Abstract
In the absence of effective pharmacological interventions for the prevention of dementia, attention has turned to lifestyle factors that contribute to cognitive reserve. Although cognitive reserve cannot prevent the occurrence of disease, the trajectory is different for high reserve and low reserve patients, giving more time for independent living to high reserve individuals. We argue that lifelong bilingual experience meets the criteria for an experience that confers cognitive reserve, although neural reserve, a related concept, is more difficult to validate. Bilingual patients show symptoms at a later stage of disease and decline more rapidly than comparable monolingual patients. These patterns are considered in terms of evidence from behavioural, imaging and epidemiological studies. Finally, the role of bilingualism in protecting against symptoms of some forms of dementia are discussed in the context of other protective factors and the limits of this reserve approach in dealing with the consequences of dementia.
Keywords: cognitive reserve, bilingualism, biomarkers, retrospective studies, prospective studies
Alzheimer’s disease (AD) is a neurodegenerative disease for which symptoms develop over time, including memory loss, language impairment, and in later stages, loss of some bodily functions such as walking or swallowing, and is ultimately fatal.1-3 As populations age and live longer, the prevalence of AD also increases. Globally, dementia (including of the Alzheimer’s type but also others) affects ∼50 million people, with this number rising to a projected 82 million in 2030 and 152 million by 2050. 4 Considering only the USA, an estimated 5.8 million Americans live with AD, a number that is projected to rise to close to 14 million by 2050. 5 As of 2018, AD was the fifth leading cause of death in individuals aged 65 or older in the USA. Care provided by family members was estimated at around $250 billion unpaid hours, without factoring in the associated mental and physical costs of taking care of a loved one with dementia. Total costs associated with health care and long-term care for adults aged 65 and older with AD were an estimated $305 billion in 2020. There is extensive physical and emotional stress on both personal and systemic levels for everyone involved in a diagnosis of AD. Pharmaceutical treatments have limited effectiveness in slowing progression of the disease or treating symptoms,6,7 but there is some evidence that the course of the disease could be delayed by non-pharmaceutical, cognitive-based methods that build ‘reserve’. 8 It has been estimated that a delay of 5 years of AD onset would lead to an ∼50% decrease in overall disease frequency. 9 Thus, delaying the development of AD symptoms would be the most beneficial course of action for reducing the associated costs.
Defining and Creating Reserve
Reserve, broadly speaking, is one theory that provides a framework for how to delay AD symptom onset and subsequent dementia. The concept of reserve is used to describe the individual differences that exist in cognitive level, clinical status and functional ability in ageing and brain disease such that specific levels of brain health can have variable associations with cognitive or functional outcomes. Although the precise mechanism is unknown, 10 multiple factors may be responsible and include neuroprotective mechanisms (i.e. factors preventing cognitive decline or neurodegeneration) and compensatory mechanisms (i.e. factors that allow individuals to adapt to declining neural health). These factors work both alone and in tandem to preserve cognitive performance during ageing and neural decline. 11 This disconnect between preserved function and neurodegeneration is the hallmark of reserve and expressed through the specific concepts of brain reserve, brain maintenance and cognitive reserve. Moreover, since cognitive reserve and brain reserve are likely based on different mechanisms, we speculate that it is possible to have one without the other.
Stern et al. 12 proposed individual definitions for these component concepts to clarify the differences among them, and our definitions and claims are derived from their work. Brain reserve is generally thought of as ‘neurobiological capital’; that is, it refers to cortical thickness, total brain volume, quantity of neurons, and the like, at a given point in time. Individuals with high brain reserve are expected to deal with ageing and neurodegeneration better than those with low brain reserve as a result of this built up ‘capital’ prior to decline; there is more neural matter available to lose before cognitive difficulties are observed. In this sense, brain reserve is often considered a passive model of reserve in which cognitive impairment is imminent once a simple threshold of brain deterioration has occurred. The reasons for high brain reserve compared to low brain reserve may be impacted by the related concepts, brain maintenance and cognitive reserve, which, in turn, are impacted by genetic and lifestyle factors.
Brain maintenance is complementary to brain reserve, but whereas brain reserve refers to neural capital at a given point in time, brain maintenance reflects reduced age- or disease-related neural degeneration over time. Individuals with high brain maintenance will show slower development of neuronal plaques or grey matter deterioration compared to those with low brain maintenance. Thus, whereas brain reserve is measured at a given point in time, brain maintenance is best measured longitudinally by examining deterioration over time. Brain reserve may help protect against the effects of pathology, but brain maintenance is posited to prevent this pathology in the first place. Both genetic factors (e.g. allelic variation in genes) and lifestyle factors (e.g. stimulating leisure activities) are thought to influence brain maintenance.
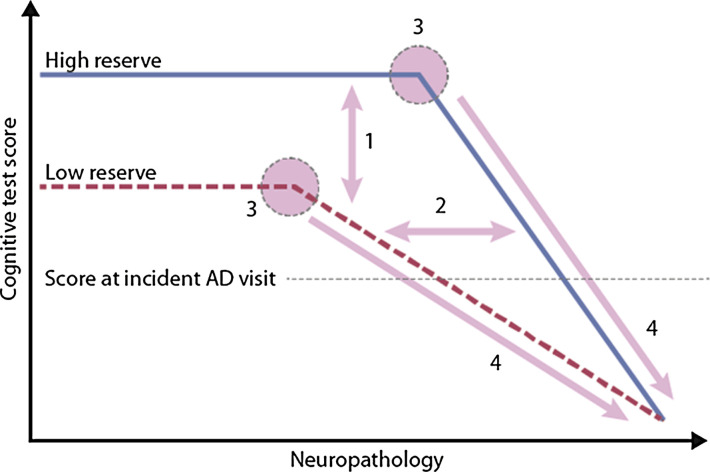
Cognitive reserve posits that cognitive processes are adaptable, and it is this adaptability that helps explain individual discrepancies in cognitive functioning despite neurodegeneration and pathology. Cognitive reserve is thought of as an active process of reserve, such that individuals dynamically cope with or adjust to ageing and pathology using compensatory mechanisms or functional brain processes. For these reasons, there are several outcomes to be expected when comparing high cognitive reserve individuals against low cognitive reserve individuals (depicted in Figure 1). First, individuals with high cognitive reserve should show better cognitive performance than low cognitive reserve individuals at similar levels of neuropathology. Second, individuals with high cognitive reserve should show greater amounts of neuropathology than individuals with low cognitive reserve at comparable levels of cognitive performance: high cognitive reserve individuals are better able to cope with the effects of neurodegeneration than their low cognitive reserve peers. Third, the point of inflection, or where memory begins to be affected by AD, should be later for high cognitive reserve individuals than low cognitive reserve individuals. Fourth, once symptoms of cognitive decline appear, disease progression should proceed faster in high cognitive reserve than low cognitive reserve individuals. These dynamics that distinguish between high and low reserve individuals (and groups) are shown in Figure 1. 10
Figure 1.
Theoretical depiction of cognitive performance as a function of increasing neuropathology in high and low cognitive reserve individuals, with numbered predictions as outlined in the text (adapted from Stern, 2012 10 ).
It is not possible to directly assess cognitive reserve, so it can only be investigated through examining proxies that covary with and contribute to reserve more broadly. Commonly cited sources of cognitive reserve are several socio-behavioural proxies, including formal education, occupational complexity, stimulating leisure activities and physical activity.10,11,13,14 Two common threads emerge from research examining these proxy factors. The first is that individuals with higher levels of one or more of these factors (e.g. more formal education and/or more physical exercise and activity) show better clinical and cognitive outcomes in ageing than their peers with low levels of these factors. These benefits extend to an individual both when the activity occurred early in life (such as education) and when the activity is currently ongoing (such as stimulating physical and leisure activities). The second common thread is that these factors of cognitive reserve are effortful and engaging. Theoretically, therefore, any sufficiently challenging and continuous activity should be a source of cognitive reserve, although what qualifies as ‘sufficiently challenging’ is a matter of ongoing discussion. 15
Bilingualism as a Source of Reserve
Bilingualism has been posited as another potential proxy for cognitive reserve. 16 Of all engaging activities, language use is the most sustained throughout the day and throughout our lives. A surprising finding from psycholinguistic research is that both languages in the bilingual mind are jointly activated, so successful language production requires monitoring and selective attention to the required language. 17 Language use activates essentially the entire brain, except for some posterior regions, 18 making it a prime candidate for reserve because of its scope. This joint language activation in bilinguals has been posited to have extensive effects in shaping brain structure and cognitive ability, on brain regions and processes beyond language processing to include nonverbal domains and cognitive performance. 19
Research investigating the cognitive implications of bilingual language use across the lifespan have reported that, on average, bilinguals outperform their monolingual peers on cognitive tests of executive function.19,20 This assertion is not without controversy as some researchers have challenged this proposition both in young adults21-23 and in children24,25 by claiming not to replicate the central findings. The debate is ongoing, and current discussion focuses on specific factors of bilingualism that lead to these effects such as age of language acquisition, continuous language use, or interactional language context. However, performance on cognitive tasks in older adults more reliably shows effects of bilingualism such that bilinguals more consistently outperform monolinguals than is often found in the younger groups.26,27
Evidence supporting the proposition that bilingualism is a proxy for cognitive reserve has been reported for all 4 predictions indicated in Figure 1. 16 The first prediction is that high reserve individuals will show better cognitive performance than low reserve individuals at similar levels of neuropathology. In a study by Berkes et al, 28 32 cognitively healthy bilingual participants were recruited from the community and diffusion tensor imaging (DTI) brain scans were collected. Brain health was determined through principal component analysis of white matter measures (fractional anisotropy, axial diffusivity and radial diffusivity). These participants were then matched to monolingual participants from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database using one-to-one propensity score matching based on age, education, and sex to a group of 32 individuals with similar brain health scores calculated from DTI. All the bilinguals were cognitively normal, so the question was to evaluate the cognitive status of the monolinguals who were similar in brain health and demographic variables. The first difference was evidence from MMSE scores, where it was found that bilinguals (M = 29.4) obtained significantly higher scores than monolinguals (M = 26.7). More important, however, was the clinical determination of cognitive status. Unlike the bilinguals who were all cognitively normal, the monolinguals revealed a variety of cognitive status outcomes such that 41% of the matched monolinguals had a clinical diagnosis of impairment, specifically either mild cognitive impairment (MCI) or AD. These data can also be compared to the overall probability of cognitive impairment by monolinguals in the larger unmatched sample from the same database. In this case, the population distribution indicated a clinical impairment rate of 27%, a rate that was significantly lower than the rate of 41% observed in the matched sample of monolinguals that was equated to the cognitively healthy bilinguals on brain measures. Put another way, the same level of white matter structural integrity was significantly associated with cognitive impairment in monolinguals but not in bilinguals. This finding that monolinguals had poorer cognitive and clinical outcomes than expected by chance and poorer than those for bilinguals at similar levels of neuropathology supports the role of bilingualism in supporting healthy ageing.
The second prediction from the model in Figure 1 is that bilingual individuals should have more neuropathology than monolingual individuals at similar levels of cognition. In the first study to demonstrate this point, Schweizer et al. 29 measured brain atrophy in monolingual and bilingual patients diagnoses with probable AD who were matched on clinical level. Using computer tomography and several linear measurements of atrophy, the researchers found greater atrophy for bilingual patients than monolingual patients in regions typically associated with AD, such as the radial width of the temporal horn and the temporal horn ratio. A similar pattern was reported by Perani et al. 30 who used PET to study synaptic function and density in an older adult patient population comprised of monolinguals and bilinguals. Patients in both language groups were diagnosed with probable AD and matched on disease severity and duration, yet bilingual patients showed greater cerebral hypometabolism. In both studies, monolingual and bilingual patients matched on clinical level revealed greater neurodegeneration for bilinguals than monolinguals at similar levels of disease progression. These results suggest that bilinguals are able to withstand greater amounts of neurodegeneration than monolinguals without showing increasing symptoms of clinical impairment.
The third prediction from cognitive reserve is that high reserve individuals will show symptoms of impairment at a later point than those with low reserve. A seminal retrospective study showed this result for bilinguals in a clinical sample in which bilingual older adult patients presented with symptoms of dementia ∼4.5 years later than monolingual patients, an effect that was seen even after accounting for education, MMSE performance, and immigration status. 31 These effects have been replicated across other studies in other countries including India, 32 Belgium 33 and China 34 and confirmed in meta-analyses.35-37
The final prediction from the cognitive reserve model in Figure 1 is that once symptoms of dementia appear, they progress more rapidly in high reserve than low reserve individuals. Although this seems counterintuitive, the endpoint of disease progression remains the same regardless of high or low reserve: high reserve individuals who are coping with greater neuropathology at time of symptom onset decline more rapidly when this protective threshold has been breached. Support for this idea comes from a study by Berkes et al. 38 In a clinical sample of bilingual and monolingual patients attending a memory clinic, the time between a diagnosis of MCI and conversion of that diagnosis to AD was shorter for bilingual patients than monolingual patients. Specifically, bilingual patients converted from MCI to AD in a mean of 1.9 years whereas the interval for monolinguals was 2.6 years, a difference that was significant. From one perspective, this finding seems to contradict the previous positive findings showing maintained cognitive function despite neuropathology, similar clinical outcomes with greater neuropathology, and delayed onset of clinical symptoms; in contrast, more rapid decline at advanced stages of disease appears to be a negative consequence. However, this difference in trajectory is inevitable given the common endpoint for these patients. The positive aspect of this finding is that it has created more time in earlier stages for higher quality of life by postponing the decline. The overall impact of high cognitive reserve is that it allows for the best cognitive outcomes despite the continued process of neurodegeneration, an effect that has been shown to be associated with bilingual experience.
Does Bilingualism Protect Against Dementia?
The argument to this point has been that bilingualism is a proxy for cognitive reserve, with evidence that shows that bilinguals (high reserve) are better able to cope with neurodegeneration than monolinguals (low reserve). However, there are limits to what bilingualism can do in terms of healthy ageing. Importantly, bilingualism does not prevent the accumulation of disease pathology but rather allows individuals to cope with that pathology for a longer period before it interferes with cognition. The retrospective studies that have been described above indicate an older age of dementia symptom onset or diagnosis for bilinguals than monolinguals but provide no evidence that bilinguals can avoid the disease entirely. The question of the likelihood of developing the disease requires a different methodology.
Prospective studies, in contrast to retrospective ones, attempt to determine risk of disease development prior to symptom onset. These studies typically follow a cohort of healthy individuals and track the rate at which individuals or groups develop the disease. Prospective studies examining bilingualism in the context of dementia have largely found no differences between monolingual and bilingual individuals for the likelihood that they will develop AD or other dementias. One of the first studies to show this was conducted in a large sample of 1067 older adults who lived in the Washington/Hamilton Heights community of New York. 39 The researchers examined cognitive test scores and clinical diagnoses of Spanish–English bilinguals and Spanish monolinguals who were tested at 18–24-month intervals over the course of approximately 2 decades. By the completion of the study, 31% of monolinguals and 20% of bilinguals had developed dementia; however, this difference was not significant when it was included in a comprehensive model that accounted for other possible predictors. Being female and attaining higher education were associated with reduced risk of dementia and older age was associated with increased risk of dementia, but bilingualism had no further association with dementia risk. This absence of any unique protective effect of bilingualism on the likelihood of developing of dementia has been reported in other prospective studies across the US and in Sweden,40,41 although another study in the US showed decreased incidence of MCI among individuals who had studied a foreign language to a high level. 42 One limitation in these studies is that bilingualism is often determined from subjective (e.g. self-report) rather than objective (e.g. vocabulary tests) measures which limits the interpretability of conclusions drawn regarding language use specifically. Furthermore, some of the studies had too few participants to draw meaningful conclusions. For example, the study by Ljungberg et al. 41 reported that 102 out of 736 monolinguals developed dementia over the course of 10 years, but 10 out of only 72 bilinguals developed dementia over the same time. Because the proportions are similar in the two language groups, the data suggest that bilingualism is not a protective factor for the development of dementia. It is possible that prospective studies fail to find effects of bilingualism because they typically rely on a narrow categorical definition of bilingualism. However, the question addressed by prospective studies is different from that addressed by retrospective ones. There have never been any claims that bilingualism prevents dementia; the claim from retrospective studies is that the symptoms appear later in the disease. Since prospective studies examine the likelihood that individuals or groups will develop the disease, there is no basis for predicting different incidence rates, only that when the disease does appear, bilingual patients will be older. Most prospective studies do not report the age of diagnosis.
Although the imbalance in language group size was particularly apparent in the Ljungberg et al. study, 41 all the prospective studies are based on convenience samples and possibly too small for subtler effects to emerge. An interesting alternative to community-based samples, therefore, is to use population level statistics as the unit of analysis instead of individuals. 43 The researchers calculated language use data and incident AD data for 93 different countries. In a generalised additive model, the researchers found that incidence of senile dementia declined as the mean number of languages used in the population increased up to 2. That is, the countries that were more bilingual (mean languages = 2) showed lower incidence of dementia than the more monolingual countries (mean languages = 1). This result was seen after weighting for population size and life expectancy. Although the model was not significant for countries with mean number of languages spoken greater than 2, the reason is likely because there were too few of these countries to contribute systematic explanation to the model. The authors concluded that bilingualism may contribute to cognitive reserve that in turn protects against AD, but also cautioned that comprehensive and thorough data about these factors are needed to make appropriate evaluations.
Related Factors and Next Steps
Bilingualism, and cognitive reserve more broadly, are not the sole determinants of clinical outcomes in ageing. Other factors that have been shown to be related to clinical outcomes include biomarkers, such as the amyloid-β (Aβ) peptide and tau microtubules in cerebrospinal fluid (CSF), and genetic factors, such as the presence of the apolipoprotein E (APOE) ε4 allele on chromosome 19. However, these biomarkers and genetic risk factors have rarely been discussed in tandem with language use as relating to ageing and cognitive decline. Considering that these ‘hidden’ genetic factors may influence clinical outcomes, studying the interactions between these exogenous and endogenous factors is essential to furthering our understanding of both cognitive and clinical outcomes in ageing.
Amyloid-β has historically been thought to play a role in neuronal loss and cognitive impairment through the ‘amyloid cascade hypothesis’,44,45 which posits Aβ as the primary cause of AD due to accumulation of senile plaques and intercellular deposition of neurofibrillary tau tangles. As such, levels of Aβ42 found in CSF show an inverse relationship with disease progression – as plaques accumulate in the parenchymal tissue of the brain, less Aβ is found in CSF. 46 A study using education as a proxy for cognitive reserve to examine levels of CSF Aβ in cognitively normal and impaired individuals found no effect of reserve nor interaction with age, 47 although the authors did not examine cognitive decline as a factor. Soldan et al. 48 used a composite score of reading, vocabulary, and education to determine cognitive reserve in middle-aged cognitively normal individuals to then determine risk of developing symptoms of preclinical AD. In contrast to Almeida et al., this study examined decline and found main effects of cognitive reserve and baseline Aβ42 levels such that higher levels of reserve as indicated by the language and education measures were associated with lower risk of developing symptoms, and lower levels of Aβ42 were associated with higher risk of developing symptoms. Another study used bilingualism, rather than education, as a measure of cognitive reserve to examine the impact on CSF biomarkers but found no differences between cognitively normal monolinguals and bilinguals in CSF Aβ levels. 49 This study, however, included middle-aged participants rather than older adults and also did not investigate cognitive decline as a factor. These studies, therefore, provide conflicting reports on how cognitive reserve may modify decline and Aβ levels and none of the studies has examined the interaction between bilingualism as the reserve proxy and CSF biomarkers for their effect on decline.
Tau, the major microtubule-associated protein in developed neurons, is another biomarker of interest in AD. The presence of phosphate in the brain leads to phosphorylation of tau, which is necessary for tau to bind with microtubules and stimulate their assembly. However, excess levels of phosphate, or hyperphosphorylation, depresses the biological activity of tau. In the brain of an AD patient, tau is three-to four-fold more hyperphosphorylated than that found in a neurotypical adult brain 50 and so is a prime indicator of brain health and disease progression. Tau’s function in neurons is of such importance that recent research posits tau pathology, and not Aβ, as the primary driver of AD.51-53
The study by Almeida et al. 47 used education as a proxy for cognitive reserve and investigated CSF levels of total tau (t-tau) and phosphorylated tau (p-tau) in both cognitively intact and impaired older adults, whereas their previously mentioned results regarding Aβ showed no effect of reserve, they found a significant interaction effect between tau and cognitive reserve, such that both t-tau and p-tau levels were higher in older adults with low education than in age-matched peers with high education. Further, tau levels were higher for cognitively impaired adults than for cognitively normal adults. This is in contrast to Aβ findings that usually see the reverse, that is, lower CSF Aβ values in cognitively impaired individuals due to greater accumulation of neuropathology staying in the brain. The finding by Almeida et al. is somewhat supported by the previously described study by Estanga et al. 49 who also investigated CSF t-tau and p-tau in middle-aged monolingual and bilingual individuals. Unlike Almeida et al., the study by Estanga et al. used bilingualism as their measure of cognitive reserve. They found lower t-tau values in early bilinguals than in monolinguals and late bilinguals, an effect they attributed to increased cognitive reserve. These are promising results for the hypothesis that bilingualism contributes to reserve, but participants in this study were young adults, so no symptoms or diagnoses of AD had been met. It is difficult to compare these results in a meaningful way to studies that use older or clinically impaired adults, but the results provide a framework upon which future studies can build.
In addition to Aβ and tau, one of the largest genetic risk factors of AD is the presence of the APOE ε4 allele. APOE exists as three polymorphic alleles, ε2, ε3, and ε4, but only the ε4 allele is associated with an increased risk of developing AD. The ε4 allele has a worldwide frequency of ∼14% yet jumps to ∼40% frequency in patients diagnosed with AD, 54 (see 55 for differences between countries). Additionally, an older study by Seshadri et al. 56 used a Bayesian calculation to show that adults with at least 1 APOE ε4 allele had a 29% risk of developing AD in their lifetime compared to a 9% risk in those adults with no ε4 allele. This predictive aspect of the ε4 allele has been recognised for over 2 decades and found to be reliable. 55 One mechanism by which APOE influences development of AD is that the APOE genotype strongly affects deposition rates of Aβ in the brain, such that APOE ε4 carriers show greater abundance of senile plaques compared to noncarriers.57-60
Ferrari et al. conducted a long-term follow up study of a population-based cohort and showed that higher education and greater physical activity reduced incidence of AD in APOE ε4 carriers. 61 The effect was significant enough that carriers of the ε4 allele with high education had similar hazard ratios of developing AD as noncarriers. Although the study did not specify education as a proxy for cognitive reserve, the authors posited brain and cognitive reserve as the possible mechanisms for this effect. This finding follows from previous work done by the same group showing the protective effects of high education on development of dementia and AD, both with and without the context of APOE information.62,63 Using bilingualism as the proxy for cognitive reserve, Crane et al. examined cognitive decline in Japanese-American men aged ∼75 years and found no effect of speaking or writing Japanese (in addition to English) on rates of decline after accounting for APOE allele status. 64 Their conclusion was that multilingualism does not contribute to the cognitive reserve hypothesis. This conclusion was similarly reached by Hack et al. 65 who investigated dementia onset and the effects of multilingualism using data from the Nun Study. 66 No effect of bilingualism was apparent on dementia onset times after accounting for APOE status, despite independent effects of APOE and written linguistic ability. The authors of this study did find that individuals who spoke four or more languages were significantly less likely to develop dementia than monolinguals, but this effect was minimised once linguistic ability was included in the model alongside APOE status and education. Cognitive reserve, broadly speaking, has been shown to reduce the rates and odds of developing dementia, but in the presence of APOE, the results are less clear. Interpreting the findings is confounded further depending on how cognitive reserve is measured, that is, in terms of education, aerobic exercise or multilingualism. Given that the presence of APOE ε4 is possibly the largest risk factor for developing dementia, it is possible that the risk from carrying an ε4 allele outweighs any positive effects that may be gained from higher cognitive reserve.
A recent study from our lab attempted to account for Aβ, tau, and APOE ε4 alongside potential effects of bilingualism in a patient population from the ADNI database. 67 The database was searched for monolingual and bilingual participants with available neuropsychometric assessments, biomarker assays, MRI scans, clinical diagnoses and demographic information. Monolinguals were classified as such if their primary language and preferred language of testing were both English and participants were neither Latino nor Hispanic to rule out participants with a strong likelihood of using or being exposed to Spanish. There were no racial criteria for monolinguals. Bilinguals were classified as such if the language used for testing was English but the home language was any language other than English. There were no ethnic or racial criteria for bilinguals. In total, 577 monolinguals were found with relatively complete records, but significantly fewer bilinguals with records that had missing data at random, producing 64 participants.
Due to this disparity of available information and participant numbers between groups, it was necessary to identify a methodology that could address these issues in order to assess the data. Functional gradient boosting, or gradient boosted regression modelling (GBM), is one such method. In brief, GBM is a machine-learning algorithm that determines a predictive model of best fit for a given dataset. Gradient boosted regression modelling accomplishes this prediction by building the model in sequential stage-wise fashion from iterative ‘decision trees’ – regression models that are rough and gradually increase in predictive accuracy as they focus on minimising errors from previous models. Because decision trees are added sequentially, GBM is a slow process that increases in accuracy as more trees are considered. Different boosting techniques have been introduced over the years.68,69 These processes account for missing data and are highly customisable with remarkable success in practical applications. 70 The current analyses made use of boosting from the R package Twang 71 to estimate propensity scores for each language group. Propensity scores are a measure that indicates how likely a participant is to be a member of a particular group (e.g. how likely it is that a monolingual is in fact a monolingual). These propensity scores were then used to weight variables of interest before analyses.
The model that was used to estimate language group propensity scores included the predictive covariates of demographic data, such as age and sex, and biomarker and genetic factors, such as CSF Aβ, tau, and the presence of the APOE ε4 allele. When using GBM to estimate propensity scores, multiple diagnostic plots and balance measures are produced to ensure model fit worked as theoretically intended. One such balance measure revealed the level of influence each predictive covariate in the model had on propensity score estimation. In this model, t-tau and p-tau accounted for ∼66% and ∼20%, respectively, of propensity score estimation. In other words, estimating language group status was largely determined by differences in CSF levels of t-tau and p-tau between language groups. This finding, however, does not differentiate across clinical groups which includes cognitively normal, MCI and AD patients. After weighting participants by propensity scores, test scores on the AD Assessment Scale were compared between language groups by diagnosis. There was no difference in performance for cognitively normal participants, nor for AD participants, but bilinguals with MCI performed significantly better than monolinguals with MCI. The interpretation is that cognition is comparable both pre-symptom onset and at the end of decline (AD), but during decline bilinguals are able to function at a level that is better than expected. These findings and methodology are preliminary and need to be validated on a sample that includes more bilinguals, is better balanced in terms of participant numbers across clinical groups, and includes more comprehensive language measures. Nonetheless, it is the first study to evaluate how the cognitive reserve proxy of bilingualism might interact with the biomarkers associated with clinical cognitive decline.
Concluding Thoughts
The reviewed literature points to strong evidence that bilingualism is a proxy for cognitive reserve. Bilinguals have better clinical outcomes than expected by chance when matched to monolinguals on brain health but have more neurodegeneration when matched on clinical levels. Bilinguals present with symptoms of dementia at a later time than monolinguals, but as a result also decline in cognition and subsequent clinical diagnosis at a faster rate than monolingual peers. Bilingualism provides resilience so the effects of neurodegeneration on cognition are reduced with ageing. However, bilingualism does not prevent this neurodegeneration and associated diseases from occurring. Bilingualism, as a lifestyle factor, can lead to better cognitive and clinical outcomes in older age than occurs from single language use. The impact of the delay of symptoms found for bilinguals is better quality of life for longer than expected, with positive outcomes for the individual, their families and society.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Preparation of this paper was supported by grant A2559 from the Natural Sciences and Engineering Research Council, Canada, to EB.
ORCID iD
Ellen Bialystok https://orcid.org/0000-0001-9844-2082
References
- 1.Horner J, Alberts MJ, Dawson DV, Cook GM. Swallowing in Alzheimer’s disease. Alzheimer Dis Assoc Disord. 1994;8(3):177-189. [DOI] [PubMed] [Google Scholar]
- 2.Adams RD, Ropper VM, Daroff RB. Principles of Neurology. 6th ed. New York, London: McGraw-Hill; 1997. [Google Scholar]
- 3.Chouinard J. Dysphagia in Alzheimer disease: A review. J Nutr Health Aging. 2000;4(4):214-217. [PubMed] [Google Scholar]
- 4.Organization, WH . Dementia. 2020. http://www.who.int/en/news-room/fact-sheets/detail/dementia.
- 5.Association, As . 2020 Alzheimer's disease facts and figures. Alzheimer's Dementia. 2020;16:391-460. [Google Scholar]
- 6.Mehta D, Jackson R, Paul G, Shi J, Sabbagh M. Why do trials for Alzheimer’s disease drugs keep failing? A discontinued drug perspective for 2010–2015. Expet Opin Invest Drugs. 2017;26(6):735-739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Becker RE, Greig NH, Giacobini E. Why do so many drugs for Alzheimer's disease fail in development? Time for new methods and new practices? J Alzheim Dis. 2008;15(2):303-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baumgart M, Snyder HM, Carrillo MC, Fazio S, Kim H, Johns H. Summary of the evidence on modifiable risk factors for cognitive decline and dementia: A population-based perspective. Alzheimer's Dement. 2015;11(6):718-726. [DOI] [PubMed] [Google Scholar]
- 9.Association, As . Changing the Trajectory of Alzheimer's Disease: How a Treatment by 2025 Saves Lives and Dollars. Chicago: Alzheimer's Association; 2015. [Google Scholar]
- 10.Stern Y. Cognitive reserve in ageing and Alzheimer's disease. Lancet Neurol. 2012;11(11):1006-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barulli D, Stern Y. Efficiency, capacity, compensation, maintenance, plasticity: Emerging concepts in cognitive reserve. Trends Cognit Sci. 2013;17(10):502-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stern Y, Arenaza‐Urquijo EM, Bartrés‐Faz D, et al. Whitepaper: Defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimer's Dementia. 2020;16:1305-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc. 2002;8:448-460. [PubMed] [Google Scholar]
- 14.Valenzuela MJ, Sachdev P. Brain reserve and dementia: A systematic review. Psychol Med. 2006;36:441-454. [DOI] [PubMed] [Google Scholar]
- 15.Scarmeas N, Stern Y. Cognitive reserve and lifestyle. J Clin Exp Neuropsychol. 2003;25:625-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bialystok E. Bilingualism: Pathway to cognitive reserve. Trends Cognit Sci. 2021;25(5):355-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kroll JF, Bobb SC, Hoshino N. Two languages in mind: Bilingualism as a tool to investigate language, cognition, and the brain. Curr Dir Psychol Sci. 2014;23:159-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friederici AD. The brain basis of language processing: From structure to function. Physiol Rev. 2011;91:1357-1392. [DOI] [PubMed] [Google Scholar]
- 19.Bialystok E. The bilingual adaptation: How minds accommodate experience. Psychol Bull. 2017;143(3):233-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barac R, Bialystok E, Castro DC, Sanchez M. The cognitive development of young dual language learners: A critical review. Early Child Res Q. 2014;29:699-714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paap KR, Greenberg ZI. There is no coherent evidence for a bilingual advantage in executive processing. Cognit Psychol. 2013;66(2):232-258. [DOI] [PubMed] [Google Scholar]
- 22.Paap KR, Johnson HA, Sawi O. Are bilingual advantages dependent upon specific tasks or specific bilingual experiences? J Cognit Psychol. 2014;26(6):615-639. [Google Scholar]
- 23.von Bastian CC, Souza AS, Gade M. No evidence for bilingual cognitive advantages: A test of four hypotheses. J Exp Psychol Gen. 2016;145:246-258. [DOI] [PubMed] [Google Scholar]
- 24.Dick AS, Garcia NL, Pruden SM, et al. No evidence for a bilingual executive function advantage in the ABCD study. Nature human behaviour. 2019;3:692-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duñabeitia JA, Hernández JA, Antón E, et al. The inhibitory advantage in bilingual children revisited: Myth or reality? Exp Psychol. 2014;61(3):234-251. [DOI] [PubMed] [Google Scholar]
- 26.Zhang H, Wu YJ, Thierry G. Bilingualism and aging: A focused neuroscientific review. J Neurolinguistics. 2020;54:100890. [Google Scholar]
- 27.Ware AT, Kirkovski M, Lum JAG. Meta-analysis reveals a bilingual advantage that is dependent on task and age. Front Psychol. 2020;11:1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berkes M, Calvo N, Anderson JAE, Bialystok E. Poorer clinical outcomes for older adult monolinguals when matched to bilinguals on brain health. Brain Struct Funct. 2021;226:415-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schweizer TA, Ware J, Fischer CE, Craik FIM, Bialystok E. Bilingualism as a contributor to cognitive reserve: Evidence from brain atrophy in Alzheimer's disease. Cortex. 2012;48(8):991-996. [DOI] [PubMed] [Google Scholar]
- 30.Perani D, Farsad M, Ballarini T, et al. The impact of bilingualism on brain reserve and metabolic connectivity in Alzheimer's dementia. Proc Natl Acad Sci Unit States Am. 2017;114(7):1690-1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bialystok E, Craik FIM, Freedman M. Bilingualism as a protection against the onset of symptoms of dementia. Neuropsychologia. 2007;45(2):459-464. [DOI] [PubMed] [Google Scholar]
- 32.Alladi S, Bak TH, Duggirala V, et al. Bilingualism delays age at onset of dementia, independent of education and immigration status. Neurology. 2013;81:1938-1944. [DOI] [PubMed] [Google Scholar]
- 33.Woumans E, Santens P, Sieben A, Versijpt J, Stevens M, Duyck W. Bilingualism delays clinical manifestation of Alzheimer’s disease. Biling Lang Cognit. 2015;18:568-574. [Google Scholar]
- 34.Zheng Y, Wu Q, Su F, Fang Y, Zeng J, Pei Z. The protective effect of Cantonese/Mandarin bilingualism on the onset of Alzheimer's disease. Dement Geriatr Cognit Disord. 2018;45:210-219. [DOI] [PubMed] [Google Scholar]
- 35.Anderson JAE, Hawrylewicz K, Grundy JG. Does bilingualism protect against dementia? A meta-analysis. Psychonomic Bull Rev. 2020;27(5):952-965. [DOI] [PubMed] [Google Scholar]
- 36.Paulavicius AM, Mizzaci CC, Tavares DRB, et al. Bilingualism for delaying the onset of Alzheimer's sisease: A systematic review and meta-analysis. European Geriatric Medicine; 2020;11(4):651-658. [DOI] [PubMed] [Google Scholar]
- 37.Brini S, Sohrabi HR, Hebert JJ, et al. Bilingualism is associated with a delayed onset of dementia but not with a lower risk of developing it: A systematic review with meta-analyses. Neuropsychol Rev. 2020;30(1):1-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berkes M, Bialystok E, Craik FIM, Troyer A, Freedman M. Conversion of mild cognitive impairment to Alzheimer disease in monolingual and milingual patients. Alzheimer Dis Assoc Disord. 2020;34:225-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zahodne LB, Schofield PW, Farrell MT, Stern Y, Manly JJ. Bilingualism does not alter cognitive decline or dementia risk among Spanish-speaking immigrants. Neuropsychology. 2014;28(2):238-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lawton DM, Gasquoine PG, Weimer AA. Age of dementia diagnosis in community dwelling bilingual and monolingual Hispanic Americans. Cortex. 2015;66:141-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ljungberg JK, Hansson P, Adolfsson R, Nilsson L-G. The effect of language skills on dementia in a Swedish longitudinal cohort. Linguistic Approaches to Bilingualism. 2016;6:190-204. [Google Scholar]
- 42.Wilson RS, Boyle PA, Yang J, James BD, Bennett DA. Early life instruction in foreign language and music and incidence of mild cognitive impairment. Neuropsychology. 2015;29:292-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klein RM, Christie J, Parkvall M. Does multilingualism affect the incidence of Alzheimer’s disease?: A worldwide analysis by country. SSM - Population Health. 2016;2:463-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hensley K, Carney JM, Mattson MP, et al. A model for beta-amyloid aggregation and neurotoxicity based on free radical generation by the peptide: Relevance to Alzheimer disease. Proc Natl Acad Sci Unit States Am. 1994;91:3270-3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Selkoe DJ. Normal and abnormal biology of the β-amyloid precursor protein. Annu Rev Neurosci. 1994;17:489-517. [DOI] [PubMed] [Google Scholar]
- 46.Blennow K, Hampel H, Weiner M, Zetterberg H. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nat Rev Neurol. 2010;6:131-144. [DOI] [PubMed] [Google Scholar]
- 47.Almeida RP, Schultz SA, Austin BP, et al. Effect of cognitive reserve on age-related changes in cerebrospinal fluid biomarkers of Alzheimer disease. JAMA Neurol. 2015;72:699-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Soldan A, Pettigrew C, Li S, et al. Relationship of cognitive reserve and CSF biomarkers to emergence of clinical symptoms in preclinical Alzheimer’s Disease. Neurobiol Aging. 2013;34:2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Estanga A, Ecay-Torres M, Ibañez A, et al. Beneficial effect of bilingualism on Alzheimer's disease CSF biomarkers and cognition. Neurobiol Aging. 2017;50:144-151. [DOI] [PubMed] [Google Scholar]
- 50.Iqbal K, Liu F, Gong C-X, Grundke-Iqbal I. Tau in Alzheimer disease and related tauopathies. Curr Alzheimer Res. 2010;7:656-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ossenkoppele R, Schonhaut DR, Schöll M, et al. Tau PET patterns mirror clinical and neuroanatomical variability in Alzheimer’s disease. Brain. 2016;139:1551-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brier MR, Gordon B, Friedrichsen K, et al. Tau and Aβ imaging, CSF measures, and cognition in Alzheimer’s disease. Sci Transl Med. 2016;8:338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kametani F, Hasegawa M. Reconsideration of amyloid hypothesis and tau hypothesis in Alzheimer’s Disease. Front Neurosci. 2018;12:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Farrer LA, Cupples LA, Haines JL, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. JAMA. 1997;278:1349-1356. [PubMed] [Google Scholar]
- 55.Ward A, Crean S, Mercaldi CJ, et al. Prevalence of apolipoprotein e4 genotype and homozygotes (APOE e4/4) among patients diagnosed with Alzheimer’s Disease: A systematic review and meta-analysis. Neuroepidemiology. 2012;38:1-17. [DOI] [PubMed] [Google Scholar]
- 56.Seshadri S, Drachman DA, Lippa CF. Apolipoprotein E e4 allele and the lifetime risk of Alzheimer’s Disease. Arch Neurol. 1995;52:1074-1079. [DOI] [PubMed] [Google Scholar]
- 57.Schmechel DE, Saunders AM, Strittmatter WJ, et al. Increased amyloid beta-peptide deposition in cerebral cortex as a consequence of apolipoprotein E genotype in late-onset Alzheimer disease. Proc Natl Acad Sci Unit States Am. 1993;90:9649-9653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kok E, Haikonen S, Luoto T, et al. Apolipoprotein E-dependent accumulation of Alzheimer disease-related lesions begins in middle age. Ann Neurol. 2009;65:650-657. [DOI] [PubMed] [Google Scholar]
- 59.Morris JC, Roe CM, Xiong C, et al. APOE predicts amyloid-beta but not tau Alzheimer pathology in cognitively normal aging. Ann Neurol. 2010;67:122-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Polvikoski T, Sulkava R, Haltia M, et al. Apolipoprotein E, dementia, and cortical deposition of beta-amyloid protein. N Engl J Med. 1995;333:1242-1248. [DOI] [PubMed] [Google Scholar]
- 61.Ferrari C, Xu W-L, Wang H-X, et al. How can elderly apolipoprotein E ε4 carriers remain free from dementia? Neurobiol Aging. 2013;34:13-21. [DOI] [PubMed] [Google Scholar]
- 62.Qiu C, Bäckman L, Winblad B, Agüero-Torres H, Fratiglioni L. The influence of education on clinically diagnosed dementia incidence and mortality data from the Kungsholmen Project. Arch Neurol. 2001;58:2034-2039. [DOI] [PubMed] [Google Scholar]
- 63.Wang HX, Gustafson DR, Kivipelto M, et al. Education halves the risk of dementia due to apolipoprotein ε4 allele: A collaborative study from the Swedish brain power initiative. Neurobiol Aging. 2012;33:1007.e1. [DOI] [PubMed] [Google Scholar]
- 64.Crane PK, Gruhl JC, Erosheva EA, et al. Use of spoken and written Japanese did not protect Japanese-American men from cognitive decline in late life. J Gerontol B Psychol Sci Soc Sci. 2010;65B(6):654-666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hack EE, Dubin JA, Fernandes MA, Costa SM, Tyas SL. Multilingualism and dementia risk: Longitudinal analysis of the nun study. J Alzheim Dis. 2019;71:201-212. [DOI] [PubMed] [Google Scholar]
- 66.Snowdon DA, Greiner LH, Mortimer JA, Riley KP, Greiner PA, Markesbery WR. Brain infarction and the clinical expression of Alzheimer disease. The nun study. J Am Med Assoc. 1997;277:813-817. [PubMed] [Google Scholar]
- 67.Berkes M. Bilingualism as a Proxy of Cognitive Reserve. York University; 2021. [Google Scholar]
- 68.Friedman JH. Greedy function approximation: A gradient boosting machine. Ann Stat. 2001;29(5):1189-1232. [Google Scholar]
- 69.Freund Y, Schapire RE. A decision-theoretic generalization of on-line learning and an application to boosting. J Comput Syst Sci. 1997;55:119-139. [Google Scholar]
- 70.Natekin A, Knoll A. Gradient boosting machines, a tutorial. Front Neurorob. 2013;7:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McCaffrey DF, Ridgeway G, Morral AR. Propensity score estimation with boosted regression for evaluating causal effects in observational studies. Psychol Methods. 2004;9(4):403-425. [DOI] [PubMed] [Google Scholar]