Abstract
Introduction:
Oral health is an essential non-integrated part of general health that plays a vital role in preventing chronic diseases. The oral cavity acts as a suitable environment for the proliferation of bacteria by forming a connecting link to invade the tissues through direct contact from outside. For the past few decades, there has been increased resistance of human pathogenic bacteria to the currently used antibiotics and chemotherapeutics for tooth decay, gingivitis, periodontitis and fungal infection among different age groups. Hence, the search has shifted to traditional plants and natural products, which are a good alternative. To create oral hygiene solutions for the prevention of oral infections, several ayurvedic ingredients, including Andrographis paniculata and Mimusops elengi, have been tested for their effectiveness against dental pathogens. The present investigation's purpose is to determine the minimum inhibitory concentration-based antibacterial efficiency of Andrographis paniculata and Mimusops elengi against Streptococcus mutans, Lactobacillus acidophilus, Actinomyces and Candida albicans.
Methodology:
Antimicrobial activity of herbal extracts was determined using the agar well diffusion method. Ethanolic extracts were prepared using a cold extraction method whereas Dimethyl sulfoxide and water were used as dissolution solvents. The diluted herbal extract sample was used as the test sample, while the positive control used was an antibiotic solution and the negative control used was dissolution solvents. The samples were implanted, the bacteria along with the culture media were incubated, and the zone of inhibition was measured.
Results:
The Minimum inhibitory concentration and zones of inhibition of Andrographis Paniculata and Mimusops Elengi showed significant antibacterial efficacy when compared with standards.
Conclusion:
Andrographis Paniculata and Mimusops Elengi may be used as an efficient addition to conventional care in the management of oral disorders, according to their antimicrobial efficacy.
Keywords: Andrographis paniculata, antimicrobial activity, mimusops elengi, oral microflora
INTRODUCTION
More than 500 diverse species, both aerobic and anaerobic, are found in the oral cavity and are linked to a variety of oral illnesses. Since oral health is a vital and integral component of overall health, it is crucial in preventing chronic diseases like diabetes, endocarditis and other conditions.[1] The most common lesion affecting the oral cavity is dental caries and candidiasis, which are seen frequently in all age groups.
Microorganisms play a key role in the enhancement of cariogenicity and production of extracellular polysaccharides, facilitating their adhesion to oral and dental tissues and causing massive destruction. The long-standing inhabitation of microorganisms like Streptococcus mutans, Lactobacillus acidophilus, Actinomyces and Candida albicans compromises oral hygiene and promotes systemic complications. The participation of microorganisms in both oral and non-oral diseases has created a global public health problem whose control requires the introduction of new herbal products for prevention.[2]
Ayurveda is an age-old science of life using herbs with medicinal properties as an effective source of treatment of various diseases and excluding the possibility of side effects.[3] The correlation of dental lesions with Ayurvedic treatment is an exclusive topic of discussion in the present era. Among all dental diseases, dental caries and candidiasis are elusively epidemiological diseases, the eradication of which is a far-fetched dream.
The untouched and more potential ayurvedic substances[4] are yet to be evaluated, among which Andrographis paniculata and Mimusops elengi are yet to be explored in the field of dentistry. Andrographis paniculata, an herb (Nilavembu), belongs to the genus Andrographis and has been widely used for decades due to its known biological activities like anti-viral and immune-stimulant properties. Mimusops elengi, locally named “Magizham”, possesses several medicinal properties viz., astringent, analgesic, anti-Pyretic and anti-inflammatory properties.
The purpose of the study was to evaluate the antibacterial efficacy as well as the minimum inhibitory concentration (MIC) of Andrographis paniculata and Mimusops elengi against streptococcus mutans, lactobacillus acidophilus, actinomyces and candida albicans.
METHODOLOGY
Randomly, 5 adult patients (aged 17–50 yrs) were recruited and consented to collection of saliva samples from the Department of Oral Pathology and Microbiology, Chettinad Dental College and Research Institute, Chennai. Inclusion criteria stipulated that subjects should be healthy, not under any antibiotic coverage within 2 weeks prior to the sample collection and with all permanent molars fully erupted in the oral cavity. This study was approved by the Institutional Human ethics committee, Chettinad Academy of Research and Education (205/IHEC/1-19).
Bacterial isolates were obtained from patients with high caries risk. Saliva samples were collected from patients with high-risk caries using the Navazesh method.[5] Bacterial culturing and identification of species were done at the Department of Microbiology, Chettinad Dental College and Research Institute using selective growth media. Andrographis paniculata leaves and Mimusops elengi flowers were collected and left to dry at room temperature for 24 hours [Figure 1]. Later they were ground to powder and stored in dry containers. The extracts were prepared by a cold extraction method by mixing 100 gm of powder with 300 ml of ethanol and subjected to cold maceration process. The flask was kept for 3 days with occasional shaking. The sample was then filtered using a sterile muslin cloth. The filtrate was subjected to drying till the solvent was completely evaporated. The dried extract (Total yield 2g) was obtained and used further for antimicrobial activity.
Figure 1.

Andrographis paniculata and Mimusops elengi
Preparation of stock solution of the extract
To get an extract concentration of 10%, one gram of extract was diluted in 10 ml of dimethylformamide. To create various extract concentrations, the residual extract was then further diluted with dimethylformamide (0.5%, 1%, 2%, 5% and 10%).
Antimicrobial activity test
The antimicrobial activity of herbal extracts was determined using the Agar well diffusion method.[6] Mullar Hinton agar (Himedia – M173) was used with different diluted extract concentrations (0.5%, 1%, 2%, 5% and 10%). On the agar, 0.1 ml with 105 CFU/ml (0.5 McFarland) were dispersed. The antimicrobial activity of herbal extracts was indicated by a zone of inhibition measured using a Vernier caliper [Figures 2 and 3]. In this method, 0.5 McFarland standard culture (Himedia – R092) (1.5 × 108 CFU mL − 1) for each test microbe was prepared in Muller Hinton broth. MIC was determined using Maki's method, 1985.[7] The MIC value was determined to be the lowest concentration at which a colour change occurred [Figure 4].
Figure 2.

Zone of inhibition of herbal extracts against S. mutans and Lactobacillus
Figure 3.
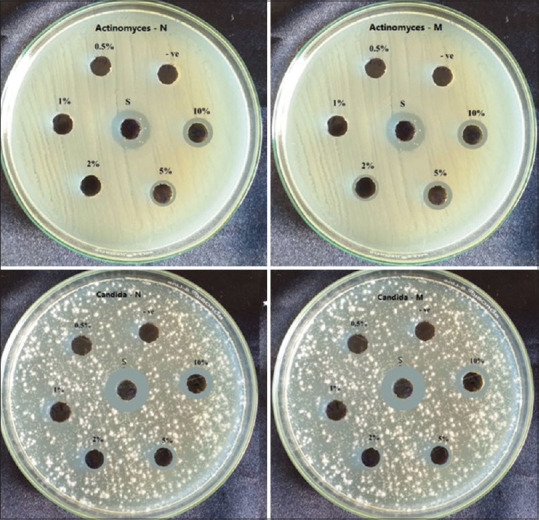
Zone of inhibition of herbal extracts against Actinomyces and Candida albicans
Figure 4.
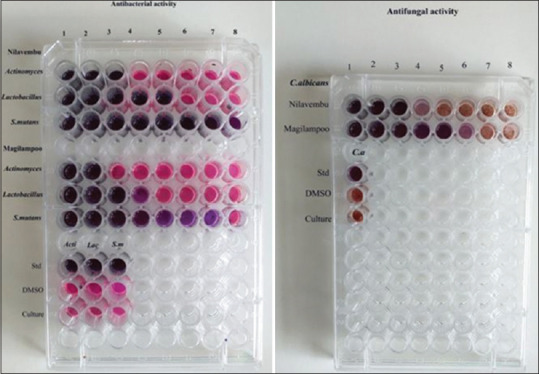
Minimum inhibitory concentration of extracts
Statistical analysis
The experimental results were performed in triplicate and the results were expressed as mean ± Standard Deviation (SD) for 4 isolates of every type of bacterium. The calculation was done using Microsoft Excel 2010 software.
All the experimental analyses were performed in triplicate and the results were expressed as the mean for every type of bacteria on both the extracts. The calculation was done using Microsoft Excel 2010 software. The descriptive analysis of numerical variables encompasses the calculation of the mean (M). Descriptive statistics for antimicrobial activity of the ethanolic extracts in terms of zone of inhibition and MIC against the positive controls are tabulated [Tables 1 and 2].
Table 1.
Growth of inhibition
| Microorganisms/ Sample | Zone of Inhibition in mm |
||||||
|---|---|---|---|---|---|---|---|
| 0.50% | 1% | 2% | 5% | 10% | DMSO (15 µl) | Streptomycin (15 µl) | |
| Streptococcus mutans | |||||||
| Nilavembu | 0 | 2 | 5 | 8 | 12 | 0 | 20 |
| Magilampoo | 0 | 1 | 2 | 6 | 10 | 0 | 15 |
| Actinomyces | |||||||
| Nilavembu | 0 | 0 | 0 | 2 | 4 | 0 | 8 |
| Magilampoo | 0 | 0 | 2 | 5 | 8 | 0 | 12 |
| Lactobacillus | |||||||
| Nilavembu | 0 | 2 | 4 | 8 | 15 | 0 | 20 |
| Magilampoo | 0 | 0 | 2 | 4 | 8 | 0 | 20 |
| Candida albicans | |||||||
| Nilavembu | 0 | 0 | 2 | 5 | 8 | 0 | 12 |
| Magilampoo | 0 | 0 | 1 | 4 | 7 | 0 | 10 |
Table 2.
MIC values
| Microorganisms/sample | MIC Value (µg) |
|---|---|
| Nilavembu | |
| Actinomyces | 250 |
| Lactobacillus | 62.5 |
| Streptococcus mutans | 7.8 |
| Candida albicans | 250 |
| Magilampoo | |
| Actinomyces | 500 |
| Lactobacillus | 125 |
| Streptococcus mutans | 15.6 |
| Candida albicans | 32.1 |
RESULTS
The inhibition growth zones of tested microbes measured for each extract were presented in Figures 2-4. The least zones of inhibition were displayed by the negative control and streptomycin exhibited the widest zones of inhibition against all microbial species. Both extracts showed increasing zones of inhibition with increasing concentration against all species.
Andrographis paniculata and Mimusops elengi displayed wide inhibition zones at 5% and 10%. Both extracts were further evaluated to know their MIC. MIC for Andrographis paniculata was observed at concentrations of 250 μg, 62.5 μg, 7.8 μg and 250 μg against Actinomyces, Lactobacillus, S. mutans and Candida, respectively, and for Mimusops elengi was at 500 μg, 125 μg, 15.6 μg and 32.1μg, respectively.
DISCUSSION
Dental caries is a multifactorial infectious disease with Streptococcus mutans playing a central role in the initiation of caries, with further disease progression of dissolution, decalcification and eventually ending in decay happens with the help of Lactobacillus acidophilus and Actinomycetes. In recent studies, they have proposed candida species as one of the carcinogenic microbiota though it is not the real pathogen indirectly helps in biofilm formation.[8] Candida albicans, opportunistic dimorphic fungi are known to cause candidiasis and denture stomatitis. Candida species are able to colonize several surfaces of the oral cavity where occlusal tooth surfaces are a site of high frequency.[9] This property of candida allows it to adhere to tooth and bind to the cariogenic species to cause further tooth destruction.
Given the literature gathered highlighting the association of candida, streptococcus, lactobacillus and actinomyces, saliva sample of a high caries risk index patient was collected and subjected to bacterial and fungal culture. Hence, the search has shifted to traditional plants and natural products, which are a good alternative to conventional antibiotics to fight against these pathogens.
Andrographolide is a major bioactive phytoconstituent found in the leaves of A. paniculata. It is reported to be highly stable and soluble in organic solvents with high antimicrobial activity.[10] A. paniculata has been long perceived as safe for use in Traditional Chinese Medicine (TCM), India and Thailand. Previous studies have shown that 60 μg/mL of A. paniculata is non-toxic to mammalian cells[11] and causes no change in vital signs suggesting its safety for use for medicinal purposes. Moreover, A. paniculata extract offered protection against hepatotoxicity induced by paracetamol[12] and prevented thioacetamide-induced liver cirrhosis in rats. Moreover, due to its antioxidant property, A. paniculata has the ability to scavenge the free radicals produced by the engulfment of microbes by polymorphs which eventually damages the normal healthy cells.
Various parts of the Mimusops elengi have been used in traditional medicine to manage pain, inflammation due to the presence of major active phytochemicals like triterpenoids, cardiac glycosides, quercetin, quercitol, Anthraquinone glycosides, Saponins and 2 antibacterial compounds i.e. 2,3-dihyro-3,3'4'5,7-pentahydroxyflavone and 3,3',4', 5, 7-pentahydroxyflavone responsible for providing several benefits like anti-inflammatory, antihyperglycemic, antioxidant, antibacterial, anticarcinogenic, etc.[13] In Ayurveda, M. elengi has been reported to be used for arresting bleeding gums.[14]
Many studies conducted by Mishra US et al.,[15] Singha PK et al.,[16] Sircar B et al.,[17] Mistry KS et al.[18] have all shown the antimicrobial properties of Andrographis paniculata and Mimusops elengi against different organisms claiming that these extracts can be used as an effective therapy against various systemic infections. This study was conducted to prove these assumptions and further investigate the antimicrobial effect of A. paniculata and M. elengi against dental pathogens.
At 5% and 10% extracts showed the highest zone of inhibition against lactobacillus than other species [Table 1]. When further evaluated for MIC, Andrographis paniculata showed inhibitory activity at a minimum concentration of 7.8μg/ml and 250 μg/ml and Mimusops elengi of 15.6μg/ml and 32.1 μg/ml against bacteria and fungi, respectively. The tested compounds showed the highest antimicrobial activity at minimum concentration indicating that these extracts can be incorporated in oral rinses, varnishes and dentifrices to improve oral hygiene. The main limitation of this study includes that it was conducted in vitro with the extracts. The response of microorganisms in the oral cavity in vivo with direct contact with the extracts is not clear, hence further clinical studies can help to elucidate the picture.
However, despite the boundaries of the current study, it may be said that Andrographis paniculata and Mimusops elengi could be used as an adjunct to routine care in the management of oral hygiene and cleanliness “if” further research reveals that they are both safe and effective.
CONCLUSION
Clinicians, researchers and the general public are well aware of the danger posed by antibiotic resistance. The proliferation and evolution of antibiotic-resistant components in bacterial infections have increased the virulence of the diseases. Ayurvedic medications can be utilised as a therapy option since they eliminate the root of the ailment, have no side effects and are inexpensive for patients.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Vaou N, Stavropoulou E, Voidarou C, Tsigalou C, Bezirtzoglou E. Towards advances in medicinal plant antimicrobial activity: A review study on challenges and future perspectives. Microorganisms. 2021;9:2041. doi: 10.3390/microorganisms9102041. 10.3390/microorganisms9102041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mallikarjun S, Rao A, Rajesh G, Shenoy R, Pai M. Antimicrobial efficacy of Tulsi leaf (Ocimum sanctum) extract on periodontal pathogens: An in vitro study. J Indian Soc Periodontol. 2016;20:145–50. doi: 10.4103/0972-124X.175177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta R, Ingle NA, Kaur N, Yadav P, Ingle E, Charania Z. Ayurveda in dentistry: A review. J Int Oral Health. 2015;7:141–3. [PMC free article] [PubMed] [Google Scholar]
- 4.Choi J-O, Choi Y-R, Nam S-H. Antibacterial effect of Forsythiae fructus extract in dental caries. Biomed Res. 2019;30:465–8. [Google Scholar]
- 5.Bellagambi FG, Lomonaco T, Salvo P, Vivaldi F, Hangouët M, Ghimenti S, et al. Saliva sampling: Methods and devices. An overview. Trends Analyt Chem. 2020;124:115781. [Google Scholar]
- 6.Khan ZA, Siddiqui MF, Park S. Current and emerging methods of antibiotic susceptibility testing. Diagnostics. 2019;9:49. doi: 10.3390/diagnostics9020049. 10.3390/diagnostics9020049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maki HA. M. Sc. Thesis. College of Medicine, Univ. Baghdad; 1983. Isolation and Identification of pathogenic bacteria encountered in cases of wound infection with their Antibiogram pattern. [Google Scholar]
- 8.Thomas A, Mhambrey S, Chokshi K, Chokshi A, Jana S, Thakur S, et al. Association of oral candida albicans with severe early childhood caries-A pilot study. J Clin Diagn Res. 2016;10:ZC109–12. doi: 10.7860/JCDR/2016/19387.8357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al-Amad SH, Rahman B, Khalifa N, Awad MA. Oral candidal carriage and its association with dental carious lesions in asymptomatic adults: A cross-sectional study from the UAE. BMC Oral Health. 2021;21:197. doi: 10.1186/s12903-021-01559-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hossain S, Urbi Z, Karuniawati H, Mohiuddin RB, Moh Qrimida A, Allzrag AMM, et al. Andrographis paniculata (Burm. f.) Wall. ex Nees: An updated review of phytochemistry, antimicrobial pharmacology, and clinical safety and efficacy. Life (Basel) 2021;11:348. doi: 10.3390/life11040348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jarukamjorn K, Nemoto N. Pharmacological aspects of Andrographis paniculata on health and its major diterpenoid constituent andrographolide, J Health Sci. 2008;54:370–81. [Google Scholar]
- 12.Bothiraja C, Pawar AP, Shende VS, Joshi PP. Acute and subacute toxicity study of andrographolide bioactive in rodents: Evidence for the medicinal use as an alternative medicine. Comp Clin Path. 2013;22:1123–8. [Google Scholar]
- 13.Gupta PC. Mimusops elengi Linn.(Bakul)-A potential medicinal plant: A review. Int J Pharm Phytopharmacol Res. 2013;2:332–9. [Google Scholar]
- 14.Murudkar A, Mundhada SS, Tatke P. Antibacterial activity of Mimusops elengi l. bark against dental pathogens. Ind J Pharm Educ Res. 2007;41:114–20. [Google Scholar]
- 15.Alojid AA, Kub TN, Miran H, Mohamad S, Tuan TN, Yusoff ME, et al. Antibacterial activity of andrographis paniculata aqueous extract against oral pathogens. Malays Appl Biol. 2021;50:163–7. [Google Scholar]
- 16.Pandey J, Saini V, Tiwari S, Raja W. A study of antibacterial activity of andrographis paniculata leaf and stem bark extracts against some clinical pathogenic bacteria's. Asian J Pharm Biol Res. 2019;7:65–70. [Google Scholar]
- 17.Salve AV, Malik R, Ansari S, Uike S. Mimusops elengi (Linn.): An effective aid in dental care. J Glob Oral Health. 2022;5:54–7. [Google Scholar]
- 18.Mistry KS, Sanghvi Z, Parmar G, Shah S, Pushpalatha K. Antibacterial efficacy of Azadirachta indica, Mimusops elengi and 2% CHX on multispecies dentinal biofilm. J Conserv Denti. 2015;18:461. doi: 10.4103/0972-0707.168810. [DOI] [PMC free article] [PubMed] [Google Scholar]


