Abstract
Objective
There is a need for evidence on the best sedative agents in children undergoing open heart surgery for congenital heart disease. This study aimed to evaluate the feasibility and safety of dexmedetomidine in this group compared with midazolam.
Design
Double blinded, pilot randomized controlled trial.
Setting
Cardiac operating theatre and paediatric intensive care unit in Brisbane, Australia.
Participants
Infants (≤12 months of age) undergoing their first surgical repair of a congenital heart defect.
Interventions
Dexmedetomidine (up to 1.0mcg/kg/hr) versus midazolam (up to 80mcg/kg/hr), commenced in the cardiac operating theatre prior to surgery.
Main outcome measures
The primary outcome was the time spent in light sedation (Sedation Behavior Scale [SBS] -1 to +1); Co-primary feasibility outcome was recruitment, retention and protocol adherence. Secondary outcomes were use of supplemental sedatives, ventilator free days, delirium, vasoactive drug support, and adverse events. Neurodevelopment and health-related quality of life (HRQoL) were assessed at 12 months post-surgery.
Results
Sixty-six participants were recruited. The number of SBS scores in the light sedation range were greater in the dexmedetomidine group at 24 hours, 48 hours, and overall study duration (0-14 days) versus the midazolam group (24hr: 76/170 [45%] vs 60/178 [34%], aOR 4.14 [95% CI 0.48, 35.92]; 48hr: 154/298 [52%] vs 122/314 [39%], aOR 6.95 [95% CI 0.77, 63.13]; 0-14 days: 597/831 [72%] vs 527/939 [56%], aOR 3.93 [95% CI 0.62, 25.03]). Feasibility was established with no withdrawals or loss to follow-up at 14 days and minimal protocol deviations. There were no differences between the groups relating to clinical, safety, neurodevelopment or HRQoL outcomes.
Conclusions
The use of dexmedetomidine was associated with more time spent in light sedation when compared with midazolam. The feasibility of conducting a blinded RCT of midazolam and dexmedetomidine in children undergoing open heart surgery was also established. The findings justify further investigation in a larger trial.
Clinical trial registration
ACTRN12615001304527.
Keywords: Anaesthesia and intensive care, Cardiology and cardiac surgery, Paediatrics, Sedation
1. Introduction
Every year, approximately 1.35 million infants are diagnosed with congenital heart disease (CHD) worldwide.1 In Australia and New Zealand, 1500 cases of cardiac surgery requiring cardiopulmonary bypass (CPB) are performed in children every year.2 These children, all of whom are admitted to paediatric intensive care units (PICUs) postoperatively, are at a risk of significant postsurgery complications and sequelae. The physiological strain induced by surgery and CPB is followed by endothelial injury due to an inflammatory response and coagulopathy, which can lead to delayed convalescence and increased postoperative morbidity and mortality.3
Sedation is commonly used to manage the postoperative phase; however, the historical approach to sedation during mechanical ventilation in children after surgery for CHD lacks standardisation, with midazolam still being the most widely used sedative agent.4,5 Recent guidelines on the prevention of agitation in critically ill children recommended that dexmedetomidine should be considered as a primary sedative for sedation in postoperative cardiac surgical patients.6 However, evidence used for these guidelines is mostly retrospective in nature or based on small clinical trials that do not directly compare dexmedetomidine with midazolam. Despite its ongoing use, midazolam usage in children has also been shown to have a negative impact on the developing brain.7,8 In the recent years, dexmedetomidine, an alpha-2 adrenergic sedative with anxiolytic and analgesic characteristics, has been successfully used.9,10 Emerging animal model data also suggest that dexmedetomidine may have neuroprotective effects.11,12 A recent meta-analysis of dexmedetomidine in paediatric cardiac surgery suggests that use of dexmedetomidine during the perioperative phase may shorten the duration of mechanical ventilation and PICU and hospital length of stay; 13 however, none of the studies included the use of midazolam in the control group, with most controls using placebo.
In view of the limited available high-quality evidence, there is an urgent need to further determine the short- and long-term safety and feasibility of dexmedetomidine for sedation in paediatric cardiac surgery. The aim of this pilot randomised clinical trial (RCT) was to determine the feasibility of a definitive RCT evaluating dexmedetomidine compared to midazolam for sedation after paediatric cardiac surgery with CPB. We hypothesised that conducting a pragmatic blinded RCT of dexmedetomidine and midazolam is feasible in the CHD cohort.
2. Materials and methods
2.1. Study design
The study design incorporated a single-centre, double-blind, pilot RCT in infants requiring cardiac surgery to correct CHD. The trial was prospectively registered with the Australian New Zealand Clinical Trials Registry (ACTRN12615001304527), and the protocol at commencement of the study is provided in Appendix 1. Approval to undertake the trial was received from the Children's Health Services (HREC/15/QRCH/119) Human Research Ethics Committee. This trial is reported according to the CONSORT 2010 statement: extension to randomised pilot and feasibility trials.14
2.2. Study setting
The trial was conducted within the cardiac operating theatre and PICU at the Queensland Children's Hospital, Australia. The centre incorporates a quaternary level 36-bed PICU and over 2000 medical and surgical admissions annually, of which 700 are cardiac surgical procedures.2
2.3. Study participants
2.3.1. Inclusion criteria
Infants (≤12 m of age) undergoing their first surgical repair of a congenital heart defect were eligible for enrolment if (i) the surgical repair required CPB; (ii) the patient required mechanical ventilation via an endotracheal tube postoperatively; and (iii) the patient required immediate postoperative and ongoing sedative medication for comfort and safety and to facilitate the delivery of life-support measures.
2.3.2. Exclusion criteria
Patients with a documented allergy to dexmedetomidine were ineligible for trial enrolment.
Variable block size randomisation (4,6,8) was generated on a 1:1 ratio for the study groups and loaded into a password-protected, encrypted, web-based interface (Griffith University: http://151.griffith.edu.au/random). Patients, clinical staff members, and investigators were blinded to the group allocation.
2.4. Interventions
Clinical research nurses daily screened all patients who were booked for PICU admission after a cardiac surgical repair, proceeding to obtain written informed consent from parents or legal guardians if the child was eligible. Parents were approached about the study in their child's planned presurgical consultation or via phone and email, and all consents were signed prior to surgery.
Following randomisation, a research nurse not providing clinical care to the participant prepared the blinded study drug syringe according to a predetermined guideline and delivered the syringe to the cardiac anaesthetist prior to surgery or thereafter to the PICU. Concentrations of each study drug were prepared as detailed in the following and labelled as ‘Cardiac Baby SPICE Study Drug’. The study drug was administered via syringe driver in an mL/h delivery mode.
Participants were randomised to receive one of two primary sedative agents:
Intervention: A dexmedetomidine intravenous infusion (concentration, 200mcg in 50 mL of either 5% glucose or 0.9% sodium chloride) was administered in the operating room as the primary sedative agent (blinded to the operator) and titrated at a dose range of 0.25–1.0 mcg/kg/h to achieve the target sedation up to an age-dependent maximum dose.15,16 Clonidine was prohibited as a sedative agent, with its use reserved only for management of suspected dexmedetomidine withdrawal (after cessation for 96 h). Benzodiazepine use was permitted only for rescue treatment of refractory agitation.
Standard care: A midazolam intravenous infusion (concentration, 16 mg midazolam in 50 mL of either 5% glucose or 0.9% sodium chloride) was administered in the operating room as the primary sedative agent (blinded to the operator) and titrated at a dose range of 20–80 mcg/kg/h. Dexmedetomidine could be used at the discretion of the treating intensivist if the target sedation range was not achieved despite maximum titration of conventional sedative agents (except when clonidine had been used).
The default sedation target for all patients (unless otherwise clinically indicated) was light sedation, defined by the state Behavioural scale (SBS)17 range of −1 to +1. Treating clinicians were provided with a weight-adjusted study drug calculation sheet which outlined volume–dose equivalent rates for both study arms (Appendix 1). Titration of the blinded study drug was according to an established study algorithm,4 adapted for use in this study (Appendix 2). To optimise blinding, clinicians titrated each study drug in mL/h in each arm and every increase in 0.625 ml/h resulted in an increase of 0.25 mcg/kg/h of dexmedetomidine or 20 mcg/kg/h of midazolam.
Loading doses or bolus infusions of the study drug were prohibited. Postoperatively, if the primary sedative agent was titrated to the maximum dose and additional sedative agents were required, additional agents including chloral hydrate, sedating antihistamines, phenobarbital, propofol (maximum dose 4 mg/kg/h, maximum duration 24 h), or ketamine were considered, based on the treating physician’s preference. Blinded dexmedetomidine or midazolam infusions were continued until sedation was no longer required or to a maximum of 14 d after enrolment. If sedation was necessary beyond 14 d after enrolment, the choice of sedative regimen and weaning plan was at the discretion of the treating clinician. Study drug cessation was at the discretion of the treating clinician if they considered that the patient safety was compromised by administration of the blinded study drug.
2.5. Perioperative and concurrent treatments
Induction and maintenance of anaesthesia was performed as per local policy and standard site practice for cardiac surgery on CPB. The study drug was started prior to commencement of CPB at a rate equivalent to 1.0 mcg/kg/h of dexmedetomidine or 80 mcg/kg/h of midazolam. During PICU care, if additional sedative agents were required, open-label intravenous midazolam rescue boluses of 0.05–0.1 mg/kg were allowed in either treatment arm. Clonidine was prohibited as a sedative agent in both arms. The provision of pain relief for all children (e.g., morphine, fentanyl, ketamine, paracetamol, or remifentanil) was provided by an infusion or opioid bolus at the discretion of the treating clinician. Patients (in either arm) who required neuromuscular blockade postoperatively (intermittent or continuous) received adequate sedation by keeping the study drug at maximum clinically tolerable dose and use of additional sedatives to achieve an SBS17 of −2 to −3 to prevent awareness during neuromuscular blockade. Once there was no need for further neuromuscular blockade, sedative management continued as per protocol.
All patients had a sedation target score charted in their electronic medical record, as determined by the treating clinician, and reviewed at least 12 hourly. Sedation infusions were titrated to the lowest dose needed to achieve the desired sedation. All patients had SBS assessment every 4 h and on demand, along with pain assessments (by bedside nurse). Withdrawal was assessed daily using the Withdrawal Assessment Tool 18 after 72 h following randomisation or during sedative weaning. Daily delirium assessments were conducted if SBS ≥−1, using the Cornell Assessment of Pediatric Delirium.19
2.6. Intervention fidelity
To promote intervention fidelity and protocol adherence, a comprehensive education package was delivered to the relevant clinical staff. Prior to commencement, we ensured that 80% of nursing staff had completed online education TEACHQ is the Children's Health Qld Learning Management Platform (TEACHQ) and had the opportunity for clinical feedback from a nurse educator. All sedation, delirium, and withdrawal assessments were used as a part of standard clinical care. Protocol violation was defined as ‘the randomised intervention was never performed’. Protocol deviation was defined as ‘the incorrect intervention was used for a portion of the study enrolment’.20
2.7. Outcome measures
The study included primary sedation and feasibility outcomes. The sedation outcome assessed was the proportion of time spent in light sedation, defined as time spent in the light sedation range (−1 to +1) of the SBS during study enrolment. Feasibility outcomes comprised eligibility, enrolment, acute retention and attrition at 12 m, protocol adherence, and missing data. End points of the secondary outcomes were clinical outcome, daily cumulative weight-adjusted doses of intravenous sedative agents and opioids given, incidence of delirium assessed using the Cornell Assessment of Pediatric Delirium, incidence of withdrawal using the Withdrawal Assessment Tool, duration of PICU and hospital length of stay, and PICU and hospital mortality. Ventilator-free days were defined as the measurement period (d) that the patient was both alive and free of invasive mechanical ventilation, censored at 14 d; death within the study period was weighted as 0. Other outcomes included neurodevelopmental outcomes at 12 m, as measured by the Ages and Stages Questionnaire (ASQ)21 and health-related quality of life, as measured by the Pediatric Quality of Life Inventory (PedsQL).22
Safety outcomes included incidence of cardiac arrhythmias, bradycardia, and/or hypotension, defined as heart rate or blood pressure more than two standard deviations (SDs) below the mean for age, which led to study drug cessation; cumulative and daily dose of vasopressor/inotropic infusions; and adverse events including unplanned extubation, loss of invasive lines (e.g., vascular access devices), and cardiac arrest. Primary and secondary outcome definitions are provided in Appendix 3. Safety outcomes were reviewed and verified daily via the clinical information system (MetaVision).
2.8. Study procedures
Perioperative and PICU data were collected by a member of the research team from the clinical information system (CIS: MetaVision) and entered into the electronic data platform REDCap (Research Electronic Data CAPture) (http://project-redcap.org/).23 This included data on outcomes and clinical and demographic factors including diagnosis, surgical intervention, anaesthetic data, and PICU assessment medical management. At 12 m after the surgery, child survival status was checked prior to ringing the parents about completion of the ASQ and PedsQL questionnaires.
2.9. Statistical analysis
Data were analysed using StataSE v17.0 (StataCorp Pty Ltd, College Station, Texas). Descriptive statistics were used to report patient characteristics; amount of light and deep sedation; the sedative, analgesic, and vasopressor used; and clinical outcomes. The patient was the unit of analysis, apart from SBS assessments, where each individual assessment was the unit of analysis. All analyses were conducted on an intention-to-treat basis. End points were compared between groups using logistic regression (binary outcomes; reporting odds ratio), quantile regression (non-normally distributed continuous outcomes; reporting estimate of difference), and linear regression (normally distributed outcomes; reporting the estimate of difference), along with 95% confidence intervals (CIs). For the primary outcome, the odds of achieving light sedation at various timepoints, adjusted for repeated patient observations and time of SBS assessment, are reported. Repeated SBS assessments were analysed using multilevel modelling, accounting for both the patient as well as the timepoint of the assessment. All comparisons are considered exploratory, and no adjustment for multiple comparisons was undertaken.
A pragmatic sample size of 60 infants was required with an attrition rate of 10% (total n = 66), based on recommendations for pilot studies.24 This would also be sufficient to provide enough sedation assessments to allow meaningful evaluation of feasibility and safety.4
2.10. Ethical considerations
This study was approved by the Children's Health Queensland Hospital and Health Services HREC and undertaken according to ICH-GCP, Declaration of Helsinki. Consent was obtained from the legal guardian before participation.
3. Results
3.1. Study participants
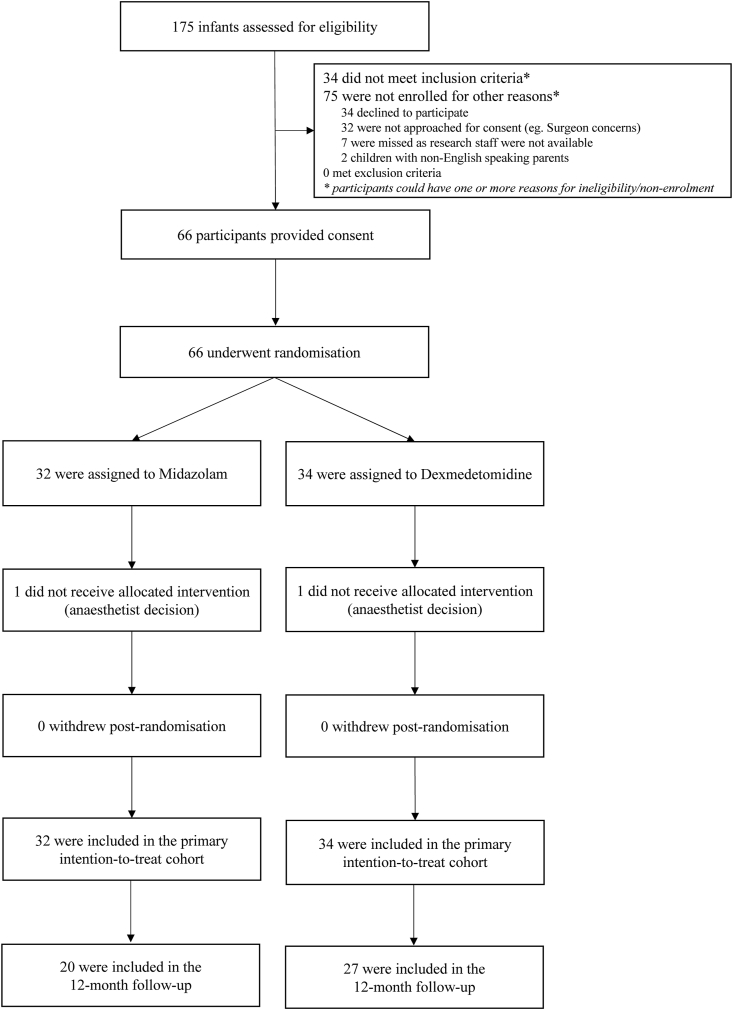
Between March 2017 and September 2018, 175 patients were screened for eligibility, of which 139 were eligible and 66 (47.5%) participants were randomised into the study: 34 to the dexmedetomidine arm and 32 into the midazolam arm. In each arm, one patient did not receive the intervention but was included in the primary analysis. Participant flow through the study is outlined in Fig. 1. At the beginning of recruitment, 32 children were missed due to initial staff concerns with a blinded sedative. After a period of recruiting children in lower Risk Adjustment for Congenital Heart Surgery (RACHS) categories and no demonstration of safety concerns, the recruitment rate increased.
Fig. 1.
Flow of participants through the study.
Participant baseline characteristics are outlined in Table 1. Most patient characteristics and clinical features were evenly distributed throughout the research groups, whereas several exhibited a higher than 10% imbalance (e.g., type of surgical repair). The median number of SBS assessments per patient (dexmedetomidine: 15, interquartile range [IQR]: 6–41; midazolam: 17.5, IQR: 8.5–47.5) and median duration of assessments (dexmedetomidine: 79 h, IQR: 31–169; midazolam: 84 h, IQR: 34–180) were similar between the groups.
Table 1.
Patient characteristics.
| Patient characteristics | Midazolam sedation, N = 32 |
Dexmedetomidine sedation, N = 34 |
|---|---|---|
| Age (m), median (IQR) | 2.5 (0.3, 5.5) | 3.0 (1.0, 8.0) |
| Male, n (%) | 20 (63) | 17 (50) |
| Weight (kg), median (IQR) | 4.6 (3.6, 5.7) | 5.3 (3.6, 6.5) |
| Gestational age (weeks), median (IQR) | 38 (36, 39) | 38 (36, 40) |
| Surgical repair, n (%) | ||
| ASD/VSD | 10 (31) | 13 (38) |
| ASO | 7 (22) | 0 (0) |
| TOF repair | 6 (19) | 9 (26) |
| Other | 9 (28) | 12 (35) |
| Ventilated preop, n (%) | 1 (3) | 1 (3) |
| RACHS, n (%) | ||
| 1 | 1 (3) | 0 (0) |
| 2 | 15 (47) | 21 (62) |
| 3 | 11 (34) | 6 (18) |
| 4 | 3 (9) | 2 (9) |
| 6 | 2 (6) | 5 (15) |
| Aristotle, median (IQR) | 10.0 (7.0, 11.8) | 9.7 (8.0, 12.0) |
| Cardiac pathophysiology, n (%) | ||
| Univentricular | 1 (3) | 5 (15) |
| Biventricular | 31 (97) | 29 (85) |
| PIM 3, median (IQR) | 0.4 (0.3, 0.6) | 0.4 (0.3, 0.6) |
| Baseline ASQ-3 subscalesa | ||
| Communication below cut-off, n (%) | 5 (24) | 7 (24) |
| Gross motor below cut-off, n (%) | 10 (48) | 16 (55) |
| Fine motor below cut-off, n (%) | 4 (19) | 6 (21) |
| Problem solving below cut-off, n (%) | 6 (29) | 9 (31) |
| Personal–social below cut-off, n (%) | 4 (19) | 11 (38) |
IQR = interquartile range; kg = kilograms; RACHS = Risk Adjustment for Congenital Heart Surgery; PIM = Paediatric Index of Mortality; ASD = atrial septum defect; VSD = ventricular septum defect; ASO = arterial switch operation; TOF = tetralogy of Fallot.
Does not include infants <1 month of age.
3.1.1. Primary feasibility outcome
Following randomisation, no patients withdrew from the study or were lost to follow-up for hospital data (or similar). A protocol violation occurred in two participants (1 in the midazolam arm; 1 in the dexmedetomidine arm) who did not receive the randomised sedative agent, based on the treating anaesthetist's decision to not proceed with the blinded study drug due to perioperative instability (both received open-label dexmedetomidine). Sixteen patients experienced a protocol deviation, with one patient experiencing two protocol deviations (incorrect intervention used for a portion of study enrolment) (Appendix 4). Twenty-nine patients were lost to follow-up at 12 m.
3.1.2. Primary sedation outcome
For the entire study duration, the number of SBS measurements in the light sedation range in the dexmedetomidine group was 597 of 831 (71.8%) compared to 527 of 939 (56.1%) in the midazolam arm (adjusted OR: 3.93, 95% CI: 0.62, 25.03) (Table 2).
Table 2.
Comparison of SBS assessments in the light and deep sedation range in the first 24 h, 48 h after surgery, and across the study duration (0–14 d) between the study groups.
| Light/deep sedation | Midazolam sedation |
Dexmedetomidine sedation |
Intervention |
|
|---|---|---|---|---|
| n (%) | n (%) | aORa | 95% CIa | |
| First 24 h | N = 178 | N = 170 | ||
| Light sedation (−1 to +1) | 60 (33.7%) | 76 (44.7%) | 4.14 | 0.48, 35.92 |
| Deep sedation (−2 to −3)b | 118 (66.3%) | 94 (55.3%) | ||
| First 48 h | N = 314 | N = 298 | ||
| Light sedation (−1 to +1) | 122 (38.9%) | 154 (51.7%) | 6.95 | 0.77, 63.13 |
| Deep sedation (−2 to −3)b | 192 (61.2%) | 144 (48.3%) | ||
| Study duration (0–14 days) | N = 939 | N = 831 | ||
| Light sedation (−1 to +1) | 527 (56.1%) | 597 (71.8%) | 3.93 | 0.62, 25.03 |
| Deep sedation (−2 to −3)b | 412 (43.9%) | 234 (28.2%) | ||
aOR = adjusted odds ratio; CI = confidence interval; IQR = interquartile range; SBS = state behavioural scale.
adjusted for repeated patient observations and time of SBS assessment.
deep sedation includes paralysis with neuromuscular blockade.
3.1.3. Clinical and safety outcomes
There were minimal differences in the cumulative dosages of other intravenous sedatives or opioids administered during the trial period (Table 3). Morphine was the most used opiate paired with the study drug, with the median dose in the dexmedetomidine group at 1459.2 mcg/kg (IQR: 711.2–3866.7) compared to 2258.1 mcg/kg in the midazolam group (IQR: 1197.8–4896.2).
Table 3.
Cumulative dose/kg and duration of treatment with sedatives and analgesic agents throughout the study period.
| Treatmenta | Midazolam sedation, N = 32 |
Dexmedetomidine sedation, N = 34 |
||||
|---|---|---|---|---|---|---|
| n (%) | Median | IQR | n (%) | Median | IQR | |
| Operating room | ||||||
| Dexmedetomidine (mcg/kg) | 2 (6) | 2.8 | 2.1, 3.7 | 34 (100) | 3.8 | 2.3, 3.8 |
| Midazolam (mcg/kg) | 5 (16) | 161.3 | 142.9, 188.7 | 3 (9) | 156.3 | 117.0, 156.3 |
| Midazolam – rescue sedation, open label (mcg/kg) | – | – | – | – | – | – |
| Morphine (mcg/kg) | 27 (84) | 100.0 | 60.3, 141.7 | 32 (94) | 64.7 | 36.4, 120.3 |
| Fentanyl (mcg/kg) | 28 (87) | 38.1 | 24.5, 51.3 | 34 (100) | 33.3 | 19.5, 50.0 |
| Ketamine (mcg/kg) | – | – | – | 1 (3) | 2500 | – |
| Paediatric intensive care unit | ||||||
| Dexmedetomidine (mcg/kg) | 7 (22) | 4.2 | 1.7, 19.0 | 34 (100) | 14.0 | 6.7, 38.2 |
| Midazolam (mcg/kg) | 31 (97) | 2082.9 | 988.3, 6489.8 | 10 (29) | 892.2 | 354.6, 1435.0 |
| Midazolam – rescue sedation, open label (mcg/kg) | 8 (25) | 100.0 | 74.5, 177.9 | 6 (18) | 123.3 | 74.4, 200.0 |
| Morphine (mcg/kg) | 32 (100) | 2148.6 | 1164.9, 4854.2 | 33 (97) | 1309.2 | 595.8, 3796.9 |
| Fentanyl (mcg/kg) | 25 (78) | 13.2 | 8.5, 44.4 | 22 (65) | 9.4 | 4.0, 45.4 |
| Chloral hydrate (mg/day) | 12 (38) | 57.1 | 15.3, 110.2 | 15 (44) | 30.7 | 12.0, 96.2 |
| Ketamine (mcg/kg) | 18 (56) | 759.6 | 500, 2857.1 | 12 (35) | 721.0 | 500, 1909.7 |
| Combined (operating room and paediatric intensive care unit) | ||||||
| Dexmedetomidine (mcg/kg) | 8 (25) | 3.4 | 1.9, 18.1 | 34 (100) | 16.2 | 9.5, 41.9 |
| Days on dexmedetomidine | 1.0 | 1.0, 2.5 | 2.0 | 2.0, 4.0 | ||
| Midazolam (mcg/kg) | 31 (97) | 2503.2 | 1160.6, 6760.5 | 13 (38) | 720.0 | 203.6, 1260.7 |
| Days on midazolam | 2.0 | 2.0, 5.0 | 2.0 | 1.0, 3.0 | ||
| Midazolam – rescue sedation, open label (mcg/kg) | 8 (25) | 100.0 | 74.5, 177.9 | 6 (18) | 123.3 | 74.4, 200.0 |
| Days on midazolam – open label | 1.0 | 1.0, 1.5 | 1.0 | 1.0–2.0 | ||
| Morphine (mcg/kg) | 32 (100) | 2258.1 | 1197.8, 4896.2 | 33 (97) | 1459.2 | 711.2, 3866.7 |
| Days on morphine | 4.0 | 3.0, 7.0 | 4.0 | 2.0, 5.0 | ||
| Fentanyl (mcg/kg) | 30 (94) | 45.8 | 38.9, 73.6 | 34 (100) | 44.6 | 24.5, 72.8 |
| Days on fentanyl | 2.0 | 1.0, 4.0 | 1.5 | 1.0, 3.0 | ||
| Chloral hydrate (mg/d) | 12 (38) | 57.1 | 15.3, 110.2 | 15 (44) | 30.7 | 12.0, 96.2 |
| Days on chloral hydrate | 2.5 | 1.5, 4.0 | 2.0 | 1.0, 3.0 | ||
| Ketamine (mcg/kg) | 18 (56) | 759.6 | 500.0, 2857.1 | 13 (38) | 937.5 | 500.0, 1943.4 |
| Days on ketamine | 1.0 | 1.0, 2.0 | 1.0 | 1.0, 1.0 | ||
IQR = interquartile range; mcg = micrograms; kg = kilograms.
Includes bolus doses, where allowed.
Total CPB times were shorter in the dexmedetomidine group (68.5 min, IQR: 52.4–83.0), compared to those receiving midazolam (120.5 min, IQR: 66.0–161.5 [−55 min, 95% CI: -85.4, −24.6]) (Table 4). The median time to achieve light sedation was 9.8 h (IQR: 3.6–32.6) in the dexmedetomidine group compared to 16.1 h (IQR: 6.2–56.8) in the midazolam group (−6.7, 95% CI: -26.5, 13.1). Median time to extubation was 21.1 h in the dexmedetomidine group compared to 37.9 h in the midazolam group (IQR: 12.2–116.6) (IQR: 7.0–66.2; difference: −21.3, 95% CI: -62.5, 19.9). Four children (13%) in the midazolam group died in hospital (RACHS scores: 3, 3, 4, 6), compared with none in the dexmedetomidine group. There was variation in the amount of vasopressors administered across groups; however, withdrawal symptoms were not reported in either group. Overall, five participants in each arm received clonidine >96 h following cessation of the study drug, with more clonidine administered in the dexmedetomidine group (median: 18.2 mcg/kg, IQR: 7–19.3) than in the midazolam group (median: 2.6 mcg/kg, IQR: 2.1–7.6).
Table 4.
Secondary outcomes.
| Outcome | Midazolam sedation, N = 32 |
Dexmedetomidine sedation, N = 34 |
Estimate of difference (95% CI) |
|---|---|---|---|
| Efficacy | |||
| Intubated >24 h post surgery, n (%) | 17 (53) | 15 (44) | 0.70 (0.26, 1.84)a |
| CPB time (min), median (IQR) | 120.5 (66.0, 161.5) | 68.5 (52.4, 83.0) | −55.0 (−85.4, −24.6)b |
| Cross clamp time (min), median (IQR) | 81.0 (46.0, 112.0) | 53.0 (36.0, 64.0) | −28.0 (−51.9, −4.1)b |
| MUF time (min), median (IQR) | 12.0 (9.0, 14.0) | 9.0 (8.0, 12.0) | −3.0 (−5.3, −0.7)b |
| Time to first light sedation, median (IQR) (h) | 16.1 (6.2, 56.8) | 9.8 (3.6, 32.6) | −6.7 (−26.5, 13.1)b |
| CAP-D + ve, n (%) | 27 (93) | 24 (89) | 0.59 (0.09, 3.85)a |
| Delirium coma-free days, median (IQR) | 11.0 (9.0, 12.5) | 12 (9.0, 13.0) | 1.0 (−0.8, 2.8)b |
| Time to extubation after surgery (hours), median (IQR) | 37.9 (12.2, 116.6) | 21.1 (7.0, 66.2) | −21.3 (−62.5, 19.9)b |
| Ventilator-free days, at day 14, median (IQR) | 12.5 (9.0, 13.5) | 13.0 (11.0, 14.0) | 1.0 (−0.8, 2.8)b |
| PICU length of stay (h), median (IQR) | 81.1 (46.9, 253.4) | 73.2 (38.4, 190.3) | −16.9 (−104.3, 70.5)b |
| Hospital LOS (d), median (IQR) | 17.6 (8.5, 34.0) | 12.6 (7.3, 34.2) | −5.1 (−16.6, 6.5)b |
| Neuromuscular blockade medication use, n (%) | 17 (53) | 15 (44) | 0.70 (0.26, 1.84)a |
| Clonidine given >96 h post study drug cease, n (%) | 5 (16) | 5 (15) | 0.93 (0.24, 3.58)a |
| Clonidine >96 h after study drug cease (mcg/kg), median (IQR) | 2.6 (2.1, 7.6) | 18.2 (7.0, 19.3) | 15.55 (3.56, 27.54)b |
| Clonidine duration >96 h post study drug cease, median (IQR) | 2 (1, 4) | 4 (3, 5) | 2.00 (−1.23, 5.23)b |
| Safety | |||
| Bradycardia, n (%) | 1 (3) | 1 (3) | 0.94 (0.06, 15.68)a |
| Hypotension, n (%) | 1 (3) | 3 (9) | 3.00 (0.30, 30.44)a |
| Arrhythmias in OR, n (%) | 4 (13) | 2 (6) | 0.44 (0.07, 2.57)a |
| Arrhythmias post surgery, n (%) | 9 (28) | 11 (32) | 1.22 (0.43, 3.50)a |
| SAE, n (%) | 2 (6) | 2 (6) | 0.94 (0.12, 7.08)a |
| ECLS, n (%) | 2 (6) | 1 (3) | 0.45 (0.04, 5.27)a |
| RRT, n (%) | 2 (6) | 2 (6) | 0.94 (0.12, 7.08)a |
| PICU mortality, n (%) | 2 (6) | 0 (0) | – |
| Hospital mortality, n (%) | 4 (13) | 0 (0) | – |
H = hours; Min = minutes; IQR = interquartile range; CPB = cardiopulmonary bypass; CAP-D = Cornell Assessment of Pediatric Delirium; PICU = paediatric intensive care unit; LOS = length of stay; SAE = serious adverse event; ECLS = extracorporeal life support; RRT = renal replacement therapy.
odds ratio reported.
coefficient reported.
Across the study period, there was no difference in the incidence of cardiac arrhythmias, bradycardia, and hypotension between study arms intraoperatively and postoperatively. There were two serious adverse events identified in each arm, which were not considered to be related to the study drugs, including emergency chest reopening for bleeding, intraoperative gastrointestinal injury, sepsis, and pericardial effusion.
Appendix 5 shows the cumulative doses of vasopressors used during the study period. In the PICU, children in the dexmedetomidine group received a cumulative dose of milrinone (median: 1533 mcg/kg, IQR: 583–3212) compared to children sedated with midazolam (median: 2703 mcg/kg, IQR: 865 to 4835). No other differences in vasopressor use were identified between groups across the study period. At 12 m post surgery, 77% of patients’ parents completed outcome questionnaires (n = 47). There was a higher proportion of children in the dexmedetomidine group who fell below the cut-off (>2 SD below mean) in the fine motor domain of the ASQ, adjusting for the baseline ASQ (41% vs 5%, difference: 10.32 [1.14, 93.22]). There were no differences between the groups in the remaining ASQ domains. There were no differences in the total or subscale PedsQL z-scores between the two treatment groups (Table 5).
Table 5.
Association between sedation and morbidity at 12 m.
| Outcome | Midazolam sedation |
Dexmedetomidine sedation | Estimate of difference (95% CI) |
|---|---|---|---|
| ASQ-3 Subscalea | N = 20 | N = 27 | |
| Communication below cut-off, n (%) | 6 (30) | 8 (30) | 1.07 (0.27, 4.25) |
| Gross motor below cut-off, n (%) | 9 (45) | 12 (45) | 0.77 (0.18, 3.37) |
| Fine motor below cut-off, n (%) | 1 (5) | 11 (41) | 10.32 (1.14, 93.22) |
| Problem-solving below cut-off, n (%) | 3 (15) | 7 (26) | 1.60 (0.33, 7.69) |
| Personal–social below cut-off, n (%) | 3 (15) | 6 (22) | 1.00 (0.19, 5.38) |
| PedsQL z-Scoreb | N = 19 | N = 26 | |
| Total, mean (SD)b | −1.5 (2.2) | −1.4 (1.7) | 0.1 (−1.1, 1.2) |
| Physical subscale, mean (SD)b | −1.2 (2.2) | −1.4 (2.4) | −0.2 (−1.6, 1.2) |
| Psychosocial subscale, mean (SD)b | −1.4 (2.1) | −1.2 (1.2) | 0.2 (−0.8, 1.2) |
ASQ-3 = Ages and Stages Questionnaire, 3rd Edition; CI = confidence interval; PedsQL = Pediatric Quality of Life Inventory; SD = standard deviation.
odds ratio reported; model adjusted for subscale ASQ at baseline.
coefficient reported.
4. Discussion
The feasibility of the proposed double-blinded trial design comparing dexmedetomidine to standard care was demonstrated in this pilot study. Our overall recruitment rate was lower than expected, mainly due to the blinded nature of the trial. This was managed by gaining staff buy-in by recruiting children in lower RACHS categories and increasing the recruitment rate once there was no demonstration of safety concerns. In addition, to aid blinding and administration of the study drugs, significant education and resources were provided to all the treating clinicians, including the use of a previously developed PICU sedation algorithm. The feasibility of the project was thus impacted by increasing staff education and gaining staff trust and buy-in. The lack of safety concerns in our pilot trial could lead to improved recruitment in future trials. No patients were lost to follow-up, and missing data were less than 5% at 14 d. Our follow-up rate at 12 m following surgery was consistent with other follow-up studies in the PICU, so it may be comparable to other studies of children with CHD in this age range.25 Whilst the median gestational age was 38 weeks, there were several infants who were not able to have their baseline ASQ recorded due to corrected age on PICU admission being <1 month. This makes interpretation difficult as often these infants are those undergoing higher risk surgeries, with a theoretical higher risk of neurodevelopmental impairment. As a brief online screener for neurodevelopment however, administration of the parent-completed questionnaire via email was easily administered and well received by caregivers.
Dexmedetomidine could be safely administered throughout open-heart surgery and during PICU admission. There were no observed differences in safety outcomes, including cardiac arrhythmias, bradycardia, or hypotension. There were two serious adverse events identified in each arm, which were not considered to be related to the study drugs. Many studies have documented the increased incidence of bradycardia and hypotension with dexmedetomidine use, with rates up to 26%.26 A recent observational study also demonstrated that a loading dose and doses greater than 1.2 mcg/kg/h of dexmedetomidine increased the odds of haemodynamic changes.27 Although we did experience some episodes of bradycardia and hypotension across the two groups, we only used doses up to 1.0 mcg/kg/h of dexmedetomidine and only a few required interventions (dose reduction or temporary cessation). Furthermore, our protocol did not allow boluses of dexmedetomidine on commencement in the operation theatre or whilst in the PICU. Despite this, we acknowledge that bradycardia and hypotension are managed by the administration of fluids, blood products, and vasopressors and may have been used to correct symptoms <2 SD below the mean. Therefore, the true incidence of bradycardia and hypotension in this cohort may have been masked by the concurrent use of these therapies, and future studies should monitor administration of fluids and blood products use closely. Further due to the use of various vasoactive drugs, calculation of vasoactive inotrope scores would be a useful addition to future trials. Additionally, although we did not observe an incidence of withdrawal in our study, clonidine was used in both groups >96 h after study cessation, and the dose of clonidine used was higher in the dexmedetomidine group. Dexmedetomidine has been reported to be associated with rebound effects in 0–27% of patients; 26 therefore, a strong safety focus needs to remain on withdrawal assessment and management.
Children who underwent surgery for CHD and received dexmedetomidine achieved a higher proportion of time spent in light sedation during their first 14 d following surgery than those treated with midazolam. Time taken to achieve light sedation was also shorter in patients treated with dexmedetomidine. Children in the dexmedetomidine arm also received less morphine and shorter time to extubation than children who received midazolam. Previous studies have evaluated levels of sedation between drugs; however, it is difficult to draw comparisons as various control arms and sedation assessment tools were used.[28], [29], [30] A recent study demonstrated the ability to achieve light sedation quicker with dexmedetomidine in a general PICU cohort 4 and however found that one-third of the dexmedetomidine group also required additional sedatives to achieve light sedation.31 In the current study, we did not observe an increase in the use of additional sedatives in the dexmedetomidine group.
The morphine-sparing properties of dexmedetomidine have been reported in other paediatric surgery studies32,33 and congenital heart surgeries34 alike. Historically, high-dose opioid agents have been used to attenuate the significant neuroendocrine stress response experienced during and after surgery. However, there is mounting evidence to suggest that reducing opioid exposure during the postoperative period could enhance postoperative recovery by circumventing prolonged sedation and mechanical ventilation.35,36 Despite dexmedetomidine and midazolam having different pharmacokinetic and pharmacodynamic profiles, the off-label use of dexmedetomidine has emerged as an encouraging adjunct or alternative to opioids in the perioperative and postoperative setting for children undergoing cardiac surgery. As a strong sympatholytic agent, dexmedetomidine also attenuates the neuroendocrine response and the need for morphine. The analgesic properties of dexmedetomidine are unique compared to other non-opioid-sparing sedative agents and make it particularly useful as an opioid-sparing agent. Given that a few studies have also shown that increasing opiate doses are related to poorer neurodevelopmental outcome, this could be a potentially meaningful candidate for the primary outcome in a larger definitive trial.25,37 A previous meta-analysis of clinical trials examining dexmedetomidine in paediatric cardiac surgery found that dexmedetomidine significantly reduced the postoperative duration of mechanical ventilation and PICU length of stay; 13 however, none of the studies used midazolam as the comparison and most infusions did not continue into the PICU. In an open-label randomised trial, Garisto et al. compared low-dose dexmedetomidine and half-dose opioids and benzodiazepines to standard-dose opioids and benzodiazepines and found no difference in mechanical ventilation time.38 Given that dexmedetomidine causes sedation without severe respiratory depression and is linked to lower opioid usage, this may lead to reduced mechanical ventilation duration but only when benzodiazepines are not used in conjunction.
In this study, we found that the proportion of children who scored below the cut-off in the fine motor domain of the ASQ screener was higher in the dexmedetomidine group. To date, very few studies have evaluated the impact of dexmedetomidine on neurodevelopment, following paediatric cardiac surgery, and results remain inconclusive.39,40 Li et al.41 argue that prenatal injury is the primary cause of neurodevelopmental delay in infants with CHD, with cardiac surgery potentially resulting in only mild brain abnormalities. However, perioperative brain injury is still widespread in CHD and is associated with neurodevelopmental delays, and perioperative variables are thought to still have a role in outcomes.
To our knowledge, this study is the first double-blinded randomised controlled trial of sedation in paediatric open-heart surgery. We have demonstrated that sedation medications can be administered across multidisciplinary teams of clinicians in a blinded fashion; however, we recommend the inclusion of an implementation science framework in any future trial, which includes exploring the barriers and enablers to administering blinded study drugs with all stakeholders. Other trials have determined that blinding in sedation studies would be difficult and a potential safety risk.4 We also incorporated short- and long-term outcomes, which provide a more comprehensive picture of the patient's journey. Although some may argue that sedation would play a very little role in the neurodevelopmental outcome of CHD patients, with an increasing focus on survivorship, every opportunity to optimise brain health should be explored.
Our findings have multiple significant implications. The web-based randomisation and double-blinding processes of the study were performed well and should be replicated in a larger trial. However, due to the heterogenous nature of CHD defects, a future trial would benefit from stratification of the underlying cardiac pathophysiology to ensure that higher risk surgeries do not bias the outcomes. Further internal validity of the sedation process and side effect management are required and will likely require refinement of the sedation algorithm and haemodynamic protocol, including drug cessation rules, in consultation with the wider multidisciplinary team. To ensure high levels of fidelity, protocol checks of the study algorithm will need to be checked on a regular basis throughout a larger trial, and targeted education will need to be provided to sites where adherence is not at a high level. Although we provided extensive online education and bedside training around sedation assessments, we acknowledge that these practices require ongoing audits and updates to ensure the consistent and reliable capture of patient sedation status. This study demonstrated that a higher percentage of time spent in light sedation could be achieved with dexmedetomidine. Recent literature has discouraged deep sedation as this increases the risk of delirium, mortality, length of mechanical ventilation, and length of ICU stay.42 While there are no data on the depth of sedation and long-term outcomes, both ICU evidence and PICU evidence have highlighted these areas as risk factors for post–intensive care syndrome. Although midazolam has been reported as potentially damaging to the developing brain,7,8 there are still indications for this drug in the PICU. Despite this, further research around sedation and neurodevelopment is warranted in the CHD and broader PICU population.
5. Limitations
This study has several limitations. The study was conducted at a single site, which limits its generalisability to other settings and populations. Despite a robust randomisation process, we observed an imbalance in the CPB times between the groups, possibly due to zero children undergoing arterial switch operation allocated to the dexmedetomidine group. Despite this, we did not observe major differences in RACHS distribution, and most univentricular repairs were allocated to the dexmedetomidine group. A future definitive trial would benefit from a stratification by underlying CHD pathology. In addition, this study did not determine the impact of drug sequestration from the CPB circuitry for dexmedetomidine or midazolam. Future studies are required to determine the impact of CPB on drug concentrations. Further, the ASQ questionnaire is only a validated parent-reported screener and does not provide the comprehensive assessment with a neurodevelopment that a face-to-face report to an independent clinician would provide. Protocol violation occurred in cases where medical staff members felt uncomfortable with the administration of a blinded sedation drug. Despite the use of a sedation algorithm, sedation practices and assessments can be biased. Despite these limitations, this study demonstrated that all study concepts were safe and feasible.
Sedation is a modifiable factor in the postoperative care of children, and it is critical to have a greater understanding of the unique contributions of these medicines to relevant outcomes.
6. Conclusion
Administration of perioperative dexmedetomidine was associated with more time spent in light sedation than that of midazolam. The feasibility of conducting a blinded RCT of midazolam and dexmedetomidine in children undergoing open-heart surgery was also established. Further definitive, innovative, and multicentre trials are required to understand optimal sedation and recovery in this vulnerable cohort.
Funding
This study was supported by a Pfizer Essential Health Investigator Initiated Grant. The organisation was not involved in the design, undertaking, analysis, or reporting of the study including manuscript preparation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Credit authorship statement
DL conceived the study, wrote grant, developed protocol and funding application and approved the final draft. AS and YS assisted with study conception, protocol development and funding application, reviewed the manuscript and approved the final draft. CS, MR, NA, and CG assisted with protocol development, contributed in data collection, assistance with study management and primary end point assignment, reviewed the manuscript and approved the final draft. JS assisted with data collection, manuscript draft and approved the final draft. KG and RLM led data management, cleaning and analysis, contributed to draft manuscript, and approved the final draft.
Tweet
Dexmedetomidine commenced prior to open heart surgery may be more effective in providing light sedation than midazolam in #CHD kids. #PedsICU.
Acknowledgements
The authors gratefully acknowledge the contributions of clinicians and researchers working in the anaesthetic, cardiac, surgical, and paediatric intensive care units at the Queensland Children's Hospital, Brisbane, who assisted in the preparation and completion of this research. The authors would also like to thank the children and their families who participated in this study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ccrj.2023.04.007.
Contributor Information
Debbie A. Long, Email: da.long@qut.edu.au.
Yahya Shehabi, Email: yahya.shehabi@monash.edu.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.van der Linde D., Konings E.E., Slager M.A., Witsenburg M., Helbing W.A., Takkenberg J.J., et al. Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. J Am Coll Cardiol. 2011;58(21):2241–2247. doi: 10.1016/j.jacc.2011.08.025. [DOI] [PubMed] [Google Scholar]
- 2.ANZICS Centre for Outcome and Resource Evaluation . ANZICS CORE; 2017. Report of the Australian and New Zealand paediatric intensive care Registry 2017 victoria, AUS; p. 28. [Google Scholar]
- 3.Fudulu D., Angelini G. Oxidative stress after surgery on the immature heart. Oxid Med Cell Longev. 2016;2016 doi: 10.1155/2016/1971452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Erickson S.J., Millar J., Anderson B.J., Festa M.S., Straney L., Shehabi Y., et al. Dexmedetomidine sedation in mechanically ventilated critically ill children: a pilot randomized controlled trial. Pediatr Crit Care Med. 2020;21(9):e731–e739. doi: 10.1097/PCC.0000000000002483. [DOI] [PubMed] [Google Scholar]
- 5.Egbuta C., Mason K.P. Current state of analgesia and sedation in the pediatric intensive care unit. J Clin Med. 2021;10(9):1847. doi: 10.3390/jcm10091847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith H.A., Besunder J.B., Betters K.A., Johnson P.N., Srinivasan V., Stormorken A., et al. 2022 society of critical care medicine clinical practice guidelines on prevention and management of pain, agitation, neuromuscular blockade, and delirium in critically ill pediatric patients with consideration of the ICU environment and early mobility. Pediatr Crit Care Med. 2022;23(2):e74–e110. doi: 10.1097/PCC.0000000000002873. [DOI] [PubMed] [Google Scholar]
- 7.Anand K.J., Barton B.A., McIntosh N., Lagercrantz H., Pelausa E., Young T.E., et al. Analgesia and sedation in preterm neonates who require ventilatory support: results from the NOPAIN trial. Neonatal Outcome and Prolonged Analgesia in Neonates. Arch Pediatr Adolesc Med. 1999;153(4):331–338. doi: 10.1001/archpedi.153.4.331. [DOI] [PubMed] [Google Scholar]
- 8.Andropoulos D.B., Easley R.B., Brady K., McKenzie E.D., Heinle J.S., Dickerson H.A., et al. Changing expectations for neurological outcomes after the neonatal arterial switch operation. Ann Thorac Surg. 2012;94(4):1250–1255. doi: 10.1016/j.athoracsur.2012.04.050. discussion 5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Czaja A.S., Zimmerman J.J. The use of dexmedetomidine in critically ill children. Pediatr Crit Care Med. 2009;10(3):381–386. doi: 10.1097/PCC.0b013e3181a3191f. [DOI] [PubMed] [Google Scholar]
- 10.Chrysostomou C., Di Filippo S., Manrique A.M., Schmitt C.G., Orr R.A., Casta A., et al. Use of dexmedetomidine in children after cardiac and thoracic surgery. Pediatr Crit Care Med. 2006;7(2):126–131. doi: 10.1097/01.PCC.0000200967.76996.07. [DOI] [PubMed] [Google Scholar]
- 11.Tachibana K., Hashimoto T., Kato R., Uchida Y., Ito R., Takita K., et al. Neonatal administration with dexmedetomidine does not impair the rat hippocampal synaptic plasticity later in adulthood. Pediatric Anesthesia. 2012;22(7):713–719. doi: 10.1111/j.1460-9592.2012.03810.x. [DOI] [PubMed] [Google Scholar]
- 12.Sanders R.D., Sun P., Patel S., Li M., Maze M., Ma D. Dexmedetomidine provides cortical neuroprotection: impact on anaesthetic-induced neuroapoptosis in the rat developing brain. Acta Anaesthesiol Scand. 2010;54(6):710–716. doi: 10.1111/j.1399-6576.2009.02177.x. [DOI] [PubMed] [Google Scholar]
- 13.Liu Y., Bian W., Liu P., Zang X., Gu X., Chen W. Dexmedetomidine improves the outcomes in paediatric cardiac surgery: a meta-analysis of randomized controlled trials. Interact Cardiovasc Thorac Surg. 2018;26(5):852–858. doi: 10.1093/icvts/ivy043. [DOI] [PubMed] [Google Scholar]
- 14.Eldridge S., Chan C., Campbell M., Bond C., Hopewell S., Thabane L., et al. CONSORT statement: extension to randomised pilot and feasibility trials. BMJ. 2010;2016:355. doi: 10.1136/bmj.i5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Potts A.L., Anderson B.J., Warman G.R., Lerman J., Diaz S.M., Vilo S. Dexmedetomidine pharmacokinetics in pediatric intensive care--a pooled analysis. Paediatr Anaesth. 2009;19(11):1119–1129. doi: 10.1111/j.1460-9592.2009.03133.x. [DOI] [PubMed] [Google Scholar]
- 16.Potts A.L., Warman G.R., Anderson B.J. Dexmedetomidine disposition in children: a population analysis. Paediatr Anaesth. 2008;18(8):722–730. doi: 10.1111/j.1460-9592.2008.02653.x. [DOI] [PubMed] [Google Scholar]
- 17.Curley M.A., Harris S.K., Fraser K.A., Johnson R.A., Arnold J.H. State Behavioral Scale: a sedation assessment instrument for infants and young children supported on mechanical ventilation. Pediatr Crit Care Med. 2006;7(2):107–114. doi: 10.1097/01.PCC.0000200955.40962.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Franck L.S., Scoppettuolo L.A., Wypij D., Curley M.A.Q. Validity and generalizability of the Withdrawal Assessment Tool-1 (WAT-1) for monitoring iatrogenic withdrawal syndrome in pediatric patients. Pain. 2012;153(1):142–148. doi: 10.1016/j.pain.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silver G., Traube C., Kearney J., Kelly D., Yoon M.J., Nash Moyal W., et al. Detecting pediatric delirium: development of a rapid observational assessment tool. Intensive Care Med. 2012;38(6):1025–1031. doi: 10.1007/s00134-012-2518-z. [DOI] [PubMed] [Google Scholar]
- 20.Schults J.A., Cooke M., Long D.A., Schibler A., Ware R.S., Mitchell M.L. Normal saline instillation versus no normal saline instillation and lung Recruitment versus no lung recruitment with paediatric Endotracheal Suction: the NARES trial. A study protocol for a pilot, factorial randomised controlled trial. BMJ Open. 2018;8(1) doi: 10.1136/bmjopen-2017-019789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Squires J., Potter L., Bricker D. Paul H Brookes Publishing; 1995. The ASQ user's guide for the Ages & Stages Questionnaires: a parent-completed, child-monitoring system. [Google Scholar]
- 22.Varni J.W., Seid M., Kurtin P.S. PedsQL™ 4.0: reliability and validity of the pediatric quality of life Inventory™ version 4.0 generic core scales in healthy and patient populations. Med Care. 2001:800–812. doi: 10.1097/00005650-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inf. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Teare M.D., Dimairo M., Shephard N., Hayman A., Whitehead A., Walters S.J. Sample size requirements to estimate key design parameters from external pilot randomised controlled trials: a simulation study. Trials. 2014;15(1):264. doi: 10.1186/1745-6215-15-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Zellem L., Utens E.M., de Wildt S.N., Vet N.J., Tibboel D., Buysse C. Analgesia-sedation in PICU and neurological outcome: a secondary analysis of long-term neuropsychological follow-up in meningococcal septic shock survivors. Pediatr Crit Care Med. 2014;15(3):189–196. doi: 10.1097/PCC.0000000000000044. [DOI] [PubMed] [Google Scholar]
- 26.Daverio M., Sperotto F., Zanetto L., Coscini N., Frigo A.C., Mondardini M.C., et al. Dexmedetomidine for prolonged sedation in the PICU: a systematic review and meta-analysis. Pediatr Crit Care Med. 2020;21(7):e467–e474. doi: 10.1097/PCC.0000000000002325. [DOI] [PubMed] [Google Scholar]
- 27.Sperotto F., Mondardini M.C., Dell'Oste C., Vitale F., Ferrario S., Lapi M., et al. Efficacy and safety of dexmedetomidine for prolonged sedation in the PICU: a prospective multicenter study (PROSDEX) Pediatr Crit Care Med. 2020;21(7):625–636. doi: 10.1097/PCC.0000000000002350. [DOI] [PubMed] [Google Scholar]
- 28.Garisto C., Rizza A., Ricci Z. Springer; 2018. Sedation in pediatric critically ill patients; pp. 213–244. (Critical care sedation). [Google Scholar]
- 29.Gulla K.M., Sankar J., Jat K.R., Kabra S.K., Lodha R. Dexmedetomidine vs midazolam for sedation in mechanically ventilated children: a randomized controlled trial. Indian Pediatr. 2021;58(2):117–122. [PubMed] [Google Scholar]
- 30.RamachandRan R., Pariyarath N., Ponnarmeni S., Jain P., Subramanian M. Prospective observational cohort study on dexmedetomidine and midazolam in mechanically ventilated children. J Clin Diagn Res. 2020;14(8) [Google Scholar]
- 31.Viana K.A., Daher A., Maia L.C., Costa P.S., Martins CdC., Paiva S.M., et al. What is the level of evidence for the amnestic effects of sedatives in pediatric patients? A systematic review and meta-analyses. PLoS One. 2017;12(7) doi: 10.1371/journal.pone.0180248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adler A.C., Daszkowski A., Tan J.C., Poliner A.D., Wei E.Z., Nathanson B.H., et al. The association of dexmedetomidine on perioperative opioid consumption in children undergoing adenotonsillectomy with and without obstructive sleep apnea. Anesth Analg. 2021;133(5):1260–1268. doi: 10.1213/ANE.0000000000005410. [DOI] [PubMed] [Google Scholar]
- 33.Cater D.T., Rogerson C.M., Hobson M.J., Ackerman L.L., Rowan C.M. The association of postoperative dexmedetomidine with pain, opiate utilization, and hospital length of stay in children post-Chiari malformation decompression. J Neurosurg Pediatr. 2021;1(aop):1–7. doi: 10.3171/2021.10.PEDS21291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frankel W.C., Maul T.M., Chrysostomou C., Wearden P.D., Lowry A.W., Baker K.N., et al., editors. Seminars in thoracic and cardiovascular surgery. Elsevier; 2020. A minimal opioid postoperative management protocol in congenital cardiac surgery: safe and effective. [DOI] [PubMed] [Google Scholar]
- 35.Penk J.S., Lefaiver C.A., Brady C.M., Steffensen C.M., Wittmayer K. Intermittent versus continuous and intermittent medications for pain and sedation after pediatric cardiothoracic surgery; A randomized controlled trial. Crit Care Med. 2018;46(1):123–129. doi: 10.1097/CCM.0000000000002771. [DOI] [PubMed] [Google Scholar]
- 36.Amula V., Vener D.F., Pribble C.G., Riegger L., Wilson E.C., Shekerdemian L.S., et al. Changes in anesthetic and postoperative sedation-analgesia practice associated with early extubation following infant cardiac surgery: experience from the pediatric heart network collaborative learning study. Pediatr Crit Care Med. 2019;20(10):931–939. doi: 10.1097/PCC.0000000000002005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Watson R.S., Asaro L.A., Hutchins L., Bysani G.K., Killien E.Y., Angus D.C., et al. Risk factors for functional decline and impaired quality of life after pediatric respiratory failure. Am J Respir Crit Care Med. 2019;200(7):900–909. doi: 10.1164/rccm.201810-1881OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garisto C., Ricci Z., Tofani L., Benegni S., Pezzella C., Cogo P. Use of low-dose dexmedetomidine in combination with opioids and midazolam in pediatric cardiac surgical patients: randomized controlled trial. Minerva Anestesiol. 2018;84(9):1053–1062. doi: 10.23736/S0375-9393.18.12213-9. [DOI] [PubMed] [Google Scholar]
- 39.Gong J., Zhang R., Shen L., Xie Y., Li X. The brain protective effect of dexmedetomidine during surgery for paediatric patients with congenital heart disease. J Int Med Res. 2019;47(4):1677–1684. doi: 10.1177/0300060518821272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang J., Gou B., Rong F., Wang W. Dexmedetomidine improves neurodevelopment and cognitive impairment in infants with congenital heart disease. Per Med. 2020;17(1):33–41. doi: 10.2217/pme-2019-0003. [DOI] [PubMed] [Google Scholar]
- 41.Li Y., Yin S., Fang J., Hua Y., Wang C., Mu D., et al. Neurodevelopmental delay with critical congenital heart disease is mainly from prenatal injury not infant cardiac surgery: current evidence based on a meta-analysis of functional magnetic resonance imaging. Ultrasound Obstet Gynecol. 2015;45(6):639–648. doi: 10.1002/uog.13436. [DOI] [PubMed] [Google Scholar]
- 42.Stephens R.J., Dettmer M.R., Roberts B.W., Ablordeppey E., Fowler S.A., Kollef M.H., et al. Practice patterns and outcomes associated with early sedation depth in mechanically ventilated patients: a systematic review and meta-analysis. Crit Care Med. 2018;46(3):471–479. doi: 10.1097/CCM.0000000000002885. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.