Abstract
Background
The effect of conservative vs. liberal oxygen therapy on 90-day in-hospital mortality in adults with sepsis receiving unplanned invasive mechanical ventilation in the intensive care unit (ICU) is uncertain.
Objective
The objective of this study was to summarise the protocol and statistical analysis plan for the Mega-ROX Sepsis trial.
Design, setting, and participants
The Mega-ROX Sepsis trial is an international randomised clinical trial that will be conducted within an overarching 40,000-patient registry-embedded clinical trial comparing conservative and liberal ICU oxygen therapy regimens. We anticipate that between 10,000 and 13,000 patients with sepsis who are receiving unplanned invasive mechanical ventilation in the ICU will be enrolled in this trial.
Main outcome measures
The primary outcome is in-hospital all-cause mortality up to 90 days from the date of randomisation. Secondary outcomes include duration of survival, duration of mechanical ventilation, ICU length of stay, hospital length of stay, and the proportion of patients discharged home.
Results and conclusions
Mega-ROX Sepsis will compare the effect of conservative vs. liberal oxygen therapy on 90-day in-hospital mortality in adults with sepsis who are receiving unplanned invasive mechanical ventilation in the ICU. The protocol and a prespecified approach to analyses are reported here to mitigate analysis bias.
Keywords: Sepsis, Oxygen therapy, Intensive care, Critical care, Hyperoxaemia, Hypoxaemia
1. Introduction
Sepsis occurs commonly in intensive care unit (ICU) patients.1 It causes or contributes to between one-third and one-half of all deaths in hospital2 and is responsible for more than six million deaths worldwide annually.3 For ICU patients with sepsis receiving invasive mechanical ventilation, supplemental oxygen therapy is ubiquitous. Liberal provision of oxygen to patients with sepsis who are receiving invasive mechanical ventilation has potential advantages. In addition to providing a greater margin of safety against the development of hypoxaemia, liberal oxygen use may enhance oxidative killing of bacteria and aid in wound healing. Experimental evidence suggests that oxygen tension is often low in infected tissues4 and that neutrophil superoxide production is enhanced when oxygen tension is high.5 For patients with infected wounds, liberal provision of oxygen may enhance wound healing through enhanced re-epithelialization, blood vessel angiogenesis, and tissue collagen synthesis.6,7
In a post hoc analysis of patients with sepsis who were enrolled in the ICU randomised trial that compared two approaches to giving oxygen (ICU-ROX),8 the observed 90-day mortality rate for patients treated with usual (liberal) oxygen was 7 percentage points lower (95% confidence interval [CI], −4.6 percentage points to 18.6 percentage points) than it was for patients who received conservative oxygen therapy.9 Despite a plausible basis for benefit with liberal oxygen therapy in patients with sepsis who receive invasive mechanical ventilation in the ICU, further research is required to establish the optimal oxygen therapy regimen for patients with sepsis.
To address the uncertainty, we are conducting the Mega-ROX Sepsis trial. This trial compares conservative oxygen therapy with liberal oxygen therapy in adults with sepsis who receive invasive mechanical ventilation in the ICU and will test the hypothesis that liberal oxygen therapy reduces 90-day in-hospital mortality. Here we present the protocol and statistical analysis plan for Mega-ROX Sepsis.
2. Methods
2.1. Trial design
Mega-ROX Sepsis is a phase 3 international, multicentre, randomised two-sided superiority trial designed to test the hypothesis that among adult ICU patients with sepsis who receive unplanned invasive ventilation, liberal oxygen therapy compared to conservative oxygen therapy reduces in-hospital all-cause mortality up to 90 days from the date of randomisation. Mega-ROX Sepsis is one of three nested trials being conducted within an overall 40,000-participant sample size envelope as part of the Mega-ROX trial research program. Protocol and statistical analysis plan manuscripts for the overarching Mega-ROX trial10 and for the two other nested randomised trials are published separately with this manuscript. We plan to present data for the sepsis subgroup in a stand-alone manuscript because this nested study has sufficient size to detect a plausible treatment effect. Moreover, given that we are comparing treatment strategies that fall within the spectrum of usual care, we submit that subgroup data may provide a reasonable basis to individualise oxygen therapy, even if subgroup interaction terms are not statistically significant.11
Trial registration: Australian and New Zealand Clinical Trials Registry (ACTRN12620000391976)
2.2. Setting and population
Mega-ROX Sepsis will be conducted in around 100 ICUs worldwide and is expected to include patients from a range of low-, middle-, and high-income countries. Patients aged ≥18 years with confirmed or strongly suspected sepsis prior to randomisation who receive invasive mechanical ventilation in the ICU following an emergency (unplanned) ICU admission or when mechanical ventilation starts in the ICU (i.e., endotracheal intubation occurs in the ICU) will be eligible for inclusion. Sepsis will be defined as a life-threatening organ dysfunction due to a dysregulated host response to infection.12
Where enrolment is not considered in a particular patient's best interests by the treating clinician, that patient will be excluded. Operationally, this criterion will exclude all patients where either of the oxygen regimens being tested are considered clinically indicated or contraindicated or patients where death is deemed imminent and inevitable. Patients who have previously been enrolled in the study will also be excluded. Patients must be enrolled within 12 h of fulfilling the eligibility criteria. When a patient is not enrolled within this timeframe, they will be counted as “eligible but missed” rather than “excluded” for the purposes of describing participant flow.
2.3. Randomisation and blinding
Treatment assignment will be performed using a secure, centralised, web-based, randomisation interface. Participants will be enrolled in the study by ICU doctors, nurses, and research staff. The assigned intervention will be communicated to the bedside nurse and/or respiratory therapist who will implement the study intervention. One novel feature of this trial is that it will use adaptive randomisation to subtly increase the probability that trial participants are allocated to the oxygen regimen that appears to be associated with the lowest mortality risk based on accumulated trial data. Randomisation ratios of 1.05:1 in favour of liberal oxygen therapy, 1:1.05 in favour of conservative oxygen therapy, and 1:1 may all be used at different times of the trial. Other randomisation ratios will not be used. The initial randomisation ratio will favour liberal oxygen therapy because observed mortality was lower in patients with sepsis who were enrolled in the ICU-ROX.8 The randomisation ratio will be adapted at interim analyses as described in the protocol manuscript for the overarching Mega-ROX trial program.10
2.4. Study treatments
The Mega-ROX trial program is designed to compare two approaches to oxygen therapy that are within the spectrum of current usual practice. For the Mega-ROX Sepsis trial, liberal oxygen therapy is defined as the intervention and will be compared with a control arm of conservative oxygen therapy. The details of these approaches have been outlined in the protocol manuscript for the overarching Mega-ROX trial program.10 In brief, for patients allocated to the liberal oxygen therapy, there will be no protocol-defined upper SpO2 alarm limits and patients will receive a minimum FIO2 of 0.30 while they are invasively mechanically ventilated. Patients allocated to the conservative oxygen therapy will receive the lowest possible FIO2 to achieve an SpO2 of ≥91%. In such patients, SpO2 levels of greater than 94% will be strictly avoided and an upper SpO2 alarm limit of 95% will apply whenever supplemental oxygen is being administered in the ICU to minimise the risk of hyperoxaemia.
The duration of study therapy will be until ICU discharge or 90 days, whichever is sooner. The study intervention will be applied in the ICU only. If, during the course of their ICU admission, patients are transported outside of the ICU for radiological or other investigations or for procedures or operations, they will receive standard (nonstudy) treatment. Similarly, if an increase in FIO2 is required for procedures performed in the ICU including (but not limited to) bronchoscopy, suctioning, tracheostomy, or preparation for extubation, this is permitted in both groups. There are no restrictions to concomitant treatments provided to patients such as the amount of positive end-expiratory pressure used.
2.5. Outcomes
The primary outcome is in-hospital all-cause mortality up to 90 days from the date of randomisation. All patients who survive the index hospital admission and are discharged from that hospital within 90 days of randomisation will be defined as alive.
Secondary outcomes are duration of survival time up until the last follow-up, ICU length of stay, hospital length of stay, duration of invasive mechanical ventilation, the proportion of patients discharged home, and 90-day all-cause mortality, which will be reported for patients where vital status after hospital discharge can be obtained from registry data source (for example from a national death registry).
2.6. Data collection and management
The Mega-ROX Sepsis trial will use a combination of trial-specific data and existing registry data sources. Specific details of data sources that will be used and data management process are reported in the protocol manuscript for the overarching Mega-ROX trial program.10 Study data will be retained for the minimum period required by local regulations after the completion or discontinuation of the study.
2.7. Ethics approval
Research ethics approval will be obtained prior to the start of the study at each institution from the responsible local and/or national human research ethics committee. Specific consent processes that will be used are described in the protocol manuscript for the overarching Mega-ROX trial program.10
2.8. Data monitoring committee
An independent data monitoring committee (DMC) consisting of experts in intensive care medicine clinical research and biostatistics was established before the first trial participant was enrolled. The DMC members are Prof Anders Perner (Chair), Prof Manu Shankar-Hari, and Prof Laurent Billot (DMC statistician). The specific responsibilities of the DMC are outlined in a set of DMC guidelines and a DMC Charter which was prepared by the study management committee and signed by the members of the DMC before the trial commenced.
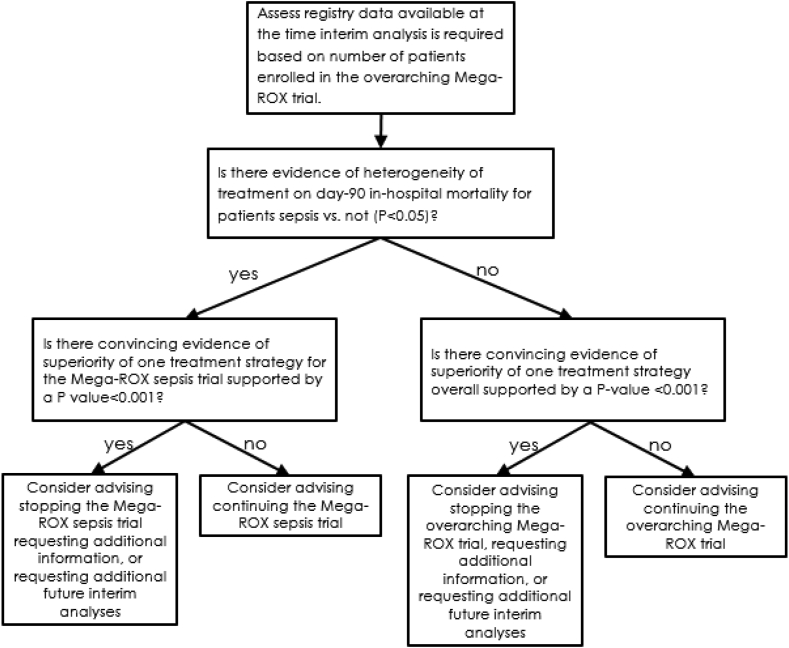
The timing of interim analyses for the Mega-ROX Sepsis trial will be determined by the overall recruitment rate in the overarching trial program. In particular, interim analyses for efficacy will occur after every 8000 trial participants are enrolled in the overarching trial. These interim analyses will require the DMC to provide advice to the management committee about both the overarching Mega-ROX trial and about the Mega-ROX Sepsis trial specifically. However, as shown in Fig. 1, an interim analysis for Mega-ROX Sepsis patients specifically will only occur where there is evidence of heterogeneity of treatment response (P < 0.05). If such an analysis is undertaken, stopping rules will be determined by a Haybittle–Peto boundary of p < 0.001. This approach implies that the hypothesis tested at the final analysis can be tested at the traditional 0.05 level.
Fig. 1.
Overview of steps undertaken by the data monitoring committee at interim analyses
Abbreviations: Mega-ROX: Mega randomised registry trial comparing two approaches to oxygen therapy in the intensive care unit.
2.9. Sample size and power
The specific sample size of the Mega-ROX Sepsis trial will be determined by the proportion of patients in the overarching Mega-ROX trial who are identified as having confirmed or strongly suspected sepsis at baseline. Assuming a control mortality event rate of 36.2%, we have reported previously that to detect an effect on 90-day mortality with liberal oxygen therapy of 3.5 percentage points absolute (i.e. a reduction to 32.7%; half the magnitude suggested by point estimates of treatment effect in patients with sepsis included in the ICU-ROX study), a sample size of 7744 would be required for a trial with 90% power with a two-sided alpha of 0.05.9 Based on the proportion of patients with sepsis included in the ICU-ROX trial,8 we would expect ≈10,000 patients to be included in the Mega-ROX sepsis trial. A sample size of 10,000 participants provides 90% power at an alpha of 0.05 to detect 3.1 percentage point difference in mortality between groups (a reduction from 36.2% to 33.1%) using a two-sided test and allowing for a loss to follow-up rate of 1%. Around 30.4% of the first 6000 participants enrolled in the Mega-ROX trial had sepsis, a recruitment rate which, if sustained, would translate to a final Mega-ROX sepsis sample of ≈12,000 participants. Table 1 summarises a range of potential scenarios for sample size and power for the Mega-ROX Sepsis trial. We will update the Australian and New Zealand Clinical Trials Registry Mega-ROX trial registration with the anticipated final sample size, based on the proportion of patients with sepsis recruited at the time of the fourth interim analysis.
Table 1.
Potential scenarios for sample size and power for Mega-ROX Sepsis.
| Control event ratea | Sample size | Absolute mortality effect detectable with 90% power and 2-sided significance level of 0.05 |
|---|---|---|
| 20% | 10000 | 2.53 |
| 20% | 11000 | 2.41 |
| 20% | 12000 | 2.31 |
| 20% | 13000 | 2.23 |
| 25% | 10000 | 2.75 |
| 25% | 11000 | 2.63 |
| 25% | 12000 | 2.52 |
| 25% | 13000 | 2.42 |
| 30% | 10000 | 2.93 |
| 30% | 11000 | 2.79 |
| 30% | 12000 | 2.68 |
| 30% | 13000 | 2.57 |
| 35% | 10000 | 3.06 |
| 35% | 11000 | 2.92 |
| 35% | 12000 | 2.79 |
| 35% | 13000 | 2.69 |
| 40% | 10000 | 3.15 |
| 40% | 11000 | 3.01 |
| 40% | 12000 | 2.88 |
| 40% | 13000 | 2.77 |
The control event rate is assumed in-hospital all-cause mortality up to 90 days from the date of randomisation in patients allocated to conservative oxygen therapy (the comparator arm). No loss to follow-up is assumed.
2.10. Overview of planned statistical analyses
2.10.1. Analysis and reporting principles
We will analyse data on an intention-to-treat basis, whereby all patients assigned to a treatment group will be analysed according to the group to which they were assigned, without imputation of missing data except where prespecified. The intention-to-treat population will be defined as all patients enrolled in the trial except for those where consent for use of study data is either not provided or withdrawn. A P value less than 0.05 (two-tailed) will be used to indicate statistical significance for the primary outcome variable. For the six secondary clinical outcomes, we will control the family-wise error rate by applying a Holm–Bonferroni correction. All analyses will be performed using Stata v17.0 or later (Stata Statistical Software, College Station, TX, USA). Reporting of the study will align with the CONSORT statement.13
The study team includes a blinded statistician who is a member of the study management committee and an unblinded statistician who is independent of the study management committee. The unblinded statistician will conduct interim analyses and will provide these to the DMC. Once study data are available for the entire study population, the unblinded study statistician will assign mock treatment codes to study participants. Analyses using actual study data but with mock treatment codes will be run by the blinded statistician using the general approach outlined in this document. Any data queries that arise from these initial analyses will be addressed. Any changes that are needed to the approach outlined here will be specified in the formal stand-alone statistical analysis plan which will be publicly available prior to final study database lock or unmasking of actual study treatment assignments. Analyses of the final study dataset will be untaken by two study statisticians independently, with any discrepancies between findings resolved through consensus and discussion with the management committee when required.
2.10.2. Analyses of the primary outcome
Analysis of the primary outcome and other binary outcomes will be via log-binomial models, adjusting for hypoxic ischaemic encephalopathy and acute brain injuries or conditions. These characteristics will be included in the model because patients with these diagnoses will also be included in the other Mega-ROX substudies, so there is potential for imbalance in these characteristics across arms of the Mega-ROX Sepsis trial. The numbers at risk in each group and the number and proportion of events observed will be reported, as well as the equivalent absolute risk difference and relative risk ratio and corresponding 95% CI. Sensitivity analyses accounting for differences across sites and any clinically meaningful baseline imbalances will be performed using log-binomial regression. In addition, we will incorporate adjustment for the independent covariates of age, sex, and illness severity. The main sensitivity analyses for the impact of missing primary outcomes will involve imputing outcomes under “worst-best” and “best-worst” case scenarios. In the “worst-best” scenario, a “worst” outcome event (i.e., in-hospital death within 90 days) is assigned to all patients missing the outcome in one treatment group, and a “best” outcome event (i.e. survival to hospital discharge within 90 days) is assigned to all patients missing the outcome in the other treatment group. The “best-worst” scenario is the exact opposite assignment of outcomes. If substantively different conclusions do not arise from these two analyses, then no further missing data assessments will be performed for that outcome. If a substantively different conclusion does arise, then multiple imputation will be undertaken. Missing outcomes will be imputed separately by a randomised group, using chained equations and predictive mean matching, using the five nearest neighbours.
In some low- and middle-income countries participating in this study, patients are sometimes discharged from the ICU (to home) when discharge is not considered medically indicated (e.g., because of the high cost of care and/or because death is anticipated). We will undertake two sensitivity analyses to account for patients categorised as discharged from the ICU when discharge was not considered medically indicated. In the first analysis, these patients, when assigned to conservative oxygen, will be defined as dead and, when assigned to liberal oxygen, will be defined as alive. In the second analysis, these patients, when assigned to conservative oxygen, will be defined as alive and, when assigned to liberal oxygen, will be defined as dead.
2.10.3. Analyses of secondary outcomes
The effect of treatment allocation on the proportion of patients discharged home will be assessed in the same way as the primary outcome. To account for the competing risk of death, ICU and hospital lengths of stay will be analysed using subdistribution hazard regression models and presented using cumulative incidence functions. As lengths of stay are typically well approximated by log-normal distributions, for increased transparency, they will also be reported as geometric means (95% CI), with additional stratification for survival and differences between groups reported as a ratio (95% CI). Survival time according to the treatment group will be displayed as Kaplan–Meier curves and analysed using a log-rank test. Estimates of hazard ratios for survival, with corresponding 95% CI and P values, will be obtained from the Cox proportional hazards models incorporating treatment group and hypoxic ischaemic encephalopathy and acute brain injuries or conditions, and additionally using independent covariates used in the multivariate logistic models described in relation to the primary outcome. The assumption of proportional hazards will be assessed, and if violated, the log-rank test will be used to compare survival times between treatment groups.
2.10.4. Analyses of oxygen exposure metrics
For analyses that compare differences in the median percentage of hours per participant and the median number of hours per participant above and below specific PaO2 thresholds and those that compared the median percentage of hours per participant and the median number of hours spent breathing an FiO2 of 0.21 while in the ICU, we will calculate differences and medians and 95% CIs using quantile regression.
Analyses that compare the proportion of patients with at least one PaO2 recording less than 60 mmHg and with at least one PaO2 recording greater than 100 mmHg will be conducted via log-binomial models. The numbers at risk in each group and the number and proportion of events observed will be reported, as well as the relative risk and corresponding 95% CIs.
2.11. Presentation of outcome data
The planned presentation of baseline data is shown in Table 2. Exposure to oxygen by treatment group will be described as shown in Table 3. Primary and secondary outcome data will be presented as shown in Table 4. A complete set of mock tables and figures (including those that will appear in the Supplementary Appendix) is available online at http://www.wellingtonicu.com/PubResPres/Protocols.
Table 2.
Proposed presentation of baseline characteristics.
| Characteristic | Conservative oxygen therapy (n = xxxx) | Liberal oxygen therapy (n = xxxx) |
|---|---|---|
| Age, yr | xx.x ± xx | xx.x ± xx |
| Male sex, no. (%) | xxx (xx.x) | xxx (xx.x) |
| Body mass index | xx.x ± xx | xx.x ± xx |
| Clinical frailty score | xxx (xx.x) | xxx (xx.x) |
| Source of admission to ICU, no. (%) | ||
| Emergency department | xxx (xx.x) | xxx (xx.x) |
| Hospital ward | xxx (xx.x) | xxx (xx.x) |
| Transfer from another ICU | xxx (xx.x) | xxx (xx.x) |
| Transfer from another hospital (except from another ICU) | xxx (xx.x) | xxx (xx.x) |
| From OT following surgery | xxx (xx.x) | xxx (xx.x) |
| Hours from hospital admission to randomisation | xx.x ± xx | xx.x ± xx |
| Hours from ICU admission to randomisation | xx.x ± xx | xx.x ± xx |
| APACHE-II scorea | xx.x ± xx | xx.x ± xx |
| SAPS-III scoreb | ||
| Sepsis source, no. (%) | ||
| Respiratory | xxx (xx.x) | xxx (xx.x) |
| Gastrointestinal | xxx (xx.x) | xxx (xx.x) |
| Genitourinary | xxx (xx.x) | xxx (xx.x) |
| Musculoskeletal and skin | xxx (xx.x) | xxx (xx.x) |
| Other | xxx (xx.x) | xxx (xx.x) |
| COVID-19, no. (%) | xxx (xx.x) | xxx (xx.x) |
| Baseline oxygen data | ||
| FiO2 | xx.x ± xx | xx.x ± xx |
| PaO2, mmHg | xx.x ± xx | xx.x ± xx |
| PaO2/FiO2 ratio, mmHg | xx.x ± xx | xx.x ± xx |
Plus-minus values will be expressed as mean ± SD (where the distribution of the data is not symmetric, median [IQR] will be reported instead of mean ± SD). To facilitate meaningful interpretation of categorical variables, categories with small numbers (<10) will be collapsed for analysis.
Abbreviations: APACHE: Acute Physiology and Chronic Health Evaluation; CNS: central nervous system; ICU: intensive care unit; IQR: interquartile range; OT: operating theatre; SpO2: arterial oxygen saturation on pulse oximetry; PaO2: arterial partial pressure of oxygen; FiO2: fraction of inspired oxygen; PaCO2: arterial partial pressure of carbon dioxide; PEEP: positive end-expiratory pressure; SD: standard deviation.
Scores on the APACHE-II range from 0 to 71, with higher scores indicating more severe disease and a higher risk of death.
Scores on the SAPS-III range from 0 to 217, with higher scores indicating more severe disease. The SAPS-III score was collected from trial participants from Brazil.
Table 3.
Proposed presentation of oxygen exposure by treatment group.
| Oxygen exposure metric – n (%) | Conservative oxygen therapy | Liberal oxygen therapy (n = xxxx) | Between-group difference (95% CI) |
|---|---|---|---|
| Median [IQR] percentage of hours per patient SpO2 ≥97% | xx (xx–xx) | xx (xx–xx) | xx (xx to xx) |
| Median [IQR] number of hours per patient SpO2 ≥97% | xx (xx–xx) | xx (xx–xx) | xx (xx to xx) |
| Median [IQR] percentage of hours per patient SpO2 <88% | xx (xx–xx) | xx (xx–xx) | xx (xx to xx) |
| Median [IQR] number of hours per patient SpO2 <88% | xx (xx–xx) | xx (xx–xx) | xx (xx to xx) |
| Proportion of patients with at least one PaO2 recording <60 mmHg | xx (xx.x) | xx (xx.x) | xx (xx to xx) |
| Proportion of patients with at least one PaO2 recording >100 mmHg | xx (xx.x) | xx (xx.x) | xx (xx to xx) |
| Median [IQR] percentage of hours per patient FIO2 0.21 | xx (xx–xx) | xx (xx–xx) | xx (xx to xx) |
| Median [IQR] number of hours per patient FIO2 0.21 | xx (xx–xx) | xx (xx–xx) | xx (xx to xx) |
Abbreviations: IQR: interquartile range; CI: confidence Interval.
Table 4.
Proposed presentation of outcomes.
| Conservative oxygen therapy (n = xxxx) | Liberal oxygen therapy (n = xxxx) | Estimate (95% CI) | |
|---|---|---|---|
| Primary outcomea | |||
| Died at the hospital by day 90, no. (%) | xxxx (xx.x) | xxxx (xx.x) | Relative risk xx (xx to xx) Risk difference xx (xx to xx) |
| Secondary outcomes | |||
| Hours until liberated from invasive mechanical ventilation alive | Subhazard ratio of time to extubationc | ||
| Number of patients | xxxx | xxxx | |
| Median (IQR)b | xx (xx–xx) | xx (xx–xx) | xx (xx to xx) |
| Days until discharged alive from ICU | Subhazard ratio of time to ICU dischargec | ||
| Number of patients | xxxx | xxxx | |
| Median (IQR)b | xx (xx–xx) | xx (xx–xx) | xx (xx to xx) |
| Days until discharged alive from hospital | Subhazard ratio of time to Hospital dischargec | ||
| Number of patients | xxxx | xxxx | |
| Median (IQR)b | xx (xx–xx) | xx (xx–xx) | xx (xx to xx) |
| Discharged home, no. (%) | xxxx (xx.x) | xxxx (xx.x) | Relative risk xx (xx to xx) Risk difference xx (xx to xx) |
| 90-Day mortality, no. (%) | xxx (xx.x) | xxx (xx.x) | Relative risk xx (xx to xx) Risk difference xx (xx to xx) |
Abbreviations: IQR: interquartile range; CI: confidence interval.
A P value for the primary outcome comparison will be shown in a footnote. The absolute difference in 90-day mortality and corresponding relative risk will be adjusted for the presence or absence of each of the following at randomisation: suspected hypoxic ischaemic encephalopathy following resuscitation from a cardiac arrest and acute brain pathologies other than hypoxic ischaemic encephalopathy.
Duration of invasive mechanical ventilation and ICU and hospital length of stay will be calculated from cumulative incidence functions with mortality regarded as a competing risk.
Ratios of median time to discharge (or extubation) will be estimated using censored linear regression with logarithm of time to discharge (or extubation) as the dependent variable. Adjustment will be made for the same variables as for the primary outcome.
2.12. Subgroup analyses
Analyses will be performed on predefined subgroups irrespective of whether there is evidence of a mortality treatment effect. Heterogeneity between subgroups will be determined by fitting an interaction between treatment and subgroup for the primary outcome (90-day in-hospital mortality). The subgroups will be as follows:
-
•
Patients with a respiratory source of sepsis vs. gastrointestinal source vs genitourinary source vs. another source of sepsis.
-
•
Patients admitted to the ICU following surgery vs. those with another ICU admission source.
2.13. Summary
Mega-ROX Sepsis is an approximately phase 3 international, multicentre, randomised two-sided superiority trial designed to test the hypothesis that among adult ICU patients with sepsis who receive unplanned invasive ventilation, liberal oxygen therapy compared to conservative oxygen therapy, reduces in-hospital all-cause mortality up to 90 days from the date of randomisation. This protocol and statistical analysis plan article was submitted for publication before recruitment was completed.
Funding and support
Mega-ROX Sepsis is funded by grants from the Health Research Council of New Zealand (ref 20/084) and by an unrestricted donation from the Alpha Charitable Trust. In Canada, Mega-ROX has received funding from the Pragmatic Trials Platform – Alberta Strategy for Patient-Oriented Research (SPOR) Support Unit. The funding bodies have had no input into the design or conduct of the trial or into the statistical analysis plan and will have no input into analysis or reporting of the results. The study is coordinated in New Zealand by the Medical Research Institute of New Zealand and in Australia by the Australian and New Zealand Intensive Care Research Centre. The study is coordinated in Ireland by the Irish Critical Care Clinical Trials Network, which is supported by the Health Research Board. The study is coordinated in Canada by the University of Alberta. The study is coordinated in Japan by Jikei University. The study is coordinated in Asia by the Critical Care Asia Network and in Africa by the Critical Care Africa Network (parts of the National Intensive Care Surveillance, Mahidol–Oxford Tropical Medicine Research Unit [NICS-MORU] collaboration), which are supported by a Wellcome Innovations grant (215522). This study is endorsed by the Australia and New Zealand Intensive Care Society Clinical Trials Group, the Irish Critical Care Clinical Trials Group, and the Alberta Health Services Critical Care Strategic Clinical Network and approved by the Japanese Intensive Care Research Group.
CRediT authorship contribution statement
Young: Conceptualisation, Methodology, Writing - original draft, Funding acquisition. Al-Fares, Aryal, Arabi, Ashraf, de Oliveira Manoelo, Fujii, Hodgson, Landoni, Maia, Mangal, Mazlan, Nichol, Tirupakuzhi Vijayaraghavan: Writing - review and editing. Beane, Dullawe, Fazla, Haniffa, Hunt, Lawrence, Mackle, Olatunji, A Rashan, S Rashan: Writing - review and editing, Project administration. Bagshaw: Funding acquisition, Writing - review and editing. Kasza: Methodology, Writing - review and editing.
Conflict of interest
None declared.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ccrj.2023.04.008.
Appendix A. Supplementary data
The following is the supplementary data to this article.
References
- 1.Kaukonen K.M., Bailey M., Suzuki S., Pilcher D., Bellomo R. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000-2012. JAMA. 2014;311:1308–1316. doi: 10.1001/jama.2014.2637. [DOI] [PubMed] [Google Scholar]
- 2.Liu V., Escobar G.J., Greene J.D., Soule J., Whippy A., Angus D.C., et al. Hospital deaths in patients with sepsis from 2 independent cohorts. JAMA. 2014;312:90–92. doi: 10.1001/jama.2014.5804. [DOI] [PubMed] [Google Scholar]
- 3.Fleischmann C., Scherag A., Adhikari N.K., Hartog C.S., Tsaganos T., Schlattmann P., et al. Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. Am J Respir Crit Care Med. 2016;193:259–272. doi: 10.1164/rccm.201504-0781OC. [DOI] [PubMed] [Google Scholar]
- 4.Hays R.C., Mandell G.L. PO2, pH, and redox potential of experimental abscesses. Proc Soc Exp Biol Med. 1974;147:29–30. doi: 10.3181/00379727-147-38275. [DOI] [PubMed] [Google Scholar]
- 5.Allen D.B., Maguire J.J., Mahdavian M., Wicke C., Marcocci L., Scheuenstuhl H., et al. Wound hypoxia and acidosis limit neutrophil bacterial killing mechanisms. Arch Surg. 1997;132:991–996. doi: 10.1001/archsurg.1997.01430330057009. [DOI] [PubMed] [Google Scholar]
- 6.Knighton D.R., Hunt T.K., Scheuenstuhl H., Halliday B.J., Werb Z., Banda M.J. Oxygen tension regulates the expression of angiogenesis factor by macrophages. Science. 1983;221:1283–1285. doi: 10.1126/science.6612342. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez P.G., Felix F.N., Woodley D.T., Shim E.K. The role of oxygen in wound healing: a review of the literature. Dermatol Surg. 2008;34:1159–1169. doi: 10.1111/j.1524-4725.2008.34254.x. [DOI] [PubMed] [Google Scholar]
- 8.ICU-ROX investigators and the Australian and New Zealand intensive care society clinical trials group Conservative oxygen therapy during mechanical ventilation in the ICU. N Engl J Med. 2020;382:989–998. doi: 10.1056/NEJMoa1903297. [DOI] [PubMed] [Google Scholar]
- 9.Young P., Mackle D., Bellomo R., Bailey M., Beasley R., Deane A., et al. Conservative oxygen therapy for mechanically ventilated adults with sepsis: a post hoc analysis of data from the intensive care unit randomized trial comparing two approaches to oxygen therapy (ICU-ROX) Intensive Care Med. 2020;46:17–26. doi: 10.1007/s00134-019-05857-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Young P.J., Arabi Y.M., Bagshaw S.M., Bellomo R., Fujii T., Haniffa R., et al. Protocol and statistical analysis plan for the mega randomised registry trial research program comparing conservative versus liberal oxygenation targets in adults receiving unplanned invasive mechanical ventilation in the ICU (Mega-ROX) Crit Care Resus. 2022;24:137–149. doi: 10.51893/2022.2.OA4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Young P.J., Nickson C.P., Perner A. When should clinicians act on non-statistically significant results from clinical trials? JAMA. 2020;323:2256–2257. doi: 10.1001/jama.2020.3508. [DOI] [PubMed] [Google Scholar]
- 12.Singer M., Deutschman C.S., Seymour C.W., Shankar-Hari M., Annane D., Bauer M., et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schulz K.F., Altman D.G., Moher D., Group C. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c332. doi: 10.1136/bmj.c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.