.5 on hypothalamic monoamines and corticotrophin releasing hormone in lean and obese rats. Neurotoxicology. 2013;36:106-111. doi: 10.1016/j.neuro.2012.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Park H, Poo MM. Neurotrophin regulation of neural circuit development and function. Nat Rev Neurosci. 2013;14(1):7-23. doi: 10.1038/nrn3379 [DOI] [PubMed] [Google Scholar]
- 53.Autry AE, Monteggia LM. Brain-derived neurotrophic factor and neuropsychiatric disorders. Pharmacol Rev. 2012;64(2):238-258. doi: 10.1124/pr.111.005108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Murugan M, Boison D. Ketogenic diet, neuroprotection, and antiepileptogenesis. Epilepsy Res. 2020;167:106444. doi: 10.1016/j.eplepsyres.2020.106444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baik SH, Rajeev V, Fann DY, Jo DG, Arumugam TV. Intermittent fasting increases adult hippocampal neurogenesis. Brain Behav. 2020;10(1):e01444. doi: 10.1002/brb3.1444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marosi K, Kim SW, Moehl K, et al. 3-Hydroxybutyrate regulates energy metabolism and induces BDNF expression in cerebral cortical neurons. J Neurochem. 2016;139(5):769-781. doi: 10.1111/jnc.13868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim SW, Marosi K, Mattson M. Ketone beta-hydroxybutyrate up-regulates BDNF expression through NF-κB as an adaptive response against ROS, which may improve neuronal bioenergetics and enhance neuroprotection. Neurology. 2017;88(16):P3.090. [Google Scholar]
- 58.Walsh JJ, Myette-Côté E, Little JP. The effect of exogenous ketone monoester ingestion on plasma BDNF during an oral glucose tolerance test. Front Physiol. 2020;11:1094. doi: 10.3389/fphys.2020.01094 [DOI] [PMC free article] [PubMed] [Google Scholar]