ABSTRACT
With the global prevalence of Varroa mites, more and more beekeepers resort to confining the queen bee in a queen cage to control mite infestation or to breed superior and robust queen bees. However, the impact of such practices on the queen bee remains largely unknown. Therefore, we subjected the queen bees to a 21-day egg-laying restriction treatment (from the egg stage to the emergence of adult worker bees) and analyzed the queen bees’ ovarian metabolites and gut microbiota after 21 days, aiming to assess the queen bees’ quality and assist beekeepers in better hive management. Our findings revealed a significant reduction in the relative expression levels of Vg and Hex110 genes in the ovaries of egg laying-restricted queen bees compared to unrestricted egg-laying queens. The diversity of gut microbiota in the queen bee exhibited a notable decrease, accompanied by corresponding changes in the core bacteria of the microbial community, the relative abundance of Lactobacillus and Bifidobacterium increased from 22.34% to 53.14% (P = 0.01) and from 0.053% to 0.580% (P = 0.04), respectively. The relative abundance of Bombella decreased from 25.85% to 1.720% (P = 0.002). Following egg-laying restriction, the activity of the queen bee’s ovaries decreased, while the metabolism of glycerophospholipids remained or stored more lipid molecules, awaiting environmental changes for the queen bee to resume egg laying promptly. Furthermore, we observed that Bombella in the queen bee’s gut may regulate the queen’s ovarian metabolism through tryptophan metabolism. These findings provide novel insights into the interplay among queen egg laying, gut microbiota, and ovarian metabolism.
IMPORTANCE
With Varroa mite infestation, beekeepers often confine the queen bee in cages for control or breeding. However, the impact on the queen bee is largely unknown. We evaluated queen bee quality by restricting egg laying and analyzing ovarian metabolites and gut microbiota. In this study, we provided a comprehensive explanation of the expression of ovarian genes, the diversity of gut microbiota, and changes in ovarian metabolism in the queen bee. Through integrated analysis of the queen bee’s gut microbiota and ovarian metabolism, we discovered that the gut microbiota can regulate the queen bee’s ovarian metabolism. These findings provide valuable insights into the interplay among egg laying, gut microbiota, and the reproductive health of the queen bee. Understanding these relationships can contribute to the development of better strategies for Varroa mite control and queen bee breeding.
KEYWORDS: gut microbiota, ovary, queen bee, oviposition restriction, metabolomics
INTRODUCTION
Honeybees are highly social insects. A honeybee colony usually consists of a queen, tens of thousands of worker bees, and a few males who are interdependent and work together to form an organic whole (1). Honeybees are also one of the most important pollinators, pollinating 87.5% of angiosperms. A honeybee visits approximately 2,000 flowers per day while foraging for nectar and pollen (2). In addition, honeybees produce valuable products such as honey, propolis, pollen, beeswax, and royal jelly.
However, honeybee colonies have declined dramatically worldwide in recent years (3), and the factors contributing to this situation are multiple and complex. Pathogens, bacteria, and fungi can cause colony collapse disorder (4 – 6). Additionally, the widespread use of pesticides and herbicides, as well as the prevalence of parasites such as Varroa mites, can all contribute to the decline of honeybee populations (7 – 9). Moreover, viruses are one of the major threats to honeybee colony decline (10).
In addition to the above factors, queen failure and loss are also considered major causes of colony decline (11, 12). The queen is the central part of the colony and plays a vital role. The queen regulates the colony’s population and potential by releasing pheromones and laying eggs (13), and these pheromones also inhibit worker bees from laying eggs (14, 15). Queen fertility is a critical factor for the colony, and colony productivity is most directly related to the overall reproductive health of the queen. A healthy queen that is in the best egg-laying period is crucial for the existence of the colony (16). This is not only because the queen can lay many high-quality eggs but also because a young and strong queen has the most control over the colony. Therefore, beekeepers usually use young, strong queens to manage the colony for better beekeeping (17).
However, if a serious disease occurs in the colony or the number of Varroa mites threatens the colony’s health, the colony will soon collapse if the situation is not controlled. Nevertheless, the use of acaricides in beekeeping should be minimized to avoid chemical residues that can accumulate in honey and beeswax (18). Today, in order to eliminate diseases and control Varroa mite populations, beekeepers typically confine the queen in a cage for 21 days (from the egg stage to the emergence of adult workers). They also use oxalic acid, a natural, non-toxic organic acid, as an acaricide to control the mite population in the colony and prevent the queen from laying eggs or spreading viruses in the colony (19 – 21).
Meanwhile, restricting queen egg production significantly increases egg weight and size; egg weight and size have a significant effect on the weight, thoracic length, and thoracic width of the queen (22). The expression level of the vitellogenin (Vg) gene in the queen’s abdomen also significantly increases with increasing restriction time. However, the expression level of the vitellogenin receptor (Vgr) in the ovary remains constant to provide vitellogenin for oviposition immediately after environmental improvement (23). The use of oviposition restriction to improve queen laying productivity has been widely used in production practices. In the early years of nectar abundance, beekeepers use queen confinement to obtain a large number of foragers and thus obtain high yields. The use of oviposition restriction to produce a large number of overwintering bees is also one of the important measures for honey bee management in all seasons (24). Recent research has shown that using oviposition restriction to treat queen bees results in larger eggs, which in turn produce high-quality queens (25).
In addition to the above types of artificial restrictions on queen egg production, the queen bees will also reduce egg production on their own. As the optimum temperature range for bees to live is between 15 and 25℃ (26), bees can come out of the hive, the queen can lay eggs, and worker bees can nurse larvae when the temperature is between 5 and 35℃ (27). This period can be called the breeding period and is also the time for producing various bee products. When the temperature is below 10°C for a long time, the queen stops laying eggs; the bees reduce their activities outside the hive and form clusters inside the hive; and the colony enters the overwintering period (28).
As the colony enters the reproductive period, some queens may leave the hive to find a new home due to environmental changes and lack of food. During this period, the worker bees will feed the queen less frequently (29), or the queen may actively refuse to be fed by the worker bees; the queen will lose weight; the queen will reduce the number of eggs she lays; and egg production will drop dramatically (30). Therefore, the queen can easily fly and take the worker bees out of the hive. Certainly, beekeepers also refer to this phenomenon as “swarming.”
Many beekeepers also sell queens, which are placed in a small cage and escorted safely to their destination by a few worker bees (31). Upon arrival, the queen often becomes depressed and does not lay eggs, and will only try to start laying after a few days of acclimatization in the colony (32). Whether this restriction of queen laying or reduction in queen laying affects the queens themselves has not been studied.
In this study, we placed the queen bee in a queen cage and restricted the queen bee from laying eggs and left the queen bee’s ovaries in a closed state. To investigate whether the queen ovaries change and whether this change affects the queen ovarian development and egg production, we used metabolomics to identify pathways and differential metabolites that were significantly affected in ovaries after spawning restriction. Moreover, we will also analyze this change in terms of gut bacteria. Previous studies have shown that the diversity of the queen’s gut flora is relatively homogeneous compared to that of the worker bees, and that the queen’s gut microbiota changes, depending on the environment (33). Therefore, we analyzed the gut microbiota of queen bees and found that the gut microbiota of queen bees changed after oviposition restriction; the richness of the gut microbiota of queen bees decreased (34); and the two groups were significantly separated. In addition, we determined the expression of important genes in the ovaries after the queen was restricted to laying eggs. These differences and variations help us to investigate whether the queen’s ovaries, when in a closed state, affect the queen and thus the development of the whole colony, causing losses to the colony and the beekeeper.
MATERIALS AND METHODS
Sample collection
This experiment was conducted in the apiary of Kunming University of Science and Technology in May 2022. Twenty healthy, disease-free colonies of honey bees (Apis mellifera) were selected, and their colony strength, sealed brood area, and food supply were equal. The queen of each colony was a mated queen of the same species and age, healthy, and in egg-laying condition; the age of all 20 queen bees is 1 year. Ten colonies were randomly selected as one group, and each queen was placed in the queen cage (CQ), while the other groups of 10 colonies were left with the queens untreated (FQ). After 21 days of captivity (35), all 20 queens were removed from the colonies and stored at −80°C for subsequent experiments.
Dissection of the queen bee intestine and ovary
All experiments are performed in the ultra-clean table. First, take the queen bee out of the −80°C refrigerator and place it on ice to thaw. After thawing, soak the queen bee sample in 75% alcohol for two minutes, then place the queen bee sample in phosphate-buffered saline (PBS) for cleaning. Then, a pair of forceps was used to hold the queen bee’s body in place. Scissors were used to lift the scales off the queen bee’s abdomen starting from the caudal end of the stomach and along the sides of the abdomen. At that point, we could see the ovaries and intestinal tissues in the abdominal cavity of the queen bee. As described in Prešern and Smodiš Škerl (36), the scales of the queen bee’s abdomen were then fixed with a needle and the ovaries and entire intestinal tissues were removed with another pair of forceps but excluding the honey crop. Finally, the intestinal and ovarian tissues were snap frozen with liquid nitrogen and stored at −80°C.
Intestinal DNA extraction and sequencing
The dissected queen bee gut was transferred to a 1.5-mL microcentrifuge tube containing 100 µL of double-distilled water and ceramic beads (0.1 mm) for subsequent DNA extraction.
Queen bee gut samples were homogenized in a tissue lyser and the lysed samples were then subjected to DNA extraction using the Insect DNA Kit (Do926-02; Omega, Inc., USA). Total DNA was eluted in 50-µL elution buffer according to the manufacturer’s instructions, and the quality of the extracted DNA was assessed using NanoDrop 2000 (Thermo Scientific, Wilmington, USA) and 2% agarose gel electrophoresis to measure and evaluate the concentration and quality of the extracted DNA.
The hypervariable region V3-V4 of the bacterial 16S rRNA gene was amplified with primer pairs 338F (5′-ACTCCTACGGGAGGCAGCAG-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′) (37) by T100 Thermal Cycler PCR thermocycler (Bio-Rad, USA). The PCR reaction mixture including 4-µL 5× Fast Pfu buffer, 2-µL 2.5-mM deoxy-ribonucleoside triphosphate (dNTP), 0.8 µL of each primer (5 µM), 0.4-µL Fast Pfu polymerase, 10 ng of template DNA, and ddH2O to a final volume of 20 µL. PCR amplification cycling conditions were as follows: initial denaturation at 95℃ for 3 min, followed by 27 cycles of denaturing at 95℃ for 30 s, annealing at 55℃ for 30 s and extension at 72℃ for 45 s, single extension at 72℃ for 10 min, and end at 4℃. The PCR product was extracted from 2% agarose gel and purified using the PCR Clean-Up Kit (YuHua, Shanghai, China) according to manufacturer’s instructions and was quantified using Qubit (version 4.0; Thermo Fisher Scientific, USA). Purified amplicons were pooled in equimolar amounts and paired-end sequenced on an Illumina PE300 platform (Illumina, San Diego, USA) according to the standard protocols by Majorbio Bio-Pharm Technology Co. Ltd. (Shanghai, China).
After demultiplexing, the resulting sequences were quality filtered with fastp (0.19.6) and merged with FLASH (version 1.2.11). Then, the high-quality sequences were de-noised using DADA2 (38) plugin in the Qiime2 (version 2020.2) pipeline with recommended parameters, which obtains single-nucleotide resolution based on error profiles within samples. DADA2 de-noised sequences are usually called amplicon sequence variants (ASVs). Remove all sequences annotated as chloroplasts and mitochondria from all samples. To minimize the effects of sequencing depth on alpha and beta diversity measure, the number of sequences from each sample was rarefied to 20,000, which still yielded an average Good’s coverage of 97.90%. The ASV abundance represents the abundance value of each ASV in each sample (i.e., the corresponding count of all sequences). Typically, when analyzing the microbial composition among samples, the ASV or species sequence count in each sample is divided by the total sequence count of the sample to obtain the relative abundance (proportion) of each ASV or species. Taxonomic assignment of ASVs was performed using the naive Bayes consensus taxonomy classifier implemented in Qiime2 and the SILVA 16S rRNA database (version 138).
Sample preparation for metabolite extraction
Fifty milligrams of the queen ovary sample was weighed into a 2-mL centrifuge tube, and a 6-mm diameter grinding bead was added. Extract (400 µL; methanol:acetonitrile = 1:1, vol/vol) containing four internal standards (L-2-chlorophenylalanine, etc.) was added. Cryogenic tissue grinder was used for 6 min (−10°C, 50 Hz). Ultra-sonic extraction was performed at low temperature for 30 min (5°C, 40 KHz). The samples were placed at −20°C for 30 min, centrifuged for 15 min (13,000 g, 4°C), and the supernatant was transferred to an injection vial with an internal cannula for analysis. In addition, 20 µL of supernatant was removed from each sample, mixed, and used as a quality control (QC) sample.
Untargeted metabolomics profiling of queen bee ovary
The instrumental platform for this LC-MS analysis was an ultra-high-performance liquid chromatography-tandem Fourier transform mass spectrometry UHPLC-Q Exactive HF-X system (Thermo Scientific).
Chromatographic conditions were as follows: the column was an ACQUITY UPLC HSS T3 (100 mm × 2.1 mm i.d., 1.8 µm; Waters Corporation, Milford, USA); mobile phase A was 95% water + 5% acetonitrile (containing 0.1% formic acid); mobile phase B was 47.5% acetonitrile + 47.5% isopropanol + 5% water (containing 0.1% formic acid), and the injection volume was 3 µL. The column temperature was 40°C.
Mass spectrometry conditions were as follows: samples were subjected to electrospray ionization, and mass spectra were acquired in positive and negative ion scanning modes. The scan range was 70–1,050 m/z; the sheath gas flow rate was 50 arb; the auxiliary gas flow rate was 13 arb; the heating temperature was 425°C; the capillary temperature was 325°C; the spray voltage (+) was 3500 V; the spray voltage (−) was −3500 V; and the S-lens voltage was 50.
We first injected three QC samples to balance the system and column. In the analysis process, one QC sample was injected after every three samples to monitor instrument stability.
RNA extraction and RT-PCR
Ovary samples were removed from −80°C and placed on ice, and the entire procedure was performed on ice. RNA was extracted from the ovaries of queen bees using Trizol (Invitrogen, Carlsbad, CA, USA), and the concentration of RNA was measured using NonDrop 2000 (Thermo Scientific). The extracted RNA was then reverse transcribed using Takara kit (TaKaRa, Dalian, China). The reverse transcribed cDNA is frozen at −20°C until use.
We selected six genes [Vgr, Vg, juvenile hormone acid methyltransferase (Jhamt), Hex110, Tor, and Egfr] (Table S1) using Actin as a reference gene and performed a 10-fold dilution of the cDNA. A total of 1.6-µL forward and reverse primers, 2-µL CDNA solution, 10-µL TB Green Premix Ex Taq, and the addition of sterilized water to 20 µL. Amplification was performed using the following cycling conditions: 95°C for 30 s, 40 cycles of 95°C for 5 s, 60°C for 30 s, then 95°C for 15 s, 60°C for 1 min, and 95°C for 15 s. We used the 2−ΔΔCT method and calculated the mean of three technical replicates (39). Calculation of relative differential gene expression in queen ovaries was performed after limiting queen oviposition.
Statistical analysis
Independent samples t-tests were used to calculate the variability of ovary weight between the two groups of queens using the built-in method of GraphPad Prism version 8.0.2. The chao and sobs indices of alpha diversity were used in the same way.
The raw metabolomic data were imported into the metabolomics software Progenesis QI (Waters Corporation) for baseline filtering, peak identification, integration, retention time correction, and peak alignment, resulting in a data matrix containing retention time, mass-to-charge ratio, and peak intensity information. The MS (mass spectrum) and MS/MS (tandem mass spectrometry) mass spectra were matched to the metabolism database with the MS mass error set to less than 10 ppm, and the metabolites were identified based on the secondary mass spectra matching score. Databases included METLIN (https://metlin.scripps.edu/), the human metabolome database (http://www.hmdb.ca), and Lipid Maps (http://www.lipidmaps.org).
RESULTS
Gene expression in queen ovaries and ovary weight
Fig. 1A shows the changes in queen ovary weight after 21 days of oviposition restriction. The results show that the weight of the queen ovaries changed significantly after 21 days of oviposition restriction, and the weight of the ovaries of the restricted queen (CQ) was almost half that of the unrestricted queen (FQ) compared to the unrestricted queen (P < 0.001).
FIG 1.
(A) Weight of queen bee ovaries. (B–G) Gene expression in the ovaries of queen bees. (H) Chao indices of alpha diversity in the queen bee gut. *P < 0.05, **P < 0.01, ****, P < 0.0001.
After restricting the oviposition, there was a significant reduction in the expression of the Vg gene in queen ovaries (Fig. 1B). However, the relative expression of Vgr did not show any difference between the two groups (Fig. 1C). Additionally, the relative expression of Jhamt, a gene related to queen hierarchical differentiation, did not show any difference either (Fig. 1D). On the other hand, the expression of Hex110, a gene related to queen ovary development, was significantly higher in FQ ovaries compared to CQ ovaries (Fig. 1E). The expression of two nutrition-related genes, Tor and Egfr, did not differ between the two groups, but their expression was higher in FQ ovaries compared to CQ ovaries (Fig. 1F and G).
The results indicate that the development of the queen’s ovaries may be impeded and even regressed when the queen is restricted from laying eggs. However, it appears that the nutritional stress and access to food for the queen may not be as severe as initially anticipated.
Changes in the gut flora of the queen bee
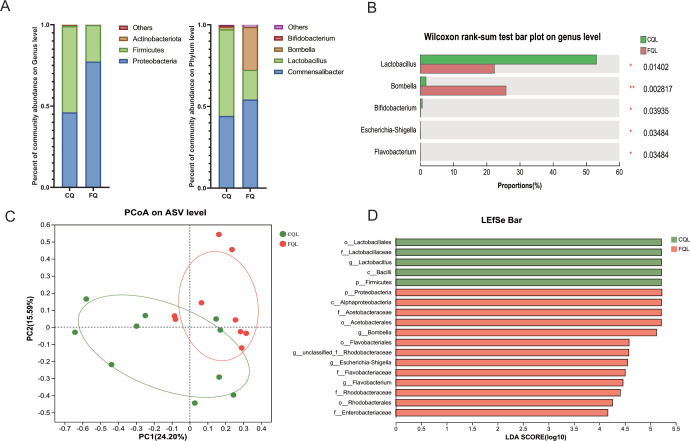
The richness of the gut microbiota in queen bees was reflected by the Chao index. Independent sample t-test analysis showed no significant difference in the Chao index between the two groups. However, compared to the CQ group, the FQ group exhibited higher gut microbiota richness. This result indicates that egg-laying restriction affects the abundance of gut microbiota in queen bees (Fig. 1H). At the phylum and genus level, oviposition restriction significantly altered the queen gut flora. The core phylum of the CQ gut flora changed from Proteobacteria to Firmicutes after queen oviposition restriction; the dominant flora of the gut flora also changed from Commensalibacter to Lactobacillus, and the diversity of the CQ gut flora decreased (Fig. 2A). The core of the microbiota changed significantly; the relative abundance of Lactobacillus increased from 22.34% to 53.14% (P = 0.03); the relative abundance of Bombella decreased from 25.85% to 1.72% (P = 0.008); the relative abundance of Bifidobacterium increased from 0.053% to 0.580% (P = 0.04); and the relative abundance of Commensalibacter did not change significantly (Fig. 2B).
FIG 2.
(A) The community distribution map of the queen bee’s gut microbiota at the phylum and genus levels. (B) At the genus level, the Wilcoxon rank-sum test was used to detect the differentially abundant genera in the gut microbiota of queen bees based on the abundance data of each genus in the samples. (C) PCoA of the queen bee gut microbiota at the ASV level. (D) LDA discriminant bar chart to summarize the microbial taxa with significant effects in each group of queen gut microbiota. *P < 0.05, **P < 0.01. LDA, linear discriminant analysis; LEfSe, linear discriminant analysis effect size; PCoA, principal coordinate analysis.
Principal coordinate analysis (PCoA) is a non-restricted data dimensionality reduction method used to study the similarity or dissimilarity in the composition of sample communities. We employed PCoA to analyze the gut microbiota of two groups of queen bees. The results revealed that egg-laying restriction altered the composition and distribution of the gut microbiota in queen bees. There was a clear trend of separation between the gut microbiota of CQ and FQ groups, with a decrease in gut microbiota richness observed in the CQ group after egg-laying restriction (Fig. 2C).
Furthermore, a linear discriminant analysis effect size analysis was conducted to identify differential gut flora between the two groups (Fig. 2D). The findings revealed that Lactobacillus was more abundant in CQ, while Bombella was more abundant in FQ and significantly differed from the other group. Additionally, the diversity of gut bacteria was found to be greater in FQ compared to CQ. These results suggest that the queen’s gut flora diversity may decrease after oviposition restriction and that the queen’s core flora may undergo changes as a defensive mechanism against external environmental effects.
Metabolomic analysis and metabolite identification
Ultra-high performance liquid chromatography tandem mass spectrometry (UHPLC-MS) was used to obtain ovarian metabolite information of CQ and FQ. After processing, a total of 841 metabolites were identified in the two sample sets, including coenzyme factors, amino acids, nucleic acids, lipids, hormones, conducting substances, and carbohydrates (Table S2). Based on this metabolite information, an unsupervised model, principal component analysis (PCA), was constructed. The PCA score plot showed a clear separation trend between CQ and FQ in both the anionic and cationic modes, indicating that the two groups of queen bees differed in the composition of ovarian metabolites (Fig. 3A and B).
FIG 3.
(A and B) The PCA score plots are shown separately in positive and negative ion modes. (C and D) The OPLS-DA score plots are shown in positive and negative ion modes, respectively. (E and F) The OPLS-DA permutation test plots are shown in positive and negative ion modes, respectively. OPLS-DA, orthogonal partial least squares discriminant analysis; PCA, principal component analysis.
To further identify these metabolites, orthogonal partial least squares discriminant analysis (OPLS-DA) was performed. The OPLS-DA score plot was orthogonally rotated to filter out irrelevant information and allow better differentiation between the groups. The OPLS-DA score plot showed clear separation between CQ and FQ in both anionic and cationic modes (Fig. 3C and D). In addition, to avoid overfitting, the OPLS-DA results were evaluated using the OPLS-DA substitution test, with an R2 of 0.9451 in the cationic mode and 0.9526 in the anionic mode (Fig. 3E and F). These results suggest that the OPLS-DA model is reliable in both the cationic and anionic modes and can be used for further analysis of differential metabolites.
Differential metabolite identification
These differential metabolites were selected based on a combination of a statistically significant threshold of variable influence on projection (VIP) values obtained from the OPLS-DA model and P values from a two-tailed Student t-test on normalized peak areas. Metabolites with VIP values greater than 1.0 and P values less than 0.05 were considered statistically significant.
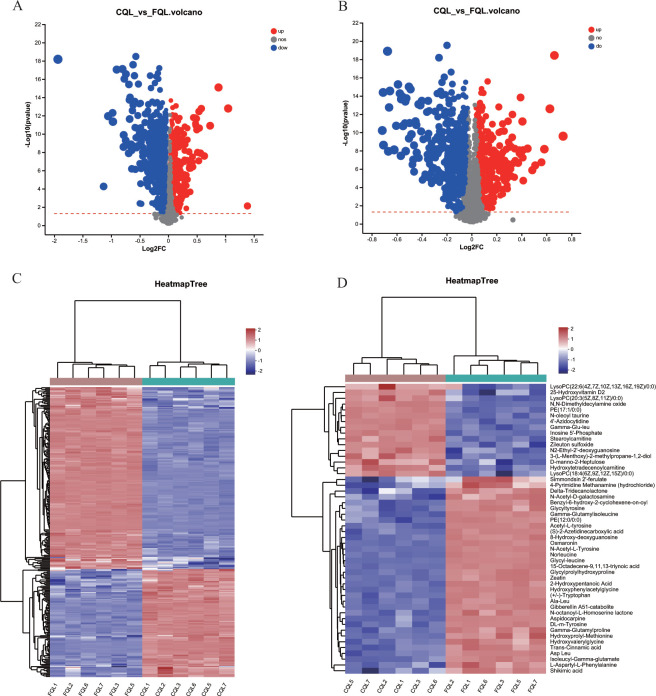
Based on these differential metabolites, volcano plots were constructed to specifically display the metabolites within the two groups. In the cationic mode volcano plot, a total of 877 differential ion peaks were detected, with only 157 metabolites identified (Fig. 4A). In the anionic mode volcano plot, a total of 860 differential ion peaks were detected, with only 106 metabolites identified (Fig. 4B). The volcano plot revealed that the fold change values of metabolite expression differences between the two groups were higher in the cationic mode than in the anionic mode, but the statistical test values of the differences in metabolite expression changes were not significantly different between the two groups. A total of 263 named metabolites were detected (Table S3), of which 165 were upregulated and 98 were downregulated.
FIG 4.
(A and B) The volcano plots are shown separately in positive and negative ion mode. (C) Cluster analysis of each sample with all metabolites, where red represents positive correlation and blue represents negative correlation. (D) The clustering analysis of each sample with the top 50 abundant metabolites, with red indicating positive correlation and blue indicating negative correlation.
A clustering heat map was constructed based on these significantly different metabolites, and the distribution of each metabolite in the two groups was visualized in the heat map (Fig. 4C). The clustering analysis showed that restricting queen oviposition caused changes in metabolites in CQ ovaries, with a clear separation of metabolites between CQ and FQ, and that restricting queen oviposition caused a decrease in the expression of most metabolites in CQ ovaries and an increase in the expression of only a small fraction of metabolites. To better understand the changes in ovarian metabolites, we selected the 50 most abundant metabolites to construct a clustering heat map (Fig. 4D). The results showed that the expression of 34 metabolites was downregulated, and only 16 metabolites were upregulated compared to the control, which was similar to the results of the previous clustering heat map. This suggests that when the queen ovary is in a closed state, the ovary itself decreases some metabolic activities to adapt to this change.
Metabolic pathway analysis
To further understand the changes occurring in the ovary, pathway enrichment analysis of differential metabolites was performed, and 79 pathways were enriched (Table S4), including tryptophan metabolism, glycerophospholipid metabolism, purine metabolism, and tyrosine metabolism (Fig. 5A), where tryptophan metabolism (P < 0.001) and glycerophospholipid metabolism (P < 0.01) were significantly affected.
FIG 5.
(A) Bubble plot of Kyoto Encyclopedia of Genes and Genomes (KEGG) topological analysis, where the size of the bubble indicates the importance of the pathway. (B) The changes of differential metabolites identified in phenylalanine, tyrosine, and tryptophan biosynthesis, Tryptophan metabolism, serotonergic synapse, and glycerophospholipid metabolism are shown with a red background indicating metabolites at higher levels, and a green background indicating metabolites at lower levels. (C) The specific expression of differential metabolites in phenylalanine, tyrosine, and tryptophan biosynthesis, tryptophan metabolism, serotonergic synapse, and glycerophospholipid metabolism. **P < 0.01, ****P < 0.0001. TCA, tricarboxylic acid cycle; CHAYI, the name of the metabolome set.
In addition, specific changes in metabolites involved in tryptophan and glycerophospholipid metabolism were investigated using non-targeted metabolomics (Fig. 5B). In the tryptophan pathway, 5-hydroxy-L-tryptophan, serotonin, indole-3-acetaldehyde, 5-hydroxyindoleacetic acid, indole, 3-methyldioxyindole, and kynurenine metabolites were significantly lower (P < 0.001), while 5-hydroxyindoleacetylglycine was significantly higher (P < 0.001) in CQ ovaries after restriction of queen oviposition. However, in glycerophospholipid metabolism, metabolites such as phosphatidylcholine (PC) (P < 0.01), phosphoethanolamine (P < 0.001), and 1-acyl-sn-glycero-3-phosphocholine (P < 0.001) were significantly increased in the ovaries, whereas sn-glycero-3-phosphocholine was significantly decreased (P < 0.001).
Based on the complete metabolite ensemble of the KEGG database, the metabolic network of changes occurring in the ovary was mapped using metabolomics data, which can visualize the interactions between different metabolic pathways (Fig. 5C). The figure reveals that shikimate in the phenylalanine, tyrosine, and tryptophan biosynthesis pathway can promote the production of indole, which in turn stimulates the production of 5-hydroxy-L-tryptophan in the tryptophan pathway, leading to the production of 5-hydroxytryptamine (5-HT). The 5-HT can participate in various metabolic activities through serotonergic synapse, assisting the host in regulating metabolic activities to adapt to external changes. Moreover, the metabolic correlation network map can provide specific information about changes in the ovary’s metabolome, aiding in a better understanding of the queen’s changes and reducing risks for beekeepers and apiculture.
Gut microbiota regulates queen bee ovarian metabolism
To study the potential dependence between queen gut flora and ovarian metabolism, we used the Pearson correlation algorithm to calculate the correlation between the two data sets in volume and constructed a heat map of the proportional relationship between bacteria in the gut and the top 50 metabolites identified in abundance, and the top 50 association features in abundance were selected to form a correlation analysis-correlation heat map (Fig. 6A). The figure shows that there is a robust correlation between Bifidobacterium, Bombella, and Lactobacillus with numerous metabolites, while other gut bacteria exhibit partial involvement in modulating the metabolic activity of the queen bee’s ovary, although lacking a strong correlation with metabolites. These results imply that the gut microbiota of queen bees can influence the metabolic activity of the ovary, yet its impact appears to be relatively limited, with the ovaries themselves playing a more dominant regulatory role.
Fig 6.
(A) Correlation analysis was performed between the top 50 metabolites and 16S rRNA data using Pearson’s correlation algorithm, and a heatmap was constructed using the top 50 associated features. (B) Correlation analysis was performed between the five core bacteria in the queen bee gut and all differential metabolites, and the darker the color, the greater the correlation coefficient.
We selected Commensalibacter, Lactobacillus, Bombella, Bifidobacterium, and Acinetobacter from the queen’s gut flora to gain further insights into the relationship between the queen’s core gut bacteria and ovarian metabolism. The correlations between these five bacteria and 263 differential metabolites were calculated using the Pearson correlation algorithm, and a correlation heat map was constructed. The results revealed that the correlation between Commensalibacter, Lactobacillus, and Acinetobacter with ovarian metabolites was not particularly strong, while the correlation between Bombella and Bifidobacterium with ovarian metabolites was robust (Fig. 6B). This suggests that Bombella and Bifidobacterium may have the ability to regulate metabolites in the queen’s ovaries through metabolic pathways, helping the queen to safely navigate challenging periods and supporting the queen during egg laying due to environmental changes.
DISCUSSION
In this study, we found that after restricting oviposition, the weight of CQ ovaries changed significantly, with the weight of the ovaries almost half of that of FQ. This is similar to previous reports that the weight of queen bee ovaries significantly decreased within 10 days after restricting oviposition (40). The longer the queen bee is restricted from laying eggs, the smaller the ovary becomes. When the queen bee is unable to lay eggs, the oocytes produced accumulate in the ovary, and the excess oocytes may in turn inhibit the rate of oocyte production. This leads to a reduction in the weight and size of the ovary of the queen bee (41).
A critical process in the maturation of most insect oocytes is the accumulation of Vg (42). Vitellogenin is synthesized primarily in the fat body of the honey bee abdomen and then transferred to the oocyte via the Vgr (43). When the queen bee is restricted from laying eggs, the number of oocyte cells in her ovaries may decrease, leading to a reduction in the amount of Vg in her ovaries. However, the expression level of Vgr remains constant and is not lowered due to oviposition restriction (40). In a closed state of the ovaries, the expression level of Vgr in the queen bee’s ovaries tends to remain stable to provide support when the queen bee begins to lay eggs due to changes in the environment.
Jhamt is an important enzyme involved in the biosynthesis of juvenile hormone (JH) in bees. JH is a known primary regulatory factor in the caste differentiation of bees (44). Previous studies have shown that applying a combination of sugar-rich royal jelly and JH to the larvae cells of queen bees is more likely to produce high-quality queen bees compared to larvae without this food supplement (45). Hexamerins would participate in the synthesis and utilization of amino acids during insect development. They may also function as JH-binding proteins. In addition, there is circumstantial evidence to support the hypothesis that larval hexamers are targeted for egg production (46, 47). Hex110 is one of the four hexamerins in the honeybee. Due to the fact that Hex110 is synthesized in large quantities by larval fat bodies and is widely secreted and stored in hemolymph, it meets the criteria for storage proteins in insects (48). However, there is also research showing that Hex110 is located in the cytoplasm and nucleus of honey bee ovarian cells (49). Furthermore, the expression level of Hex110 in the ovaries of mated egg-laying queen bees is higher than that in young virgin queen bees (50). Our results indicate that the expression of the Jhamt gene in the queen bee’s ovary does not change significantly after egg-laying restriction. Previous studies have shown that Jhamt is more abundant in queen bees than in worker bees (51). It is evident that the queen bee does not undergo caste differentiation due to the egg-laying restriction. Interestingly, after limiting oviposition, the expression level of Hex110 in the queen’s ovary significantly decreased. When the queen stops laying eggs, the number of oocyte cells decreases, which may lead to a significant decrease in the expression of Hex110 in the queen’s ovary. Oviposition not only causes changes in the behavior of the queen but also may affect differential gene expression.
In our study, when the queen bees were restricted from egg laying, they were also confined in a cage and only fed by worker bees that entered the cage. This resulted in increased nutritional stress for the queen bees (52). Gene expression of Tor and Egfr, which are related to nutritional stress, may be different under such conditions. However, our results showed that after egg-laying restriction, the expression of Tor and Egfr genes in the queen bee’s ovary only slightly decreased and did not significantly decrease. We believe that the expression of the Tor and Egfr genes may have significantly changed in the early stage of the queen bee’s restriction. However, after 21 days of restriction, the queen bees may have adjusted themselves through a series of metabolic activities, which led to no significant difference in the expression of Tor and Egfr genes between the two groups.
The gut microbiota of queen bees has rarely been studied. Our research found that the dominant bacteria in the gut microbiota of queen bees are Commensalibacter, Lactobacillus, and Bombella, and the diversity of the gut microbiota in queen bees is much lower compared to worker bees (33, 34). Interestingly, our study also revealed significant alterations in the gut microbiota of egg-limited queens. The gut microbiota of egg-limited queens exhibited striking similarity to that of aged queens. Specifically, there was a decrease in the abundance of Alpha 2.1 and other dominant bacteria in the gut of aged queens, while the relative abundance of core hindgut bacteria, such as Lactobacillus and Bifidobacterium, which are commonly considered probiotic, increased (33, 53). The highest relative abundance of bacteria in the CQ gut changed to Lactobacillus, which increased from 22.13% to 46.29%, and Bombella, which significantly decreased from 15.35% to 1.907%. The relative abundance of Commensalibacter did not change significantly, but it also decreased compared to FQ. This may be due to the fact that after the queen is restricted from laying eggs, the only source of food for the queen is the worker bees that enter the cage through the gaps in the cage to feed the queen (54). The queen has a single access to food, which can lead to a reduction in the diversity of the queen’s gut flora. In addition, the colonization of the queen’s gut flora is not completed in a few days as in the case of worker bees; it takes weeks or months to complete the colonization of the queen’s gut flora (55).
After egg-laying restriction, the gut microbiota of the queen bee changed, and we found that the gut microbiota of the queen bee with older age and lower productivity was extremely similar to that of the CQ (53). Previous studies have suggested that Bombella and Commensalibacter in the gut of queen bees may be related to their longevity (56), and both of these strains are part of the Acetobacter branch. There have been many reports on the reasons for the longevity of queen bees, but they all mention royal jelly. The significant difference between queen bees and worker bees is nutrition, and queen bees mainly eat royal jelly throughout their lives (57). The existing studies have shown that Acetobacteriaceae are abundant in the pharyngeal gland of nurse bees, royal jelly, and larvae fed with royal jelly, but can be negligible in the midgut and viscera of nurse bees and foraging bees (58). The queen bee’s gut is the digestive organ where royal jelly can enter, indicating that royal jelly may promote the proliferation of Acetobacteriaceae in the queen bee’s gut. Moreover, overwintering honey bee colonies with high abundance of Acetobacteriaceae show lower overwintering losses, indicating that Acetobacteriaceae plays a positive health role inside bees (59). Our results indicated that although there was no difference in the expression level of nutrition-related genes between the two groups, the expression level of nutrition-related genes in the queen bee did decrease after being subjected to egg-laying restriction. This suggests that the nutritional stress on the queen bee increased, which may lead to a decrease in the abundance of Acetobacteraceae in the queen bee gut, resulting in a decrease in queen bee lifespan and a decrease in the ability to adapt to changes in the external environment, causing irreversible damage to the bee colony. Moreover, after the queen bee restricts egg laying, the relative abundance of certain bacteria in the queen bee’s gut microbiota decreases, particularly a significant decline in Bombella. It is worth considering whether these bacteria are involved in the reproductive regulation of the queen bee.
The vitellogenin is mainly synthesized in the fat body and then transferred to the oocyte through the vitellogenin receptor (60). The queen’s ovaries utilize these lipid molecules to produce eggs, so the distribution of lipid molecules in the ovaries is crucial for the queen (61). Our results indicate that the glycerophospholipid metabolism pathway in CQ ovaries was significantly disturbed after limiting the queen’s oviposition. Glycerophospholipids are important lipid components in queen ovaries and play a crucial role in cell membrane structure and function. Previous studies have shown that glycerophospholipid metabolism in queen ovaries is closely related to their reproductive capacity (62).
The levels of phosphoethanolamine (PE), PC, and 1-acyl-sn-glycero-3-phosphocholine in the ovarian glycerophospholipid metabolism pathway were significantly increased in CQ compared to FQ. PE and PC are important components of biological membranes (63). Research suggests that PC in ovarian tissue prepares for egg production, promoting the maturation of oocytes and the development of the ovary (64). After restricting egg laying, some metabolic activities in the ovaries of queen bees begin to change, helping the queen to adapt to changes in the external environment. The levels of some metabolites in the glycerophospholipid metabolic pathway in the queen bee’s ovaries increase. These metabolites may promote the maturation of oocytes by regulating the composition and function of ovarian cell membranes, among other aspects. PC and PE accumulate during the growth process of oocytes, leading to an increase in the levels of these two metabolites.
The impact of tryptophan metabolism is most significant after restricting egg laying in queen bees. Many research results have shown that tryptophan metabolism plays an important role in animal ovaries; for example, tryptophan can promote ovarian development (65), possibly affecting hormone secretion (66) and promoting the maturation and development of ovarian follicles (67). Tryptophan is a precursor for serotonin (5-HT) synthesis, and tryptophan metabolism is an important mechanism for regulating serotonin. 5-HT has been detected in the oviduct, uterus, and ovary of various animals (including mice and hamsters) (68, 69). It has been shown that when 5-HT is reduced in the fallopian tubes and uterus of rats and this treatment is applied early in gestation, rats cannot produce neonates normally but can produce neonates when 5-HT levels return to normal (70). 5-HT can also induce ovulation, and serotonin injected directly into the gonads of clams can induce ovulation and enhance the fertilization capacity of sperm on oocytes (71). These results suggest that 5-HT is one of the important signaling molecules that regulate ovarian function, regulating ovarian development and function. 5-HT is able to regulate ovarian physiological functions, such as follicle development and maturation, by binding to receptors in the ovary, thus affecting egg production. This is similar to our findings; after the queen bee restricted her egg laying, the content of 5-hydroxy-L-tryptophan in the tryptophan metabolism pathway in the ovary decreased significantly, leading to a decrease in the content of 5-HT. 5-HT regulates the physiological activity of the queen bee through serotonergic synapses, adapting to changes in the environment. The metabolism of glycerophospholipids can maintain or store more lipid molecules, allowing the queen bee to quickly lay eggs when environmental conditions change. At this time, the queen bee will produce eggs with higher content of nutrients such as vitellogenin, which promotes egg development and leads to the formation of high-quality queen bees.
There is growing evidence that gut microbiota can provide essential amino acids and proteins to the host to maintain protein balance (72). Gut microbiota can also help the host resist external invasion by regulating the host’s immune response (73). Bartonella in worker bees can regulate the biosynthesis of amino acids in the bee body during winter, including several essential amino acids such as phenylalanine, tryptophan, and methionine. Providing essential amino acids in winter can help bees maintain their health (74). The core bacterium Gilliamella in bees can regulate the host’s carbohydrate metabolism and help the host obtain energy from it (75). Lactobacillus Firm4 and Firm5 can significantly alter the amino acid metabolic pathways of bees (76). Bifidobacterium acts as a core bacterium in the bee gut, helping the host digest polysaccharides (75). The research results indicate that the gut microbiota plays an important role in regulating the metabolic activity and immune response of honey bees. Different bacteria can provide essential amino acids, proteins, and energy for honey bees and also regulate the metabolic pathways inside the honey bees’ body to help them adapt to changes in the external environment. Our results suggest that Bifidobacterium and Bombella, which have a high correlation with differential metabolites in queen ovaries, may affect ovarian metabolic pathways by regulating some metabolic activities in the queen’s gut.
We believe that in the early stages of queen bee egg-laying restriction, Bombella (previously named Parasaccharibacter apium or Alpha 2.2) (77, 78) in the queen bee’s gut may help the queen bee adapt to environmental changes by regulating tryptophan metabolism in the queen bee’s ovaries (Fig. 5B). Once the queen bee has adapted to this change and her ovaries are always closed, Bombella in the queen’s gut may be further replaced by Lactobacillus, which becomes the dominant bacterium in the queen’s intestine. As mentioned earlier, when the queen bee is restricted from laying eggs, the relative abundance of Bombella in the CQ gut significantly decreases, and the relative abundance of Lactobacillus significantly increases. Moreover, we also found that the gut flora of older and less productive queens was very similar to that of the CQ gut flora. This result further supports the possibility that Bombella in the gut of queen bees may regulate ovarian development and function.
Conclusion
In this study, we analyzed the gut microbiota and ovarian metabolites of queen bees. We found that when the queen bee’s egg laying was restricted, there was a decrease in gut microbiota diversity and a change in the core gut microbiota. Specifically, the relative abundance of Bombella decreased from 15.35% to 1.907%. This reduction weakened the queen’s resistance to environmental changes. From the perspective of ovarian metabolites, the queen’s ovaries exhibited decreased metabolic activity after egg-laying restriction, particularly in glycerophospholipid metabolism and tryptophan metabolism. Through integrated analysis, we discovered that Bombella in the queen bee’s gut microbiota may regulate the queen’s ovarian metabolism through tryptophan metabolism.
These findings provide new insights into the interaction between queen bee egg laying, gut microbiota, and ovarian metabolism. Further research can explore the precise role of Bombella and tryptophan metabolism in the physiological activities of queen bees, as well as better management and protection of bee colonies. Additionally, the methods used in this study can be applied to investigate the gut microbiota and metabolome of other insects or animals, providing new approaches for understanding the interactions between microbiota and hosts within organisms.
ACKNOWLEDGMENTS
The work was supported by the National Natural Science Foundation of China (32260863) , National Key R&D Program of China (2022YFD1600200), and Yunnan Provincial Fundamental Research Projects (CB22052C156A).
J.G. and K.W. designed this study. W.-L.L., Q.H., and J.-L.L. wrote the manuscript. W.-L.L. and X.-J.L. performed the experiments. W.-L.L. analyzed the data and wrote the manuscript. W.-z.Z., Q.-H.T., J.X., J.Z., C.-H.Z., Z.C., and X.L. reviewed the manuscript. J.G. and W.-L.L. revised the manuscript.
Contributor Information
Wen-zheng Zhao, Email: rurosezwz@163.com.
Kai Wang, Email: wangkai@caas.cn.
Jun Guo, Email: guojun0591@126.com.
Beile Gao, South China Sea Institute of Oceanology, Chinese Academy of Sciences, Guangzhou, Guangdong, China .
DATA AVAILABILITY
Raw data for 16S rRNA of queen bee have been deposited under BioProject PRJNA949168 in the NCBI database. Metabolomic raw data have been uploaded to MetaboLights under the number MTBLS7576.
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/spectrum.02145-23.
Stacked bar graph of queen bee ovary weights.
Primer sequences for real-time fluorescence quantitative PCR of queen bee ovaries.
841 metabolites in queen bee ovaries.
263 metabolites with names from queen bee ovaries.
79 metabolic pathways in the queen bee ovary.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Page RE, Peng CYS. 2001. Aging and development in social insects with emphasis on the honey bee. Exp Gerontol 36:695–711. doi: 10.1016/s0531-5565(00)00236-9 [DOI] [PubMed] [Google Scholar]
- 2. Abbasi KH, Jamal M, Ahmad S, Ghramh HA, Khanum S, Khan KA, Ullah MA, Aljedani DM, Zulfiqar B. 2021. Standardization of managed honey bee (Apis mellifera) hives for pollination of sunflower (Helianthus annuus) crop. J King Saud Univ Sci 33:101608. doi: 10.1016/j.jksus.2021.101608 [DOI] [Google Scholar]
- 3. Vanengelsdorp D, Meixner MD. 2010. A historical review of managed honey bee populations in Europe and the United States and the factors that may affect them. J Invertebr Pathol 103:S80–S95. doi: 10.1016/j.jip.2009.06.011 [DOI] [PubMed] [Google Scholar]
- 4. Cornman RS, Tarpy DR, Chen Y, Jeffreys L, Lopez D, Pettis JS, vanEngelsdorp D, Evans JD, Highlander SK. 2012. Pathogen webs in collapsing honey bee colonies. PLoS ONE 7:e43562. doi: 10.1371/journal.pone.0043562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dainat B, Evans JD, Chen YP, Gauthier L, Neumann P. 2012. Predictive markers of honey bee colony collapse. PLoS One 7:e32151. doi: 10.1371/journal.pone.0032151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Genersch E. 2010. Honey bee pathology: current threats to honey bees and Beekeeping. Appl Microbiol Biotechnol 87:87–97. doi: 10.1007/s00253-010-2573-8 [DOI] [PubMed] [Google Scholar]
- 7. Di Prisco G, Annoscia D, Margiotta M, Ferrara R, Varricchio P, Zanni V, Caprio E, Nazzi F, Pennacchio F. 2016. A mutualistic symbiosis between a parasitic mite and a pathogenic virus undermines honey bee immunity and health. Proc Natl Acad Sci U S A 113:3203–3208. doi: 10.1073/pnas.1523515113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dong ZX, Tang QH, Li WL, Wang ZW, Li XJ, Fu CM, Li D, Qian K, Tian WL, Guo J.. 2022. Honeybee (Apis mellifera) resistance to deltamethrin exposure by modulating the gut microbiota and improving immunity. Environ Pollut 314:120340. [DOI] [PubMed] [Google Scholar]
- 9. Tang QH, Li WL, Wang JP, Li XJ, Li D, Cao Z, Huang Q, Li JL, Zhang J, Wang ZW, Guo J, Li JL. 2022. Effects of spinetoram and glyphosate on physiological biomarkers and gut microbes in Bombus Terrestris. Front Physiol 13:1054742. doi: 10.3389/fphys.2022.1054742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McMenamin AJ, Genersch E. 2015. Honey bee colony losses and associated viruses. Curr Opin Insect Sci 8:121–129. doi: 10.1016/j.cois.2015.01.015 [DOI] [PubMed] [Google Scholar]
- 11. Spleen AM, Lengerich EJ, Rennich K, Caron D, Rose R, Pettis JS, Henson M, Wilkes JT, Wilson M, Stitzinger J, Lee K, Andree M, Snyder R, vanEngelsdorp D. 2013. A national survey of managed honey bee 2011–12 winter colony losses in the United States: results from the bee informed partnership. J Apic Res 52:44–53. doi: 10.3896/IBRA.1.52.2.07 [DOI] [Google Scholar]
- 12. vanEngelsdorp D, Tarpy DR, Lengerich EJ, Pettis JS. 2013. Idiopathic brood disease syndrome and queen events as precursors of colony mortality in migratory beekeeping operations in the eastern United States. Prev Vet Med 108:225–233. doi: 10.1016/j.prevetmed.2012.08.004 [DOI] [PubMed] [Google Scholar]
- 13. Kocher SD, Richard F-J, Tarpy DR, Grozinger CM. 2009. Queen reproductive state modulates pheromone production and queen-worker interactions in honeybees. Behav Ecol 20:1007–1014. doi: 10.1093/beheco/arp090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nino EL, Malka O, Hefetz A, Teal P, Hayes J, Grozinger CM. 2012. Effects of honey bee (Apis mellifera L.) queen insemination volume on worker behavior and physiology. J Insect Physiol 58:1082–1089. doi: 10.1016/j.jinsphys.2012.04.015 [DOI] [PubMed] [Google Scholar]
- 15. Richard FJ, Tarpy DR, Grozinger CM. 2007. Effects of insemination quantity on honey bee queen physiology. PLoS One 2:e980. doi: 10.1371/journal.pone.0000980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Amiri E, Strand MK, Rueppell O, Tarpy DR. 2017. Queen quality and the impact of honey bee diseases on queen health: potential for interactions between two major threats to colony health. Insects 8:48. doi: 10.3390/insects8020048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tang J, Ma C, Shi W, Chen X, Liu Z, Wang H, Chen C. 2020. A national survey of managed honey bee colony winter losses (Apis mellifera) in China (2013–2017). Diversity 12:318. doi: 10.3390/d12090318 [DOI] [Google Scholar]
- 18. Wallner K. 1999. Varroacides and their residues in bee products. Apidologie 30:235–248. doi: 10.1051/apido:19990212 [DOI] [Google Scholar]
- 19. Gregorc A, Alburaki M, Werle C, Knight PR, Adamczyk J. 2017. Brood removal or queen caging combined with oxalic acid treatment to control varroa mites (Varroa destructor) in honey bee colonies (Apis mellifera). Apidologie 48:821–832. doi: 10.1007/s13592-017-0526-2 [DOI] [Google Scholar]
- 20. Büchler R, Uzunov A, Kovačić M, Prešern J, Pietropaoli M, Hatjina F, Pavlov B, Charistos L, Formato G, Galarza E, Gerula D, Gregorc A, Malagnini V, Meixner M, Nedić N, Puškadija Z, Rivera-Gomis J, Rogelj Jenko M, Smodiš Škerl MI, Vallon J, Vojt D, Wilde J, Nanetti A. 2020. Summer brood interruption as integrated management strategy for effective Varroa control in Europe. J Apic Res 59:764–773. doi: 10.1080/00218839.2020.1793278 [DOI] [Google Scholar]
- 21. Gabel M, Scheiner R, Büchler R. 2023. Immediate and long-term effects of induced brood interruptions on the reproductive success of Varroa destructor. Apidologie 54:20. doi: 10.1007/s13592-023-00998-x [DOI] [Google Scholar]
- 22. Mattiello S, Rizzi R, Cattaneo M, Martino PA, Mortarino M. 2022. Effect of queen cell size on morphometric characteristics of queen honey bees (Apis mellifera ligustica). Ital J Anim Sci 21:532–538. doi: 10.1080/1828051X.2022.2043790 [DOI] [Google Scholar]
- 23. Aamidor SE, Cardoso-Júnior CAM, Harianto J, Nowell CJ, Cole L, Oldroyd BP, Ronai I. 2022. Reproductive plasticity and oogenesis in the queen honey bee (Apis mellifera). J Insect Physiol 136:104347. doi: 10.1016/j.jinsphys.2021.104347 [DOI] [PubMed] [Google Scholar]
- 24. Knoll S, Pinna W, Varcasia A, Scala A, Cappai MG. 2020. The honey bee (Apis mellifera L., 1758) and the seasonal adaptation of productions. highlights on summer to winter transition and back to summer metabolic activity. A review. Livest Sci 235:104011. doi: 10.1016/j.livsci.2020.104011 [DOI] [Google Scholar]
- 25. Wei H, He XJ, Liao CH, Wu XB, Jiang WJ, Zhang B, Zhou LB, Zhang LZ, Barron AB, Zeng ZJ. 2019. A maternal effect on queen production in honeybees. Curr Biol 29:2208–2213.e3. doi: 10.1016/j.cub.2019.05.059 [DOI] [PubMed] [Google Scholar]
- 26. Heinrich B. 1981. The mechanisms and energetics of honeybee swarm temperature regulation. J. Exp. Biol 91:25–55. doi: 10.1242/jeb.91.1.25 [DOI] [Google Scholar]
- 27. Becher MA, Scharpenberg H, Moritz RFA. 2009. Pupal developmental temperature and behavioral specialization of honeybee workers (Apis mellifera L.). J Comp Physiol A 195:673–679. doi: 10.1007/s00359-009-0442-7 [DOI] [PubMed] [Google Scholar]
- 28. Rousseau A, Giovenazzo P. 2021. Successful indoor mass storage of honeybee queens (Apis mellifera) during winter. Agriculture 11:402. doi: 10.3390/agriculture11050402 [DOI] [Google Scholar]
- 29. Pierce AL, Lewis LA, Schneider SS. 2007. The use of the vibration signal and worker piping to influence queen behavior during swarming in honey bees, Apis mellifera. Ethology 113:267–275. doi: 10.1111/j.1439-0310.2006.01314.x [DOI] [Google Scholar]
- 30. Grozinger CM, Richards J, Mattila HR. 2014. From molecules to societies: mechanisms regulating swarming behavior in honey bees (Apis spp.). Apidologie 45:327–346. doi: 10.1007/s13592-013-0253-2 [DOI] [Google Scholar]
- 31. Given K. 2021. Queen rearing and bee breeding, p 363-366, honey bee medicine for the veterinary practitioner. doi: 10.1002/9781119583417 [DOI]
- 32. Rhodes JW, Somerville DC, Harden S. 2004. Queen honey bee introduction and early survival? effects of Queen age at introduction. Apidologie 35:383–388. doi: 10.1051/apido:2004028 [DOI] [Google Scholar]
- 33. Copeland Duan C, Anderson Kirk E, Mott Brendon M. 2022. Early queen development in honey bees: social context and queen breeder source affect gut microbiota and associated metabolism. Microbiol Spectr 10:e0038322. doi: 10.1128/spectrum.00383-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Powell JE, Eiri D, Moran NA, Rangel J. 2018. Modulation of the honey bee queen microbiota: effects of early social contact. PLoS One 13:e0200527. doi: 10.1371/journal.pone.0200527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Toomemaa K, Kaart T. 2022. Caging the queen with and without a comb section to achieve high efficacy of Varroa destructor control. J Apic Res:1–9. doi: 10.1080/00218839.2022.2036310 [DOI] [Google Scholar]
- 36. Prešern J, Smodiš Škerl MI. 2019. Parameters influencing Queen body mass and their importance as determined by machine learning in honey bees (Apis Mellifera Carnica). Apidologie 50:745–757. doi: 10.1007/s13592-019-00683-y [DOI] [Google Scholar]
- 37. Liu C, Zhao D, Ma W, Guo Y, Wang A, Wang Q, Lee D-J. 2016. Denitrifying sulfide removal process on high-salinity wastewaters in the presence of Halomonas sp. Appl Microbiol Biotechnol 100:1421–1426. doi: 10.1007/s00253-015-7039-6 [DOI] [PubMed] [Google Scholar]
- 38. Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP. 2016. DADA2: high-resolution sample inference from Illumina amplicon data. Nat Methods 13:581–583. doi: 10.1038/nmeth.3869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408. doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 40. Aamidor SE, Cardoso-Júnior CAM, Harianto J, Nowell CJ, Cole L, Oldroyd BP, Ronai I. 2022. Reproductive plasticity and oogenesis in the queen honey bee (Apis mellifera). J Insect Physiol 136:104347. doi: 10.1016/j.jinsphys.2021.104347 [DOI] [PubMed] [Google Scholar]
- 41. Eppig JJ, Wigglesworth K, Pendola FL. 2002. The mammalian oocyte orchestrates the rate of ovarian follicular development. Proc. Natl. Acad. Sci. USA 99:2890–2894. doi: 10.1073/pnas.052658699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tufail M, Takeda M. 2008. Molecular characteristics of insect vitellogenins. J Insect Physiol 54:1447–1458. doi: 10.1016/j.jinsphys.2008.08.007 [DOI] [PubMed] [Google Scholar]
- 43. Wu Z, Yang L, He Q, Zhou S. 2021. Regulatory mechanisms of vitellogenesis in insects. Front. Cell Dev. Biol 8. doi: 10.3389/fcell.2020.593613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Li W, Huang ZY, Liu F, Li Z, Yan L, Zhang S, Chen S, Zhong B, Su S, Palli SR. 2013. Molecular cloning and characterization of juvenile hormone acid methyltransferase in the honey bee, Apis mellifera, and its differential expression during caste differentiation. PLoS ONE 8:e68544. doi: 10.1371/journal.pone.0068544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. De Souza DA, Huang MH, Tarpy DR. 2019. Experimental improvement of honey bee (Apis mellifera) queen quality through nutritional and hormonal supplementation. Apidologie 50:14–27. doi: 10.1007/s13592-018-0614-y [DOI] [Google Scholar]
- 46. Hahn DA, Wheeler DE. 2003. Presence of a single abundant storage hexamerin in both larvae and adults of the grasshopper, Schistocerca americana. J Insect Physiol 49:1189–1197. doi: 10.1016/j.jinsphys.2003.09.002 [DOI] [PubMed] [Google Scholar]
- 47. Zakharkin SO, Headley VV, Kumar NK, Buck NA, Wheeler DE, Beneš H. 2001. Female-specific expression of a hexamerin gene in larvae of an autogenous mosquito. Eur. J. Biochem. 268:5713–5722. doi: 10.1046/j.0014-2956.2001.02514.x [DOI] [PubMed] [Google Scholar]
- 48. Bitondi MMG, Nascimento AM, Cunha AD, Guidugli KR, Nunes FMF, Simões ZLP. 2006. Characterization and expression of the hex 110 gene encoding a glutamine-rich hexamerin in the honey bee, Apis mellifera. Arch Insect Biochem Physiol 63:57–72. doi: 10.1002/arch.20142 [DOI] [PubMed] [Google Scholar]
- 49. Martins JR, Bitondi MMG, Bridger JM. 2016. The HEX 110 hexamerin is a cytoplasmic and nucleolar protein in the ovaries of Apis mellifera. PLoS ONE 11:e0151035. doi: 10.1371/journal.pone.0151035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Martins JR, Nunes FM, Cristino AS, Simões ZL, Bitondi MM. 2010. The four hexamerin genes in the honey bee: structure, molecular evolution and function deduced from expression patterns in queens, workers and drones. BMC Molecular Biol 11:23. doi: 10.1186/1471-2199-11-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Guo X, Su S, Geir S, Li W, Li Z, Zhang S, Chen S, Chen R. 2016. Differential expression of miRNAs related to caste differentiation in the honey bee, Apis mellifera. Apidologie 47:495–508. doi: 10.1007/s13592-015-0389-3 [DOI] [Google Scholar]
- 52. Costa CP, Fisher K, Guillén BM, Yamanaka N, Bloch G, Woodard SH. 2021. Care-giver identity impacts offspring development and performance in an annually social bumble bee. BMC Ecol Evol 21:20. doi: 10.1186/s12862-021-01756-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Anderson KE, Ricigliano VA, Mott BM, Copeland DC, Floyd AS, Maes P. 2018. The queen’s gut refines with age: longevity phenotypes in a social insect model. Microbiome 6:108. doi: 10.1186/s40168-018-0489-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Vojvodic S, Johnson BR, Harpur BA, Kent CF, Zayed A, Anderson KE, Linksvayer TA. 2015. The Transcriptomic and evolutionary signature of social interactions regulating honey bee caste development. Ecol Evol 5:4795–4807. doi: 10.1002/ece3.1720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Corby-Harris V, Maes P, Anderson KE. 2014. The bacterial communities associated with honey bee (Apis mellifera) foragers. PLoS One 9:e95056. doi: 10.1371/journal.pone.0095056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wang H, Lei L, Chen W, Chi X, Han K, Wang Y, Ma L, Liu Z, Xu B. 2022. The comparison of antioxidant performance, immune performance IIS activity and gut microbiota composition between queen and worker bees revealed the mechanism of different lifespan of female casts in the honeybee. Insects 13:772. doi: 10.3390/insects13090772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Crailsheim K. 1991. Interadult feeding of jelly in honeybee (Apis mellifera L.) colonies. J Comp Physiol B 161:55–60. doi: 10.1007/BF00258746 [DOI] [Google Scholar]
- 58. Anderson KE, Sheehan TH, Mott BM, Maes P, Snyder L, Schwan MR, Walton A, Jones BM, Corby-Harris V, Gerardo NM. 2013. Microbial ecology of the hive and pollination landscape: bacterial associates from floral nectar, the alimentary tract and stored food of honey bees (Apis mellifera). PLoS ONE 8:e83125. doi: 10.1371/journal.pone.0083125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wang H, Liu C, Liu Z, Wang Y, Ma L, Xu B. 2020. The different dietary sugars modulate the composition of the gut microbiota in honeybee during overwintering. BMC Microbiol. 20:61. doi: 10.1186/s12866-020-01726-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Excels W. 1974. Occurrence and significance of vitellogenins in female castes of social hymenoptera. Am Zool 14:1229–1237. doi: 10.1093/icb/14.4.1229 [DOI] [Google Scholar]
- 61. Liu Z, Liu F, Li G, Chi X, Wang Y, Wang H, Ma L, Han K, Zhao G, Guo X, Xu B. 2020. Metabolite support of long-term storage of sperm in the spermatheca of honeybee (Apis mellifera) queens. Front. Physiol 11. doi: 10.3389/fphys.2020.574856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zhao H, Mashilingi SK, Liu Y, An J. 2021. Factors influencing the reproductive ability of male bees: current knowledge and further directions. Insects 12:529. doi: 10.3390/insects12060529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Niebergall LJ, Vance DE. 2012. The ratio of phosphatidylcholine to phosphatidylethanolamine does not predict integrity of growing MT58 Chinese hamster ovary cells. Biochim Biophys Acta 1821:324–334. doi: 10.1016/j.bbalip.2011.10.018 [DOI] [PubMed] [Google Scholar]
- 64. Zhan X, Fletcher L, Dingle S, Baracuhy E, Wang B, Huber L-A, Li J. 2021. Choline supplementation influences ovarian follicular development. Front Biosci (Landmark Ed) 26:1525–1536. doi: 10.52586/5046 [DOI] [PubMed] [Google Scholar]
- 65. Maezawa T, Ishikawa M, Sekii K, Nagamatsu G, Furukawa R, Kobayashi K. 2021. d-Tryptophan enhances the reproductive organ-specific expression of the amino acid transporter homolog Dr-SLC38A9 involved in the sexual induction of planarian Dugesia ryukyuensis. Zoological Lett 7:4. doi: 10.1186/s40851-021-00173-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Fouad AM, El-Senousey HK, Ruan D, Wang S, Xia W, Zheng C. 2021. Tryptophan in poultry nutrition: impacts and mechanisms of action. J Anim Physiol Anim Nutr 105:1146–1153. doi: 10.1111/jpn.13515 [DOI] [PubMed] [Google Scholar]
- 67. Jiang SQ, Gou ZY, Lin XJ, Li L. 2018. Effects of dietary tryptophan levels on performance and biochemical variables of plasma and intestinal mucosa in yellow-feathered broiler breeders. J Anim Physiol Anim Nutr 102:e387–e394. doi: 10.1111/jpn.12757 [DOI] [PubMed] [Google Scholar]
- 68. Amenta F, Vega JA, Ricci A, Collier WL.. 1992. Localization of 5-hydroxytryptamine-like immunoreactive cells and nerve fibers in the rat female reproductive system. Anat Rec 233:478-484. [DOI] [PubMed] [Google Scholar]
- 69. Il'ková G, Rehák P, Veselá J, Čikoš š, Fabian D, Czikková S, Koppel J.. 2004. Serotonin localization and its functional significance during mouse preimplantation embryo development. Zygote 12:205-213. [DOI] [PubMed] [Google Scholar]
- 70. Acharya SB, Goswami NG, Debnath PK. 1989. Uterine and placental 5-HT profile in different gestational period of albino rats. Indian J Exp Biol 27:505–509. [PubMed] [Google Scholar]
- 71. Masseau I, Bannon P, Anctil M, Dubé F. 2002. Localization and quantification of gonad serotonin during gametogenesis of the surf clam, Spisula solidissima. Biol Bull 202:23–33. doi: 10.2307/1543219 [DOI] [PubMed] [Google Scholar]
- 72. Bauer E, Lampert N, Mikaelyan A, Köhler T, Maekawa K, Brune A. 2015. Physicochemical conditions, metabolites and community structure of the bacterial microbiota in the gut of wood-feeding cockroaches (Blaberidae: Panesthiinae). FEMS Microbiol Ecol 91:1–14. doi: 10.1093/femsec/fiu028 [DOI] [PubMed] [Google Scholar]
- 73. Wang K, Zhu L, Rao L, Zhao L, Wang Y, Wu X, Zheng H, Liao X. 2022. Nano- and micro-polystyrene plastics disturb gut microbiota and intestinal immune system in honeybee. Sci Total Environ 842:156819. doi: 10.1016/j.scitotenv.2022.156819 [DOI] [PubMed] [Google Scholar]
- 74. Li C, Tang M, Li X, Zhou X. 2022. Community dynamics in structure and function of honey bee gut bacteria in response to winter dietary shift. mBio 13:e01131-22. doi: 10.1128/mbio.01131-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Zheng H, Perreau J, Powell JE, Han B, Zhang Z, Kwong WK, Tringe SG, Moran NA. 2019. Division of labor in honey bee gut microbiota for plant polysaccharide digestion. Proc Natl Acad Sci USA 116:25909–25916. doi: 10.1073/pnas.1916224116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Zhang Z, Mu X, Shi Y, Zheng H. 2022. Distinct roles of honeybee gut bacteria on host metabolism and neurological processes. Microbiol Spectr 10:e02438-21. doi: 10.1128/spectrum.02438-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Cox-Foster DL, Conlan S, Holmes EC, Palacios G, Evans JD, Moran NA, Quan P-L, Briese T, Hornig M, Geiser DM, Martinson V, vanEngelsdorp D, Kalkstein AL, Drysdale A, Hui J, Zhai J, Cui L, Hutchison SK, Simons JF, Egholm M, Pettis JS, Lipkin WI. 2007. A metagenomic survey of microbes in honey bee colony collapse disorder. Science 318:283–287. doi: 10.1126/science.1146498 [DOI] [PubMed] [Google Scholar]
- 78. Corby-Harris V, Snyder Lucy A, Schwan Melissa R, Maes P, McFrederick Quinn S, Anderson Kirk E. 2014. Origin and effect of alpha 2.2 Acetobacteraceae in honey bee larvae and description of Parasaccharibacter apium gen. nov., sp. nov. Appl Environ Microbiol 80:7460–7472. doi: 10.1128/AEM.02043-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Stacked bar graph of queen bee ovary weights.
Primer sequences for real-time fluorescence quantitative PCR of queen bee ovaries.
841 metabolites in queen bee ovaries.
263 metabolites with names from queen bee ovaries.
79 metabolic pathways in the queen bee ovary.
Data Availability Statement
Raw data for 16S rRNA of queen bee have been deposited under BioProject PRJNA949168 in the NCBI database. Metabolomic raw data have been uploaded to MetaboLights under the number MTBLS7576.