Abstract
Background
Alzheimer’s disease (AD) is an age-related neurodegenerative disease and exercises might mitigate the progression of AD. This investigation aimed to manifest the potential mechanism of exercises in AD.
Methods
Morris water maze (MWM) test was conducted to evaluate the cognitive function in APP/PS1 mice. Quantitative real-time PCR was performed to detect the expression of HOTAIR and miR-130a-3p. The enzyme-linked immunosorbent assay was applied to appraise the concentration of IL-1β, IL-6, and TNF-α. A luciferase report experiment was implemented to substantiate the relationship between miR-130a-3p and HOTAIR.
Results
Exercises contributed to the elevated expression of HOTAIR. The findings of MWM implied HOTAIR inhibited the impacts of voluntary exercises on escape latency, distance moved, percentage of time spent in the target quadrant, platform crossing times, and inflammation. MiR-130a-3p mediated the function of HOTAIR on cognitive ability and inflammation.
Conclusion
HOTAIR participated in the regulation of exercises on AD by sponging miR-130a-3p.
Keywords: Alzheimer’s disease, HOTAIR, miR-130a-3p, cognition, inflammation
Introduction
Alzheimer’s disease (AD) is a burdensome dysfunction that is conducive to degeneration of brain and dementia. 1 In the clinic, AD is often viewed as progressive amnesia, aphasia, apraxia, and visual space disorder. 2 Beyond that, AD is accompanied by mental and behavioral symptoms, such as anxiety, depression, agitation, and impulse, and it exerts a serious impact on the normal life and social activity of the elderly.3,4 For pathology, two main neuropathological hallmarks including amyloid plaques and neurofibrillary tangles trigger the development of AD. 5 In current days, there is no specific treatment for AD in the clinic, but early diagnosis and intervention can enhance patients’ quality of life and relieve economic pressure and family burden. 6 The worldwide incidence of AD keeps rising and the number of AD patients in the world will exceed .1 billion in 2050.7,8 Although AD has become a major health hazard, the pathogenesis of AD remains obscure.
Massive evidence supports the significance of regular physical exercises on inhibiting thinking degeneration in AD-affected patients. 9 A study about AD provides treadmill walking can restrict the inflammatory situation and reinforce psychological happiness for AD patients. 10 Another evidence by Lu et al 11 indicates that exercises can exert multifactorial influence to hold back several pathological processes of AD rat models, involving the cognitive function and inflammation. From all these studies, physical exercise may effectively intervene in neurodegenerative diseases by delaying their progress and preventing their occurrence. Besides, several lncRNAs participate in the regulation of main AD pathways. 12 In amyloid β1-42 (Aβ)-induced SH-SY5Y cells, lncRNA NEAT1 is highly expressed and mediates the influence of Aβ on neurons via miR-107. 13 The expression of lncRNA BACE1-AS is elevated in the AD transgenic mice and it might play essential roles in the progression of AD via miR-214-3p/ATG5 axis. 14 More importantly, the importance of HOTAIR on inflammation and cognition has been reported widely. It is reported that silenced lncRNA HOTAIR may attenuate the brain function impairment in AD rat models by facilitating cognitive responses. 15 What’s more, HOTAIR is a critical player in inflammatory and immune response in macrophages and H9C2 cells treated by lipopolysaccharide.16,17 As a result, the impacts of HOTAIR regarding cognitive impairment and its possible mechanism attract our attention. In our previous study, we found that HOTAIR was an alternative diagnostic biomarker for AD patients and it is associated with the cognitive situation. At the same time, the exercise should be an independent maker of HOTAIR expression in AD patients.
Considering the possibility of HOTAIR in AD and the necessity of exercises for AD patients, we hypothesized HOTAIR might play roles in APP/PS1 models. First and foremost, the function of exercises on AD models was assessed from two aspects, including inflammation and cognitive situation. Secondly, the influence of HOTAIR on voluntary exercise (VE) was evaluated. Also, the putative target of HOTAIR was investigated in our study.
Materials and Methods
AD Mice Model Treatment and Transfection
The design of this investigation was demonstrated by the experimental animal ethics committee of the First People’s Hospital of Yancheng City. All methods were conducted based on the ARRIVE guidelines. Eight-month-old double transgenic APP/PS1 mice (B6. Cg-Tg (APPswe, PSEN1dE9) 85Dbo/J; MRRC-034832-JAX) from the Jackson Laboratory (Ba Harbor, ME, USA) were purchased. All animals were increased in an animal room at a temperature of 22 ± 2°C and humidity of about 50%. The APP/PS1 mice were free to food and drink to get used to the new environment for one week.
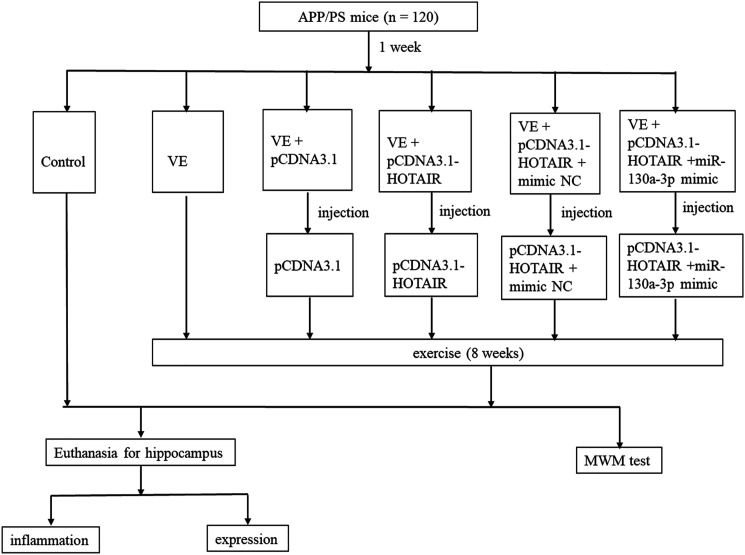
The sequences of HOTAIR, miR-130a-3p mimic, and mimic negative control (mimic-NC) were purchased from GenePharma (Shanghai, China). The pCDNA3.1 vectors carrying HOTAIR were obtained by cloning HOTAIR sequences into pCDNA3.1 blank vectors. To detect the function of HOTAIR, a total of four groups were set up, including the control group, VE group, VE + pCDNA3.1 group, and VE + pCDNA3.1-HOTAIR group. To detect the mediated function of miR-130a-3p, the VE group, VE + pCDNA3.1 group, VE + pCDNA3.1-HOTAIR group, VE + pCDNA3.1-HOTAIR + mimic NC group, and VE + pCDNA3.1-HOTAIR +miR-130a-3p mimic group were constructed. All these experimental mice except those in the control group went through exercises in the cages with flexible wheels for four weeks. In the control group, the mice were not treated. Additionally, pCDNA3.1 blank vectors and vector particles expressing HOTAIR were stereotaxically injected into the bilateral hippocampus of the mice in the VE + pCDNA3.1 group and the VE + pCDNA3.1-HOTAIR group, respectively. A similar method was adopted in the VE + pCDNA3.1-HOTAIR + mimic NC group and VE + pCDNA3.1-HOTAIR +miR-130a-3p mimic group for the delivery of relative interference reagents. Each group consisted of twenty mice. Half of the mice in each group were anesthetized to separate their hippocampus tissues. All mice were single-housed and given free access to water and food. These tissues obtained were put into liquid nitrogen immediately for total RNA extraction and inflammatory detection. The protocol was illustrated in Figure 1.
Figure 1.
The protocol of our experiments on mice.
Morris Water-Maze (MWM) Test
The experimental pool was subdivided into four quadrants on average, including northeast, northwest, southwest, and southeast. A platform was fixed at the center of the pool. The mice were trained four times every day, with an interval of 60 minutes each time. During the training process, the mice were put into any water entry point at random in the pool by facing the pool wall. If the mice did not find the platform within the specified time (60 seconds), they should be led to the platform to stay for 20 seconds. The escape latency and distance moved of each mouse were recorded and summarized. After five days of training, the platform was removed. Each mouse was put into the pool facing the wall to delve into the percentage of time staging in the target quadrant and crossing times.
RNA Extraction and Quantitative Real-Time PCR (qRT-PCR)
Total RNA samples were extracted from hippocampal tissues using an RNA easy fast tissue/cell kit (TIANGEN, Beijing, China). The purity and concentration were detected by the DanoDrop 2000. The first line of cDNA of HOTAIR was synthesized through 1st strand cDNA synthesis superMix (Yeason, Shanghai, China). For miR-130a-3p, 1st strand cDNA synthesis kit (Vazyme, Nanjing, Jiangsu, China) was adopted to synthesize cDNA. The qRT-PCR assay was performed using the Toyobo SYBR qPCR mix (Toyobo, Osaka, Japan). With GAPDH or U6 as the internal references, the relative content of HOTAIR or miR-130a-3p was calculated by the 2−ΔΔ Ct method.
Enzyme-Linked Immunosorbent Assay (ELISA)
The hippocampus was extracted from each mouse and washed in pre-cooled PBS. Then, the hippocampus was put into the fresh lysis buffer solution and the supernatant was obtained after centrifuging. According to the requirements of the specification, 100 μl prepared sample was added to each well for 1 hour at 37°C and then the detection solutions A and B were added and incubated in turn. Afterward, the TMB substrate solution was added for color development without light for 20 minutes. In the end, a termination reagent was added and the optical density (OD) value of each well was measured immediately with a microplate reader at 450 nm wavelength. All ELISA kits used for detecting IL-1β, IL-6, and TNF-α were from CLOID-CLONE CORP (Wuhan, Hubei, China). The sensitivity of the ELISA kit for IL-1β was 15.6–1000 pg/mL, for IL-6 was 7.8–500 pg/mL, and for TNF-α was 15.6–1000 pg/mL.
Luciferase Activity Assay
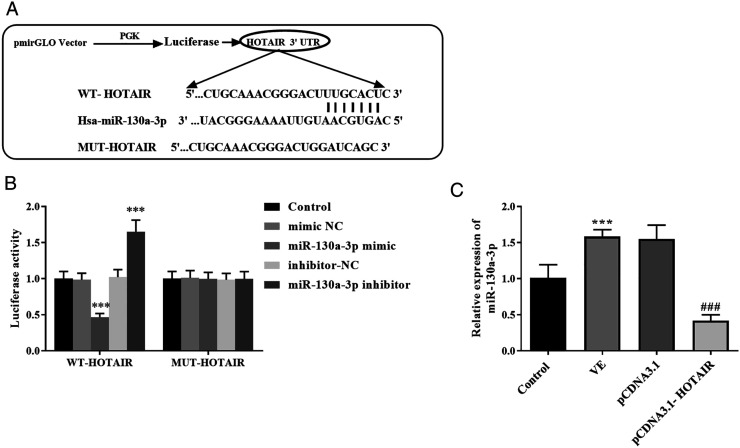
The wide type (WT) and mutation type (MUT) of HOTAIR, mimic negative control (NC), miR-130a-3p mimic, inhibitor-NC, and miR-130a-3p inhibitor were obtained from GenePharma (Shanghai, China). The sequences of WT-HOTAIR and MUT-HOTAIR were cloned into pmirGLO vectors. HEK293 cells were inoculated into 12-well plates with 1×106 cells per well. These cells were incubated overnight and transiently transfected by Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) when cell density reached 70%–80%. For the WT-HOTAIR group, the vectors carrying HOTAIR were co-transfected with mimic NC, miR-130a-3p mimic, inhibitor-NC, and miR-130a-3p inhibitor to HEK293 cells. The vectors carrying MUT-HOTAIR as well as different miR-130a-3p sequences were also co-transfected into HEK293 cells. After 48 h of transfection, we performed a luciferase reporter assay by following the instructions of the double luciferase reporter gene detection kit (Yeason, Shanghai, China). Ultimately, the synergy H4 microplate reader (Biotek Winooski, VT, USA) was applied to get the fluorescence value.
Statistical Analysis
The experimental data were dissected through GraphPad Prism 6.0 software. The numerical data were expressed as mean ± standard deviation. T-test was applied to figure up differences between the two groups. The one-way analysis of variance followed by Tukey’s post hoc test and two-way analysis of variance followed by a Sidak post hoc analysis were applied for differences among multiple groups. The number of mice was twenty in each group: half mice were used for the MWM test and the other for euthanasia. P < .05 represented statistical significance.
Results
VE Contributed to the Recovery of Cognition and Inflammation
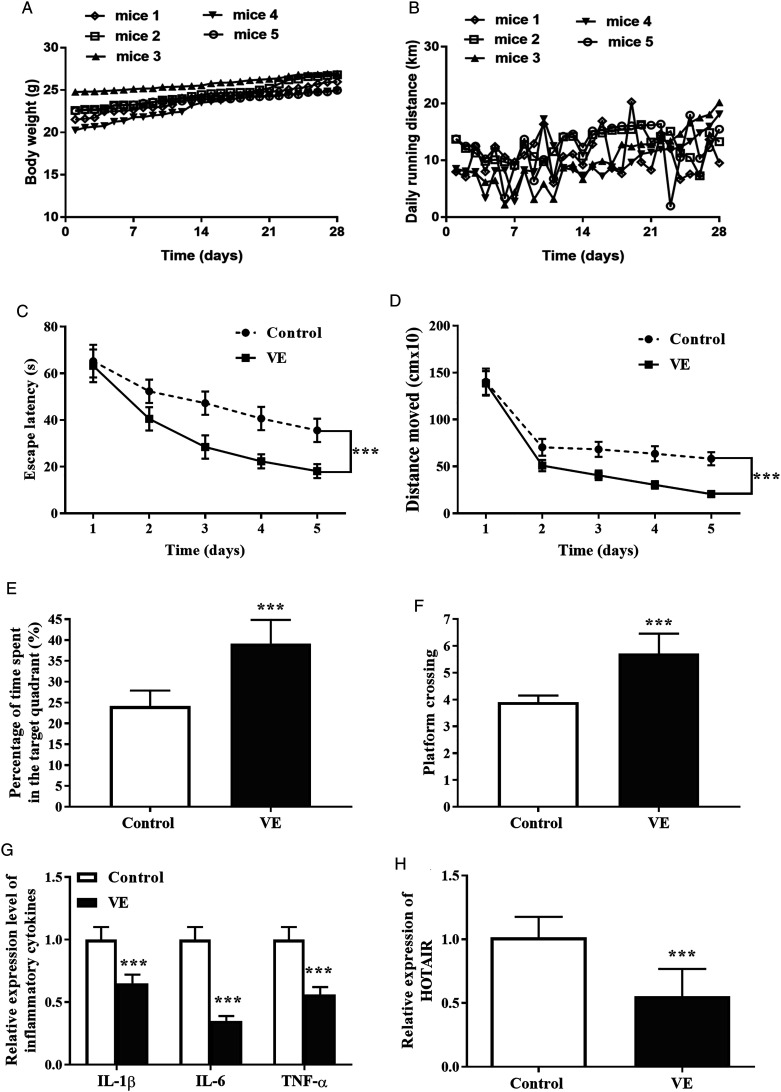
With the purpose of detecting the movement of mice, five mice were randomly chosen, and their daily movement distance and weight were recorded and analyzed, as shown in Figures 2A and 2B.
Figure 2.
The impacts of VE on cognition, inflammation, and the expression of HOTAIR in APP/PS1 mice. The mice in the control group were mutant mice without exercise and the mice in the VE group were mutant mice with exercise. (A) The body weights of mice. (B) The daily running distances of mice. (C) The influence of VE on escape latency. (D) Distance moved in the VE group was lessened compared with the control group. (E) The percentage of time spent in the target quadrant in the VE group was increased compared with the control group. (F) VE elevated the platform crossing times. (G) The secretion of IL-1β, IL-6, and TNF-α was inhibited in the VE group. (H) VE contributed to the declined expression of HOTAIR. ***P < .001, compared with the control group.
After a 4-week exercise, an MWM test was performed to test the cognitive function of APP/PS1 mice. As seen in Figure 2C and Figure 2D, the escape latency and distance moved on the fifth day were obviously decreased in the VE group compared with the control group, indicating exercises might enhance the cognitive situation (P < .001). The percentage of time spent in the target quadrant and target platform crossing of the sixth day in the VE group were higher than those in the control group (Figures 2E and 2F, (p) < .001). Results of inflammatory factors manifested that exercises could inhibit the concentration of IL-1β, IL-6, and TNF-α (Figure 2G, (p) < .001).
VE Reduced the Level of HOTAIR in APP/PS1 Mice
The level of HOTAIR in the hippocampal tissues from APP/PS1 mice was evaluated by qRT-PCR. As shown in Figure 2H, HOTAIR was lowly expressed in the VE group compared with the control group (P < .001), implying that exercises might affect the expression of HOTAIR in APP/PS1 mice.
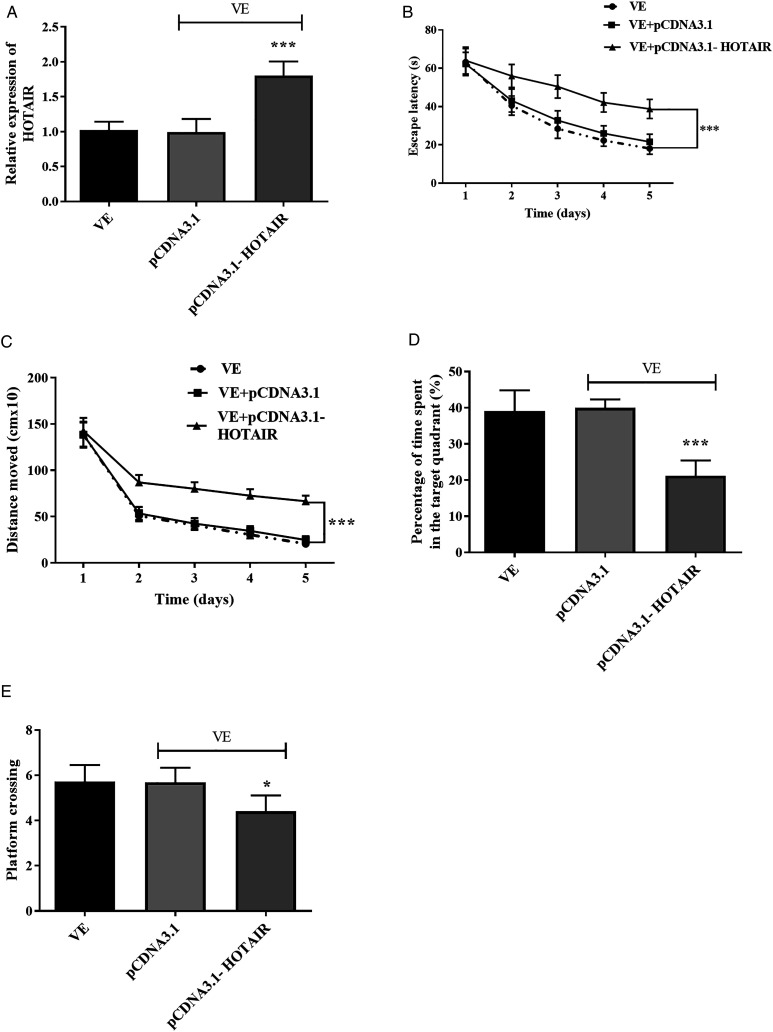
Increased HOTAIR Level Reversed the Beneficial Influence of VE
The pCDNA3.1-HOTAIR was adopted in this study to regulate the expression of HOTAIR in mice and to examine the function of HOTAIR. The transfection efficiency of pCDNA3.1-HOTAIR was detected by qRT-PCR and expressed in Figure 3A. As exhibited in Figure 3A, the expression of HOTAIR in the pCDNA3.1-HOTAIR group was upregulated compared with the VE group (P < .001), which indicated that HOTAIR was successfully transfected in mice. The enhanced HOTAIR expression in the pCDNA3.1-HOTAIR group strengthened the escape latency, led to the promotion of distance moved, inhibited the percentage time staying in the target area, and repressed the platform crossing times compared with the VE group (Figures 3B-3E, (p) < .05). These findings verified that overexpression of HOTAIR could abrogate the recovery of learning and cognitive ability induced by VE.
Figure 3.
The expression and roles of HOTAIR. (A) The expression of HOTAIR was increased in the pcDNA3.1-HOTAIR group. The impacts of VE on (B) escape latency, (C) distance moved, (D) percentage of time spent in the target area, and (E) platform crossing times were attenuated by the overexpression of HOTAIR. *P < .05, ***P < .001, compared with the VE group.
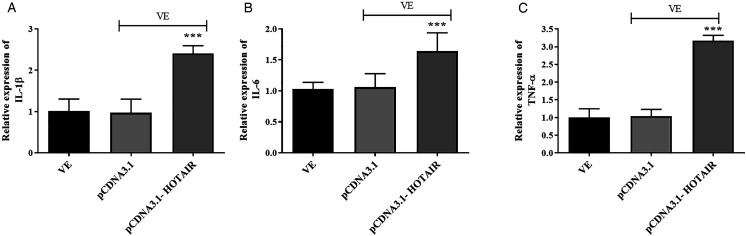
Furthermore, the detection of inflammatory indicators found that the increased HOTAIR expression contributed to the enhanced levels of IL-1β, IL-6, and TNF-α compared with the VE group (Figures 4A-C, (p) < .001).
Figure 4.
The concentration of (A) IL-1β, (B) IL-6, and (C) TNF-α in the pCDNA3.1-HOTAIR group was increased. ***P < .001, compared with the VE group.
MiR-130a-3p Acted as a ceRNA of HOTAIR
The potential mechanism of HOTAIR was further analyzed. As observed in Figure 5A, the putative target sites between miR-130a-3p and HOTAIR were proven. Results of the luciferase report experiment implied that in the group of WT-HOTAIR, miR-130a-3p mimic induced the lessened luciferase activity and miR-130a-3p inhibitor facilitated the luciferase activity (Figure 5B, P < .001). Beyond that, the expression of miR-130a-3p was increased in the VE group compared with the control group (Figure 5C, P < .001). Accordingly, the overexpression of HOTAIR decreased the level of miR-130a-3p compared with the VE group (Figure 5C, P < .001).
Figure 5.
(A) The putative sequence between HOTAIR and miR-130a-3p. (B) Overexpression of miR-130a-3p inhibited the luciferase activity and silenced miR-130a-3p expression elevated luciferase activity in the WT-HOTAIR group. (C) The expression of miR-130a-3p was increased in the VE group and decreased in the pCDNA3.1-HOTAIR group. ***P < .001, compared with the control group; ###P < .001, compared with the VE group.
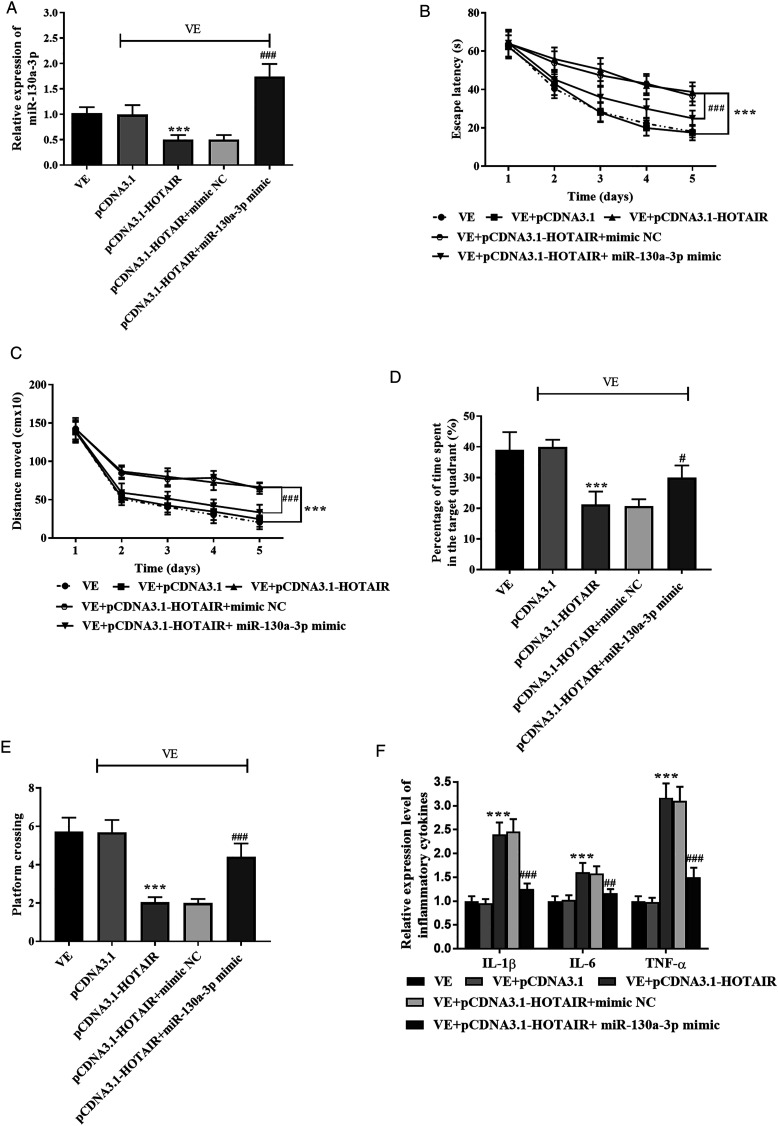
MiR-130a-3p Mediated the Function of HOTAIR
Considering the possible functions of miR-130a-3p, we co-transfected pCDNA-HOTAIR and miR-130a-3p mimic to AD models. The declined expression of miR-130a-3p in the pCDNA3.1-HOTAIR group was reversed in the pCDNA3.1-HOTAIR + miR-130a-3p mimic group, indicating the co-transfection of miR-130a-3p and pCDNA3.1-HOTAIR could regulate the expression of miR-130a-3p (Figure 6A, (p) < .001).
Figure 6.
The function of miR-130a-3p. (A) The expression of miR-130a-3p was decreased in the pCDNA3.1 group and increased in the pCDNA3.1-HOTAIR + miR-130a-3p group. The overexpression of miR-130a-3p inhibited the influence of HOTAIR on (B) escape latency, (C) distance moved, (D) percentage of time spent in the target area, and (E) platform crossing times. (F) The concentration inflammatory indicators were increased by overexpression of HOTAIR and inhibited by the enhancement of miR-130a-3p. ***P < .001, compared with the VE group; #P < .05, ##P < .01, ###P < .001, compared with the pCDNA3.1-HOTAIR group.
What’s more, the presence of miR-130a-3p mimics in the HOTAIR + miR-130a-3p mimic group reversed the function of HOTAIR on the escape latency, distance moved, percentage of time spent in the target quadrant, and platform crossing times, indicating miR-130a-3p might mediate the function of HOTAIR on cognition (Figures 6B-6E, (p) < .05). Meanwhile, the concentration of IL-1β, IL-6, and TNF-α was inhibited in the HOTAIR + miR-130a-3p mimic group relative to the pCDNA3.1-HOTAIR group (Figure 6F, (p) < .01).
Discussion
AD is a common form of dementia of growing old. 18 With the problem of aging, the incidence of AD has shown a rapid growth trend and its diagnosis and treatment have aroused widespread concern from governments and medical circles around the world. 19 Studies have found that physical activities show a protective effect on brain structure and cognitive function. 20 Aerobic exercises can drive cardiorespiratory fitness in patients with mild AD and may contribute to altering disease development and cognitive function. 21 Exercises may recover the cognitive function that occurs in participants who suffer risks of or have AD. 22 However, more clarity about the potential mechanism should be still necessary.
In the current study, we conducted an MWM test to analyze the cognitive function in mice with/without exercises. Our findings indicated that the exercises could be good for cognitive recovery by inhibiting escape latency, repressing distance moved, promoting the percentage of time spent in the target area, and elevating platform crossing times. Additionally, the inflammatory cytokines were inhibited in mice undergoing exercises. The beneficial function of exercise on learning and inflammation has been studied in several kinds of research. A meta-analysis performed by Du et al 23 offered the beneficial functions of physical activities to cognitive dysfunction in AD patients. Another study certifies that regular exercises can ameliorate spatial learning ability, and thus exercises showed a therapeutic possibility for AD. 24 All these elucidates exercises might be beneficial to AD patients by reinforcing learning and cognitive ability.
As known, pCDNA 3.1 was used as a common carrier for transfection.25,26 Thus, an overexpression vector of HOTAIR was constructed. In the in vivo assay, we found the level of HOTAIR in the mice with exercises was lessened compared with the control mice, suggesting exercise might inhibit the expression of HOTAIR. The expression of HOTAIR was artificially regulated by the pCDNA3.1-HOTAIR to evaluate the effects of HOTAIR in mice. In contrast to the beneficial roles of VE in AD, the overexpression of HOTAIR restricted the recovery of spatial exploration, implying the disadvantageous influence of HOTAIR in AD. Wang et al 15 also report HOTAIR develops an essential role in neurodegenerative disorders by mediating the cognitive responses. What’s more, we found the elevated HOTAIR expression suppressed the amelioration of IL-6, IL-1β, and TNF-α caused by VE, indicating that HOTAIR might facilitate the inflammatory situation. In a study about sepsis, the upregulation of HOTAIR can trigger the enhancement of IL-1β, IL-6, and TNF-α by inhibiting miR-211, suggesting the positive association between HOTAIR and inflammation. 27 In hepatocytes, elevated expression of HOTAIR also facilitates the secretion of IL-1β, IL-6, and TNF-α, implying that HOTAIR may participate in the development of inflammation. 28
Several investigations on the mechanism of HOTAIR have been performed recently. In rheumatoid arthritis, miR-138 regulates the impacts of HOTAIR on inflammation by acting as a ceRNA. 29 In Parkinson’s disease, the expression of HOTAIR is increased and it may influence disease progression by sponging miR-126-5p. 30 In our current study, we certified that miR-130a-3p might be a ceRNA of HOTAIR by luciferase report. Additionally, the expression of miR-130a-3p was enhanced in the VE group, while the overexpression of HOTAIR reversed the trend, further confirming the association between HOTAIR and miR-130a-3p. In knee osteoarthritis, HOTAIR can repress the expression of miR-130a-3p in chondrocytes. 31 In hepatocellular carcinoma, Hu et al 32 also verify the target relationship between HOTAIR and miR-130a-3p. Furthermore, the effects of miR-130a-3p were explored. We found that the overexpression of miR-130a-3p reversed the function of HOTAIR on cognitive function and inflammation, showing that HOTAIR might regulate the progression of AD by miR-130a-3p. The expression and effects of miR-130a-3p have been examined by many researchers. For instance, miR-130a-3p is lowly expressed in the AD mice, and upregulation of miR-130a-3p benefits the recovery of the cognitive situation. 33 In aging rats, voluntary wheel running moderates rats’ aging by elevating the expression of miR-130a-3p. 34 One limitation of this publication was the lack of other types of cognitive tests.
Collectively, we found that exercise could mitigate the learning ability, qualify inflammation, and inhibit HOTAIR expression in AD mice. Overexpression of HOTAIR restricted the effects of VE on cognitive function and inflammation. MiR-130a-3p was a ceRNA of HOTAIR. The abundance of miR-130a-3p expression ameliorated spatial learning ability and inflammation influenced by the HOTAIR.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by National Key Research and Development Program "China’s Elderly Disability Prevention and Intervention Management Network and Technology Research” [No.2020YFC2008505].
Significance Statement: Exercise contributed to the elevated expression of HOTAIR.
MiR-130a-3p mediated the function of HOTAIR on cognitive ability and inflammation.
ORCID iD
Zhongli Jiang https://orcid.org/0000-0002-7989-6502
References
- 1.Breijyeh Z, Karaman R. Comprehensive review on Alzheimer's disease: causes and treatment. Molecules. 2020;25(24):5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guo X, Ma T. Effects of acupuncture on neurological disease in clinical- and animal-based research. Front Integr Neurosci. 2019;13:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yin Z, Wang X, Zheng S, et al. Long Sheng Zhi capsule attenuates alzheimer-like pathology in app/ps1 double transgenic mice by reducing neuronal oxidative stress and inflammation. Front Aging Neurosci. 2020;12:582455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cajanus A, Solje E, Koikkalainen J, et al. The association between distinct frontal brain volumes and behavioral symptoms in mild cognitive impairment, alzheimer's disease, and frontotemporal dementia. Front Neurol. 2019;10:1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abeysinghe AADT, Deshapriya RDUS, Udawatte C. Alzheimer's disease; a review of the pathophysiological basis and therapeutic interventions. Life Sci. 2020;256:117996. [DOI] [PubMed] [Google Scholar]
- 6.Vaz M, Silvestre S. Alzheimer's disease: recent treatment strategies. Eur J Pharmacol. 2020;887:173554. [DOI] [PubMed] [Google Scholar]
- 7.Fan L, Mao C, Hu X, et al. New insights into the pathogenesis of alzheimer’s disease. Front Neurol. 2019;10:1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khoury R, Patel K, Gold J, Hinds S, Grossberg GT. Recent progress in the pharmacotherapy of alzheimer’s disease. Drugs Aging. 2017;34(11):811-820. [DOI] [PubMed] [Google Scholar]
- 9.Valenzuela PL, Castillo-García A, Morales JS, et al. Exercise benefits on alzheimer’s disease: state-of-the-science. Ageing Res Rev. 2020;62:101108. [DOI] [PubMed] [Google Scholar]
- 10.Abd El-Kader SM, Al-Jiffri OH. Aerobic exercise improves quality of life, psychological well-being and systemic inflammation in subjects with Alzheimer's disease. Afr Health Sci. 2016;16(4):1045-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu Y, Dong Y, Tucker D, et al. Treadmill exercise exerts neuroprotection and regulates microglial polarization and oxidative stress in a streptozotocin-induced rat model of sporadic alzheimer’s disease. J Alzheim Dis. 2017;56(4):1469-1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Idda ML, Munk R, Abdelmohsen K, Gorospe M. Noncoding RNAs in alzheimer’s disease. Wiley interdisciplinary reviews. RNA. 2018;9(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ke S, Yang Z, Yang F, Wang X, Tan J, Liao B. Long noncoding RNA NEAT1 aggravates Aβ-induced neuronal damage by targeting miR-107 in Alzheimer’s Disease. Yonsei Med J. 2019;60(7):640-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou Y, Ge Y, Liu Q, et al. LncRNA BACE1-AS promotes autophagy-mediated neuronal damage through The miR-214-3p/ATG5 signalling axis in alzheimer’s disease. Neuroscience. 2021;455:52-64. [DOI] [PubMed] [Google Scholar]
- 15.Wang J-y., Feng Y, Fu Y-h., Liu G-l. Effect of sevoflurane anesthesia on brain is mediated by lncRNA HOTAIR. J Mol Neurosci. 2018;64(3):346-351. [DOI] [PubMed] [Google Scholar]
- 16.Obaid M, Udden SMN, Deb P, Shihabeddin N, Zaki MH, Mandal SS. LncRNA HOTAIR regulates lipopolysaccharide-induced cytokine expression and inflammatory response in macrophages. Sci Rep. 2018;8(1):15670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ni S-Y, Xu W-T, Liao G-Y, Wang Y-L, Li J. LncRNA HOTAIR promotes LPS-induced inflammation and apoptosis of cardiomyocytes via lin28-mediated PDCD4 stability. Inflammation. 2021;44(4):1452-1463. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Yu F, Bao S, Sun J. Systematic characterization of circular RNA-associated CeRNA network identified novel circRNA biomarkers in alzheimer’s disease. Front Bioeng Biotechnol. 2019;7:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cocchiara RA, De Lucia F, Koci L, Lisanti E, Petruccini G, La Torre G. Management of the early stage of Alzheimer's disease: a systematic review of literature over the past 10 years. La Clinica terapeutica. 2020;171(4):e357-e368. [DOI] [PubMed] [Google Scholar]
- 20.Vesperman CJ, Pozorski V, Dougherty RJ, et al. Cardiorespiratory fitness attenuates age-associated aggregation of white matter hyperintensities in an at-risk cohort. Alzheimer's Res Ther. 2018;10(1):97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sobol NA, Dall CH, Høgh P, et al. Change in fitness and the relation to change in cognition and neuropsychiatric symptoms after aerobic exercise in patients with mild alzheimer’s disease. J Alzheim Dis. 2018;65(1):137-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Panza GA, Taylor BA, MacDonald HV, et al. Can exercise improve cognitive symptoms of alzheimer’s disease? J Am Geriatr Soc. 2018;66(3):487-495. [DOI] [PubMed] [Google Scholar]
- 23.Du Z, Li Y, Li J, Zhou C, Li F, Yang X. Physical activity can improve cognition in patients with Alzheimer’s disease: a systematic review and meta-analysis of randomized controlled trials. Clin Interv Aging. 2018;13:1593-1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pang R, Wang X, Pei F, et al. Regular exercise enhances cognitive function and intracephalic GLUT expression in alzheimer’s disease model mice. J Alzheim Dis. 2019;72(1):83-96. [DOI] [PubMed] [Google Scholar]
- 25.Liang Q, Asila A, Deng Y, Liao J, Liu Z, Fang R. Osteopontin‐induced lncRNA HOTAIR expression is involved in osteoarthritis by regulating cell proliferation. BMC Geriatr. 2021;21(1):57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang YF, Li CS, Zhou Y, Lu XH. Effects of propofol on colon cancer metastasis through STAT3/HOTAIR axis by activating WIF‐1 and suppressing Wnt pathway. Cancer Med. 2020;9(5):1842-1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen J, Gu X, Zhou L, et al. Long non-coding RNA-HOTAIR promotes the progression of sepsis by acting as a sponge of miR-211 to induce IL-6R expression. Exp Ther Med. 2019;18(5):3959-3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang J, Chen M, Zhai Y, Fu Y. Retracted : HOTAIR regulates lipopolysaccharide‐induced inflammatory response in hepatocytes. J Cell Physiol. 2020;235(5):4247-4255. [DOI] [PubMed] [Google Scholar]
- 29.Zhang H-j, Wei Q-f, Wang S-j, et al. RETRACTED: LncRNA HOTAIR alleviates rheumatoid arthritis by targeting miR-138 and inactivating NF-κB pathway. Int Immunopharm. 2017;50:283-290. [DOI] [PubMed] [Google Scholar]
- 30.Lin Q, Hou S, Dai Y, Jiang N, Lin Y. LncRNA HOTAIR targets miR-126-5p to promote the progression of Parkinson's disease through RAB3IP. Biol Chem. 2019;400(9):1217-1228. [DOI] [PubMed] [Google Scholar]
- 31.He B, Jiang D. HOTAIR‐induced apoptosis is mediated by sponging miR‐130a‐3p to repress chondrocyte autophagy in knee osteoarthritis. Cell Biol Int. 2020;44(2):524-535. [DOI] [PubMed] [Google Scholar]
- 32.Hu M, Fu Q, Jing C, Zhang X, Qin T, Pan Y. LncRNA HOTAIR knockdown inhibits glycolysis by regulating miR-130a-3p/HIF1A in hepatocellular carcinoma under hypoxia. Biomed Pharmacother. 2020;125:109703. [DOI] [PubMed] [Google Scholar]
- 33.Wang Y, Shi M, Hong Z, Kang J, Pan H, Yan C. MiR-130a-3p has protective effects in alzheimer's disease via targeting DAPK1. Am J Alzheimer's Dis Other Dementias. 2021;36:15333175211020572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shen K, Liu X, Chen D, Chang J, Zhang Y, Kou X. Voluntary wheel-running exercise attenuates brain aging of rats through activating miR-130a-mediated autophagy. Brain Res Bull. 2021;172:203-211. [DOI] [PubMed] [Google Scholar]