Abstract
Dementia is one of neurodegenerative disease without preventive medicine currently. Dextromethorphan (DXM) has been reported to reduce neuronal damage and neurodegeneration in animal and human models. The effect of DXM on the dementia has not been fully examined. We examined the medical records over 40 years old in Taiwan’s National Health Insurance Research Database between 2000 and 2015 to establish matched cohorts. We used a Cox regression hazard model to identify risk factors of dementia during 16 years of follow-up, and the results indicate that a significantly lower percentage of subjects with DXM use (P < .001) developed dementia compared with those without DXM use (11.38%, 4541/39 895 vs 18.66%, 29 785/159 580). After adjustment for age and other variables [adjusted hazard ratio: .567 (95% confidence interval: .413-.678, P < .001)], this study also demonstrated that DXM use appeared to reduce the risk of developing dementia. DXM use may potentially provide a protective effect against dementia.
Keywords: dextromethorphan, dementia, Alzheimer disease, cognition decline
Introduction
Dementia is a chronic, progressive neurodegenerative disease characterized by a decrease of cognitive function. Around 55 million people worldwide suffer from dementia, and there are nearly 10 million new cases every year, with Asia estimated to account for 59% of the cases worldwide in 2050.1,2 The consequent cost and demand for treatment and care of the cognitive declined patients are posing considerable socio-economic impact.
There are many different forms of dementia, with Alzheimer disease being the most common form and possibly contributing to 60-70% of cases. 2 Other major forms include vascular dementia, dementia with Lewy bodies, and frontotemporal dementia listed in the order of prevalence. 2 Without drugs to cure or reverse the progression of dementia, prevention is still an important strategy in reducing numbers of individuals affected. Elderlies are correlated with multiple drugs use, therefore it is crucial to survey the relationship between these commonly prescribed drugs and dementia.
Dextromethorphan (DXM) has been used extensively and safely as a nonprescription antitussive for about 50 years and is commonly prescribed for treating chronic cough in older population. 3 It is a weak, uncompetitive N-methyl-D-aspartate (NMDA) receptor antagonist, a sigma-1 receptor agonist, a serotonin and norepinephrine reuptake inhibitor, and an α3β4 neuronal nicotinic receptor antagonist.4,5
Over the past years, accumulating evidence suggests that DXM has a neuroprotective effect in many central nerve system (CNS) injuries including epilepsy, cerebral ischemia, traumatic brain injury (TBI), and neurodegenerative diseases.3,6,7 Memantine, which is also a NMDA receptor antagonist, has been approved to treat moderate to severe Alzheimer’s disease. 8 Dextromethorphan and quinidine, has also been approved to treat pseudobulbar affect (PBA) in the United States and European Union.9,10
To our knowledge, there are no previous or prospective research that focuses on the relation between DXM and its effect on dementia. We conducted a study investigating DXM and its possible association with dementia by utilizing Taiwan’s National Health Insurance Research Database (NHIRD) to detect the development of dementia in a Taiwanese population with and without DXM use over a 16-year follow-up.
Materials and Methods
Data Sources and Ethical Consideration
Our data was extracted from the NHIRD of medical claims that were registered from 2000 to 2015. The National Health Insurance (NHI) is a compulsory social insurance health program for all citizens of Taiwan, starting from birth and lasts an entire lifetime. The dataset comprises patient medical information, including demographics, outpatient diagnoses, admission dates, discharge diagnoses, and prescriptions and it covers nearly the entire population. The Institutional Review Board of Tri-Service General Hospital approved this study (TSGHIRB No. 2-106-05-029). Since this is a deidentified database, the IRB waived the requirement of informed consent for this study.
Study Design and Participants Selection
We gathered data from 2000 to 2015, with the International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM) as the diagnosis system used by the NHI.
We divided the participants into two groups, with DXM use as our study cohort and without DXM use as our reference cohort. The reference group was matched to our control group with 4-fold propensity score by age and index date.
Outcome Measures
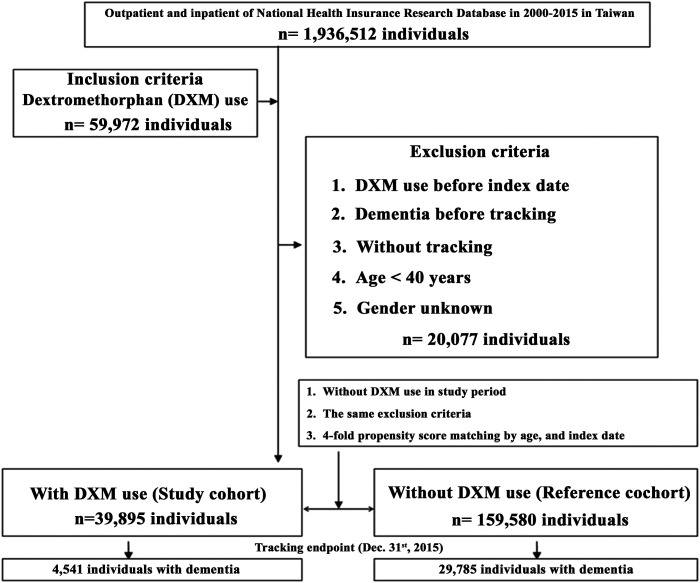
All the study participants were followed from the index date until the onset of dementia (ICD-9-CM codes: 290.xx, 294.1 and 331.0∼331.2). The date of prescription was set as our index date, and the diagnosis of dementia was set as our primary endpoint. The death of patients, disenrollment from the NHI and the end of the study date (December 31, 2015) were also set as endpoints. For both groups, we excluded patients with use of DXM before index date, dementia before tracking, without tracking, age <40 years and gender unknown. Data from 1998-1999 were first examined and set as a washout period to determine if the dementia was new and if the patient received DXM before index date. We did not obtain data before 1998 so we assumed that if the patient did not have a diagnosis of dementia between year 1998-2000, it is considered a new diagnosis. The same applies for DXM use. If the person had records of DXM prescription during 1998-2000, he will be excluded from the cohort. We also excluded the participants with age <40 years. After the exclusion, we identified a total of 39 895 participants in DXM group and 159 580 participants in non-DXM group. We received 4541 and 29 785 records of dementia, in DXM and non-DXM groups, respectively.
Dose and Duration of Dextromethorphan
The dosage of DXM was calculated by Defined Daily Dose (DDD), an assumed average maintenance dose per day for a drug used for its main indication in adults. 11 The DDD is 90 mg for dextromethorphan. The duration of the usage of DXM was calculated by dividing the cumulative doses by the DDD of DXM. We categorized DXM into three groups (low dose 1-30 DDD, intermediate 31-90 DDD, and high dose ≥91 DDD per year), to survey the potential effects on the risk of dementia.
Statistical Analysis
All data analyses were performed using IBM SPSS for Windows, version 22.0 (IBM Corp, Armonk, NY, USA). The Chi-square test and Fisher’s exact test were used to compare the difference of categorical variables, and Student’s t test was used to compare the difference of continuous variables between with DXM use and without DXM use. Multivariate Cox proportional hazards regression was used to determine the risk of dementia, and the results are presented as a hazard ratio (HR) with 95% confidence interval (CI). The difference in risk of dementia for patients with or without DXM use was estimated using the Kaplan–Meier method with a log-rank test. The cumulative effects of prescribing DXM were calculated according to DDD, using Cox regression. A 2-tailed P value <.05 was considered statistically significant.
Results
Characteristics of Participants
The study population consisted of 199 475 participants, including 39 895 individuals with DXM use and 159 580 individuals without DXM use (Figure 1). The mean follow-up time was 9.96 ± 9.35 years in the cohort and 9.88 ± 9.26 years in the control cohort. At the time of baseline, the two groups of participants present similarities in age and gender distribution. By the time of endpoint, 4541 in DXM group (11.38%) and 29 785 in non-DXM group (18.66%) was diagnosed with dementia. The study group showed a lower rate of developing dementia at the end of follow-up than the control group (P < .001) in Table 1.
Figure 1.
The flowchart of study sample selection.
Table 1.
Characteristics of Study in the Endpoint.
| Dextromethorphan | Total | With | Without | P | |||
|---|---|---|---|---|---|---|---|
| Variables | n | % | n | % | n | % | |
| Total | 199 475 | 39 895 | 20.00 | 159 580 | 80.00 | ||
| Dementia | 34 326 | 17.21 | 4541 | 11.38 | 29 785 | 18.66 | <.001 |
| Gender | .999 | ||||||
| Male | 100 605 | 50.43 | 20 121 | 50.43 | 80 484 | 50.43 | |
| Female | 98 870 | 49.57 | 19 774 | 49.57 | 79 096 | 49.57 | |
| Age (years) | 54.82 ± 25.70 | 55.28 ± 26.45 | 54.70 ± 25.51 | <.001 | |||
| Insured premium (NT$) | .094 | ||||||
| <18 000 | 111 348 | 55.82 | 22 451 | 56.28 | 88 897 | 55.71 | |
| 18 000-34 999 | 55 417 | 27.78 | 11 012 | 27.60 | 44 405 | 27.83 | |
| ≥35 000 | 32 710 | 16.40 | 6432 | 16.12 | 26 278 | 16.47 | |
| Catastrophic illness | 14 775 | 7.41 | 2671 | 6.70 | 12 104 | 7.58 | <.001 |
| Diabetes mellitus | 19 895 | 9.97 | 3884 | 9.74 | 16 011 | 10.03 | .076 |
| Hypertension | 44 254 | 22.19 | 9112 | 22.84 | 35 142 | 22.02 | <.001 |
| Depression | 5145 | 2.58 | 1097 | 2.75 | 4048 | 2.54 | .016 |
| Insomnia | 8667 | 4.34 | 1845 | 4.62 | 6822 | 4.27 | .002 |
| Stroke | 10 816 | 5.42 | 2111 | 5.29 | 8705 | 5.45 | .197 |
| Chronic kidney disease | 21 379 | 10.72 | 4344 | 10.89 | 17 035 | 10.67 | .217 |
| Hyperlipidaemia | 5683 | 2.85 | 1184 | 2.97 | 4499 | 2.82 | .111 |
| Epilepsy | 1804 | .90 | 429 | 1.08 | 1375 | .86 | <.001 |
| Autoimmune disease | 16 646 | 8.34 | 3245 | 8.13 | 13 401 | 8.40 | .088 |
| Ischemic heart disease | 15 279 | 7.66 | 2675 | 6.71 | 12 604 | 7.90 | <.001 |
| Chronic obstructive pulmonary disease | 15 774 | 7.91 | 2973 | 7.45 | 12 801 | 8.02 | <.001 |
| Pneumonia | 26 425 | 13.25 | 5671 | 14.21 | 20 754 | 13.01 | <.001 |
| Head injury | 33 160 | 16.62 | 6881 | 17.25 | 26 279 | 16.47 | <.001 |
| Asthma | 22 200 | 11.13 | 4213 | 10.56 | 17 987 | 11.27 | <.001 |
| Alcohol abuse/dependence | 1656 | .83 | 381 | .96 | 1275 | .80 | .002 |
| Tobacco abuse/dependence | 1539 | .77 | 291 | .73 | 1248 | .78 | .282 |
| Chronic liver disease | 20 165 | 10.11 | 4041 | 10.13 | 16 124 | 10.10 | <.001 |
| Parkinson’s disease | 4151 | 2.08 | 900 | 2.26 | 3251 | 2.04 | .006 |
| Urbanization level | <.001 | ||||||
| 1 (The highest) | 56 147 | 27.75 | 12 121 | 30.38 | 44 026 | 27.10 | |
| 2 | 68 507 | 33.86 | 13 986 | 35.06 | 54 521 | 33.57 | |
| 3 | 25 893 | 12.80 | 5111 | 12.81 | 20 782 | 12.79 | |
| 4 (The lowest) | 51 778 | 25.59 | 8677 | 21.75 | 43 101 | 26.54 | |
| Level of care | <.001 | ||||||
| Hospital center | 60 690 | 30.42 | 14 562 | 36.50 | 46 128 | 28.91 | |
| Regional hospital | 80 873 | 40.54 | 15 756 | 39.49 | 65 117 | 40.81 | |
| Local hospital | 57 912 | 29.03 | 9577 | 24.01 | 48 335 | 30.29 | |
P: Chi-square/Fisher exact test on category variables and t-test on continue variables.
Factors with higher risk of dementia includes male, higher age, catastrophic illness, diabetes mellitus (DM), hypertension (HTN), depression, stroke, chronic kidney disease (CKD), epilepsy, autoimmune disease (AID), ischemic heart disease (IHD), pneumonia, head injury, alcohol use/dependence, chronic liver disease (CLD), Parkinson’s disease, higher urbanization level (Table 2).
Table 2.
Factors of Dementia by Using Cox Regression.
| Variables | Crude HR | 95% CI | 95% CI | P | Adjusted HR | 95% CI | 95% CI | P |
|---|---|---|---|---|---|---|---|---|
| Dextromethorphan | .672 | .565 | .789 | <.001 | .567 | .413 | .678 | <.001 |
| Gender (male/female) | 1.682 | 1.029 | 2.501 | .022 | 1.523 | 1.007 | 2.452 | .043 |
| Age (yrs) | 2.010 | 1.345 | 2.394 | <.001 | 1.972 | 1.226 | 2.420 | <.001 |
| Insured premium (NT$) | ||||||||
| <18 000 | Reference | Reference | ||||||
| 18 000-34,999 | .903 | .598 | 1.385 | .501 | .895 | .688 | 1.265 | .462 |
| ≥35 000 | .786 | .401 | 1.211 | .568 | .723 | .562 | 1.172 | .588 |
| Catastrophic illness | 2.972 | 1.865 | 3.565 | <.001 | 1.863 | 1.424 | 2.784 | <.001 |
| Diabetes mellitus | 2.012 | 1.269 | 2.970 | <.001 | 1.952 | 1.112 | 2.401 | <.001 |
| Hypertension | 2.301 | 1.386 | 2.984 | <.001 | 1.801 | 1.436 | 2.165 | <.001 |
| Depression | 1.485 | 1.223 | 1.706 | <.001 | 1.386 | 1.065 | 1.565 | <.001 |
| Insomnia | 1.298 | .449 | 2.131 | .482 | 1.682 | .897 | 2.765 | .304 |
| Stroke | 2.597 | 2.101 | 3.401 | <.001 | 2.245 | 1.264 | 2.872 | <.001 |
| Chronic kidney disease | 2.561 | 1.572 | 3.450 | <.001 | 2.706 | 1.897 | 3.801 | <.001 |
| Hyperlipidaemia | 1.786 | 1.001 | 2.301 | .049 | 1.562 | .801 | 1.886 | .288 |
| Epilepsy | 5.986 | 3.232 | 7.889 | <.001 | 1.240 | 1.016 | 1.456 | .032 |
| Autoimmune disease | 2.154 | 1.642 | 2.976 | <.001 | 2.295 | 1.785 | 2.459 | <.001 |
| Ischemic heart disease | 1.976 | 1.333 | 2.487 | <.001 | 1.865 | 1.235 | 1.996 | <.001 |
| COPD | 1.343 | .976 | 3.010 | .073 | 1.298 | .896 | 2.975 | .187 |
| Pneumonia | 2.101 | 1.301 | 2.899 | <.001 | 2.456 | 1.597 | 3.024 | <.001 |
| Head injury | 10.597 | 5.124 | 27.978 | <.001 | 1.862 | 1.452 | 2.459 | <.001 |
| Asthma | 1.004 | .349 | 1.7.2 | .483 | 1.026 | .452 | 1.896 | .372 |
| Alcohol abuse/dependence | 1.976 | 1.035 | 2.701 | .026 | 1.765 | 1.009 | 2.652 | .040 |
| Tobacco abuse/dependence | 1.452 | .845 | 1.986 | .114 | 1.234 | .786 | 1.897 | .385 |
| Chronic liver disease | 1.565 | 1.028 | 1.983 | .033 | 1.798 | 1.111 | 2.068 | <.001 |
| Parkinson’s disease | 3.301 | 1.986 | 4.865 | <.001 | 2.701 | 1.852 | 3.498 | <.001 |
| Urbanization level | ||||||||
| 1 (The highest) | 1.792 | 1.459 | 2.506 | <.001 | 1.686 | 1.452 | 2.452 | <.001 |
| 2 | 1.676 | 1.235 | 2.418 | <.001 | 1.587 | 1.101 | 2.334 | <.001 |
| 3 | 1.489 | 1.026 | 2.121 | .022 | 1.331 | .956 | 2.098 | .072 |
| 4 (The lowest) | Reference | Reference | ||||||
| Level of care | ||||||||
| Hospital center | 1.725 | 1.308 | 2.140 | <.001 | 2.010 | 1.452 | 2.701 | <.001 |
| Regional hospital | 1.501 | 1.176 | 1.997 | <.001 | 1.865 | 1.226 | 2.154 | <.001 |
| Local hospital | Reference | Reference | ||||||
Abbreviations: Adjusted HR, Adjusted hazard ratio: Adjusted variables listed in the table; CI, confidence interval.
Cumulative Risk of Dementia
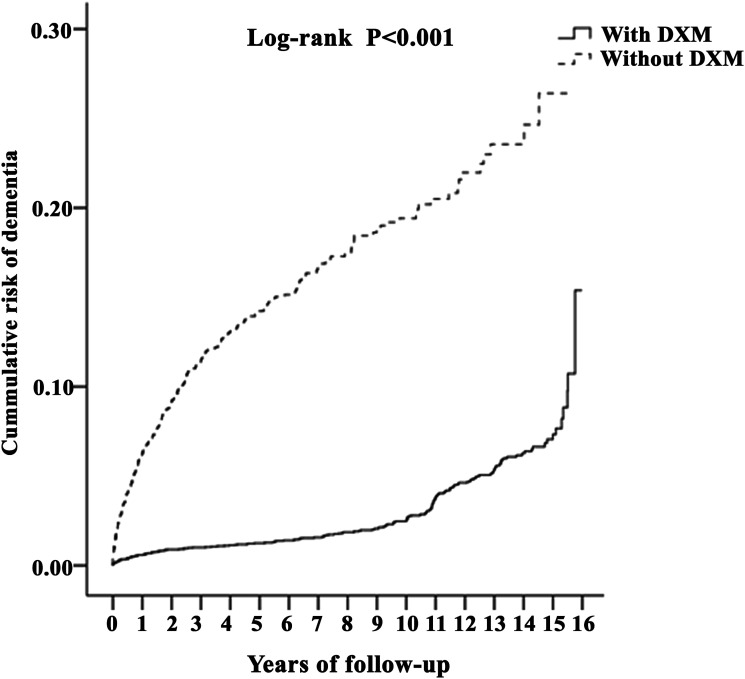
Overall, the cumulative incidence of dementia showed overall increase as years of follow-up progress. The risk in DXM group is lower compared to non-DXM group with log-rank (P < .001) starting from year one and in every year of follow-up (Figure 2).
Figure 2.
Kaplan-Meier for cumulative risk of dementia among aged 40 and over stratified by dextromethorphan with log-rank test.
Different DXM Dose and Risk of Dementia
To observe then relationship between different dosage and the cumulative risk of dementia, patients were separated into three groups, using Defined Daily Dose (DDD) as criteria. The multivariable-adjusted hazard ratios for dementia compared with non-DXM group, were .75 (95% CI, .54 to .90) for a dose of 1-30 DDD, .56 (95% CI, .40 to .67) for 31-90 DDD, and .44 (95% CI, .32 to .53) for higher than 90 DDD (Table 3). The data indicated that patients using DXM, independent of dosage, exhibited a reduced risk of dementia rates compared with the non-DXM group. Furthermore, there is a dose dependent pattern, where higher dosage of DXM relates with a lower risk of dementia.
Table 3.
Factors of Dementia Among Different Dose of Dextromethorphan by Using Cox Regression.
| Dextromethorphan Dose | Population | Events | PYs | Rate (per 105 PYs) | Adjusted HR | 95% CI | 95% CI | P |
|---|---|---|---|---|---|---|---|---|
| Without | 159 580 | 29 785 | 1 845 032.15 | 1614.34 | Reference | |||
| With | 39 895 | 4541 | 489 281.51 | 928.10 | .567 | .413 | .678 | <.001 |
| 1-30 DDD | 8423 | 1261 | 102 435.27 | 1231.02 | .754 | .541 | .897 | <.001 |
| 31-90 DDD | 19 450 | 2214 | 240 978.55 | 918.75 | .563 | .404 | .673 | <.001 |
| ≥91 DDD | 12 022 | 1066 | 145 867.69 | 730.80 | .442 | .321 | .531 | <.001 |
Abbreviations: PYs, Person-years; Adjusted HR, Adjusted Hazard ratio: Adjusted for the variables listed in Table 2; CI, confidence interval; DDD, defined daily dose.
Selected Subgroup Analyses
The patients were stratified by the variables presented in Table 1, and adjusted hazard ratio of different subgroups were calculated (Table 4). DXM group encountered 4541 medical events due to first diagnosed dementia in the 489 281 person-years (PY) observed, representing a rate of 928 per 105 PYs, while non-DXM patients encountered 29 785 medical events in the 1 845 032 person-years (PY) observed, representing a rate of 1614 per 105 PYs. Overall, patients with DXM use shows significant lower multivariable-adjusted hazard ratio .567 (95% CI, .413 to .678) compared to non-DXM group (P < .001).
Table 4.
Factors of Dementia Stratified by Variables Listed in the Table by Using Cox Regression.
| Dextromethorphan | With | Without | With vs Without (Reference) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Stratified | Events | PYs | Rate (per 105 PYs) | Events | PYs | Rate (per 105 PYs) | Adjusted HR | 95% CI | 95% CI | P |
| Total | 4541 | 489 281.51 | 928.10 | 29 785 | 1 845 032.15 | 1614.34 | .567 | .413 | .678 | <.001 |
| Gender | ||||||||||
| Male | 2971 | 246 768.60 | 1203.96 | 19 151 | 930 539.96 | 2058.05 | .577 | .420 | .690 | <.001 |
| Female | 1570 | 242 512.91 | 647.39 | 10 634 | 914 492.18 | 1162.83 | .549 | .400 | .657 | <.001 |
| Insured premium (NT$) | ||||||||||
| <18 000 | 2973 | 275 344.26 | 1079.74 | 20 124 | 1 027 809.39 | 1957.95 | .544 | .396 | .650 | <.001 |
| 18 000-34,999 | 1323 | 135 053.72 | 979.61 | 8123 | 513 401.76 | 1582.19 | .611 | .445 | .730 | <.001 |
| ≥35 000 | 245 | 78 883.54 | 310.58 | 1538 | 303 821.00 | 506.22 | .605 | .441 | .724 | <.001 |
| Catastrophic illness | ||||||||||
| Without | 1640 | 456 523.75 | 359.24 | 15 583 | 1 705 088.11 | 913.91 | .388 | .282 | .464 | <.001 |
| With | 2901 | 32 757.76 | 8855.92 | 14 202 | 139 944.04 | 10 148.34 | .861 | .627 | 1.029 | .083 |
| Diabetes mellitus | ||||||||||
| Without | 2530 | 441 647.24 | 572.86 | 16 664 | 1 659 916.16 | 1003.91 | .563 | .410 | .673 | <.001 |
| With | 2011 | 47 634.27 | 4221.75 | 13 121 | 185 115.99 | 7087.99 | .587 | .428 | .702 | <.001 |
| Hypertension | ||||||||||
| Without | 2555 | 377 529.83 | 676.77 | 18 539 | 1 438 727.35 | 1288.57 | .518 | .377 | .619 | <.001 |
| With | 1986 | 111 751.68 | 1777.15 | 11 246 | 406 304.80 | 2767.87 | .633 | .461 | .757 | <.001 |
| Depression | ||||||||||
| Without | 2696 | 475 827.65 | 566.59 | 19 642 | 1 798 229.98 | 1092.30 | .512 | .373 | .612 | <.001 |
| With | 1845 | 13 453.86 | 13 713.53 | 10 143 | 46 802.17 | 21 672.07 | .624 | .455 | .746 | <.001 |
| Insomnia | ||||||||||
| Without | 2591 | 466 654.00 | 555.23 | 20 943 | 1 766 157.54 | 1185.79 | .462 | .336 | .552 | <.001 |
| With | 1950 | 22 627.51 | 8617.83 | 8842 | 78 874.60 | 11 210.20 | .758 | .552 | .907 | <.001 |
| Stroke | ||||||||||
| Without | 2515 | 463 391.72 | 542.74 | 16 644 | 1 744 386.67 | 954.15 | .561 | .409 | .671 | <.001 |
| With | 2026 | 25 889.79 | 7825.48 | 13 141 | 100 645.47 | 13 056.72 | .591 | .431 | .707 | <.001 |
| Chronic kidney disease | ||||||||||
| Without | 2588 | 436 005.69 | 593.57 | 19 643 | 1 648 076.87 | 1191.87 | .491 | .358 | .587 | <.001 |
| With | 1953 | 53 275.82 | 3665.83 | 10 142 | 196 955.27 | 5149.39 | .702 | .511 | .840 | <.001 |
| Hyperlipidaemia | ||||||||||
| Without | 3450 | 474 760.66 | 726.68 | 24 113 | 1 793 015.61 | 1344.83 | .533 | .388 | .637 | <.001 |
| With | 1091 | 14 520.85 | 7513.33 | 5672 | 52 016.54 | 10 904.22 | .680 | .495 | .813 | <.001 |
| Epilepsy | ||||||||||
| Without | 1696 | 484 020.15 | 350.40 | 14 743 | 1 829 134.67 | 806.01 | .429 | .312 | .513 | <.001 |
| With | 2845 | 5261.36 | 54 073.52 | 15 042 | 15 897.48 | 94 618.79 | .564 | .411 | .674 | <.001 |
| Autoimmune disease | ||||||||||
| Without | 2569 | 449 484.08 | 571.54 | 17 684 | 1 690 092.46 | 1046.33 | .539 | .392 | .644 | <.001 |
| With | 1972 | 39 797.43 | 4955.09 | 12 101 | 154 939.69 | 7810.14 | .626 | .456 | .748 | <.001 |
| Ischemic heart disease | ||||||||||
| Without | 2655 | 456 474.69 | 581.63 | 18 681 | 1 699 307.21 | 1099.33 | .522 | .380 | .624 | <.001 |
| With | 1886 | 32 806.82 | 5748.80 | 11 104 | 145 724.94 | 7619.84 | .744 | .542 | .890 | <.001 |
| COPD | ||||||||||
| Without | 3545 | 452 819.95 | 782.87 | 23 887 | 1 697 029.54 | 1407.58 | .549 | .400 | .656 | <.001 |
| With | 996 | 36 461.56 | 2731.64 | 5898 | 148 002.61 | 3985.06 | .676 | .492 | .808 | <.001 |
| Pneumonia | ||||||||||
| Without | 3231 | 419 731.05 | 769.78 | 23 313 | 1 605 078.54 | 1452.45 | .523 | .381 | .625 | <.001 |
| With | 1310 | 69 550.46 | 1883.52 | 6472 | 239 953.61 | 2697.19 | .689 | .502 | .824 | <.001 |
| Head injury | ||||||||||
| Without | 2525 | 404 891.33 | 623.62 | 18 476 | 1 541 199.59 | 1198.81 | .513 | .374 | .613 | <.001 |
| With | 2016 | 84 390.18 | 2388.90 | 11 309 | 303 832.56 | 3722.12 | .633 | .461 | .757 | <.001 |
| Asthma | ||||||||||
| Without | 3088 | 437 612.30 | 705.65 | 22 733 | 1 637 070.04 | 1388.64 | .501 | .365 | .599 | <.001 |
| With | 1453 | 51 669.21 | 2812.12 | 7052 | 207 962.11 | 3391.00 | .818 | .596 | .978 | .022 |
| Alcohol abuse/dependence | ||||||||||
| Without | 4155 | 484 608.84 | 857.39 | 27 783 | 1 830 290.85 | 1517.96 | .557 | .406 | .666 | <.001 |
| With | 386 | 4672.67 | 8260.80 | 2002 | 14 741.30 | 13 580.90 | .600 | .437 | .717 | <.001 |
| Tobacco abuse/dependence | ||||||||||
| Without | 4224 | 485 712.62 | 869.65 | 27 800 | 1 830 603.02 | 1518.63 | .565 | .411 | .675 | <.001 |
| With | 317 | 3568.89 | 8882.31 | 1985 | 14 429.13 | 13 756.90 | .637 | .464 | .761 | <.001 |
| Ischemic heart disease | ||||||||||
| Without | 4091 | 439 721.75 | 930.36 | 27 704 | 1 658 609.68 | 1670.31 | .549 | .400 | .657 | <.001 |
| With | 450 | 49 559.76 | 907.99 | 2081 | 186 422.47 | 1116.28 | .802 | .584 | .959 | .008 |
| Parkinson’s disease | ||||||||||
| Without | 2940 | 478 243.70 | 614.75 | 21 984 | 1 807 444.74 | 1216.30 | .498 | .363 | .596 | <.001 |
| With | 1601 | 11 037.81 | 14 504.69 | 7801 | 37 587.41 | 20 754.29 | .689 | .502 | .824 | <.001 |
| Urbanization level | ||||||||||
| 1 (The highest) | 1644 | 148 654.75 | 1105.92 | 7015 | 500 088.56 | 1402.75 | .778 | .566 | .930 | <.001 |
| 2 | 1510 | 171 527.54 | 880.33 | 8144 | 619 300.61 | 1315.03 | .660 | .481 | .789 | <.001 |
| 3 | 678 | 62 682.49 | 1081.64 | 6255 | 236 061.43 | 2649.73 | .403 | .293 | .481 | <.001 |
| 4 (The lowest) | 709 | 106 416.74 | 666.25 | 8371 | 489 581.55 | 1709.83 | .384 | .280 | .460 | <.001 |
| Level of care | ||||||||||
| Hospital center | 1983 | 178 591.74 | 1110.35 | 9012 | 533 322.74 | 1689.78 | .648 | .472 | .775 | <.001 |
| Regional hospital | 1245 | 193 235.23 | 644.29 | 8842 | 752 869.77 | 1174.44 | .541 | .394 | .647 | <.001 |
| Local hospital | 1313 | 117 454.54 | 1117.88 | 11 931 | 558 839.63 | 2134.96 | .516 | .376 | .617 | <.001 |
PYs, Person-years; Adjusted HR, Adjusted Hazard ratio: Adjusted for the variables listed in Table 3; CI, confidence interval.
Every subgroup except patients with catastrophic illness showed significant lower hazard ratio in the DXM group. In both male and female, the hazard risk was similar, .577 (95% CI, .42 to .69) in male and .549 (95% CI, .400 to .657) in female. The hazard ratio in patients with catastrophic illness .861 (95% CI, .627 to 1.029) showed no statistical significance when DXM group compared to non-DXM group.
Note that in each subgroup, patients with comorbidities have higher hazard ratio compared to patients without comorbidities. For example, in patients without depression, the hazard ratio is .512 (95% CI, .373 to .612) and in patients with depression, .624 (95% CI, .455 to .746). This pattern can be observed in all subgroups. What is also interesting is that the higher urbanization and the higher level of care correlates with higher hazard ratio, with .778 (95% CI, .566 to .93) in urbanization level 1 (the highest), .384 (95% CI, .28 to .46) in urbanization level 4 (the lowest); and .648 (95% CI, .472 to .775) in hospital center and .516 (95% CI, .376 to .617) in regional hospital.
Discussion
The results revealed that DXM use is related with a significantly decreased risk of developing dementia when compared to non-DXM use, which is consistence with previous evidence that DXM could exert neuroprotective effect in many CNS diseases including epilepsy, cerebral ischemia, TBI and neurodegenerative diseases. 3 The data indicated that DXM use may provide potential benefits for the management and prevention of dementia.
In Taiwan, several community studies revealed that Alzheimer-type dementia is the most common cause of dementia (40-60% in all dementias), followed by vascular dementia (20-30% in all dementias), and mixed or other dementias (7-15%).12-14 In our study, 17.21% of our subjects developed dementia at the endpoint; 11.38% in DXM group and 18.66% in non-DXM group, which is higher than the prevalence of 2-3% for the population age among 65-69 years, but lower than the prevalence of >30% for the population age >80 years in community studies. 15 It is possibly because Taiwan has become an aged society with the highest rate of aging compared to the rest of the world and the increased incidence of young onset dementia before the age of 65. 2 Another possibility is our use of ICD-9 code as the diagnosis of dementia, which may lead to including patients with other cognitive impairments and causing overdiagnosis.
The risk factors for dementia includes less education, hypertension, hearing impairment, smoking, obesity, depression, physical inactivity, diabetes, low social contact, excessive alcohol consumption, TBI, and air pollution. 16 Most of the risk factors are similar with our study. Interestingly, hearing loss in the midlife has been associated with an 8% increased risk for dementia. 16 Preventing or treating hearing loss may reduce the consequent risk of dementia. In our previous study, DXM use may have a potential protective effect against sensorineural hearing loss. 17 Thus, this study further supports the additive effect of DXM treatment for dementia related to hearing loss.
DXM has been found to be neuroprotective in many preclinical studies and CNS injuries models. One of the mechanisms is its role as a NMDA receptor (NMDAR) antagonist. 3 Excessive glutamate NMDAR activity causes excitotoxicity and promotes cell death, underlying a mechanism in the pathophysiology of neurodegenerative disorders.18-20 However, past clinical studies are few and yielded poor results.21,22 One of the suspected reasons is due to significant lower dosage than the required dosage of neuroprotective effect. DXM’s neuroprotective effect is only seen at a dosage higher than that used for cough suppression due to its rapid first-pass metabolism in the liver into dextrophan through cytochrome P450 2D6 (CYP2D6) isoenzyme.7 Dextrophan has a similar pharmacological profile to DXM and has been found to have neuroprotective effects in glutamate/NMDA toxicity and ischemia models as well.3,5,23 However, dextrophan is associated with unwanted phencyclidine-like psychotomimetic side effects.24,25 To decrease DXM metabolization, quinidine, which is an inhibitor of CYP2D6, was used combinedly to block the drugs first-pass metabolization and increase DXM plasma concentration thus increasing the bioavailability.
Our study demonstrated a decreased prevalence of dementia associated with DXM use without the coadministration of quinidine, which is more promising than past studies. The reason of this may be due to the polymorphism of CYP2D6 enzyme across different ethnic groups. On a population basis, Asians exhibit a marked shift toward overall slower CYP2D6 activity due to the high frequencies (up to 64%, averaging 42%) of the reduced function allele CYP2D6∗10. 26 In other populations, the frequencies of CYP2D6∗10 range between 3 and 7% and the frequency is the lowest in white Europeans. 27 Due to this, DXM may be much more potent in Asian population.26,27
DXM has long been used to treat chronic cough and was recently approved by the FDA for the treatment of PBA in dementia patients with quinidine as a complex drug (Neudexa). 28 Despite being approved for treating PBA, DXM/Quinidine was prescribed by many doctors on dementia patients. A study advocates a more cautious attitude toward off-label use of this drug due to the lack of evidence on its effectiveness on other behavioral symptoms of dementia. 29 Our study may provide evidence that DXM use may decrease the risk of dementia in real world population.
The strength of this study is that it is a national cohort study based on Taiwan’s NHIRD, which contains data From Taiwan’s compulsory and universal healthcare system. This allowed us to perform the analysis in a real-life setting in an unselected patient population. In addition, patient dropout was avoided and selection bias or recall bias minimized because of the use of routine database records.
The study also has several limitations. First, our study did not include the data of DXM/quinidine use due to unavailability of this drug in the country. Second, the diagnosis of dementia was based on NHIRD dataset using ICD-9 code instead of validated assessment tools (such as Clinical Dementia Rating and Mini-Mental State Examination), thus, data on the severity, scales and stage of dementia, and impact on their caregivers were not available. Third, the subtypes of dementia were not assessed to clarify the association between the risk of different types of dementia and DXM use. Another limitation is NHIRD does not have complete environmental status including smoking habits, dietary habits, exercise habits and others which are all risk factors for dementia.
Conclusion
This population-based cohort study suggested that DXM use may reduce the risk for developing dementia and that cumulative DXM dose showed a more reduced risk for dementia development. Our result required further prospective randomized clinical trials to clarify the preventive effect against dementia to face the future coming hyper-aged population.
Acknowledgments
We acknowledge the technical support in the form of the National Health Insurance research data provided by the National Health Insurance Administration, the Ministry of Health and Welfare.
Footnotes
Author Contributions: C.-Y. C. initiated the study and wrote the first draft of the manuscript. C.-H. C and W.-C. C. analyzed and interpreted the data. H.-C. C critically revised the manuscript. All authors read and approved the final manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the Ministry of Science and Technology, Taiwan (MOST 110-2314-B-016-043 to H.C. Chen), the Medical Affairs Bureau, the Ministry of Defense of Taiwan (MND-MAB-D-111117 to H.C. Chen), the Tri-Service General Hospital Research Foundation (TSGH-A-111003 to H.C. Chen and TSGH-B-111018 to W.C. Chien) and based in part on data from the Health and Welfare Data Science Center, Ministry of Health and Welfare.
Data Availability: The data that support the findings of this study are available from the Health and Welfare Data Science Center, Ministry of Health and Welfare (HWDC, MOHW) but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are, however, available from the authors upon reasonable request and with permission of HWDC (https://dep.mohw.gov.tw/DOS/cp-5119-59201-113.html).
ORCID iDs
Chia-Yuan Chen https://orcid.org/0000-0001-8314-9799
Hsin-Chien Chen https://orcid.org/0000-0002-5498-5621
References
- 1.Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM. Forecasting the global burden of Alzheimer’s disease. Alzheimers Dement. 2007;3:186-191. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Dementia . https://www.who.int/news-room/fact-sheets/detail/dementia2/Sep/2021
- 3.Werling LL, Lauterbach EC, Calef U. Dextromethorphan as a potential neuroprotective agent with unique mechanisms of action. Neurologist. 2007;13:272-293. [DOI] [PubMed] [Google Scholar]
- 4.Hernandez SC, Bertolino M, Xiao Y, Pringle KE, Caruso FS, Kellar KJ. Dextromethorphan and its metabolite dextrorphan block alpha3beta4 neuronal nicotinic receptors. J Pharmacol Exp Ther. 2000;293:962-967. [PubMed] [Google Scholar]
- 5.Taylor CP, Traynelis SF, Siffert J, Pope LE, Matsumoto RR. Pharmacology of dextromethorphan: Relevance to dextromethorphan/quinidine (Nuedexta®) clinical use. Pharmacology & Therapeutics. 2016;164:170-182. [DOI] [PubMed] [Google Scholar]
- 6.Oestreicher MK, Desmeules J, Piguet V, Allaz AF, Dayer P. Genetic and environmental effects on neuromodulation and the antinociceptive effect of dextromethorpha]. Schweiz Med Wochenschr. 1998;128:212-215. [PubMed] [Google Scholar]
- 7.Shin EJ, Bach JH, Lee SY, et al. Neuropsychotoxic and neuroprotective potentials of dextromethorphan and its analogs. J Pharmacol Sci. 2011;116:137-148. [DOI] [PubMed] [Google Scholar]
- 8.McShane R, Areosa Sastre A, Minakaran N. Memantine for dementia. Cochrane Database Syst Rev. 2006;3:CD003154. [DOI] [PubMed] [Google Scholar]
- 9.Cummings JL, Lyketsos CG, Peskind ER, et al. al. Effect of dextromethorphan-quinidine on agitation in patients with Alzheimer disease dementia: A randomized clinical trial. JAMA. 2015;314:1242-1254. [DOI] [PubMed] [Google Scholar]
- 10.Nguyen L, Thomas KL, Lucke-Wold BP, Cavendish JZ, Crowe MS, Matsumoto RR. Dextromethorphan: An update on its utility for neurological and neuropsychiatric disorders. Pharmacol Ther. 2016;159:1-22. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization . WHO collaborating centre for drug statistics methodology. https://www.whocc.no/atc_ddd_methodology/who_collaborating_centre/8/Mar/2022
- 12.Liu HC, Lin KN, Teng EL, et al. Prevalence and subtypes of dementia in Taiwan: A community survey of 5297 individuals. J Am Geriatr Soc. 1995;43:144-149. [DOI] [PubMed] [Google Scholar]
- 13.Liu CK, Lai CL, Tai CT, Lin RT, Yen YY, Howng SL. Incidence and subtypes of dementia in southern Taiwan: Impact of socio-demographic factors. Neurology. 1998;50:1572-1579. [DOI] [PubMed] [Google Scholar]
- 14.Lin RT, Lai CL, Tai CT, Liu CK, Yen YY, Howng SL. Prevalence and subtypes of dementia in southern Taiwan: Impact of age, sex, education, and urbanization. J Neurol Sci. 1998;160:67-75. [DOI] [PubMed] [Google Scholar]
- 15.Fiest KM, Jette N, Roberts JI, et al. al. The prevalence and incidence of dementia: A systematic review and meta-analysis. Can J Neurol Sci. 2016;43(suppl 1):S3-S50. [DOI] [PubMed] [Google Scholar]
- 16.Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396:413-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen HC, Wang CH, Chien WC, et al. Dextromethorphan attenuates sensorineural hearing loss in an animal model and population-based cohort study. Int J Environ Res Public Health. 2020;17:6336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hynd MR, Scott HL, Dodd PR. Glutamate-mediated excitotoxicity and neurodegeneration in Alzheimer’s disease. Neurochem Int. 2004;45:583-595. [DOI] [PubMed] [Google Scholar]
- 19.Wang R, Reddy PH. Role of Glutamate and NMDA receptors in Alzheimer’s disease. J Alzheimers Dis. 2017;57:1041-1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arundine M, Tymianski M. Molecular mechanisms of glutamate-dependent neurodegeneration in ischemia and traumatic brain injury. Cell Mol Life Sci. 2004;61:657-668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walker FO, Hunt VP. An open label trial of dextromethorphan in Huntington's disease. Clin Neuropharmacol. 1989;12:322-330. [DOI] [PubMed] [Google Scholar]
- 22.Gredal O, Werdelin L, Bak S, et al. A clinical trial of dextromethorphan in amyotrophic lateral sclerosis. Acta Neurol Scand. 1997;96:8-13. [DOI] [PubMed] [Google Scholar]
- 23.Tortella FC, Pellicano M, Bowery NG. Dextromethorphan and neuromodulation: Old drug coughs up new activities. Trends Pharmacol Sci. 1989;10:501-507. [DOI] [PubMed] [Google Scholar]
- 24.Székely JI, Sharpe LG, Jaffe JH. Induction of phencyclidine-like behavior in rats by dextrorphan but not dextromethorphan. Pharmacol Biochem Behav. 1991;40:381-386. [DOI] [PubMed] [Google Scholar]
- 25.Dematteis M, Lallement G, Mallaret M. Dextromethorphan and dextrorphan in rats: Common antitussives--different behavioural profiles. Fundam Clin Pharmacol. 1998;12:526-537. [DOI] [PubMed] [Google Scholar]
- 26.Kitada M. Genetic polymorphism of cytochrome P450 enzymes in Asian populations: Focus on CYP2D6. Int J Clin Pharmacol Res. 2003;23:31-35. [PubMed] [Google Scholar]
- 27.Gaedigk A. Complexities of CYP2D6 gene analysis and interpretation. Int Rev Psychiatry. 2013;25:534-553. [DOI] [PubMed] [Google Scholar]
- 28.Hammond FM, Alexander DN, Cutler AJ, et al. PRISM II: An open-label study to assess effectiveness of dextromethorphan/quinidine for pseudobulbar affect in patients with dementia, stroke or traumatic brain injury. BMC Neurol. 2016;16:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fralick M, Sacks CA, Kesselheim AS. Assessment of use of combined dextromethorphan and quinidine in patients with dementia or parkinson disease after US food and drug administration approval for pseudobulbar affect. JAMA Intern Med. 2019;179:224-230. [DOI] [PMC free article] [PubMed] [Google Scholar]