ABSTRACT
The emergence of multidrug-resistant fungal pathogens is a significant concern for global public health. Candida auris poses a considerable threat as a multidrug-resistant fungal pathogen. Our recent study revealed that the adenylyl cyclase Cyr1 and protein kinase A (PKA) pathways play distinct and redundant roles in drug resistance and pathogenicity of C. auris. However, the upstream and negative feedback regulatory mechanisms of C. auris are not yet fully understood. In this study, we discovered that the small GTPase Ras1, along with its nucleotide exchange factor Cdc25 and GTPase-activating protein Ira2, plays a major role in regulating cAMP/PKA-dependent traits, while G-protein-coupled receptor Gpr1 and heterotrimeric G-protein α subunit Gpa2 play a minor role. Pde2 plays a major role in negative feedback regulation of the cAMP/PKA pathway, while Pde1 plays a minor role. Hyperactivation of the Ras/cAMP/PKA pathway by deleting PDE2 or BCY1 renders C. auris cells thermosensitive and susceptible to nutrient deficiency, which leads to attenuated virulence. Our study demonstrates the distinct contributions of hyperactivation of the Ras/cAMP/PKA signaling pathway to C. auris pathogenesis and suggests potential therapeutic targets for C. auris-mediated candidiasis.
IMPORTANCE
Candida auris is a major concern as a multidrug-resistant fungal pathogen. While our previous studies highlighted the crucial roles of the cAMP/protein kinase A (PKA) pathway in regulating drug resistance, stress responses, morphogenesis, ploidy change, biofilm formation, and pathogenicity in this pathogen, their regulatory mechanism remains unclear. In our study, we provided evidence that the cAMP/PKA signaling pathway in C. auris is primarily governed by the small GTPase RAS rather than a G-protein-coupled receptor. Additionally, we discovered that the negative feedback regulation of cAMP, controlled by phosphodiesterases, is vital for C. auris virulence by promoting resistance to high temperatures and nutrient deficiencies. These findings underscore the diverse pathobiological significance of the Ras/cAMP/PKA signaling pathway in C. auris, shedding light on potential therapeutic targets and strategies for combating this multidrug-resistant fungal pathogen.
KEYWORDS: C. auris, Ras1, Cdc25, Ira1, Gpr1, Gpa2, Pde1, Pde2, biofilm, glycogen, nutrient starvation, virulence
INTRODUCTION
The emergence of multidrug-resistant pathogenic fungi is a significant threat to global public health, with over 150 million people experiencing severe fungal infections annually, resulting in approximately 1.5 million fatalities (1). Candidiasis alone affects about 9 million people and continues to increase (2). Candida auris, a multidrug-resistant pathogenic fungus, has been causing candidemia in healthcare environments worldwide since 2009 (3). It displays high resistance to current antifungal drugs, particularly azoles, with over 90% of strains in the United States being resistant to fluconazole (FCZ) (4). Therefore, there is a pressing need for novel therapeutic strategies to combat this pathogenic fungus.
The clinical symptoms of invasive C. auris infection are similar to those caused by other invasive Candida species, making it difficult to distinguish between them (5). The primary treatment for candidiasis caused by C. auris involves using caspofungin, micafungin, and anidulafungin due to their relatively low resistance rates in the echinocandin class of antifungal drugs (6). Secondary therapy may include amphotericin B (AMB), itraconazole, and posaconazole (PCZ), depending on the patient’s condition. The mortality rate associated with C. auris infection is estimated to be between 30% and 60% (7). Thus, the Centers for Disease Control and Prevention (CDC) has assigned a higher threat level to C. auris owing to its significant antifungal drug resistance. Furthermore, the prevalence of C. auris co-infection in immunocompromised COVID-19 patients is increasing (8). Therefore, there is a pressing need to comprehensively understand the mechanisms underlying antifungal drug resistance and the pathogenicity of C. auris.
The cAMP pathway is a well-known signaling network that significantly contributes to the growth, drug resistance, and virulence of various pathogenic fungi (9). It is activated by two upstream signaling branches, depending on the fungal species. One involves a G-protein-coupled receptor (GPCR) that undergoes a conformation change in response to carbohydrates and amino acids, releasing the active α subunit of the heterotrimer G-protein complex which then activates adenylyl cyclase (10 – 12). The second branch involves small GTPase RAS, which is a membrane-bound protein that undergoes lipid modification and is positively activated by guanine nucleotide exchange factor (GEF) and negatively regulated by GTPase-activating protein (GAP) (13). Upon activation, adenylyl cyclase catalyzes the conversion of ATP to cAMP, which binds to the regulatory subunit of protein kinase A (PKA), releasing its catalytic subunits. These catalytic subunits then translocate to the nucleus and activate a cascade of downstream transcription factors (14).
To maintain cAMP homeostasis and prevent overaccumulation, cyclic nucleotide phosphodiesterases (PDEs) play a crucial role. There are two types of PDEs: Pde1 and Pde2, which convert cAMP to AMP with low and high affinity, respectively (15, 16). In most pathogenic fungi, inhibition of the Ras/cAMP/PKA pathway reduces virulence, while hyperactivation of the cAMP/PKA pathway attenuates virulence in some cases (17 – 20). Thus, balanced modulation of the cAMP/PKA pathway is crucial for fungal pathogenicity.
Our previous research showed that adenylyl cyclase (Cyr1) and PKA subunits (Bcy1 regulatory subunit and Tpk1/2 catalytic subunits) play redundant and distinct roles in various aspects of C. auris, including growth, stress response, drug and disinfectant resistance, pseudohyphae and biofilm formation, ploidy switch, and virulence (21). Interestingly, we discovered that Tpk1/2 in C. auris has both Cyr1-dependent and independent functions, distinguishing it from other pathogenic fungi. Furthermore, we discovered that hyperactivation of the cAMP/PKA pathway (by BCY1 deletion), rather than its inactivation (by CYR1 or TPK1/2 deletion), reduces virulence in C. auris (21). However, the upstream signaling branches and negative feedback regulatory systems of the Ras/cAMP/PKA pathways in C. auris are still unknown.
In this study, our primary objective was to determine the upstream signaling branch that governs the cAMP/PKA pathway in C. auris. To achieve this, we investigated two potential upstream candidates, GPCR and RAS, which are well-known regulators of fungal adenylyl cyclases, using reverse genetic approaches. Additionally, we aimed to unravel the PDE-dependent negative feedback regulatory mechanism of the cAMP/PKA pathway and evaluate its significance in the pathobiology of this fungal pathogen. By addressing these objectives, our study provides a comprehensive understanding of the regulatory mechanisms and pathobiological functions associated with the cAMP/PKA pathway in C. auris. These findings promise to develop innovative therapeutic strategies targeting this pathway for treating C. auris-mediated candidiasis.
RESULTS
Ras1 is the primary upstream regulator of the cAMP/PKA pathway
To determine potential upstream signaling branches of the cAMP/PKA pathway in C. auris, we searched its genome database for orthologous proteins that may be involved in GPCR and RAS signaling branches. Using this approach, we identified C. auris proteins with the highest orthology to Gpr1, Gpa2, and Ras1 in other Candida species: Gpr1 (B9J08_002066), Gpa2 (B9J08_002635), and Ras1 (B9J08_003446) (Fig. S1). These C. auris proteins all contain conserved domains that are typical of GPCR, Gα, or Ras proteins, as determined by a conserved protein domain search using InterPro analysis (https://www.ebi.ac.uk/interpro/) (Fig. S1). To investigate the functional roles of these genes, we generated deletion mutants for RAS1, GPR1, and GPA2 in the clade I wild-type strain (B8441) background using a split-gene disruption cassette containing the nourseothricin resistance marker (CaNAT) and heat shock transformation method (Fig. S2). We also generated complemented strains to verify the phenotypic traits of the deletion mutants. These complemented strains were constructed by reintegrating the wild-type allele into its native locus (gpr1Δ::GPR1) or through ectopic integration (ras1Δ + RAS1, gpa2Δ + GPA2). These complemented strains exhibited a fully restored wild-type phenotype (Fig. S2). Additionally, we constructed ras1Δ gpa2Δ double deletion mutants to investigate whether the RAS and GPCR branches activate Cyr1 independently (Fig. S2).
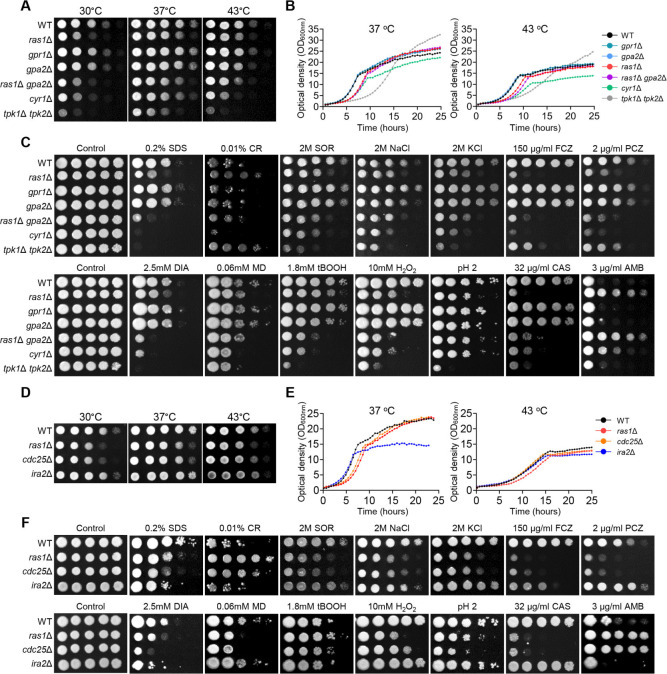
We conducted a comprehensive analysis of phenotypic traits in constructed mutants of Ras1, Gpr1, and Gpa2 in comparison to mutants of adenylyl cyclase (cyr1Δ) and PKA catalytic subunit (tpk1Δ tpk2Δ) in C. auris. We measured the growth rate of all mutants qualitatively and quantitatively at various temperatures (Fig. 1A and B ). The ras1Δ mutant showed a growth defect that was less severe than that of cyr1Δ, while gpr1Δ and gpa2Δ mutants displayed growth rates similar to that of the wild type at all tested temperatures. The ras1Δ gpa2Δ double mutant had a slightly more severe growth defect than every single mutant at 43°C, although not as severe as cyr1Δ (Fig. 1A and B). Regarding the stress response, ras1Δ and cyr1Δ exhibited similar susceptibility patterns to most stress conditions. Intriguingly, they displayed heightened susceptibility to FCZ, PCZ, and caspofungin, while exhibiting increased tolerance to AMB compared to the wild-type strain. However, they showed contrasting responses to congo red and low pH stress (Fig. 1C). These results suggest that Ras may regulate certain signaling pathways independent of Cyr1. Meanwhile, gpr1Δ and gpa2Δ were almost indistinguishable from the wild type under all tested stress conditions, while ras1Δ gpa2Δ was slightly more susceptible to diamide (DIA) and H2O2 than ras1Δ (Fig. 1C). Overall, our results suggest that Ras1 serves as a primary upstream regulator of Cyr1, while GPCR and other undefined upstream branches serve as secondary Cyr1 regulators in C. auris.
Fig 1.
The role of the Ras1/cAMP/PKA signaling pathway in the growth and stress response of C. auris. (A and D) Qualitative spot assays for measuring the growth and thermotolerance of the C. auris wild type and mutants. (B and E) Quantitative growth rates of wild type and mutants. (C and F) Spot analysis of the C. auris wild type and mutants under various stress-inducing conditions, including sodium dodecyl sulfate (SDS), congo red (CR), sorbitol (SOR), sodium chloride (NaCl), potassium chloride (KCl), FCZ, PCZ, DIA, menadione (MD), tert-butyl hydroperoxide (tBOOH), hydrogen peroxide (H2O2), acidic pH, caspofungin (CAS), and AMB.
Ras1 is positively and negatively regulated by Cdc25 and Ira2
Ras1 is regulated by GEF and GAP in Saccharomyces cerevisiae, where GEF (Cdc25) activates Ras1 by stimulating GDP release and GTP binding, and GAP (Ira1/2) regulates the intrinsic GTPase activity of RAS (22, 23). In C. auris, we found orthologs of Cdc25 and Ira1/2, with B9J08_003305 and B9J08_003924 being orthologous to Cdc25 and Ira2, respectively (Fig. S1). Candida auris Cdc25 contains the Ras-GEF, while Ira2 has Ras-GAP domains (Fig. S1). To investigate the functional roles of Cdc25 and Ira2, we generated cdc25Δ and ira2Δ mutants in C. auris (Fig. S2). Furthermore, we constructed complemented strains and verified the restoration of wild-type phenotypic characteristics (Fig. S2).
We compared the phenotypic traits of cdc25Δ and ira2Δ mutants with those of the ras1Δ mutant. As expected, ras1Δ and cdc25Δ mutants showed similar growth defects at varying temperatures, with similar growth patterns observed in qualitative spot assays at 30°C–43°C (Fig. 1D). The quantitative growth rate measurement showed that ras1Δ and cdc25Δ entered the stationary phase approximately 2 h later than the wild type at 37° (Fig. 1E). Additionally, cdc25Δ showed susceptibility to stress-inducing agents similar to that of ras1Δ (Fig. 1F). These results indicate that Cdc25 is the positive regulator of Ras in C. auris. However, IRA2 deletion did not lead to discernable phenotypic variation, except for increased sensitivity to SDS, FCZ, and DIA, compared to the wild type (Fig. 1D through F).
Roles of Pde1 and Pde2 for the negative feedback regulation of the cAMP/PKA pathway
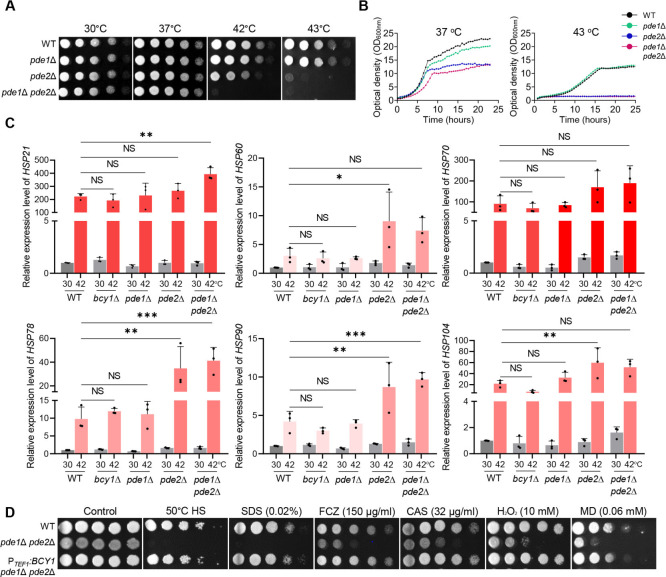
The cAMP/PKA pathway is negatively regulated by low-affinity and high-affinity PDEs, Pde1 and Pde2, which can degrade cAMP to AMP (15). Lack of PDEs can cause cell toxicity due to hyperaccumulation of intracellular cAMP (24 – 28). In the C. auris genome database, we found Pde1 (B9J08_002549) and Pde2 (B9J08_003265) orthologs (Fig. S1). To investigate the roles of Pde1 and Pde2 in regulating the cAMP/PKA pathway, we generated pde1Δ, pde2Δ, and pde1Δ pde2Δ mutants and analyzed their phenotypic traits (Fig. S2). We additionally constructed complemented strains and validated the restoration of the wild-type phenotype (Fig. S2). Previous studies have reported that PDE2 deletion decreases thermotolerance in S. cerevisiae and Candida albicans, whereas PDE1 deletion has no effect (25, 26). Consistent with these findings, we observed that pde1Δ was as thermotolerant as the wild type, whereas pde2Δ showed markedly decreased thermotolerance compared to the wild type (Fig. 2A and B). The pde1Δ pde2Δ mutant was even more defective in thermotolerance than pde2Δ (Fig. 2A). Moreover, we found that pde2Δ and pde1Δ pde2Δ mutants were unable to grow at 43°C (Fig. 2B). We confirmed that heat shock at 42°C for 2 h increased PDE1 and PDE2 expression relative to the basal condition of 30°C (Fig. S3). We also measured the ability of the mutants to recover from heat stress (at 50°C and 53°C for 10 min) and found that pde1Δ recovered to a similar extent as the wild type, whereas pde2Δ failed to recover properly. The pde1Δ pde2Δ mutant recovered more poorly than pde2Δ (Fig. S3). The heat shock-sensitive phenotype of pde2Δ was similar to that of bcy1Δ, which has a hyperactivated cAMP/PKA pathway (Fig. S3).
Fig 2.
The role of PDE in thermotolerance of C. auris. (A) Qualitative spot assay and (B) quantitative growth rate measurement of the C. auris wild type and mutants. (C) Quantitative reverse transcription-PCR (RT-PCR) analysis of thermotolerance-related genes in wild type and mutants. Statistical analysis was performed using one-way analysis of variance with Bonferroni’s multiple-comparison tests (*P < 0.05, **P < 0.01, and ***P < 0.001 ). (D) Spot analysis of the wild type and mutants under various stress-inducing conditions.
We next investigated whether PDEs regulate the expression levels of major heat shock proteins involved in thermotolerance. Under heat shock conditions, the expression of HSP genes was more strongly induced in pde2Δ and pde1Δ pde2Δ mutants than in the wild type or bcy1Δ and pde1Δ strains. The expression of HSP60, HSP78, and HSP90 was induced by approximately threefold to fourfold in pde2Δ and pde1Δ pde2Δ mutant strains relative to wild type or bcy1Δ and pde1Δ strains (Fig. 2C), suggesting that the increased thermosensitivity of PDE mutants may be compensated by induction of HSP genes.
To confirm whether Bcy1 acts downstream of PDEs, we overexpressed BCY1 in the pde1Δ pde2Δ background using the constitutively active TEF1 promoter (Fig. S3). BCY1 overexpression restored thermotolerance and several other stress-resistant phenotypes in pde1Δ pde2Δ (Fig. 2D), suggesting that Pde1 and Pde2 play minor and major roles, respectively, in negatively regulating the Ras/cAMP/PKA signaling pathway, and their deletion can decrease the thermotolerance of C. auris.
Hyperactivated cAMP/PKA pathway decreases the survival rate of C. auris under nutrient-starved conditions
A hallmark of fungal cells with a hyperactivated cAMP/PKA pathway is a reduction in intracellular glycogen levels (29, 30). Glycogen is crucial as a carbon and energy source and serves as the primary reservoir of glucose in yeast cells (31). Pathogenic fungi typically reside in nutrient-poor environments, such as the host’s skin surface and organs, where the conversion of glucose into glycogen and its storage are essential for their long-term survival (32).
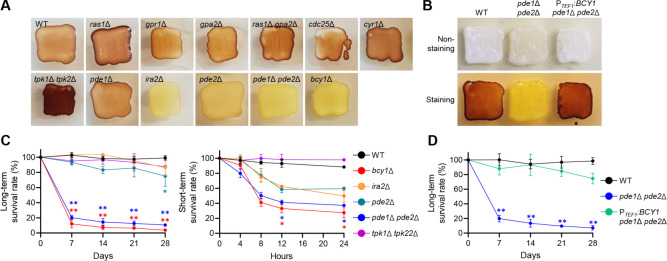
To evaluate glycogen accumulation, we streaked wild type and mutant strains onto solid yeast extract-peptone-dextrose (YPD) media and incubated them at 30°C for 2 days, followed by staining the colonies with an iodine/iodide solution (Fig. 3A). The Ras/cAMP/PKA inactivated mutants generally exhibited darker brown staining than the wild type, with tpk1Δ tpk2Δ exhibiting the darkest brown staining. Conversely, Ras/cAMP/PKA hyperactivated mutants, such as bcy1Δ, ira2Δ, pde2Δ, and pde1Δ pde2Δ, did not show brown staining (Fig. 3A). Additionally, BCY1 overexpression restored wild-type levels of intracellular glycogen in pde1Δ pde2Δ (Fig. 3B). These results suggest that the hyperactivation of the Ras/cAMP/PKA pathway in C. auris reduced glycogen accumulation.
Fig 3.
The role of Ras/cAMP/PKA signaling pathway in nutritional susceptibility of C. auris. (A and B) Glycogen accumulation in wild type and Ras/cAMP/PKA mutants. (C and D) The survival rate of C. auris wild type and mutants in nutrient starvation conditions. Statistical analysis was performed using one-way ANOVA with Bonferroni’s multiple-comparison test (*P < 0.05 and **P < 0.01).
We then assessed how altered glycogen accumulation levels affect the survival rate of C. auris under nutrient-starved conditions. To measure the long-term survival rate, we spotted wild-type and mutant strains onto solid nutrient-deprived media (yeast nitrogen base [YNB] without ammonium sulfate and amino acid) and incubated plates at 25°C for 28 days, assessing viability every 7 days. The survival rates of bcy1Δ and pde1Δ pde2Δ mutants declined sharply in the first 7 days, followed by a gradual decline over the next 21 days, with only 3.7% of bcy1Δ and 10.5% of pde1Δ pde2Δ mutants surviving at the end of the 28-day incubation period (Fig. 3C). The pde2Δ mutant showed a significant decrease, with only 75% of the strains surviving, while the ira2Δ mutant showed no statistically significant decrease with 87% of the strains surviving (Fig. 3C). For short-term survival rate measurement, wild type and each mutant were grown to the stationary phase in liquid nutrient-deprived medium at 30°C for 2 days and subsequently resuspended in phosphate-buffered saline (PBS). The results indicated that bcy1Δ and pde1Δ pde2Δ mutants were more vulnerable to nutrient deprivation than both wild-type and other mutant strains (Fig. 3C). Following inoculation in PBS, bcy1Δ, and pde1Δ pde2Δ mutants exhibited a rapid decline in survival rates, with only 27.4% and 37.2% of the strains surviving after 24 h, respectively (Fig. 3C). The survival rates of pde2Δ and ira2Δ mutants were 59.2% and 49.7%, respectively, after 24-h incubation in PBS, but the decrease was not statistically significant (Fig. 3C). BCY1 overexpression completely restored the wild-type survival rate in pde1Δ pde2Δ under nutrient-starved conditions (Fig. 3D), consistent with the data shown in Fig. 3B. These findings suggest that the Ras/cAMP/PKA signaling pathway plays a crucial role in regulating intracellular glycogen accumulation and sensitivity to nutrient starvation in C. auris.
Hyperactivation of the Ras/cAMP/PKA signaling pathway leads to increased biofilm formation ability of C. auris
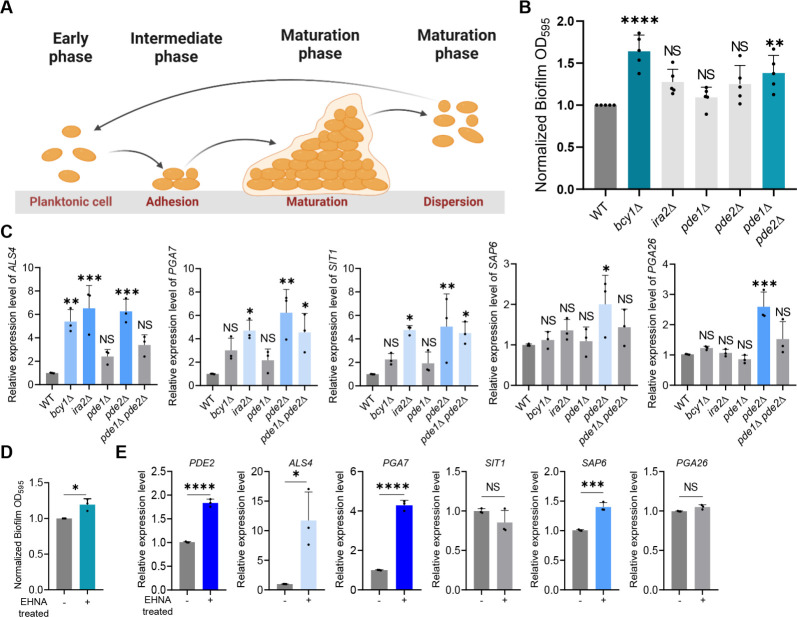
Biofilm formation is a crucial component of virulence for Candida and other pathogenic fungi, as it confers resistance to antifungal medications and environmental stresses (33 – 35). The process of biofilm formation involves the adhesion of planktonic cells to solid surfaces, followed by biofilm maturation and dispersal of cells to form new biofilms (Fig. 4A). Previous research has shown that the bcy1Δ mutant has an enhanced capacity for biofilm formation relative to the wild type, whereas the tpk1Δ tpk2Δ mutant has a decreased biofilm capacity (21). We found that pde1Δ pde2Δ exhibited increased biofilm formation ability compared to the wild type (Fig. 4B), suggesting that the Ras/cAMP/PKA pathway promotes the biofilm formation in C. auris.
Fig 4.
The role of Ras/cAMP/PKA signaling pathway in biofilm formation of C. auris. (A) Schematic representation of biofilm development phases in Candida species. (B) Biofilm formation assay of C. auris wild type and mutants. (C) qRT-PCR analysis of cell adhesion-related genes in wild type and mutants. (B and C) Statistical analysis was performed using one-way ANOVA with Bonferroni’s multiple-comparison test (*P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001). (D) Biofilm formation assay of C. auris wild type with EHNA [erythro-9-(2-hydroxy-3-nonly)adenine] treatment. (E) qRT-PCR analysis of cell adhesion-related genes in wild type with EHNA treatment. (D and E) Statistical analysis was performed using Student’s t test (*P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001).
We also investigated the expression of genes involved in cell adhesion, which is the initial stage of biofilm formation, namely ALS4, PGA7, SIT1, SAP6, and PGA26 (36 – 39). Our results showed that ALS4 was upregulated in bcy1Δ, ira2Δ, and pde2Δ, while PGA7 and SIT1 were significantly increased in ira2Δ, pde2Δ, and pde1Δ pde2Δ (Fig. 4C). We observed an antagonistic effect of Pde1 and Pde2, as the expression levels of ALS4, PGA7, and SIT1 were consistently increased in pde2Δ but only slightly increased in pde1Δ with no statistical significance, leading to a relatively lower expression in pde1Δ pde2Δ compared to pde2Δ (Fig. 4C). However, the expression levels of SAP6 and PGA26, which act as negative regulators, were enhanced in pde2Δ, which could explain why pde2Δ formed less biofilm than pde1Δ pde2Δ and other mutants. Our findings suggest that cell adhesion, promoted by the Ras/cAMP/PKA pathway, is critical for the biofilm formation in C. auris.
To further explore the effect of PDE2 inhibition on biofilm formation, we treated the wild-type strain of C. auris with the PDE2 inhibitor EHNA [erythro-9-(2-hydroxy-3-nonyl)adenine]. We found that treatment with 50 µM EHNA resulted in a 20% increase in biofilm formation compared to the nontreated wild-type control group (Fig. 4D). The expression of PDE2 increased by 60% upon treatment with EHNA, indicating a compensating response to the inhibitor (Fig. 4E). The expression levels of genes involved in cell adhesion were also altered, with the positive regulator ALS4 increasing approximately 10-fold and PGA7 increasing approximately fourfold compared to the nontreated wild-type group (Fig. 4E). However, SIT1 did not show a significant difference, and the negative regulator SAP6 increased by approximately 1.4-fold, while PGA26 did not show a statistical difference (Fig. 4E). Our results suggest that hyperactivation of the Ras/cAMP/PKA pathway in C. auris can increase the expression levels of multiple genes associated with biofilm formation, leading to enhancing biofilm formation in C. auris.
Hyperactivation of the Ras/cAMP/PKA signaling pathway attenuates the virulence of C. auris
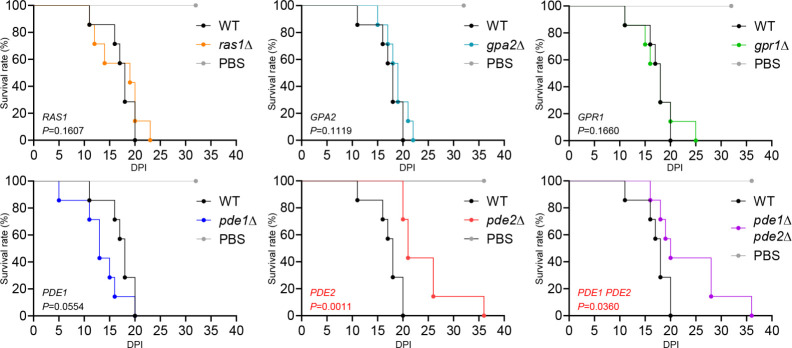
We established that disrupting the PKA regulatory subunit Bcy1 attenuates C. auris virulence, while disrupting the adenylate cyclase Cyr1 and PKA catalytic subunits Tpk1/2 does not (21). This suggests that the hyperactivated Ras/cAMP/PKA pathway can reduce C. auris virulence. We hypothesized that disrupting Pde1 and Pde2 would lead to decreased virulence, while disrupting Ras1, Gpa2, and Gpr1 would not affect C. auris virulence. The virulence of ras1Δ, gpa2Δ, gpr1Δ, and pde1Δ was similar to that of the wild-type strain, with mice inoculated with these strains dying mostly within 20–25 days of infection (Fig. 5). However, pde2Δ and pde1Δ pde2Δ exhibited reduced virulence compared to the wild type, with mice inoculated with these mutants surviving up to 16 days longer than those inoculated with the wild type (Fig. 5). These results support our hypothesis that hyperactivated Ras/cAMP/PKA pathway attenuates C. auris virulence.
Fig 5.
The role of Ras/cAMP/PKA signaling pathway in pathogenicity of C. auris. Female BALB/c mice (7-week-old) were injected intravenously with 1 × 107 cells of strains of C. auris wild type and mutants. Survival was monitored daily, and statistical analysis was performed with Log-rank (Mantel-Cox) test by using Prism 8.0.
DISCUSSION
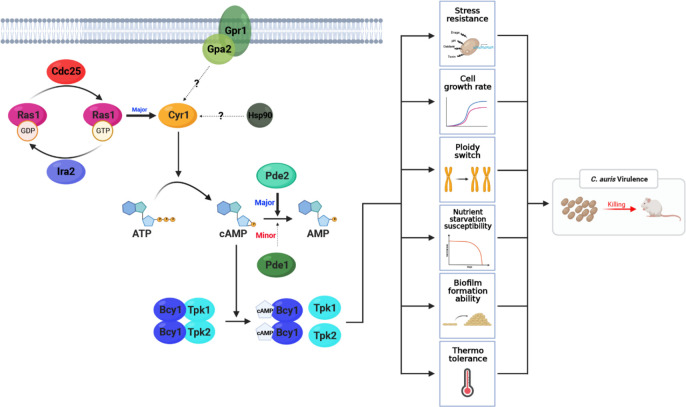
In this study, we investigated the upstream signaling and negative feedback regulatory networks of the cAMP/PKA pathway required for the pathobiological process of C. auris. Our results demonstrate that the RAS signaling branch, consisting of Ras1, Cdc25, and Ira2, is a primary upstream regulator of the adenylyl cyclase Cyr1 in C. auris. In contrast, GPCR and other undefined signaling branches play minor roles. Regarding homeostatic regulation of the Ras/cAMP/PKA pathway, Pde2 and Pde1 are the primary and secondary regulators, respectively, that hydrolyze cAMP (Fig. 6). Furthermore, our findings indicate that hyperactivation of the Ras/cAMP/PKA signaling pathway can decrease the virulence of C. auris, while inactivation of the pathway only affects drug resistance and has no direct effect on virulence.
Fig 6.
Proposed pathobiological functions and regulatory mechanisms of the Ras/cAMP/PKA signaling pathway in C. auris. The principal regulation of Cyr1 is conducted by Ras1, while cAMP degradation is handled by Pde1 and Pde2, serving minor and major roles, respectively. The Ras/cAMP/PKA pathway influences several phenotypes, including stress resistance, cell growth rate, ploidy change, nutrient starvation susceptibility, biofilm formation, and thermotolerance. These combined effects contribute to the virulence of C. auris. Notably, the inhibition of Bcy1 and Pde2 significantly heightens susceptibility to nutrient starvation and high temperature, underscoring their crucial role in C. auris pathogenicity.
Fungal adenylyl cyclases contain multiple protein-interaction domains, including the Ras-associating and Gα-binding domains, and are primarily regulated by the GPCR and RAS signaling branches. Adenylyl cyclases in C. albicans, S. cerevisiae, and Aspergillus fumigatus contain both domains and are activated by GPCRs and Ras (Fig. S4). However, in Cryptococcus neoformans, the adenylyl cyclase Cac1 lacks the Ras-associating domain and is solely activated by the GPCR signaling branch (Fig. S4). In this study, we found that Cyr1 in C. auris is mainly activated by Ras1. Supporting it, we observed that C. auris Cyr1 lacks a Gα-binding domain, suggesting that it may not sense signals through GPCRs and Gα subunits (Fig. S4). As a result, ras1Δ and cyr1Δ displayed similar phenotypes, while gpr1Δ and gpa2Δ were phenotypically distinct from cyr1Δ. The divergent upstream signaling branches of the cAMP/PKA pathways among fungi may reflect their need to respond and adapt to corresponding biological niches. In C. albicans, known external cues for Ras1 activation include serum, amino acids, and N-acetyl glucosamine (40, 41). Although our data indicate that C. auris Ras1 may sense various stress signals, the exact external cues remain to be elucidated in future studies.
Our study suggests the presence of an alternative pathway that activates Cyr1 in C. auris, which is distinct from Ras and GPCR signaling. This is evident from our observation that cyr1Δ mutants exhibit more severe phenotypes than ras1Δ gpa2Δ double mutants. One possible candidate for this alternative pathway is an Hsp90-dependent signaling pathway. In C. albicans, the leucine-rich domain of Cyr1 interacts with Hsp90 to regulate temperature-dependent hyphal growth (41). Considering that C. auris Cyr1 also possesses this domain, it is reasonable to hypothesize that Hsp90 may serve as an additional upstream regulator of Cyr1 in C. auris. Supporting this idea, we found that cyr1Δ mutants were more sensitive to high temperature and heat shock than ras1Δ gpa2Δ. Another potential candidate includes other Ras-like small GTPases in C. auris. Our analysis of the C. auris genome revealed the presence of more than 20 small GTPases. Their role in the cAMP/PKA pathway should be further investigated in future studies.
The cAMP/PKA pathway in fungi requires negative feedback regulation to maintain biological balance. PDEs, which can hydrolyze cAMP to AMP, are key negative feedback regulators of the cAMP pathway in many fungal species. Ascomyctoa fungi, including C. albicans, S. cerevisiae, and Aspergillus flavus, primarily employ high-affinity PDEs (15, 26, 28). However, some Basidiomycota fungi, such as C. neoformans, use low-affinity PDEs as their primary regulator (27). In this study, we show that C. auris, an Ascomycota fungus, primarily employs high-affinity PDE (Pde2) as the primary negative regulator of the cAMP/PKA pathway, while low-affinity PDE (Pde1) plays a secondary role. Notably, Ustilago maydis, a Basidiomycota fungus, uses both high-affinity (Umpde1) and low-affinity (Umpde2) PDEs to regulate various biological processes, with Umpde1 being primarily required for virulence (42). Interestingly, heterologous expression of Umpde1 in pde1Δ mutants of C. neoformans and pde1Δ pde2Δ mutants of S. cerevisiae can restore their wild-type phenotypes (42), suggesting redundant roles for high-affinity and low-affinity PDEs across diverse fungal lineages. However, our study also revealed antagonistic actions between Pde1 and Pde2 in regulating the expression of some biofilm-related genes, such as ALS4, SAP6, and PGA26, in C. auris. Therefore, further investigation is needed to elucidate the redundant and distinct roles of these two PDEs in C. auris.
Our study revealed that hyperactivation of the Ras/cAMP/PKA pathway has both positive and negative effects on the pathogenicity of C. auris. Deletion of Bcy1, Ira2, Pde1, and Pde2 led to decreased glycogen accumulation and reduced survival under nutrient-starved conditions, which may reduce the virulence of C. auris. Previous studies have demonstrated that deletion of PDEs in C. albicans leads to impaired glycogen accumulation, reduced survival rates under nutrient-starved conditions, and attenuated virulence (29). A similar observation was also made in A. flavus (28). The mammalian host environment is characterized by nutrient scarcity, affecting the competitive fitness of a pathogen with an impaired accumulation of glycogen as a carbon source. In addition, the thermotolerance of bcy1Δ, pde2Δ, and pde1Δ pde2Δ mutants was significantly reduced, which may also decrease the virulence. In contrast, hyperactivation of the Ras/cAMP/PKA pathway by deletion of Bcy1, Ira2, Pde1, or Pde2 increased biofilm formation, which could enhance the virulence of C. auris, because cells in biofilm could be more tolerant to the host immune system (43). Therefore, these compounding effects by hyperactivation of the Ras/cAMP/PKA pathway may lead to the less pronounced virulence attenuation in pde2Δ and pde1Δ pde2Δ mutants.
In conclusion, we have comprehensively analyzed the Ras/cAMP/PKA pathway and established its role in various aspects of C. auris pathobiology. We have demonstrated that hyperactivation of this pathway is linked to key virulence factors, such as thermotolerance, biofilm formation, and nutrient susceptibility. Although the specific functions of the Ras/cAMP/PKA pathway may vary depending on the clade, its importance in the various pathobiological functions of C. auris is widely recognized. Therefore, both aspects of this pathway must be taken into account when developing treatments for patients infected with C. auris. One promising approach is to combine an Ras/cAMP/PKA pathway inhibitor with existing antifungal drugs. Although inactivation of this pathway may increase resistance to AMB, it can significantly reduce resistance to other drugs such as azoles or echinocandins. Therefore, combination therapy with a PKA inhibitor is expected to be highly effective in treating C. auris infections. A second strategy involves using a Pde2 inhibitor to hyperactivate the Ras/cAMP/PKA pathway. Although it may lead to increased biofilm formation, it could significantly reduce thermotolerance and glycogen accumulation, resulting in decreased virulence in the host. By considering both of these methods, we can more effectively treat patients infected with C. auris in the future.
MATERIALS AND METHODS
Candida auris strains and growth media
Candida auris strains used in this study are listed in Table S1 in the supplemental material. The parental wild-type strain, B8441 (AR0387), was obtained from the CDC. These isolates and the constructed mutant strains were stored as frozen stocks in 20% glycerol at −80°C until further use. Yeast strains were routinely cultured on YPD agar plates (2% agar in YPD broth: 1% yeast extract, 2% peptone, and 2% D-glucose) at 30°C for 24–48 h. For liquid cultures, cells were grown in YPD broth at 30°C with shaking at 200 rpm. For experimental assays, cells were inoculated into fresh YPD broth and grown to mid-log phase (optical density at 600 nm [OD600] = 0.6–0.8) before being subjected to various treatments.
Gene deletion and complementation
To generate gene deletion mutants, we used the nourseothricin resistance marker (CaNAT), hygromycin B resistance marker (CaHYG), or G481 resistance marker (CaNEO) flanked by 0.5–1.0 kb 5′ and 3′ regions of each target gene, including RAS1, GPR1, GPA2, CDC25, IRA2, PDE1, and PDE2. Each gene disruption cassette containing a selection marker was constructed using double-joint PCR. To amplify the flanking regions of a target gene, we used L1-L2, and R1-R2 primer pairs in the first round of PCR. The CaNAT selection marker was amplified by PCR using the plasmid pV1025 containing the CaNAT gene as a template and the primer pairs listed in Table S1 in the supplemental material. The first round of PCR products of the flanking regions and CaNAT marker were purified together and used as templates for the second round of double-joint PCR. In the second round of PCR, 5′- and 3′-gene disruption cassettes containing split CaNAT selection markers were amplified by L1-split primer 2 and R2-split primer 1, respectively (Table S1 in the supplemental material).
For the transformation of C. auris with gene disruption cassettes, we used a lithium acetate/heat-shock protocol with modifications. Cells were cultured overnight at 30°C in 50 mL YPD broth with shaking. We centrifuged 1.2 mL of cultured cells, washed them with dH2O and lithium acetate buffer (100 mM lithium acetate, 10 mM Tris, 1 mM EDTA, pH 7.5), and resuspended them in 300 µL of lithium acetate buffer. The transformation was set up with 10 µL of denatured salmon sperm DNA (Sigma, cat no. D9156), 100 µL of the competent cells, 500 µL of 50% PEG4000 (Sigma, cat no. P4338), and 50 µL of the amplified gene deletion cassette. The transformation mixture was incubated at 30°C for 6 h with intermittent vortexing. Subsequently, the cells were subjected to a 20-min heat shock at 42°C followed by 1 min of cooling on ice. The cells were then pelleted, resuspended in 1 mL of YPD medium, and incubated at 30°C for 1 h with shaking. After the incubation, the cells were washed twice with fresh liquid YPD medium and then spread onto selective YPD agar plates supplemented with 600 µg/mL nourseothricin, 1.8 mg/mL hygromycin B, or 2.4 mg/mL G418. The plates were then incubated at 37°C for 2–3 days. We confirmed the desired genotype of each positive nourseothricin-, hygromycin B-, or G418-resistant transformant by diagnostic PCR and Southern blot (Fig. S2).
To confirm the phenotypes of the ras1Δ, gpr1Δ, gpa2Δ, cdc25Δ, ira2Δ, pde1Δ, and pde2Δ mutants, we constructed corresponding complemented strains, in which each wild-type allele was either ectopically integrated (ras1Δ + RAS1, gpa2Δ + GPA2, and cdc25Δ + CDC25) or re-integrated into its native locus (gpr1Δ::GPR1, pde1Δ::PDE1, pde2Δ::PDE2, and ira2Δ::IRA2). To generate each full-length gene fragment, Phusion-PCR was performed using genomic DNA from the wild-type B8441 strain as a template and each primer pair listed in Table S1 in the supplemental material. The resulting fragments were directly cloned into the TOPO vector (Invitrogen) to generate the plasmids pTOP-RAS1, pTOP-GPR1, pTOP-GPA2, pTOP-CDC25, pTOP-IRA2, pTOP-PDE1, and pTOP-PDE2. After confirming the target sequence, the CaHYG inserts were sub-cloned into each pTOP vector to produce the pTOP-RAS1-HYG, pTOP-GPR1-HYG, pTOP-GPA2-HYG, pTOP-CDC25-HYG, pTOP-IRA2-HYG, pTOP-PDE1-HYG, and pTOP-PDE2-HYG. For the targeted re-integration into its native locus, pTOP-RAS1-HYG, pTOP-GPR1-HYG, pTOP-GPA2-HYG, pTOP-CDC25-HYG, pTOP-IRA2-HYG, pTOP-PDE1-HYG, and pTOP-PDE2-HYG were linearized by NsiI, BsmI, AfeI, AvrII, PmlI, AfeI, and AgeI, respectively, and introduced into each mutant by the lithium acetate heat-shock method. The correct genotype of the complemented strain was confirmed by diagnostic PCR (Fig. S2).
Total RNA preparation and quantitative RT-PCR
Total RNA was extracted from C. auris wild-type and Ras/cAMP/PKA mutant strains cultured overnight at 30°C in YPD broth. Briefly, cells were collected by centrifugation after reaching an OD600 of 0.6–0.8, frozen in liquid nitrogen, and lyophilized. For stress conditions, 10 mL of the culture was sampled for the basal state, and the remaining 30 mL was further incubated with stress agents. Total RNA was isolated by the Trizol extraction method with Easy-blue (Intron). Complementary DNA was synthesized from purified total RNA using reverse transcriptase (Thermo Scientific). Quantitative PCR was performed using specific primer pairs for each gene and the CFX96TM Real-Time system (Bio-Rad). ACT1 expression was used for normalization. Statistical analysis was performed using one-way ANOVA, followed by Bonferroni’s multiple-comparison test. All experiments were conducted in triplicate and repeated thrice biologically.
Growth and stress sensitivity spot assay
To assess the growth and stress sensitivity of wild type and Ras/cAMP/PKA mutant strains of C. auris, cells were grown overnight at 30°C and serially diluted 10-fold, four times (final dilution 1:104). The diluted cells were spotted onto YPD plates, which were then incubated at various temperatures (30°C, 37°C, 42°C, and 43°C). Growth was qualitatively assessed by photographing the plates after 1 day. To impose various stresses on the cells, different chemical agents were added to the media, including sorbitol (osmotic stress agent), NaCl or KCl (cation and salt stress), hydrogen peroxide, tert-butyl hydroperoxide, MD, or DIA (oxidative stress), SDS (membrane destabilizing stress), congo red (cell-wall destabilizing stress), and various antifungal agents (fludioxonil, flucytosine, FCZ, itraconazole, PCZ, caspofungin, or AMB). Cells were incubated at 30°C and photographed for 3 days after stress treatment.
Construction of BCY1 overexpression strains
To generate the BCY1 constitutive overexpression strain, we replaced the native promoter of BCY1 with the TEF1 promoter using an amplified homologous recombination cassette (Fig. S3). In the first round of PCR, we amplified the native promoter and 5′ coding regions (from ATG) of BCY1 using primer pairs B17979/B17980 and B17981/B17982, respectively. We also amplified the G418 resistance gene (NEO)-TEF1 promoter region with the primer pair B16376/B17918. In the second-round PCR, we used double joint-PCR to amplify the 5′ region of the P TEF1:BCY1 cassette using the mixed templates of the native promoter region of BCY1 and the 5′ region of the NEO-TEF1 promoter with the primer pair B17979/B16379 (Table S1). We similarly amplified the 3′ region of the P TEF1:BCY1 cassette using the mixed templates of the 5′ coding region of BCY1 and the 3′ region of the NEO-TEF1 promoter with the primer pair B16378/B17982. Next, we then introduced the combined split P TEF1:BCY1 cassettes into the pde1Δ pde2Δ (YSBA70) strain by heat shock transformation. Stable transformants were selected on a YPD medium containing 2.4 mg/mL G418. Positive transformants were confirmed using diagnostic PCR with a primer pair B10742/B16380.
Glycogen accumulation measurement assay
To measure the glycogen levels in each strain, iodine/iodide staining was performed as follows. All C. auris strains were streaked onto YPD agar plates and incubated at 30°C for 2 days. The colonies were then gently covered with 1 mL of Lugol solution (Sigma, cat no: 62650), and the plates were photographed 2 min later to visualize the iodine/iodide staining pattern.
Survival rate measurement
To measure the long-term survival rate, C. auris strains were cultured overnight in 2 mL YPD broth at 30°C, washed twice with dH2O, spotted onto solid YNB media lacking amino acids and ammonium sulfate, and incubated at 25°C for 28 days. Every 7 days, cells were collected from the plate, resuspended in dH2O, and the cell count was determined. Approximately 300 cells were then spread onto a solid YPD medium, and the percentage survival rate was calculated at each time point using the formula: survival rate = (number of colonies at each time point/number of colonies on the control plate) × 100.
For short-term survival rate measurement, strains were grown to stationary phase in liquid YNB medium lacking amino acids and ammonium sulfate at 30°C for 2 days, washed, resuspended in 1× PBS at a concentration of 104 cells/mL, and incubated at 25°C for 24 h. Aliquots were taken at 0, 4, 8, 12, 16, and 24 h of incubation, and approximately 300 cells were spread onto YPD plates. The plates were incubated at 37°C for 24 h, and the number of colonies was counted. The percentage survival rate of each strain at each time point was calculated as described above.
Biofilm assay
To evaluate the biofilm formation of wild-type and Ras/cAMP/PKA mutant strains of C. auris, cells were cultured overnight in 2 mL YPD broth at 30°C. Cells were then suspended in RPMI-1640 media to an OD600 of 0.5, and 200 µL of the suspension was added to flat-bottomed 96-well plates. To reduce evaporation and prevent cross-contamination between wells, plates were sealed with Breathe-Easy sealing membranes. Plates were incubated at 37°C with shaking at 250 rpm for 90 min. The RPMI medium was then removed, and the wells were washed with 200 µL PBS before adding 200 µL of fresh RPMI-1640 medium. The plates were then re-sealed and incubated at 37°C with shaking at 250 rpm for 24 h. After incubation, the RPMI-1640 medium was removed, and OD595 was measured using a microplate reader. The average density of adhered cells was calculated from the optical density readings from three independent wells in the plate. Data were normalized by subtracting the average blank well value from the experimental and control well values, and then dividing the resulting value by the mean blank-subtracted OD595 for the control wells.
Virulence study
To evaluate the virulence of C. auris wild type and Ras/cAMP/PKA mutant strains in vivo, we used a murine systemic infection model with reference to previous studies (21, 44). Fungal cells were incubated overnight at 30°C in YPD broth and washed three times with PBS. Cell concentrations were measured and adjusted to 108 cells/mL in PBS. To confirm CFUs and viability of the inoculum, the diluted cells were plated onto YPD agar plates and incubated at 37°C for 24 h. Specific pathogen-free/viral antibody-free (SPF/VAF) inbred 6-week-old female mice of the BALB/c (AnNCrlOri) strain were used for this study (Orient Bio Inc., South Korea), and they were habituated for 1 week before the experiment. For infection, the mice were restrained, and their tails were placed in warm (40°C) water to expand the lateral veins. The 100 µL of cell suspension was injected intravenously. Daily monitoring of survival was performed and abnormal behavior (head tilt or body spinning) was judged as a symptom of infection (44), and the mice were sacrificed as a humane endpoint for the experiment. The survival curves were analyzed using the Log-rank (Mantel-Cox).
ACKNOWLEDGMENTS
This work was financially supported by the Korean government through two programs: the Bio & Medical Technology Development Program of the National Research Foundation (NRF) (no. 2021R1A2B5B03086596 to Y.-S.B.; 2022R1A4A3022401 and 2022R1C1C2003274 to K.-T.L.), funded by the Korean government (MSIT), and the Korea Health Technology R&D Project via the Korea Health Industry Development Institute (KHIDI) (HI22C1987 and HI20C0326), funded by the Ministry of Health & Welfare.
The project was conceptualized by Y.-S.B., while J.-S.K. and K.-T.L. conducted the experiments and/or analyzed the data. Y.-S.B. supervised the experimental analysis and the manuscript was written by Y.-S.B. and J.-S.K.
The authors declare no conflict of interest.
Contributor Information
Kyung-Tae Lee, Email: lee.kt@jbnu.ac.kr.
Yong-Sun Bahn, Email: ysbahn@yonsei.ac.kr.
Gustavo H. Goldman, Universidade de Sao Paulo, Ribeirao Preto, Sao Paulo, Brazil
DATA AVAILABILITY
We will provide any strain and materials used in this study upon request.
ETHICS APPROVAL
Animal care and research were approved after deliberation by the Institutional Animal Care and Use Committee of the Experimental Animal Center at Jeonbuk National University (approval number: JBNU 2022-092). All experiments followed the experimental ethics guidelines. Animal experiments were conducted at the Core Facility Center for Zoonosis Research (Jeonbuk National University, South Korea).
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/spectrum.02152-23.
Figures S1 to S4.
C. auris strains and primers used in this study.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Kainz K, Bauer MA, Madeo F, Carmona-Gutierrez D. 2020. Fungal infections in humans: the silent crisis. Microb Cell 7:143–145. doi: 10.15698/mic2020.06.718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rhodes J, Fisher MC. 2019. Global epidemiology of emerging Candida auris. Curr Opin Microbiol 52:84–89. doi: 10.1016/j.mib.2019.05.008 [DOI] [PubMed] [Google Scholar]
- 3. Lee WG, Shin JH, Uh Y, Kang MG, Kim SH, Park KH, Jang H-C. 2011. First three reported cases of nosocomial fungemia caused by Candida auris. J Clin Microbiol 49:3139–3142. doi: 10.1128/JCM.00319-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sharma C, Kadosh D. 2023. Perspective on the origin, resistance, and spread of the emerging human fungal pathogen Candida auris. PLoS Pathog 19:e1011190. doi: 10.1371/journal.ppat.1011190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ahmad S, Alfouzan W. 2021. Candida auris: epidemiology, diagnosis, pathogenesis, antifungal susceptibility, and infection control measures to combat the spread of infections in healthcare facilities. Microorganisms 9:807. doi: 10.3390/microorganisms9040807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Caceres DH, Forsberg K, Welsh RM, Sexton DJ, Lockhart SR, Jackson BR, Chiller T. 2019. Candida auris: a review of recommendations for detection and control in healthcare settings. J Fungi (Basel) 5:111. doi: 10.3390/jof5040111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Du H, Bing J, Hu T, Ennis CL, Nobile CJ, Huang G. 2020. Candida auris: epidemiology, biology, antifungal resistance, and virulence. PLoS Pathog 16:e1008921. doi: 10.1371/journal.ppat.1008921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Janniger EJ, Kapila R. 2021. Public health issues with Candida auris in COVID-19 patients. World Med Health Policy 13:766–772. doi: 10.1002/wmh3.472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Borges-Walmsley MI, Walmsley AR. 2000. cAMP signaling in pathogenic fungi: control of dimorphic switching and pathogenicity. Trends Microbiol 8:133–141. doi: 10.1016/s0966-842x(00)01698-x [DOI] [PubMed] [Google Scholar]
- 10. Xue C, Hsueh Y-P, Heitman J. 2008. Magnificent seven: roles of G protein-coupled receptors in extracellular sensing in fungi. FEMS Microbiol Rev 32:1010–1032. doi: 10.1111/j.1574-6976.2008.00131.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lorenz MC, Pan X, Harashima T, Cardenas ME, Xue Y, Hirsch JP, Heitman J. 2000. The G protein-coupled receptor Gpr1 is a nutrient sensor that regulates pseudohyphal differentiation in Saccharomyces cerevisiae. Genetics 154:609–622. doi: 10.1093/genetics/154.2.609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lemaire K, Van de Velde S, Van Dijck P, Thevelein JM. 2004. Glucose and sucrose act as agonist and mannose as antagonist ligands of the G protein-coupled receptor Gpr1 in the yeast Saccharomyces cerevisiae. Mol Cell 16:293–299. doi: 10.1016/j.molcel.2004.10.004 [DOI] [PubMed] [Google Scholar]
- 13. Hennig A, Markwart R, Esparza-Franco MA, Ladds G, Rubio I. 2015. Ras activation revisited: role of GEF and GAP systems. Biol Chem 396:831–848. doi: 10.1515/hsz-2014-0257 [DOI] [PubMed] [Google Scholar]
- 14. Sassone-Corsi P. 2012. The cyclic AMP pathway. Cold Spring Harb Perspect Biol 4:a011148. doi: 10.1101/cshperspect.a011148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ma P, Wera S, Van Dijck P, Thevelein JM. 1999. The PDE1-encoded low-affinity phosphodiesterase in the yeast Saccharomyces cerevisiae has a specific function in controlling agonist-induced cAMP signaling. Mol Biol Cell 10:91–104. doi: 10.1091/mbc.10.1.91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hoyer LL, Cieslinski LB, McLaughlin MM, Torphy TJ, Shatzman AR, Livi GP. 1994. A Candida albicans cyclic nucleotide phosphodiesterase: cloning and expression in Saccharomyces cerevisiae and biochemical characterization of the recombinant enzyme. Microbiology (Reading) 140:1533–1542. doi: 10.1099/13500872-140-7-1533 [DOI] [PubMed] [Google Scholar]
- 17. Hogan DA, Sundstrom P. 2009. The Ras/cAMP/PKA signaling pathway and virulence in Candida albicans. Future Microbiol 4:1263–1270. doi: 10.2217/fmb.09.106 [DOI] [PubMed] [Google Scholar]
- 18. Caza M, Kronstad JW. 2019. The cAMP/protein kinase a pathway regulates virulence and adaptation to host conditions in Cryptococcus neoformans. Front Cell Infect Microbiol 9:212. doi: 10.3389/fcimb.2019.00212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liebmann B, Gattung S, Jahn B, Brakhage AA. 2003. cAMP signaling in Aspergillus fumigatus is involved in the regulation of the virulence gene pksP and in defense against killing by macrophages. Mol Genet Genomics 269:420–435. doi: 10.1007/s00438-003-0852-0 [DOI] [PubMed] [Google Scholar]
- 20. Krüger J, Loubradou G, Wanner G, Regenfelder E, Feldbrügge M, Kahmann R. 2000. Activation of the cAMP pathway in Ustilago maydis reduces fungal proliferation and teliospore formation in plant tumors. Mol Plant Microbe Interact 13:1034–1040. doi: 10.1094/MPMI.2000.13.10.1034 [DOI] [PubMed] [Google Scholar]
- 21. Kim J-S, Lee K-T, Lee MH, Cheong E, Bahn Y-S. 2021. Adenylyl cyclase and protein kinase A play redundant and distinct roles in growth, differentiation, antifungal drug resistance, and Pathogenicity of Candida Auris. mBio 12:e0272921. doi: 10.1128/mBio.02729-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tamanoi F. 2011. Ras signaling in yeast. Genes Cancer 2:210–215. doi: 10.1177/1947601911407322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tanaka K, Nakafuku M, Satoh T, Marshall MS, Gibbs JB, Matsumoto K, Kaziro Y, Toh-e A. 1990. S. cerevisiae genes IRA1 and IRA2 encode proteins that may be functionally equivalent to mammalian Ras GTPase activating protein. Cell 60:803–807. doi: 10.1016/0092-8674(90)90094-u [DOI] [PubMed] [Google Scholar]
- 24. Wilson RB, Renault G, Jacquet M, Tatchell K. 1993. The pde2 gene of Saccharomyces cerevisiae is allelic to rca1 and encodes a phosphodiesterase which protects the cell from extracellullar cAMP. FEBS Lett 325:191–195. doi: 10.1016/0014-5793(93)81071-7 [DOI] [PubMed] [Google Scholar]
- 25. Park JI, Grant CM, Dawes IW. 2005. The high-affinity cAMP phosphodiesterase of Saccharomyces cerevisiae is the major determinant of cAMP levels in stationary phase: involvement of different branches of the Ras-cyclic AMP pathway in stress responses. Biochem Biophys Res Commun 327:311–319. doi: 10.1016/j.bbrc.2004.12.019 [DOI] [PubMed] [Google Scholar]
- 26. Wilson D, Tutulan-Cunita A, Jung W, Hauser NC, Hernandez R, Williamson T, Piekarska K, Rupp S, Young T, Stateva L. 2007. Deletion of the high-affinity cAMP phosphodiesterase encoded by PDE2 affects stress responses and virulence in Candida albicans. Mol Microbiol 65:841–856. doi: 10.1111/j.1365-2958.2007.05788.x [DOI] [PubMed] [Google Scholar]
- 27. Hicks JK, Bahn Y-S, Heitman J. 2005. Pde1 phosphodiesterase modulates cyclic AMP levels through a protein kinase A-mediated negative feedback loop in Cryptococcus neoformans. Eukaryot Cell 4:1971–1981. doi: 10.1128/EC.4.12.1971-1981.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yang K, Liu Y, Liang L, Li Z, Qin Q, Nie X, Wang S. 2017. The high-affinity phosphodiesterase PdeH regulates development and aflatoxin biosynthesis in Aspergillus flavus. Fungal Genet Biol 101:7–19. doi: 10.1016/j.fgb.2017.02.004 [DOI] [PubMed] [Google Scholar]
- 29. Bahn Y-S, Staab J, Sundstrom P. 2003. Increased high-affinity phosphodiesterase PDE2 gene expression in germ tubes counteracts CAP1-dependent synthesis of cyclic AMP, limits hypha production and promotes virulence of Candida albicans. Mol Microbiol 50:391–409. doi: 10.1046/j.1365-2958.2003.03692.x [DOI] [PubMed] [Google Scholar]
- 30. Kronstad J, De Maria AD, Funnell D, Laidlaw RD, Lee N, de Sá MM, Ramesh M. 1998. Signaling via cAMP in fungi: interconnections with mitogen-activated protein kinase pathways. Arch Microbiol 170:395–404. doi: 10.1007/s002030050659 [DOI] [PubMed] [Google Scholar]
- 31. Wilson WA, Roach PJ, Montero M, Baroja-Fernández E, Muñoz FJ, Eydallin G, Viale AM, Pozueta-Romero J. 2010. Regulation of glycogen metabolism in yeast and bacteria. FEMS Microbiol Rev 34:952–985. doi: 10.1111/j.1574-6976.2010.00220.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Silljé HH, Paalman JW, ter Schure EG, Olsthoorn SQ, Verkleij AJ, Boonstra J, Verrips CT. 1999. Function of trehalose and glycogen in cell cycle progression and cell viability in Saccharomyces cerevisiae. J Bacteriol 181:396–400. doi: 10.1128/JB.181.2.396-400.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pereira R, Dos Santos Fontenelle RO, de Brito EHS, de Morais SM. 2021. Biofilm of Candida albicans: formation, regulation and resistance. J Appl Microbiol 131:11–22. doi: 10.1111/jam.14949 [DOI] [PubMed] [Google Scholar]
- 34. Liu S, Le Mauff F, Sheppard DC, Zhang S. 2022. Filamentous fungal biofilms: conserved and unique aspects of extracellular matrix composition, mechanisms of drug resistance and regulatory networks in Aspergillus fumigatus. NPJ Biofilms Microbiomes 8:83. doi: 10.1038/s41522-022-00347-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ramage G, Rajendran R, Sherry L, Williams C. 2012. Fungal biofilm resistance. Int J Microbiol 2012:528521. doi: 10.1155/2012/528521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhao X, Oh S-H, Yeater KM, Hoyer LL. 2005. Analysis of the Candida albicans Als2p and Als4p adhesins suggests the potential for compensatory function within the Als family. Microbiology (Reading) 151:1619–1630. doi: 10.1099/mic.0.27763-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Murillo LA, Newport G, Lan C-Y, Habelitz S, Dungan J, Agabian NM. 2005. Genome-wide transcription profiling of the early phase of biofilm formation by Candida albicans. Eukaryot Cell 4:1562–1573. doi: 10.1128/EC.4.9.1562-1573.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nobile CJ, Fox EP, Nett JE, Sorrells TR, Mitrovich QM, Hernday AD, Tuch BB, Andes DR, Johnson AD. 2012. A recently evolved transcriptional network controls biofilm development in Candida albicans. Cell 148:126–138. doi: 10.1016/j.cell.2011.10.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Laforet L, Moreno I, Sánchez-Fresneda R, Martínez-Esparza M, Martínez JP, Argüelles J-C, de Groot PWJ, Valentín-Gomez E. 2011. Pga26 mediates filamentation and biofilm formation and is required for virulence in Candida albicans. FEMS Yeast Res 11:389–397. doi: 10.1111/j.1567-1364.2011.00727.x [DOI] [PubMed] [Google Scholar]
- 40. Pentland DR, Piper-Brown E, Mühlschlegel FA, Gourlay CW. 2017. Ras signalling in pathogenic yeasts. Microb Cell 5:63–73. doi: 10.15698/mic2018.02.612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Huang G, Huang Q, Wei Y, Wang Y, Du H. 2019. Multiple roles and diverse regulation of the Ras/cAMP/protein kinase A pathway in Candida albicans. Mol Microbiol 111:6–16. doi: 10.1111/mmi.14148 [DOI] [PubMed] [Google Scholar]
- 42. Agarwal C, Aulakh KB, Edelen K, Cooper M, Wallen RM, Adams S, Schultz DJ, Perlin MH. 2013. Ustilago maydis phosphodiesterases play a role in the dimorphic switch and in pathogenicity. Microbiology (Reading) 159:857–868. doi: 10.1099/mic.0.061234-0 [DOI] [PubMed] [Google Scholar]
- 43. Eix EF, Nett JE. 2020. How biofilm growth affects Candida-host interactions. Front Microbiol 11:1437. doi: 10.3389/fmicb.2020.01437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gao J, Chow EWL, Wang H, Xu X, Cai C, Song Y, Wang J, Wang Y. 2021. LncRNA DINOR is a virulence factor and global regulator of stress responses in Candida auris. Nat Microbiol 6:842–851. doi: 10.1038/s41564-021-00915-x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figures S1 to S4.
C. auris strains and primers used in this study.
Data Availability Statement
We will provide any strain and materials used in this study upon request.