ABSTRACT
The 2022 World Health Organization guidelines recommend use of two core anti-tuberculosis (TB) drugs, bedaquiline (BDQ) and clofazimine (CFZ), for treatment of drug-resistant (DR)-TB. However, several mutated Mycobacterium tuberculosis (MTB) genes, conferring BDQ and CFZ resistance, have been reported that predominantly arose from sporadic mutations that have not been comprehensively characterized. Herein, MTB clinical isolates collected from drug-susceptible (DS)-, multidrug-resistant (MDR)-, and extensively drug-resistant (XDR)-TB patients were cultured in vitro with BDQ or CFZ to generate progeny strains with resistance to these drugs. Progeny strains exposed to CFZ exhibited increased CFZ minimum inhibitory concentrations (MICs) that exceeded MIC increases of BDQ-exposed progeny strains. Notably, mmpR and pepQ mutations accounted for 83% and 17% of BDQ-induced spontaneous gene mutations, respectively, and 86% and 14% of CFZ-induced spontaneous gene mutations, respectively. Analyses of predicted mutation-induced changes in amino acid sequences and structures of MmpR and PepQ mutants revealed several point mutations affected sequence conversation and functionality as an underlying mechanism for observed acquired BDQ/CFZ resistance. Moreover, our results revealed differences in patterns of BDQ- and CFZ-induced acquired spontaneous mutations that may enhance our understanding of MTB BDQ/CFZ-resistance mechanisms.
IMPORTANCE
This study of MTB drug resistance mechanisms revealed patterns of spontaneous MTB mutations associated with acquired BDQ and CFZ resistance that arose after clinical MTB isolates were cultured in vitro with BDQ or CFZ. Results of protein sequence and structural analyses provided insights into potential mechanisms underlying associations between MTB gene mutations and DR phenotypes. Taken together, these results revealed differences in acquired BDQ and CFZ resistance mechanisms as a new perspective that may enhance our understanding of BDQ/CFZ resistance mechanisms and facilitate the development of new methods for detecting MTB drug resistance genes.
KEYWORDS: Mycobacterium tuberculosis, bedaquiline, clofazimine, resistance
INTRODUCTION
Tuberculosis (TB), an infectious disease caused by Mycobacterium tuberculosis (MTB), remains a major public health issue and one of the leading causes of human deaths worldwide (1). Notably, the emergence of drug-resistant TB (DR-TB), especially multidrug-resistant TB (MDR-TB), has greatly hindered global TB control efforts, as have the following factors: low DR-TB treatment success rates, long DR-TB treatment durations, and interrupted anti-TB treatment administration during the COVID-19 epidemic (2 – 4). Therefore, new anti-TB drugs are urgently needed to stop the spread of DR-TB.
Bedaquiline (BDQ) and clofazimine (CFZ) are core anti-TB drugs recommended by 2022 World Health Organization (WHO) guidelines for the treatment of DR-TB cases, with both drugs predominantly used to treat multidrug-resistant/rifampicin-resistant TB (MDR/RR-TB) (5). BDQ is a member of one of only two new anti-TB drug classes that have been approved for clinical use in over half a century. Notably, results of studies conducted in South Africa have demonstrated that administration of the 6-mo BPaL (bedaquiline + pretomanid + linezolid) regimen to extensively drug-resistant TB (XDR-TB) and MDR-TB patients with histories of poor treatment outcomes achieved high treatment success rates of about 90% (6). Moreover, results of other clinical studies have shown that successful treatment of DR-TB cases with BDQ-containing regimens could be completed in shorter periods of time than conventional treatments, while incorporation of BDQ in all-oral treatment regimens simplified anti-TB treatment and enhanced patient compliance (6). Meanwhile, CFZ, a key drug used to treat leprosy patients (7), has been shown to significantly inhibit MTB growth both in vitro and in vivo (8, 9). In fact, results of multiple clinical studies have demonstrated high success rates of CFZ-containing chemotherapy regimens that ranged from approximately 84% to 89% (10 – 13). Furthermore, results of other studies have shown that treatment of MDR-TB patients with such regimens could achieve successful outcomes when administered for shorter periods of time than are required for completion of conventional anti-MDR-TB treatments (14, 15). Based on the abovementioned results, BDQ and CFZ are currently under evaluation in several clinical trials, including the ongoing Phase III BEAT-TB clinical trial.
Nevertheless, in spite of improved TB cure rates of BDQ- and CFZ-containing anti-TB regimens, in recent years, TB cases with resistance to BDQ and CFZ and cross-resistance between the two drugs have been reported. Consequently, investigations of mechanisms underlying resistance to BDQ and CFZ have been conducted that have led to discoveries of several genes associated with phenotypic resistance to these drugs. For example, mutations of atpE (Rv1305), mmpR (Rv0678), and pepQ (Rv2535c) have been associated with phenotypic resistance to BDQ, while mutations of the latter two genes have been implicated in cross-resistance to CFZ and BDQ (16 – 18). However, due to the fact that these drugs have only been clinically used for a few years, few clinical isolates with resistance to these drugs have been reported. More recently, these isolates have been shown to mainly harbor sporadic gene mutations that have yet been comprehensively characterized as an explanation for why high-confidence BDQ or CFZ resistance variants have not yet been announced by WHO (19). Nonetheless, the emergence of BDQ and CFZ resistance-inducing mutations has led to declining effectiveness of these drugs (20 – 25). To address this issue, researchers are urgently studying mechanisms underlying MTB resistance to BDQ and CFZ toward developing rapid DR-TB early detection methods and new drugs to prevent DR-TB emergence and transmission.
In this study, clinical MTB isolates were cultured with BDQ or CFZ in vitro to generate MTB progeny strains with acquired resistance to these drugs. Thereafter, drug-treated progeny strains were analyzed to identify drug resistance-associated gene mutations, then gene mutation-induced changes in protein sequences and structures were predicted in order to identify mutations associated with observed drug resistance. The results of this study provide new clues to guide future research efforts toward development of new methods for detecting MTB anti-TB drug resistance genes.
MATERIALS AND METHODS
Clinical strains
The strains in this study were classified as drug-susceptible (DS)-, MDR-, and XDR-TB strains according to the pre-2021 WHO definition (26). Ultimately, 15 DS, 15 MDR and 15 XDR MTB strains derived from clinical isolates (collected from March to September 2016) were obtained from the Biobank of Beijing Chest Hospital in China. Each MTB strain was isolated from a unique TB patient. Phenotypic resistance to first- and second-line drugs was determined using the absolute concentration method, as described in the aforementioned WHO recommendations (27).
Minimal inhibitory concentration determinations
The resazurin microtiter assay (REMA) was conducted to determine BDQ and CFZ minimal inhibitory concentrations (MICs) for the abovementioned clinical MTB strains (28). Concentrations of initial stock solutions of CFZ and BDQ (dissolved in dimethyl sulfoxide solvent) were both 6,400 µg/mL. To prepare each MTB inoculum, a cell suspension of each isolate with turbidity equivalent to that of a McFarland 1.0 standard was diluted 1:20 in Middlebrook 7H9 broth containing 10% oleic acid-albumin-dextrose-catalase (OADC). Serial twofold dilutions of BDQ or CFZ in 100 µL volumes of 7H9 broth were directly prepared in wells of 96-well plates in concentrations ranging from 0.016 μg/mL to 16 μg/mL. Next, 100 µL of the diluted inoculum was added to each well, then plates were incubated at 37°C for 7 days. After incubation, 40 µL of 0.01% fresh resazurin solution was pipetted into each well, then results were read after an additional 24 h incubation at 37°C. The MIC for each isolate was determined as the lowest drug concentration that prevented a color change from blue to pink, with the MTB H37Rv strain (ATCC 27294) serving as a control for assay performance that was included in all assay runs. Clinical isolates with MICs below the tentative critical drug concentration (BDQ: 0.125 µg/mL, CFZ: 1 µg/mL) were scored as DS results (29, 30); isolates with MICs that were equal to the tentative critical concentration fell within the area of technical uncertainty, meaning that sensitivity or resistance could not be determined (31); strains with MICs that were greater than the abovementioned tentative critical concentrations were scored as DR strains.
Isolation of spontaneous mutants
We further adapted the method of Ismail et al. to generate spontaneous mutants of clinical strains (21). Briefly, a fresh culture of each clinical strain was harvested, then cultured in order to generate an inoculum with turbidity equivalent to that of a McFarland 1.0 standard. Next, 100 µL of the inoculum was inoculated onto two 7H10 agar plates, of which one plate contained BDQ and the other contained CFZ. For BDQ, 0.25 µg/mL was selected as the tentative critical concentration for 7H10 agar screening, as based on results of previous studies (32, 33). Due to the fact that the 7H10/7H11 agar method has not been reported as a method for determining CFZ critical concentration, 1 µg/mL was chosen as the tentative CFZ critical concentration (as based on our REMA results). Thereafter, two concentrations (0.5× and 1× of the tentative critical concentration) of each drug (BDQ: 0.125 µg/mL and 0.25 µg/mL; CFZ: 0.5 µg/mL and 1 µg/mL) were incorporated in solid medium within culture plates, then a total of 12 plates was inoculated (three plates for each of the two concentrations for each drug). Four weeks after inoculation, progeny strains growing on drug-containing plates were harvested, then MICs for all strains were determined. Genomic DNA was isolated from each strain, then subjected to DNA sequence-based analysis.
Amplification and sequencing of resistant genes conferring BDQ and/or CFZ resistance
Genomic DNA extraction and sequencing were performed using DNA sequencing and amplification primers as reported previously (24). Coding regions of atpE, mmpR, and pepQ were amplified and sequenced for BDQ-treated progeny strains and corresponding parent strains, while coding regions of mmpR, pepQ, and Rv1979c were amplified and sequenced for CFZ-treated progeny strains and corresponding parent strains. Each PCR was performed in a final volume of 50 µL containing 5 µL PCR buffer, 2 mM MgCl2, 200 µM of each dNTP, 0.2 µM of each primer set, and 1 U HotStar Taq polymerase (Qiagen). Amplified products were sent to the Tsingke Company (Beijing, China) for DNA sequence analysis. DNA sequences were aligned to corresponding sequences of the standard laboratory MTB strain H37Rv using BioEdit Sequence Alignment Editor version 7.1 (https://bioedit.software.informer.com/7.1/).
Spoligotyping
Genotyping of MTB was performed using a MeltPro Mycobacterium tuberculosis McSpoligotyping Kit (Xiamen Zeesan Biotech, Xiamen, P. R. China) as described in previous studies (34). Each amplification reaction was prepared in a final volume of 25 µL that contained 19.75 µL of McSpoligotyping PCR Mix (A/B/C), 0.25 µL of McSpoligotyping enzyme, and 5 µL of DNA. Thermal cycling was conducted using a Zeesan SLAN96 real-time fluorescent PCR instrument under PCR cycling conditions as specified by the instructions provided with the kit. The melt curve analysis procedure was performed under the following conditions: 95°C for 3 min, then 35°C for 1 min followed by a temperature increase from 35°C to 90°C at a rate of 0.04°C/s to acquire fluorescence signals. The results were submitted to the SITVIT2 database (http://www.pasteur-guadeloupe.fr:8081/SITVIT2/submit.jsp) and used to assign spoligotype international types and corresponding clades to our MTB isolates.
Analysis of protein sequences and structures
We first performed BLAST against the UniProt database (https://www.uniprot.org/blast/) to find orthologs of MmpR and PepQ. Sequence alignment was performed using ClustalO (35), then results were formatted and visualized using ESPript (36). The PROVEAN score (37), a mutation impact score based on a multiple sequence alignment of protein sequences against the non-redundant protein sequence database, was calculated for each mutation using TBvar3D (https://swissmodel.expasy.org/var3d/).
Structures of mutant proteins were modeled using SWISS-MODEL (http://swissmodel.expasy.org/), and templates were obtained from Protein Data Bank (PDB) (https://www.rcsb.org/) and AlphaFold Protein Structure Database (http://alphafold.ebi.ac.uk/). Templates used to model mutant MmpR and PepQ proteins included PDB file 4NB5 and AlphaFold file AF-I6YDN6-F1, respectively, while the PDB file 1Z9C template was used to model the MmpR-DNA complex.
Free energy changes were calculated using Eris (38) and PremPS (39) to predict impacts of point mutation-induced amino acid sequence changes on protein stability. Detailed local interaction changes were analyzed using the “Structure Monitor” module of Discovery Studio Visualizer v.4.5 (BIOVIA, Dassault Systèmes, San Diego, CA, USA).
RESULTS
Characterization of parent strains
Due to culture contamination or initial BDQ- or CFZ-resistance, nine strains were excluded. Of the remaining 36 strains, 30 strains with MICs that fell below tentative critical MIC values indicative of BDQ or CFZ drug resistance (10 DS, 10 MDR, and 10 XDR) were randomly selected to serve as parent strains, with MIC values of the 30 parent strains shown in Table 1. Results of spoligotyping analysis indicated that 27 (90%) of the 30 parent strains belonged to the Beijing MTB family, including all 20 MDR and XDR strains and 7 of the DS strains (70%), while 3 of the DS strains (30%) belonged to the T1 MTB family (Table S1).
TABLE 1.
MIC distribution of parent strains
| Drugs | Strains | No. of strains with different MICs (µg/mL) | Breakpoint (µg/mL) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≤0.016 | 0.03 | 0.06 | 0.125 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | |||
| BDQ | DS a | 1 | 5 | 3 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.125 |
| MDR b | 3 | 5 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| XDR c | 1 | 3 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| CFZ | DS | 0 | 0 | 0 | 0 | 5 | 5 | 0 | 0 | 0 | 0 | 0 | 1 |
| MDR | 0 | 0 | 0 | 1 | 3 | 6 | 0 | 0 | 0 | 0 | 0 | ||
| XDR | 0 | 0 | 0 | 1 | 5 | 4 | 0 | 0 | 0 | 0 | 0 | ||
DS: drug susceptible.
MDR: multidrug resistant.
XDR: extensively drug resistant.
After 4 wk of culture, progeny strains treated with BDQ or CFZ were harvested and analyzed; BDQ-treated strains could only be obtained from plates with 0.125 µg/mL BDQ (0.5× of the tentative critical BDQ MIC), not from plates with 0.25 µg/mL BDQ (the tentative critical BDQ MIC). Ultimately, progeny strains were obtained from nine parent strains (30%) that included four DS strains (DS-1, DS-3, DS-6, and DS-10), two MDR strains (MDR-7 and MDR-8), and three XDR strains (XDR-1, XDR-7, and XDR-9). After CFZ treatment, 21 parent strains (70%) yielded progeny strains that grew on plates containing 0.5 µg/mL CFZ (0.5× of the tentative critical CFZ MIC), while 11 parent strains (37%) yielded progeny strains that grew on plates containing 1 µg/mL CFZ (the tentative critical CFZ MIC). The 21 parent strains that yielded progeny strains when cultured on CFZ plates included seven DS strains (DS-2 to DS-6, DS-8, and DS-9), seven MDR strains (MDR-1 to MDR-4 and MDR-7 to MDR-9), and seven XDR strains (XDR-1, XDR-3 to XDR-6, XDR-9, and XDR-10). Notably, six parent strains (DS-3, DS-6, MDR-7, MDR-8, XDR-1, and XDR-9) yielded progeny strains when cultured on both BDQ plates and CFZ plates (Table S2).
Progeny strain drug resistance gene mutations and MICs
After BDQ exposure, 13 progeny strains were obtained, of which only one strain lacked mutations within sequenced regions of mmpR, pepQ, and atpE genes. Of the remaining 12 progeny strains, 10 strains (83%) possessed mutations within mmpR and 2 strains (17%) possessed mutations within pepQ, while no atpE mutation was detected. Analysis of these mutations (five point mutations, two frameshift mutations, and two stop codon-generating mutations) indicated that they would induce nine different types of amino acid sequence alterations (Table 2). After progeny strains were exposed to BDQ, their BDQ MICs ranged from 0.125 µg/mL to 0.25 µg/mL and exhibited four- to eightfold BDQ MIC increases relative to corresponding parental strain MICs. Interestingly, all BDQ-induced mutations led to CFZ resistance, as reflected by CFZ MICs of these strains that ranged from 2 µg/mL to 4 µg/mL. In addition, all mutant MmpR and PepQ proteins (except for MmpR G24C and G65E mutants) were associated with greater fold changes in CFZ MICs relative to parent MICs as compared to corresponding fold changes observed for BDQ MICs (Table 2).
TABLE 2.
Mutations in BDQ resistance-conferring genes
| Genes | Nucleotide change | Amino acid change | MIC (µg/mL) | No. of strains | MIC change fold e | ||
|---|---|---|---|---|---|---|---|
| BDQ | CFZ | BDQ | CFZ | ||||
| mmpR | 32delG a | G11fs c | 0.125 | 2 | 2 | 4 | 16 |
| mmpR | 70G > T | G24C | 0.25 | 2 | 1 | 8 | 8 |
| mmpR | 134T > G | V45G | 0.125 | 4 | 1 | 4 | 16 |
| mmpR | 194G > A | G65E | 0.25 | 2 | 1 | 4 | 4 |
| mmpR | 233G > C | G78A | 0.25 | 2 | 2 | 4 | 8 |
| mmpR | 253G > T | V85P | 0.125 | 2 | 1 | 4 | 8 |
| mmpR | 466C > T | R156 d | 0.25 | 2 | 2 | 4 | 8 |
| pepQ | 347_348insG b | D116fs | 0.25 | 4 | 1 | 4 | 16 |
| pepQ | 735G > A | W245 d | 0.25 | 4 | 1 | 4 | 16 |
del: deletion.
ins: insertion.
fs: frame shift.
stop codon.
MIC change fold relative to parent MIC.
After CFZ exposure, a total of 186 progeny strains was obtained, of which approximately one-fourth (46 strains) was randomly selected for culture by taking one progeny strain colony from each plate. During the culturing of progeny strains, four strains were excluded due to contamination, yielding 42 progeny strains that were subjected to further analyses. Of these, 20 progeny strains lacked atpE, pepQ, and mmpR mutations, while 19 of the remaining 22 strains (86%) possessed mmpR mutations and 3 strains (14%) possessed pepQ mutations. Analysis of strains with mutations within sequenced regions of atpE, pepQ, and mmpR revealed a total of 21 distinct types of amino acid sequence-altering mutations that included 12 point mutations, 5 frameshift mutations, 1 insertion, and 3 stop codon-generating mutations (Table 3). For CFZ-exposed progeny strains, BDQ and MICs ranged from 0.03 µg/mL to 0.5 µg/mL and CFZ MICs ranged from 1 µg/mL to 4 µg/mL, while progeny strain MICs were 2- to 16-fold greater than corresponding parent strain MIC values.
TABLE 3.
Mutations in CFZ resistance-conferring genes
| Genes | Nucleotide change | Amino acid change | MIC (µg/mL) | No. of strains | MIC change fold | ||
|---|---|---|---|---|---|---|---|
| BDQ | CFZ | BDQ | CFZ | ||||
| mmpR | 32delG a | G11fs c | 0.125 | 2 | 1 | 4 | 16 |
| mmpR | 109G > C | G37R | 0.25 | 1 | 1 | 8 | 2 |
| mmpR | 116T > C | L39S | 0.06 | 1 | 1 | 2 | 2 |
| mmpR | 119T > G | L40W | 0.5 | 4 | 1 | 4 | 8 |
| mmpR | 124T > C | W42R | 0.25 | 4 | 1 | 4 | 8 |
| mmpR | 134T > G | V45G | 0.125 | 4 | 2 | 4 | 16 |
| mmpR | 151C > T | Q51 d | 0.125 | 2 | 1 | 2 | 4 |
| mmpR | 194G > A | G65E | 0.25 | 2 | 1 | 16 | 4 |
| mmpR | 198delG | G66fs | 0.5 | 4 | 1 | 8 | 16 |
| mmpR | 226C > T | Q76 d | 0.125 | 1 | 1 | 4 | 2 |
| mmpR | 248T > C | L83P | 0.5 | 2 | 1 | 16 | 4 |
| mmpR | 257C > T | A86V | 0.125 | 2 | 1 | 8 | 8 |
| mmpR | 278T > C | F93S | 0.5 | 4 | 1 | 4 | 8 |
| mmpR | 302C > A | A101E | 0.03 | 1 | 1 | ≥2 | 4 |
| mmpR | 412_413insG b | E138fs | 0.5 | 4 | 1 | 8 | 16 |
| mmpR | 425T > C | L142P | 0.125 | 2 | 1 | 4 | 4 |
| mmpR | 435_436ins GCGGATTTC ACAAAGCAG |
Y145_M146 insADFTKQ |
0.125 | 1 | 1 | 4 | 2 |
| mmpR | 436delA, 437delT | M146fs | 0.25 | 2 | 1 | 8 | 8 |
| pepQ | 735G > A | W245 d | 0.125 | 2 | 1 | 2 | 8 |
| pepQ | 947T > G | L316R | 0.25 | 2 | 1 | 4 | 8 |
| pepQ | 1060delA | T354fs | 0.5 | 2 | 1 | 8 | 8 |
del: deletion.
ins: insertion.
fs: frame shift.
stop codon.
Contributions of mutations to drug resistance
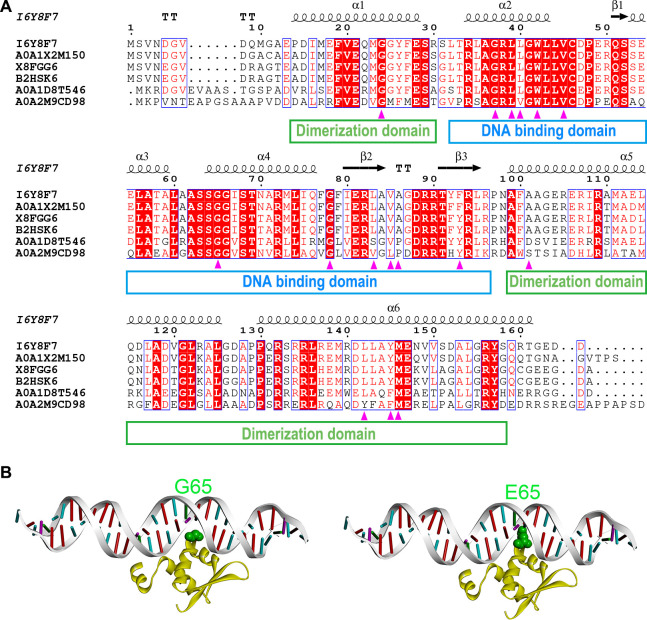
MmpR is a transcriptional repressor of MmpL5 and MmpS5, whereby mutations that inactivate MmpR may lead to increased MmpL5 and MmpS5 levels and elevated CFZ and BDQ MICs (18). To evaluate impacts of point mutations on MmpR function, we first performed MmpR sequence analysis, with results shown in Fig. 1A. Notably, MmpR residues affected by these mutations (G24, G37, L39, W42, V45, G65, and G78) were highly conserved, while residues 86 and 101 were not conserved. In addition, all MmpR mutation-induced amino acid substitutions (except for V85F, A86V, and A101E) yielded markedly reduced Protein Variation Effect Analyzer (PROVEAN) scores (below −2.282), which indicated that these mutations reduced protein sequence similarities between MmpR and sequences of many other functionally orthologous proteins (Table 4). Moreover, an analysis of predicted effects of point mutation-induced MmpR structural alterations on protein stability provided insights into MTB BDQ/CFZ drug resistance mechanisms. The L-shaped MmpR monomer structure includes a shorter domain (residues 32–97) that participates in DNA binding and a longer dimerization domain (residues 14–30 and 99–158). Interestingly, of the 14 BDQ/CFZ-induced MmpR mutations detected in this study, 11 mutations led to amino acid substitutions (G37R, L39S, L40W, W42R, V45G, G65E, G78A, L83P, V85F, A86V, and F93S) within the DNA-binding domain, while the other 3 mutations (G24C, A101E, and L142P) were found in the dimerization domain (Fig. 1A). Of the DNA-binding domain mutant, only the G65E amino acid substitution was predicted to directly influence DNA binding by inducing the formation of a bump that would disrupt binding between the MmpR glutamic acid side chain and the DNA molecule (Fig. 1B); the other mutation-induced amino acid sequence alterations may indirectly influence DNA binding or domain dimerization through effects on local protein structures. Meanwhile, results of detailed structural analysis suggested that mutation-induced amino acid substitutions G24C, G37R, L40W, and V85F cause structural instability by promoting formation of protein structure-disrupting bumps, while L39S, W42R, V45G, and F93S substitutions would lead to loss of hydrophobic interactions. Furthermore, substitutions of non-proline amino acids with proline residues may alter the directional orientation of the protein backbone to reduce hydrogen bonding interactions that might disrupt α-helix (L142P) and β-sheet (L83P) structures. Taken together, our predicted results indicate that all of the abovementioned mmpR amino acid changes except for A86V may lead to protein instability (Table 4) and that all MmpR point mutation-induced amino acid substitutions other than A86V and A101E may contribute to MTB BDQ and CFZ resistance (Table 4).
Fig 1.
Sequence and structural analyses of MTB MmpR. (A) Multiple sequence alignments of MTB MmpR and its orthologs. According to BLAST results, the five orthologs of MmpR (UniProt entry I6Y8F7) with highest BLAST scores included a transcriptional regulator from Mycobacterium decipiens (UniProt entry A0A1 × 2M150), the putative regulatory protein MarR from Mycobacterium ulcerans (UniProt entry X8FGG6), a transcriptional regulator from Mycobacterium marinum (UniProt entry B2HSK6), a transcriptional regulator from Rhodococcus sp. WMMA185 (UniProt entry A0A1D8T546), and a MarR family protein from Sediminihabitans luteus (UniProt entry A0A2M9CD98). (B) Structures of wild-type MmpR (left) and MmpR G65E mutant (right) within MmpR-DNA complexes. MmpR protein (yellow) and DNA (gray) molecular structures are displayed in cartoon mode with residue 65 indicated in green, as based on the Corey–Pauling–Koltun color convention.
TABLE 4.
Protein sequence and structure analysis of point mutations
| Gene | Amino acid change | Sequence conservation | PROVEAN score a | ΔΔG (kcal/mol) b | Changes of interaction | Predicted contribution to drug resistance | MIC (µg/mL) | No. of strains | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Eris | PremPS | Local structure | DNA binding | Dimerization | BDQ | CFZ | ||||||
| mmpR | G24C | High | −3.79 | NA | 0.88 | + Bump c | None | None | Resistance | 0.25 | 2 | 1 |
| mmpR | G37R | High | −7.03 | 1.13 | 0.98 | + Bump | None | None | Resistance | 0.25 | 1 | 1 |
| mmpR | L39S | High | −3.14 | 5.72 | 2.92 | − Hydrophobic | None | None | Resistance | 0.06 | 1 | 1 |
| mmpR | L40W | Mediate | −3.54 | 9.31 | 1.26 | + Bump | None | None | Resistance | 0.5 | 4 | 1 |
| mmpR | W42R | High | −5.93 | >10 | 0.82 | − Hydrophobic | None | None | Resistance | 0.25 | 4 | 1 |
| mmpR | V45G | High | −6.17 | 5.77 | 1.53 | − Hydrophobic | None | None | Resistance | 0.125 | 4 | 3 |
| mmpR | G65E | High | −4.5 | 8.39 | 0.03 | None | + Bump | None | Resistance | 0.25 | 2 | 2 |
| mmpR | G78A | High | −4.15 | 0.67 | 0.93 | None | None | None | Resistance | 0.25 | 2 | 2 |
| mmpR | L83P | Mediate | −2.88 | 5.41 | 1.87 | − β-Sheet | None | None | Resistance | 0.5 | 2 | 1 |
| mmpR | V85F | Mediate | −0.61 | 1.15 | 0.24 | + Bump | None | None | Resistance | 0.125 | 2 | 1 |
| mmpR | A86V | Low | −1.42 | 1.28 | −0.05 | None | None | None | Uncertain | 0.125 | 2 | 1 |
| mmpR | F93S | Mediate | −5.8 | 3.2 | 2.73 | − Hydrophobic | None | None | Resistance | 0.5 | 4 | 1 |
| mmpR | A101E | Low | 0.25 | 1.65 | 0.04 | None | NA | None | Uncertain | 0.03 | 1 | 1 |
| mmpR | L142P | Mediate | −4.08 | >10 | 1.5 | − α-Helix | NA | None | Resistance | 0.125 | 2 | 1 |
| pepQ | L316R | High | −5.41 | >10 | 3.01 | − Hydrophobic | NA | NA | Resistance | 0.25 | 2 | 1 |
A mutation impact score based on a multiple sequence alignment of the protein sequence against the non-redundant protein sequence database, a score of lower than −2.282 was considered to have a deleterious effect. The values higher than −2.282 were indicated in bold.
The free energy (ΔΔG) was calculated for point mutations by using two endpoint methods, namely, Eris and PremPS. ΔΔG value higher than 0 indicated that the mutation might destabilize the protein structure. The value lower than 0 was indicated in bold.
+ Bump, introduction of unfavorable bump; − Hydrophobic, loss of hydrophobic interaction; − α-Helix, disruption of α-helix; − β-Sheet, disruption of β-sheet. Mutations without any change of interaction were indicated in bold.
The function of PepQ is still unclear. However, BLAST results revealed that the PepQ L316 residue was highly conserved. Meanwhile, the PepQ mutation that induced the L316R substitution markedly reduced the PROVEAN score to below −2.282 as an indication that this mutation reduced the similarity between the mutant MTB PepQ sequence and sequences of MTB PepQ orthologs. Moreover, predicted free energy changes (calculated using Eris and PremPS) suggested that this amino acid substitution would potentially cause structural instability of PepQ, as consistent with results of a detailed structural analysis suggesting that the PepQ L316R substitution led to loss of hydrophobic interaction(s) (Table 4). Taken together, the abovementioned sequence and structural analysis results indicate that the PepQ L316R amino acid substitution may contribute to drug resistance (Table 4).
DISCUSSION
In the present study, we performed in vitro experiments to generate progeny strains with increased BDQ and/or CFZ resistance that were derived from DS, MDR, and XDR-TB clinical isolates (parent strains). Subsequent sequencing of coding regions of several genes associated with progeny strain phenotypic resistance to these drugs resulted in the identification of novel gene mutations. Results of analyses of predicted amino acid sequence and protein structural changes induced by detected BDQ/CFZ-induced mmpR and pepQ mutations revealed potential impacts of these point mutations on MTB phenotypic expression of BDQ and/or CFZ resistance.
Results of previous studies have confirmed that phenotypic cross-resistance between BDQ and CFZ can occur in clinical MTB isolates with resistance to these drugs, including MTB strains with in vitro-induced BDQ and CFZ resistance and strains with BDQ/CFZ resistance acquired in vivo during MTB infection of mice (16, 18, 20, 22, 24, 40). Similarly, cross-resistance between BDQ and CFZ was observed in this work, as evidenced by elevated MICs observed after in vitro exposures of progeny strains to either of the two drugs. Specifically, all mutations induced by BDQ treatment resulted in CFZ resistance, as reflected by CFZ MICs within the range of 2‒4 µg/mL. In addition, each mutation-induced mutant protein (except for G24C and G65E) exhibited a fold change in CFZ MIC (relative to that of the parent strain) that was greater than that observed for its corresponding BDQ MIC.
In this work, 1 BDQ-treated progeny strain (8%) and 20 CFZ-treated progeny strains (48%) harbored no gene mutations in sequenced regions of mmpR, pepQ, and atpE, as consistent with results of previous studies (41 – 43). There are several possible explanations for the lack of resistance mutations within gene sequences of isolates with phenotypic resistance to these drugs, such as the presence of undetected mutations within other drug resistance genes, as well as false-positive drug resistance results. Nevertheless, spontaneous BDQ-induced mmpR mutations accounted for the highest proportion of detected mutations (83%), while the remaining mutations (17%) were detected within pepQ. Importantly, we did not identify any mutations within atpE, as consistent with results of previously reported studies showing that such mutations were infrequently detected in clinical samples (23, 44). Meanwhile, spontaneous CFZ-induced mutations detected here within mmpR and pepQ accounted for 86% and 14% of detected mutations, respectively. In other studies, in vitro exposure of the MTB H37Rv strain to BDQ or CFZ led to the generation of progeny strains with resistance to these drugs, of which 93% (157/168) of BDQ-resistant strains and 97% (93/96) of CFZ-resistant strains harbored mmpR mutations (25, 45). Similarly, most gene mutations detected in clinical isolates obtained from patients exposed to CFZ and/or BDQ occurred within the mmpR gene (71–80%) (24, 40, 46, 47). Taken together, the abovementioned results suggest that mmpR mutations play a major role in BDQ and CFZ resistance. Intriguingly, most MmpR mutant amino acid substitutions associated with drug resistance in this study (79%, 11/14) were located within the MmpR DNA-binding domain (residues 32–97), thus indicating this domain is a potential hotspot where drug resistance-induced amino acid changes with low fitness costs are frequently found. Similarly, results described in a recent systematic review of mutations associated with BDQ and CFZ resistance revealed that amino acid sequence changes resulting from BDQ/CFZ-induced mmpR mutations occurred most frequently within three MmpR DNA-binding domain regions (residues 64–66, 46–48, and 70–72) (48).
The most important limitation of the present study pertains to the fact that some mutations induced by in vitro drug treatments of our clinical isolates may have remained undetected. Nevertheless, parent strains studied herein were derived from clinical isolates and thus were more closely related to clinically relevant organisms than standard laboratory strains. However, previously reported Q76*, G78A, and F93S MmpR mutants (25, 44, 47), and mutants reported here for the first time (to our knowledge), should be verified for clinical relevance through analysis of additional drug-resistant clinical MTB isolates. Furthermore, it has been suggested that the development of BDQ resistance in vitro is a dynamic process, such that mmpR mutation-induced acquisition of low-level BDQ resistance may be an initial transient, but required, step for subsequent acquisition of high-level BDQ resistance through mutation of atpE (49). Nonetheless, we did not engage in multiple exposure-based screening processes that might have enabled us to observe the abovementioned dynamic phenomenon. Moreover, the MIC breakpoint used here to classify MTB strains as BDQ resistant has not been independently validated, while WHO-endorsed BDQ MIC breakpoints that were chosen to minimize false-positive resistance results may lead to missed detection of BDQ-resistant TB cases associated with only small BDQ MIC increases. Finally, we did not conduct whole-genome sequencing (WGS) of BDQ/CFZ-resistant progeny strains that lacked detected mutations, thus warranting additional WGS-based studies to explore additional novel drug resistance-inducing MTB gene mutations.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (81401739), Beijing Natural Science Foundation (7232060), and Beijing Key Clinical Specialty Project (20201214).
J.L. and Y.P. developed the concept and designed the experiments; J.S. and M.G. assisted with the design; J.S., T.W., Y.L., and L.L. conducted the in vitro MIC experiment; J.S., Y.L., and T.W. carried out selection of spontaneous-resistant mutants; J.S., T.W., and Y.L. sequenced and analyzed the spontaneous mutants; J.L. performed sequence and structure analysis; S.H., X.P., Y.S., and Y.G. analyzed the results. J.S., Y.L., and J.L. wrote the manuscript; all the authors modified the manuscript.
The authors declare that there are no conflicts of interest.
Contributor Information
Jie Lu, Email: lujiebch@163.com.
Alexandra Aubry, AP-HP, Paris, France .
DATA AVAILABILITY
The mmpR and pepQ nucleotide sequences of all mutant strains were deposited in GenBank with accession numbers OR188353 to OR188386.
ETHICS APPROVAL
All clinical isolates and study protocols used in this research study were approved by the Ethics Committee of Beijing Chest Hospital, a hospital affiliated with Capital Medical University (No. 2016KY05).
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/spectrum.00090-23.
Tables S1 to S2.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. WHO . 2022. Global tuberculosis report 2022. WHO, Geneva, Switzerland. https://www.who.int/publications/i/item/9789240061729. [Google Scholar]
- 2. Cox V, Wilkinson L, Grimsrud A, Hughes J, Reuter A, Conradie F, Nel J, Boyles T. 2020. Critical changes to services for TB patients during the COVID-19 pandemic. Int J Tuberc Lung Dis 24:542–544. doi: 10.5588/ijtld.20.0205 [DOI] [PubMed] [Google Scholar]
- 3. McQuaid CF, McCreesh N, Read JM, Sumner T, CMMID COVID-19 Working Group, Houben R, White RG, Harris RC. 2020. The potential impact of COVID-19-related disruption on tuberculosis burden. Eur Respir J 56:2001718. doi: 10.1183/13993003.01718-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pang Y, Liu Y, Du J, Gao J, Li L. 2020. Impact of COVID-19 on tuberculosis control in China. Int J Tuberc Lung Dis 24:545–547. doi: 10.5588/ijtld.20.0127 [DOI] [PubMed] [Google Scholar]
- 5. WHO . 2022. WHO consolidated guidelines on tuberculosis. Module 4: treatment - drug-resistant tuberculosis treatment, 2022 update. WHO, Geneva, Switzerland. Available from: https://www.who.int/publications/i/item/9789240063129 [PubMed] [Google Scholar]
- 6. Conradie F, Diacon AH, Ngubane N, Howell P, Everitt D, Crook AM, Mendel CM, Egizi E, Moreira J, Timm J, McHugh TD, Wills GH, Bateson A, Hunt R, Van Niekerk C, Li M, Olugbosi M, Spigelman M. 2020. Treatment of highly drug-resistant pulmonary tuberculosis. N Engl J Med 382:893–902. doi: 10.1056/NEJMoa1901814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Browne SG, Hogenzeil LM. 1962. "B 663" in the treatment of leprosy. Supplementary report of the pilot trial. Lepr Rev 33:182–184. [PubMed] [Google Scholar]
- 8. Swanson RV, Ammerman NC, Ngcobo B, Adamson J, Moodley C, Dorasamy A, Moodley S, Mgaga Z, Bester LA, Singh SD, Almeida DV, Grosset JH. 2016. Clofazimine contributes sustained antimicrobial activity after treatment cessation in a mouse model of tuberculosis chemotherapy. Antimicrob Agents Chemother 60:2864–2869. doi: 10.1128/AAC.00177-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van Rensburg CE, Jooné GK, Sirgel FA, Matlola NM, O’Sullivan JF. 2000. In vitro investigation of the antimicrobial activities of novel tetramethylpiperidine-substituted phenazines against Mycobacterium tuberculosis. Chemotherapy 46:43–48. doi: 10.1159/000007255 [DOI] [PubMed] [Google Scholar]
- 10. Aung KJM, Van Deun A, Declercq E, Sarker MR, Das PK, Hossain MA, Rieder HL. 2014. Successful '9-month Bangladesh regimen' for multidrug-resistant tuberculosis among over 500 consecutive patients. Int J Tuberc Lung Dis 18:1180–1187. doi: 10.5588/ijtld.14.0100 [DOI] [PubMed] [Google Scholar]
- 11. Kuaban C, Noeske J, Rieder HL, Aït-Khaled N, Abena Foe JL, Trébucq A. 2015. High effectiveness of a 12-month regimen for MDR-TB patients in Cameroon. Int J Tuberc Lung Dis 19:517–524. doi: 10.5588/ijtld.14.0535 [DOI] [PubMed] [Google Scholar]
- 12. Trébucq A, Schwoebel V, Kashongwe Z, Bakayoko A, Kuaban C, Noeske J, Hassane S, Souleymane B, Piubello A, Ciza F, Fikouma V, Gasana M, Ouedraogo M, Gninafon M, Van Deun A, Cirillo DM, Koura KG, Rieder HL. 2018. Treatment outcome with a short multidrug-resistant tuberculosis regimen in nine African countries. Int J Tuberc Lung Dis 22:17–25. doi: 10.5588/ijtld.17.0498 [DOI] [PubMed] [Google Scholar]
- 13. Van Deun A, Maug AKJ, Salim MAH, Das PK, Sarker MR, Daru P, Rieder HL. 2010. Short, highly effective, and inexpensive standardized treatment of multidrug-resistant tuberculosis. Am J Respir Crit Care Med 182:684–692. doi: 10.1164/rccm.201001-0077OC [DOI] [PubMed] [Google Scholar]
- 14. Ammerman NC, Swanson RV, Bautista EM, Almeida DV, Saini V, Omansen TF, Guo H, Chang YS, Li S-Y, Tapley A, Tasneen R, Tyagi S, Betoudji F, Moodley C, Ngcobo B, Pillay L, Bester LA, Singh SD, Chaisson RE, Nuermberger E, Grosset JH. 2018. Impact of clofazimine dosing on treatment shortening of the first-line regimen in a mouse model of tuberculosis. Antimicrob Agents Chemother 62:e00636-18. doi: 10.1128/AAC.00636-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tyagi S, Ammerman NC, Li SY, Adamson J, Converse PJ, Swanson RV, Almeida DV, Grosset JH. 2015. Clofazimine shortens the duration of the first-line treatment regimen for experimental chemotherapy of tuberculosis. Proc Natl Acad Sci U S A 112:869–874. doi: 10.1073/pnas.1416951112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Almeida D, Ioerger T, Tyagi S, Li S-Y, Mdluli K, Andries K, Grosset J, Sacchettini J, Nuermberger E. 2016. Mutations in pepQ confer low-level resistance to bedaquiline and clofazimine in Mycobacterium tuberculosis. Antimicrob Agents Chemother 60:4590–4599. doi: 10.1128/AAC.00753-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cholo MC, Mothiba MT, Fourie B, Anderson R. 2017. Mechanisms of action and therapeutic efficacies of the lipophilic antimycobacterial agents clofazimine and bedaquiline. J Antimicrob Chemother 72:338–353. doi: 10.1093/jac/dkw426 [DOI] [PubMed] [Google Scholar]
- 18. Hartkoorn RC, Uplekar S, Cole ST. 2014. Cross-resistance between clofazimine and bedaquiline through upregulation of MmpL5 in Mycobacterium tuberculosis. Antimicrob Agents Chemother 58:2979–2981. doi: 10.1128/AAC.00037-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Walker TM, Miotto P, Köser CU, Fowler PW, Knaggs J, Iqbal Z, Hunt M, Chindelevitch L, Farhat M, Cirillo DM, Comas I, Posey J, Omar SV, Peto TE, Suresh A, Uplekar S, Laurent S, Colman RE, Nathanson C-M, Zignol M, Walker AS, CRyPTIC Consortium, Seq&Treat Consortium, Crook DW, Ismail N, Rodwell TC. 2022. The 2021 WHO catalogue of Mycobacterium tuberculosis complex mutations associated with drug resistance: a genotypic analysis. Lancet Microbe 3:e265–e273. doi: 10.1016/S2666-5247(21)00301-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Andries K, Villellas C, Coeck N, Thys K, Gevers T, Vranckx L, Lounis N, de Jong BC, Koul A. 2014. Acquired resistance of Mycobacterium tuberculosis to bedaquiline. PLoS One 9:e102135. doi: 10.1371/journal.pone.0102135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ismail N, Omar SV, Ismail NA, Peters RPH. 2018. In vitro approaches for generation of Mycobacterium tuberculosis mutants resistant to bedaquiline, clofazimine or linezolid and identification of associated genetic variants. J Microbiol Methods 153:1–9. doi: 10.1016/j.mimet.2018.08.011 [DOI] [PubMed] [Google Scholar]
- 22. Ismail N, Peters RPH, Ismail NA, Omar SV. 2019. Clofazimine exposure in vitro selects efflux pump mutants and bedaquiline resistance. Antimicrob Agents Chemother 63:e02141-18. doi: 10.1128/AAC.02141-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu Y, Gao M, Du J, Wang L, Gao J, Shu W, Wang Y, Xue Z, Li L, Pang Y. 2021. Reduced susceptibility of Mycobacterium tuberculosis to bedaquiline during antituberculosis treatment and its correlation with clinical outcomes in China. Clin Infect Dis 73:e3391–e3397. doi: 10.1093/cid/ciaa1002 [DOI] [PubMed] [Google Scholar]
- 24. Pang Y, Zong Z, Huo F, Jing W, Ma Y, Dong L, Li Y, Zhao L, Fu Y, Huang H. 2017. In vitro drug susceptibility of bedaquiline, delamanid, linezolid, clofazimine, moxifloxacin, and gatifloxacin against extensively drug-resistant tuberculosis in Beijing, China. Antimicrob Agents Chemother 61:e00900-17. doi: 10.1128/AAC.00900-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang S, Chen J, Cui P, Shi W, Zhang W, Zhang Y. 2015. Identification of novel mutations associated with clofazimine resistance in Mycobacterium tuberculosis. J Antimicrob Chemother 70:2507–2510. doi: 10.1093/jac/dkv150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. WHO . 2020. Meeting report of the WHO expert consultation on the definition of extensively drug-resistant tuberculosis. Geneva, Switzerland: WHO. https://www.who.int/publications/i/item/9789240018662. [Google Scholar]
- 27. WHO . 2008. Guidelines for the programmatic management of drug-resistant tuberculosis. WHO, Geneva, Switzerland. Available from: https://www.who.int/publications/i/item/9789241547581 [Google Scholar]
- 28. Palomino J-C, Martin A, Camacho M, Guerra H, Swings J, Portaels F. 2002. Resazurin microtiter assay plate: simple and inexpensive method for detection of drug resistance in Mycobacterium tuberculosis. Antimicrob Agents Chemother 46:2720–2722. doi: 10.1128/AAC.46.8.2720-2722.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lopez B, Siqueira de Oliveira R, Pinhata JMW, Chimara E, Pacheco Ascencio E, Puyén Guerra ZM, Wainmayer I, Simboli N, Del Granado M, Palomino JC, Ritacco V, Martin A. 2019. Bedaquiline and linezolid MIC distributions and epidemiological cut-off values for Mycobacterium tuberculosis in the Latin American region. J Antimicrob Chemother 74:373–379. doi: 10.1093/jac/dky414 [DOI] [PubMed] [Google Scholar]
- 30. Park S, Jung J, Kim J, Han SB, Ryoo S. 2022. Investigation of clofazimine resistance and genetic mutations in drug-resistant Mycobacterium tuberculosis isolates. J Clin Med 11:1927. doi: 10.3390/jcm11071927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Georghiou SB, Rodwell TC, Korobitsyn A, Abbadi SH, Ajbani K, Alffenaar J-W, Alland D, Alvarez N, Andres S, Ardizzoni E, Aubry A, Baldan R, Ballif M, Barilar I, Böttger EC, Chakravorty S, Claxton PM, Cirillo DM, Comas I, Coulter C, Denkinger CM, Derendinger B, Desmond EP, de Steenwinkel JEM, Dheda K, Diacon AH, Dolinger DL, Dooley KE, Egger M, Ehsani S, Farhat MR, Fattorini L, Finci I, Le Ray LF, Furió V, Groenheit R, Gumbo T, Heysell SK, Hillemann D, Hoffmann H, Hsueh P-R, Hu Y, Huang H, Hussain A, Ismail F, Izumi K, Jagielski T, Johnson JL, Kambli P, Kaniga K, Eranga Karunaratne GHR, Sharma MK, Keller PM, Kelly EC, Kholina M, Kohli M, Kranzer K, Laurenson IF, Limberis J, Grace Lin S-Y, Liu Y, López-Gavín A, Lyander A, Machado D, Martinez E, Masood F, Mitarai S, Mvelase NR, Niemann S, Nikolayevskyy V, Maurer FP, Merker M, Miotto P, Omar SV, Otto-Knapp R, Palaci M, Palacios Gutiérrez JJ, Peacock SJ, Peloquin CA, Perera J, Pierre-Audigier C, Pholwat S, Posey JE, Prammananan T, Rigouts L, Robledo J, Rockwood N, Rodrigues C, Salfinger M, Schechter MC, Seifert M, Sengstake S, Shinnick T, Shubladze N, Sintchenko V, Sirgel F, Somasundaram S, Sterling TR, Spitaleri A, Streicher E, Supply P, Svensson E, Tagliani E, Tahseen S, Takaki A, Theron G, Torrea G, Van Deun A, van Ingen J, Van Rie A, van Soolingen D, Vargas Jr R, Venter A, Veziris N, Villellas C, Viveiros M, Warren R, Wen S, Werngren J, Wilkinson RJ, Yang C, Yılmaz FF, Zhang T, Zimenkov D, Ismail N, Köser CU, Schön T, Antimycobacterial Susceptibility Testing Group . 2022. Updating the approaches to define susceptibility and resistance to anti-tuberculosis agents: implications for diagnosis and treatment. Eur Respir J 59:2200166. doi: 10.1183/13993003.00166-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kaniga K, Aono A, Borroni E, Cirillo DM, Desmaretz C, Hasan R, Joseph L, Mitarai S, Shakoor S, Torrea G, Ismail NA, Omar SV. 2020. Validation of bedaquiline phenotypic drug susceptibility testing methods and breakpoints: a multilaboratory, multicountry study. J Clin Microbiol 58:e01677-19. doi: 10.1128/JCM.01677-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kaniga K, Cirillo DM, Hoffner S, Ismail NA, Kaur D, Lounis N, Metchock B, Pfyffer GE, Venter A. 2016. A multilaboratory, multicountry study to determine bedaquiline MIC quality control ranges for phenotypic drug susceptibility testing. J Clin Microbiol 54:2956–2962. doi: 10.1128/JCM.01123-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pan J, Ye H, Wu Z, Xiao J, Liu J. 2021. Correction to: one-step melting curve analysis-based McSpoligotyping reveals genotypes of Mycobacterium tuberculosis in a coastal city, China. Arch Microbiol 203:5277. doi: 10.1007/s00203-021-02475-4 [DOI] [PubMed] [Google Scholar]
- 35. Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, Lopez R, McWilliam H, Remmert M, Söding J, Thompson JD, Higgins DG. 2011. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol 7:539. doi: 10.1038/msb.2011.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Robert X, Gouet P. 2014. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res 42:W320–W324. doi: 10.1093/nar/gku316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Choi Y, Sims GE, Murphy S, Miller JR, Chan AP, de Brevern AG. 2012. Predicting the functional effect of amino acid substitutions and indels. PLoS ONE 7:e46688. doi: 10.1371/journal.pone.0046688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yin S, Ding F, Dokholyan NV. 2007. Eris: an automated estimator of protein stability. Nat Methods 4:466–467. doi: 10.1038/nmeth0607-466 [DOI] [PubMed] [Google Scholar]
- 39. Chen Y, Lu H, Zhang N, Zhu Z, Wang S, Li M. 2020. PremPS: predicting the impact of missense mutations on protein stability. PLoS Comput Biol 16:e1008543. doi: 10.1371/journal.pcbi.1008543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ismail NA, Omar SV, Joseph L, Govender N, Blows L, Ismail F, Koornhof H, Dreyer AW, Kaniga K, Ndjeka N. 2018. Defining bedaquiline susceptibility, resistance, cross-resistance and associated genetic determinants: a retrospective cohort study. EBioMedicine 28:136–142. doi: 10.1016/j.ebiom.2018.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kardan-Yamchi J, Kazemian H, Battaglia S, Abtahi H, Foroushani AR, Hamzelou G, Cirillo DM, Ghodousi A, Feizabadi MM. 2020. Whole genome sequencing results associated with minimum inhibitory concentrations of 14 anti-tuberculosis drugs among rifampicin-resistant isolates of Mycobacterium tuberculosis from iran. J Clin Med 9:465. doi: 10.3390/jcm9020465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Saeed DK, Shakoor S, Razzak SA, Hasan Z, Sabzwari SF, Azizullah Z, Kanji A, Nasir A, Shafiq S, Ghanchi NK, Hasan R. 2022. Variants associated with Bedaquiline (BDQ) resistance identified in Rv0678 and efflux pump genes in Mycobacterium tuberculosis isolates from BDQ naïve TB patients in Pakistan. BMC Microbiol 22:62. doi: 10.1186/s12866-022-02475-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yang JS, Kim KJ, Choi H, Lee SH. 2018. Delamanid, bedaquiline, and linezolid minimum inhibitory concentration distributions and resistance-related gene mutations in multidrug-resistant and extensively drug-resistant tuberculosis in Korea. Ann Lab Med 38:563–568. doi: 10.3343/alm.2018.38.6.563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nimmo C, Millard J, van Dorp L, Brien K, Moodley S, Wolf A, Grant AD, Padayatchi N, Pym AS, Balloux F, O’Donnell M. 2020. Population-level emergence of bedaquiline and clofazimine resistance-associated variants among patients with drug-resistant tuberculosis in Southern Africa: a phenotypic and phylogenetic analysis. Lancet Microbe 1:e165–e174. doi: 10.1016/S2666-5247(20)30031-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Guo Q, Bi J, Lin Q, Ye T, Wang Z, Wang Z, Liu L, Zhang G. 2022. Whole genome sequencing identifies novel mutations associated with bedaquiline resistance in Mycobacterium tuberculosis. Front Cell Infect Microbiol 12:807095. doi: 10.3389/fcimb.2022.807095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Andrés S, Merker M, Heyckendorf J. 2020. Bedaquiline-resistant tuberculosis: dark clouds on the horizon. Am J Respir Crit Care Med 201:1564–1568. doi: 10.3390/ani10122364 [DOI] [PubMed] [Google Scholar]
- 47. Zimenkov DV, Nosova Ey, Kulagina EV, Antonova OV, Arslanbaeva LR, Isakova AI, Krylova Ly, Peretokina IV, Makarova MV, Safonova SG, Borisov SE, Gryadunov DA. 2017. Examination of bedaquiline- and linezolid-resistant Mycobacterium tuberculosis isolates from the moscow region. J Antimicrob Chemother 72:1901–1906. doi: 10.1093/jac/dkx094 [DOI] [PubMed] [Google Scholar]
- 48. Kadura S, King N, Nakhoul M, Zhu H, Theron G, Köser CU, Farhat M. 2020. Systematic review of mutations associated with resistance to the new and repurposed Mycobacterium tuberculosis drugs bedaquiline, clofazimine, linezolid, delamanid and pretomanid. J Antimicrob Chemother 75:2031–2043. doi: 10.1093/jac/dkaa136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ismail N, Ismail NA, Omar SV, Peters RPH. 2019. In vitro study of stepwise acquisition of Rv0678 and atpE mutations conferring bedaquiline resistance. Antimicrob Agents Chemother 63. doi: 10.1128/AAC.00292-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1 to S2.
Data Availability Statement
The mmpR and pepQ nucleotide sequences of all mutant strains were deposited in GenBank with accession numbers OR188353 to OR188386.