Abstract
Objectives
This article aims to critically review the literature on continuous electroencephalography (cEEG) monitoring in the intensive care unit (ICU) from an Australian and New Zealand perspective and provide recommendations for clinicians.
Design and review methods
A taskforce of adult and paediatric neurologists, selected by the Epilepsy Society of Australia, reviewed the literature on cEEG for seizure detection in critically ill neonates, children, and adults in the ICU. The literature on routine EEG and cEEG for other indications was not reviewed. Following an evaluation of the evidence and discussion of controversial issues, consensus was reached, and a document that highlighted important clinical, practical, and economic considerations regarding cEEG in Australia and New Zealand was drafted.
Results
This review represents a summary of the literature and consensus opinion regarding the use of cEEG in the ICU for detection of seizures, highlighting gaps in evidence, practical problems with implementation, funding shortfalls, and areas for future research.
Conclusion
While cEEG detects electrographic seizures in a significant proportion of at-risk neonates, children, and adults in the ICU, conferring poorer neurological outcomes and guiding treatment in many settings, the health economic benefits of treating such seizures remain to be proven. Presently, cEEG in Australian and New Zealand ICUs is a largely unfunded clinical resource that is subsequently reserved for the highest-impact patient groups. Wider adoption of cEEG requires further research into impact on functional and health economic outcomes, education and training of the neurology and ICU teams involved, and securement of the necessary resources and funding to support the service.
Keywords: Seizure, Epilepsy, Hypoxic-ischaemic encephalopathy, Electroencephalography
1. Introduction
Patients in the intensive care unit (ICU) with nonpharmacologically depressed conscious state, particularly those with acute neurological disorders and brain injuries, are at risk of seizures, often without overt clinical manifestations. Electrographic or EEG-only seizures (ESz) and electroclinical seizures (ECSz) are more prevalent in ICU patients who have prior clinical seizures (acute symptomatic or epilepsy related), infective and autoimmune encephalitis, stroke, traumatic brain injury (TBI), hypoxic-ischaemic encephalopathy (HIE), and extracorporeal membrane oxygenation (ECMO) therapy. Seizures are theoretically an indicator of cerebral cortical injury in neurological and cranial insults and may impact adversely on neurological outcome, over and above that of the underlying neurological disorder. Additionally, affected patients may benefit from treatment with antiseizure medication (ASM). In some patients, electrographic status epilepticus (ESE) is the cause of their altered consciousness and prompt treatment with ASM reverses the coma. Conversely, episodic nonepileptic movements and autonomic changes in ICU patients are commonly misinterpreted and unnecessarily treated as seizures, prolonging ICU stay and impacting adversely on outcome.[1], [2], [3], [4], [5], [6], [7], [8], [9], [10]
EEG with video is essential for accurate diagnosis of seizures and nonepileptic phenomena and is the only way ESz and ESE can be identified. Video-EEG recordings of relatively brief duration (0.5–3 h), herein referred to as routine EEG, are often sufficient to answer clinical questions, including diagnosis of ESE and identifying those patients at risk of ESz. However, if the clinical episodes are not recorded, or if clinical and EEG risk factors indicate a high likelihood of infrequent ESz or intermittent ESE, then longer duration (continuous) EEG with video, herein referred to as continuous electroencephalography (cEEG), is required for diagnosis. Additionally, if ESz are discovered, cEEG may help determine seizure burden, monitor disease progress, and allow assessment of response to therapy.11 Other potential applications of cEEG include monitoring for vasospasm in subarachnoid haemorrhage and ensuring maintenance of burst suppression during pharmacological management of raised intracranial pressure and ESE.12
cEEG is not utilised routinely in ICUs in Australia and New Zealand (ANZ), unlike in North America and many centres in Europe.1 The availability of inpatient EEG services varies around our countries, particularly between city and rural, and paediatric and adult hospitals. Impediments to cEEG in ANZ include the following: it is a resource- and time-intensive investigation, well-trained staff members to perform and interpret cEEG in a timely fashion are few, the clinical significance of some cEEG findings remains uncertain, the cost-effectiveness of cEEG and treatment of ESz remains to be demonstrated, and specific financial reimbursement is lacking.
This critical review and position statement attempts to (i) summarise the evidence for, practicalities of, and controversies in cEEG for detection of ESz and ESE in ICU patients with depressed conscious state and (ii) provide recommendations for clinical practice, research, and reimbursement of cEEG in ANZ ICUs.
2. Methods
The Epilepsy Society of Australia tasked a working group to review the literature, determine current practices in ANZ, and establish consensus recommendations for the use of cEEG in ICUs for the detection and management of seizures. Taskforce members had a range of clinical expertise in neonatal, paediatric, and adult neurology and ICU settings and were all skilled in EEG. For this review and statement, we considered cEEG to be eight or more channels (usually standard 10–20 systems in adults and children) of scalp EEG recording for typically greater than 12 h duration, with simultaneous video recording, being reviewed either continuously or intermittently. The terminology and acronyms used in this document are those recommended by the American Clinical Neurophysiology Society (ACNS)13 (Table 1). Unless stated otherwise, seizure includes both ESz and ECSz.
Table 1.
EEG-related terminology, acronyms, synonyms, and definitions.
| Terminology (synonym) | Acronym | Definition |
|---|---|---|
| Amplitude-integrated EEG | aEEG | Compressed (amplitude is logarithmic and time is linear) recording of EEG using 2 or several channels, typically of greater than 12 h duration and in ICU settings, with the aim of detecting background changes indicative of encephalopathy or suggestive of seizures. |
| Continuous EEG monitoring | cEEG | ≥16 channels (≥8 in neonates) of EEG recording with simultaneous video recording, typically of greater than 12 h duration and in ICU settings, with the aim of detecting and monitoring seizures, including electrographic (EEG only) seizures. |
| EEG background | The predominant EEG activity, in ICU recordings typically categorised as: normal or sedated sleep; slow and disorganised; discontinuous or burst suppression; or attenuated and featureless.[166], [167], [168], [103] | |
| Electroclinical seizure (clinical seizure, convulsive seizure) | ECSz | A seizure with clinical manifestations and time-locked to an EEG pattern (note: EEG pattern does not need to fulfil electrographic seizure criteria) OR an electrographic seizure and subsequent clinical improvement attributable to suppression of seizures with an ASM.98,169 |
| Electroclinical status epilepticus (clinical or convulsive status epilepticus) | ECSE | An uninterrupted electroclinical seizure lasting 10 min or longer OR recurrent seizures totalling 12 min in any 1-h period (hourly seizure burden ≥20%) OR ≥ 5 min of a convulsive (i.e. with bilateral tonic-clonic motor activity) seizure.13,98,169 |
| Electrographic seizure (EEG seizure) | ESz | An abnormal paroxysmal electrographic event that differs from the background activity, lasts longer than 10 s (briefer if associated with clinical change), has a plausible electrographic field, typically has a frequency of >2.5 Hz, and evolves in frequency, morphology, or spatial distribution (except for neonatal seizures which may not evolve).13,170 |
| Electrographic status epilepticus | ESE | An uninterrupted electrographic seizure lasting 10 min or longer OR recurrent electrographic seizures totalling 12 min in any 1-h period (hourly seizure burden ≥20%)13,169 |
| Ictal-interictal continuum | IIC | An EEG pattern that does not qualify as an electrographic seizure or electrographic status epilepticus, but there is a reasonable chance that it may be contributing to coma, causing other clinical symptoms, and/or contributing to neuronal injury.13 |
| Routine EEG | ≥16 channels (≥8 in neonates) of EEG recording, typically 20–60 min duration with simultaneous video recording, with the aim of detecting abnormalities of EEG background, interictal epileptiform discharges, seizures, and status epilepticus. In ICU settings, routine EEG is considered a screening tool. | |
| Sporadic epileptiform discharges (interictal epileptiform discharges) | SEDs | Non-rhythmic and non-periodic (intermittent) interictal EEG phenomena that are intermixed with the background and are associated with seizures e.g. spikes, polyspikes, sharp waves.13 |
| Total seizure burden | The proportion of time occupied by seizures during cEEG.99 | |
| Video-EEG monitoring | ≥16 channels (≥8 in neonates) of EEG recording with simultaneous video recording, typically greater than 3 h duration and in ward or ambulatory settings, with the aim of recording seizures and other episodic phenomena. In ICU, video-EEG monitoring is generally referred to as cEEG. |
The methodology and issues of interest were defined during regular teleconference sessions of the Epilepsy Society of Australia taskforce, commencing in May 2020. Literature searches were conducted with the goal of identifying relevant clinical studies and international practice guidelines on cEEG in the ICU. Studies reporting on the valuable role of shorter duration routine EEG in the ICU, and on animal research, were not reviewed. Review of the English language literature was done following searches in PubMed, Embase, CINAHL, Web of science, and Cochrane databases until June 2022 for each topic area using Medical Subject Headings keywords (see Electronic Supplementary Material).
Taskforce members considered the strength of evidence behind the outcomes of interest, as well as issues pertaining to risk-to-benefit ratio and cost.[14], [15], [16] Given the paucity of high-quality evidence in the field, the taskforce issued recommendations that were primarily based on consensus opinion. Formal grading of evidence was not undertaken given the few randomised controlled trials (RCTs) comparing cEEG to routine EEG for the detection of seizures in ICU. Additionally, some important outcomes of cEEG are difficult to evaluate in RCTs or cohort studies (e.g., distinguishing epileptic vs. nonepileptic events).
The draft review, position statement, and recommendations were reviewed in 2021 by several ANZ societies representing neonatal, paediatric, and adult neurology and intensive care medicine, with incorporation of their feedback into subsequent drafts. In summary, this document presents (i) a summary of clinical research studies and international guidelines on cEEG for seizures in different ICU settings (reported in the Results) and (ii) the authors’ expert opinions on cEEG in ANZ (reported in the Discussion). Commentary and recommendations about cEEG are made broadly, recognising the sometimes significant differences in patients and practice in neonatal, paediatric, and adult ICU settings.
3. Results
3.1. cEEG in the neonatal ICU
Seizure burden in neurologically sick neonates is higher than at any other time in life, with neonatal seizures occurring in 1–4/1000 live births.[17], [18], [19], [20] Evidence suggests that seizures contribute to secondary brain injury in neonates, especially in neonates with HIE.[21], [22], [23], [24], [25], [26], [27] Delay in recognition and treatment of seizures in neonates is associated with poorer response to treatment and poorer outcomes.28,29
Neonatal seizures are challenging to manage as many paroxysmal phenomena mimic seizures, neonatal seizures have variable and subtle clinical manifestations, and most neonatal seizures are ESz, especially in neonates with moderate to severe HIE[30], [31], [32], [33], [34], [35], [36] and those on ASMs. Even experienced neonatologists and neurologists are unreliable at distinguishing seizures and nonepileptic movements at the bedside.30,31,37 Studies indicate that 20–46% of neonates receiving ASM for seizures identified on clinical assessment do not have ESz on cEEG, exposing them to the risk of adverse neurodevelopmental effects of ASM.[37], [38], [39], [40], [41], [42], [43] Conversely, under-recognition and undertreatment of neonatal seizures, including ESE, is associated with worse neurodevelopmental outcomes.21,[22], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54]
Amplitude integrated EEG (aEEG) is used frequently in neonatal ICUs (NICUs), aEEG background being a useful indicator of neurological prognosis, especially in term and near-term infants. However, aEEG has low sensitivity and specificity for the detection of ESz, especially ESz of low amplitude, brief duration, and occurring at a distance from the 2–4 recording electrodes.[55], [56], [57], [58], [59]
An RCT performed in Australian NICUs, randomising encephalopathic term or near-term neonates to management of aEEG detected plus clinical seizures versus management of clinical seizures only, found no difference in mortality or severe neurological morbidity at age 2 years.60 Limitations of the trial may be that aEEG was not commenced early enough in some infants, aEEG findings are not necessarily influencing ASM dosing and timing, and aEEG has a low sensitivity and specificity for detection of neonatal seizures.61,62 Nevertheless, the trial highlighted the possibility that potentially neurotoxic GABAergic ASM like phenobarbitone and midazolam mitigated the potential benefits from reduced seizure burden.40,41,63 Levetiracetam, topiramate, and lacosamide have been proposed as safer ASMs[64], [65], [66], [67], [68], [69], [70] although less effective in one trial.29
Video-EEG remains the gold standard for diagnosis of seizures in neonates, either during short-duration routine EEG or cEEG.[71], [72], [73], [74] cEEG is widely used in NICUs in North America (see Fig. 1 for cEEG set-up).10,75
Fig. 1.
Typical cEEG set-up in the NICU, PICU, and ICU. Abbreviations: cEEG, continuous electroencephalography monitoring; ICU, intensive care unit; NICU, neonatal ICU; PICU, paediatric ICU.
cEEG studies in the NICU report seizures in 20–60% of high-risk neonates (80–90% being ESz) and ESE in up to 40%.31,48,72,73 High-risk groups include neonates with HIE, metabolic or genetic disorders, stroke, meningitis, and neonates on ECMO.7,21,48,76
cEEG impacts clinical care in up to 75% neonates, including the early detection of seizures and encephalopathy, the appropriate and prompt use of ASM for seizures, and the curtailing of ASM when episodes are not seizures.9,21,48,76 Both under-recognition and overestimation of seizures occur when cEEG is unavailable in the NICU, with undertreatment and overtreatment contributing to adverse neurodevelopmental outcomes.[40], [41], [42],72,77,78 Studies suggest improved long-term outcomes and cost-effectiveness for neonates with HIE or ESE when managed with cEEG.44,10,[79], [80], [81], [82], [83] However, when it comes to the issue of which ESz or how much seizure burden to treat, and which ASM to utilise, opinion is divided; aetiology might determine management and outcome just as much as seizure burden.31,48,[84], [85], [86]
3.2. cEEG in the PICU
cEEG detects seizures in up to 40% of children in the PICU with unexplained coma and risk factors.6,48,[87], [88], [89], [90], [91], [92], [93], [94], [95], [96], [97] Risk factors include age <2 years, prior clinical seizures, HIE, stroke, head injury, encephalitis, ECMO, cardiac surgery, and routine EEG showing discontinuous background activity and epileptiform discharges.[98], [99], [100], [101], [102], [103], [104], [105], [106], [107], [108], [109], [110]
cEEG is widely used in North American and European PICUs, with some variation in indications.94 The role of cEEG in PICU patients with refractory ESE is well established.48,98,[111], [112], [113], [114], [115], [116], [117], [118] There is evidence from prospective cohort studies for cEEG to detect seizures in children with unexplained, persistently depressed or fluctuating consciousness and children with acute cerebral injury.92,104,119 Other indications include children under pharmacological paralysis and children with paroxysmal events suspected to be seizures, though evidence supporting these practices is weaker[108], [109], [110],117,120,121 and routine EEG may suffice if there are no additional clinical or EEG risk factors.
Several cohort studies of children in the PICU have shown an association between ESE and increased mortality and poor neurologic outcome, when corrected for aetiology.88,89,92,93,100,104,114 Greater seizure burden, defined as the maximum percentage of any given hour occupied by ESz,122 and the presence of ESE are associated with worse outcomes.119,122 Delay in the identification and treatment of ESE in children is associated with decreased effectiveness of ASM and a lower likelihood of seizure termination.97 Improved detection and treatment of ESz have been shown to improve patient outcomes, including reduced mortality, reduced length of stay, and improved short-term neurologic outcomes in children admitted to the PICU with an altered level of consciousness due to all causes.93,99,102,111,116 Impact on longer-term outcomes, including likelihood of subsequent epilepsy and effects on cognition, is being explored.117,119
The impact of cEEG on resource utilisation depends on selection of patients.123 Health economic modelling suggests that cost-effectiveness of cEEG of 24–48 h duration in high-risk groups in ESz identification and management improves patient outcomes by as little as 3–7% (see Fig. 2 for EEG interpretation resourcing).123,124
Fig. 2.
Continuous electroencephalography interpretation.
3.3. cEEG in the adult ICU
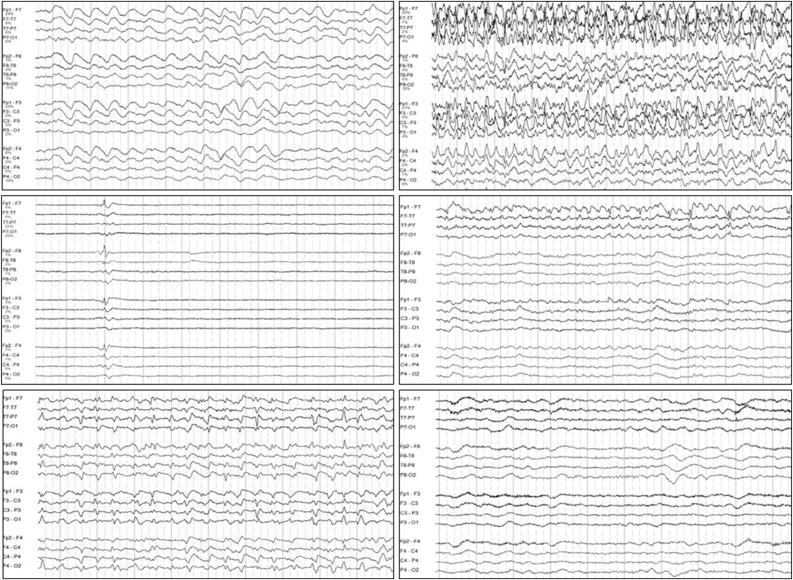
ESz and ESE are detected on cEEG in 10–30% of adults with critical illness in the ICU, particularly following electroclinical or convulsive status epilepticus (ECSE) and in adults with an acute CNS insults such as TBI, cerebral infection, inflammation, and intracranial haemorrhage (e.g., in Fig. 3).118,[118], [125], [126], [127], [128], [129], [130], [131], [132], [133], [134], [135], [136], [137], [138], [139]
Fig. 3.
Electroencephalography example.
Clinical predictors most consistently associated with ESz in adults are the presence of persistent coma and clinical seizures.125,140 Additionally, epileptiform abnormalities and periodic or rhythmic patterns on routine EEG are associated with a 2- to 3-fold increase in the likelihood of detecting ESz on cEEG.132,141,142 A simple algorithm that combines clinical and routine EEG features (“2HELPS2B” score) identifies patients most likely to have ESz, this potentially being useful to guide the rational use of cEEG in adults.143,144
There is evidence that ESz have harmful secondary effects on cerebral physiology in adults, in addition to the underlying aetiology. Evidence of inflammation, elevated neuron-specific enolase, low brain oxygen, high lactate, and increased intracranial pressure is more commonly identified in adults with acute brain insults such as TBI who have ESz than in matched patients without seizures.126,[145], [146], [147], [148], [149] Supporting this are case series showing increased mortality and morbidity in adults with ESz, and particularly ESE, compared to matched cohorts without seizures.133,135,139,145,[150], [151], [152], [153] Furthermore, two large registry studies of critically ill adults admitted to the ICU reported those undergoing cEEG compared with only routine EEG had a lower mortality rate.134 However, there is some evidence that suggests cEEG-identified ESz and ESE do not confer a worse prognosis, independent of the underlying aetiology.134,[152], [153], [154], [155], [156], [157]
Only one RCT of cEEG has been performed, in which critically ill adult patients with impaired consciousness and no recent seizures were randomised to cEEG or routine EEG.158 Unsurprisingly, seizures were more frequently detected and ASM more frequently escalated in patients undergoing cEEG, but no difference in mortality was shown. This was a pragmatic study, with enrolment dependent on need for EEG, the reasons for which were not specified. Furthermore, the prevalence of seizures was low, patients with prior clinical seizures were excluded, and detailed functional outcomes in survivors were not reported.
3.4. Guidelines and recommendations for cEEG monitoring
Whilst there are no universally accepted international guidelines for the use of cEEG, the Consensus Summary Statement of the International Multidisciplinary Consensus Conference on Multimodality Monitoring in Neurocritical Care is published,5,159 and there are several country-specific consensus statements with evidence-based recommendations. The ACNS produced standardised, critical care EEG terminology, with a recent update,13,160 as well as consensus statements on the practice and technical standards for cEEG in neonates,7,9 children,6,161 and adults.6,161 Guidelines and consensus statements have also been published by several other neurocritical care societies.2,4,[7], [8], [9],10
The ICU patient subgroups and clinical problems recommended or suggested by different international societies for cEEG are summarised in Table 2. These publications all acknowledge insufficient, high-quality evidence to support many of the recommendations and are therefore written as consensus statements rather than guidelines. They describe an ideal system that may help guide cEEG but do not cover issues regarding implementation into clinical practice.
Table 2.
Summary of international guidelines and consensus statements on cEEG in the ICU.
| Society/body | Summary of recommendations |
|---|---|
| American Clinical Neurophysiology Society6,7,9,161 |
|
| International Multidisciplinary Consensus Conference on Multimodality Monitoring in Neurocritical Care5,159 |
|
| European Academy of Neurology3 |
|
| Neurointensive care section (European Society of Intensive Care Medicine)2 |
|
4. Discussion
Seizures, predominantly ESz, may occur in ICU patients with depressed conscious state, and ESE is present in a proportion of these patients. The prevalence of ESz in ICU patients with depressed conscious state depends on age (neonates > children > adults), the presence of prior clinical seizures, and aetiology (highest risk in patients with stroke, encephalitis, TBI, HIE, and intracerebral haemorrhage). Reported prevalence rates for ESz and ESE are 10–60% and 5–10%, respectively, in at-risk patients in the ICU, higher rates being reported in neonates. There is some evidence, particularly in neonates and children, indicating ESE and high seizure burden have adverse effects on outcome, over and above that of the underlying condition. However, there is a paucity of high-quality evidence that detection and treatment of such seizures improves health or economic outcomes.
Routine EEG, ideally with neurological consultation, is widely available and funded in ANZ. Routine EEG is effective for diagnosis of many seizure-related issues in the ICU, such as detection of frequent ESz or ESE, and clarification of clinical or aEEG-identified phenomena. Additionally, routine EEG may show background abnormalities, epileptiform discharges, or rhythmic/periodic patterns, indicating a higher likelihood of subsequent ESz and potential benefit of cEEG.
cEEG allows characterisation of infrequently occurring events, detection of infrequent or variable frequency ESz and intermittent periods of ESE, determination of seizure burden, and assessment of the response to treatment of seizures. There is evidence suggesting cEEG is cost-effective and contributes to improved mortality, neurological outcomes, and economic benefits in certain age groups and aetiologies.
4.1. Controversies and gaps in evidence with cEEG monitoring
While there is no debate that ESz are present in a significant proportion of critically ill patients in the ICU, well-designed, large-scale, prospective trials to evaluate the impact on morbidity and mortality of detecting and treating ESz are few. Additionally, some cEEG studies in adults include findings that could be determined and actioned from routine EEG.
Many observational studies of cEEG reporting poorer outcomes for patients with recorded ESz failed to distinguish the impact of seizures from the impact of the underlying condition and the effects of ASM and anaesthesia. However, several prospective cohort studies in the PICU and NICU suggest a seizure burden greater than 20% contributes to morbidity, over and above the underlying aetiology.99,[114], [115], [116], [117]
The only RCT of cEEG versus routine EEG,158 in selected adult patients at low risk of seizures, showed no reduction of mortality despite the higher rate of seizure detection and escalation of ASMs. Although this study suggests routine EEG is a reasonable approach for patients with impaired consciousness who require an EEG, the findings cannot be extrapolated to patients with prior seizures and does not adequately address potential reductions in neurological morbidity.162 A similarly pragmatic RCT in the NICU,60 assessing mortality and developmental outcomes in term and near-term infants with HIE, found no difference in neonates whose treatment was escalated for ESz diagnosed on aEEG versus those whose management was only of ECSz. These two studies highlight some of the difficulties in performing EEG-related research in the ICU, notably that care did not change in either arm of the NICU study, suggesting the incorporation of EEG into care is not sufficiently “protocoled.” They also highlight obtaining long-term health outcome data in cEEG research is challenging; cohort study evidence obtained mainly in the PICU suggests feasibility and impact.22,44,83,110,[114], [115], [116], [117],124,163,164
The goals of treatment (e.g., abolition of all seizures vs. reduction of seizure burden) and whether infrequent brief seizures need to be treated are areas of ongoing investigation. Treatment impact may differ based on aetiology, seizure duration, and management approach.88,104 Given it may be considered unethical not to treat cEEG identified ESz and ESE in a randomised study, novel study designs have been suggested.88,165 Furthermore, focus on treatment of specific and potentially uncertain cEEG patterns, as well as a minimal dataset and defined outcome sets, is needed.
Inter-rater reliability and training issues regarding the interpretation of cEEG patterns have only been partially addressed by the introduction of internationally agreed and standardised adult ICU EEG terminology.13 Furthermore, there remains no clear international consensus on the definition of ESz and ESE, although criteria have been proposed by the ACNS to address this issue in adults, along with clarification of cEEG patterns.13
Cost–benefit analysis of cEEG has not been adequately investigated. Cost assessments need to include, in addition to the costs of cEEG, the costs of treatments to which cEEG findings often lead, with associated prolongation of ICU stay.134,139 Benefit analyses need to include, in addition to health economic costs. The impact of the underlying neurological condition or brain injury on long-term outcomes in patients often confounds interpretation of potential benefits of interventions.
Whilst consensus statements suggest indications for patient selection and ideal cEEG monitoring set-up, there are no recommendations for resource-poor settings or indications for transfer of patients to cEEG-capable centres.
The Critical Care EEG Monitoring Research Consortium is a large international collaboration of experts in cEEG committed to addressing uncertainties in these areas. Tertiary centres in ANZ could commit to contribute to the evidence base.
4.2. Challenges with implementation of cEEG monitoring in Australian and New Zealand ICUs
Considerable challenges exist to providing cEEG monitoring services in ANZ in a coordinated and equitable manner, both at single centres and nationwide. As such, the uptake of cEEG in ICUs has been limited.1,72,107
cEEG would typically be provided by the EEG department of a major teaching hospital, potentially as an extension of their video-EEG service for patients with epilepsy. cEEG would be impossible in small, maternity, private, and rural hospitals without partnerships with a major neurological centre, access to neurophysiology personnel and equipment, and a high-bandwidth telemedicine platform. More likely, patients in peripheral ICUs and high-dependency units would be transferred to major centres for cEEG.
The additional equipment, staff, and time required for most well-resourced neurology departments to provide cEEG as a new service would include the following: one or more portable, digital video-EEG recording units; neurophysiology scientists with experience in cEEG, the ICU environment, and ICU EEG patterns; neurologists with expertise in EEG patterns related to coma, cerebral injury, anaesthesia, and seizures, in different age groups; after-hours availability of neurophysiology scientists to commence, maintain, troubleshoot, and cease cEEG recordings; after-hours availability of neurologists to interpret cEEG and to assist ICU staff in seizure management; network connectivity with the neurology department's EEG server and archive storage (local or cloud based); remote access from neurology clinics and offices in the hospital and from home to live and archived cEEG recordings; funded time for neurophysiology scientists and neurologists to review, report, and archive cEEG studies in a timely fashion each day; online analysis software with compressed EEG display, trend analysis, and seizure detection capabilities; and cEEG training and continuing education programs across neurology, neurophysiology, and ICU teams.
Paramount to successful implementation of cEEG is a good working relationship between medical, nursing, and technical staff in the neurology, ICU and medical imaging departments, and the development of appropriate clinical standards and protocols. Additionally, specific training would be necessary for ICU nurses, staff neurologists and intensivists, rotating neurology and ICU trainees, and neurophysiology scientists, in cEEG technology, the capabilities and caveats of cEEG, and the management of cEEG detected seizures. An appropriately credentialed neurologist/neurophysiologist with experience in cEEG and ICU medicine would ideally oversee, supervise, and coordinate the service; collaborate in national and international research; and conduct regular audits.
Lack of dedicated funding or reimbursement is a major challenge for implementation of cEEG in ANZ. Inpatient EEG services are poorly reimbursed in general, and some EEG services are not reimbursed by funding bodies. For public inpatients, reimbursement is typically by activity-based funding to hospitals according to patient diagnoses. A minority of patients in ICUs of major teaching hospitals are privately insured or compensable, and even then, reimbursement is meagre. For example, the Australian Medicare items 11003/4/5 used for prolonged EEG monitoring attract a scheduled fee of only A$344/day. Such reimbursement would barely cover the salary of a neurophysiology scientist performing cEEG, let alone the equipment, consumables, and neurologist time. While case-mix funding for ICU patients is significantly greater than for typical ward patients, based on their complexity, complications, comorbidities, and protracted length of stay, this funding is not directly shared with ancillary departments providing care in the ICU, such as neurology. Furthermore, it would be inadequate to support the interdisciplinary, neurocritical care team required for implementation of this new service.
Providing cEEG without the appropriate resources, training, and reimbursement risks poor clinical outcomes for patients, cost overruns for neurology departments, staff burnout, and diversion of care away from established epilepsy services. Attempts to convince hospital administrators and health funding bodies to support cEEG would require detailed “new technology” submissions from neurology and ICU departments for block funding, with submissions supported by clinical and health economic evidence of improved patient outcomes and cost benefits.
5. Conclusions and recommendations
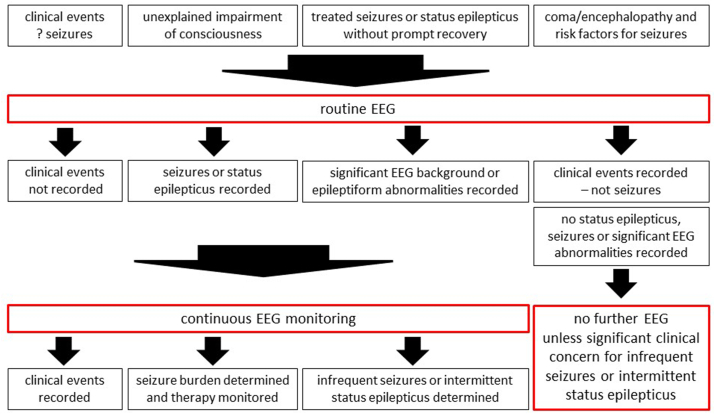
cEEG should be considered in ICU patients with clinical or routine EEG risk factors for seizures and in patients in whom routine EEG fails to clarify the clinical question (Fig. 4).
Fig. 4.
Suggested clinical application of routine EEG and continuous EEG monitoring in the ICU. EEG, electroencephalography; ICU, intensive care unit.
cEEG should only be undertaken by appropriately trained neurophysiology scientists and neurologists or neurophysiologists with ICU EEG experience. The resources and funding required to implement and maintain a cEEG service in the ICU are substantial. Although video-EEG monitoring capabilities exist at most tertiary hospitals, these are primarily for epilepsy assessment on neurology wards, not for cEEG in ICUs. Furthermore, remuneration for EEG services is poor and insufficient for many hospitals to establish or maintain a cEEG service. A case should be made by neurologists and intensivists to hospitals and health departments for specific funding of targeted cEEG of appropriate duration in high-risk ICU patients. Advocacy by professional societies would help promote awareness of the value of targeted cEEG in the ICU.
One important, presently unmet need for cEEG is for neonates and infants in ICUs, where onsite neurological and neurophysiological service provision is often limited, and seizure management is typically undertaken by neonatologists and intensivists using aEEG and other “cerebral function monitors.”
High-quality clinical research (multicentre, age-specific, prospective, randomised, pragmatic) including ANZ-specific health economic evaluations is required to better define the appropriate patient groups for cEEG, the ideal duration of monitoring, and the outcomes of detection and treatment of cEEG-based seizures. Until that time, and until adequate resourcing and funding is provided, neurologists and intensivists will need to rely upon targeted use of existing clinical and EEG services. Coma and seizure management protocols in the ICU should include EEG-based criteria to improve diagnosis and management, where resources are available.
Author contributions
The concept was conceived by the EEG and Executive Committees of the ESA. All authors contributed equally to the content, drafted sections of the manuscript, and provided subsequent edits.
Conflict of interest
All authors have no conflicts of interest to declare.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethics approval
Not required.
Acknowledgements
The authors thank the representatives of the Epilepsy Society of Australia (ESA), Australian and New Zealand Association of Neurologists (ANZAN), Australian and New Zealand Child Neurology Society (ANZCNS), Australian and New Zealand Intensive Care Society (ANZICS), and Australian and New Zealand Neonatal Network (ANZNN) who reviewed an early draft of the manuscript and provided insightful comments and suggestions.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ccrj.2023.04.004.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.Laing J., Lawn N., Perucca P., Kwan P., O'brien T.J. Continuous EEG use and status epilepticus treatment in Australasia: a practice survey of Australian and New Zealand epileptologists. BMJ Neurol Open. 2020;2:1–7. doi: 10.1136/bmjno-2020-000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Claassen J., Taccone F.S., Horn P., Holtkamp M., Stocchetti N., Oddo M., et al. Recommendations on the use of EEG monitoring in critically ill patients: consensus statement from the neurointensive care section of the ESICM. Intensive Care Med. 2013;39:1337–1351. doi: 10.1007/s00134-013-2938-4. [DOI] [PubMed] [Google Scholar]
- 3.Meierkord H., Boon P., Engelsen B., Göcke K., Shorvon S., Tinuper P., et al. EFNS guideline on the management of status epilepticus in adults. Eur J Neurol. 2010;17:348–355. doi: 10.1111/j.1468-1331.2009.02917.x. [DOI] [PubMed] [Google Scholar]
- 4.Hong Z., Su Y.Y. Recommendations for electroencephalography monitoring in neurocritical care units. Chin Med J Engl. 2017;130:1851–1855. doi: 10.4103/0366-6999.211559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Le Roux P., Menon D.K., Citerio G., Vespa P., Bader M.K., Brophy G.M., et al. Consensus summary statement of the International Multidisciplinary Consensus Conference on Multimodality Monitoring in Neurocritical Care: a statement for healthcare professionals from the Neurocritical Care Society and the European Society of Intensive Care Medicine. Neurocrit Care. 2014;21:1–26. doi: 10.1007/s12028-014-0041-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herman S.T., Abend N.S., Bleck T.P., Chapman K.E., Drislane F.W., Emerson R.G., et al. Consensus statement on continuous EEG in critically ill adults and children, part I. J Clin Neurophysiol. 2015;32:87–95. doi: 10.1097/WNP.0000000000000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herman S.T., Abend N.S., Bleck T.P., Chapman K.E., Drislane F.W., Emerson R.G., et al. The American Clinical Neurophysiology Society's guideline on continuous electroencephalography monitoring in neonates. J Clin Neurophysiol. 2011;28:611–617. doi: 10.1097/WNP.0b013e31823e96d7. [DOI] [PubMed] [Google Scholar]
- 8.Shellhaas R.A., Chang T., Tsuchida T., Scher M.S., Riviello J.J., Abend N.S., et al. Consensus protocol for EEG and amplitude-integrated EEG assessment and monitoring in neonates. Clin Neurophysiol. 2021;132:886–903. doi: 10.1016/j.clinph.2021.01.012. [DOI] [PubMed] [Google Scholar]
- 9.Dilena R., Raviglione F., Cantalupo G., Cordelli D.M., de Liso P., di Capua M., et al. The American Clinical Neurophysiology Society's guideline on continuous EEG monitoring in neonates. ACNS Guidel. 2012;28:1–17. [Google Scholar]
- 10.Buttle S.G., Sell E., Webster R., Varin M., Lemyre B., Hahn C., et al. Continuous electroencephalography monitoring for critically ill neonates: a Canadian perspective. Can J Neurol Sci. 2019;46:394–402. doi: 10.1017/cjn.2019.36. [DOI] [PubMed] [Google Scholar]
- 11.Gilmore E.J. Continuous electroencephalogram—necessity or luxury? JAMA Neurol. 2020 doi: 10.1001/jamaneurol.2020.1483. [DOI] [PubMed] [Google Scholar]
- 12.Claassen J., Hirsch L.J., Kreiter K.T., Du E.Y., Connolly E.S., Emerson R.G., et al. Quantitative continuous EEG for detecting delayed cerebral ischemia in patients with poor-grade subarachnoid hemorrhage. Clin Neurophysiol. 2004;115:2699–2710. doi: 10.1016/j.clinph.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 13.Hirsch L.J., Fong M.W.K., Leitinger M., LaRoche S.M., Beniczky S., Abend N.S., et al. Critical care EEG terminology: 2021 version. J Clin Neurophysiol. 2021;38 doi: 10.1097/WNP.0000000000000806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guyatt G.H., Oxman A.D., Schünemann H.J., Tugwell P., Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol. 2011;64:380–382. doi: 10.1016/j.jclinepi.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 15.Andrews J.C., Schünemann H.J., Oxman A.D., Pottie K., Meerpohl J.J., Coello P.A., et al. GRADE guidelines: 14. Going from evidence to recommendations: the significance and presentation of recommendations. J Clin Epidemiol. 2013;66:719–725. doi: 10.1016/j.jclinepi.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 16.Andrews J.C., Schünemann H.J., Oxman A.D., Pottie K., Meerpohl J.J., Coello P.A., et al. GRADE guidelines: 15. Going from evidence to recommendation – determinants of a recommendation's direction and strength. J Clin Epidemiol. 2013;66:726–735. doi: 10.1016/j.jclinepi.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 17.Chapman K.E., Raol Y.H., Brooks-Kayal A. Neonatal seizures: controversies and challenges in translating new therapies from the lab to the isolette. Eur J Neurosci. 2012;35:1857–1865. doi: 10.1111/j.1460-9568.2012.08140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saliba R.M., Annegers J.F., Waller D.K., Tyson J.E., Mizrahi E.M. Incidence of neonatal seizures in Harris County, Texas, 1992–1994. Am J Epidemiol. 1999;150:763–769. doi: 10.1093/oxfordjournals.aje.a010079. [DOI] [PubMed] [Google Scholar]
- 19.Tsuchida T.N., Wusthoff C.J., Shellhaas R.A., Abend N.S., Hahn C.D., Sullivan J.E., et al. American Clinical Neurophysiology Society standardized EEG terminology and categorization for the description of continuous EEG monitoring in neonates: report of the American Clinical Neurophysiology Society Critical Care Monitoring Committee. J Clin Neurophysiol. 2013;30:161–173. doi: 10.1097/WNP.0b013e3182872b24. [DOI] [PubMed] [Google Scholar]
- 20.Pellegrin S., Munoz F.M., Padula M., Heath P.T., Meller L., Top K., et al. Neonatal seizures: case definition & guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine. 2019;37:7596–7609. doi: 10.1016/j.vaccine.2019.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glass H.C., Glidden D., Jeremy R.J., Barkovich A.J., Ferriero D.M., Miller S.P. Clinical neonatal seizures are independently associated with outcome in infants at risk for hypoxic-ischemic brain injury. J Pediatr. 2009;155:318–323. doi: 10.1016/j.jpeds.2009.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Rooij L.G.M., Toet M.C., van Huffelen A.C., Groenendaal F., Laan W., Zecic A., et al. Effect of treatment of subclinical neonatal seizures detected with aEEG: randomized, controlled trial. Pediatrics. 2010;125 doi: 10.1542/peds.2009-0136. [DOI] [PubMed] [Google Scholar]
- 23.Wietstock S.O., Bonifacio S.L., McCulloch C.E., Kuzniewicz M.W., Glass H.C. Neonatal neurocritical care service is associated with decreased administration of seizure medication. J Child Neurol. 2015;30:1135–1141. doi: 10.1177/0883073814553799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rakhade S.N., Jensen F.E. Epileptogenesis in the immature brain: emerging mechanisms. Nat Rev Neurol. 2009;5:380–391. doi: 10.1038/nrneurol.2009.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shah D.K., Wusthoff C.J., Clarke P., Wyatt J.S., Ramaiah S.M., Dias R.J., et al. Electrographic seizures are associated with brain injury in newborns undergoing therapeutic hypothermia. Arch Dis Child Fetal Neonatal Ed. 2014;99 doi: 10.1136/archdischild-2013-305206. [DOI] [PubMed] [Google Scholar]
- 26.Dunne J.M., Wertheim D., Clarke P., Kapellou O., Chisholm P., Boardman J.P., et al. Automated electroencephalographic discontinuity in cooled newborns predicts cerebral MRI and neurodevelopmental outcome. Arch Dis Child Fetal Neonatal Ed. 2017;102:F58–F64. doi: 10.1136/archdischild-2015-309697. [DOI] [PubMed] [Google Scholar]
- 27.Srinivasakumar P., Zempel J., Trivedi S., Wallendorf M., Rao R., Smith B., et al. Treating EEG seizures in hypoxic ischemic encephalopathy: a randomized controlled trial. Pediatrics. 2015;136:e1302–e1309. doi: 10.1542/peds.2014-3777. [DOI] [PubMed] [Google Scholar]
- 28.Toet M.C., Groenendaal F., Osredkar D., Van Huffelen A.C., De Vries L.S. Postneonatal epilepsy following amplitude-integrated EEG-detected neonatal seizures. Pediatr Neurol. 2005;32:241–247. doi: 10.1016/j.pediatrneurol.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 29.Sharpe C., Reiner G.E., Davis S.L., Nespeca M., Gold J.J., Rasmussen M., et al. Levetiracetam versus phenobarbital for neonatal seizures: a randomized controlled trial. Pediatrics. 2020;145 doi: 10.1542/peds.2019-3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boylan G.B., Stevenson N.J., Vanhatalo S. Monitoring neonatal seizures. Semin Fetal Neonatal Med. 2013:202–208. doi: 10.1016/j.siny.2013.04.004l. [DOI] [PubMed] [Google Scholar]
- 31.Pressler R.M., Cilio M.R., Mizrahi E.M., Moshé S.L., Nunes M.L., Plouin P., et al. The ILAE classification of seizures and the epilepsies: modification for seizures in the neonate. Position paper by the ILAE Task Force on Neonatal Seizures. Epilepsia. 2021;62:615–628. doi: 10.1111/epi.16815. [DOI] [PubMed] [Google Scholar]
- 32.Rennie J.M., de Vries L.S., Blennow M., Foran A., Shah D.K., Livingstone V., et al. Characterisation of neonatal seizures and their treatment using continuous EEG monitoring: a multicentre experience. Arch Dis Child Fetal Neonatal Ed. 2019;104:F493–F501. doi: 10.1136/archdischild-2018-315624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clancy R.R., Legido A., Lewis D. Occult neonatal seizures. Epilepsia. 1988;29:256–261. doi: 10.1111/j.1528-1157.1988.tb03715.x. [DOI] [PubMed] [Google Scholar]
- 34.Nagarajan L. Classification of clinical semiology in epileptic seizures in neonates. Eur J Paediatr Neurol. 2012;16:118–125. doi: 10.1016/j.ejpn.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 35.Scher M.S., Alvin J., Gaus L., Minnigh B., Painter M.J. Uncoupling of EEG-clinical neonatal seizures after antiepileptic drug use. Pediatr Neurol. 2003;28:277–280. doi: 10.1016/s0887-8994(02)00621-5. [DOI] [PubMed] [Google Scholar]
- 36.Biagioni E., Ferrari F., Boldrini A., Roversi M.F., Cioni G. Electroclinical correlation in neonatal seizures. Eur J Paediatr Neurol. 1998;2:117–125. doi: 10.1016/s1090-3798(98)80027-5. [DOI] [PubMed] [Google Scholar]
- 37.Bye A.M., Flanagan D. Spatial and temporal characteristics of neonatal seizures. Epilepsia. 1995;36:1009–1016. doi: 10.1111/j.1528-1157.1995.tb00960.x. [DOI] [PubMed] [Google Scholar]
- 38.Nagarajan L. Ictal electroencephalograms in neonatal seizures: characteristics and associations. Pediatr Neurol. 2011;45:11–16. doi: 10.1016/j.pediatrneurol.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 39.Nagarajan L. Brief electroencephalography rhythmic discharges (BERDs) in the neonate with seizures: their significance and prognostic implications. J Child Neurol. 2011;26:1529–1533. doi: 10.1177/0883073811409750. [DOI] [PubMed] [Google Scholar]
- 40.Forcelli P.A., Janssen M.J., Vicini S., Gale K. Neonatal exposure to antiepileptic drugs disrupts striatal synaptic development. Ann Neurol. 2012;72:363–372. doi: 10.1002/ana.23600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maitre N.L., Smolinsky C., Slaughter J.C., Stark A.R. Adverse neurodevelopmental outcomes after exposure to phenobarbital and levetiracetam for the treatment of neonatal seizures. J Perinatol. 2013;33:841–846. doi: 10.1038/jp.2013.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bittigau P., Sifringer M., Ikonomidou C. Antiepileptic drugs and apoptosis in the developing brain. Ann N Y Acad Sci. 2003;114:103–114. doi: 10.1111/j.1749-6632.2003.tb07517.x. [DOI] [PubMed] [Google Scholar]
- 43.Fitzgerald M.P., Kessler S.K., Abend N.S. Early discontinuation of antiseizure medications in neonates with hypoxic-ischemic encephalopathy. Epilepsia. 2017;58:1047–1053. doi: 10.1111/epi.13745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kharoshankaya L., Stevenson N.J., Livingstone V., Murray D.M., Murphy B.P., Aheame C.E., et al. Seizure burden and neurodevelopmental outcome in neonates with hypoxic-ischemic encephalopathy. Dev Med Child Neurol. 2016;58:1242–1248. doi: 10.1111/dmcn.13215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Murray D.M., Boylan G.B., Ali I., Ryan C.A., Murphy B.P., Connolly S. Defining the gap between electrographic seizure burden, clinical expression and staff recognition of neonatal seizures. Arch Dis Child Fetal Neonatal Ed. 2008;93:F187–F191. doi: 10.1136/adc.2005.086314. [DOI] [PubMed] [Google Scholar]
- 46.Uria-Avellanal C., Marlow N., Rennie J.M. Outcome following neonatal seizures. Semin Fetal Neonatal Med. 2013;18:224–232. doi: 10.1016/j.siny.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 47.Toet M.C., Groenendaal F., Osredkar D. Postneonatal epilepsy following amplitude-integrated EEG-detected neonatal seizures. Pediatr Neurol. 2005;32:241–247. doi: 10.1016/j.pediatrneurol.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 48.Abend N.S., Wusthoff C.J., Goldberg E.M., Dlugos D.J. Electrographic seizures and status epilepticus in critically ill children and neonates with encephalopathy. Lancet Neurol. 2013;12:1170–1179. doi: 10.1016/S1474-4422(13)70246-1. [DOI] [PubMed] [Google Scholar]
- 49.Kang S.K. Neonatal seizures: impact on neurodevelopmental outcomes. Front Pediatr. 2015;3:101. doi: 10.3389/fped.2015.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van Rooij L.G., de Vries L.S., Handryastuti S. Neurodevelopmental outcome in term infants with status epilepticus detected with amplitude-integrated electroencephalography. Pediatrics. 2007;120:e354–e363. doi: 10.1542/peds.2006-3007. [DOI] [PubMed] [Google Scholar]
- 51.Iyer K.K., Roberts J.A., Hellström-Westas L., Wikström S., Pupp I.H., Ley D., et al. Early detection of preterm intraventricular hemorrhage from clinical electroencephalography. Crit Care Med. 2015;43:2219–2227. doi: 10.1097/CCM.0000000000001190. [DOI] [PubMed] [Google Scholar]
- 52.Fitzgerald M.P., Massey S.L., Fung F.W., Kessler S.K., Abend N.S. High electroencephalographic seizure exposure is associated with unfavorable outcomes in neonates with hypoxic-ischemic encephalopathy. Seizure. 2018;61:221–226. doi: 10.1016/j.seizure.2018.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pisani F., Facini C., Pelosi A., Mazzotta S., Spagnoli C., Pavlidis E. Neonatal seizures in preterm newborns: a predictive model for outcome. Eur J Paediatr Neurol. 2016;20:243–251. doi: 10.1016/j.ejpn.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 54.McBride M.C., Laroia N., Guillet R. Electrographic seizures in neonates correlate with poor neurodevelopmental outcome. Neurology. 2000;55:506–513. doi: 10.1212/wnl.55.4.506. [DOI] [PubMed] [Google Scholar]
- 55.Rakshasbhuvankar A., Rao S., Palumbo L., Ghosh S., Nagarajan L. Amplitude-integrated EEG for detection of neonatal seizures: a systematic review. Seizure. 2015;33:90–98. doi: 10.1016/j.seizure.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 56.Rakshasbhuvankar A., Rao S., Palumbo L., Ghosh S., Nagarajan L. Amplitude integrated electroencephalography compared with conventional video EEG for neonatal seizure detection: a diagnostic accuracy study. J Child Neurol. 2017;32:815–822. doi: 10.1177/0883073817707411. [DOI] [PubMed] [Google Scholar]
- 57.Buttle S.G., Lemyre B., Sell E., Redpath S., Bulusu S., Webster R.J., et al. Combined conventional and amplitude-integrated EEG monitoring in neonates: a prospective study. J Child Neurol. 2019;34:313–320. doi: 10.1177/0883073819829256. [DOI] [PubMed] [Google Scholar]
- 58.Rakshasbhuvankar A.A., Wagh D., Athikarisamy S.E., Davis J., Nathan E.A., Palumbo L., et al. Inter-rater reliability of amplitude-integrated EEG for the detection of neonatal seizures. Early Hum Dev. 2020;143 doi: 10.1016/j.earlhumdev.2020.105011. [DOI] [PubMed] [Google Scholar]
- 59.Lawrence R., Mathur A., Tich S.N.T., Zempel J., Inder T. A pilot study of continuous limited-channel aEEG in term infants with encephalopathy. J Pediatr. 2009;154:835–841.e1. doi: 10.1016/j.jpeds.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 60.Hunt R.W., Liley H.G., Wagh D., Schembri R., Lee K.J., Shearman A.D., et al. Effect of treatment of clinical seizures vs electrographic seizures in full-term and near-term neonates: a randomized clinical trial. JAMA Netw Open. 2021;4:1–12. doi: 10.1001/jamanetworkopen.2021.39604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vanhatalo S., Stevenson N.J., Pressler R.M., Abend N.S., Auvin S., Brigo F., et al. Why monitor the neonatal brain—that is the important question. Pediatr Res. 2023;93:19–21. doi: 10.1038/s41390-022-02040-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Soul J.S., Glass H.C., Mohammad K., Ment L.R., Smyser C.D., Bonifacio S.L., et al. Continuous EEG monitoring still recommended for neonatal seizure management: commentary on NEST trial. Pediatr Res. 2022:9–10. doi: 10.1038/s41390-022-02138-0. [DOI] [PubMed] [Google Scholar]
- 63.Duerden E.G., Guo T., Dodbiba L., Chakravarty M.M., Chau V., Poskitt K.J., et al. Midazolam dose correlates with abnormal hippocampal growth and neurodevelopmental outcome in preterm infants. Ann Neurol. 2016;79:548–559. doi: 10.1002/ana.24601. [DOI] [PubMed] [Google Scholar]
- 64.Slaughter L.A., Patel A.D., Slaughter J.L. Pharmacological treatment of neonatal seizures: a systematic review. J Child Neurol. 2013;28:351–364. doi: 10.1177/0883073812470734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Morin L., Enderlin J., Leger P.L., Perrotte G., Bonnin P., Dupuis N., et al. Different response to antiepileptic drugs according to the type of epileptic events in a neonatal ischemia-reperfusion model. Neurobiol Dis. 2017;99:145–153. doi: 10.1016/j.nbd.2016.12.023. [DOI] [PubMed] [Google Scholar]
- 66.Abend N.S., Gutierrez-Colina A.M., Monk H.M., Dlugos D.J., Clancy R.R. Levetiracetam for treatment of neonatal seizures. J Child Neurol. 2011;26:465–470. doi: 10.1177/0883073810384263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Noh M.-R., Kim S.K., Sun W., Park S.K., Choi H.C., Lim J.H., et al. Neuroprotective effect of topiramate on hypoxic ischemic brain injury in neonatal rats. Exp Neurol. 2006;201:470–478. doi: 10.1016/j.expneurol.2006.04.038. [DOI] [PubMed] [Google Scholar]
- 68.Glass H.C., Poulin C., Shevell M.I. Topiramate for the treatment of neonatal seizures. Pediatr Neurol. 2011;44:439–442. doi: 10.1016/j.pediatrneurol.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Riesgo R., Winckler M.I., Ohlweiler L., Ranzan J., Becker M., Salvador S., et al. Treatment of refractory neonatal seizures with topiramate. Neuropediatrics. 2012;43:353–356. doi: 10.1055/s-0032-1327771. [DOI] [PubMed] [Google Scholar]
- 70.Venkatesan C., Young S., Schapiro M., Thomas C. Levetiracetam for the treatment of seizures in neonatal hypoxic ischemic encephalopathy. J Child Neurol. 2017;32:210–214. doi: 10.1177/0883073816678102. [DOI] [PubMed] [Google Scholar]
- 71.Nagarajan L., Ghosh G. Neonatal seizures current management and future challenges. MacKeith Press; London: 2016. The role of the video EEG in neonates with seizures; pp. 12–29. [Google Scholar]
- 72.Sharpe C.M., Davis S.L., Reiner G.E., Lee L.I., Gold J.J., Nespeca M., et al. Assessing the feasibility of providing a real-time response to seizures detected with continuous long-term neonatal electroencephalography monitoring. J Clin Neurophysiol. 2019;36:9–13. doi: 10.1097/WNP.0000000000000525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fitzgerald M.P., Massey S.L., Fung F.W., Puopolo K.M., Posencheg M., Allen-Napoli L., et al. Expanding access to continuous EEG monitoring in neonatal intensive care units. J Clin Neurophysiol. 2021;38:525–529. doi: 10.1097/WNP.0000000000000730. [DOI] [PubMed] [Google Scholar]
- 74.Macdonald-Laurs E., Sharpe C., Nespeca M., Rismanchi N., Gold J.J., Kuperman R., et al. Does the first hour of continuous electroencephalography predict neonatal seizures? Arch Dis Child Fetal Neonatal Ed. 2021;106:162–167. doi: 10.1136/archdischild-2020-318985. [DOI] [PubMed] [Google Scholar]
- 75.Boylan G., Burgoyne L., Moore C., O'Flaherty B., Rennie J. An international survey of EEG use in the neonatal intensive care unit. Acta Paediatr. 2010;99:1150–1155. doi: 10.1111/j.1651-2227.2010.01809.x. [DOI] [PubMed] [Google Scholar]
- 76.Nunes M.L., Yozawitz E.G., Zuberi S., Mizrahi E.M., Roberta Cilio M., Moshé S.L., et al. Neonatal seizures: is there a relationship between ictal electroclinical features and etiology? A critical appraisal based on a systematic literature review. Epilepsia Open. 2019;4:10–29. doi: 10.1002/epi4.12298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rakshasbhuvankar A., Rao S., Ghosh S., Nathan E.A., Nagarajan L. Why do neonates receive antiseizure medications? J Matern Neonatal Med. 2020;0:1–5. doi: 10.1080/14767058.2020.1819976. [DOI] [PubMed] [Google Scholar]
- 78.Pisani F., Fusco C., Nagarajan L., Spagnoli C. Acute symptomatic neonatal seizures, brain injury, and long-term outcome: the role of neuroprotective strategies. Expert Rev Neurother. 2021;21:189–203. doi: 10.1080/14737175.2021.1848547. [DOI] [PubMed] [Google Scholar]
- 79.Bashir R.A., Espinoza L., Vayalthrikkovil S., Buchhalter J., Irvine L., Bello-Espinosa L., et al. Implementation of a neurocritical care program: improved seizure detection and decreased antiseizure medication at discharge in neonates with hypoxic-ischemic encephalopathy. Pediatr Neurol. 2016;64:38–43. doi: 10.1016/j.pediatrneurol.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 80.Massey S.L., Jensen F.E., Abend N.S. Electroencephalographic monitoring for seizure identification and prognosis in term neonates. Semin Fetal Neonatal Med. 2018;23:168–174. doi: 10.1016/j.siny.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 81.Hellstrom-Westas L., Blennow G., Lindroth M., Rosen I., Svenningsen N.W. Low risk of seizure recurrence after early withdrawal of antiepileptic treatment in the neonatal period. Arch Dis Child Fetal Neonatal Ed. 1995;72:F97–F101. doi: 10.1136/fn.72.2.f97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gray J., Geva A., Zheng Z., Zupancic J.A.F. CoolSim: using industrial modeling techniques to examine the impact of selective head cooling in a model of perinatal regionalization. Pediatrics. 2008;121:28–36. doi: 10.1542/peds.2007-0633. [DOI] [PubMed] [Google Scholar]
- 83.Harris M.L., Malloy K.M., Lawson S.N., Rose R.S., Buss W.F., Mietzsch U., et al. Standardized treatment of neonatal status epilepticus improves outcome. J Child Neurol. 2016;31:1546–1554. doi: 10.1177/0883073816664670. [DOI] [PubMed] [Google Scholar]
- 84.Yozawitz E., Stacey A., Pressler R.M. Pharmacotherapy for seizures in neonates with hypoxic ischemic encephalopathy. Pediatr Drugs. 2017:1–15. doi: 10.1007/s40272-017-0250-4. [DOI] [PubMed] [Google Scholar]
- 85.Pavel A.M., Rennie J.M., de Vries L.S., Blennow M., Foran A., Shah D.K., et al. Neonatal seizure management: is the timing of treatment critical? J Pediatr. 2022;243:61–68.e2. doi: 10.1016/j.jpeds.2021.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Aslam S., Strickland T., Molloy E.J. Neonatal encephalopathy: need for recognition of multiple etiologies for optimal management. Front Pediatr. 2019;7 doi: 10.3389/fped.2019.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chong D.J., Hirsch L.J. Which EEG patterns warrant treatment in the critically ill? Reviewing the evidence for treatment of periodic epileptiform discharges and related patterns. J Clin Neurophysiol. 2005;22:79–91. doi: 10.1097/01.wnp.0000158699.78529.af. [DOI] [PubMed] [Google Scholar]
- 88.Sánchez S.M., Arndt D.H., Carpenter J.L., Chapman K.E., Cornett K.M., Dlugos D.J., et al. Electroencephalography monitoring in critically ill children: current practice and implications for future study design. Epilepsia. 2013;54:1419–1427. doi: 10.1111/epi.12261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sanchez S.M., Carpenter J., Chapman K.E., Dlugos D.J., Gallentine W.B., Giza C.C., et al. Pediatric ICU EEG monitoring. J Clin Neurophysiol. 2013;30:156–160. doi: 10.1097/WNP.0b013e31827eda27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fung F.W., Fan J., Vala L., Jacobwitz M., Parikh D.S., Donnelly M., et al. EEG monitoring duration to identify electroencephalographic seizures in critically ill children. Neurology. 2020 doi: 10.1212/WNL.0000000000010421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vlachy J., Jo M., Li Q., Ayer T., Keskinocak P., Swann J., et al. Risk factors for seizures among young children monitored with continuous electroencephalography in intensive care unit: a retrospective study. Front Pediatr. 2018;6:1–7. doi: 10.3389/fped.2018.00303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Abend N.S., Topjian A.A., Gutierrez-Colina A.M., Donnelly M., Clancy R.R., Dlugos D. Impact of continuous EEG monitoring on clinical management in critically ill children. Neurocrit Care. 2011;15:70–75. doi: 10.1007/s12028-010-9380-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kirkham F.J., Wade A.M., McElduff F., Boyd S.G., Tasker R.C., Edwards M., et al. Seizures in 204 comatose children: incidence and outcome. Intensive Care Med. 2012;38:853–862. doi: 10.1007/s00134-012-2529-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gaínza-Lein M., Sánchez Fernández I., Loddenkemper T. Use of EEG in critically ill children and neonates in the United States of America. J Neurol. 2017 doi: 10.1007/s00415-017-8510-3. [DOI] [PubMed] [Google Scholar]
- 95.Abend N.S., Gutierrez-Colina A.M., Topjian A.A., Zhao H., Guo R., Donnelly M., et al. Nonconvulsive seizures are common in critically ill children. Neurology. 2011;76:1071–1077. doi: 10.1212/WNL.0b013e318211c19e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Abend N.S., Dlugos D.J., Clancy R.R. A review of long-term EEG monitoring in critically ill children with hypoxic-ischemic encephalopathy, congenital heart disease, ECMO, and stroke. J Clin Neurophysiol. 2013;30:134–142. doi: 10.1097/WNP.0b013e3182872af9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Williams R.P., Banwell B., Berg R.A., Dlugos D.J., Donnelly M., Ichord R., et al. Impact of an ICU EEG monitoring pathway on timeliness of therapeutic intervention and electrographic seizure termination. Epilepsia. 2016;57:786–795. doi: 10.1111/epi.13354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Brophy G.M., Bell R., Claassen J., Alldredge B., Bleck T.P., Glauser T., et al. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care. 2012;17:3–23. doi: 10.1007/s12028-012-9695-z. [DOI] [PubMed] [Google Scholar]
- 99.Payne E.T., Zhao X.Y., Frndova H., McBain K., Sharma R., Hutchison J.S., et al. Seizure burden is independently associated with short term outcome in critically ill children. Brain. 2014 doi: 10.1093/brain/awu042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Schreiber J.M., Zelleke T., Gaillard W.D., Kaulas H., Dean N., Carpenter J.L. Continuous video EEG for patients with acute encephalopathy in a pediatric intensive care unit. Neurocrit Care. 2012;17:31–38. doi: 10.1007/s12028-012-9715-z. [DOI] [PubMed] [Google Scholar]
- 101.McCoy B., Sharma R., Ochi A., Go C., Otsubo H., Hutchison J.S., et al. Predictors of nonconvulsive seizures among critically ill children. Epilepsia. 2011;52:1973–1978. doi: 10.1111/j.1528-1167.2011.03291.x. [DOI] [PubMed] [Google Scholar]
- 102.Gwer S., Idro R., Fegan G., Chengo E., Garrashi H., White S., et al. Continuous EEG monitoring in Kenyan children with non-traumatic coma. Arch Dis Child. 2012;97:343–349. doi: 10.1136/archdischild-2011-300935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ramantani G., Schmitt B., Plecko B., Pressler R.M., Wohlrab G., Klebermass-Schrehof K., et al. Neonatal seizures—are we there yet? Neuropediatrics. 2019;50:280–293. doi: 10.1055/s-0039-1693149. [DOI] [PubMed] [Google Scholar]
- 104.Abend N.S., Arndt D.H., Carpenter J.L., Chapman K.E., Cornett K.M., Gallentine W.B., et al. Electrographic seizures in pediatric ICU patients: cohort study of risk factors and mortality. Neurology. 2013;81:383–391. doi: 10.1212/WNL.0b013e31829c5cfe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yang A., Arndt D.H., Berg R.A., Carpenter J.L., Chapman K.E., Dlugos D.J., et al. Development and validation of a seizure prediction model in critically ill children. Seizure. 2015;25:104–111. doi: 10.1016/j.seizure.2014.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fung F.W., Jacobwitz M., Parikh D.S., Vala L., Donnelly M., Fan J., et al. Development of a model to predict electroencephalographic seizures in critically ill children. Epilepsia. 2020;61:498–508. doi: 10.1111/epi.16448. [DOI] [PubMed] [Google Scholar]
- 107.Shahwan A., Bailey C., Shekerdemian L., Harvey A.S. The prevalence of seizures in comatose children in the pediatric intensive care unit: a prospective video-EEG study. Epilepsia. 2010;51:1198–1204. doi: 10.1111/j.1528-1167.2009.02517.x. [DOI] [PubMed] [Google Scholar]
- 108.Gutierrez-Colina A.M., Topjian A.A., Dlugos D.J., Abend N.S. Electroencephalogram monitoring in critically ill children: indications and strategies. Pediatr Neurol. 2012;46:158–161. doi: 10.1016/j.pediatrneurol.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fernández I.S., Sansevere A.J., Gaínza-Lein M., Buraniqi E., Tasker R.C., Loddenkemper T., et al. Time to continuous electroencephalogram in repeated admissions to the pediatric intensive care unit. Seizure. 2018;54:19–26. doi: 10.1016/j.seizure.2017.11.013. [DOI] [PubMed] [Google Scholar]
- 110.Wilson C.A. Continuous electroencephalogram detection of non-convulsive seizures in the pediatric intensive care unit: review of the utility and impact on management and outcomes. Transl Pediatr. 2015;4:283–289. doi: 10.3978/j.issn.2224-4336.2015.10.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Topjian A.A., Gutierrez-Colina A.M., Sanchez S.M., Berg R.A., Friess S.H., Dlugos D.J., et al. Electrographic status epilepticus is associated with mortality and worse short-term outcome in critically III children. Crit Care Med. 2013;41:215–223. doi: 10.1097/CCM.0b013e3182668035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Greiner H.M., Holland K., Leach J.L., Horn P.S., Hershey A.D., Rose D.F., et al. Nonconvulsive status epilepticus: the encephalopathic pediatric patient. Pediatrics. 2012;129:e748–e755. doi: 10.1542/peds.2011-2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jette N., Claassen J., Emerson R.G., Hirsch L.J. Frequency and predictors of nonconvulsive seizures during continuous electroencephalographic monitoring in critically ill children. Arch Neurol. 2006;63:1750–1755. doi: 10.1001/archneur.63.12.1750. [DOI] [PubMed] [Google Scholar]
- 114.Pinchefsky E.F., Hahn C.D. Outcomes following electrographic seizures and electrographic status epilepticus in the pediatric and neonatal ICUs. Curr Opin Neurol. 2017;30:156–164. doi: 10.1097/WCO.0000000000000425. [DOI] [PubMed] [Google Scholar]
- 115.Fernández I.S., Sansevere A.J., Guerriero R.M., Buraniqi E., Pearl P.L., Tasker R.C., et al. Time to electroencephalography is independently associated with outcome in critically ill neonates and children. Epilepsia. 2017;58:420–428. doi: 10.1111/epi.13653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Abend N.S., Wagenman K.L., Blake T.P., Schultheis M.T., Radcliffe J., Berg R.A., et al. Electrographic status epilepticus and neurobehavioral outcomes in critically ill children. Epilepsy Behav. 2015;49:238–244. doi: 10.1016/j.yebeh.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lalgudi Ganesan S., Hahn C.D. Electrographic seizure burden and outcomes following pediatric status epilepticus. Epilepsy Behav. 2019;101 doi: 10.1016/j.yebeh.2019.07.010. [DOI] [PubMed] [Google Scholar]
- 118.Wickstrom R., Taraschenko O., Dilena R., Payne E.T., Specchio N., Nabbout R., et al. International consensus recommendations for management of New Onset Refractory Status Epilepticus (NORSE) including Febrile Infection-Related Epilepsy Syndrome (FIRES): summary and clinical tools. Epilepsia. 2022:1–13. doi: 10.1111/epi.17391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fung F.W., Jacobwitz M., Vala L., Parikh D., Donnelly M., Xiao R., et al. Electroencephalographic seizures in critically ill children: management and adverse events. Epilepsia. 2019;60:2095–2104. doi: 10.1111/epi.16341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fernández I.S., Abend N.S., Agadi S., An S., Arya R., Brenton J.N., et al. Time from convulsive status epilepticus onset to anticonvulsant administration in children. Neurology. 2015;84:2304–2311. doi: 10.1212/WNL.0000000000001673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Abend N.S., Chapman K.E., Gallentine W.B., Goldstein J., Hyslop A.E., Loddenkemper T., et al. Electroencephalographic monitoring in the pediatric intensive care unit. Curr Neurol Neurosci Rep. 2013;13:330. doi: 10.1007/s11910-012-0330-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Payne E.T., Zhao X.Y., Frndova H., McBain K., Sharma R., Hutchison J.S., et al. Seizure burden is independently associated with short term outcome in critically ill children. Brain. 2014;137:1429–1438. doi: 10.1093/brain/awu042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Abend N.S., Topjian A.A., Williams S. How much does it cost to identify a critically ill child experiencing electrographic seizures? J Clin Neurophysiol. 2015;32:257–264. doi: 10.1097/WNP.0000000000000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Abend N.S., Topjian A.A., Williams S. Could EEG monitoring in critically ill children be a cost-effective neuroprotective strategy? J Clin Neurophysiol. 2015;32:486–494. doi: 10.1097/WNP.0000000000000198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Claassen J., Albers D., Michael Schmidt J., De Marchis G.M., Pugin D., Maria Falo C., et al. Nonconvulsive seizures in subarachnoid hemorrhage link inflammation and outcome. Ann Neurol. 2014;75:771–781. doi: 10.1002/ana.24166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Vespa P.M., Miller C., McArthur D., Eliseo M., Etchepare M., Hirt D., et al. Nonconvulsive electrographic seizures after traumatic brain injury result in a delayed, prolonged increase in intracranial pressure and metabolic crisis. Crit Care Med. 2007;35:2830–2836. [PMC free article] [PubMed] [Google Scholar]
- 127.Freund B., Probasco J.C., Ritzl E.K. Seizure incidence in the acute postneurosurgical period diagnosed using continuous electroencephalography. J Neurosurg. 2019;130:1203–1209. doi: 10.3171/2018.1.JNS171466. [DOI] [PubMed] [Google Scholar]
- 128.Towne A.R., Waterhouse E.J., Boggs J.G., Garnett L.K., Brown A.J., Smith J.R., et al. Prevalence of nonconvulsive status epilepticus in comatose patients. Neurology. 2000;54:340–345. doi: 10.1212/wnl.54.2.340. [DOI] [PubMed] [Google Scholar]
- 129.Alvarez V., Ruiz A.A.R., LaRoche S., Hirsch L.J., Parres C., Voinescu P.E., et al. The use and yield of continuous EEG in critically ill patients: a comparative study of three centers. Clin Neurophysiol. 2017;128:570–578. doi: 10.1016/j.clinph.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 130.Limotai C., Ingsathit A., Thadanipon K., McEvoy M., Attia J., Thakkinstian A., et al. How and whom to monitor for seizures in an ICU: a systematic review and meta-analysis. Crit Care Med. 2019;47:e366–e373. doi: 10.1097/CCM.0000000000003641. [DOI] [PubMed] [Google Scholar]
- 131.DeLorenzo R.J., Waterhouse E.J., Towne A.R., Boggs J.G., Ko D., DeLorenzo G.A., et al. Persistent nonconvulsive status epilepticus after the control of convulsive status epilepticus. Epilepsia. 1998;39:833–840. doi: 10.1111/j.1528-1157.1998.tb01177.x. [DOI] [PubMed] [Google Scholar]
- 132.Westover M.B., Shafi M.M., Bianchi M.T., Moura L.M.V.R., O'Rourke D., Rosenthal E.S., et al. The probability of seizures during EEG monitoring in critically ill adults. Clin Neurophysiol. 2015;126:463–471. doi: 10.1016/j.clinph.2014.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Oddo M., Carrera E., Claassen J., Mayer S.A., Hirsch L.J. Continuous electroencephalography in the medical intensive care unit. Crit Care Med. 2009;37:2051–2056. doi: 10.1097/CCM.0b013e3181a00604. [DOI] [PubMed] [Google Scholar]
- 134.Ney J.P., Van Der Goes D.N., Nuwer M.R., Nelson L., Eccher M.A. Continuous and routine EEG in intensive care. Neurology. 2013;81:2002–2008. doi: 10.1212/01.wnl.0000436948.93399.2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Young B.G., Jordan K.G., Doig G.S. An assessment of nonconvulsive seizures in the intensive care unit using continuous EEG monitoring. Neurology. 1996;47:83–89. doi: 10.1212/wnl.47.1.83. [DOI] [PubMed] [Google Scholar]
- 136.Vespa P.M., Nuwer M.R., Nenov V., Ronne-Engstrom E., Hovda D.A., Bergsneider M., et al. Increased incidence and impact of nonconvulsive and convulsive seizures after traumatic brain injury as detected by continuous electroencephalographic monitoring. J Neurosurg. 1999;91:750–760. doi: 10.3171/jns.1999.91.5.0750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kubota Y., Nakamoto H., Egawa S., Kawamata T. Continuous EEG monitoring in ICU. J Intensive Care. 2018;6:1–8. doi: 10.1186/s40560-018-0310-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Newey C.R., Kinzy T.G., Punia V., Hantus S. Continuous electroencephalography in the critically ill: clinical and continuous electroencephalography markers for targeted monitoring. J Clin Neurophysiol. 2018;35:325–331. doi: 10.1097/WNP.0000000000000475. [DOI] [PubMed] [Google Scholar]
- 139.Hill C.E., Blank L.J., Thibault D., Davis K.A., Dahodwala N., Litt B., et al. Continuous EEG is associated with favorable hospitalization outcomes for critically ill patients. Neurology. 2018 doi: 10.1212/WNL.0000000000006689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Struck A.F., Osman G., Rampal N., Biswal S., Legros B., Hirsch L.J., et al. Time-dependent risk of seizures in critically ill patients on continuous electroencephalogram. Ann Neurol. 2017;82:177–185. doi: 10.1002/ana.24985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Shafi M.M., Westover M.B., Cole A.J., Kilbride R.D., Hoch D.B., Cash S.S., et al. Absence of early epileptiform abnormalities predicts lack of seizures on continuous EEG. Neurology. 2012;79:1796–1801. doi: 10.1212/WNL.0b013e3182703fbc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ruiz A.R., Vlachy J., Lee J.W., Gilmore E.J., Ayer T., Haider H.A., et al. Association of periodic and rhythmic electroencephalographic patterns with seizures in critically ill patients. JAMA Neurol. 2017;74:181–188. doi: 10.1001/jamaneurol.2016.4990. [DOI] [PubMed] [Google Scholar]
- 143.Struck A.F., Ustun B., Ruiz A.R., Lee J.W., LaRoche S.M., Hirsch L.J., et al. Association of an electroencephalography-based risk score with seizure probability in hospitalized patients. JAMA Neurol. 2017;74:1419–1424. doi: 10.1001/jamaneurol.2017.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Struck A.F., Tabaeizadeh M., Schmitt S.E., Ruiz A.R., Swisher C.B., Subramaniam T., et al. Assessment of the validity of the 2HELPS2B score for inpatient seizure risk prediction. JAMA Neurol. 2020;77:500–507. doi: 10.1001/jamaneurol.2019.4656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.De Marchis G.M., Pugin D., Meyers E., Velasquez A., Suwatcharangkoon S., Park S., et al. Seizure burden in subarachnoid hemorrhage associated with functional and cognitive outcome. Neurology. 2016;86:253–260. doi: 10.1212/WNL.0000000000002281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Vespa P., Tubi M., Claassen J., Buitrago-Blanco M., McArthur D., Velazquez A.G., et al. Metabolic crisis occurs with seizures and periodic discharges after brain trauma. Ann Neurol. 2016;79:579–590. doi: 10.1002/ana.24606. [DOI] [PubMed] [Google Scholar]
- 147.Vespa P.M., Martin N.A., Nenov V., Glenn T., Bergsneider M., Kelly D., et al. Delayed increase in extracellular glycerol with post-traumatic electrographic epileptic activity: support for the theory that seizures induce secondary injury. Acta Neurochir Suppl. 2002:355–357. doi: 10.1007/978-3-7091-6738-0_90. [DOI] [PubMed] [Google Scholar]
- 148.Claassen J., Perotte A., Albers D., Kleinberg S., Schmidt J.M., Tu B., et al. Nonconvulsive seizures after subarachnoid hemorrhage: multimodal detection and outcomes. Ann Neurol. 2013;74:53–64. doi: 10.1002/ana.23859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Witsch J., Frey H.P., Schmidt J.M., Velazquez A., Falo C.M., Reznik M., et al. Electroencephalographic periodic discharges and frequency-dependent brain tissue hypoxia in acute brain injury. JAMA Neurol. 2017;74:301–309. doi: 10.1001/jamaneurol.2016.5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kurtz P., Gaspard N., Wahl A.S., Bauer R.M., Hirsch L.J., Wunsch H., et al. Continuous electroencephalography in a surgical intensive care unit. Intensive Care Med. 2014;40:228–234. doi: 10.1007/s00134-013-3149-8. [DOI] [PubMed] [Google Scholar]
- 151.Waterhouse E.J., Vaughan J.K., Barnes T.Y., Boggs J.G., Towne A.R., Kopec-Garnett L., et al. Synergistic effect of status epilepticus and ischemic brain injury on mortality. Epilepsy Res. 1998;29:175–183. doi: 10.1016/s0920-1211(97)00071-5. [DOI] [PubMed] [Google Scholar]
- 152.Claassen J., Jetté N., Chum F., Green R., Schmidt M., Choi H., et al. Electrographic seizures and periodic discharges after intracerebral hemorrhage. Neurology. 2007;69:1356–1365. doi: 10.1212/01.wnl.0000281664.02615.6c. [DOI] [PubMed] [Google Scholar]
- 153.Sculier C., Gaínza-Lein M., Sánchez Fernández I., Loddenkemper T. Long-term outcomes of status epilepticus: a critical assessment. Epilepsia. 2018;59:155–169. doi: 10.1111/epi.14515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Khawaja A.M., Wang G., Cutter G.R., Szaflarski J.P. Continuous electroencephalography (cEEG) monitoring and outcomes of critically ill patients. Med Sci Mon Int Med J Exp Clin Res. 2017;23:649–658. doi: 10.12659/MSM.900826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Alvarez V., Drislane F.W., Westover M.B., Dworetzky B.A., Lee J.W. Characteristics and role in outcome prediction of continuous EEG after status epilepticus: a prospective observational cohort. Epilepsia. 2015;56:933–941. doi: 10.1111/epi.12996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Crepeau A.Z., Fugate J.E., Mandrekar J., White R.D., Wijdicks E.F., Rabinstein A.A., et al. Value analysis of continuous EEG in patients during therapeutic hypothermia after cardiac arrest. Resuscitation. 2014;85:785–789. doi: 10.1016/j.resuscitation.2014.01.019. [DOI] [PubMed] [Google Scholar]
- 157.Rossetti A.O., Hirsch L.J., Drislane F.W. Nonconvulsive seizures and nonconvulsive status epilepticus in the neuro ICU should or should not be treated aggressively: a debate. Clin Neurophysiol Pract. 2019;4:170–177. doi: 10.1016/j.cnp.2019.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Rossetti A.O., Schindler K., Sutter R., Rüegg S., Zubler F., Novy J., et al. Continuous vs routine electroencephalogram in critically ill adults with altered consciousness and no recent seizure. JAMA Neurol. 2020:1–8. doi: 10.1001/jamaneurol.2020.2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Le Roux P., Menon D.K., Citerio G., Vespa P., Bader M.K., Brophy G., et al. The International Multidisciplinary Consensus Conference on Multimodality Monitoring in Neurocritical Care: evidentiary tables: a statement for healthcare professionals from the Neurocritical Care Society and the European Society of Intensive Care Medicine. Neurocrit Care. 2014;21:297–361. doi: 10.1007/s12028-014-0081-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hirsch L.J., LaRoche S.M., Gaspard N., Gerard E., Svoronos A., Herman S.T., et al. American Clinical Neurophysiology Society's standardized critical care EEG terminology: 2012 version. J Clin Neurophysiol. 2013;30:1–27. doi: 10.1097/WNP.0b013e3182784729. [DOI] [PubMed] [Google Scholar]
- 161.Herman S.T., Abend N.S., Bleck T.P., Chapman K.E., Drislane F.W., Emerson R.G., et al. Consensus statement on continuous EEG in critically ill adults and children, part II: personnel, technical specifications, and clinical practice. J Clin Neurophysiol. 2015;32 doi: 10.1097/WNP.0000000000000165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Beuchat I., Rossetti A.O., Novy J., Schindler K., Rüegg S., Alvarez V., et al. Continuous versus routine standardized electroencephalogram for outcome prediction in critically ill adults: analysis from a randomized trial. Crit Care Med. 2022;50:329–334. doi: 10.1097/CCM.0000000000005311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sansevere A.J., Duncan E.D., Libenson M.H., Loddenkemper T., Pearl P.L., Tasker R.C., et al. Continuous EEG in pediatric critical care: yield and efficiency of seizure detection. J Clin Neurophysiol. 2017;34:421–426. doi: 10.1097/WNP.0000000000000379. [DOI] [PubMed] [Google Scholar]
- 164.Duncan E., Sansevere A., Tasker R. Electrographic seizures in pediatric critical illness: systematic review of outcome. Crit Care Med. 2015;43:135. [Google Scholar]
- 165.Struck A.F., Rodriguez-Ruiz A.A., Osman G., Gilmore E.J., Haider H.A., Dhakar M.B., et al. Comparison of machine learning models for seizure prediction in hospitalized patients. Ann Clin Transl Neurol. 2019;6:1239–1247. doi: 10.1002/acn3.50817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Westhall E., Rossetti A.O., van Rootselaar A.-F., Kjaer T.W., Horn J., Ullén S., et al. Standardized EEG interpretation accurately predicts prognosis after cardiac arrest. Neurology. 2016;86:1482–1490. doi: 10.1212/WNL.0000000000002462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Mandel R., Martinot A., Delepoulle F., Lamblin M., Laureau E., Vallee L., et al. Prediction of outcome after hypoxic-ischemic encephalopathy: a prospective clinical and electrophysiologic study. J Pediatr. 2002;141:45–50. doi: 10.1067/mpd.2002.125005. [DOI] [PubMed] [Google Scholar]
- 168.Leitinger M., Beniczky S., Rohracher A., Gardella E., Kalss G., Qerama E., et al. Salzburg consensus criteria for non-convulsive status epilepticus – approach to clinical application. Epilepsy Behav. 2015;49:158–163. doi: 10.1016/j.yebeh.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 169.Trinka E., Cock H., Hesdorffer D., Rossetti A.O., Scheffer I.E., Shinnar S., et al. A definition and classification of status epilepticus – report of the ILAE Task Force on Classification of Status Epilepticus. Epilepsia. 2015;56:1515–1523. doi: 10.1111/epi.13121. [DOI] [PubMed] [Google Scholar]
- 170.Gaspard N., Hirsch L.J., LaRoche S.M., Hahn C.D., Brandon M. Interrater agreement for critical care EEG terminology. Epilepsia. 2014;55:1366–1373. doi: 10.1111/epi.12653. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.