Abstract
A significant portion of COVID-19 patients and survivors display marked clinical signs of neurocognitive impairments. SARS-CoV-2-mediated peripheral cytokine storm and its neurotropism appear to elicit the activation of glial cells in the brain proceeding to neuroinflammation. While adult neurogenesis has been identified as a key cellular basis of cognitive functions, neuroinflammation-induced aberrant neuroregenerative plasticity in the hippocampus has been implicated in progressive memory loss in ageing and brain disorders. Notably, recent histological studies of post-mortem human and experimental animal brains indicate that SARS-CoV-2 infection impairs neurogenic process in the hippocampus of the brain due to neuroinflammation. Considering the facts, this article describes the prominent neuropathogenic characteristics and neurocognitive impairments in COVID-19 and emphasizes a viewpoint that neuroinflammation-mediated deterioration of hippocampal neurogenesis could contribute to the onset and progression of dementia in COVID-19. Thus, it necessitates the unmet need for regenerative medicine for the effective management of neurocognitive deficits in COVID-19.
Keywords: COVID-19, cytokine storm, neuroinflammation, hippocampal neurogenesis, dementia
Introduction
The severe acute respiratory syndrome coronavirus (SARS-CoV)-2 infection accountable for coronavirus disease (COVID)-19 has become one of the leading causes of death worldwide. 1 Notably, elderly people are at a high risk of being infected with SARS-CoV-2, mainly due to the progressive physiological deficits and reduced immunogenic competence. 2 Moreover, individuals with late-onset comorbid pathogenic conditions like hypertension, diabetes and coronary heart disease are highly vulnerable to SARS-CoV-2 infection and severity of the disease. 3 While SARS-CoV-2 infection mediates cytokine storm responsible for neuroinflammation and oxidative stress in the brain, COVID-19-associated long-term neurological consequences have become increasingly evident.4-9 A significant portion of COVID-19 patients and survivors display marked clinical signs of stress, depression, anxiety, endocrine disruption and neurodegenerative disorders accounting for a wide array of cognitive deficits ranging from mild cognitive impairment to irreversible dementia. 10 To note, the neuropathogenic signatures of COVID-19 appear to be overlapped with many brain disorders like stroke, multiple sclerosis, seizure, Alzheimer’s disease (AD), Parkinson’s disease (PD) and Huntington’s disease (HD) in which progressive sensory-motor impairments, cognitive decline and memory loss in association with neuroinflammation are highly evident.6,11,12 Besides, the incidence of SARS-CoV-2-mediated prion-like disease with cognitive impairments has become prominent in post-recovery state.13,14 In addition to post-COVID-19 dementia and other brain abnormalities, rapidly progressive dementia and autoimmune encephalitis have also been reported in individuals immunized with adenovirus-based vaccines encoding SARS-CoV-2 spike protein.15,16 While the hippocampus of the brain plays an important role in the regulation of emotions, cognitive functions and long-term potentiation, some viral infection-induced hippocampal atrophy results in neurocognitive impairments and memory loss.17,18 Considering the facts, SARS-CoV-2-mediated neuropathogenic alterations and neuroinflammation might be linked to abnormal hippocampal plasticity leading to cognitive impairments, mood disorders and dementia. Thus, understanding the structural and functional deterioration of the hippocampus responsible for neurocognitive impairments resulting from neuropathogenic events of COVID-19 might be highly important. The hippocampus is a key neurogenic area of the adult brain that harbours neural stem cells (NSCs).19,20 NSCs in the hippocampus are multipotent in nature that give rise to new neurons throughout life.17,20 While NSC-derived neurogenesis in the hippocampus represents a key cellular foundation for regenerative plasticity accounting for various neurocognitive functions including learning and memory in adulthood,17,19,21,22 impaired hippocampal neurogenesis has been established as a potential cause of progressive memory loss in ageing and many neurodegenerative disorders.23-28 Notably, ageing, stress, depression, anxiety and neurodegenerative disorders have been characterized by various neurocognitive impairments including chronic memory deficits due to neuroinflammation-mediated pathogenic defects in hippocampal neurogenesis regardless of neuronal dysfunction and synaptic loss.19,22 Therefore, the establishment of a potential link between SARS-CoV-2-mediated neuropathogenic changes and the regulation of hippocampal regenerative plasticity appears to be unequivocally important with reference to the incidence and degree of dementia in COVID-19 patients and survivors.
COVID-19 and Mental Disorders
Due to the expanding COVID-19 pandemic, most people worldwide suffer from different mental problems like stress, anxiety, depression and panic attacks.10,29,30 Anxiety, stress and depression-related issues have been reported to be escalated dramatically not only in COVID-19 patients but also in frontline workers, healthcare professionals and caretakers due to unrelenting workload and for being at great risk of SARS-CoV-2 infection.30-32 Notably, elderly people, breadwinners of the family, students and children also experience a significant level of mental health issues due to lockdown, unmanageable socioeconomic status, online mode of education, reduced physical activities, social withdrawal, loneliness and overall uncertainty.10,33 Many cross-sectional studies and meta-analyses have revealed that a significant portion of COVID-19 survivors and the high-risk population, especially from the containment zone, have frequently been experiencing insomnia, anxiety, depression and post-traumatic stress disorder.29,30,34-37 Considering the aforementioned facts, it is obvious that unmanaged mental health issues will lead to increased levels of stress hormones like cortisol, corticosteroid releasing hormone (CRH), epinephrine and norepinephrine in the circulation that could alter the hypothalamic–pituitary–adrenal (HPA) axis, exacerbate neuroinflammation and deteriorate neuroplasticity of the brain.35,38,39 Therefore, identifying the COVID-19-related pathogenic determinants that affect the functional regulation of neuroplasticity has become very crucial in this unprecedented pandemic situation.
COVID-19 and Neuroinflammation
SARS-CoV-2 enters the human body via the angiotensin-converting enzyme (ACE)-2 receptor which has been found to be expressed by airway epithelia, lungs, and various subpopulations of the brain cells including the endothelial cells of the cerebral microvascular system.40-42 In addition to ACE2, neuropilin - 1 (NRP1), a transmembrane receptor, has also been identified to facilitate the entry of SARS-CoV-2 via the olfactory epithelium. 43 Likewise, other molecular mediators that aid the entry of SARS-CoV-2 include angiotensin II receptor type (AGTR)-2, receptor for advanced glycation end products (RAGE), transmembrane protease, serine (TMPRSS)-2, cluster of differentiation (CD)-147 and peripheral olfactory receptors.44-48 Upon infection, SARS-CoV-2 radically replicates in the tissues and organs and induces peripheral and local cytokine storm that potentially deteriorates the innate immune system.49,50 Based on the experimental data derived from immunological assays in the plasma samples of COVID-19 patients, elevated levels of key proinflammatory determinants including different interleukins (ILs), fibroblast growth factor (FGF), interferon-gamma (IFN-γ), tumour necrosis factor alpha (TNF-α) and vascular endothelial growth factor (VEGF) have become evident. 50 Among them, the surplus levels of IFN-γ, TNF-α, IL-1 and IL-6 have been known to be associated with the dysfunction of the blood–brain barrier (BBB) as a part of priming the neuroinflammatory process in the brain.17,51 In the neuropathological study conducted in a hamster model and post-mortem humans, disruption of the BBB has been reported in different parts of the brain, with maximum disruption in the hippocampus. 52 It is well known that peripheral immune response exerts an influence on the activation of microglial cells resulting in the surplus discharge of proinflammatory cytokines in the brain through various mechanisms including sensitising the afferent vagus nerve, stimulation of neurovascular endothelial cells and antibody-mediated humoural immune response.53-55 As SARS-CoV-2 and peripheral proinflammatory cytokines enter the brain, microglial cells get activated and contribute to the proinflammatory secretome in the brain. 56 Moreover, the gut–brain axis has been proposed to facilitate the neuroinvasive properties of SARS-CoV-2 which could also be a pathophysiological basis for microglial activation accounting for the pathogenic establishment of neuroinflammation. 57 Moreover, a study by Awogbindin I O et al. haves suggested that microglia could act in the clearance of the virus, but at the same time, unregulated activated forms of microglia can trigger robust neuroinflammation which could contribute to neuronal dysfunction, neurodegeneration and impaired neuroplasticity similar to PD. 58 Notably, pathological signs of microglial activation have been detected in the post-mortem brains of COVID-19 victims. 59 The SARS-CoV-2 infection has also been identified to result in pathogenic activation of microglia and neuroinflammation leading to demyelination diseases like multiple sclerosis.60,61 Besides, enhanced levels of IL-8 and TNF-α have been reported in the cerebrospinal fluid (CSF) of a COVID-19 patient. 62 Moreover, a significant increase in the neurofilament light chain (NF-L), a molecular determinant indicating the presence of neurological disorders, has been observed in the critical COVID-19 group compared to other groups. 63 Similar to non-COVID-19 stroke controls, a prominent increase in the levels of TNF-α, IL-6 and IL-12p70 has been found in COVID-19 subjects. 63 Notably, various forms of dementia including AD have been characterized by elevated levels of TNF-α, IL-6 and IL-1.24,64,65 To note, the hippocampus is highly vulnerable to neuroinflammation,66,67 while previously, Jacomy H et al. demonstrated that the endemic human coronavirus HCoV-OC43 affects many brain areas and induces neurodegeneration in the hippocampus. 68 Further, based on co-immunolabeling experiments and readout of caspase signalling, it has become apparent that HCoV-OC43 infects hippocampal-derived astrocytes, microglia and neurons in vitro and induces apoptosis predominantly in primary cultures of neurons. 68 Considering neuroinflammation as a major detrimental factor for neuroplasticity, various viral infections have been reported to affect hippocampal functions due to the abnormal activation of glial cells.69-71 Thus, SARS-CoV-2-mediated hippocampal pathology needs distinct scientific attention as it may govern dementia-related issues.
Neuroimaging Observations and Hippocampal Atrophy in COVID-19
The hippocampus is one of the important functional regions of the limbic system of the brain that contributes to the neuroregenerative process, long-term potentiation, learning process, memory formation and regulation of emotion.19,21,72 Defects in the hippocampal structure and functions due to ageing and neurological illnesses have been directly linked to emotional disorders and memory loss.17,73 Many neuroimaging studies have clearly established that SARS-CoV-2-mediates pathogenic alterations in various functional regions of the brain accounting for comorbid neurological deficits.74-78 Recently, a diffusion tensor imaging (DTI)-based study on COVID-19 survivors revealed abnormal microstructural changes and hypertrophy in different brain regions including the olfactory cortex and hippocampus in parallel with memory loss. 79 A magnetic resonance imaging (MRI) based investigation on the brain of a COVID-19 patient showed hyperintensities in the unilateral ventricle and temporal lobe along with hippocampal atrophy indicating the clinical signs of encephalitis. 78 Similarly, a nuclear magnetic resonance (NMR) based case report by Chiveri L et al. reported a pathogenic lesion in the posterior portion of the hippocampus of a SARS-CoV-2 positive elderly woman with neurological deficits. 80 Meanwhile, a number of neuroimaging findings of COVID-19 patients revealed abnormalities in the medial temporal lobe in association with cerebral haemorrhage, stroke, encephalitis and seizure.74,76,77,81,82 Based on previous retrospective studies and meta-analysis, de Erausquin GA et al. indicated that one in five recovered individuals from the previous outbreak of SARS-CoV displayed memory loss.83,84 Presently, ample scientific evidence points towards the potential link between COVID-19 and AD as both conditions appear to share a similar pattern in the changes of the neuroinflammatory molecules like TNF-α and IL-1.11,85 Taken together, SARS-CoV-2-mediated noticeable hippocampal pathology appears to be an underlying basis of neurocognitive impairments in COVID-19 survivors.86-88 Thus, the neuropathogenic findings on the hippocampus of COVID-19 subjects need to be extended to understand the underlying cellular basis of dementia, as the chances for the deterioration of neurogenesis in the hippocampus of COVID-19 positive cases are highly possible, which could further result in cognitive decline in the post-recovery state regardless of neurodegeneration.
Neuropathogenic signatures in post-mortem brain samples and experimental models of COVID-19
The post-mortem analysis of the brains of COVID-19 victims revealed prominent histopathological signatures of neuronal loss in different areas of the brain including the cerebral cortex, cerebellar Purkinje layer and hippocampus. 89 Haemorrhage, acute hypoxic injury and neuroinflammation have been commonly noticed in the post-mortem brains of COVID-19 victims.90,91 Brain ischaemic injury has also been identified in a post-mortem study conducted in SARS-CoV-2-affected individuals. 92 The histological brain slices derived from the SARS‐CoV‐2 positive cases have been characterized by widened gyri, narrowed sulci, flattened surface and congested meninges.91,92 Acute neuronal injury was observed to be prominent in the hippocampal cornu ammonis (CA)-1 region, the parahippocampal gyrus and the cerebellar Purkinje cells. 92 A study by Boroujeni ME et al. revealed a reduction in the number of neurons and an increase in the number of ionized calcium binding adaptor molecule (Iba)-1-positive microglial cells and glial fibrillary acidic protein (GFAP)-positive astrocytes in the cerebral cortex of COVID-19 victims. 93 Moreover, upregulation of inflammation and immune-related genes and reduction in glutathione levels have also been reported in the cerebral cortex derived from the COVID-19 victims. 93 Neuropathological findings by Colombo D et al. have also indicated vascular changes in the cerebral cortex, neuronal loss in the cerebellum and neuronal injury in the pons and medulla. 94 Thakur and colleagues indicated the prominent vasculature pathology, hypoxic neuronal damage and presence of activated microglia in the hippocampus of COVID-19 victims and correlated these neuropathological outcomes with dementia. 95 In addition, post-mortem brains of COVID-19 patients have been characterized by meningitis like pathology, lymphocytic panencephalitis, microinfarcts and indication for cerebral stroke.96,97
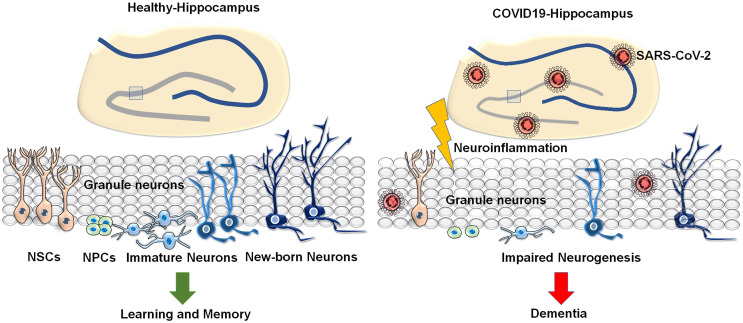
Besides, experimental animal models of COVID-19 have been characterized by stress and various neurological illnesses.97,98 A mouse model injected with the S1 subunit of the spike protein displayed stress-related behaviours and increased levels of caspase-3, C5b-9, TNF-α and IL-6 in association with neuroendothelial damage in the brain. 99 Further, murine motorneuron (MN)-1 cell line incubated with SARS-CoV-2 spike protein showed its infection and cell death. 99 Recent studies have also suggested that the S1 subunit of SARS-CoV-2 interacts with prion protein leading to the formation of homo- or hetero-polymers which results in the protein misfolding in prion disorders. 16 This could be due to the direct toxicity as a result of prolonged exposure to the spike protein of SARS-CoV-2. 16 While interaction between the spike protein and aggregation-prone proteins in the brain can leadto neurodegeneration, another possible mechanism is by the cross-reaction of anti-spike protein antibodies with the neural tissue antigens. 16 Neurotropism by pseudo SARS-CoV-2 has clearly been demonstrated in studies using human-induced pluripotent stem cell (hiPSC) derived BrainSphere model and human embryonic stem cell-derived brain organoids.100,101 A study conducted in hiPSC-derived monolayer brain cells and brain organoids revealed that SARS-CoV-2 strongly infects epithelial cells of choroid plexus than that of cortical neurons and astrocytes. 102 Besides, a parallel study by McMahon CL et al. also showed higher infectivity of SARS-CoV-2 in glial cells and choroid plexus than neurons. 103 Meanwhile, Ramani A et al. demonstrated that SARS-CoV-2 targets neurons and causes an altered distribution and hyperphosphorylation of Tau, and neuronal cell death in the brain organoids. 104 Moreover, a recent study through two independent immunolocalization experiments has also provided the evidence for the reduced neurogenesis in the hippocampus in brains of SARS-CoV-2-infected humans and hamsters. 52 Though the reports on the regulation of neurogenesis in COVID-19 are limited now, chances for the occurrence of impairment of hippocampal neurogenesis in subjects with COVID-19 might be highly relevant to various neurocognitive impairments and dementia (Figure 1).
Figure 1.
Schematic representation of the hippocampus in healthy vs COVID-19 condition in association with the respective cognitive status: The figure represents the neural stem cell-mediated neurogenic process in the hippocampus in healthy condition responsible for learning and memory and impaired neurogenesis in the hippocampus in COVID-19 state due to neuroinvasion of SARS-CoV-2 and neuroinflammation leading to dementia.
Possibilities for the occurrence of neuroregenerative failure in the hippocampus as a potential cause of dementia in COVID-19
Adult neurogenesis is the NSC-based neuroregenerative process that appears to be a key cellular basis for the regulation of neuroplasticity of the brain.24,25,105 The occurrence of adult neurogenesis appears to be highly prominent in the subgranular zone (SGZ) of the hippocampal dentate gyrus, subventricular zone (SVZ) of the lateral ventricles, and hypothalamus.20,24,25,105,106 The continuous generation and integration of new neurons in the hippocampus of the adult brain have been functionally linked to learning and memory.20,107 However, scientific facts of neurogenesis in the adult brain have been a longstanding subject of debate. During the 1960s, the early reports on the possibilities of the occurrence of mitotic activities and neurogenesis in the dentate gyrus and olfactory bulb of the adult brain of experimental animals had largely been ignored. 108 A few decades later, with technical advancement, concurrent experimental studies validated previous data on adult neurogenesis and concluded the ongoing neurogenic process in the adult brains of rodents, songbirds and nonhuman primates.109-112 Moreover, adult neurogenesis in the hippocampus has been linked to pattern separation, emotions and learning and memory. 113 Though there exist controversies on previous reports that highlight neurogenesis in the adult human brain due to various technical drawbacks and limitations in the availability of experimental post-mortem human brain tissue samples, recent studies have established the experimental proofs for the existence of NSCs and neurogenesis in the hippocampus of the human brain using bromodeoxyuridine (BrdU), carbon dating, neurosphere culture and immunohistochemical methods, and further, adult neurogenesis has also been identified to occur in the hypothalamus, cortex, amygdala and striatum.20,106,114-117 Markedly, hippocampal neurogenesis has been known to be positively regulated by many factors including physical activity, enriched environment, nutrients, cell cycle stimulating cytokines, neurotrophic factors and some antipsychotic drugs.118-120 However, ageing, stress, depression, anxiety-like disorders and neurodegenerative disorders have been known to suppress the proliferative and differentiation capacities of NSCs in the hippocampus leading to progressive memory loss.19,22,24,28,121 Notably, cognitive decline and memory loss noticed in AD, PD and HD have been known to be the result of impaired neurogenesis.22,122-124 Besides, ample reports indicate that stress, neuroinflammation and viral infections lead to the inactivation of the proliferative potential of NSCs, thereby suppressing neurogenesis and cognitive function.120,125,126 Though cerebral stroke, epileptic seizure, neurodegenerative disorders and psychiatric problems induce reactive neurogenesis in the hippocampus at an early stage of these diseases, survival and the functional integration of new neurons appear to be diminished as the disease progresses.19,22,24,127-130 The elevated levels of proinflammatory cytokines resulting from the pathogenic process of neurological diseases and abnormal levels of stress hormones have been proposed to inhibit the proliferation and neuronal differentiation of NSCs and interfere with the functional integration of new-born neurons in the hippocampus.24,25,27,118 Further, the decline in the level of neurogenesis has been known to be associated with memory impairment in the aforementioned neuropathogenic condition due to neuroinflammation 17 which might be highly relevant to COVID-19. Notably, experimental evidence strongly indicates that SARS-CoV-2 has the potential to infect the hiPSC-derived NSCs and the brain organoids.101,103,131 Moreover, recent immunohistochemical examinations suggest that SARS-CoV-2 infection leads to impaired hippocampal neurogenesis in post-mortem brain samples of COVID-19 victims and SARS-CoV-2-infected experimental animals, where increased levels of cytokines like IL-1β and increased microglial activation have also been reported in brain regions including the hippocampus. 52 The increased levels of cytokines like transforming growth factor beta (TGF)-β and IFN-γ and ILs are responsible for cytokine storm and neuroinflammation in COVID-19. 132 Previously, Kandasamy et al. have demonstrated that elevated level of TGF-β signalling disrupts the proliferation and differentiation potentials of NSCs leading to aberrant neurogenesis in the hippocampus of experimental animal brains.17,26,106 Taken together, neuroinflammation has been clearly known to affect the neurogenic potential of NSCs and integration of the neuroblast in the hippocampus, contributing to abnormal regenerative plasticity, ultimately leading to cognitive impairments.17,106,133 While hippocampal neurogenesis is responsible for learning and memory, occurrence and progression of cognitive deficits and dementia have clearly been attributed to impairment in hippocampal neurogenesis.20,123,134 Considering the aforementioned facts, it can be proposed that the SARS-CoV-2-mediated stress, depression, emotional and psychological trauma, sequence of comorbid neuropathological alterations and neuroinflammation might drastically alter the neurogenic potential of NSCs in the hippocampus of the brain. Further, the resulting aberrant neurogenesis in the hippocampus can be a potential cause of dementia in a significant portion of COVID-19 patients and survivors as the dysfunctional and degenerating neurons are least likely to be replenished (Fig 1). Thus, there is an urgent need of detailed scientific attention on abnormal regulation of hippocampal neurogenesis relating to cognitive impairment in COVID-19.
Conclusion
While the rising mortality resulting from COVID-19 worldwide has become an issue of serious concern, a significant portion of COVID-19 survivors appears to have an increased risk of various neurological deficits and dementia. As elevated levels of stress hormones and proinflammatory molecules in the brain have been reported to impair hippocampal neuroplasticity, clinical signs of comorbid neuropathogenic condition noticed in subjects with COVID-19 might be linked to defects in the NSC potentials accounting for aberrant neurogenesis in the hippocampus. As the failure in neuroregenerative process in the hippocampus has been identified as a major pathogenic determinant of cognitive decline , COVID-19 might represent a potential risk factor for mental health issues and dementia due to neuroinflammation and deterioration of hippocampal neurogenesis. Thus, therapeutic strategies and implementation of regenerative medicine to prevent and defend the neuroregenerative failure in the hippocampus is highly crucial to manage the possible occurrence of dementia in COVID-19 patients and survivors.
Abbreviations
ACE2, angiotensin-converting enzyme 2; AD, Alzheimer’s disease; AGTR2, angiotensin II receptor type 2; BBB, blood–brain barrier; BrdU, bromodeoxyuridine; CA1, cornu ammonis 1; CD-147, Cluster of differentiation 147; COVID-19, coronavirus disease 2019; CRH, corticosteroid releasing hormone; CSF, cerebrospinal fluid; DTI, diffusion tensor imaging; FGF, fibroblast growth factor; GFAP, glial fibrillary acidic protein; HCoV-OC43, human coronavirus OC43 strain; HD, Huntington’s disease; hiPSC, human-induced pluripotent stem cell; HPA, hypothalamic–pituitary–adrenal axis; Iba-1, ionized calcium binding adaptor molecule 1; IFN-γ, interferon-gamma; ILs, interleukins; MN-1, murine motorneuron; MRI, magnetic resonance imaging; NF-L, neurofilament light chain; NMR, nuclear magnetic resonance; NRP1, neuropilin 1; NSC, neural stem cell; PD, Parkinson’s disease; RAGE, receptor for advanced glycation end products; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SGZ, subgranular zone; SVZ, subventricular zone; TMPRSS2, transmembrane protease serine 2; TNFα, tumour necrosis factor alpha; TGF-β, transforming growth factor beta; VEGF, vascular endothelial growth factor
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: M.K. has been supported by the Faculty Recharge Programme, University Grants Commission (UGC-FRP), New Delhi, India. M.K. received a research grant (SERB-EEQ/2016/000639) and an Early Career Research Award (SERB-ECR/2016/000741) from the Science and Engineering Research Board (SERB), government of India. The authors acknowledge, RUSA2.0, Biological Sciences, Bharathidasan University for the financial support, UGC-SAP, DST-FIST for the infrastructure of the Department of Animal Science, Bharathidasan University. R.K.R. was supported as JRF from the project grant-ECR/2016/000741, SERB.
ORCID iD
Mahesh Kandasamy https://orcid.org/0000-0002-6720-9082
References
- 1.Lai C-C, Shih T-P, Ko W-C, Tang H-J, Hsueh P-R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents. 2020;55(3):105924. doi: 10.1016/j.ijantimicag.2020.105924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perrotta F, Corbi G, Mazzeo G, et al. COVID-19 and the elderly: insights into pathogenesis and clinical decision-making. Aging Clin Exp Res. 2020;32:1599-1608. doi: 10.1007/s40520-020-01631-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guzik TJ, Mohiddin SA, Dimarco A, et al. COVID-19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc Res. 2020;116(10):1666-1687. doi: 10.1093/cvr/cvaa106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhaskar S, Sinha A, Banach M, et al. Cytokine Storm in COVID-19-Immunopathological Mechanisms, Clinical Considerations, and Therapeutic Approaches: The REPROGRAM Consortium Position Paper. Front Immunol. 2020;11:1648. doi: 10.3389/fimmu.2020.01648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heneka MT, Golenbock D, Latz E, Morgan D, Brown R. Immediate and long-term consequences of COVID-19 infections for the development of neurological disease. Alzheimer’s Res Ther. 2020;12(1):69. doi: 10.1186/s13195-020-00640-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rethinavel HS, Ravichandran S, Radhakrishnan RK, Kandasamy M. COVID-19 and Parkinson's disease: defects in neurogenesis as the potential cause of olfactory system impairments and anosmia. J Chem Neuroanat. 2021;115:101965. doi: 10.1016/j.jchemneu.2021.101965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Selvaraj K, Ravichandran S, Krishnan S, Radhakrishnan RK, Manickam N, Kandasamy M. Testicular Atrophy and Hypothalamic Pathology in COVID-19: possibility of the incidence of male infertility and HPG axis abnormalities. Reprod Sci. 2021;28:2735-2742. doi: 10.1007/s43032-020-00441-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kandasamy M. Perspectives for the use of therapeutic Botulinum toxin as a multifaceted candidate drug to attenuate COVID-19. Med Drug Discov. 2020;6:100042. doi: 10.1016/j.medidd.2020.100042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kandasamy M. NF-κB signalling as a pharmacological target in COVID-19: potential roles for IKKβ inhibitors. Naunyn-Schmiedeberg’s Arch Pharmacol. 2021;394(3):561-567. doi: 10.1007/s00210-020-02035-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xiong J, Lipsitz O, Nasri F, et al. Impact of COVID-19 pandemic on mental health in the general population: a systematic review. J Affect Disord. 2020;277:55-64. doi: 10.1016/j.jad.2020.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Achar A, Ghosh C. COVID-19-Associated Neurological Disorders: the potential route of CNS invasion and blood-brain barrier relevance. Cells. 2020;9(11):2360. doi: 10.3390/cells9112360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferini-Strambi L, Salsone M. COVID-19 and neurological disorders: are neurodegenerative or neuroimmunological diseases more vulnerable? J Neurol. 2020;268:409-419. doi: 10.1007/s00415-020-10070-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Young MJ, O'Hare M, Matiello M, Schmahmann JD. Creutzfeldt-Jakob disease in a man with COVID-19: SARS-CoV-2-accelerated neurodegeneration? Brain Behav Immun. 2020;89:601-603. doi: 10.1016/j.bbi.2020.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nasiri E, Naseri A, Yazdchi M, Talebi M. Is there a link between COVID-19 and Creutzfeldt-Jakob disease? a case report. J Res Clin Med. 2021;9(1):26. doi: 10.34172/jrcm.2021.026 [DOI] [Google Scholar]
- 15.Kwon H, Kim T. Autoimmune encephalitis following ChAdOx1-S SARS-CoV-2 vaccination. Neurol Sci. 2021;30:1-3. doi: 10.1007/s10072-021-05790-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chakrabarti SS, Tiwari A, Jaiswal S, et al. Rapidly progressive dementia with asymmetric rigidity following ChAdOx1 nCoV-19 vaccination. Aging Dis;3:2021. doi: 10.14336/AD.2021.1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kandasamy M, Anusuyadevi M, Aigner KM, et al. TGF-β Signaling: a therapeutic target to reinstate regenerative plasticity in vascular dementia? Aging Dis. 2020;11(4):828-850. doi: 10.14336/AD.2020.0222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Popkirov S, Ismail FS, Grönheit W, Kapauer M, Wellmer J, Bien CG. Progressive hippocampal sclerosis after viral encephalitis: potential role of NMDA receptor antibodies. Seizure. 2017;51:6-8. doi: 10.1016/j.seizure.2017.07.006 [DOI] [PubMed] [Google Scholar]
- 19.Kandasamy M, Aigner L. Neuroplasticity, limbic neuroblastosis and neuro-regenerative disorders. Neural Regen Res. 2018;13(8):1322-1326. doi: 10.4103/1673-5374.235214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ming G-l., Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 2011;70(4):687-702. doi: 10.1016/j.neuron.2011.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kempermann G, Song H, Gage FH. Neurogenesis in the adult hippocampus. Cold Spring Harb Perspect Biol. 2015;7(9):a018812. doi: 10.1101/cshperspect.a018812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kandasamy M, Aigner L. Reactive Neuroblastosis in Huntington’s disease: a putative therapeutic target for striatal regeneration in the adult brain. Front Cell Neurosci. 2018;12:37. doi: 10.3389/fncel.2018.00037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klempin F, Kempermann G. Adult hippocampal neurogenesis and aging. Eur Arch Psychiatry Clin Neurosci. 2007;257(5):271-280. doi: 10.1007/s00406-007-0731-5 [DOI] [PubMed] [Google Scholar]
- 24.Kandasamy M, Couillard-Despres S, Raber KA, et al. Stem cell quiescence in the hippocampal neurogenic niche is associated with elevated transforming growth factor-β Signaling in an animal model of huntington disease. J Neuropathol Exp Neurol. 2010;69(7):717-728. doi: 10.1097/NEN.0b013e3181e4f733 [DOI] [PubMed] [Google Scholar]
- 25.Kandasamy M, Reilmann R, Winkler J, Bogdahn U, Aigner L. Transforming growth factor-beta signaling in the neural stem cell niche: a therapeutic target for Huntington’s disease. Neurol Res Int. 2011;2011:1-13. doi: 10.1155/2011/124256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kandasamy M, Radhakrishnan RK, Poornimai Abirami GP, et al. Possible existence of the hypothalamic-pituitary-hippocampal (HPH) axis: a reciprocal relationship between hippocampal specific neuroestradiol synthesis and neuroblastosis in ageing brains with special reference to menopause and neurocognitive disorders. Neurochem Res. 2019;44(8):1781-1795. doi: 10.1007/s11064-019-02833-1 [DOI] [PubMed] [Google Scholar]
- 27.Shohayeb B, Diab M, Ahmed M, Ng DCH. Factors that influence adult neurogenesis as potential therapy. Transl Neurodegener. 2018;7:4. doi: 10.1186/s40035-018-0109-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Toda T, Parylak SL, Linker SB, Gage FH. The role of adult hippocampal neurogenesis in brain health and disease. Mol Psychiatry. 2019;24(1):67-87. doi: 10.1038/s41380-018-0036-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mazza MG, De Lorenzo R, Conte C, et al. Anxiety and depression in COVID-19 survivors: role of inflammatory and clinical predictors. Brain Behav Immun. 2020;89:594-600. doi: 10.1016/j.bbi.2020.07.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salari N, Hosseinian-Far A, Jalali R, et al. Prevalence of stress, anxiety, depression among the general population during the COVID-19 pandemic: a systematic review and meta-analysis. Glob Health. 2020;16(57):1-11. doi: 10.1186/s12992-020-00589-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luo M, Guo L, Yu M, Jiang W, Wang H. The psychological and mental impact of coronavirus disease 2019 (COVID-19) on medical staff and general public - A systematic review and meta-analysis. Psychiatry Res. 2020;291:113190. doi: 10.1016/j.psychres.2020.113190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun N, Wei L, Shi S, et al. A qualitative study on the psychological experience of caregivers of COVID-19 patients. Am J Infect Control. 2020;48(6):592-598. doi: 10.1016/j.ajic.2020.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh S, Roy D, Sinha K, Parveen S, Sharma G, Joshi G. Impact of COVID-19 and lockdown on mental health of children and adolescents: a narrative review with recommendations. Psychiatry Res. 2020;293:113429. doi: 10.1016/j.psychres.2020.113429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ivbijaro G, Brooks C, Kolkiewicz L, Sunkel C, Long A. Psychological impact and psychosocial consequences of the COVID 19 pandemic Resilience, mental well-being, and the coronavirus pandemic. Indian J Psychiatry. 2020;62(suppl 3):S395-S403. doi: 10.4103/psychiatry.IndianJPsychiatry_1031_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramezani M, Simani L, Karimialavijeh E, Rezaei O, Hajiesmaeili M, Pakdaman H. The role of anxiety and cortisol in outcomes of patients with Covid-19. Basic Clin Neurosci. 2020;11(2):179-184. doi: 10.32598/bcn.11.covid19.1168.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rehman U, Shahnawaz MG, Khan NH, et al. Depression, anxiety and stress among Indians in Times of Covid-19 lockdown. Community Ment Health J. 2021;57(1):42-48. doi: 10.1007/s10597-020-00664-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taquet M, Luciano S, Geddes JR, Harrison PJ. Bidirectional associations between COVID-19 and psychiatric disorder: retrospective cohort studies of 62 354 COVID-19 cases in the USA. Lancet Psychiatr. 2021;8(2):130-140. doi: 10.1016/S2215-0366(20)30462-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Herman JP, McKlveen JM, Ghosal S, et al. Regulation of the hypothalamic‐pituitary‐adrenocortical stress response. Compr Physiol. 2016;6(2):603-621. doi: 10.1002/cphy.c150015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tan T, Khoo B, Mills EG, et al. Association between high serum total cortisol concentrations and mortality from COVID-19. Lancet Diabetes Endocrinol. 2020;8(8):659-660. doi: 10.1016/S2213-8587(20)30216-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hamming I, Timens W, Bulthuis M, Lely A, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203(2):631-637. doi: 10.1002/path.1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buzhdygan TP, DeOre BJ, Baldwin-Leclair A, et al. The SARS-CoV-2 spike protein alters barrier function in 2D static and 3D microfluidic in-vitro models of the human blood-brain barrier. Neurobiol Dis. 2020;146:105131. doi: 10.1016/j.nbd.2020.105131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gheblawi M, Wang K, Viveiros A, et al. Angiotensin-Converting Enzyme 2: SARS-CoV-2 Receptor and Regulator of the Renin-Angiotensin System. Circ Res. 2020;126(10):1456-1474. doi: 10.1161/CIRCRESAHA.120.317015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Davies J, Randeva H, Chatha K, et al. Neuropilin-1 as a new potential SARS-CoV-2 infection mediator implicated in the neurologic features and central nervous system involvement of COVID-19. Mol Med Rep. 2020;22(5):4221-4226. doi: 10.3892/mmr.2020.11510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cui C, Huang C, Zhou W, et al. AGTR2, one possible novel key gene for the entry of SARS-CoV-2 into Human cells. IEEE/ACM Trans Comput Biol Bioinform. 2021;18(4):1230-1233. doi: 10.1109/TCBB.2020.3009099 [DOI] [PubMed] [Google Scholar]
- 45.Kerkeni M, Gharbi J. RAGE receptor: may be a potential inflammatory mediator for SARS-COV-2 infection? Med Hypotheses. 2020;144:109950. doi: 10.1016/j.mehy.2020.109950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kerslake R, Hall M, Randeva H, et al. Co-expression of peripheral olfactory receptors with SARS-CoV-2 infection mediators: potential implications beyond loss of smell as a COVID-19 symptom. Int J Mol Med. 2020;46(3):949-956. doi: 10.3892/ijmm.2020.4646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xia P, Dubrovska A. Tumor markers as an entry for SARS‐CoV‐2 infection? FEBS J. 2020;287(17):3677-3680. doi: 10.1111/febs.15499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271-280. e8. doi: 10.1016/j.cell.2020.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen G, Wu D, Guo W, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130(5):2620-2629. doi: 10.1172/JCI137244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506. doi: 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yarlagadda A, Alfson E, Clayton AH. The Blood Brain Barrier and the Role of Cytokines in Neuropsychiatry. Psychiatry Edgmont. 2009;6(11):18-22. [PMC free article] [PubMed] [Google Scholar]
- 52.Klein R, Soung A, Sissoko C, et al. COVID-19 induces neuroinflammation and loss of hippocampal neurogenesis. Res Sq. Published online October 29, 2021:rs.3.rs-1031824. doi: 10.21203/rs.3.rs-1031824/v1. [DOI]
- 53.Banerjee A, Khemka VK, Roy D, et al. Role of Pro-Inflammatory Cytokines and Vitamin D in Probable Alzheimer’s Disease with depression. Aging Dis. 2017;8(3):267-276. doi: 10.14336/AD.2016.1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lim A, Krajina K, Marsland AL. Peripheral inflammation and cognitive aging. Mod Trends Pharmacopsychiatry. 2013;28:175-187. doi: 10.1159/000346362 [DOI] [PubMed] [Google Scholar]
- 55.Tracey KJ. The inflammatory reflex. Nature. 2002;420(6917):853-859. doi: 10.1038/nature01321 [DOI] [PubMed] [Google Scholar]
- 56.Bossù P, Toppi E, Sterbini V, Spalletta G. Implication of aging related chronic neuroinflammation on COVID-19 pandemic. J Pers Med. 2020;10(3):102. doi: 10.3390/jpm10030102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shi Y, Li Z, Yang C, Liu C. The role of gut-brain axis in SARA-CoV-2 neuroinvasion: Culprit or innocent bystander? Brain Behav Immun. 2021;94:476-477. doi: 10.1016/j.bbi.2021.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Awogbindin IO, Ben-Azu B, Olusola BA, et al. Microglial Implications in SARS-CoV-2 Infection and COVID-19: lessons from Viral RNA Neurotropism and Possible Relevance to Parkinson’s Disease. Front Cell Neurosci. 2021;15:670298. doi: 10.3389/fncel.2021.670298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Matschke J, Lütgehetmann M, Hagel C, et al. Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. Lancet Neurol. 2020;19(11):919-929. doi: 10.1016/S1474-4422(20)30308-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dziedzic A, Saluk-Bijak J, Miller E, Niemcewicz M, Bijak M. The Impact of SARS-CoV-2 Infection on the Development of Neurodegeneration in Multiple Sclerosis. Int J Mol Sci. 2021;22(4):1804. doi: 10.3390/ijms22041804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Khateb M, Bosak N, Muqary M. Coronaviruses and central nervous system manifestations. Front Neurol. 2020;11:715. doi: 10.3389/fneur.2020.00715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pilotto A, Odolini S, Masciocchi S, et al. Steroid‐Responsive Encephalitis in Coronavirus disease 2019. Ann Neurol. 2020;88(2):423-427. doi: 10.1002/ana.25783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Garcia MA, Barreras PV, Lewis A, et al. Cerebrospinal fluid in COVID-19 neurological complications: no cytokine storm or neuroinflammation. medRxiv. 2021;12. Published online January. doi: 10.1101/2021.01.10.20249014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang WY, Tan MS, Yu JT, Tan L. Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Ann Transl Med. 2015;3(10):136. doi: 10.3978/j.issn.2305-5839.2015.03.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ng A, Tam WW, Zhang MW, et al. IL-1β, IL-6, TNF- α and CRP in elderly patients with Depression or Alzheimer’s disease: systematic Review and Meta-Analysis. Sci Rep. 2018;8(1):12050. doi: 10.1038/s41598-018-30487-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Barrientos RM, Kitt MM, Watkins LR, Maier SF. Neuroinflammation in the normal aging hippocampus. Neuroscience. 2015;309:84-99. doi: 10.1016/j.neuroscience.2015.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hueston CM, O’Leary JD, Hoban AE, et al. Chronic interleukin-1β in the dorsal hippocampus impairs behavioural pattern separation. Brain Behav Immun. 2018;74:252-264. doi: 10.1016/j.bbi.2018.09.015 [DOI] [PubMed] [Google Scholar]
- 68.Jacomy H, Fragoso G, Almazan G, Mushynski WE, Talbot PJ. Human coronavirus OC43 infection induces chronic encephalitis leading to disabilities in BALB/C mice. Virology. 2006;349(2):335-346. doi: 10.1016/j.virol.2006.01.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Poluektova L, Meyer V, Walters L, Paez X, Gendelman HE. Macrophage-induced inflammation affects hippocampal plasticity and neuronal development in a murine model of HIV-1 encephalitis. Glia. 2005;52(4):344-353. doi: 10.1002/glia.20253 [DOI] [PubMed] [Google Scholar]
- 70.Jurgens HA, Amancherla K, Johnson RW. Influenza infection induces neuroinflammation, alters hippocampal neuron morphology, and impairs cognition in adult mice. J Neurosci. 2012;32(12):3958-3968. doi: 10.1523/JNEUROSCI.6389-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hosseini S, Wilk E, Michaelsen-Preusse K, et al. Long-term neuroinflammation induced by influenza a virus infection and the impact on hippocampal neuron morphology and function. J Neurosci. 2018;38(12):3060-3080. doi: 10.1523/JNEUROSCI.1740-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Baptista P, Andrade JP. Adult hippocampal neurogenesis: regulation and possible functional and clinical correlates. Front Neuroanat. 2018;12:44. doi: 10.3389/fnana.2018.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ball MJ, Hachinski V, Fox A, et al. A new definition of Alzheimer’s disease: a Hippocampal Dementia. Lancet. 1985;325(8419):14-16. doi: 10.1016/s0140-6736(85)90965-1 [DOI] [PubMed] [Google Scholar]
- 74.Coolen T, Lolli V, Sadeghi N, et al. Early postmortem brain MRI findings in COVID-19 non-survivors. Neurology. 2020;95(14):e2016-e2027. doi: 10.1212/WNL.0000000000010116 [DOI] [PubMed] [Google Scholar]
- 75.Helms J, Kremer S, Merdji H, et al. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. 2020;382(23):2268-2270. doi: 10.1056/NEJMc2008597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li Y, Li M, Wang M, et al. Acute cerebrovascular disease following COVID-19: a single center, retrospective, observational study. Stroke Vasc Neurol. 2020;5(3):279-284. doi: 10.1136/svn-2020-000431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683-689. doi: 10.1001/jamaneurol.2020.1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Moriguchi T, Harii N, Goto J, et al. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int J Infect Dis. 2020;94:55-58. doi: 10.1016/j.ijid.2020.03.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lu Y, Li X, Geng D, et al. Cerebral micro-structural changes in COVID-19 Patients - An MRI-based 3-month follow-up study. EClinicalMedicine. 2020;25:100484. doi: 10.1016/j.eclinm.2020.100484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chiveri L, Verrengia E, Muscia F, et al. Limbic encephalitis in a COVID-19 patient? J Neurovirol. 2021;31:1-3. doi: 10.1007/s13365-021-00971-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Filatov A, Sharma P, Hindi F, Espinosa PS. Neurological complications of Coronavirus Disease (COVID-19): encephalopathy. Cureus. 2020;12(3):e7352. doi: 10.7759/cureus.7352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Poyiadji N, Shahin G, Noujaim D, Stone M, Patel S, Griffith B. COVID-19-associated Acute Hemorrhagic Necrotizing Encephalopathy: imaging features. Radiology. 2020;296:E119-E120. doi: 10.1148/radiol.2020201187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rogers JP, Chesney E, Oliver D, et al. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatr. 2020;7(7):611-627. doi: 10.1016/S2215-0366(20)30203-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Erausquin GA, Snyder H, Carrillo M, Hosseini AA, Brugha TS, Seshadri S. The chronic neuropsychiatric sequelae of COVID‐19: the need for a prospective study of viral impact on brain functioning. Alzheimer’s Dementia. 2021;17:1056-1065. doi: 10.1002/alz.12255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xia X, Wang Y, Zheng J. COVID-19 and Alzheimer’s disease: how one crisis worsens the other. Transl Neurodegener. 2021;10(1):15. doi: 10.1186/s40035-021-00237-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Abate G, Memo M, Uberti D. Impact of COVID-19 on Alzheimer’s disease risk: viewpoint for research action. Healthcare. 2020;8(3):286. doi: 10.3390/healthcare8030286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Miners S, Kehoe PG, Love S. Cognitive impact of COVID-19: looking beyond the short term. Alzheimer’s Res Ther. 2020;12(1):170. doi: 10.1186/s13195-020-00744-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ciaccio M, Lo Sasso B, Scazzone C, et al. COVID-19 and Alzheimer’s disease. Brain Sci. 2021;11(3):305. doi: 10.3390/brainsci11030305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Solomon IH, Normandin E, Bhattacharyya S, et al. Neuropathological features of Covid-19. N Engl J Med. 2020;383:989-992. doi: 10.1056/NEJMc2019373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mukerji SS, Solomon IH. What can we learn from brain autopsies in COVID-19? Neurosci Lett. 2021;742:135528. doi: 10.1016/j.neulet.2020.135528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Machhi J, Herskovitz J, Senan AM, et al. The natural history, pathobiology, and clinical manifestations of SARS-CoV-2 infections. J Neuroimmune Pharmacol. 2020;15(3):359-386. doi: 10.1007/s11481-020-09944-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fabbri VP, Foschini MP, Lazzarotto T, et al. Brain ischemic injury in COVID‐19‐infected patients: a series of 10 post‐mortem cases. Brain Pathol. 2020;31(1):205-210. doi: 10.1111/bpa.12901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Boroujeni ME, Simani L, Bluyssen HAR, et al. Inflammatory response leads to neuronal death in human post-mortem cerebral cortex in patients with COVID-19. ACS Chem Neurosci. 2021;12(12):2143-2150. doi: 10.1021/acschemneuro.1c00111 [DOI] [PubMed] [Google Scholar]
- 94.Colombo D, Falasca L, Marchioni L, et al. Neuropathology and inflammatory cell characterization in 10 Autoptic COVID-19 brains. Cells. 2021;10(9):2262. doi: 10.3390/cells10092262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Thakur KT, Miller EH, Glendinning MD, et al. COVID-19 neuropathology at Columbia University Irving Medical Center/New York Presbyterian Hospital. Brain J Neurol. 2021;144:2696-2708. doi: 10.1093/brain/awab148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.von Weyhern CH, Kaufmann I, Neff F, Kremer M. Early evidence of pronounced brain involvement in fatal COVID-19 outcomes. Lancet. 2020;395(10241):e109. doi: 10.1016/S0140-6736(20)31282-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Song E, Zhang C, Israelow B, et al. Neuroinvasion of SARS-CoV-2 in human and mouse brain. bioRxiv. Published online September 8, 2020. doi: 10.1101/2020.06.25.169946 [DOI]
- 98.Shou S, Liu M, Yang Y, et al. Animal Models for COVID-19: Hamsters, Mouse, Ferret, Mink, Tree Shrew, and Non-human Primates. Front Microbiol. 2021;12:626553. doi: 10.3389/fmicb.2021.626553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nuovo GJ, Magro C, Shaffer T, et al. Endothelial cell damage is the central part of COVID-19 and a mouse model induced by injection of the S1 subunit of the spike protein. Ann Diagn Pathol. 2021;51:151682. doi: 10.1016/j.anndiagpath.2020.151682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bullen CK, Hogberg HT, Bahadirli-Talbott A, et al. Infectability of Human BrainSphere Neurons Suggests Neurotropism of SARS-CoV-2*. ALTEX. 2020;37(4):665-671. doi: 10.14573/altex.2006111 [DOI] [PubMed] [Google Scholar]
- 101.Yi SA, Nam KH, Yun J, et al. Infection of Brain Organoids and 2D Cortical Neurons with SARS-CoV-2 Pseudovirus. Viruses. 2020;12(9):1004. doi: 10.3390/v12091004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jacob F, Pather SR, Huang W-K, et al. Human pluripotent stem cell-derived neural cells and brain Organoids reveal SARS-CoV-2 Neurotropism Predominates in Choroid Plexus Epithelium. Cell Stem Cell. 2020;27(6):937-950. e9. doi: 10.1016/j.stem.2020.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.McMahon CL, Staples H, Gazi M, Carrion R, Hsieh J. SARS-CoV-2 targets glial cells in human cortical organoids. Stem Cell Rep. 2021;16(5):1156-1164. doi: 10.1016/j.stemcr.2021.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ramani A, Müller L, Ostermann PN, et al. SARS ‐CoV‐2 targets neurons of 3D human brain organoids. EMBO J. 2020;39(20):e106230. doi: 10.15252/embj.2020106230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kandasamy M, Lehner B, Kraus S, et al. TGF‐beta signalling in the adult neurogenic niche promotes stem cell quiescence as well as generation of new neurons. J Cell Mol Med. 2014;18(7):1444-1459. doi: 10.1111/jcmm.12298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fares J, Bou Diab Z, Nabha S, Fares Y. Neurogenesis in the adult hippocampus: history, regulation, and prospective roles. Int J Neurosci. 2019;129(6):598-611. doi: 10.1080/00207454.2018.1545771 [DOI] [PubMed] [Google Scholar]
- 107.Deng W, Aimone JB, Gage FH. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 2010;11(5):339-350. doi: 10.1038/nrn2822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124(3):319-335. doi: 10.1002/cne.901240303 [DOI] [PubMed] [Google Scholar]
- 109.Goldman SA, Nottebohm F. Neuronal production, migration, and differentiation in a vocal control nucleus of the adult female canary brain. Proc Natl Acad Sci Unit States Am. 1983;80(8):2390-2394. doi: 10.1073/pnas.80.8.2390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255(5052):1707-1710. doi: 10.1126/science.1553558 [DOI] [PubMed] [Google Scholar]
- 111.Meskenaite V, Krackow S, Lipp H-P. Age-dependent neurogenesis and neuron numbers within the olfactory bulb and hippocampus of Homing Pigeons. Front Behav Neurosci. 2016;10:126. doi: 10.3389/fnbeh.2016.00126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kornack DR, Rakic P. Continuation of neurogenesis in the hippocampus of the adult macaque monkey. Proc Natl Acad Sci U S A. 1999;96(10):5768-5773. doi: 10.1073/pnas.96.10.5768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cameron HA, Glover LR. Adult neurogenesis: beyond learning and memory. Annu Rev Psychol. 2015;66:53-81. doi: 10.1146/annurev-psych-010814-015006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Eriksson PS, Perfilieva E, Björk-Eriksson T, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4(11):1313-1317. doi: 10.1038/3305 [DOI] [PubMed] [Google Scholar]
- 115.Bergmann O, Spalding KL, Frisén J. Adult neurogenesis in humans. Cold Spring Harb Perspect Biol. 2015;7(7):a018994. doi: 10.1101/cshperspect.a018994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kempermann G, Gage FH, Aigner L, et al. Human adult neurogenesis: evidence and remaining questions. Cell Stem Cell. 2018;23(1):25-30. doi: 10.1016/j.stem.2018.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sierra A, Encinas JM, Maletic-Savatic M. Adult human neurogenesis: from microscopy to magnetic resonance imaging. Front Neurosci. 2011;5:47. doi: 10.3389/fnins.2011.00047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Aimone JB, Li Y, Lee SW, Clemenson GD, Deng W, Gage FH. Regulation and Function of adult neurogenesis: from genes to cognition. Physiol Rev. 2014;94(4):991-1026. doi: 10.1152/physrev.00004.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kohl Z, Kandasamy M, Winner B, et al. Physical activity fails to rescue hippocampal neurogenesis deficits in the R6/2 mouse model of Huntington’s disease. Brain Res. 2007;1155:24-33. doi: 10.1016/j.brainres.2007.04.039 [DOI] [PubMed] [Google Scholar]
- 120.Torner L, Karg S, Blume A, et al. Prolactin prevents chronic stress-induced decrease of adult hippocampal neurogenesis and promotes neuronal fate. J Neurosci Off J Soc Neurosci. 2009;29(6):1826-1833. doi: 10.1523/JNEUROSCI.3178-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kang E, Wen Z, Song H, Christian KM, Ming G-l. Adult neurogenesis and psychiatric disorders. Cold Spring Harb Perspect Biol. 2016;8(9):a019026. doi: 10.1101/cshperspect.a019026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Marxreiter F, Regensburger M, Winkler J. Adult neurogenesis in Parkinson’s disease. Cell Mol Life Sci CMLS. 2013;70(3):459-473. doi: 10.1007/s00018-012-1062-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ransome MI, Renoir T, Hannan AJ. Hippocampal neurogenesis, cognitive deficits and affective disorder in Huntington’s disease. Neural Plast. 2012;2012:1-7. doi: 10.1155/2012/874387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Choi SH, Tanzi RE. Is Alzheimer’s disease a neurogenesis disorder? Cell Stem Cell. 2019;25(1):7-8. doi: 10.1016/j.stem.2019.06.001 [DOI] [PubMed] [Google Scholar]
- 125.Filgueira L, Larionov A, Lannes N. The influence of virus infection on microglia and accelerated brain aging. Cells. 2021;10(7):1836. doi: 10.3390/cells10071836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wang T, Rumbaugh JA, Nath A. Viruses and the brain: from inflammation to dementia. Clin Sci Lond Engl 1979. 2006;110(4):393-407. doi: 10.1042/CS20050278 [DOI] [PubMed] [Google Scholar]
- 127.Dhikav V, Anand K. Hippocampus in health and disease: an overview. Ann Indian Acad Neurol. 2012;15(4):239-246. doi: 10.4103/0972-2327.104323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cho K-O, Lybrand ZR, Ito N, et al. Aberrant hippocampal neurogenesis contributes to epilepsy and associated cognitive decline. Nat Commun. 2015;6:6606. doi: 10.1038/ncomms7606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Velusamy T, Panneerselvam AS, Purushottam M, et al. Protective effect of antioxidants on neuronal dysfunction and plasticity in Huntington’s disease. Oxid Med Cell Longev. 2017;2017:1-15. doi: 10.1155/2017/3279061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Manickam N, Radhakrishnan RK, Vergil Andrews JF, Selvaraj DB, Kandasamy M. Cell cycle re-entry of neurons and reactive neuroblastosis in Huntington’s disease: possibilities for neural-glial transition in the brain. Life Sci. 2020;263:118569. doi: 10.1016/j.lfs.2020.118569 [DOI] [PubMed] [Google Scholar]
- 131.Zhang B-Z, Chu H, Han S, et al. SARS-CoV-2 infects human neural progenitor cells and brain organoids. Cell Res. 2020;30(10):928-931. doi: 10.1038/s41422-020-0390-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ghazavi A, Ganji A, Keshavarzian N, Rabiemajd S, Mosayebi G. Cytokine profile and disease severity in patients with COVID-19. Cytokine. 2021;137:155323. doi: 10.1016/j.cyto.2020.155323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Pang Y, Fan L-W. Dysregulation of neurogenesis by neuroinflammation: key differences in neurodevelopmental and neurological disorders. Neural Regen Res. 2017;12(3):366-371. doi: 10.4103/1673-5374.202926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Moreno-Jiménez EP, Flor-García M, Terreros-Roncal J, et al. Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer’s disease. Nat Med. 2019;25(4):554-560. doi: 10.1038/s41591-019-0375-9 [DOI] [PubMed] [Google Scholar]