ABSTRACT
California serogroup viruses (CSGVs) of medical importance in the United States include La Crosse virus, Jamestown Canyon virus (JCV), California encephalitis virus, and snowshoe hare virus. Current diagnosis of CSGVs relies heavily on serologic techniques for detecting immunoglobulin M (IgM), an indication of a recent CSGV infection. However, human-positive control sera reactive to viruses in the serogroup are scarce because detection of recent infections is rare. Here, we describe the development of new murine monoclonal antibodies (MAbs) reactive to CSGVs and the engineering of a human-murine chimeric antibody by combining the variable regions of the broadly CSGV cross-reactive murine MAb, 3-3B6/2-3B2 and the constant region of the human IgM. MAb 3-3B6/2-3B2 recognizes a tertiary epitope on the Gn/Gc heterodimer, and epitopes important in JCV neutralization were mapped to the Gc glycoprotein. This engineered human IgM constitutively expressed in a HEK-293 stable cell line can replace human-positive control sera in diagnostic serological techniques such as IgM antibody capture enzyme-linked immunosorbent assay (MAC-ELISA). Compared to the parent murine MAbs, the human-chimeric IgM antibody had identical serological activity to CSGVs in ELISA and demonstrated equivalent reactivity compared to human immune sera in the MAC-ELISA.
Importance
Orthobunyaviruses in the California serogroup cause severe neurological disease in children and adults. While these viruses are known to circulate widely in North America, their occurrence is rare. Serological testing for CSGVs is hindered by the limited availability and volumes of human-positive specimens needed as controls in serologic assays. Here, we described the development of a murine monoclonal antibody cross-reactive to CSGVs engineered to contain the variable regions of the murine antibody on the backbone of human IgM. The chimeric IgM produced from the stably expressing HEK293 cell line was evaluated for use as a surrogate human-positive control in a serologic diagnostic test.
KEYWORDS: La Crosse virus, Jamestown Canyon virus, immunoglobulin M, serology, immunodiagnostics, California serogroup viruses, ELISA, humanized antibodies, murine antibodies
INTRODUCTION
The California serogroup viruses (CSGVs) are a group of viruses in the genus Orthobunyavirus, family Peribunyaviridae of the order Bunyavirales (1, 2). Eight of these viruses are known to cause human neuroinvasive disease, including La Crosse virus (LACV), Jamestown Canyon virus (JCV), California encephalitis virus (CEV), Keystone virus, Trivitattus virus, and snowshoe hare virus (SSHV), which circulate in the United States (3, 4). JCV and SSHV have also been detected in Canada, whereas Tahyna virus (TAHV) has a widespread distribution throughout Europe and Asia, while Inkoo virus has caused a few cases of neuroinvasive disease in Scandinavia (5 – 9). While CSGVs have been detected across the globe, some viruses have only been isolated a few times and, therefore, may not be actively circulating (5).
Orthobunyaviruses have a tripartite negative single-stranded RNA genome encoding three structural proteins. The nucleocapsid (N) protein is approximately 23–27 kDa in size and encapsidates the segmented genome and several copies of the viral RNA-dependent RNA polymerase. A lipid bilayer surrounds the nucleocapsid, and glycoproteins, Gc (108–125 kDa) and Gn (29–41 kDa), are displayed on the surface of the virion. While the nucleocapsid is involved in viral replication, the glycoproteins are involved in receptor-mediated endocytosis and viral-cell membrane fusion (10).
CSGVs are maintained in a zoonotic cycle between mosquitoes (primarily Aedes sp.) and small mammals such as squirrels, chipmunks, and hares (11 – 15). Transovarial transmission of CSGVs likely contributes to overwintering (6, 13 – 23). Isolation of virus in hares during winter indicates an alternative overwintering strategy for SSHV (13). JCV is the only CSGV that is known to infect large mammalian hosts, such as white-tailed deer and other ungulates, as well as some livestock (16, 24 – 27).
Human diseases from CSGV infections range in severity from asymptomatic and mild symptoms, such as headache, fever, and vomiting, to severe neuroinvasive complications such as encephalitis, meningoencephalitis, and meningitis (5, 8, 28 – 30). LACV is the leading cause of pediatric arboviral encephalitis in the United States with approximately 50–150 cases per year (28). LACV has a reported case fatality rate of 1.9% in children (31). Conversely, JCV infection is more commonly seen in adults with approximately 25 cases of JCV per year, although increasing case numbers have been recorded since 2013, which could be indicative of more testing by public health labs (32). From 2017 to 2019, epidemic levels of JCV were reported (81 cases) by the U.S. Centers for Disease Control and Prevention (CDC) (33, 34). Half of all JCV cases were reported in Wisconsin from 2010 to 2019, which is likely the result of enhanced surveillance within the state (35). Fatalities from JCV infection are rare; however, disease in immunosuppressed individuals may result in a progressive fatal infection (36, 37). SSHV, like LACV, is more commonly seen in children with several cases per year in Canada (8, 38, 39). TAHV infections manifest as flu-like illness, called Valtice fever, commonly observed in children but rarely causing neuroinvasive disease (6, 40, 41). Due to asymptomatic or mild illness, testing for CSGVs is not routinely performed unless an arboviral infection is suspected; therefore, disease from CSGVs may be underreported (5). In areas considered to be endemic for CSGVs, seroprevalence can range from 27% for LACV, 15%–54% for JCV, 42% for SSHV, and up to 80% for TAHV (5, 6, 26, 42 – 47). In 2020, the United States saw an increase in the incidence of rare arboviruses, including a 22% increase in LACV infections, the highest increase in cases since 2011 (48).
Diagnosis of CSGV infection relies mostly on serology (36, 49, 50). Molecular-based diagnostic methods, such as RT-PCR, are typically not used due to the low or transient viremia observed in CSGV infected patients usually before symptoms manifest (1, 49, 50). Molecular-based methods may be applied in diagnosing immunocompromised individuals (1, 37). The most commonly used serologic assay is the IgM antibody capture enzyme-linked immunosorbent assay (MAC-ELISA), which tests virus-reactive IgM that may be present during acute viral infection and early convalescence. Plaque reduction neutralization test (PRNT) is used for subsequent confirmation of MAC-ELISA results (49). Serodiagnosis of CSGVs is hindered by the limited availability and volumes of human-positive specimens needed as controls in the MAC-ELISA. In 2021, only seven LACV-endemic states in the United States reported using the CDC MAC-ELISA, while historically 23 states have reported LACV cases since 2003 (51). Likewise, only five JCV-endemic states reported using the CDC MAC-ELISA, while 18 states have reported JCV cases since 2011 (52).
To meet the need for a better human-positive control supply, we first developed murine hybridomas expressing CSGV cross-reactive monoclonal antibodies (MAbs). A subsequent murine-human chimeric IgM antibody expressing the variable regions of the murine MAbs on the human IgM backbone was expressed constitutively in HEK-293 cells. In this report, we describe the evaluation of the CSGV group-reactive chimeric IgM antibody in the standard MAC-ELISA, and its relative performance was compared to antibody-positive human control specimens against all CSGVs tested.
MATERIALS AND METHODS
Cells, viruses, and diagnostic specimens
LACV strain AR6319, JCV strain 61V2235, TAHV strain Bardos 92, and SSHV strain Original were obtained from the Arboviral Diseases Branch, Arbovirus Reference Collection (ARC, Fort Collins, CO, USA). Stocks of virus were grown in African green monkey Vero cells and Aedes albopictus C6/36 cells in Dulbecco's modified Eagle medium (DMEM) (Cat# 11965118, Invitrogen, Waltham, MA, USA) supplemented with 2% fetal bovine serum (FBS) (Cat# 1500-500G, VWR, Radnor, PA, USA), 2 mM L-glutamine (Cat# 25030-081 Invitrogen, Waltham, MA, USA), 0.15% sodium bicarbonate (Cat# 25080-094, Gibco, Waltham, MA, USA), 100 U/mL penicillin G sodium, and 100 µg/mL streptomycin (Cat # 15140-122, Gibco, Waltham, MA, USA) at 37°C in 5% CO2. LACV and JCV were purified on glycerol tartrate gradients and protein concentrations were determined by Bradford assay (Cat# 5000006, BioRad, Hercules, CA, USA) as previously described (53). These purified stocks were used in subsequent serologic assays. Human embryonic kidney-293 (HEK-293) cells (HEK-293.2sus CRL-1573.3, ATCC, VA, USA) were grown in a 1:1 mixture of Expi-293 media (ThermoFisher, MA, USA) and Ex-Cell media (Millipore Sigma, MA, USA) with 1% (vol/vol) penicillin-streptomycin (ThermoFisher Scientific, Waltham, MA, USA). Cells were incubated at 37°C with 5% CO2 in suspension with constant mixing at 130 rpm on orbital shaking platforms (ThermoFisher, MA, USA).
Recombinant nucleoprotein (N) protein expression and purification for animal studies
Viral RNA was extracted from infected cell supernatant using a QIAmp viral RNA minikit (Cat# 52904, Qiagen, Hilden, Germany) according to the manufacturer’s instructions. LACV and JCV N genes were amplified by RT-PCR and cloned into the pMAL-c5X vector (Cat# E8200, New England Biolabs, Ipswich, MA, USA) using the NEBuilder High-Fidelity DNA Assembly Cloning kit (Cat# E5520S, New England Biolabs, Ipswich, MA, USA) with virus sequence-specific primers according to the manufacturer’s protocol (Table S1). Confirmation of N genes into the constructed plasmid was performed by Sanger sequencing on an ABI 3130 instrument (Applied Biosystems, Waltham, MA, USA). Recombinant plasmids (JCV-N-pMAL and LACV-N-pMAL) expressing the JCV or LACV N protein fused to the maltose-binding protein (MBP) were transformed into Escherichia coli ER2523 cells. LACV-N-MBP or JCV-N-MBP protein production was induced with isopropyl β-D-1-thiogalactopyranoside and purified from cell lysate according to the manufacturer’s protocol for pMAL protein fusion and purification (Cat# E8200, New England Biolabs, Ipswich, MA, USA). Recombinant proteins were separated by sodium dodecyl sulphate polyacrylamide gel electrophoresis on a 4%–12% Bis-Tris gel (Cat# NP0335PK2, ThermoFisher Scientific, Waltham, MA, USA) under reduced conditions with dithiothreitol (DTT) (Cat# NP0004, ThermoFisher Scientific, Waltham, MA, USA) and stained with SimplyBlue SafeStain (Cat#LC6060, ThermoFisher Scientific, Waltham, MA, USA).
Animal studies
AG129 mice deficient in interferon α/β and γ receptors were originally obtained from B&K Universal (Hull, United Kingdom) and bred in-house. Virulence of JCV in 5-week-old AG129 mice was evaluated by inoculating three groups of mice (n = 6) intraperitoneally with 10-fold dilutions of virus (10–10−1 PFU/0.1 mL). After challenge, mice were monitored four times per day for signs of morbidity (hunched posture, ruffled fur, and neurological signs) for 3 weeks. Mice that showed signs of morbidity were euthanized immediately. The first immunization of surviving mice (n = 3) inoculated in the 1 PFU group was done with a combination of 10 µg of recombinant LACV or JCV N protein coupled to MBP and Magic Mouse adjuvant (Cat# CDN-A001, Creative Diagnostics, Shirley, NY, USA) 20 days after viral challenge. A second boost was performed 40 days later, and the final boost of 10 PFU/0.1 mL of LACV before hybridoma fusions were performed 60 days after JCV infection. Mice were handled as specified by the Division of Vector-Borne Diseases Institutional Animal Care and Use Committee recommendations (Protocol #19-003).
Indirect ELISA
ELISAs were performed in 96-well plates (Cat# 44-2404-21, Nunc Maxisorp plates, ThermoFisher Scientific, Waltham, MA, USA). Plates were coated with 0.25 µg/well of either purified LACV or JCV, diluted in carbonate/bicarbonate buffer (50 mM sodium carbonate and 50 mM sodium bicarbonate, pH 9.6), and incubated overnight at 4°C. Plates were washed five times with phosphate buffered saline (PBS)/0.1% Tween-20 wash buffer with an automatic plate washer. Non-specific binding sites were blocked with Pierce Starting Block (PBS formulation) blocking buffer (Cat# 37538, ThermoFisher Scientific, Waltham, MA, USA) (100 µL/well) and immediately removed. Mouse sera tested in duplicate and diluted in PBS were added in fourfold dilutions (50 µL/well) and incubated for 1 h at 37°C. Plates were washed five times before the addition of goat anti-mouse antibody conjugated to horseradish peroxidase (Cat# 115-035-062, Jackson ImmunoResearch, West Grove, PA, USA) (50 µL/well), diluted 1:5,000 in PBS. After an incubation period of 1 h at 37°C, plates were washed again 10 times. Enhanced K-blue tetramethylbenzidine (TMB) substrate (Cat# 308175, Neogen, Lansing, MI, USA) was added to each well of the plate (100 µL/well) and incubated in the dark at room temperature for 10 min. The reaction was stopped with the addition of 2N H2SO4 (50 µL/well), and the plates were read at 450 nm. Virus binding curves were generated by a four-parameter nonlinear logistic regression model used to calculate the half-maximal effective concentration (EC50) values of sera at each timepoint using GraphPad Prism V6 with the bottom of the curve constrained to 0.
Hybridoma development
Five days after the last inoculation, splenocytes from vaccinated mice were harvested. Electrofusions with the mouse myeloma cell line P3 × 63Ag8.653 were performed with the ECM2001 electrofusion system (BTX, Holliston, MA, USA) in glass microslides with a 10 mm gap according to the manufacturer’s protocol. After 48 h of recovery, fused cells were plated in ClonaCell-HY hybridoma semi-solid selection medium (Cat# 03804, StemCell Technologies, Vancouver, BC, Canada) containing anti-mouse immunoglobulin G (IgG) (H + L) CloneDetect (Cat# K8220, Molecular Devices, Sunnyvale, CA, USA) and incubated for 10 days at 37°C in 5% CO2. Clones expressing anti-mouse Ig were identified and picked with the ClonePix2 automated colony picker (Molecular Devices, Sunnyvale, CA, USA) according to the manufacturer’s protocol. Clones were grown in 96-well plates in Clona-Cell-HY hybridoma medium containing hypoxanthine and thymidine (Cat# 03805, StemCell Technologies, Vancouver, BC, Canada). Positive clones were selected after screening hybridoma supernatant by ELISA and expanded in CD hybridoma medium (Cat#11279023, ThermoFisher Scientific, Waltham, MA, USA) supplemented with 2% low IgG FBS (Cat# 16250078, ThermoFisher Scientific, Waltham, MA, USA). Up to 500 mL of hybridoma supernatant was concentrated using centrifugal concentrators (Cat# UFC710008, Millipore Sigma, Burlington, MA, USA) with a 100 kDa cut off and dialyzed overnight in 0.1M phosphate buffer.
MAb isotyping and purification
MAb isotypes were determined using the Pierce Rapid Mouse Antibody Isotyping kit (Cat# 26178, ThermoFisher Scientific, Waltham, MA, USA). Murine MAbs were purified using protein-A Sepharose (Cat# 101041, GE Healthcare, Pittsburgh, PA, USA) for IgG purification, and chimeric IgM antibodies were purified using the Pierce IgM purification kit (Cat# 44897, ThermoFisher Scientific, Waltham, MA, USA) according to the manufacturer’s instructions. Protein concentrations were determined by Bradford assay (Cat# 5000006, BioRad, Hercules, CA, USA) as previously described (53).
PRNT
Purified MAbs were tested in duplicate in PRNT with CSGVs: LACV, JCV, SSHV, and TAHV. Approximately, 100 PFU of each CSGV were incubated with equal amounts of serial twofold dilutions of purified MAb in BA1 media [Hanks M-199 salts (Cat# M9163, Sigma-Aldrich, St. Louis, MO, USA)], 0.05M Tris pH 7.6 (Cat# 15567027, Gibco, Waltham, MA, USA), 1% bovine serum albumin (Cat# 81-066-4, Millipore, Burlington, MA, USA), 0.35 g/L sodium bicarbonate (Cat#25080-094, Gibco, Waltham, MA, USA), 100 U/mL penicillin, 100 mg/L streptomycin (Cat #15140-122, Gibco, Waltham, MA, USA), 1 mg/L fungizone (Cat# SV30078.01, Hyclone, Marlborough, MA, USA) starting at 104 ng/mL and incubated overnight at 4°C. Six-well plates of Vero cells were then inoculated with the virus–MAb mixtures and incubated at 37°C with 5% CO2 for 1 h, after which the cells were overlaid with 3 mL of medium containing 1% SeaKem LE agarose (Cat# 50004, Lonza, Rockland, ME, USA) in nutrient medium [0.165% lactalbumin hydrolysate (Cat# 259962, VWR, Radnor, PA, USA)], 0.033% yeast extract (Cat#212750, ThermoFisher Scientific, Waltham, MA, USA), Earle’s balanced salt solution, and 2% FBS. After incubation at 37°C for 2 days, a second overlay containing an additional 80 µg/mL of neutral red vital stain (Cat# 091691149, MP Biomedicals, Santa Ana, CA, USA) was added. Plaques were counted 3–5 days after infection and percent neutralization was calculated based on input virus titer. Virus neutralization curves were generated by a four-parameter nonlinear logistic regression model used to calculate the half-maximal inhibitory concentration (IC50) values of each MAb using GraphPad Prism V6 with the bottom and top of the curves constrained to 0 and 100, respectively.
Flow cytometry
HEK-293 cells were grown under standard growth conditions prior to infection with CSGVs LACV, JCV, and SSHV, at a multiplicity of infection (MOI) of 0.1, and TAHV at an MOI of 0.03. LACV-infected cells were incubated for 24 h, while JCV- and SSHV-infected cells were incubated for 48 h, and TAHV-infected cells were incubated for 72 h. To harvest, cells were broken up by pipetting and transferred to 50 mL conical tubes for centrifugation at 2,000 rpm for 2 min. Cells were first washed with PBS to remove residual cell culture media and then stained with a viability stain (Zombie dye NIR, BioLegend, San Diego, CA, USA) for 30 min at room temperature in the dark. After viability staining, the cells were washed once with 10% goat serum (diluted in PBS, Jackson ImmunoResearch, PA, USA). Cells were then fixed in 4% paraformaldehyde (ThermoFisher Scientific, Waltham, MA, USA) for 20 min. All incubation steps were carried out on a shaker plate set to 600 rpm, in the dark, at room temperature. Non-specific binding sites on fixed cells were blocked with the 1:1 blocking buffer: paraformaldehyde mixture and incubated for 30 min. CSGV MAbs were serially diluted in blocking buffer, and cells were resuspended in dilutions of MAb before a 30-min incubation. Cells were washed in MACS rinsing buffer (Cat# 130-091-222, Miltenyi Biotec, Auburn, CA, USA) in a Curiox laminar cell washer according to the manufacturer’s instructions (Curiox, Woburn, MA, USA). The secondary antibody, goat anti-mouse IgG H + L conjugated to R-PE (Cat# PA1-84395, ThermoFisher Scientific, Waltham, MA, USA) was diluted 1:100 in blocking buffer and incubated on cells for 30 min. Cells were washed with MACS buffer before final resuspension in 200 µL MACS buffer and placed in a 96-well plate for analysis on the Guava EasyCyte cytometer (Luminex, Austin, TX, USA). Data analysis was conducted in GuavaSoft V4.0, and data collected in duplicate was fit by a four-parameter nonlinear logistic regression dose response used to calculate the half-maximal efficacy concentration (EC50) values of each MAb using GraphPad Prism V6 with the bottom and top of the curves constrained to 0 and 100, respectively.
Selection of neutralization escape variant virus
Neutralization escape variants of JCV were selected using MAbs 3-3B6 and 2-3B2 in a manner previously described (51, 52). Briefly, varying amounts (−50, 100, and 200 µg/mL) of purified MAb (six replicates each) were incubated with 100 PFU of virus overnight at 4°C before being plated on Vero cell monolayers in six-well plates with a nutrient overlay. Viral plaques were cored, eluted overnight at 4°C, and mixed again with 100 µg/mL of MAb. Virus-MAb mixtures were incubated and plated on Vero cell monolayers as described above. Viral plaques were again cored, eluted overnight at 4°C, and allowed to adsorb to Vero cell monolayers containing 500 µg/mL of MAb in culture medium. Cells were monitored for cytopathic effect, and supernatant was collected for viral RNA isolation.
RNA extraction, complementary DNA (cDNA), and high-throughput sequencing library preparation
Hybridoma cells were harvested, and RNA was extracted from 1 × 106 hybridoma cells using the Total RNA Purification kit (Cat# 17200, Norgen Biotek Corp., Thorold, ON, Canada). Messenger RNA (mRNA) was selected using the RNeasy Pure mRNA bead kit (Cat# 180244 Qiagen). cDNA was generated using the Ovation RNA-seq system V2 (Cat# 7102-08, Tecan, Redwood City, CA, USA). Libraries were made using the Ion Xpress Plus Fragment Library preparation kit (Cat# 4471269, ThermoFisher Scientific, Waltham, MA, USA), barcoded using Ion Xpress Barcodes (Cat# 4474517, ThermoFisher Scientific, Waltham, MA, USA), and quantified using the Ion Library TaqMan Quantitation kit (Cat# 4468802, ThermoFisher Scientific, Waltham, MA, USA). For viral cDNA generation, viral RNA was extracted using the QIAamp viral RNA mini kit (Cat# 52904, Qiagen, Germantown, MD, USA) and libraries were generated as described above.
Sequencing and analysis
Sequencing was performed on the Ion GeneStudio S5 (ThermoFisher Scientific, Waltham, MA, USA). Ion 530 sequencing chips were prepared on the Ion Chef instrument with the Ion 510, 520, and 530 Chef kit (Cat# A34461, ThermoFisher Scientific, Waltham, MA, USA). FastQ sequences (quality Phred >20) were exported and used for de novo assembly in CLC Genomics Workbench v22 (Qiagen, Germantown, MD, USA). Contigs were subjected to NCBI BLAST analysis (www.ncbi.nlm.nih.gov). Each consensus contig generated in Genomics Workbench was subjected to a final reference guided assembly in SeqMan NGen v15 (DNAStar, Madison, WI, USA). Completeness of open reading frames were checked using EditSeq (DNAStar, Madison, WI, USA), and heavy and kappa variable regions were identified using IMGT/V Quest alignment (www.imgt.org) (54, 55).
Cloning and Gibson assembly of dual expression vectors
Primers for amplification and cloning (56) are listed in Table S2 and were designed to be 40 nucleotides in length with 20 nucleotides complementary to the antibody variable regions and 20 nucleotides complementary to the vector. To generate murine-human MAb clones, the heavy and light chain transcription units from pVITRO1 vectors (57, 58) were swapped for murine heavy and light chain transcription units. Fragments for assembly were amplified by using the Q5 High-Fidelity 2× Master mix kit (Cat#M0492, New England Biolabs, Ipswich, MA, USA) following the manufacturer’s instructions.
The four fragments (two vector fragments, one heavy chain fragment, and one light chain fragment) were assembled using the NEBuilder HiFi DNA assembly reaction (Cat# E5520, New England, Biolabs, Ipswich, MA, USA) following the manufacturer’s instructions. Plasmids were extracted from transformed, chemically competent E. coli DH5α cells using Qiaprep Spin Miniprep kit (Cat# 27106 × 4, Qiagen, Germantown, MD, USA), and heavy and light chain inserts were verified by Sanger sequencing on an ABI 3130 instrument (Applied Biosystems, Waltham, MA, USA).
Development of HEK-293 cells constitutively expressing human-murine chimeric antibody
HEK-293 cells were electroporated with expression vectors containing the human-murine chimeric IgM constructs as previously described (59). Briefly, 30 µg of DNA per reaction was added to 0.5 mL of cells at a cell density of 1–2 × 107 cells/mL. Cells were placed in a cuvette with a 4 mm gap (Cat# 1652081, Bio-Rad, Hercules, CA, USA), and electroporated in a Genepulser Xcell (Bio-Rad, Hercules, CA, USA) with the following settings: voltage of 250 V, capacitance of 975 µF, and resistance of infinity. Cells were recovered overnight in growth medium and reseeded the next day at 1 × 106 cells/mL. Immunofluorescence assays (IFAs) were performed after recovery to verify the expression of the gene construct as previously described using a goat-anti-human IgM Fc5µ antibody conjugated to fluorescein (Cat#109-095-043, Jackson Immunoresearch, West Grove, PA, USA) (59). The process for the selection of human-murine immunoglobulin expressing HEK-293 cells was adapted from previous methodology used to develop HEK-293 cell lines expressing flavivirus diagnostic antigens using 3% carboxymethyl cellulose in growth media (59). Cells were grown for 7–14 days, and colonies were picked using the ClonePix2 (Molecular Devices, Sunnyvale, CA, USA) and placed into 96-well plates (Corning, Tewksbury, MA, USA) containing growth media. Clones were grown for 7–10 days before screening with IFA to identify clones with at least 50% cells expressing human IgM.
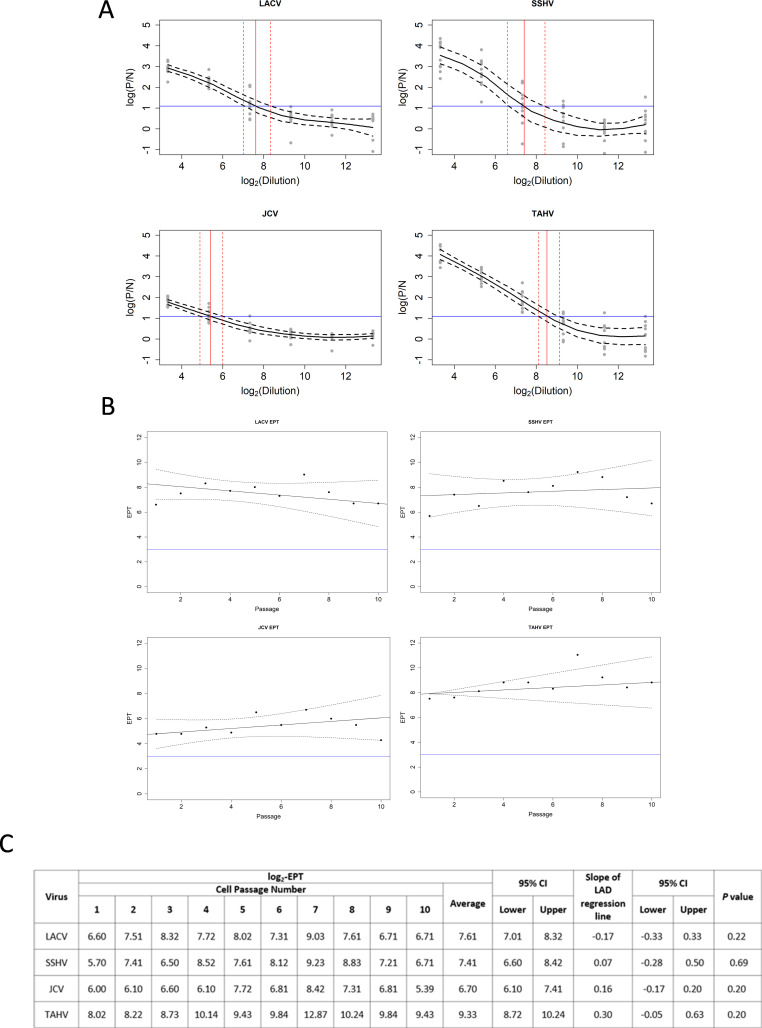
CSGV hIgM stable expression from HEK-293 cells was determined as previously described (59). Cells were passaged 10 times, and cell-culture supernatants from each passage were tested in the MAC-ELISA in duplicate. Regression spline fits of expression (P/N ratio) against log-dilution were fitted to all passages and a mean curve was estimated using methods of Heckman et al. (60) and Powers et al. (59). The endpoint titer (EPT) was estimated from the spline curves for each passage as the lowest dilution that resulted in a positive (P/N) ratio of 3. P/N ratios were determined by taking the average optical density (OD) of supernatant reacted on viral antigen divided by the average OD of normal human serum control (diluted 1:400) reacted on viral antigen. Figure 3A shows the individual predicted mean curves [95% pointwise confidence intervals (CI)] for each passage and cell line (60). The EPTs estimated using the mean curves (95% CIs) shown in Fig. 3B are shown in Fig. 3C. The EPTs of each passage on four viral antigens were analyzed using least absolute deviation (LAD) regression to evaluate the slope of the regression line of EPT passage sequence number using the Wald test (Fig. 3B). Analyses were performed in the R software package (www.r-project.org).
Fig 3.
Performance of hCSG-IgM antibody from a recombinant cell line. (A) Predicted mean values of hCSG-IgM EPT (black line) from the cell line were plotted as log(P/N) vs. log2(dilution) with confidence bands as dashed black lines. P/N represents the ratio of the OD of hCSG-IgM on viral antigen over the ratio of negative human serum on viral antigen tested in duplicate by ELISA. The reference line in blue for determining the EPT is shown as log2. The estimated EPT (95% CI) is indicated with vertical red lines with confidence bands as dashed red lines. (B) EPT against passage number evaluated by LAD regression test (black line) with confidence bands (dashed black lines) shows stable secretion of hCSG-IgM from the cell line tested by MAC-ELISA on four viral antigens. The reference line in blue shows the cutoff for a positive P/N at 3.0 in the MAC-ELISA (C) Individual and average log2 EPTs (95% CIs) tested on CSGV antigen over 10 passages. Slopes of the LAD regression line plotted in (B) were calculated with 95% CI and P values are indicated.
Transient expression of LACV structural proteins in HEK-293 cells
pVAX plasmids containing the Japanese encephalitis signal sequence and the genes encoding LACV Gc, Gn, or N genes were transiently expressed in HEK-293 cells as described above (61). Twenty-four hours after transfection, cells were dried onto slides and fixed with 3:1 acetone and PBS for 10 min. Cells were stained with CSGV MAbs 2-3B2, 3-3B6, 2-3C2, and 10G5.4 at a concentration of 10 µg/mL in PBS, and LACV-specific mouse hyperimmune ascitic fluid (MHIAF) (1:100 in PBS, M30177ABY, ARC DRT CDC). Normal HEK-293 cells stained with MHIAF were used as a negative control. Slides were incubated with each primary antibody for 30 min at 37°C in a humid chamber, and then washed in PBS, before the secondary antibody [goat anti-mouse IgG H + L conjugated to fluorescein (FITC)] (Cat# 115-005-003, Jackson Immunoresearch, West Grove, PA, USA) diluted 1:100 in PBS, and diamidino-2-phenylindole stain (Cat# 62248, ThermoFisher Scientific, Waltham, MA, USA) was added and incubated for 30 min at 37°C in a humid chamber. The slides were washed in PBS and then mounted with Prolong Gold Anti-Fade mountant (Cat# P10144, ThermoFisher Scientific, Waltham, MA, USA) and cured overnight in the dark at room temperature. Slides were imaged on a LSM 800 confocal microscope (Zeiss, Germany).
MAC-ELISA
The CDC MAC-ELISA was used for evaluation, as previously described (49). Briefly, purified hCSG-IgM was serially titrated twofold in duplicate, from 104 ng/mL to 310 ng/mL, and tested against the following inactivated CSGV antigens used most often by ADB’s diagnostic laboratory: La Crosse virus (LACV) antigen lot TC00598 diluted 1:320, LACV antigen lot 92-0033L diluted 1:320, JCV antigen lot TC01484 diluted 1:200, SSHV antigen lot TC01700 diluted 1:240, SSHV antigen lot B76104 diluted 1:640, TAHV antigen lot TC00877 diluted 1:40, and BHK-21 normal antigen lot TC01684 diluted 1:40 (62). A positive corresponding CSGV human reference serum sample was diluted 1:400 on all antigens except for LACV antigen lot TC00598 and 92-0033L in which the human reference serum was diluted 1:3,000. The human reference serum sample was used as comparative CSGV control in the MAC-ELISA; and negative human reference serum was diluted 1:400. A horseradish peroxidase-conjugated cross-reactive CSGV monoclonal antibody, 10G5.4 (63), was used at 1:4,000. Results were expressed as a P/N ratio, calculated as the mean OD450 value of the hCSG-IgM or positive human reference serum sample reacted on CSGV antigen divided by the mean OD450 value of the negative human serum reacted on the corresponding CSGV antigen, where P/N ≤ 1.9 is negative, P/N between 2.0 and 2.9 is equivocal, and P/N ≥ 3.0 is positive.
RESULTS
Development of LACV and JCV recombinant nucleoprotein for animal inoculations
Our objective was to isolate hybridoma clones with cross-reactivity to CSGVs of public health importance in the United States; therefore, we produced N-MBP recombinant proteins of LACV and JCV because the S segment of CSGVs shares high nucleotide and amino acid sequence homology (64). The N protein of LACV and JCV was expressed together with MBP and purified from E. coli cells. The recombinant protein had a molecular weight of approximately 75 KDa corresponding to the estimated molecular size of LACV-N or JCV-N and MBP (Fig. S1A). We confirmed the antigenicity of the recombinant N proteins in an indirect ELISA with LACV MHIAF and showed reactivity with EC50 values of 67 µg/well and 205 µg/well, respectively (Fig. S1B).
Virulence of JCV and immunogenicity of CSGV recombinant N protein in AG129 mice
Five-week-old AG129 mice were inoculated with 10, 1, and 0.1 PFU/0.1 mL of JCV. The first signs of illness began on day 7 post-infection (PI) (Fig. 1A). All animals inoculated with 10 PFU/0.1 mL of JCV and one mouse inoculated with 1 PFU/0.1 mL of JCV succumbed to infection by 11 days PI. Surviving mice were bled on day 20 to determine the level of anti-JCV antibodies, and only one of the five surviving mice inoculated with 1 PFU/0.1 mL of JCV had detectable anti-JCV antibodies with an EC50 ELISA reciprocal dilution value of 17,214 (Fig. 1B and C). Anti-LACV reactivity was also detected with an EC50 ELISA reciprocal dilution value of 215 (Fig. 1B and C). The mouse with detectable anti-CSGV antibody (mouse 3) and two surviving mice (mice 1 and 2) in the same infection group were subsequently boosted with a combination of LACV and JCV-N-MBP (Fig. 1A). At 40 days PI, mouse 3 had increased levels of anti-CSGV antibodies with EC50 ELISA reciprocal dilution values of 45,107 and 2,088 on JCV and LACV antigen, respectively (Fig. 1B and C). Mice were re-boosted with LACV or JCV N-MBP and tested for anti-CSGV antibodies at 55 days PI (Fig. 1A). At this time, only mouse 3 had reactive anti-CSGV antibodies with EC50 ELISA reciprocal dilution values of 49,202 and 6,061 to JCV and LACV antigens, respectively (Fig. 1B and C). These mice were infected with 10 PFU/0.1 mL of LACV before euthanasia, and spleens were harvested for hybridoma fusions (Fig. 1A).
Fig 1.
Anti-LACV and anti-JCV specific Ab response in AG129 mice. (A) Inoculation timeline created with BioRender.com. (B) Binding of anti-LACV and anti-JCV sera from the mice initially infected with JCV and subsequently immunized with recombinant N-protein was determined by indirect ELISA with fourfold dilutions of sera. Graphs are labeled by mouse numbers (1, 2, and 3). Colors indicate day PI [day 20 (blue), day 40 (black), and day 55 (green)]. (C) EC50 values, shown as the reciprocal of the dilution of sera, were determined by nonlinear fit analysis, with the bottom of the curve constrained to 0. EC50 values were calculated for 20-, 40-, and 55 days PI (dpi); > indicates no detectable binding reactivity at a 1:200 dilution of sera.
Development of CSGV reactive hybridomas
Twenty-one hybridomas secreting antibodies to LACV, JCV, or both viruses were isolated from the fusions with immunized mice. Seven hybridomas were further evaluated based on cross-reactivity in ELISA with LACV and JCV. Four of the seven hybridomas secreted IgM antibody and three hybridomas secreted IgG antibody. The IgM clones were shown to be N-protein specific, originating from fusions with splenocytes from mice 1 and 2; however, they were not characterized further because of their low reactivity in ELISA (EC50 values to JCV and LACV were >10,000 ng/mL for all IgM MAbs).
Characterization of CSGV-reactive MAbs
The three IgG MAbs, 2-3B2, 3-3B6, and 2-3C2, showed high reactivity with CSGV-infected HEK-293 cells measured by flow cytometry (Fig. 2A). MAb 10G5.4, previously developed against LACV and shown to be CSGV cross-reactive, was included for comparison. All clones had comparable EC50 values to JCV-infected cells (2-3B2: 74 ng/mL, 3-3B6: 79 ng/mL, 2-3C2: 80 ng/mL, and 10G5.4: 167 ng/mL) (Fig. 2A and E). On LACV-infected cells, reactivity of the MAbs based on EC50 values varied with MAb 10G5.4 having the highest reactivity, while MAb 2-3C2 had the lowest (2-3B2: 306 ng/mL, 3-3B6: 375 ng/mL, 23 C-2: 1,070 ng/mL, and 10G5.4: 23 ng/mL). MAbs 2-3B2 and 3-3B6 had higher EC50 values on SSHV-infected cells, while 2-3C2 and 10G5.4 had similar EC50 values as those on LACV (2-3B2: 1,050 ng/mL, 3-3B6: 723 ng/mL, 2-3C2: 571 ng/mL, and 10G5.4: 22 ng/mL). All MAbs reacted similarly on TAHV-infected cells (2-3B2: 375 ng/mL, 3-3B6: 549 ng/mL, 2-3C2: 339 ng/mL, and 10G5.4: 226 ng/mL) (Fig. 2A and E).
Fig 2.
Binding and neutralization reactivity, protein, and epitope specificity of anti-CSGV MAbs. (A) Flow cytometry determined the binding of anti-CSGV MAbs to infected HEK-293 cells. LACV-infected cells (blue); JCV-infected cells (red); SSHV-infected cells (black); and TAHV-infected cells (purple) were fixed and stained with anti-CSGV MAbs as described in the Materials and Methods. (B) CSGV-specific neutralizing antibody responses. Percent neutralization of purified MAbs was calculated based on virus input titer. Virus neutralization curves were generated by a four-parameter nonlinear regression dose response and used to calculate each sample’s half-maximal inhibitory concentration (IC50) values. The dotted line represents 50% neutralization (IC50). (C) IFA of HEK-293 cells transiently expressing recombinant LACV structural proteins Gc (glycoprotein C), Gn (glycoprotein N), and N (nucleoprotein). Cells were stained with 10 µg of each MAb and MHIAF (1:100 dilution). Negative control cells were normal HEK-293 cells stained with MHIAF. (D) JCV neutralization escape variants (1.6, 4.3, and 4.6) were generated with selective pressure from MAb 3-3B6 to determine the epitope on JCV important in 3-3B6 neutralization. (E) EC50 and IC50 values (shown as MAb concentrations of ng/mL) in ELISA and PRNT were determined by nonlinear fit analysis. The bottom of the curve was constrained to 0 for both analyses and the top of the curve constrained to 100 for neutralization analysis; >indicates no detectable reactivity. IFA determined viral protein specificity. ELISA determined MAb isotype specificity.
In neutralization assays, MAb 2-3C2 did not neutralize any of the CSGVs tested, whereas MAbs 2-3B2 and 3-3B6 were only able to neutralize JCV with IC50 values of 6,900 and 9,070 ng/mL, respectively (Fig. 2B and E). MAb 10G5.4 neutralized LACV, JCV, and SSHV with IC50 values of 2.267, 3,107, and 3,124 ng/mL, respectively (Fig. 2B and E). None of the MAbs neutralized TAHV in vitro (Fig. 2B and E).
Viral protein specificity was determined by IFA on HEK-293 cells transiently expressing recombinant LACV Gc, Gn, or N proteins. MAb 10G5.4 detected Gc protein, while 3-3B6 and 2-3B2 detected recombinant Gn protein (Fig. 2C). MAb 2-3C2 reacted with none of the recombinant viral proteins expressed (Fig. 2C).
The dominant functional epitope of MAbs 3-3B6 was determined by selecting for neutralization escape variant JCVs in the presence of varying amounts of antibody before collecting virus supernatant for viral RNA isolation and NGS. Three neutralization escape variants were isolated and sequenced. All variants were found to have an amino acid (AA) change from E to G at position 138 in the Gc protein (Fig. 2D). Two variants had an additional AA change from K to E at position 267 in the Gc protein (Fig. 2D).
Design, construction, and expression of a CSGV cross-reactive murine-human IgM chimeric antibody
To engineer a human-murine chimeric antibody control, total RNA was extracted, and mRNA was enriched from clones 2-3B2 and 3-3B6 and sequenced by NGS. The IgBLAST IMGT annotation tool determined variable region sequences and showed that these two clones were identical (Fig. 3A and B). Primers were designed to amplify the variable regions starting at the signal sequence through framework region 4 (Table S2). Amplified heavy variable (V H ) and kappa variable (Vκ) regions replaced the variable regions of the Trastuzumab-IgM/K in the pVITRO plasmid (Fig. S2). HEK-293 cells were transformed with the plasmid, and clones expressing hCSG-IgM antibody were selected as previously described (59). The selected clone with optimal expression of hCSG-IgM monitored by IFA and ELISA showed stable antibody secretion over 10 cell passages with four CSGV antigens tested (Fig. 3). The hCSG-IgM antibody had the highest EPT with TAHV antigen (log2 EPT 9.33), while having the lowest EPT with JCV antigen (average log2 EPT 6.70) (Fig. 3). The hCSG-IgM antibody reacted similarly with LACV and SSHV antigens with average log2 EPTs of 7.61 and 7.41, respectively (Fig. 3). When analyzed by LAD regression test, antibody secreted from the cell line was stable with calculated slopes of −0.17 (95% CI −0.33,0.33), 0.07 (95% CI −0.28, 0.50), 0.16 (95% CI −0.17, 0.20), and 0.3 (95% CI −0.05, 0.63) on LACV, SSHV, JCV, and TAHV antigens, respectively (Fig. 3B and C).
Human-murine hCSG-IgM performance in diagnostic MAC-ELISA
The hCSG-IgM antibody produced in HEK-293 cells was purified from cell-culture supernatant and titrated as the positive human antibody control in the CDC diagnostic MAC-ELISA with CSGVs, LACV, JCV, SSHV, and TAHV starting at a concentration of 10,000 ng/mL (Fig. 4A). The hCSG-IgM antibody reacted positively with a P/N ratio >3.0 (a presumptive positive in the diagnostic algorithm) to all viral antigens tested from 10,000 ng/mL to 2,500 ng/mL (Fig. 4B). The hCSG-IgM antibody had the highest P/N ratios when tested on TAHV (P/N = 61.9 at 10,000 ng/mL) and SSHV TC01700 (P/N = 59.3 at 10,000 ng/mL) compared to the positive human serum (PHS) control with P/Ns of 52.8 and 51.7 for TAHV and SSHV, respectively (Fig. 4B). The hCSG-IgM antibody had the lowest reactivity with LACV 92-0033L (P/N = 10.2 at 10,000 ng/mL), which was about three times lower than the PHS (P/N = 31.4) (Fig. 4B). To determine the level of antibody non-specifically binding in the assay, non-specific binding values (NBVs) of hCSG-IgM were determined on each antigen using the ratios of the OD450 absorbance of the antibody on viral antigen to the OD450 absorbance of the antibody on BHK-21 normal antigen (BHK). The hCSG-IgM had little non-specific reactivity in the assay with NBV ratios > 3.0 for all antigens tested on antibody titrated out to 310 ng/mL, except for SSHV B76104, which had an NBV ratio >3.0 only when the antibody was titrated to 2,500 ng/mL (Fig. 4C).
Fig 4.
Reactivity of hCSG-IgM on CSGV antigen in the MAC-ELISA. (A) Binding of hCSG-IgM on CSGV antigens (LACV lot TC00598, JCV lot TC01484, SSHV lot TC01700, and TAHV, lot TC00877) and BHK-21 normal antigen (BHK) was determined by MAC-ELISA. Data were fit to a nonlinear regression model. (B) Purified hCSG-IgM was titrated in the MAC-ELISA on several CSGV antigen preparations. Results are expressed as a P/N ratio (mean OD450 value of the hCSG-IgM reacted on CSGV antigen divided by the mean OD450 value of the negative human serum reacted on the corresponding CSGV antigen). (C) Non-specific background values (the mean OD450 value of the hCSG-IgM reacted on CSGV antigen divided by the mean OD450 value of the hCSG-IgM reacted on the BHK-21 normal antigen) were determined for hCSG-IgM in the MAC-ELISA with the CSGV antigen preparations. PHS diluted 1:400 on all antigens except for TC00598 and 92-0033L, where PHS was diluted 1:3,000 in the test.
DISCUSSION
Diagnostics for CSGVs mainly rely on serologic assays like MAC-ELISA and PRNT to detect anti-viral antibodies (36, 49, 50). Molecular assays such as RT-PCR are available, but because these viruses cause transient viremia in infected individuals before symptom onset, infections may be missed when relying solely on molecular tools (1, 65). Immunoassays like MAC-ELISA and microsphere immunoassays offer a reliable method for detecting anti-viral IgM in early arboviral infections; however, the uses of these assays are limited due to the need for a PHS control containing IgM (49, 66, 67). Given the limited number of positive diagnostic CSGV IgM specimens, MAC-ELISA controls for detecting CSGVs are collected and sometimes pooled from small volumes of several positive human specimens (49). This, coupled with the rare incidence of disease caused by CSGVs, means that these controls may not be readily available for distribution to diagnostic laboratories. Lot-to-lot variation in the pooled specimens also occurs, requiring frequent recalibration of the assay (49). In this study, we sought to produce a recombinant human-murine chimeric IgM antibody cross-reactive to CSGVs circulating in the United States for use as a positive control in serology assays that can be readily distributed to diagnostic laboratories. Similar serogroup cross-reactive chimeric antibodies have previously been described for use in flaviviral and alphaviral serologic assays and are distributed across the globe and regularly used in serological diagnostic tests (68 – 70).
To make the CSGV hIgM recombinant antibody, we first generated murine MAbs cross-reactive to CSGVs using an immunization scheme that included live viral infections and boosts with recombinant N protein. The N protein is immunodominant in other orthobunyaviral infections (71, 72) and highly conserved among the viruses in the serogroup (64). We hypothesized that immunization of mice with combinations of CSGV infections and recombinant N protein would generate a highly cross-reactive antibody response allowing for the isolation of hybridoma clones secreting cross-reactive MAbs. While only one mouse showed a marked boost after JCV infection and N-protein immunization, only hybridomas secreting cross-reactive N-protein specific IgM were isolated. Immunizations with a DNA vaccine encoding the N-gene of Schmallenberg virus, another orthobunyavirus, elicited a high titer antibody response in vaccinated IFNAR-/- mice and were robust enough to protect vaccinated mice from lethal viral challenge (73). Even though the recombinant N-MBP produced for these experiments showed reactivity with LACV-immune MHIAF, the antibody response in mice vaccinated with the immunogen was minimal. In these experiments, we immunized mice with 10 µg of LACV N-MBP and JCV N-MBP using the Magic Mouse adjuvant. In previous experiments, mice were vaccinated with 50 µg per inoculation of recombinant Japanese encephalitis virus domain III of the envelope protein fused to MBP, and we were able to produce a robust antibody response in Balb/C mice that resulted in isolation of hybridomas secreting JEV domain III-specific antibodies using TiterMax Gold adjuvant (74). A higher antibody response in this study may have been obtained simply by immunizing with a higher concentration of recombinant protein over longer intervals and/or using a different adjuvant to isolate N protein-specific IgG-secreting hybridomas. Likewise, immunizing mice with recombinant N-protein of multiple CSGVs may have produced a higher cross-reactive antibody response, resulting in more hybridoma clone secreting N-protein specific IgGs.
MAbs 3-3B6 and 2-3B2 were found to have the same variable region sequence and, therefore, are considered the same clone. This MAb could bind recombinant Gn but not recombinant Gc in vitro. In contrast, propagation of neutralization escape mutants revealed the functional epitope of the MAb included residue 138 (residue 612 of the polyprotein) and residue 267 (residue 741 of the polyprotein) on Gc (Fig. 5). Similarly, MAb 10G5.4 was previously shown to bind Gn and Gc; however, in our experiments, this MAb only bound to recombinant Gc in the IFA (63). Neither MAb could detect LACV or JCV denatured glycoproteins in a Western blot (data not shown); therefore, the MAbs must be binding to an epitope dependent on the tertiary structure of the Gn/Gc heterodimer. Orthobunyaviral Gn and Gc form heterodimers on the surface of the virion, where Gc is displayed as trimer spikes protruding from the virion, and Gn forms the “floor” of the virion parallel to the viral membrane (75, 76). Epitopes important in neutralization have been mapped to the Gc protein, a class II fusion protein, in LACV (77, 78); thus, it is no surprise the functional epitope of MAb 3-3B6/2-3B2 critical in viral neutralization mapped to JCV Gc protein.
Fig 5.
MAb 3-3B6/2-3B2 binds the head domain of JCV Gc protein. (A) The image (PDB 6H3W) shows a side view of the LACV Gc head domain. The critical residue for MAb 3-3B6/2-3B2 is shown in magenta. Residues 608–611 and 613, shown in yellow spheres, are residues critical in the LACV Gc trimer interface. Disulfide bonds are shown in blue. (B) AA sequence alignment of LACV, SSHV, TAHV, JCV, and JCV NE mutants, 1.6, 4.3, and 4.6 showing mutation at AA residue 138 (left; residue 610 in the polyprotein of LACV) and residue 267 (right; residue 738 in the polyprotein of LACV) of Gc protein.
Evaluation of the high-resolution crystal structure of the LACV Gc head domain reveals that one of the functional epitopes of MAb 3-3B6/2-3B2 may be residue 612 of the JCV polyprotein (residue 610 on LACV polyprotein). This AA residue sits at the top of the head domain and is included in a cluster of AA residues critical in the Gc trimer complex (Fig. 5A). Residue 138 is also near cysteine residues critical in disulfide bond formation. The glycine at residue 138 is highly conserved among CSGVs, including LACV, SSHV, and TAHV in our analysis. The residue at 138 of the wild-type plaque-picked JCV virus stock used to produce the NE variants in our sequences was glutamate, while other sequence alignments show that the residue at this location on JCV is glycine (Fig. 5B) (76). Nevertheless, as glycine was detected in all NE mutants at this residue, it most likely plays a key role in the MAb’s ability to bind and neutralize JCV. However, because G138 is highly conserved among CSGVs, and MAb 3-3B6/2-3B2 only neutralizes JCV, another functional epitope must also be involved. Two of the three JCV NE mutants had an AA change at Gc residue 267 (residues 741 and 738 in the JCV and LACV polyprotein, respectively) from K→E (Fig. 5B). This residue is also located in the Gc head domain but is not visualized on the crystal structure of LACV Gc head domain and lies nearer the stalk of the glycoprotein spike. The K741 residue is highly conserved among CSGVs except Keystone virus and Trivittatus virus, which have a serine or aspartic acid, respectively, in the place of lysine at this location on Gc (76). Identifying the epitope of 3-3B6/2-3B2 is the information needed to determine optimal CSGV recombinant antigen development in the future. Any diagnostic assay that will utilize hCSG-IgM will need to incorporate native or recombinant antigen that includes both glycoproteins.
The HEK-293 stable cell line secreting the murine-human chimeric IgM antibody was shown to stably secrete antibody over several cell passages as evidenced by the slopes of the LAD regressions plotted from reactivity of hCSG-IgM in MAC-ELISAs with LACV (−0.17), JCV (0.16), SSHV (0.07), and TAHV (0.30) antigens. The purified chimeric IgM antibody was also tested in the MAC-ELISA with multiple CSGV antigens used in the diagnostic assay and it reacted similar to the PHS used in the assay, with similar P/Ns for each antigen when the antibody was used at a concentration of 10,000 ng/mL. Interestingly, hCSG-IgM had the highest P/N values with SSHV and TAHV antigens. As these antigen preparations are made from cell-culture supernatant, their enhanced reactivity may be due to the antigen’s quality vs. an increased antibody affinity for the viral-specific antigen. Likewise, the NBV values were much higher for purified hCSG-IgM than the PHS, which may have been an artifact of using a purified antibody preparation in the assay. For our purposes in reporting the data here, we purified hCSG-IgM to quantitatively determine the concentration of antibody needed to perform equivalently to PHS. Currently, chimeric IgMs specific to flaviviruses and alphaviruses used in the MAC-ELISA are from concentrated cell-culture supernatant, and lot-to-lot titrations are required (68, 69). The use of purified chimeric IgMs may be useful in the future by allowing for standardization of the reactivity in the assay based on protein concentration, thereby eliminating the need to titrate each antibody lot produced.
Incorporating murine-human chimeric IgM antibodies for flaviviruses and alphaviruses broadly cross-reactive across the genera has increased our serologic diagnostic assay capacity to many other rare arboviral diseases. In instances of human cases of rare arboviral infections, these antibodies may be used in the place of PHS that may be limited in volume or unavailable. Incorporating the hCSG-IgM for use in the MAC-ELISA increases local public health labs’ capacity to test for CSGVs, thereby decreasing the time from testing to reporting. It may increase our CSGV testing capacity beyond those viruses currently known to circulate in the United States, as evidenced by hCSG-IgM’s reactivity with TAHV antigen, a virus not known to circulate in North America currently. The hCSG-IgM and other murine-chimeric IgM antibodies available from the CDC for distribution to public health laboratories interested in expanding testing for these viruses will help increase our ability to detect new and emerging arboviruses by serological diagnostics.
ACKNOWLEDGMENTS
This project was partly supported by an appointment to the Research Participation Program at the Centers for Disease Control and Prevention administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and the Centers for Disease Control and Prevention.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC.
Contributor Information
Amanda E. Calvert, Email: acalvert@cdc.gov.
Juan E. Ludert, Centro de Investigacion y de Estudios Avanzados del Instituto Politecnico Nacional, Mexico City, Mexico
ETHICS APPROVAL
Ethical approval for use of previously collected human diagnostic specimens was obtained from CDC’s Human Subjects Institutional Review Board (CDC IRB# 6773).
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/spectrum.01966-23.
supplementary tables and figures.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Hughes HR, Kenney JL, Russell BJ, Lambert AJ. 2022. Laboratory validation of a real-time RT-PCR assay for the detection of Jamestown Canyon virus. Pathogens 11:536. doi: 10.3390/pathogens11050536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Calisher CH. 1996. The Bunyaviridae. Plenum Press, New York, NY, USA. [Google Scholar]
- 3. Hughes HR, Adkins S, Alkhovskiy S, Beer M, Blair C, Calisher CH, Drebot M, Lambert AJ, de Souza WM, Marklewitz M, Nunes MRT, Shí (石晓宏) X, ICTV Report Consortium . 2020. ICTV virus taxonomy profile: Peribunyaviridae. J Gen Virol 101:1–2. doi: 10.1099/jgv.0.001365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Henry CJ, Pillai AN, Lednicky JA, Morris JG, Hladish TJ. 2022. Ecology and public health burden of Keystone virus in Florida. Epidemics 39:100555. doi: 10.1016/j.epidem.2022.100555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Evans AB, Peterson KE. 2019. Throw out the map: neuropathogenesis of the globally expanding California serogroup of orthobunyaviruses. Viruses 11:794. doi: 10.3390/v11090794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hubálek Z. 2008. Mosquito-borne viruses in Europe. Parasitol Res 103:S29–S43. doi: 10.1007/s00436-008-1064-7 [DOI] [PubMed] [Google Scholar]
- 7. Cao Y, Fu S, Tian Z, Lu Z, He Y, Wang H, Wang J, Guo W, Tao B, Liang G. 2011. Distribution of mosquitoes and mosquito-borne arboviruses in inner Mongolia, China. Vector Borne Zoonotic Dis 11:1577–1581. doi: 10.1089/vbz.2010.0262 [DOI] [PubMed] [Google Scholar]
- 8. Drebot MA. 2015. Emerging mosquito-borne bunyaviruses in Canada. Can Commun Dis Rep 41:117–123. doi: 10.14745/ccdr.v41i06a01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Putkuri N, Kantele A, Levanov L, Kivistö I, Brummer-Korvenkontio M, Vaheri A, Vapalahti O. 2016. Acute human Inkoo and Chatanga virus infections, Finland. Emerg Infect Dis 22:810–817. doi: 10.3201/eid2205.151015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Soldan SS, González-Scarano F. 2014. The Bunyaviridae. Handb Clin Neurol 123:449–463. doi: 10.1016/B978-0-444-53488-0.00021-3 [DOI] [PubMed] [Google Scholar]
- 11. Sudia W, Newhouse V, Calisher C, Chamberlain R. 1971. California group arbo-viruses: isolations from mosquitoes in North America. Mosq News 31:576–600. [Google Scholar]
- 12. DeFoliart GR, Anslow RO, Thompson WH, Hanson RP, Wright RE, Sather GE. 1972. Isolations of trivittatus virus from Wisconsin mosquitoes, 1964-1968. J Med Entomol 9:67–70. doi: 10.1093/jmedent/9.1.67 [DOI] [PubMed] [Google Scholar]
- 13. LeDuc JW. 1979. The ecology of California group viruses. J Med Entomol 16:1–17. doi: 10.1093/jmedent/16.1.1 [DOI] [PubMed] [Google Scholar]
- 14. Watts DM, Morris CD, Wright RE, DeFoliart GR, Hanson RP. 1972. Transmission of LaCrosse virus (California encephalitis group) by the mosquito Aedes triseriatus. J Med Entomol 9:125–127. doi: 10.1093/jmedent/9.2.125 [DOI] [PubMed] [Google Scholar]
- 15. Pantuwatana S, Thompson WH, Watts DM, Hanson RP. 1972. Experimental infection of chipmunks and squirrels with La Crosse and trivittatus viruses and biological transmission of La Crosse virus by Aedes triseriatus. Am J Trop Med Hyg 21:476–481. doi: 10.4269/ajtmh.1972.21.476 [DOI] [PubMed] [Google Scholar]
- 16. Boromisa RD, Grimstad PR. 1986. Virus-vector-host relationships of Aedes stimulans and Jamestown Canyon virus in a northern Indiana enzootic focus. Am J Trop Med Hyg 35:1285–1295. doi: 10.4269/ajtmh.1986.35.1285 [DOI] [PubMed] [Google Scholar]
- 17. Danielova V. 1979. Laboratory demonstration of transovarial transmission of Tahyna virus in Aedes vexans and the role of this mechanism in overwintering of this arbovirus. Folia Parasitol 26:361–366. [Google Scholar]
- 18. Hammon WM, Reeves WC. 1952. California encephalitis virus, a newly described agent. Calif Med 77:303–309. [PMC free article] [PubMed] [Google Scholar]
- 19. McLintock J, Curry P, Wagner R, Leung M, Iversen J. 1976. Isolation of Snowshoe hare virus from Aedes implicatus larvae in Saskatchewan. Mosq News 36:233–237. [Google Scholar]
- 20. Moulton DW, Thompson WH. 1971. California group virus infections in small, forest-dwelling mammals of Wisconsin. Some ecological considerations. Am J Trop Med Hyg 20:474–482. doi: 10.4269/ajtmh.1971.20.474 [DOI] [PubMed] [Google Scholar]
- 21. Newhouse V, Burgdorfer W, Corwin D. 1971. Field and laboratory studies on the hosts and vectors of the Snowshoe hare strain of California virus. Mosq News 31:401–408. [Google Scholar]
- 22. Watts DM, DeFoliart GR, Yuill TM. 1976. Experimental transmission of Trivittatus virus (California virus group) by Aedes Trivittatus. Am J Trop Med Hyg 25:173–176. doi: 10.4269/ajtmh.1976.25.173 [DOI] [PubMed] [Google Scholar]
- 23. Watts DM, Pantuwatana S, DeFoliart GR, Yuill TM, Thompson WH. 1973. Transovarial transmission of LaCrosse virus (California encephalitis group) in the mosquito, Aedes triseriatus. Science 182:1140–1141. doi: 10.1126/science.182.4117.1140 [DOI] [PubMed] [Google Scholar]
- 24. Issel CJ. 1973. Isolation of Jamestown Canyon virus (a California group arbovirus) from a white-tailed deer. Am J Trop Med Hyg 22:414–417. doi: 10.4269/ajtmh.1973.22.414 [DOI] [PubMed] [Google Scholar]
- 25. Issel CJ, Trainer DO, Thompson WH. 1972. Experimental studies with white-tailed deer and four California group arboviruses (La Crosse, Trivittatus, Snowshoe hare, and Jamestown Canyon). Am J Trop Med Hyg 21:979–984. doi: 10.4269/ajtmh.1972.21.979 [DOI] [PubMed] [Google Scholar]
- 26. Zarnke RL, Calisher CH, Kerschner J. 1983. Serologic evidence of arbovirus infections in humans and wild animals in Alaska. J Wildl Dis 19:175–179. doi: 10.7589/0090-3558-19.3.175 [DOI] [PubMed] [Google Scholar]
- 27. Goff G, Whitney H, Drebot MA. 2012. Roles of host species, geographic separation, and isolation in the seroprevalence of Jamestown canyon and Snowshoe hare viruses in Newfoundland. Appl Environ Microbiol 78:6734–6740. doi: 10.1128/AEM.01351-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. CDC US. 2022. La Crosse encephalitis virus, statistics and maps. Available from: https://www.cdc.gov/lac/statistics/index.html
- 29. Teleron ALA, Rose BK, Williams DM, Kemper SE, McJunkin JE. 2016. La Crosse encephalitis: an adult case series. Am J Med 129:881–884. doi: 10.1016/j.amjmed.2016.03.021 [DOI] [PubMed] [Google Scholar]
- 30. Vahey GM, Lindsey NP, Staples JE, Hills SL. 2021. La Crosse virus disease in the United States, 2003-2019. Am J Trop Med Hyg 105:807–812. doi: 10.4269/ajtmh.21-0294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Haddow AD, Odoi A. 2009. The incidence risk, clustering, and clinical presentation of La Crosse virus infections in the eastern United States, 2003-2007. PLoS One 4:e6145. doi: 10.1371/journal.pone.0006145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. CDC US. n.d. ArboNET diseases map. Available from: https://www.cdc.gov/jamestown-canyon/statistics/index.html
- 33. Farquhar MR, Thrun NB, Tucker BJ, Bartholomay LC. 2022. Outbreak investigation: Jamestown Canyon virus surveillance in field-collected mosquitoes (Diptera: Culicidae) from Wisconsin, USA, 2018-2019. Front Public Health 10:818204. doi: 10.3389/fpubh.2022.818204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. CDC US . 2021. Jamestown Canyon virus, statistics and maps on U.S. Available from: https://www.cdc.gov/jamestown-canyon/statistics/index.html
- 35. Matkovic E, Hoang Johnson DK, Staples JE, Mora-Pinzon MC, Elbadawi LI, Osborn RA, Warshauer DM, Wegner MV, Davis JP. 2019. Enhanced arboviral surveillance to increase detection of Jamestown Canyon virus infections, Wisconsin, 2011-2016. Am J Trop Med Hyg 100:445–451. doi: 10.4269/ajtmh.18-0575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Coleman KJ, Chauhan L, Piquet AL, Tyler KL, Pastula DM. 2021. An overview of Jamestown Canyon virus disease. Neurohospitalist 11:277–278. doi: 10.1177/19418744211005948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Solomon IH, Ganesh VS, Yu G, Deng XD, Wilson MR, Miller S, Milligan TA, Mukerji SS, Mathewson A, Linxweiler J, Morse D, Ritter JM, Staples JE, Hughes H, Gould CV, Sabeti PC, Chiu CY, Piantadosi A. 2021. Fatal case of chronic Jamestown Canyon virus encephalitis diagnosed by metagenomic sequencing in patient receiving rituximab. Emerg Infect Dis 27:238–242. doi: 10.3201/eid2701.203448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fauvel M, Artsob H, Calisher CH, Davignon L, Chagnon A, Skvorc-Ranko R, Belloncik S. 1980. California group virus encephalitis in three children from Quebec: clinical and serologic findings. Can Med Assoc J 122:60–62. [PMC free article] [PubMed] [Google Scholar]
- 39. Webster D, Dimitrova K, Holloway K, Makowski K, Safronetz D, Drebot MA. 2017. California serogroup virus infection associated with encephalitis and cognitive decline, Canada, 2015. Emerg Infect Dis 23:1423–1424. doi: 10.3201/eid2308.170239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sluka F, Simkova A. 1972. Demonstration of human infection in the natural focus of the Valtice fever. Folia Parasitol (Praha) 19:358. [PubMed] [Google Scholar]
- 41. Demikhov VG, Chaitsev VG, Butenko AM, Nedyalkova MS, Morozova TN. 1991. California serogroup virus infections in the Ryazan region of the USSR. Am J Trop Med Hyg 45:371–376. doi: 10.4269/ajtmh.1991.45.371 [DOI] [PubMed] [Google Scholar]
- 42. Kosoy O, Rabe I, Geissler A, Adjemian J, Panella A, Laven J, Basile AJ, Velez J, Griffith K, Wong D, Fischer M, Lanciotti RS. 2016. Serological survey for antibodies to mosquito-borne bunyaviruses among US National Park service and US Forest service employees. Vector Borne Zoonotic Dis 16:191–198. doi: 10.1089/vbz.2015.1865 [DOI] [PubMed] [Google Scholar]
- 43. Mayo D, Karabatsos N, Scarano FJ, Brennan T, Buck D, Fiorentino T, Mennone J, Tran S. 2001. Jamestown Canyon virus: seroprevalence in connecticut. Emerg Infect Dis 7:911–912. doi: 10.3201/eid0705.017529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Walters LL, Tirrell SJ, Shope RE. 1999. Seroepidemiology of California and Bunyamwera serogroup (Bunyaviridae) virus infections in native populations of Alaska. Am J Trop Med Hyg 60:806–821. doi: 10.4269/ajtmh.1999.60.806 [DOI] [PubMed] [Google Scholar]
- 45. Grimstad PR, Barrett CL, Humphrey RL, Sinsko MJ. 1984. Serologic evidence for widespread infection with La Crosse and St. Louis encephalitis viruses in the Indiana human population. Am J Epidemiol 119:913–930. doi: 10.1093/oxfordjournals.aje.a113814 [DOI] [PubMed] [Google Scholar]
- 46. Grimstad PR, Calisher CH, Harroff RN, Wentworth BB. 1986. Jamestown Canyon virus (California serogroup) is the etiologic agent of widespread infection in Michigan humans. Am J Trop Med Hyg 35:376–386. doi: 10.4269/ajtmh.1986.35.376 [DOI] [PubMed] [Google Scholar]
- 47. Sampasa-Kanyinga H, Lévesque B, Anassour-Laouan-Sidi E, Côté S, Serhir B, Ward BJ, Libman MD, Drebot MA, Makowski K, Dimitrova K, Ndao M, Dewailly E. 2013. Zoonotic infections in communities of the James Bay Cree territory: an overview of seroprevalence. Can J Infect Dis Med Microbiol 24:79–84. doi: 10.1155/2013/370321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Soto RA, Hughes ML, Staples JE, Lindsey NP. 2022. West Nile virus and other domestic nationally notifiable arboviral diseases - United States, 2020. MMWR Morb Mortal Wkly Rep 71:628–632. doi: 10.15585/mmwr.mm7118a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Martin DA, Muth DA, Brown T, Johnson AJ, Karabatsos N, Roehrig JT. 2000. Standardization of immunoglobulin M capture enzyme-linked immunosorbent assays for routine diagnosis of arboviral infections. J Clin Microbiol 38:1823–1826. doi: 10.1128/JCM.38.5.1823-1826.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kinsella CM, Paras ML, Smole S, Mehta S, Ganesh V, Chen LH, McQuillen DP, Shah R, Chan J, Osborne M, Hennigan S, Halpern-Smith F, Brown CM, Sabeti P, Piantadosi A. 2020. Jamestown Canyon virus in Massachusetts: clinical case series and vector screening. Emerg Microbes Infect 9:903–912. doi: 10.1080/22221751.2020.1756697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. CDC . 2023. On centers for disease control and prevention. Available from: https://www.cdc.gov/lac/statistics/historic-data.html
- 52. CDC . 2023. On centers for disease control and prevention. Available from: https://www.cdc.gov/jamestown-canyon/statistics/historic-data.html
- 53. Obijeski JF, Bishop DH, Murphy FA, Palmer EL. 1976. Structural proteins of La Crosse virus. J Virol 19:985–997. doi: 10.1128/JVI.19.3.985-997.1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lefranc M-P. 2003. IMGT, the international ImMunoGeneTics database. Nucleic Acids Res 31:307–310. doi: 10.1093/nar/gkg085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Petersen TN, Brunak S, von Heijne G, Nielsen H. 2011. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat Methods 8:785–786. doi: 10.1038/nmeth.1701 [DOI] [PubMed] [Google Scholar]
- 56. Gibson DG, Young L, Chuang R-Y, Venter JC, Hutchison CA, Smith HO. 2009. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat Methods 6:343–345. doi: 10.1038/nmeth.1318 [DOI] [PubMed] [Google Scholar]
- 57. Dodev TS, Karagiannis P, Gilbert AE, Josephs DH, Bowen H, James LK, Bax HJ, Beavil R, Pang MO, Gould HJ, Karagiannis SN, Beavil AJ. 2014. A tool kit for rapid cloning and expression of recombinant antibodies. Sci Rep 4:5885. doi: 10.1038/srep05885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hackett J, Hoff-Velk J, Golden A, Brashear J, Robinson J, Rapp M, Klass M, Ostrow DH, Mandecki W. 1998. Recombinant mouse-human chimeric antibodies as calibrators in immunoassays that measure antibodies to Toxoplasma gondii. J Clin Microbiol 36:1277–1284. doi: 10.1128/JCM.36.5.1277-1284.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Powers JA, Skinner B, Davis BS, Biggerstaff BJ, Robb L, Gordon E, de Souza WM, Fumagalli MJ, Calvert AE, Chang G-J. 2022. Development of HEK-293 cell lines constitutively expressing flaviviral antigens for use in diagnostics. Microbiol Spectr 10:e0059222. doi: 10.1128/spectrum.00592-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Heckman N, Lockhart R, Nielsen JD. 2013. Penalized regression, mixed effects models and appropriate modelling. Electron J Statist 7:1517–1552. doi: 10.1214/13-EJS809 [DOI] [Google Scholar]
- 61. Chang G-J, Hunt AR, Davis B. 2000. A single intramuscular injection of recombinant plasmid DNA induces protective immunity and prevents Japanese encephalitis in mice. J Virol 74:4244–4252. doi: 10.1128/jvi.74.9.4244-4252.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Goodman CH, Russell BJ, Velez JO, Laven JJ, Nicholson WL, Bagarozzi DA, Moon JL, Bedi K, Johnson BW. 2014. Development of an algorithm for production of inactivated arbovirus antigens in cell culture. J Virol Methods 208:66–78. doi: 10.1016/j.jviromet.2014.07.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ludwig GV, Israel BA, Christensen BM, Yuill TM, Schultz KT. 1991. Monoclonal antibodies directed against the envelope glycoproteins of La Crosse virus. Microb Pathog 11:411–421. doi: 10.1016/0882-4010(91)90037-b [DOI] [PubMed] [Google Scholar]
- 64. Hughes HR, Lanciotti RS, Blair CD, Lambert AJ. 2017. Full genomic characterization of California serogroup viruses, genus Orthobunyavirus, family Peribunyaviridae including phylogenetic relationships. Virology 512:201–210. doi: 10.1016/j.virol.2017.09.022 [DOI] [PubMed] [Google Scholar]
- 65. Lambert AJ, Nasci RS, Cropp BC, Martin DA, Rose BC, Russell BJ, Lanciotti RS. 2005. Nucleic acid amplification assays for detection of La Crosse virus RNA. J Clin Microbiol 43:1885–1889. doi: 10.1128/JCM.43.4.1885-1889.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Basile AJ, Horiuchi K, Goodman CH, Kosoy O, Panella AJ, Velez JO, Pastula DM, Brault AC, Staples JE, Calvert AE. 2021. Development of diagnostic microsphere-based immunoassays for heartland virus. J Clin Virol 134:104693. doi: 10.1016/j.jcv.2020.104693 [DOI] [PubMed] [Google Scholar]
- 67. Basile AJ, Horiuchi K, Panella AJ, Laven J, Kosoy O, Lanciotti RS, Venkateswaran N, Biggerstaff BJ. 2013. Multiplex microsphere immunoassays for the detection of IgM and IgG to arboviral diseases. PLoS One 8:e75670. doi: 10.1371/journal.pone.0075670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Thibodeaux BA, Liss NM, Panella AN, Roehrig JT. 2011. Development of a human-murine chimeric immunoglobulin M for use in the serological detection of human alphavirus antibodies. Clin Vaccine Immunol 18:2181–2182. doi: 10.1128/CVI.05269-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Thibodeaux BA, Roehrig JT. 2009. Development of a human-murine chimeric immunoglobulin M antibody for use in the serological detection of human flavivirus antibodies. Clin Vaccine Immunol 16:679–685. doi: 10.1128/CVI.00354-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Thibodeaux BA, Panella AN, Roehrig JT. 2010. Development of human-murine chimeric immunoglobulin G for use in the serological detection of human flavivirus and alphavirus antibodies. Clin Vaccine Immunol 17:1617–1623. doi: 10.1128/CVI.00097-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Skinner B, Mikula S, Davis BS, Powers JA, Hughes HR, Calvert AE, Ainsworth SR. 2022. Monoclonal antibodies to Cache Valley virus for serological diagnosis. PLoS Negl Trop Dis 16:e0010156. doi: 10.1371/journal.pntd.0010156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Wernike K, Brocchi E, Cordioli P, Sénéchal Y, Schelp C, Wegelt A, Aebischer A, Roman-Sosa G, Reimann I, Beer M. 2015. A novel panel of monoclonal antibodies against Schmallenberg virus nucleoprotein and glycoprotein Gc allows specific orthobunyavirus detection and reveals antigenic differences. Vet Res 46:27. doi: 10.1186/s13567-015-0165-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Boshra HY, Charro D, Lorenzo G, Sánchez I, Lazaro B, Brun A, Abrescia NGA. 2017. DNA vaccination regimes against Schmallenberg virus infection in IFNAR(-/-) mice suggest two targets for immunization. Antiviral Res 141:107–115. doi: 10.1016/j.antiviral.2017.02.013 [DOI] [PubMed] [Google Scholar]
- 74. Calvert AE, Bennett SL, Dixon KL, Blair CD, Roehrig JT. 2019. A monoclonal antibody specific for Japanese encephalitis virus with high neutralizing capability for inclusion as a positive control in diagnostic neutralization tests. Am J Trop Med Hyg 101:233–236. doi: 10.4269/ajtmh.19-0073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Hellert J, Aebischer A, Haouz A, Guardado-Calvo P, Reiche S, Beer M, Rey FA. 2023. Structure, function, and evolution of the orthobunyavirus membrane fusion glycoprotein. Cell Rep 42:112142. doi: 10.1016/j.celrep.2023.112142 [DOI] [PubMed] [Google Scholar]
- 76. Hellert J, Aebischer A, Wernike K, Haouz A, Brocchi E, Reiche S, Guardado-Calvo P, Beer M, Rey FA. 2019. Orthobunyavirus spike architecture and recognition by neutralizing antibodies. Nat Commun 10:879. doi: 10.1038/s41467-019-08832-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Plassmeyer ML, Soldan SS, Stachelek KM, Martín-García J, González-Scarano F. 2005. California serogroup Gc (G1) glycoprotein is the principal determinant of pH-dependent cell fusion and entry. Virology 338:121–132. doi: 10.1016/j.virol.2005.04.026 [DOI] [PubMed] [Google Scholar]
- 78. Plassmeyer ML, Soldan SS, Stachelek KM, Roth SM, Martín-García J, González-Scarano F. 2007. Mutagenesis of the La Crosse virus glycoprotein supports a role for Gc (1066-1087) as the fusion peptide. Virology 358:273–282. doi: 10.1016/j.virol.2006.08.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
supplementary tables and figures.