Abstract
Finding appropriate drugs to improve cerebral autoregulation (CA) in patients with acute ischemic stroke (AIS) is necessary to improve prognosis. We aimed to investigate the effect of butylphthalide on CA in patients with AIS. In this randomized controlled trial, 99 patients were 2:1 randomized to butylphthalide or placebo group. The butylphthalide group received intravenous infusion with a preconfigured butylphthalide-sodium chloride solution for 14 days and an oral butylphthalide capsule for additional 76 days. The placebo group synchronously received an intravenous infusion of 100 mL 0.9% saline and an oral butylphthalide simulation capsule. The transfer function parameter, phase difference (PD), and gain were used to quantify CA. The primary outcomes were CA levels on the affected side on day 14 and day 90. Eighty patients completed the follow-up (52 in the butylphthalide group and 28 in the placebo group). The PD of the affected side on 14 days or discharge and on 90 days was higher in the butylphthalide group than in the placebo group. The differences in safety outcomes were not significant. Therefore, butylphthalide treatment for 90 days can significantly improve CA in patients with AIS.
Trial registration: ClinicalTrial.gov: NCT03413202
Keywords: Acute ischemic stroke, butylphthalide, cerebral autoregulation, clinical trial, prognosis
Introduction
Stroke is the second leading cause of death worldwide, with ischemic stroke accounting for approximately 62.4% of all stroke cases. 1 However, most patients with acute ischemic stroke (AIS) do not have the opportunity to receive intravenous thrombolysis or intravascular intervention and only rely on medical treatment. 2 Therefore, identifying suitable intervention targets and specific therapeutic drugs is important for improving the prognosis of patients with AIS.
Animal and clinical studies have indicated that cerebral autoregulation (CA) is an important indicator for maintaining stable cerebral blood flow despite changes in cerebral perfusion pressure and arterial blood pressure made by regulating the contraction and relaxation of cerebral small vessels and microvessels.3 –6 It has been reported that CA is closely related to the prognosis of cerebrovascular disease;4,7 –10 higher CA can maintain relatively stable and adequate cerebral perfusion after AIS, which is beneficial for the prognosis of these patients. Thus, CA may be an intervention target for improving the prognosis of AIS patients. Butylphthalide is a synthetic compound; its chemical formula is C12H14O2 and its molar mass is 190.24 g/mol. Food and Drug Administration of China approved it for treating ischemic stroke in 2002.11,12 Butylphthalide has two dosage forms, injection and capsule, and can perform sequential therapy within 90 days after AIS. Previous studies reported that butylphthalide significantly increased regional cerebral blood flow in ischemic stroke animal models13 –15 and patients with carotid artery atherosclerotic stenosis. 16 We considered that this phenomenon might be owing to the improvement of CA by butylphthalide, which keeps cerebral blood flow at a higher level and ultimately improves the prognosis of AIS patients.17,18
In the present study, we hypothesized that butylphthalide would effectively improve CA levels in patients with AIS. Accordingly, we sought to use a randomized controlled trial to compare CA levels on day 14 after butylphthalide injection and day 90 after treatment with injection + capsule.
Material and methods
The EBCAS (Effectiveness of Butylphthalide on Dynamic Cerebral Autoregulation in Patients with Acute Ischemic Stroke) study was a multicenter, randomized, controlled, blinded phase 4 clinical trial registered at ClinicalTrials.gov (NCT03413202). The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the First Hospital of Jilin University (2017-263). In this study, all participants were fully informed and provided written informed consent signed by themselves or their immediate relatives. The participants had the right to withdraw from the study at any point.
Participants
Patients with AIS at four centers, including First Hospital of Jilin University (northern China), First Hospital of Hebei North University (central China), People's Hospital of Lixin County (eastern China), and Guangdong Provincial Hospital of Traditional Chinese Medicine (southern China), who met the specific inclusion and exclusion criteria from February 2018 to December 2021 were consecutively enrolled. The inclusion criteria were as follows: (1) age ≥ 18 and < 80 years, male or female; (2) within 48 hours of symptom onset; (3) diagnosis of large-artery atherosclerosis ischemic stroke according to the Trial of Org 10172 in Acute Stroke Treatment classification (TOAST criteria); 19 (4) unilateral internal carotid artery or M1 segment of middle cerebral artery stenosis (rate of stenosis ranging from 50 to 99%); (5) baseline National Institute of Health Stroke Scale (NIHSS) score ≥ 5 and ≤25; (6) Glasgow Coma Scale score ≥8; (7) no occurrence of stroke in the past 3 months; and (8) sufficient bilateral temporal bone windows for insonation of the middle cerebral artery. The exclusion criteria were patients who: (1) had received or planned to undergo intravascular interventional treatment/thrombolytic therapy; (2) were unable to cooperate with CA monitoring; (3) had received butylphthalide treatment after stroke onset; (4) had arrhythmia, anemia, or hyperthyroidism, which may influence the stability of cerebral blood flow; (5) had other intracranial diseases, including cerebral hemorrhage (primary or secondary), intracranial neoplasm, aneurysm, and arteriovenous malformation.; (6) had alanine transaminase or glutamic-oxalacetic transaminase more than three times the upper limit of normal and continuing to rise and creatinine clearance rate <30 mL/min; (7) had Modified Rankin Scale (mRS) score ≥2 before stroke onset; (8) had malignant neoplasm and expected lifetime <2 years; (9) were pregnant or lactating; (10) were participating in other trials or had participated in other trials in the past 3 months; (11) had dementia or mental illness; (12) had CA data that could not be analyzed owing to the coherence function <0.34.
Study drugs
The active ingredient of the drug used in our study is butylphthalide, and two dosage forms of butylphthalide injection and butylphthalide capsule were used in this study. Butylphthalide injection is obtained as the marketed drug Butylphthalide and Sodium Chloride Injection (NBP injection, 25 mg butylphthalide in 100 mL 0.9% saline; CSPC-NBP Pharmaceutical Co., Ltd. Hebei, China) and butylphthalide soft capsules are also marketed products (NBP capsules, 100 mg butylphthalide; CSPC-NBP Pharmaceutical Co., Ltd. Hebei, China). Placebo consisted of identical pre-packaged 100 mL 0.9% saline and opaque capsules filled with starch.
Randomization and blinding
Patients who met all eligibility criteria were randomly assigned in a 2:1 ratio to the butylphthalide or placebo group through a stratified block randomization process using a random block size of 4. The randomization sequence was created by an independent biostatistician using SPSS statistical software 18.0 (IBM Corp., Armonk, NY, USA) and was stratified by center. The patients and researchers at each center were unaware of the block size. Butylphthalide and placebo were identical in appearance, size, smell, usage, and dosage. They were pre-packaged and numbered consecutively at the pharmaceutical factory according to the randomization sequence. Each patient was assigned an order number and received the corresponding pre-packaged medication. The patients, healthcare providers, data collectors, and outcome adjudicators were blinded to the medication assignment of the participants.
Sample size
Based on the results of our previous studies, we estimated that the phase difference (PD) of the affected side of the placebo group after 14 days was 20 ± 14 degrees. Butylphthalide was expected to increase the phase value by 10 degrees (30 ± 16 degrees). With the power set at 80% and α level at 0.05, the estimated sample size was 78 patients (52 in the butylphthalide group and 26 in the placebo group). Considering a 20% loss to follow-up, we estimated that 99 patients were needed (66 patients in the butylphthalide group and 33 patients in the placebo group).
Study design
The researchers screened potential patients in the neurology outpatient and emergency departments, followed by the completion of transcranial Doppler (EMS-9PB, Delica, Shenzhen, China) and carotid ultrasound (MVU-6500, Delica, Shenzhen, China). If the patient met the inclusion criteria, a dedicated neurologist introduced the study to the patient and their immediate relatives. Once written informed consent was obtained, patients were randomly assigned to the butylphthalide or placebo groups. The butylphthalide group received intravenous infusion with a preconfigured butylphthalide-sodium chloride solution (25 mg butylphthalide in 100 mL 0.9% saline) for 14 days (twice daily) and oral butylphthalide capsule for additional 76 days (200 mg, thrice daily). The placebo group received an intravenous infusion of 100 mL 0.9% saline for 14 days (twice daily) and an oral butylphthalide simulation capsule for an additional 76 days (200 mg, thrice daily). The transfer function parameter, PD, and gain were used to quantify the CA.
Patients in both groups received standard medical treatment according to the guidelines for the management of AIS. 20 Primary outcome measures were PD levels of the affected side on day 14 after injection and 90 days after treatment with injection + capsule. Secondary outcome measures were PD levels of the unaffected side, other CA parameters, functional independence, NIHSS scores, and Barthel scores on day 14 after injection and on day 90 after injection + capsule. Safety outcome measures were adverse events and serious adverse events within 90 days. In addition, patient data, including demographic and clinical characteristics, laboratory test results, complete trial data, and follow-up data, were collected. Functional independence was defined as an mRS score of ≤2 at 90 days.
Cerebral autoregulation assessment and data analysis
In both groups, CA was monitored thrice: at baseline, on day 14/discharge after treatment with injection, and on day 90 after treatment with injection + capsule. The measurements were performed in a special examination room with minimal visual, acoustic, and temperature stimulations. All CA measurements were performed by a doctor specialized in neurovascular ultrasound. Before the CA assessment, blood pressure and heart rate were measured at the left brachial artery using an automatic blood pressure monitor. CA was monitored using transcranial Doppler (MultiDop X4, DWL, Sipplingen, Germany) combined with a finger continuous blood pressure monitor (Finometer Model 1, FMS, Amsterdam, The Netherlands) simultaneously, and real-time cerebral blood flow velocity (CBFV) and arterial blood pressure (ABP) signals were recorded for 5 min. End-tidal CO2 levels were measured using a nasal cannula capnograph (MultiDop X4, DWL, Sipplingen, Germany). During the entire process, the patients were instructed to stay awake, breathe normally, and minimize body movements.
After the measurements were collected, the data were analyzed using MATLAB (MathWorks, Natick, MA, United States).21,22 Each patient's ABP and CBFV signals were aligned using a cross-correlation function. The resultant signals were then down-sampled to 1 Hz after applying an anti-alias filter with a cutoff frequency of 0.5 Hz. Welch's method was employed to estimate the autospectrum of ABP, , and the cross-spectrum of ABP and CBFV, , in frequency domain by averaging the periodograms of the down-sampled ABP and CBFV with a 50% overlapped hamming window of 90 s. The transfer function, , was then deviated as:
| (1) |
Gain and PD can then be calculated from (1) by equations (2) and (3), respectively, as:
| (2) |
| (3) |
where R and I denote the real and imaginary parts of the transfer function, respectively. Finally, PD, gain, and coherence in a low-frequency range, 0.06 to 0.12 Hz were calculated. Only data with coherence ≥0.34 (number of windows: 5; critical values of coherence: 5%) were included in the subsequent statistical analysis.21,22
Statistical analysis
Statistical software SPSS 26.0 (IBM Corp., Armonk, NY, USA) was used for data analysis. Data are expressed as the mean ± standard deviation or median (interquartile range) for numerical variables and were analyzed using Student's t-test or Mann–Whitney U test. Categorical variables are presented as absolute numbers and percentages and were compared using χ2 test or Fisher's precision probability test. The full analysis set and per-protocol population were used to analyze the efficacy outcomes of this study. Multiple imputations, based on five replications, were used to impute the missing values in the full analysis set. Unadjusted and adjusted associations between butylphthalide and efficacy outcomes were estimated using linear or logistic regression models, as appropriate. For safety outcomes, the differences between the two groups were compared using χ2 test or Fisher's precision probability test. Statistical significance was set at 2-tailed P < 0.05.
Results
Participant characteristics
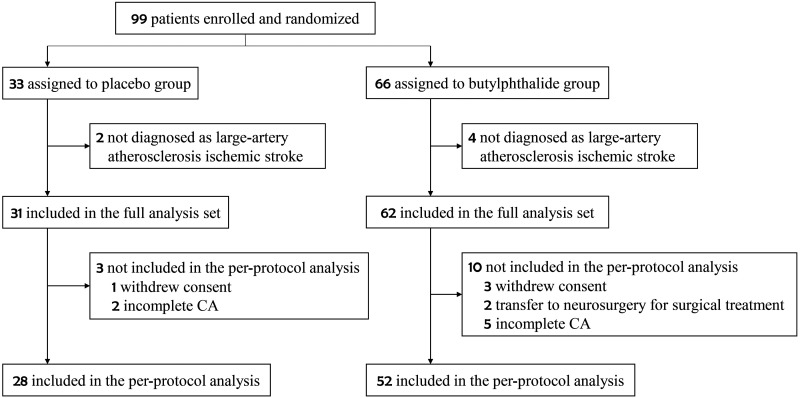
Ninety-nine patients were enrolled in this study from February 2018 to December 2021 (Figure 1). Sixty-six patients were assigned to the butylphthalide group and 33 to the placebo group. Six patients whose final diagnosis was not large-artery atherosclerotic ischemic stroke were excluded. Therefore, 93 patients were included in the full analysis set (62 in the butylphthalide group and 31 in the placebo group). A total of 80 patients completed the final follow-up process of this study and were included in the per-protocol analysis population (52 in the butylphthalide group and 28 in the placebo group); of these, CA measurements were completed at discharge for 44 patients and on day 14 for 36 patients.
Figure 1.
Flowchart of the study.
The baseline characteristics of patients in the butylphthalide group were similar to those in the placebo group (Table 1). The mean age of the 93 participants was 57.2 ± 11.6 years; 78 participants (83.9%) were male. The median NIHSS score was 7 (interquartile range, 6 to 10) in the butylphthalide group and 10 (interquartile range, 6 to 13) in the placebo group, and the median baseline PD of the affected side was 25.20 degrees (interquartile range, 8.37 to 37.81 degrees) and 25.05 degrees (interquartile range, 15.98 to 37.86 degrees), in the butylphthalide and placebo groups, respectively.
Table 1.
Characteristics of the patients between the two groups.
| Characteristic | Full analysis set (N = 93) |
Per-protocol population (N = 80) |
||||
|---|---|---|---|---|---|---|
| Butylphthalide Group (n = 62) | Placebo Group (n = 31) | P | Butylphthalide Group (n = 52) | Placebo Group (n = 28) | P | |
| Age, mean (SD), year | 57.1 ± 12.0 | 57.5 ± 11.0 | 0.886 | 55.73 ± 11.69 | 57.29 ± 10.97 | 0.564 |
| Sex, male, n (%) | 53 (85.5) | 25 (80.6) | 0.550 | 45 (86.5) | 22 (78.6) | 0.357 |
| Clinical history, n (%) | ||||||
| Smoking | 34 (54.8) | 17 (54.8) | >0.999 | 30 (57.7) | 14 (50.0) | 0.509 |
| Hypertension | 30 (48.4) | 13 (41.9) | 0.556 | 26 (50.0) | 11 (39.3) | 0.359 |
| Diabetes | 12 (19.4) | 3 (9.7) | 0.232 | 9 (17.3) | 2 (7.1) | 0.181 |
| Dyslipidemia | 3 (4.8) | 4 (12.9) | 0.165 | 3 (5.8) | 2 (7.1) | 0.577 |
| Cerebrovascular disease a | 6 (9.7) | 6 (19.4) | 0.189 | 6 (11.5) | 6 (21.4) | 0.195 |
| Concomitant medication, n (%) | ||||||
| Anti-hypertensive drugs | 21 (33.9) | 9 (29.0) | 0.638 | 20 (36.4) | 8 (28.6) | 0.478 |
| Anti-hyperlipidemic drugs | 54 (87.1) | 27 (87.1) | >0.999 | 50 (90.9) | 24 (85.7) | 0.472 |
| Anti-diabetic drugs | 10 (16.1) | 6 (19.4) | 0.698 | 10 (18.2) | 5 (17.9) | 0.971 |
| Anti-platelet drugs | 55 (88.7) | 26 (83.9) | 0.512 | 51 (92.7) | 23 (82.1) | 0.143 |
| Admission blood pressure, median (IQR), mmHg | ||||||
| SBP | 148 (132–162) | 143 (134–164) | 0.791 | 148 (132–161) | 144 (135–162) | 0.908 |
| DBP | 85 (75–99) | 84 (80–92) | 0.660 | 86 (78–100) | 85 (80–97) | 0.747 |
| Admission HR, median (IQR), times/minute | 76 (66–80) | 78 (70–84) | 0.313 | 76 (68–80) | 78 (71–84) | 0.511 |
| NIHSS score, median (IQR) | 7 (6–10) | 10 (6–13) | 0.057 | 7 (5–10) | 10 (6–13) | 0.080 |
| Baseline monitor | ||||||
| SBP, median (IQR), mmHg | 143 (124–164) | 142 (142–156) | 0.512 | 143 (123–160) | 141 (131–154) | 0.617 |
| DBP, median (IQR), mmHg | 78 (67–90) | 81 (75–92) | 0.117 | 78 (67–89) | 81 (75–91) | 0.060 |
| HR, median (IQR), times/minute | 70 (65–76) | 71 (65–78) | 0.562 | 71 (65–77) | 71 (66–79) | 0.422 |
| End-tidal CO2, median (IQR), mmHg | 40 (34–45) | 37 (34–44) | 0.511 | 41 (35–46) | 37 (35–44) | 0.330 |
| Baseline phase difference, median (IQR), degree | ||||||
| Affected side | 25.20 (8.37–37.81) | 25.05 (15.98–37.86) | 0.788 | 28.71 (9.54–40.61) | 26.60 (16.36–38.46) | 0.904 |
| Unaffected side | 32.83 (18.81–48.11) | 29.89 (19.95–41.71) | 0.453 | 32.83 (17.02–48.22) | 29.39 (20.54–41.75) | 0.579 |
| Baseline gain, median (IQR), %/mmHg | ||||||
| Affected side | 0.81 (0.60–1.14) | 1.01 (0.75–1.36) | 0.093 | 0.81 (0.60–1.13) | 1.04 (0.75–1.36) | 0.050 |
| Unaffected side | 1.05 (0.76–1.42) | 1.12 (0.91–1.53) | 0.246 | 1.00 (0.70–1.42) | 1.13 (0.92–1.63) | 0.125 |
| 14 days/discharge monitor | ||||||
| SBP, median (IQR), mmHg | 136 (125–148) | 130 (124–140) | 0.426 | 135 (124–142) | 131 (125–140) | 0.828 |
| DBP, median (IQR), mmHg | 80 (76–84) | 82 (76–85) | 0.527 | 80 (76–83) | 82 (76–86) | 0.413 |
| HR, median (IQR), times/minute | 76 (70–80) | 75 (64–82) | 0.287 | 76 (70–80) | 74 (64–82) | 0.332 |
| End-tidal CO2, median (IQR), mmHg | 39 (34–44) | 42 (37–47) | 0.164 | 39 (33–45) | 43 (37–47) | 0.078 |
| 90 days monitorb | ||||||
| SBP, median (IQR), mmHg | 130 (125–140) | 130 (123–137) | 0.601 | 130 (123–137) | 130 (122–137) | 0.933 |
| DBP, median (IQR), mmHg | 82 (75–87) | 81 (71–84) | 0.437 | 81 (76–86) | 81 (72–84) | 0.357 |
| HR, median (IQR), times/minute | 77 (71–82) | 76 (70–84) | 0.667 | 76 (71–82) | 77 (70–86) | 0.720 |
| End-tidal CO2, median (IQR), mmHg | 40 (34–45) | 43 (37–46) | 0.258 | 39 (35–45) | 44 (38–46) | 0.209 |
SD: standard deviation; IQR: interquartile range; SBP: systolic blood pressure; DBP: diastolic blood pressure; HR: heart rate.
aCerebrovascular disease was defined as all diseases involving cerebral blood vessels with an onset of more than 3 months, including ischemic stroke, hemorrhagic stroke, transient ischemic attack or subarachnoid hemorrhage.
bData on monitor at 90 days were available for 71 patients (butylphthalide group, n = 45; placebo group, n = 26).
Primary outcome
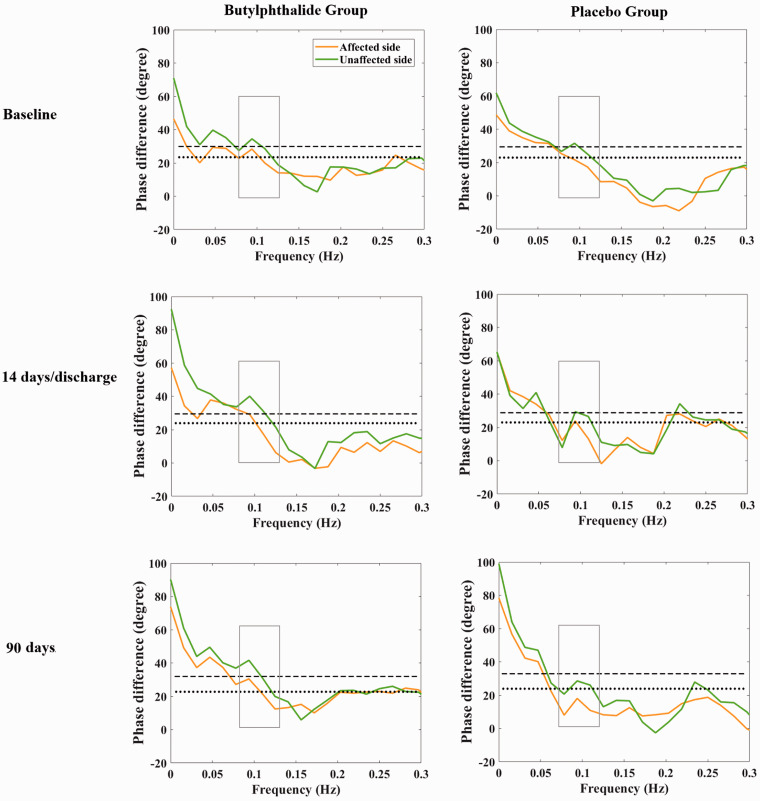
In the per-protocol analysis set, the median PD in the affected side on day 14 or at discharge was 28.4 degrees (interquartile range, 18.6 to 45.2 degrees) in the butylphthalide group and 22.0 degrees (interquartile range, 10.9 to 34.2 degrees) in the placebo group. The unadjusted beta coefficient for the PD of the affected side on day 14 or at discharge was 7.663 (95% confidence interval [CI], 0.509–14.818; P = 0.036). Furthermore, the median PD of the affected side at 90 days was 31.9 degrees (interquartile range, 21.0 to 43.1 degrees) in the butylphthalide group and 21.3 degrees (interquartile range, 8.6 to 32.4 degrees) in the placebo group. The corresponding unadjusted beta coefficient for the PD of the affected side at 90 days was 11.995 (95% CI, 3.255–20.736; P = 0.008) (Table 2 and Figure 2). The results indicated that treatment with butylphthalide increased CA on both days 14/discharge and 90. In the full analysis set, the PD of the affected side on day 90 showed similar results; however, the PD of the affected side measured on day 14 or at discharge yielded different results, with an unadjusted beta coefficient of 4.892 (95% CI, −2.440 to 12.225; P = 0.190) (Table 3).
Table 2.
Efficacy outcomes of per-protocol population.
| Outcome | Butylphthalide Group (n = 52) | Placebo Group (n = 28) | Measure of effect | Unadjusted value (95% CI) | Adjusted value (95% CI) |
|---|---|---|---|---|---|
| Primary efficacy outcomes | |||||
| Phase difference of affected side at 14 days/discharge—median (IQR), degree | 28.4 (18.6–45.2) | 22.0 (10.9–34.2) | Beta coefficient | 7.663 (0.509 to 14.818) | 6.799 (0.014 to 13.584) b |
| Phase difference of affected side at 90 days—degreea—median (IQR), degree | 31.9 (21.0–43.1) | 21.3 (8.6–32.4) | Beta coefficient | 11.995 (3.255 to 20.736) | 12.219 (3.647 to 20.791)b |
| Secondary efficacy outcomes | |||||
| Phase difference of unaffected side at 14 days/discharge—median (IQR), degree | 37.9 (21.2–54.3) | 26.8 (17.9–35.8) | Beta coefficient | 11.011 (2.640 to 19.382) | 9.905 (1.897 to 17.913)b |
| Phase difference of unaffected side at 90 days—degreea—median (IQR), degree | 35.8 (27.9–51.6) | 32.3 (18.5–42.9) | Beta coefficient | 9.177 (0.090 to 18.263) | 8.079 (−0.872 to 17.030)b |
| Gain of affected side at 14 days/discharge— median (IQR) | 1.02 (0.77–1.33) | 1.06 (0.90–1.38) | Beta coefficient | −0.062 (−0.254 to 0.130) | −0.021 (−0.218 to 0.177) c |
| Gain of affected side at 90 days—median (IQR)a | 0.98 (0.68–1.35) | 0.91 (0.73–1.41) | Beta coefficient | −0.027 (−0.268 to 0.215) | 0.018 (−0.227 to 0.263)c |
| Gain of unaffected side at 14 days/dis charge—median (IQR) | 1.18 (0.93–1.38) | 1.14 (0.88–1.58) | Beta coefficient | −0.014 (−0.271 to 0.244) | 0.052 (−0.182 to 0.287)c |
| Gain of unaffected side at 90 days—median (IQR)a | 1.12 (0.73–1.40) | 1.11 (0.86–1.50) | Beta coefficient | −0.044 (−0.291 to 0.202) | −0.029 (−0.269 to 0.210)c |
| Stroke recurrence within 90 days—n (%) | 0 | 0 | Odds ratio | NA | NA |
| Death within 90 days—n (%) | 0 | 0 | Odds ratio | NA | NA |
| Functional independence at 14 days/discharge—n (%) | 24 (46.2) | 9 (32.1) | Odds ratio | 1.810 (0.691 to 4.737) | 1.317 (0.351 to 4.942) d |
| Functional independence at 90 days—n (%)a | 33 (73.3) | 19 (73.1) | Odds ratio | 1.013 (0.341 to 3.013) | 0.630 (0.176 to 2.260)d |
| NIHSS scores at 14 days/discharge—median (IQR) | 5 (2–8) | 5 (3–9) | Beta coefficient | −0.755 (−2.537 to 1.026) | 0.458 (−0.772 to 1.689)d |
| NIHSS scores at 90 daysa—median (IQR) | 2 (0–4) | 2 (1–4) | Beta coefficient | −0.412 (−1.760 to 0.936) | 0.346 (−0.786 to 1.477)d |
| Barthel scores at 14 days/discharge— median (IQR) | 68 (36–95) | 60 (26–88) | Beta coefficient | 5.426 (−8.973 to 19.824) | −3.320 (−12.255 to 5.614) e |
| Barthel scores at 90 daysa—median (IQR) | 95 (68–100) | 95 (75–100) | Beta coefficient | −0.504 (−10.754 to 9.745) | −3.702 (−12.741 to 5.338)e |
NA denotes not applicable. IQR: interquartile range; NIHSS: National Institutes of Health Stroke Scale.
aData on outcomes at 90 days were available for 71 patients (butylphthalide group, n = 45; placebo group, n = 26).
bAdjusted for age, sex, and baseline phase difference.
cAdjusted for age, sex, and baseline Gain.
dAdjusted for age, sex, and baseline NIHSS score.
eAdjusted for age, sex, and baseline Barthel score.
Figure 2.
Phase difference in the butylphthalide and placebo groups. The dotted and dashed lines represent the median phase difference of the affected and unaffected sides in the placebo group at different time points, respectively.
Table 3.
Efficacy outcomes of full analysis set.
| Outcome | Butylphthalide Group (n = 62) | Placebo Group (n = 31) | Measure of effect | Unadjusted value (95% CI) | Adjusted value (95% CI) |
|---|---|---|---|---|---|
| Primary efficacy outcomes | |||||
| Phase difference of affected side at 14 days/ discharge—median (IQR), degree | 28.2 (18.4–44.7) | 25.6 (11.5–34.8) | Beta coefficient | 4.892 (−2.440 to 12.225) | 4.740 (−2.291 to 11.772) a |
| Phase difference of affected side at 90 days—degree—median (IQR), degree | 31.6 (18.7–41.8) | 21.5 (9.0–33.3) | Beta coefficient | 8.306 (0.138 to 16.474) | 8.802 (0.859 to 16.745)a |
| Secondary efficacy outcomes | |||||
| Phase difference of unaffected side at 14 days/discharge—median (IQR), degree | 37.1 (21.5–53.6) | 28.5 (18.8–36.0) | Beta coefficient | 9.574 (1.482 to 17.665) | 8.660 (0.945 to 16.376)a |
| Phase difference of unaffected side at 90 days—degree—median (IQR), degree | 35.8 (24.0–51.5) | 32.5 (19.8–43.1) | Beta coefficient | 6.748 (−2.068 to 15.564) | 5.517 (−3.030 to 14.063)a |
| Gain of affected side at 14 days/discharge— median (IQR) | 1.03 (0.76–1.34) | 1.11 (0.90–1.40) | Beta coefficient | −0.076 (−0.283 to 0.131) | −0.045 (−0.262 to 0.172) b |
| Gain of affected side at 90 days—median (IQR) | 0.98 (0.69–1.38) | 0.90 (0.74-1.38) | Beta coefficient | 0.013 (−0.209 to 0.236) | 0.069 (−0.145 to 0.283)b |
| Gain of unaffected side at 14 days/dis charge—median (IQR) | 1.18 (0.92–1.39) | 1.16 (0.88–1.58) | Beta coefficient | −0.029 (−0.295 to 0.237) | 0.012 (−0.223 to 0.248)b |
| Gain of unaffected side at 90 days—median (IQR) | 1.05 (0.74–1.39) | 1.10 (0.89–1.37) | Beta coefficient | −0.031 (−0.255 to 0.192) | −0.010 (−0.220 to 0.199)b |
| Stroke recurrence within 90 days—n (%) | 0 | 0 | Odds ratio | NA | NA |
| Death within 90 days—n (%) | 0 | 0 | Odds ratio | NA | NA |
| Functional independence at 14 days/dis charge—n (%) | 29.2 (47.1) | 9.6 (31.0) | Odds ratio | 1.986 (0.794 to 4.968) | 1.425 (0.446 to 4.554) c |
| Functional independence at 90 days—n (%) | 45.8 (73.9) | 19.2 (61.9) | Odds ratio | 1.737 (0.685 to 4.406) | 1.350 (0.478 to 3.810)c |
| NIHSS scores at 14 days/discharge—median (IQR) | 5 (2–8) | 5 (3–9) | Beta coefficient | −0.749 (−2.391 to 0.893) | 0.391 (−0.731 to 1.513)c |
| NIHSS scores at 90 days—median (IQR) | 2 (0–4) | 3 (1–5) | Beta coefficient | −0.749 (−2.017 to 0.519) | −0.003 (−1.028 to 1.022)c |
| Barthel scores at 14 days/discharge— median (IQR) | 58 (40–95) | 55 (25–80) | Beta coefficient | 6.539 (−6.537 to 19.616) | −1.590 (−9.677 to 6.497) d |
| Barthel scores at 90 days—median (IQR) | 94 (65–100) | 90 (75–100) | Beta coefficient | 0.363 (−8.784 to 9.511) | −3.307 (−11.164 to 4.551)d |
NA denotes not applicable. IQR: interquartile range; NIHSS: National Institutes of Health Stroke Scale.
aAdjusted for age, sex, and baseline phase difference.
bAdjusted for age, sex, and baseline Gain.
cAdjusted for age, sex, and baseline NIHSS score.
dAdjusted for age, sex, and baseline Barthel score.
Secondary outcomes
In the per-protocol analysis set, the PD of the unaffected side on day 14 or at discharge was higher in the butylphthalide group than in the placebo group, and the unadjusted beta coefficient for the PD of the unaffected side on day 14 or at discharge was 11.011 (95% CI, 2.640–19.382; P = 0.011). For the PD of the unaffected side on day 90, butylphthalide treatment was associated with an improvement in the unadjusted model; however, the association was not duplicated after adjusting for confounders. In the full analysis set, the PD of the unaffected side on day 14 or at discharge showed similar results; however, on day 90, no significant association between butylphthalide treatment and the PD of the unaffected side was observed in both unadjusted and adjusted models. No significant effect of butylphthalide on the gain was observed on either side on day 14 or at discharge and on day 90 in both per-protocol analysis and full analysis sets (Tables 2 and 3).
In the per-protocol analysis set, functional independence on day 14 or at discharge was achieved in 46.2% of the patients in the butylphthalide group and 32.1% in the placebo group. However, the unadjusted interval estimation of the odds ratio (OR) included 1 (OR, 1.810; 95% CI, 0.691–4.737; P = 0.227), which means there was no significant difference in the 14-day functional outcome between the two groups. Similarly, functional independence on day 90 was achieved in 73.3% of patients in the butylphthalide group and in 73.1% in the placebo group; the unadjusted OR was 1.013, and its 95% CI was 0.341–3.013 (P = 0.981). The results for the other secondary outcomes also did not show a significant difference between the two groups (Table 2). The full analysis yielded similar results (see Table 3).
Safety outcomes
The percentage of patients with total adverse events within 90 days did not differ significantly between the two groups (27.4% in the butylphthalide group [17 of 62 patients] and 16.1% in the placebo group [5 of 31 patients], P = 0.227; Table 4). Abnormal liver function occurred in 9.7% (6 of 62) of the patients in the butylphthalide group but in none of the patients in the placebo group. However, the difference between the two groups was not statistically significant (P = 0.173). There were no significant group differences in other types of adverse events. Only one patient experienced serious adverse events during the study period; however, according to the researcher's judgment, it was not related to the use of the study drug.
Table 4.
Safety outcomes within 90 days.
| Safety outcomes | Butylphthalide Group (n = 62) | Placebo Group (n = 31) | P |
|---|---|---|---|
| Adverse events—n (%) | |||
| Total adverse events | 17 (27.4) | 5 (16.1) | 0.227 |
| Constipation | 7 (11.3) | 2 (6.5) | 0.713 |
| Abnormal liver function | 6 (9.7) | 0 (0) | 0.173 |
| Electrolyte disorder | 1 (1.6) | 2 (6.5) | 0.257 |
| Dizziness and headache | 2 (3.2) | 1 (3.2) | >0.999 |
| Serious adverse events—n (%) | 1 (1.6) | 0 (0) | >0.999 |
Discussion
In the present study, we found that butylphthalide treatment for 90 days can significantly improve CA in patients with AIS. This indicated that butylphthalide is an effective drug for improving CA through its mechanism of action.
CA is an essential indicator of cerebrovascular function and is responsible for maintaining cerebral blood flow during fluctuations in blood pressure within a relatively constant range. 23 CA is closely related to AIS prognosis; however, few drugs have been reported to improve CA in patients with AIS. One study reported that in patients with carotid artery atherosclerotic stenosis, the butylphthalide group had higher cerebral hypoperfusion than the placebo group. 16 A possible reason for this is that the butylphthalide group had higher CA; thus, these patients were more capable of maintaining adequate cerebral blood flow. In this study, we confirmed our hypothesis and found that continuous application of butylphthalide for 90 days can improve CA, suggesting the need for continued medication use.
The mechanism by which butylphthalide improves this regulation is unclear. The normal function of cerebrovascular endothelial cells is the basis for maintaining CA because endothelial cells modulate many aspects of vascular function. Endothelial cells, for example, secrete nitric oxide, which maintains the vascular tone 24 in the blood vessels of the brain at a certain level, which is critical for maintaining CA. Thus, damage to cerebrovascular endothelial cells in patients with AIS is closely related to the impairment of CA. 25 Previous studies have reported that butylphthalide positively affects vascular endothelial cells. For example, Liu et al. showed that butylphthalide protects endothelial cells against advanced glycation end-product-induced injury by attenuating oxidative stress and inflammatory response. 26 Wei et al. found that butylphthalide reduced oxygen-glucose deprivation-induced endothelial cell damage by increasing PGC-1α. 27 Therefore, butylphthalide may improve CA in patients with AIS through its protective effects on cerebrovascular endothelial cells. In addition, previous studies have suggested that butylphthalide can improve collateral circulation, such as acting as a vasodilator and promoting collateriogenesis,14,15 which may play a role in enhancing CA.
Furthermore, the CA of the unaffected side in the butylphthalide group was higher than that of the placebo group after 14 days of treatment. This phenomenon can be explained as follows. Previous studies have found that the CA on the unaffected side is influenced by that of the affected side in patients with cerebrovascular stenosis. 28 When the CA of the affected side is damaged, part of the function of the CA of the unaffected side is also compensated. In the present study, the CA of the affected side of the butylphthalide group was higher than that of the placebo group after 14 days of medication; thus, the CA of the unaffected side of the butylphthalide group was less influenced by the affected side, masking any difference in CA of the unaffected side. This finding also suggests that butylphthalide can improve CA in patients in the acute stage.
In this study, we investigated the effects of butylphthalide on prognostic indicators. Although the proportion of functional independence at 14 and 90 days was higher in the butylphthalide group than in the placebo group, the differences were not statistically significant. This may be because of the small sample size. Simultaneously, no significant difference was found between the two groups regarding safety indicators, indicating that continuous application of butylphthalide for 90 days is safe.
It is worth mentioning that, in randomization, we chose 2:1 instead of 1:1; this is because many previous studies had demonstrated the efficacy of butylphthalide in patients with AIS. Therefore, although this study focused on the effect of butylphthalide on CA, the number of patients in the placebo group should be minimized to benefit more patients. Therefore, we revised the protocol to 2:1 randomization to ensure that more patients receive butylphthalide therapy.
Our study has a few limitations. First, we excluded patients who received endovascular treatment; thus, many patients with large vessel occlusion could not be included in this study, and whether butylphthalide can improve CA in such patients remains unknown. Second, the sample size of our study was relatively small, which may be an important reason why we did not find that butylphthalide administration improves clinical outcomes in patients with AIS.
In conclusion, butylphthalide treatment for 90 days can significantly improve CA in patients with AIS.
Acknowledgements
The authors thank Professor Yanhua Wu (Department of Clinical Research, the First Hospital of Jilin University) for guidance with the statistics.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This project was supported by the National Natural Science Foundation of China (Grant No. 81971105), the Science and Technology Department of Jilin Province (YDZJ202201ZYTS677), and Norman Bethune Program of Jilin University (2022B02) to ZNG, and the National Key R&D Program of China (2016YFC1301600), the Norman Bethune Health Science Center of Jilin University (2022JBGS03), Science and Technology Department of Jilin Province (20220303002SF), and Jilin Provincial Key Laboratory (YDZJ202302CXJD017) to YY.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions: Conception and design: YY and ZNG. Collection of data: BHY, LF, JL (Jie Liu), YZ (Yuanyuan Zhu), YZ (Yuanqi Zhao), JZ, ZL, LXL, RA, HJ and XS. Data analysis: PZ, YQ, ZS, BS, KJZ, JL (Jia Liu) and JC. Manuscript writing: ZNG and YQ. Critical and intellectual revision of manuscript: all authors. Final approval of manuscript: all authors.
ORCID iDs: Peng Zhang https://orcid.org/0000-0001-5536-0255
Junlei Chang https://orcid.org/0000-0002-0319-9022
References
- 1.Collaborators GBDS. Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet Neurol 2021; 20: 795–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ji X. Forward thinking in stroke treatment: advances in cerebrovascular reperfusion and neurorehabilitation. Brain Circul 2015; 1: 1–2. [Google Scholar]
- 3.MacGregor DG, Carswell HV, Graham DI, et al. Impaired cerebral autoregulation 24 h after induction of transient unilateral focal ischaemia in the rat. Eur J Neurosci 2000; 12: 58–66. [DOI] [PubMed] [Google Scholar]
- 4.Xiong L, Liu X, Shang T, et al. Impaired cerebral autoregulation: measurement and application to stroke. J Neurol Neurosurg Psychiatry 2017; 88: 520–531. [DOI] [PubMed] [Google Scholar]
- 5.Claassen JA. How can integrative physiology advance stroke research and stroke care? J Cereb Blood Flow Metab 2022; 42: 383–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fan JL, Nogueira RC, Brassard P, et al. Integrative physiological assessment of cerebral hemodynamics and metabolism in acute ischemic stroke. J Cereb Blood Flow Metab 2022; 42: 454–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma H, Guo ZN, Liu J, et al. Temporal course of dynamic cerebral autoregulation in patients with intracerebral hemorrhage. Stroke 2016; 47: 674–681. [DOI] [PubMed] [Google Scholar]
- 8.Ma H, Liu J, Lv S, et al. Dynamic cerebral autoregulation in embolic stroke of undetermined source. Front Physiol 2020; 11: 557408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nogueira RC, Aries M, Minhas JS, et al. Review of studies on dynamic cerebral autoregulation in the acute phase of stroke and the relationship with clinical outcome. J Cereb Blood Flow Metab 2022; 42: 430–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fan JL, Brassard P, Rickards CA, et al. Integrative cerebral blood flow regulation in ischemic stroke. J Cereb Blood Flow Metab 2022; 42: 387–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang S, Ma F, Huang L, et al. Dl-3-n-butylphthalide (NBP): a promising therapeutic agent for ischemic stroke. CNS Neurol Disord Drug Targets 2018; 17: 338–347. [DOI] [PubMed] [Google Scholar]
- 12.Chen XQ, Qiu K, Liu H, et al. Application and prospects of butylphthalide for the treatment of neurologic diseases. Chin Med J (Engl) 2019; 132: 1467–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yan CH, Feng YP, Zhang JT. Effects of dl-3-n-butylphthalide on regional cerebral blood flow in right middle cerebral artery occlusion rats. Zhongguo Yao Li Xue Bao 1998; 19: 117–120. [PubMed] [Google Scholar]
- 14.Wei ZZ, Chen D, Lee MJH, et al. DL-3-n-butylphthalide increases collateriogenesis and functional recovery after focal ischemic stroke in mice. Aging Dis 2021; 12: 1835–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qin C, Zhou P, Wang L, et al. Dl-3-N-butylphthalide attenuates ischemic reperfusion injury by improving the function of cerebral artery and circulation. J Cereb Blood Flow Metab 2019; 39: 2011–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen D, Yin Y, Shi J, et al. DL-3-n-butylphthalide improves cerebral hypoperfusion in patients with large cerebral atherosclerotic stenosis: a single-center, randomized, double-blind, placebo-controlled study. BMC Neurol 2020; 20: 212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao H, Yun W, Zhang Q, et al. Mobilization of circulating endothelial progenitor cells by dl-3-n-butylphthalide in acute ischemic stroke patients. J Stroke Cerebrovasc Dis 2016; 25: 752–760. [DOI] [PubMed] [Google Scholar]
- 18.Song K, Zeng X, Xie X, et al. Dl-3-n-butylphthalide attenuates brain injury caused by cortical infarction accompanied by cranial venous drainage disturbance. Stroke Vasc Neurol 2022; 7: 222–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adams HP, Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of org 10172 in acute stroke treatment. Stroke 1993; 24: 35–41. [DOI] [PubMed] [Google Scholar]
- 20.Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2019; 50: e344–e418. [DOI] [PubMed] [Google Scholar]
- 21.Claassen JA, Meel-van den Abeelen AS, Simpson DM, et al. Transfer function analysis of dynamic cerebral autoregulation: a white paper from the international cerebral autoregulation research network. J Cereb Blood Flow Metab 2016; 36: 665–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Panerai RB, Brassard P, Burma JS, et al. Transfer function analysis of dynamic cerebral autoregulation: a CARNet white paper 2022 update. J Cereb Blood Flow Metab 2023; 43: 3–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salinet AS, Panerai RB, Robinson TG. The longitudinal evolution of cerebral blood flow regulation after acute ischaemic stroke. Cerebrovasc Dis Extra 2014; 4: 186–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo ZN, Jin H, Sun H, et al. Antioxidant melatonin: potential functions in improving cerebral autoregulation after subarachnoid hemorrhage. Front Physiol 2018; 9: 1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nitzsche A, Poittevin M, Benarab A, et al. Endothelial S1P1 signaling counteracts infarct expansion in ischemic stroke. Circ Res 2021; 128: 363–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu CY, Zhao ZH, Chen ZT, et al. DL-3-n-butylphthalide protects endothelial cells against advanced glycation end product-induced injury by attenuating oxidative stress and inflammation responses. Exp Ther Med 2017; 14: 2241–2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wei H, Zhan LP, Zhang B, et al. dl-3n-butylphthalide reduces oxygen-glucose deprivation-induced endothelial cell damage by increasing PGC-1alpha. Eur Rev Med Pharmacol Sci 2019; 23: 4481–4490. [DOI] [PubMed] [Google Scholar]
- 28.Guo ZN, Sun X, Liu J, et al. The impact of variational primary collaterals on cerebral autoregulation. Front Physiol 2018; 9: 759. [DOI] [PMC free article] [PubMed] [Google Scholar]