Abstract
The present study investigated the role and potential mechanisms of miR-130a-3p in AD. SH-SY5Y cells were treated with Aβ 1-42 to construct AD cell models. APP/PS1 mice were used for the animal experiments. MiR-130a-3p was downregulated in Aβ-induced SH-SY5Y cells. Overexpression of miR-130a-3p attenuates Aβ induced SH-SY5Y cell apoptosis. Low miR-130a-3p expression was detected in the hippocampus tissues of AD mice. The Morris water maze (MWM) results indicated that miR-130a-3p upregulation reduced the escape latency time and increased the time of AD mice spent in the target quadrant. DAPK1 was the target gene of miR-130a-3p. High DAPK1 mRNA level was detected in Aβ treated PC 12 cells and in the hippocampus tissues of AD mice. It was concluded that overexpression of miR-130a-3p may attenuate Aβ-induced neurotoxicity and improve the cognitive function of AD mice via targeting DAPK1.
Keywords: miR-130a-3p, SH-SY5Y, APP/PS1 mice, MWM test, neurotoxicity
Significance Statement
Overexpression of miR-130a-3p may attenuate Aβ-induced neurotoxicity and improve the cognitive function of mice with AD via targeting DAPK1.
The study of miRNAs that affect the key genes of AD may bring the potential novel therapeutic approach for human AD.
Introduction
Alzheimer’s disease (AD) is a common neurodegenerative disease, it can lead to dementia in the elderly. 1 The clinical manifestations mainly include progressive memory disorder, cognitive dysfunction, personality change, and language disorder. With the aggravation of aging population, the incidence rate of AD has increased year by year. 2 The degeneration of memory and learning ability is the main pathological manifestations of AD. 3 amyloid β protein (Aβ) deposition in neurons in the brain is well known to be an initial event in AD and a key trigger of AD pathogenesis. 4 Due to the lack of effective techniques for early diagnosis, most patients are at an advanced stage when diagnosed, leading to poor outcomes. 5 Therefore, it is important to find early markers for the diagnosis of AD.
MicroRNA (miRNA) is widely found in eukaryotes, it is a kind of endogenous non-coding single-stranded small molecule RNA, with a length of 20∼24 nucleotides. 6 MiRNAs play an important role in the regulation of a multitude of physiological functions, including cell proliferation, differentiation, and apoptosis. 7 The expression of miRNAs in the central nervous system is time-and space-specific, and miRNAs can regulate the development of neurons and thus participate in the regulation of the development and function of the nervous system. 8 The occurrence of AD is closely related to miRNAs, many miRNAs have been identified to be dysregulated in AD patients, and have an important effect in AD-induced neurotoxicity and cognitive impairment, such as miR-132, miR-455-3p, and miR-28-3p. 9 -11 MiR-130a-3p has been widely reported to be involved in the progression of neurodegenerative diseases. 12,13 In Parkinson’s disease, downregulation of was identified in the serum of patients. 12 MiR-130a-3p is also reported to regulate neurotransmitter synthesis. 13 Notably, in a study of AD, miR-130a-3p is proved to be involved in the protective role of intermittent fasting against AD. 14 However, the exact role and underlying mechanism of miR-130a-3p in AD remains unknown.
Therefore, in the present study, Aβ-induced neurotoxicity in SH-SY5Y cells and APP/PS1 mice was recruited, and the role and potential mechanisms of miR-130a-3p in AD were investigated.
Materials and Methods
Cell Culture and Transfection
The SH-SY5Y cell line was purchased from the American Type Culture Collection (ATCC), and cultured in a standard growth medium (DMEM, Gibco; Thermo Fisher Scientific, Inc.). Before incubation, 10% horse serum and 5% FBS were added to the culture medium. The incubation was in a humidified incubator at 37%, containing 5% CO2 atmosphere. To mimic the condition of AD in vitro, the AD cell model was established via administration of Aβ1-42 with different concentrations. And the untreated cells incubated in the normal growth medium were used as the control group. Aβ1−42 was dissolved in 100% 1, 1, 1, 3, 3, 3-hexafluoro-2-propanol (HFIP) to a concentration of 1 mg/mL. This solution was incubated at room temperature (RT) for 1 h, then sonicated for 10 min. The HFIP/Aβ1−42 solution was subsequently dried down in a gentle stream of nitrogen, and the dried Aβ1−42 was resuspended in 1 mM DMSO. The preparation was incubated for 12 min at RT and then pipetted and stored at-80%. Before use, the Aβ1−42 preparation was rapidly thawed with PBS, and a final Aβ1−42 with different concentrations was prepared.
The sequences of miR-130a-3p mimic, miR-130a-3p inhibitor, or their negative controls (miR-NC) were synthesized and provided by the company (GenePharma, Shanghai, China). To regulate the level of miR-130a-3p in cells, miR-130a-3p mimic, miR-130a-3p inhibitor, or miR-NC were transfected into cells using Lipofectamine 3000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. Cells were collected for the next experiments 24 hours after transfection.
Animals and Grouping
6-months old APPswe/PS1dE9 (APP/PS1) transgenic AD model mice (male, 25 ± 2 g) and wild-type (WT, male, 25 ± 2 g) littermates were purchased from the Chinese Academy of Medical Science. All mice feed and water freely, and are housed in the 12h-light/12h-dark environment at room temperature. All experiments were performed in compliance with ethical requirements and under the approval of the animal ethics committee of Hangzhou seventh people’s Hospital.
All mice were divided into 4 groups (n = 16/group), WT group, AD group, AD + agomir NC, AD + miR-130a-3p agomir. miR-130a-3p agomir and the negative control (agomir NC) were purchased from GenePharma (Shanghai, China). To regulate the level of miR-130a-3p in mice, miR-130a-3p agomir or agomir NC (100 μM, 1.5 μL) mixed in PBS solution containing 5% Lipofectamine 3000 (Invitrogen, Carlsbad, CA, USA) were stereotaxic injected into both side’s hippocampus regions by a Hamilton micro-syringe at the rate of 0.2 mL/min. 15 Two weeks after injection, 8 mice in each group were sacrificed and the hippocampus tissues were collected for subsequence experiments. The rest 8 mice were used for the behavioral tests.
Morris Water Maze Test
The Morris water maze (MWM) experiment is a kind of experiment in which experimental animals (rats and mice) are forced to swim and learn to find hidden platforms in the water. The water tank was filled with water (25 ± 1%) with a platform in the center. Put the mice into the water with their head facing the wall of the pool, and randomly pick one of the 4 starting positions of east, west, south, and north. Record the time (s) when the animal finds the underwater platform. In the first few training sessions, if this time is longer than 60 seconds, the animal is guided to the platform. Keep the animal on the platform for 10 seconds. Each animal was trained 4 times a day with an interval of 15 to 20 min for 4 days. On the fifth day, the platform was removed and the exploration training of the 60 s was started. Lower the animal into the water from the opposite side of the platform quadrant. The time spent in the target quadrant (the quadrant where the platform was originally placed) and the escape latency were recorded. The recorded data were analyzed by computerized software (Wilmette, IL, USA).
qRT-PCR
TRIzol (Takara Biotechnology Co., Ltd., Dalian, China) was used for the extraction of total RNA from SH-SY5Y cells and hippocampus tissues by TRIzol. After the measurement of purity and concentration, the reverse transcription of RNA was performed by using a TaqMan Advanced miRNA cDNA Synthesis Kit (Applied Biosystems, Foster City, CA, USA). Then qRT-PCR was carried out on an ABI7900 system by employing An SYBR PrimeScript RT-PCR (Takara, Dalian, China). The primers were as follows: miR-130a-3p, forward, 5’-CCAGTGCAATGTTAAAAGGGCAT-3’, reverse: 5’-CGCTTCACGAATTTGCGTGTCAT-3’; U6, forward, 5’-GCGCGTCGTGAAGCGTTC-3’, reverse, 5’-GTGCAGGGTCCGAGGT-3’. The relative expression of miR-130a-3p was calculated by using the comparative delta CT (2−ΔΔCt) method, and U6 was utilized as the endogenous control.
CCK-8
The cell viability was determined by the cell counting kit-8 (CCK-8, Dojindo, Shanghai, China) according to the manufacturer’s instructions. In brief, the SH-SY5Y cells were incubated in the 96—well plates with a density of 1×104 cells/well, and incubated for 3 days. 10 µL CCK-8 was added into each well at the incubation time of 0 h, 24 h, 48 h and 72 h. Subsequently, the plates were incubated in the incubator for another 1 hour. The absorbance at 450 nm was measured using a microplate analyzer.
Flow Cytometry Assay
Cell apoptosis was detected by flow cytometry using Annexin V-FITC/PI Kit (4A Biotech, China). Cells were centrifuged at 1000 rpm for 3 min, and cleaned with pre-cooled PBS. The cells were resuspended by 200 µl binding buffer, then 5 µl Annexin V-FITC and PI were added and mixed. After incubation for 10 minutes at room temperature in the dark, the cell apoptosis rate was detected by flow cytometry.
Luciferase Reporter Assay
According to the TargetScan analysis, DAPK1 was identified to be a potential target gene of miR-130a-3p. The target relationship was verified by luciferase reporter assay. First, the wild-type (DAPK1-WT) and mutant-type (DAPK1-MUT) recombinant plasmid vectors of DAPK1 were constructed and provided by GenePharma (Shanghai, China). The miR-130a-3p mimic or inhibitor, and DAPK1-WT or MUT were co-transfected into SH-SY5Y cells using Lipofectamine 3000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions, and incubated for 48 h. The luciferase activity was measured by using the Dual Luciferase Reporter Assay Kit (Promega Corporation, Wisconsin, USA), and Renilla luciferase was used as an internal control for normalization.
Statistical Analysis
All data were presented as mean ± standard deviation (SD) and the data were analyzed in GraphPad Prism 7.0 software (GraphPad Software, Inc.). Differences between groups were compared by applying one-way analysis of variance (ANOVA), followed by a Tukey’s multiple comparison test. The P-value less than 0.05 was considered to be statistically significant.
Results
MiR-130a-3p Is Downregulated in Aβ-Treated SH-SY5Y Cells
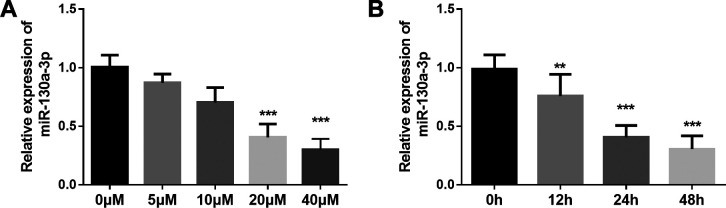
qRT-PCR was used to detect the expression level of miR-130a-3p in SH-SY5Y cells after treated with Aβ 1-42 in various concentrations and incubation times. As shown in Figure 1A, after treated with Aβ 1-42 for 24 h, the level of miR-130a-3p decreased significantly in a concentration-dependent manner in SH-SY5Y cells. And the decreasing trend of the miR-130a-3p level was obvious in 20 μM Aβ 1-42. In addition, SH-SY5Y were treated with 20 μM Aβ 1-42 for different times, and the levels of miR-130a-3p were detected. As shown in Figure 1B, after Aβ treatment for 24 hours, the level of miR-130a-3p had the obvious alteration. Therefore, SH-SY5Y cells treated with 20 μM Aβ 1-42 for 24 hours were used for the following experiments.
Figure 1.
MiR-130a-3p is downregulated in Aβ-treated SH-SY5Y cells. A. After treated with Aβ 1-42, the level of miR-130a-3p decreased significantly in a concentration dependent manner. (* P < 0.05, *** P < 0.001, compared with 0 μM group.) B. SH-SY5Y were treated with 20 μM Aβ 1-42 for different time, and after Aβ treatment for 24 hours, the level of miR-130a-3p had the obvious alteration. (** P < 0.01, *** P < 0.001, compared with 0 h group.).
Overexpression of miR-130a-3p Attenuates Aβ Induced Neurotoxicity in SH-SY5Y Cells
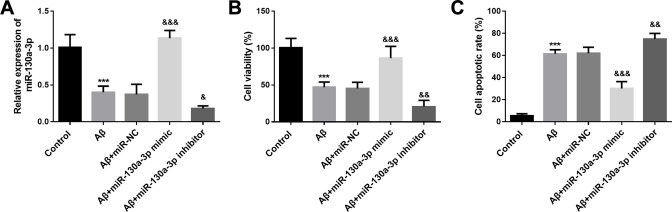
In consideration of alteration of miR-130a-3p in SH-SY5Y cells, its role in Aβ induced neurotoxicity in SH-SY5Y cells was investigated, and the cell transfection was performed to regulate the gene expression. As shown in Figure 2A, miR-130a-3p mimic transfection significantly elevated the level of miR-130a-3p, whereas miR-130a-3p inhibitor transfection had the opposite influence (P < 0.01). Furthermore, CCK-8 and flow cytometry assay were applied to evaluate the cell viability and apoptosis of SH-SY5Y cells in different groups. It was found that miR-130a-3p overexpression promoted SH-SY5Y cell viability and inhibited cell apoptosis. However, miR-130a-3p downregulation aggravated Aβ induced cell viability inhibition and apoptosis (P < 0.01, Figures 2B and C).
Figure 2.
Overexpression of miR-130a-3p attenuates Aβ induced neurotoxicity in SH-SY5Y cells. A. Levels of miR-130a-3p in different cell groups. B. SH-SY5Y cell viability under different treatment. C. Cell apoptosis rates of SY5Y cells under different treatment. *** P < 0.001, compared with control group; && P < 0.01, &&& P < 0.001, compared with Aβ group.
MiR-130a-3p Improves the Cognitive Function of AD Mice
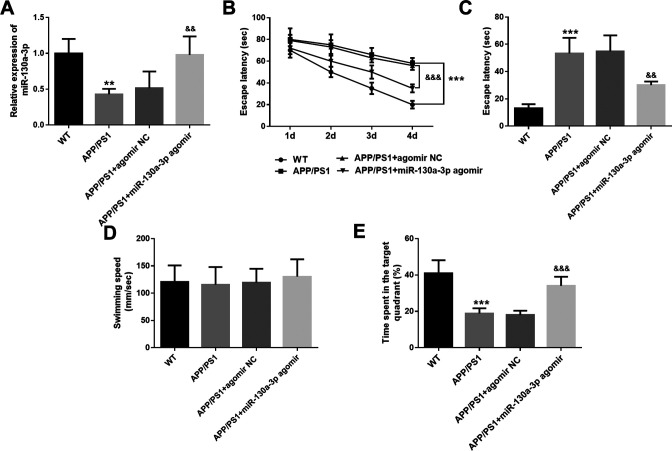
The role of miR-130a-3p was further explored in vivo, and the APP/PS1 transgenic AD mice were recruited. Consistent with the results detected in the SH-SY5Y cells, miR-130a-3p showed low expression in the hippocampus tissues of AD mice (P < 0.001, Figure 3A). MiR-130a-3p level was elevated significantly in AD mice transfected with miR-130a-3p agomir compared with that in the AD group (P < 0.01, Figure 3A). After the regulation of miR-130a-3p level in mice, the MWM test was performed to evaluate the role of miR-130a-3p in cognitive impairment. It was found that AD mice needed more escape latency time compared with the WT group (P < 0.001, Figures 3B and 3C), and spent less time in the target quadrant (P < 0.001, Figure 3E). The cognitive function of AD mice transfected with miR-130a-3p was improved significantly, showing less escape latency time (P < 0.01, Figures 3B and C) and more time spent in the target quadrant (P < 0.001, Figure 3E). However, the swimming speed of mice in different groups showed no significant difference (P > 0.05, Figure 3D).
Figure 3.
MiR-130a-3p improves the cognitive function of AD mice. A. MiR-130a-3p levels in the hippocampus tissues of mice in different groups. B. The escape latency of mice during the training days. C. The escape latency of mice in different groups on the fifth day. D. Swimming speed of mice in different groups. E. Time spent in the target quadrant of mice in different groups. ** P < 0.01, *** P < 0.001, compared with WT group; && P < 0.01, &&& P < 0.001, compared with APP/PS1 group.
DAPK1 Is the Target Gene of MiR-130a-3p
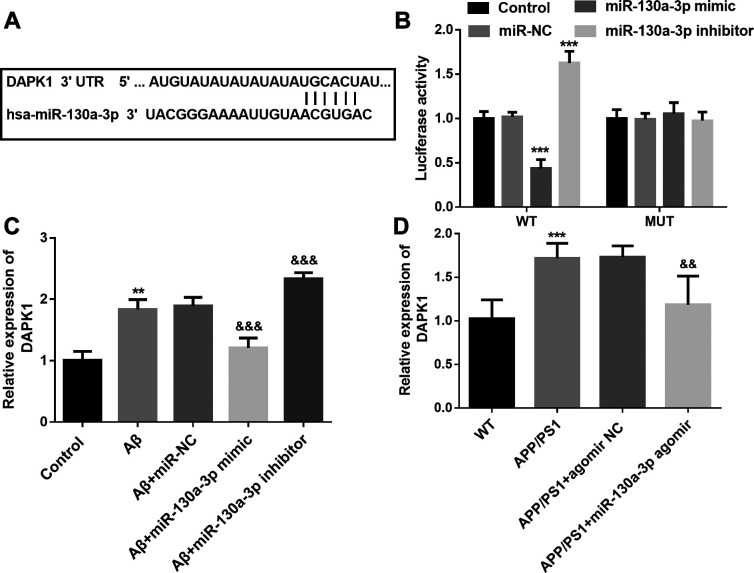
The target gene of miR-130a-3p was predicted by TargetScan online tool, as shown in Figure 4A, the complementary sequences of miR-130a-3p was identified in the 3’-UTR of DAPK1. Then the target relationship was verified by the luciferase reporter assay. The rescue experiments demonstrated that overexpression of miR-130a-3p reduced the luciferase activity in cells transfected with wild type 3’-UTR of DAPK1, whereas downregulation of miR-130a-3p increased the luciferase activity (Figure 4B). In Aβ treated SH-SY5Y cells, a high level of DAPK1 mRNA was detected, and miR-130a-3p mimic transfection reduced the level of DAPK1 mRNA, which was increased by miR-130a-3p inhibitor transfection (P < 0.01, Figure 4C). Similarly, the elevated level of DAPK1 mRNA was also detected in the hippocampus tissues of AD mice, and the rescue experiments results demonstrated that up-regulation of miR-130a-3p led to the decrease of DAPK1 mRNA level (Figure 4D).
Figure 4.
DAPK1 is the target gene of miR-130a-3p. A. Complementary sequences of miR-130a-3p in the 3’-UTR of DAPK1. B. The luciferase activity of cells in different groups. (*** P < 0.001, compared with control group.) C. DAPK1 mRNA levels in SH-SY5Y cells under different treatment. (** P < 0.01, compared with control group; && P < 0.01, compared with Aβ group.) D. DAPK1 mRNA levels in the hippocampus tissues of mice in different groups. (*** P < 0.001, compared with WT group; && P < 0.01, compared with APP-PS1 group).
Discussion
Neurodegenerative diseases are caused by structural or functional damage to neurons in the brain and spinal cord. 16 AD is a persistent neurological disorder characterized by deposition of a protein in brain tissue, neurofibrillary tangles, and diffuse cortical atrophy. Some studies have found that the abnormal expression of miRNAs is closely related to the occurrence and development of AD. Aβ, the main component of senile plaques in AD brains, is formed by cleavage of amyloid precursor protein (APP), and not easily degraded by γ-secretase. 17 The establishment of a good AD model is helpful to further study the pathogenesis and treatment of AD at the cellular and molecular level, because Aβ can simulate AD damage and is often used to induce cellular response to build AD models. 18 Aβ 1-42 induced neuronal cells have been widely used to construct AD cell models. 19,20 Therefore, in the present study, SH-SY5Y cells were treated with Aβ1-42 to establish the AD cell model. And it was found that the expression level of miR-130a-3p decreased gradually with the increase of Aβ1-42 concentration and treatment time. It was concluded that miR-130a-3p might play a potential role in the progression of AD.
Recent research in the field of oncology has focus on the concept of miRNAs, and a large amount of miRNAs have been identified to play a crucial role in the occurrence and development of a variety of tumor diseases. 21,22 In recent years, it has been found that miRNAs are closely related to the development and function of the nervous system, and a variety of miRNAs may be involved in the pathogenesis and progression of AD, which is related to early diagnosis. 23,24 In the present study, low expression of miR-130a-3p was observed in the neuron cells after Aβ1-42 treatment. Consistently, the dysregulation of miR-130a-3p has been identified in several neurodegenerative diseases. 12,13 In Parkinson’s disease, low expression of miR-130a-3p was detected, and overexpression of miR-130a-3p is involved in the neuroprotective role of laminin-511 (LM511)-Yes-associated protein 1 (YAP) signaling against neuron degeneration during Parkinson’s disease. 12 In addition, another study reported the beneficial effect of intermittent fasting against AD, and miR-130a-3p is suggested to be involved in the protective mechanisms. 13 These data all supported our hypothesis that miR-130a-3p might be involved in the development of AD.
Considering the potential role of miR-130a-3p in AD, the AD cell and mice model were constructed, and its role and potential mechanisms were explored through gain and loss function experiments. The cell experiments indicated that Aβ1-42 treatment significantly inhibited neuron cell viability and promoted cell apoptosis, indicating that the AD cell model was established successfully. Furthermore, the gain and loss function experiments demonstrated that overexpression of miR-130a-3p attenuated Aβ 1-42 induced cell viability inhibition and apoptosis, while miR-130a-3p downregulation further aggravated the cell apoptosis. In addition, a decreasing trend of miR-130a-3p level was also detected in the hippocampus of AD mice compared with the wide types. And the rescue experiments results revealed that overexpression of miR-130a-3p improved the learning and cognitive function of AD mice. Consistently, Zhang et al. have reported the downregulated expression of miR-130a-3p in the AD mice compared with wide type mice. 13 MiR-130a-3p treatment is also reported to improve neurological function and alleviate neuronal injury in intracranial hemorrhage (ICH) rats. 25 It was proved that miR-130a-3p has the neuroprotective effect during the progression of AD. Because of the small size, miRNAs can pass the blood-brain barrier (BBB), placental and glomerular filtration barrier and appear in different body fluids. 26 The identification of miR-130a-3p dysregulation may provide new therapeutic strategy for AD.
Mature miRNAs regulate post-transcriptional gene expression by complementing binding to the 3’-untranslated region (3’-UTR) of the target gene, resulting in inhibited mRNA translation. 27 In the current study, the TargetScan analysis showed that miR-130a-3p contains binding sites for Death-associated protein kinase 1 (DAPK1), therefore DAPK1 was identified to be a candidate target gene of miR-130a-3p. Consistently, the targeted relationship between miR-130a-3p and DAPK1 has been reported in previous research. 28 Moreover, the neurotoxic effect of DAPK1 has been identified in AD, a high expression pattern of DAPK1 has been found in both AD patients and mice. 9 Therefore, the involvement of DAPK1 in the protective effect of miR-130a-3p in AD was further investigated in the present study. As expected, the targeted relationship was further proved by luciferase report analysis. DAPK1 is a calcium/calmodulin-dependent serine/threonine kinase, the elevated expression of DAPK1 shows strong correlation with the neuron cell apoptosis in a variety of neurological diseases. 29 In AD patients, high expression of DAPK1 has been found in the hippocampi of patients and the dysregulation of DAPK1 is implicated in the development of AD. 30 Moreover, knockdown of DAPK1 shows cognitive benefits, mice with low level of DAPK1 have improved cognitive function compared with wide type mice. In consideration of the important role of DAPK1 in AD and its target relationship with miR-130a-3p, we speculated that miR-130a-3p might improve the cognitive function in AD via targeting DAPK1.
In conclusion, the present study demonstrated the neuroprotective effect of miR-130a-3p in AD. Overexpression of miR-130a-3p may attenuate Aβ-induced neurotoxicity and improve the cognitive function of mice with AD via targeting DAPK1. These data promote a more comprehensive understanding of the development process of AD. At the same time, study of miRNAs that affect the key genes of AD may bring the potential novel therapeutic approach for human AD.
Footnotes
Authors’ Note: All experiments were performed in compliance with ethical requirements, and under approval of the animal ethics committee of Hangzhou seventh people’s Hospital.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Ci Yan  https://orcid.org/0000-0003-2899-4708
https://orcid.org/0000-0003-2899-4708
References
- 1. Tiepolt S, Patt M, Aghakhanyan G, et al. Current radiotracers to image neurodegenerative diseases. EJNMMI Radiopharm Chem. 2019;4(1):17. doi:10.1186/s41181-019-0070-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Li X, Dai J, Zhao S, Liu W, Li H. Comparison of the value of Mini-Cog and MMSE screening in the rapid identification of Chinese outpatients with mild cognitive impairment. Medicine (Baltimore). 2018;97(22):e10966. doi:10.1097/MD.0000000000010966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Liu W, Sun F, Wan M, et al. Beta-sheet breaker peptide-HPYD for the treatment of Alzheimer’s disease: primary studies on behavioral test and transcriptional profiling. Front Pharmacol. 2018;8:969. doi:10.3389/fphar.2017.00969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bustamante HA, Gonzalez AE, Cerda-Troncoso C, et al. Interplay between the autophagy-lysosomal pathway and the ubiquitin-proteasome system: a target for therapeutic development in Alzheimer’s disease. Front Cell Neurosci. 2018;12:126. doi:10.3389/fncel.2018.00126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cai H, Ning S, Li W, Li X, Xiao S, Sun L. Patient with frontal-variant syndrome in early-onset Alzheimer’s disease. Gen Psychiatr. 2020;33(2):e100173. doi:10.1136/gpsych-2019-100173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen SN, Chang R, Lin LT, et al. MicroRNA in ovarian cancer: biology, pathogenesis, and therapeutic opportunities. Int J Environ Res Public Health. 2019;16(9):1510. doi:10.3390/ijerph16091510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wu X, Sun W, Tan M. Noncoding RNAs in steroid-induced osteonecrosis of the femoral head. Biomed Res Int. 2019;2019:8140595. doi:10.1155/2019/8140595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cho KHT, Xu B, Blenkiron C, Fraser M. Emerging roles of miRNAs in brain development and perinatal brain injury. Front Physiol. 2019;10:227. doi:10.3389/fphys.2019.00227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Deng Y, Zhang J, Sun X, et al. miR-132 improves the cognitive function of rats with Alzheimer’s disease by inhibiting the MAPK1 signal pathway. Exp Ther Med. 2020;20(6):159. doi:10.3892/etm.2020.9288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kumar S, Reddy PH. A new discovery of MicroRNA-455-3p in Alzheimer’s disease. J Alzheimers Dis. 2019;72(suppl 1):S117–S130. doi:10.3233/JAD-190583 [DOI] [PubMed] [Google Scholar]
- 11. Zhao X, Wang S, Sun W. Expression of miR-28-3p in patients with Alzheimer’s disease before and after treatment and its clinical value. Exp Ther Med. 2020;20(3):2218–2226. doi:10.3892/etm.2020.8920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang D, Yang S, Toledo EM, et al. Niche-derived laminin-511 promotes midbrain dopaminergic neuron survival and differentiation through YAP. Sci Signal. 2017;10(493). doi:10.1126/scisignal.aal4165 [DOI] [PubMed] [Google Scholar]
- 13. Greco SJ, Rameshwar P. MicroRNAs regulate synthesis of the neurotransmitter substance P in human mesenchymal stem cell-derived neuronal cells. Proc Natl Acad Sci U S A. 2007;104(39):15484–15489. doi:10.1073/pnas.0703037104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang J, Zhan Z, Li X, et al. Intermittent fasting protects against Alzheimer’s disease possible through restoring aquaporin-4 polarity. Front Mol Neurosci. 2017;10:395. doi:10.3389/fnmol.2017.00395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ji Y, Wang D, Zhang B, Lu H. MiR-361-3p inhibits beta-amyloid accumulation and attenuates cognitive deficits through targeting BACE1 in Alzheimer’s disease. J Integr Neurosci. 2019;18(3):285–291. doi:10.31083/j.jin.2019.03.1136 [DOI] [PubMed] [Google Scholar]
- 16. Michalska P, Leon R. When it comes to an end: oxidative stress crosstalk with protein aggregation and neuroinflammation induce neurodegeneration. Antioxidants (Basel). 2020;9(8):740. doi:10.3390/antiox9080740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chao AC, Lee TC, Juo SH, Yang DI. Hyperglycemia increases the production of amyloid beta-peptide leading to decreased endothelial tight junction. CNS Neurosci Ther. 2016;22(4):291–297. doi:10.1111/cns.12503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sun Y, Liang L, Dong M, Li C, Liu Z, Gao H. Cofilin 2 in serum as a novel biomarker for Alzheimer’s disease in Han Chinese. Front Aging Neurosci. 2019;11:214. doi:10.3389/fnagi.2019.00214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang N, Wang H, Li L, Li Y, Zhang R. Beta-Asarone inhibits amyloid-beta by promoting autophagy in a cell model of Alzheimer’s disease. Front Pharmacol. 2020;10:1529. doi:10.3389/fphar.2019.01529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Krishna KV, Saha RN, Dubey SK. Biophysical, biochemical, and behavioral implications of ApoE3 conjugated donepezil nanomedicine in a abeta1-42 induced Alzheimer’s disease rat model. ACS Chem Neurosci. 2020;11(24):4139–4151. doi:10.1021/acschemneuro.0c00430 [DOI] [PubMed] [Google Scholar]
- 21. Grzywa TM, Klicka K, Rak B, et al. Lineage-dependent role of miR-410-3p as oncomiR in gonadotroph and corticotroph pituitary adenomas or tumor suppressor miR in somatotroph adenomas via MAPK, PTEN/AKT, and STAT3 signaling pathways. Endocrine. 2019;65(3):646–655. doi:10.1007/s12020-019-01960-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Amatruda M, Ippolito G, Vizzuso S, Vizzari G, Banderali G, Verduci E. Epigenetic effects of n-3 LCPUFAs: a role in pediatric metabolic syndrome. Int J Mol Sci. 2019;20(9):2118. doi:10.3390/ijms20092118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liao M, Zou S, Bao Y, et al. Matrix metalloproteinases are regulated by MicroRNA 320 in macrophages and are associated with aortic dissection. Exp Cell Res. 2018;370(1):98–102. doi:10.1016/j.yexcr.2018.06.011 [DOI] [PubMed] [Google Scholar]
- 24. Swarbrick S, Wragg N, Ghosh S, Stolzing A. Systematic review of miRNA as biomarkers in Alzheimer’s disease. Mol Neurobiol. 2019;56(9):6156–6167. doi:10.1007/s12035-019-1500-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang CY, Ren XM, Li HB, et al. Effect of miR-130a on neuronal injury in rats with intracranial hemorrhage through PTEN/PI3K/AKT signaling pathway. Eur Rev Med Pharmacol Sci. 2019;23(11):4890–4897. doi:10.26355/eurrev_201906_18077 [DOI] [PubMed] [Google Scholar]
- 26. Muller-Deile J, Schroder P, Beverly-Staggs L, et al. Overexpression of preeclampsia induced microRNA-26a-5p leads to proteinuria in zebrafish. Sci Rep. 2018;8(1):3621. doi:10.1038/s41598-018-22070-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yu H, Sun J, Jiang S, Xu Y. MicroRNA-490-3p regulates cell proliferation and apoptosis in gastric cancer via direct targeting of AKT1. Exp Ther Med. 2019;17(2):1330–1336. doi:10.3892/etm.2018.7042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Feng M, Zhu X, Zhuo C. H19/miR-130a-3p/DAPK1 axis regulates the pathophysiology of neonatal hypoxic-ischemia encephalopathy. Neurosci Res. 2021;163:52–62. doi:10.1016/j.neures.2020.03.005 [DOI] [PubMed] [Google Scholar]
- 29. Su Y, Deng MF, Xiong W, et al. MicroRNA-26a/death-associated protein kinase 1 signaling induces synucleinopathy and dopaminergic neuron degeneration in Parkinson’s disease. Biol Psychiatry. 2019;85(9):769–781. doi:10.1016/j.biopsych.2018.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen D, Mei Y, Kim N, et al. Melatonin directly binds and inhibits death-associated protein kinase 1 function in Alzheimer’s disease. J Pineal Res. 2020;69(2):e12665. doi:10.1111/jpi.12665 [DOI] [PMC free article] [PubMed] [Google Scholar]