ABSTRACT
Latin-American Mediterranean (LAM) family is one of the most significant and global genotypes of Mycobacterium tuberculosis. Here, we used the murine model to study the virulence and lethality of the genetically and epidemiologically distinct LAM strains. The pathobiological characteristics of the four LAM strains (three drug resistant and one drug susceptible) and the susceptible reference strain H37Rv were studied in the C57BL/6 mouse model. The whole-genome sequencing was performed using the HiSeq Illumina platform, followed by bioinformatics and phylogenetic analysis. The susceptible strain H37Rv showed the highest virulence. Drug-susceptible LAM strain (spoligotype SIT264) was more virulent than three multidrug-resistant (MDR) strains (SIT252, SIT254, and SIT266). All three MDR isolates were low lethal, while the susceptible isolate and H37Rv were moderately/highly lethal. Putting the genomic, phenotypic, and virulence features of the LAM strains/spoligotypes in the context of their dynamic phylogeography over 20 years reveals three types of relationships between virulence, resistance, and transmission. First, the most virulent and more lethal drug-susceptible SIT264 increased its circulation in parts of Russia. Second, moderately virulent and pre-XDR SIT266 was prevalent in Belarus and continues to be visible in North-West Russia. Third, the low virulent and MDR strain SIT252 previously considered as emerging has disappeared from the population. These findings suggest that strain virulence impacts the transmission, irrespective of drug resistance properties. The increasing circulation of susceptible but more virulent and lethal strains implies that personalized TB treatment should consider not only resistance but also the virulence of the infecting M. tuberculosis strains.
IMPORTANCE
The study is multidisciplinary and investigates the epidemically/clinically important and global lineage of Mycobacterium tuberculosis, named Latin-American-Mediterranean (LAM), yet insufficiently studied with regard to its pathobiology. We studied different LAM strains (epidemic vs endemic and resistant vs susceptible) in the murine model and using whole-genome analysis. We also collected long-term, 20-year data on their prevalence in Eurasia. The findings are both expected and unexpected. (i) We observe that a drug-susceptible but highly virulent strain increased its prevalence. (ii) By contrast, the multidrug-resistant (MDR) but low-virulent, low-lethal strain (that we considered as emerging 15 years ago) has almost disappeared. (iii) Finally, an intermediate case is the MDR strain with moderate virulence that continues to circulate. We conclude that (i) the former and latter strains are the most hazardous and require close epidemiological monitoring, and (ii) personalized TB treatment should consider not only drug resistance but also the virulence of the infecting strains and development of anti-virulence drugs is warranted.
KEYWORDS: Mycobacterium tuberculosis, virulence, multidrug resistance, murine model, phylogeography
INTRODUCTION
Mycobacterium tuberculosis sensu stricto is an important human pathogen with a clonal, hierarchical, and heterogeneous population structure. Its evolutionary trajectory, genomic diversity, and phylogeography have been shaped by multiple factors, in particular, the coevolution with its human host and interaction with the human immune system. On the phylogenetically large scale, M. tuberculosis includes several lineages, while L1 to L4 are the most global and significant, although differ in their geographic patterns. In particular, Lineage 4 (Euro-American) is spread in many world regions, although remains low prevalent in most of Asia. Based on the available knowledge of phylogenetics, phylogeography, and human history, it was speculated that, most parsimoniously, Lineage 4 likely originated in the Eurasian heartland more than 3,000 years ago coinciding with the origin of the Indo-European languages (1). The most known families within Lineage 4 are LAM, Ural, Haarlem, S (all originated in Europe), L4.5 (endemic in South China), and diverse families endemic in Africa and named after different African countries where they likely originated during the colonial period.
On the global scale, Latin-American Mediterranean (LAM) family is perhaps the second most studied genotype of M. tuberculosis after the Beijing genotype. The LAM family was discovered more than 20 years ago in the seminal work of Sola et al. (2) based on the phylogenetic analysis of the large CRISPR-spoligotyping data set, and the name reflected the strains’ origins. LAM is prevalent in the Americas, Europe, Russia, and some parts of Africa. Initially defined by spoligotyping, the LAM prototype spoligotype profile is SIT42 with deleted signals 21–24 and 33–36. It should be kept in mind that SIT42 presents a stable profile because it includes genetically distant isolates, that is, this SIT is found across all LAM phylogeny and thus does not correlate with any particular subfamily within LAM. With regard to the medically important features, it should be noted that different LAM strains were shown to be associated with multidrug resistance in different settings including Russia (3 – 6), North and South America (7, 8), and Africa (9).
Inside the LAM lineage, there are sublineages mainly defined by large genomic deletions (RD, regions of difference) and other markers (Fig. S1). LAM RD-Rio sublineage was first found in Rio-de-Janeiro, Brazil, and was shown to be MDR associated (7). The LAM population in Northern Eurasia is overwhelmingly (>90%) represented by sublineage RD115/LAM-RUS, that is, isolates with RD115 deletion and specific IS6110 insertion in the plcA gene (10, 11). Several studies demonstrated the association of the Russian LAM strains with drug resistance (3, 5, 6). Drug-resistant spoligotypes SIT252 and SIT266 (both belonging to LAM-RUS) were previously termed as emerging resistant LAM clones with a potential risk of their wider spread over time hence the importance of epidemiological surveillance (12 – 14).
The spread of the particular genotypes is also impacted by external factors not related to the biological properties of the M. tuberculosis strains. LAM populations are similar in Mongolia and Eastern Siberia, Russia and are dominated by the LAM-RUS VNTR types. However, Russian but not Mongolian LAM strains are MDR associated which was attributed to insufficient health control and non-adequate TB treatment in Russia (6).
Unlike the Beijing genotype, the virulence of the LAM genotype has been little studied in animal and macrophage models to date. A Russian study by Zemskova et al. (15) demonstrated that Beijing, LAM, and reference H37Rv strains showed similar and high growth in the infected murine macrophages compared to Ural strains. The increased virulence of the Russian LAM strains similar to that of the Beijing strains in the in vivo mouse model was also demonstrated by the same group (16). It should be noted that, in spite of its certain limitations, the mouse model of tuberculosis infection is a classical one, and remains useful and meaningful (17 – 19). Recent studies of the particular Beijing subtypes demonstrated that hypervirulent and highly lethal (in murine model) strains may also lead to higher lethality in humans (20, 21).
In spite of well-known limitations, mice remain useful as model animals to study TB pathogenesis (22, 23). Here, we used the C57BL/6 mouse model and intravenous tail injection of the bacterial suspension according to the well-established and validated methodology previously used by us and others to study M. tuberculosis virulence (20, 23 – 25). We studied genetically and epidemiologically distinct strains of the LAM family in Russia that represented highly drug-resistant spoligotypes SIT252 and SIT266, low-prevalent and susceptible SIT264, and resistant and geographically widespread SIT254. The available whole-genome sequencing (WGS) data were used to gain insight into genetic variation possibly underlying the observed pathogenetic patterns. Finally, the obtained results were interpreted in light of the dynamic phylogeography of the studied genotypes.
MATERIALS AND METHODS
M. tuberculosis strains
The strains were recovered from the respiratory material of patients with infiltrative or fibrous-cavernous pulmonary TB time and place of sampling. Based on the spoligotyping and genotyping of the cluster-specific markers, they were assigned to different spoligotypes of the LAM family and its LAM-RUS branch. Spoligotyping and detection of other molecular markers of the LAM and LAM-RUS were performed as described previously (4, 7, 10).
Drug susceptibility testing of the strains was carried out using the method of absolute concentrations (Order No. 109 of the Ministry of Health of the Russian Federation) and/or using the automated system BACTEC MGIT 960.
Whole genome sequencing
The bacterial DNA was submitted to whole-genome sequencing performed using the Illumina HiSeq4000 platform. Whole-genome, paired-end sequencing on the HiSeq4000 platform was done using NEBNext Ultra, MiSeq Reagent v3, and PhiX Control v3 kits (Illumina). DNA libraries were prepared using ultrasound DNA fragmentation and NEBNext Ultra DNA Library Prep Kit for Illumina (New England Biolabs).
Data for the M. tuberculosis sequenced genomes were deposited in the NCBI Sequence Read Archive (project number PRJNA886055).
SAM-TB online tool (https://samtb.uni-medica.com/index) was used for SNP calling and phylogenetic analysis while SNPs in PE/PPE and resistance genes were excluded from phylogenetic analysis. The resulting concatenated FASTA file (678 variant nucleotide positions) was used to build a maximum likelihood tree using the MEGA7 package (https://www.megasoftware.net/), under default parameters and 1,000 bootstrap replicates.
Geneious 9.0 package (Biomatters Ltd) was additionally used for mapping the reads to the genome of reference strain H37Rv (NC_00962.3).
SAM-TB online tool was used for genotypic detection of drug resistance. MDR, pre-XDR, and XDR phenotypes were defined according to the updated World Health Organization definitions: MDR are strains resistant to isoniazid and rifampicin; pre-XDR—resistant to isoniazid, rifampicin, and fluoroquinolone; XDR—resistant to isoniazid, rifampicin, fluoroquinolone plus bedaquiline, and/or linezolid (26).
The significance of amino acid substitutions was assessed using Point Accepted Mutation 1 values calculated by PhyResSE online tool (https://bioinf.fz-borstel.de/mchips/phyresse/). The SIFT tool was used to predict whether an amino acid substitution affects protein function based on sequence homology and the physical properties of amino acids (https://sift.bii.a-star.edu.sg/index.html). As potentially biologically meaningful mutations, we defined those with significant SIFT P value or short indels. We also considered mutations in the putative promoter regions.
M. tuberculosis strains used for in vivo experiment
The mouse model study included five M. tuberculosis strains—four clinical LAM isolates and a reference laboratory strain H37Rv. The reference strain H37Rv was received from the Collection of the Scientific Center for the Expertise of Medicinal Products, Moscow, Russia (and initially received from the Institute of Hygiene and Epidemiology, Prague, Czech Republic).
The strains were cultured on Loewenstein-Jensen medium (Becton Dickinson, USA) and after 3 weeks of incubation at +35°C, the culture was suspended in a physiological solution with 15% glycerol, placed in cryovials, frozen, and stored at −80°C. Three weeks before the experiment, all strains were recultured on the Löwenstein-Jensen medium.
Experimental animals
All experimental procedures were carried out following National guidelines (Rules for working with laboratory rodents and rabbits, 2016) and were reviewed and approved by the Ethical Committee of the St. Petersburg Research Institute of Phthisopulmonology (Protocol 48.1 of 20 May 2019). In total, 210 C57BL/6 male mice (weight 16–18 g) were used in all experiments. The mice were obtained from the Andreevka laboratory animal nursery (Moscow region, Russia).
The animals were kept under the conditions of a certified animal facility at the St. Petersburg Research Institute of Phthisiopulmonology using NexGen Mouse IVC Cage & Rack system with built-in ventilation and air conditioning system. Before the start of the study, laboratory animals were quarantined for 14 days. The body weight was monitored weekly using an Adventurer electronic balance (OHAUS Corporation, USA). The criteria for inclusion of the animals in the experiment were as follows: positive dynamics of the body weight of animals during the quarantine period and the absence of visible symptoms of the disease.
Animal study design
In the animal model study, we followed the methodology that was previously described (20, 23). A mycobacterial suspension for infecting mice was prepared ex tempore from 3-week-old strains. The infecting dose was 106 CFU in 0.2 mL of saline buffer per mouse. A suspension of mycobacteria was inoculated into the lateral tail vein of the animals.
The animals were divided into two series to study the virulence of strains and survival of animals infected with mycobacteria.
In the first series, 180 mice were observed, 36 mice for each of the five studied M. tuberculosis strains. The animals were euthanized in groups of six mice at days 7, 14, 21, 28, 56, and 112 post-infection (p.i.). Next, an autopsy and a sterile sampling of the lungs and spleen were performed for further culturing of mycobacteria. Lungs and spleen were weighed for calculation of the weight coefficients. The lungs and spleens of the mice were aseptically removed. The weight of each lung was calculated and the gross anatomic picture was taken to document the extent of the lesions.
The weight coefficients for lungs and spleen were calculated based on the ratio of organ weight and animal body weight and expressed in conventional units: weight coefficient = (organ weight/animal body weight) × 100.
The second series consisted of 100 mice, that is, 20 per strain. The natural death of animals was recorded after infection. The animals that died during the study were subjected to an autopsy with an examination of the internal organs. The experiment was ended when a lethality rate of 100% was reached in the group of mice infected with the reference strain M. tuberculosis H37Rv. In our experiment, the observation period was 196 days. The lungs were visually assessed and weighed for the calculation of mass coefficients.
Mice were examined for external pathological signs, condition of the chest and abdominal cavity, and internal organs. The internal organs (lungs and spleen) were removed and weighed (to calculate biometric indicators), and the material was taken for bacteriological examination, as well as macroscopic examination (with subsequent fixation in 10% neutral formalin solution).
The body weight of the model animals in both experimental series was monitored once a week. The organ weight coefficients for the lungs and spleen were calculated based on the ratio of the organ weight to the body weight of the animal. The index of lung pathology was established based on the combined estimation of exudative and productive changes expressed in conventional units as previously described (Table S1) (20, 27).
The homogenized organs (0.1 g of lungs and whole spleen) were cultured on Loewenstein-Jensen medium (Becton Dickinson, USA) using the method of serial dilutions. The number of grown colonies of M. tuberculosis was counted after 4 weeks of incubation at +37°C. The growth of mycobacteria in cultures of lung homogenates was recalculated per organ’s weight. Results were expressed as log10 CFU per organ.
All mice in this series that survived on day 196 p.i. were euthanized to assess the severity of the course of the tuberculosis process: lethality, lung weight coefficient, and lung pathology index. Since their increase correlates with the increasing severity of the lesion, these three values were summed up to obtain a cumulative average index (CAI) that served to compare the virulence of the studied strains.
Histological examination of the lungs was performed for animals of series 1 at the final stage of the experiment (112 days). After fixation, the lung pieces were placed in special plastic cassettes, labeled, and proceeded for histological preparation using an automatic processor Tissue-TekVip 5Jr (Sakura, Japan), embedding in paraffin (using Tissue-Tek AutoTEC a120 automated embedder), cutting (using “ThermoScientific” Microm HM43 microtome) followed by placement of sections on glass slides, deparaffinization, and staining of with hematoxylin and eosin. Microscopic examination was performed on a Nikon Ci-S microscope with video digital image processing for material archiving and further processing.
For statistical analysis, Microsoft Excel 2013 and Statistica 13.0 programs were used. The significance of differences was assessed by Student’s t-test and was considered significant at P < 0.05. Lethality analysis of the model animals was performed using the Kaplan-Meier method. Pearson correlation coefficient (r) was calculated using https://www.statskingdom.com/correlation-calculator.html.
RESULTS
Genomic characterization of the studied strains
Twelve Russian LAM isolates of different spoligotypes were subjected to WGS and subsequent phylogenetic analysis (see Fig. 1 which also shows spoligotypes and resistance mutations). For the murine model study, we selected four LAM strains that were located in different parts of the tree. The isolates also differed in the drug resistance profile (see this and other information on strains in Table S2) that altogether correlated with drug resistance patterns commonly observed in strains of these spoligotypes (3 – 6, 11 – 14, 21). In particular, SIT264 is known to be mainly drug susceptible, SIT266 is XDR-associated, and SIT252 and SIT254 are MDR associated. Thus, these four LAM isolates represented the main trends in LAM population diversity in Russia: (i) sporadic susceptible strain (SIT264), (ii) potentially emerging MDR/pre-XDR strains (SIT252 and SIT266), and (iii) widespread drug-resistant strain (SIT254).
Fig 1.
WGS-based neighbor-joining tree of the Russian LAM isolates based on genome-wide SNPs (repeat regions excluded). LAM RD-Rio strain 4013 was used as an outgroup. All other isolates belong to the LAM-RUS branch. Isolates included in the murine model study are marked by an asterisk. The distance matrix for LAM-RUS strains is shown in Fig. S2.
The susceptible reference strain H37Rv was also included in the murine study as a control. H37Rv is a laboratory virulent strain that was initially isolated from a TB patient in New York, USA in 1905 (28). Thus, all five strains studied in the murine model belong to different sublineages of Lineage 4 (Euro-American lineage): L4.3 (LAM) and L4.9 (H37Rv).
Virulence study of C57BL/6 mice infected with reference and clinical isolates
The virulence of the strains was assessed by comparing the severity of the TB infection caused by different strains at days 7, 14, 21, 28, 56, and 112 p.i.
The changes in the lung and spleen weight coefficients reflect the development of specific inflammatory changes in these organs. Biometric examination of the lungs showed an overall increase in the lung weight coefficient in all groups of infected animals (Fig. 2A). On day 14 p.i., the lung weight coefficient increased significantly compared to day 7 p.i. (P < 0.05, P < 0.002) in four groups (Nos. 1, 2, 4, and 5). It was the highest at almost all periods of observation in mice infected with H37Rv and the lowest in mice infected with isolates 7074 and 3929. Detailed information on the significance of the pairwise comparisons between different strains with regard to different characteristics (lung and spleen weight coefficient, pathology index, and bacterial load) during the experiment is shown in Tables S3 through S12. The lung weight coefficient was significantly higher in group 5 (strain 4542, SIT266) compared to other clinical groups.
Fig 2.
Changes in the lung (A) and spleen (B) weight coefficients, in conventional units, of mice infected with M. tuberculosis strains determined at different time points. Here and in other figures, the same color is used to depict five studied strains. Data represent means plus standard deviations (SD) (error bars).
The pattern of changes in the spleen weight coefficient was similar in all groups and most pronounced at day 21 p.i. (Fig. 2B). Its significant increase in all groups was recorded on days 14 to 21 p.i. (P < 0.05, P < 0.001), followed by a steady decrease. The highest values, even slightly higher than H37Rv-group, were registered in group 5 (strain 4542, SIT266) at most of the time points.
The specific inflammation in the lungs assessed by the lung pathology index showed a similar increase in all groups of mice with higher scores in H37Rv and groups infected with strains 4542 (SIT266) and 306 (SIT264) (Fig. 3A) on days 14 and 21 p.i. and near the end of the experiment. Almost all animals from the experiment had single submiliary foci of productive tuberculous inflammation and small airless foci, pointing to the exudative component of the inflammatory reaction. The values of the pathology index were the highest in the H37Rv group, significantly exceeding those in other groups of mice at most of the time points (P < 0.05 to P < 0.002).
Fig 3.
Changes in lung pathology scores (A) and bacterial load in the lungs (B) of mice infected with M. tuberculosis strains determined at different time points.
Multiple submiliary and single miliary productive foci were detected on day 14 p.i. in all experimental groups, leading to a significant increase in the pathology index (P < 0.05 to P < 0.001). Exudative manifestations of infection also increased, airless areas in mice of groups 1, 2, and 4 occupied half of the lungs, while single foci were in groups 3 and 5 and occupied half of the lungs only by day 21 p.i. The confluent nature of miliary productive foci was registered earlier (day 21 p.i.) in groups infected with strains H37Rv, 306, and 4542.
In all groups, a gradual increase in the lung pathology index was noted until day 28 p.i. when it reached almost the same level in all experimental animals, followed by a decrease (Fig. 3A). A closer look at the individual data revealed that this decrease was due to the reduced severity of the exudative component of the inflammatory reaction in clinical groups, manifested by decreased area of airless foci in the lungs. In the H37Rv group, two-thirds of the lungs were airless at days 56 and 112 p.i. Extensive airlessness of the lungs in group 1 (strain H37Rv) was observed in three mice on day 112 p.i. The highest values of the lung pathology index were recorded in mice infected with H37Rv, the lowest—in mice infected with strains 7074 (SIT252) and 3929 (SIT254) which showed similar changes. In turn, strains 306 (SIT 264) and 4542 (SIT266) showed intermediate and similar lung damage indices.
The analysis of the bacterial load of the lungs confirmed the detected trends in the relative virulence of the studied strains (Fig. 3B). The lowest values were in group 2 (strain 7074, SIT252) and group 3 (strain 3929, SIT254). The highest bacterial load at most time points was noted in group 1 (H37Rv) and group 5 (strain 4542, SIT266). At day 21, the most contrasting levels of the bacterial loads were detected in mice infected with H37Rv, strains 306 and 4542 (similar and highest) vs mice infected with strains 7074 and 3929 (similar and lowest). At the end of the experiment (day 112 p.i.), the contrasting highest and lowest bacterial loads were in the groups infected with H37Rv and 3929 (SIT254), respectively, while the other three strains were similar and intermediate.
An overall similar trend was observed for changes in the bacterial load of the spleen at day 21 p.i. (the higher in H37Rv, 306, 4252 vs the lower in 7974, 3929 infected groups of mice) (Fig. S3). Also, at day 112 p.i., the lowest bacterial load was in the 7074- and 3929-infected groups, the highest in the H37Rv-group, and intermediate in the groups infected with strains 306 and 4252. Interestingly, this indicator was similar in all five groups at day 14 p.i. followed by its overall and clear decrease. This differed from much less pronounced changes in the bacterial load of the lungs (Fig. 3B).
The correlation between bacterial load and lung pathology index was estimated by Pearson correlation coefficient (r) (Table S13). In most cases, it was non-significant: very small negative for 7074 (SIT252) and 3929 (SIT254), and large positive for 306 (SIT264) and H37Rv. Only for one strain 4542 (SIT266), a significant large positive relationship was demonstrated.
Histological evaluation of lung sections
Histological examination demonstrated that all infected mice developed extensive lung damage with strain-specific features. Two types of lung lesions were observed: more widespread lung damage caused by strains H37Rv and 306, and less widespread lung damage caused by strains 7074, 3929, and 4542. We tentatively termed these states as Type I and Type II lesions, respectively. These types also differed in the nature of lesions. Predominantly, alterative-exudative changes were observed for Type I while mainly productive changes were characteristic for Type II as detailed below (Fig. 4; Fig. S10).
Fig 4.
Microscopic images at day 112 p.i. of the lung of mice infected with (A) M. tuberculosis H37Rv; note the confluent foci of specific infiltration without clear contours. (B) Strain 4542; note the focus of specific infiltration surrounded by air tissue. Stained with hematoxylin and eosin ×300.
Type I changes in the lungs included large foci of infiltration without clear boundaries, merging into conglomerates (Fig. 4A). At the same time, the airiness of the lung tissue (i.e., the ratio of the aired areas to the total area of the section) decreased by >30% in all mice infected with H37Rv, and in four out of six mice infected with strain 306 (Table S14). The interalveolar septa in the infiltration zones were noticeably thickened due to the accumulation of neutrophilic granulocytes, lymphocytes, and macrophages (Fig. S4). These cells were also located in the lumen of the alveoli, where their microscopic features were most clearly revealed. First of all, these were foamy macrophages and epithelioid cells (Fig. S5). In some alveoli, the infiltrate consisted only of these cells, closely adjacent to each other, while in others, the cells were located loosely and with serous exudate between them. There were also alveoli completely filled with serous exudate without admixture of cells, as well as alveoli containing neutrophilic granulocytes. In 4 out of 12 mice, mild perivascular and peribronchial lymphohistiocytic infiltration was determined in the lungs.
Type II changes in the lungs were characterized by clearly defined small airless areas surrounded by air tissue (Fig. 4B). A decrease in airiness by >30% was observed only in one mouse infected with strain 3929 (Fig. S6), and in two mice infected with strain 4542. In general, these changes were three times less abundant compared to the Type I changes (Table S14).
Type I and II changes also differed in the cell content of infiltrates. Thus, type II changes were predominantly productive and consisted mainly of lymphocytes, foamy macrophages, and epithelioid cells (Fig. S7). A small number of alveoli were filled with serous exudate, while neutrophilic granulocytes were found in lymphohistiocytic clusters only in one mouse infected with strain 3929 and in two mice infected with strain 4542 (Fig. S8). Finally, peribronchial and perivascular lymphohistiocytic aggregations observed in 18 mice were another striking histological characteristic of the productive response in type II changes (Fig. S9 and S10).
Survival study of C57BL/6 mice infected with reference and clinical isolates
Survival rates differed significantly between the studied groups of mice infected with different strains (Fig. 5). Mice infected with strains 7074, 3929, and 4542 had the lowest lethality that did not exceed 5% and was recorded only after day 150 p.i. By contrast, the death of mice in the groups infected with H37Rv and 306 was recorded on day 21 p.i. Survival curves in these groups followed a similar course up to day 116 p.i. with ~20% of died animals but started to change in days 117–174 p.i. with 100% lethality in H37Rv group and 50% lethality in the 306 group. Thus, in terms of survival, the studied strains can be divided into three types: high 100% lethality (H37Rv), moderate 50% lethality (strain 306, SIT264), and low 5% lethality (strains 7074, 3929, and 4542).
Fig 5.
Survival of mice after infection with M. tuberculosis strains.
An analysis of the body weight of mice showed that groups infected with 7074, 3929, and 4542 had the highest average weight values, indicating the most favorable course of infection. The lowest body weight was in mice infected with strains H37Rv and 306 (Fig. S11). A certain decrease in the body weight in mice infected with strain 306 was noted after the 14th week of the experiment, which reflects the pronounced intoxication syndrome.
A CAI was used to compare the virulence of the studied strains (Table S15). The highest value of this index (19.06) was in mice infected with strain 306 (SIT264) due to the high lung weight coefficient and lung damage. The other three clinical strains showed similar and much lower values (3.3–3.6). The group infected with strain 7074 (SIT252) had the lowest cumulative index due to concomitantly lowest lethality, lung weight coefficient, and lung damage.
Biologically meaningful mutations in strains 7074 vs 4542
This virulence study focused on four LAM isolates that represent phylogeographically and genomically distinct medically relevant spoligotypes. An in-depth genomic study of the SIT-specific genetic variation requires analysis of the enlarged collection of isolates (that is ongoing). Preliminarily, we assessed the available genomic data of the genetically closely related MDR isolates with contrasting virulence characteristics: low virulent strain 7074 (SIT252) and highly virulent strain 4542 (SIT266). In total, 13 strain-specific biologically meaningful mutations were found (Table S16).
DISCUSSION
Current views on the interplay between virulence, fitness, resistance, and transmissibility of M. tuberculosis strains suggest that (i) multiple resistance mutations reduce fitness and transmission capacity which explains the selection of the compensatory mutations in some successful strains; (ii) highly virulent strains are also highly transmissible; and (iii) the most successful strains harbor both low-cost resistance and/or compensatory mutations that restore their fitness (29 – 32). The findings of this study are partly in line with these observations. The studied isolates belong to different and epidemiologically significant subtypes of the LAM-RUS branch of M. tuberculosis. These are (i) the evolutionarily more recent and possibly emerging MDR SIT252 and SIT266, (ii) geographically diverse and drug-susceptible SIT264, and (iii) SIT254 that is the commonest LAM spoligotype in Russia and neighboring countries. Spoligotypes SIT264 and SIT266 are very similar and differ in one signal #8 that is invisible in SIT266 due to asymmetrical IS6110 insertion (12). Noteworthy, WGS analysis showed that these SIT are not directly related and differ in 92 SNPs (Fig. 1).
To the best of our knowledge, only a few articles on the LAM virulence in the murine model were published, all were in the Russian language and were published more than 10 years ago (15, 16). On the other hand, previous virulence studies of the Euro-American lineage enrolled other than LAM genotypes. For example, a study of clade-specific virulence patterns in human primary macrophages and mice infected by the aerosol route demonstrated high uptake, high cytokine induction, and high growth rates for Haarlem strains (33). It is unknown whether LAM strains would exhibit similar or partly similar properties.
Different patterns of the key virulence and lethality features
The main results on characteristic virulence features of different strains are summarized in Fig. 6 where the studied strains are listed in the descending order of their relative virulence and lethality. It appears that there is a non-straightforward relationship between different characteristics and different types of correlation may be seen. For example, pre-XDR strain SIT252 appears the least virulent while pansusceptible strain SIT264 is the most virulent, lethal, and leading to the most extensive histological changes in the lungs. The higher number of resistance mutations is in negative correlation with virulence in SIT252 but the situation was more complex with strain SIT266 which is both the most virulent and bears 10 drug resistance mutations. Finally, the highest virulence and lethality of all studied strains were observed for strain H37Rv, a control virulent strain.
Fig 6.
Summary of the main virulence and lethality characteristics of the studied strains. Observations are mostly for the period between days 14 and 56 p.i. The cumulative average index was based on lethality, lung weight coefficient, and lung damage of survived mice at 196 dpi within the survival series. In addition, the high, intermediate, and low values are marked by color (red, orange, and blue, respectively).
The histological analysis corroborated biometric results (bacterial load, pathology index). It showed that characteristic inflammation developed in the lungs of mice by the day 112 p.i. was different for different strains in terms of abundance and severity (Fig. 4; Fig. S9), thus correlating with different virulence of the strains. The most virulent strains were H37Rv and susceptible 306 (SIT264), which caused widespread pneumonic confluent changes in the lungs (that we defined as Type I changes). At the same time, strains 7074, 3929, and 4542 caused discrete small-focal lung lesions (Type I changes) and can be considered relatively less virulent. This conclusion is also supported by the microscopic picture, with a clear predominance of an alterative-exudative reaction for strains H37Rv and 306, compared to the clearly proliferative manifestation for three other strains. It may be that more virulent strains H37Rv and 306 multiply at a higher rate and accumulate more abundantly in the lungs, as evidenced by the abundance of foamy macrophages containing mycobacterial antigens.
The less virulent strains 7074, 3929, and 4542 are interesting in terms of their immunogenic properties. Indeed, in the lungs of mice infected with these strains, characteristic perivascular and peribronchial lymphohistiocytic infiltrates play a major role in the immune response. Similar reactive changes developed in the lungs during experimental infection with the BCG vaccine strain, ensuring its survival and limited reproduction in the animal body with minimal damaging effects on tissues (34).
Interestingly, in line with our findings of the striking pathogenetic differences between the closely related strains, the same was shown for LAM RD-Rio isolates in Colombia that were compared in their transcriptional response under two axenic media conditions (35). Those clinical isolates were found phenotypically different at the level of cell death, cytokine production, growth kinetics upon in vitro infection of human tissue macrophages, and membrane vesicle secretion upon culture in synthetic medium. Furthermore, RNA-seq analysis has identified different strategies to counteract the adverse condition of a carbon-poor media: (i) activation of virulence systems such as the ESX-1, synthesis of diacyl-trehalose, polyacyl-trehalose, and sulfolipids and (ii) activation of the DNA replication, cell division, and lipid biosynthesis (35).
Pathobiologically meaningful mutations suggested by in silico analysis
A comparison of WGS data for low-virulent strain 7074 (SIT252) and highly virulent strain 4542 (SIT266) revealed eight potentially biologically meaningful mutations for strain 7074 and five such mutations for strain 4542 (Table S16). Hypothetically, the former could be associated with reduced virulence while the latter—with increased virulence, respectively. Indeed, previous studies suggested the role of some of these genes/proteins in the virulence of M. tuberculosis or other pathogens (36 – 50) (Table S16). The following mutated proteins are of particular interest: (i) TatD DNase potential virulence factor in Plasmodium falciparum and Streptococcus pneumoniae involved in biofilm formation and required for virulence of Trueperella pyogenes (48), (ii) glycogen phosphorylase GlgP abundant after phagocytosis of K-strain dominant in Korea (44), and (iii) carboxylesterase A CaeA (Rv2224c) modulates innate immune response and is required for full virulence (40). In T. pyogenes, biofilms formed by TatD mutants produced a lower bacterial load in the spleen of mice and compromised virulence (48). In our study, a mutation TatD C11R in the SIT252 strain could also contribute to its reduced virulence manifested by low values of all assessed characteristics (Fig. 6 ). Similarly, Rv2224c (CaeA), a cell envelope-associated predicted protease, is critical for M. tuberculosis virulence. Disruption of Rv2224c led to prolonged survival of infected mice and highly reduced lung pathology (43). The absence of Rv2224c enhanced host innate immune responses and compromised the intracellular survival of M. tuberculosis in macrophages. While no pks8 studies were published on M. tuberculosis, the polyketide synthases are known as virulence factors and Pks8 was shown to be required for the pathogenicity of plant pathogen Pseudocercospora fijiensis (46). The true role of these mutations should be further determined in the experimental model, for example, using allele replacement experiments or knockout mutants.
Dynamic changes in the phylogeographic landscape
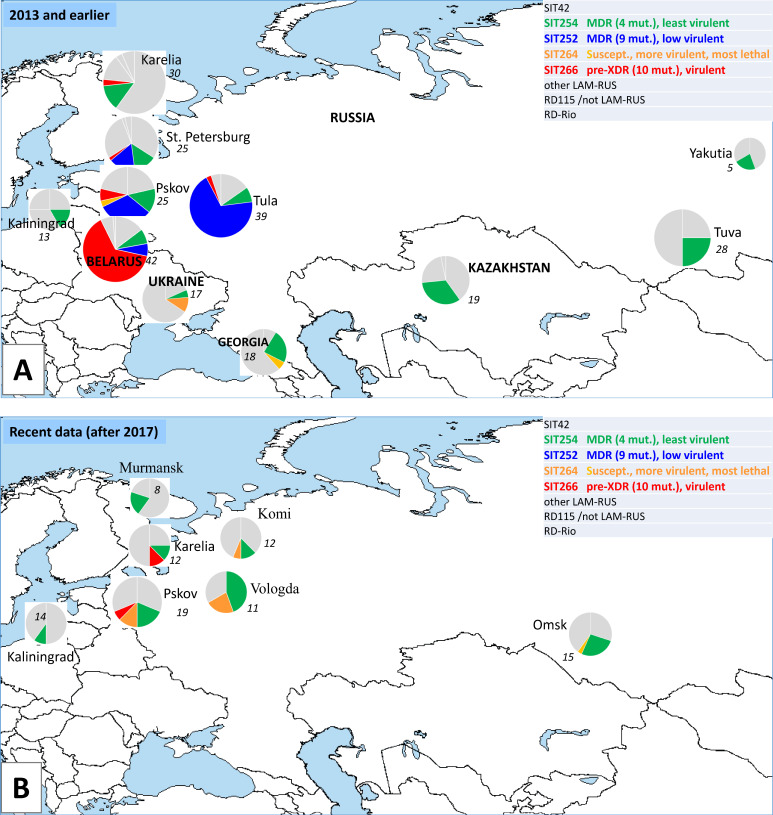
We further tried to assess the dynamic changes in the phylogeography of these four spoligotypes based on the studies published in the last 20 years from different Russian regions, Belarus and Ukraine (5, 21, 51 – 60) (Fig. 7). SIT252 was previously termed as emerging since it included MDR-associated, genetically closely related and geographically delimited isolates from European Russia (14); in our study, it was represented MDR and low-virulent strain 7074. Surprisingly, SIT252 was not described in the studies published after 2014. In contrast, rare and drug-susceptible SIT264 (virulent and moderately lethal strain 306 in the present study) slightly increased its circulation and geography. Moderately drug-resistant SIT254 (the least-virulent strain 3929 in this study) remained the most widespread LAM spoligotype. A certain caution in the interpretation of the subtype prevalence is however required. While most of the studies (all in Fig. 7B and most in Fig. 7A ) were population based, some earlier studies presented in Fig. 7A were based on convenience or small samples (St. Petersburg, Yakutia, Kazakhstan) or included only drug-resistant strains (Belarus), and thus could be biased.
Fig 7.
LAM subtypes: geography, resistance, and relative virulence in earlier (A) and recent studies (B). Circle size is roughly proportional to the percentage of LAM in a local sample (also shown in italics). The original map, freely available at https://de.wikipedia.org/wiki/Datei:A_large_blank_world_map_with_oceans_marked_in_blue.PNG, is licensed under the Creative Commons Attribution-Share Alike 3.0 Unported License. Pie charts, data labels, and legends have been overlaid by the authors.
The situation with susceptible and virulent SIT264 that is on the increase in the European part of Russia is similar to the increase in susceptible Beijing strain in Cape Town, South Africa which became visible under a longitudinal study over 12 years. The incidence of the Haarlem, LAM, Quebec, and the Low-Copy Clades remained relatively stable, in contrast to the exponentially increased incidence of the Beijing strains. This growth was exclusively attributable to drug-susceptible Beijing strains that had a greater proportion of smear-positive sputa than their non-Beijing counterparts and were less likely to be successfully treated (61). These differences likely reflected enhanced pathogenicity rather than transmissibility of the Beijing genotype in Cape Town.
Limitations
The limitation of the mice experiments comparing different genotypes of M. tuberculosis is the relatively small number of strains that can be investigated in each experiment. Only one isolate per spoligotype was included in this study. However, this limitation is almost inevitable for this kind of in vivo studies that are difficult to include multiple isolates given the already large sample size of the studied animals. Nonetheless, we believe that the enrolled clinical isolates correctly represent their spoligotypes in terms of phenotypic resistance features. The used model of intravenous tail injection was previously shown by us and others as a reliable method to study the virulence and lethality of the M. tuberculosis isolates (20, 23 – 25).
Conclusion
Putting together genomic, phenotypic, and virulence features of the LAM strains and more generally, dynamic phylogeography of their respective spoligotypes over 20 years, revealed a peculiar and partly inverse correlation and three types of manifestation. First, the most virulent, more lethal but drug-susceptible strain SIT264 increased its circulation in parts of Russia. Second, virulent and pre-XDR strain SIT266 was prevalent in Belarus and continues to be visible in North-West Russia. Third, low-virulent and MDR strain SIT252 with multiple resistance mutations was widespread in European Russia 10 years ago but drastically reduced its current circulation. The former two cases appear to be the most hazardous and require particular attention.
These observations emphasize strain virulence as an important feature associated with transmission, irrespective of drug resistance properties. Our findings suggest that increased virulence of some of the clinical isolates is associated with a fundamentally different systemic immune response, which can be detected early during infection. Virulence may correlate with the rate of reproduction of microbes in a given system (humans, mice), and if the notorious MDR strains multiply slowly, the immune system would have enough time to counteract them.
The studied strains while heterogeneous in their pathobiological features, belong to the same LAM-RUS branch, a part of the LAM family, itself a member of Lineage 4. However, Lineage 4 is heterogeneous and its other genotypes (Haarlem, Ural, S, etc.) have a different interplay of bacterial virulence and resistance, which was also modulated by coevolution with local human populations. Different lineages and sublineages, and smaller more homogeneous clusters of human M. tuberculosis isolates have developed their way of adaptation and dissemination.
Medically relevant research should focus on genetically compact epidemic clusters. Increasing circulation of the susceptible but virulent and lethal strains implies (i) the importance of their close epidemiological monitoring and (ii) that personalized TB treatment should consider not only resistance but also the virulence of the infecting strains. This highlights importance of development of the so called anti-virulence drugs.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by Russian Science Foundation (grant 19-14-00013).
Conceptualization: I.M., T.V. Investigation: M.D., N.Z., A.V., M.V., N.S. Formal analysis: I.M., T.V., B.A. Writing, original draft: I.M. Writing - Review & Editing: I.M., T.V., B.A.
Authors declare that no conflict of interest exists.
Contributor Information
Igor Mokrousov, Email: igormokrousov@yahoo.com.
Alexandra Aubry, Sorbonne Université, Paris, France .
DATA AVAILABILITY
All data of this study are presented in the article and supplementary material. Data for the M. tuberculosis genomes were deposited in the NCBI Sequence Read Archive (project number PRJNA886055).
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/spectrum.01392-23.
Fig. S1 to S11.
Tables S1 to S16.
An accounting of the reviewer comments and feedback.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Mokrousov I, Shitikov E, Skiba Y, Kolchenko S, Chernyaeva E, Vyazovaya A. 2017. Emerging peak on the phylogeographic landscape of Mycobacterium tuberculosis in West Asia: definitely smoke, likely fire. Mol Phylogenet Evol 116:202–212. doi: 10.1016/j.ympev.2017.09.002 [DOI] [PubMed] [Google Scholar]
- 2. Sola C, Filliol I, Legrand E, Mokrousov I, Rastogi N. 2001. Mycobacterium tuberculosis phylogeny reconstruction based on combined numerical analysis with Is1081, Is6110, VNTR, and DR-based spoligotyping suggests the existence of two new phylogeographical clades. J Mol Evol 53:680–689. doi: 10.1007/s002390010255 [DOI] [PubMed] [Google Scholar]
- 3. Ignatova A, Dubiley S, Stepanshina V, Shemyakin I. 2006. Predominance of multi-drug-resistant LAM and Beijing family strains among Mycobacterium tuberculosis isolates recovered from prison inmates in Tula region, Russia. J Med Microbiol 55:1413–1418. doi: 10.1099/jmm.0.46575-0 [DOI] [PubMed] [Google Scholar]
- 4. Mokrousov I, Vyazovaya A, Iwamoto T, Skiba Y, Pole I, Zhdanova S, Arikawa K, Sinkov V, Umpeleva T, Valcheva V, Alvarez Figueroa M, Ranka R, Jansone I, Ogarkov O, Zhuravlev V, Narvskaya O. 2016. Latin-American-Mediterranean lineage of Mycobacterium tuberculosis: human traces across pathogen's phylogeography. Mol Phylogenet Evol 99:133–143. doi: 10.1016/j.ympev.2016.03.020 [DOI] [PubMed] [Google Scholar]
- 5. Vyazovaya AA, Akhmedova GM, Solovieva NS, Gerasimova AA, Starkova DA, Turkin EN, Zhuravlev VY, Narvskaya OV, Mokrousov IV. 2017. Molecular epidemiology of tuberculosis in the Kaliningrad region of Russia: 10 years after. Russian Journal of Infection and Immunity 7:367–374. doi: 10.15789/2220-7619-2017-4-367-374 [DOI] [Google Scholar]
- 6. Zhdanova S, Mokrousov I, Orlova E, Sinkov V, Ogarkov O. 2022. Transborder molecular analysis of drug-resistant tuberculosis in Mongolia and Eastern Siberia, Russia. Transbound Emerg Dis 69:e1800–e1814. doi: 10.1111/tbed.14515 [DOI] [PubMed] [Google Scholar]
- 7. Gibson AL, Huard RC, Gey van Pittius NC, Lazzarini LC, Driscoll J, Kurepina N, Zozio T, Sola C, Spindola SM, Kritski AL, Fitzgerald D, Kremer K, Mardassi H, Chitale P, Brinkworth J, Garcia de Viedma D, Gicquel B, Pape JW, van Soolingen D, Kreiswirth BN, Warren RM, van Helden PD, Rastogi N, Suffys PN, Lapa e Silva J, Ho JL. 2008. Application of sensitive and specific molecular methods to uncover global dissemination of the major RDRio sublineage of the Latin American-Mediterranean Mycobacterium tuberculosis spoligotype family. J Clin Microbiol 46:1259–1267. doi: 10.1128/JCM.02231-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ordaz-Vázquez A, Torres-González P, Cruz-Hervert P, Ferreyra-Reyes L, Delgado-Sánchez G, García-García L, Kato-Maeda M, Ponce-De-León A, Sifuentes-Osornio J, Bobadilla-Del-Valle M. 2021. Genetic diversity and primary drug resistance transmission in Mycobacterium tuberculosis in Southern Mexico. Infect Genet Evol 93:104994. doi: 10.1016/j.meegid.2021.104994 [DOI] [PubMed] [Google Scholar]
- 9. Solo ES, Suzuki Y, Kaile T, Bwalya P, Lungu P, Chizimu JY, Shah Y, Nakajima C. 2021. Characterization of Mycobacterium tuberculosis genotypes and their correlation to multidrug resistance in Lusaka, Zambia. Int J Infect Dis 102:489–496. doi: 10.1016/j.ijid.2020.10.014 [DOI] [PubMed] [Google Scholar]
- 10. Dubiley S, Kirillov E, Ignatova A, Stepanshina V, Shemyakin I. 2007. Shemyakin molecular characteristics of the Mycobacterium tuberculosis LAM-RUS family prevalent in Central Russia. J Clin Microbiol 45:4036–4038. doi: 10.1128/JCM.01217-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mokrousov I, Vyazovaya A, Narvskaya O. 2014. Mycobacterium tuberculosis Latin American-Mediterranean family and its sublineages in the light of robust evolutionary markers. J Bacteriol 196:1833–1841. doi: 10.1128/JB.01485-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mokrousov I, Chernyaeva E, Vyazovaya A, Sinkov V, Zhuravlev V, Narvskaya O. 2016. Next-generation sequencing of Mycobacterium tuberculosis. Emerg Infect Dis 22:1127–1129. doi: 10.3201/eid2206.152051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zalutskaya A, Wijkander M, Jureen P, Skrahina A, Hoffner S. 2013. Multidrug-resistant Myobacterium tuberculosis caused by the Beijing genotype and a specific T1 genotype clone (SIT no. 266) is widely transmitted in Minsk. Int J Mycobacteriol 2:194–198. doi: 10.1016/j.ijmyco.2013.08.001 [DOI] [PubMed] [Google Scholar]
- 14. Vyazovaya A, Mokrousov I, Pole I, Solovieva N, Umpeleva T, Skiba Y, Al-Hajoj S, Varghese B, Ludannyy R, Alvarez Figueroa M, Skenders G, Ranka R, Jansone I, Zhuravlev V, Narvskaya O. 2016. “Multidrug-resistant clone Myсobacterium tuberculosis LAM Sit252 emerging in some regions of European Russia and Eastern Europe” 37th Annual Congress of European Society of Mycobacteriology; Catania, Italy: , p 81–82 [Google Scholar]
- 15. Zemskova ZS, Andreevskaya SN, Smirnova TG, Larionova EE, Chernousova LN. 2010. Experimental tuberculosis caused by M.Tuberculosis strains of genotypic clusters W, AI and HD. Tuberk. Bolez Legk 87:41–46. [Google Scholar]
- 16. Andreevskaia SN, Chernousova LN, Smirnova TG, Larionova EE, Kuz’min AV. 2007. Impact of M. tuberculosis genotype on survival in mice with experimental tuberculosis. Probl Tuberk Bolezn Legk, no. 7:45–50. [PubMed] [Google Scholar]
- 17. Hernández-Pando R, Marquina-Castillo B, Barrios-Payán J, Mata-Espinosa D. 2012. Use of mouse models to study the variability in virulence associated with specific genotypic lineages of Mycobacterium tuberculosis. Infect Genet Evol 12:725–731. doi: 10.1016/j.meegid.2012.02.013 [DOI] [PubMed] [Google Scholar]
- 18. Almeida FM, Ventura TL, Amaral EP, Ribeiro SCM, Calixto SD, Manhães MR, Rezende AL, Souza GS, de Carvalho IS, Silva EC, Silva J da, Carvalho ECQ, Kritski AL, Lasunskaia EB. 2017. Hypervirulent Mycobacterium tuberculosis strain triggers necrotic lung pathology associated with enhanced recruitment of neutrophils in resistant C57Bl/6 mice. PLoS One 12:e0173715. doi: 10.1371/journal.pone.0173715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mourik BC, de Steenwinkel JEM, de Knegt GJ, Huizinga R, Verbon A, Ottenhoff THM, van Soolingen D, Leenen PJM. 2019. Mycobacterium tuberculosis clinical isolates of the Beijing and East-African Indian lineage induce fundamentally different host responses in mice compared to H37Rv. Sci Rep 9:19922. doi: 10.1038/s41598-019-56300-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vinogradova T, Dogonadze M, Zabolotnykh N, Badleeva M, Yarusova I, Vyazovaya A, Gerasimova A, Zhdanova S, Vitovskaya M, Solovieva N, Pasechnik O, Ogarkov O, Mokrousov I. 2021. Extremely lethal and hypervirulent Mycobacterium Tuberculosis strain cluster emerging in Far East, Russia. Emerg Microbes Infect 10:1691–1701. doi: 10.1080/22221751.2021.1967704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mokrousov I, Vyazovaya A, Solovieva N, Sunchalina T, Markelov Y, Chernyaeva E, Melnikova N, Dogonadze M, Starkova D, Vasilieva N, Gerasimova A, Kononenko Y, Zhuravlev V, Narvskaya O. 2015. Trends in molecular epidemiology of drug-resistant tuberculosis in republic of Karelia, Russian Federation. BMC Microbiol 15:279. doi: 10.1186/s12866-015-0613-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sia KJ, Rengarajan J. 2017. Immunology of Mycobacterium tuberculosis infections. Microbiol Spectr 7. doi: 10.1128/microbiolspec.GPP3-0022-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Orme I, Gonzalez-Juarrero M. 2007. Animal models of M. tuberculosis infection. Curr Protoc Microbiol 7:10A.5.1. doi: 10.1002/9780471729259.mc10a05s7 [DOI] [PubMed] [Google Scholar]
- 24. Stukova MA, Sereinig S, Zabolotnyh NV, Ferko B, Kittel C, Romanova J, Vinogradova TI, Katinger H, Kiselev OI, Egorov A. 2006. Vaccine potential of influenza vectors expressing Mycobacterium tuberculosis ESAT-6 protein. Tuberculosis (Edinb) 86:236–246. doi: 10.1016/j.tube.2006.01.010 [DOI] [PubMed] [Google Scholar]
- 25. Fursov MV, Shitikov EA, Bespyatykh JA, Bogun AG, Kislichkina AA, Kombarova TI, Rudnitskaya TI, Grishenko NS, Ganina EA, Domotenko LV, Fursova NK, Potapov VD, Dyatlov IA. 2020. Genotyping, assessment of virulence and antibacterial resistance of the rostov strain of Mycobacterium Tuberculosis attributed to the Central Asia outbreak clade. Pathogens 9:335. doi: 10.3390/pathogens9050335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Roelens M, Battista Migliori G, Rozanova L, Estill J, Campbell JR, Cegielski JP, Tiberi S, Palmero D, Fox GJ, Guglielmetti L, Sotgiu G, Brust JCM, Bang D, Lienhardt C, Lange C, Menzies D, Keiser O, Raviglione M. 2021. Evidence-based definition for extensively drug-resistant tuberculosis. Am J Respir Crit Care Med 204:713–722. doi: 10.1164/rccm.202009-3527OC [DOI] [PubMed] [Google Scholar]
- 27. Aleksandrova AE, Ariél’ BM. 1993. Evaluation of the severity of tuberculous process in Mouse lung. Probl Tuberk, no. 3:52–53. [PubMed] [Google Scholar]
- 28. Bouwman AS, Kennedy SL, Müller R, Stephens RH, Holst M, Caffell AC, Roberts CA, Brown TA. 2012. Genotype of a historic strain of Mycobacterium tuberculosis. Proc Natl Acad Sci USA 109:18511–18516. doi: 10.1073/pnas.1209444109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Aguilar D, Hanekom M, Mata D, Gey van Pittius NC, van Helden PD, Warren RM, Hernandez-Pando R. 2010. Mycobacterium tuberculosis strains with the Beijing genotype demonstrate variability in virulence associated with transmission. Tuberculosis (Edinb) 90:319–325. doi: 10.1016/j.tube.2010.08.004 [DOI] [PubMed] [Google Scholar]
- 30. Kato-Maeda M, Shanley CA, Ackart D, Jarlsberg LG, Shang S, Obregon-Henao A, Harton M, Basaraba RJ, Henao-Tamayo M, Barrozo JC, Rose J, Kawamura LM, Coscolla M, Fofanov VY, Koshinsky H, Gagneux S, Hopewell PC, Ordway DJ, Orme IM. 2012. Beijing sublineages of Mycobacterium tuberculosis differ in pathogenicity in the guinea pig. Clin Vaccine Immunol 19:1227–1237. doi: 10.1128/CVI.00250-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ribeiro SC, Gomes LL, Amaral EP, Andrade MRM, Almeida FM, Rezende AL, Lanes VR, Carvalho ECQ, Suffys PN, Mokrousov I, Lasunskaia EB. 2014. Mycobacterium tuberculosis strains of the modern sublineage of the Beijing family are more likely to display increased virulence than strains of the ancient sublineage. J Clin Microbiol 52:2615–2624. doi: 10.1128/JCM.00498-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Merker M, Barbier M, Cox H, Rasigade JP, Feuerriegel S, Kohl TA, Diel R, Borrell S, Gagneux S, Nikolayevskyy V, Andres S, Nübel U, Supply P, Wirth T, Niemann S. 2018. Compensatory evolution drives multidrug-resistant tuberculosis in Central Asia. Elife 7:e38200. doi: 10.7554/eLife.38200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Reiling N, Homolka S, Walter K, Brandenburg J, Niwinski L, Ernst M, Herzmann C, Lange C, Diel R, Ehlers S, Niemann S. 2013. Clade-specific virulence patterns of Mycobacterium tuberculosis complex strains in human primary macrophages and aerogenically infected mice. mBio 4:e00250-13. doi: 10.1128/mBio.00250-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang L, Ru HW, Chen FZ, Jin CY, Sun RF, Fan XY, Guo M, Mai JT, Xu WX, Lin QX, Liu J. 2016. Variable virulence and efficacy of BCG vaccine strains in mice and correlation with genome polymorphisms. Mol Ther 24:398–405. doi: 10.1038/mt.2015.216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Baena A, Cabarcas F, Alvarez-Eraso KLF, Isaza JP, Alzate JF, Barrera LF. 2019. Differential determinants of virulence in two Mycobacterium Tuberculosis Colombian clinical isolates of the Lam09 family. Virulence 10:695–710. doi: 10.1080/21505594.2019.1642045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Canaan S, Maurin D, Chahinian H, Pouilly B, Durousseau C, Frassinetti F, Scappuccini-Calvo L, Cambillau C, Bourne Y. 2004. Expression and characterization of the protein Rv1399C from Mycobacterium tuberculosis. a novel carboxyl esterase structurally related to the HSL family. Eur J Biochem 271:3953–3961. doi: 10.1111/j.1432-1033.2004.04335.x [DOI] [PubMed] [Google Scholar]
- 37. Choudhary E, Bishai W, Agarwal N. 2014. Expression of a subset of heat stress induced genes of Mycobacterium tuberculosis is regulated by 3',5'-cyclic AMP. PLoS One 9:e89759. doi: 10.1371/journal.pone.0089759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kolly GS, Boldrin F, Sala C, Dhar N, Hartkoorn RC, Ventura M, Serafini A, McKinney JD, Manganelli R, Cole ST. 2014. Assessing the essentiality of the decaprenyl-phospho-d-arabinofuranose pathway in Mycobacterium tuberculosis using conditional mutants. Mol Microbiol 92:194–211. doi: 10.1111/mmi.12546 [DOI] [PubMed] [Google Scholar]
- 39. Law JD, Daniel J. 2017. The Mycobacterial Rv1551 glycerol-3-phosphate acyltransferase enhances phospholipid biosynthesis in cell lysates of Escherichia coli. Microb Pathog 113:269–275. doi: 10.1016/j.micpath.2017.10.050 [DOI] [PubMed] [Google Scholar]
- 40. Lun S, Bishai WR. 2007. Characterization of a novel cell wall-anchored protein with carboxylesterase activity required for virulence in Mycobacterium tuberculosis. J Biol Chem 282:18348–18356. doi: 10.1074/jbc.M700035200 [DOI] [PubMed] [Google Scholar]
- 41. Muttucumaru DG, Smith DA, McMinn EJ, Reese V, Coler RN, Parish T. 2011. Mycobacterium tuberculosis Rv0198C, a putative matrix metalloprotease is involved in pathogenicity. Tuberculosis (Edinb) 91:111–116. doi: 10.1016/j.tube.2010.11.010 [DOI] [PubMed] [Google Scholar]
- 42. Oh TJ, Daniel J, Kim HJ, Sirakova TD, Kolattukudy PE. 2006. Identification and characterization of Rv3281 as a novel subunit of a biotin-dependent acyl-CoA carboxylase in Mycobacterium tuberculosis H37Rv. J Biol Chem 281:3899–3908. doi: 10.1074/jbc.M511761200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rengarajan J, Murphy E, Park A, Krone CL, Hett EC, Bloom BR, Glimcher LH, Rubin EJ. 2008. Mycobacterium tuberculosis Rv2224C modulates innate immune responses. Proc Natl Acad Sci USA 105:264–269. doi: 10.1073/pnas.0710601105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ryoo SW, Park YK, Park SN, Shim YS, Liew H, Kang S, Bai GH. 2007. Comparative Proteomic analysis of virulent Korean Mycobacterium tuberculosis K-strain with other mycobacteria strain following infection of U-937 macrophage. J Microbiol 45:268–271. [PubMed] [Google Scholar]
- 45. Tallman KR, Levine SR, Beatty KE. 2016. Small-molecule probes reveal esterases with persistent activity in dormant and reactivating Mycobacterium tuberculosis. ACS Infect Dis 2:936–944. doi: 10.1021/acsinfecdis.6b00135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Thomas E, Noar RD, Daub ME. 2021. A polyketide synthase gene cluster required for pathogenicity of Pseudocercospora Fijiensis on banana. PLoS One 16:e0258981. doi: 10.1371/journal.pone.0258981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhang K, Jiang N, Chen H, Zhang N, Sang X, Feng Y, Chen R, Chen Q. 2021. TatD DNAses of African trypanosomes confer resistance to host neutrophil extracellular traps. Sci China Life Sci 64:621–632. doi: 10.1007/s11427-020-1854-2 [DOI] [PubMed] [Google Scholar]
- 48. Zhang Z, Liang Y, Yu L, Chen M, Guo Y, Kang Z, Qu C, Tian C, Zhang D, Liu M. 2021. TatD DNAses contribute to biofilm formation and virulence in Trueperella pyogenes. Front Microbiol 12:758465. doi: 10.3389/fmicb.2021.758465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Vandal OH, Roberts JA, Odaira T, Schnappinger D, Nathan CF, Ehrt S. 2009. Acid-susceptible mutants of Mycobacterium tuberculosis share hypersusceptibility to cell wall and oxidative stress and to the host environment. J Bacteriol 191:625–631. doi: 10.1128/JB.00932-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lopes Santos C, Nebenzahl-Guimaraes H, Vaz Mendes M, van Soolingen D, Correia-Neves M. 2015. To be or not to be a pseudogene: a molecular epidemiological approach to the Mclx genes and its impact in tuberculosis. PLoS One 10:e0128983. doi: 10.1371/journal.pone.0128983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Niemann S, Diel R, Khechinashvili G, Gegia M, Mdivani N, Tang YW. 2010. Mycobacterium tuberculosis Beijing lineage favors the spread of multidrug-resistant tuberculosis in the Republic of Georgia. J Clin Microbiol 48:3544–3550. doi: 10.1128/JCM.00715-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Nikolayevskyy VV, Brown TJ, Bazhora YI, Asmolov AA, Balabanova YM, Drobniewski FA. 2007. Molecular epidemiology and prevalence of mutations conferring rifampicin and isoniazid resistance in Mycobacterium tuberculosis strains from the Southern Ukraine. Clin Microbiol Infect 13:129–138. doi: 10.1111/j.1469-0691.2006.01583.x [DOI] [PubMed] [Google Scholar]
- 53. Pasechnik O, Vyazovaya A, Vitriv S, Tatarintseva M, Blokh A, Stasenko V, Mokrousov I. 2018. Major genotype families and epidemic clones of Mycobacterium tuberculosis in Omsk region, Western Siberia, Russia, marked by a high burden of tuberculosis-HIV coinfection. Tuberculosis (Edinb) 108:163–168. doi: 10.1016/j.tube.2017.12.003 [DOI] [PubMed] [Google Scholar]
- 54. Zhdanova S, Heysell SK, Ogarkov O, Boyarinova G, Alexeeva G, Pholwat S, Zorkaltseva E, Houpt ER, Savilov E. 2013. Primary multidrug-resistant Mycobacterium tuberculosis in 2 regions, Eastern Siberia, Russian Federation. Emerg Infect Dis 19:1649–1652. doi: 10.3201/eid1910.121108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Vyazovaya АA, Lebedeva IA, Ushakova NB, Pavlov VV, Gerasimova AA, Solovieva NS, Zhuravlev Vy, Narvskaya OV. 2021. Molecular and genetic analysis of Mycobacterium tuberculosis population in the Vologda region with low tuberculosis incidence. Russian Journal of Infection and Immunity 11:497–505. doi: 10.15789/2220-7619-MAG-1545 [DOI] [Google Scholar]
- 56. Vyazovaya A, Proshina E, Gerasimova A, Avadenii I, Solovieva N, Zhuravlev V, Narvskaya O, Mokrousov I. 2020. Increased transmissibility of Russian successful strain Beijing B0/W148 of Mycobacterium tuberculosis: indirect clues from history and demographics. Tuberculosis (Edinb) 122:101937. doi: 10.1016/j.tube.2020.101937 [DOI] [PubMed] [Google Scholar]
- 57. Vyazovaya AA, Gavrilova NY, Gerasimova AA, Bychkova AO, Avadenii I, Anikieva EV, Solovieva NS, Zhuravlev VY, Mokrousov IV, Narvskaya OV. 2022. Molecular-genetic monitoring of the Mycobacterium tuberculosis population in Murmansk Oblast. Mol. Genet. Microbiol. Virol 37:71–77. doi: 10.3103/S0891416822020070 [DOI] [Google Scholar]
- 58. Mokrousov I, Vyazovaya A, Otten T, Zhuravlev V, Pavlova E, Tarashkevich L, Krishevich V, Vishnevsky B, Narvskaya O. 2012. Mycobacterium tuberculosis population in Northwestern Russia: an update from Russian-EU/Latvian border region. PLoS One 7:e41318. doi: 10.1371/journal.pone.0041318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mokrousov I, Otten T, Zozio T, Turkin E, Nazemtseva V, Sheremet A, Vishnevsky B, Narvskaya O, Rastogi N. 2009. At Baltic crossroads: a molecular snapshot of Mycobacterium tuberculosis population diversity in Kaliningrad, Russia. FEMS Immunol Med Microbiol 55:13–22. doi: 10.1111/j.1574-695X.2008.00470.x [DOI] [PubMed] [Google Scholar]
- 60. Matrakshin AG, Mesko EM, Beliakova NK, Andreevskaia SN, Smirnova TG, Larionova EE, Kuzmin AV, Mokrousov IV, Pospelov LE, Chernousova LN. 2004. Genotypic characteristics of Mycobacterium tuberculosis strains from the Republic of Tyva. Probl Tuberk Bolezn Legk, no. 3:37–40. [PubMed] [Google Scholar]
- 61. van der Spuy GD, Kremer K, Ndabambi SL, Beyers N, Dunbar R, Marais BJ, van Helden PD, Warren RM. 2009. Changing Mycobacterium tuberculosis population highlights clade-specific pathogenic characteristics. Tuberculosis (Edinb) 89:120–125. doi: 10.1016/j.tube.2008.09.003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 to S11.
Tables S1 to S16.
An accounting of the reviewer comments and feedback.
Data Availability Statement
All data of this study are presented in the article and supplementary material. Data for the M. tuberculosis genomes were deposited in the NCBI Sequence Read Archive (project number PRJNA886055).