Abstract
Ameloblastoma (AM) is considered one of the most common lesions of odontogenic origin. Although it is always considered as benign neoplasm, ameloblastic carcinoma (AC) represents its malignant counterpart. It is characterized by the expansion of jaws, rapid growth, and a perforated cortex with well-defined unilocular/multilocular radiolucent lesions. To confirm the diagnosis of AM and AC is extremely crucial. Immunohistochemistry such as SOX2 and Ki67 plays a significant role in the confirmation of diagnosis. Management of these cases is from surgical excision with radical neck dissection. The prognosis is poor with only 5 years of survival. This review presents an interesting case of ex-AC, in which the patient was diagnosed at the same site with peripheral AM 1 year ago.
Keywords: Ameloblastoma, ex-ameloblastic carcinoma, Ki67, odontogenic tumors, SOX2
INTRODUCTION
Odontogenic lesions are derived from odontogenic tissue and include both odontogenic cysts and tumors. Among the odontogenic tumors, Ameloblastoma Conventional is the commonest benign odontogenic neoplasm of the jaws, affecting mainly the posterior region of the mandible. Ameloblastoma (AM) may represent mostly multilocular sometimes unilocular osteolytic lesions that frequently produce cortical expansion. If paresthesia, pain, irregular borders, and invasion of adjacent structures are present, then a malignant lesion should be suspected. Ameloblastic carcinoma (AC) is the primary odontogenic carcinoma and its malignant counterpart.[1] Nevertheless, the differential diagnosis between these two lesions can be difficult, particularly in incisional biopsies, and therefore other types of intraosseous carcinomas of the jaws should be considered.[2,3] In these cases, a marker plays an important role in these worrisome diagnostic conundrums in a pre-existing ameloblastic lesion and in a definitive AC, which would give substantial assistance in facilitating the diagnosis. The role of immunohistochemistry plays an important part to confirm the diagnosis. SOX2 and Ki67 are few markers used to confirm the diagnosis of AC.[4,5]
CASE REPORT
A 66-year-old male patient of average weight and height reported to the Department of Oral Medicine and Radiology with the chief complaint of pain and swelling in his lower left back region of the jaw for 2–3 months. History of present illness revealed patient underwent extraction of 37, 3 months back. A few days after extraction, he noticed a small swelling in the lower left posterior region (extraction site). Initially, the swelling was small, but it gradually increased in size, up to the present condition.
The patient also complained of mild pain, although no bleeding or discharge was associated with this swelling. Medical history as well as personal history was non-significant. In past dental history, the patient explained the same dental problem 1 year ago, soft tissue growth in the lower left posterior region. At that time, an excisional biopsy was done at Dept of Oral Surgery and the patient was diagnosed with peripheral AM [Figure 1]. At that time, healing was uneventful.
Figure 1.
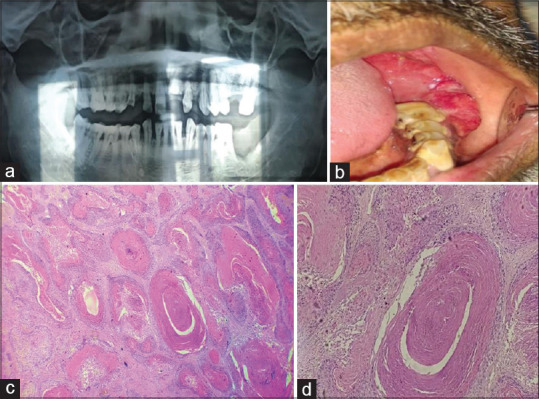
(a-d) 1 year ago, the patient was diagnosed with peripheral AM at the same site. (a) OPG: The swelling is seen in the left lower back region of the jaw extending from the first molar to the retromolar pad area. Saucer-shaped defect in the lesion of the region. (b) Intraoral growth at the same site. (c) The H and E stained tissue section shows connective tissue stroma with epithelial follicles—4×. (d) Squamous metaplasia associated with keratin formation is seen in the central portion of epithelial—10×
After 1 year, again a painful proliferative growth was observed at the same site. On inspection, growth was present in the lower left alveolar ridge region, which extends from 33 to 38 regions and the ascending border of the ramus. The size of the swelling was 4 × 4 × 3 cm in size [Figure 2].
Figure 2.

(a and b) A soft tissue proliferative growth was present in the lower left alveolar ridge region. Growth was extending from 33 to 38 regions and the ascending border of the ramus
Based on the previous history provisional diagnosis was made as “peripheral ameloblastoma.” Ortho Pantogram (OPG) was taken, and it reveals a large radiolucency extending from the 34 region posteriorly to the ascending border of the ramus. Bony erosion of the alveolus is seen. Obliteration of cortical bone presents with ragged borders. Jawbone shows Moth eaten appearance—indicative of carcinoma [Figure 3].
Figure 3.

OPG reveals a large radiolucency extending from the 34 region posteriorly to the ascending border of the ramus. Bony erosion of the alveolus is seen. Obliteration of cortical bone present with ragged borders jawbone shows Moth eaten appearance—indicative of carcinoma
To confirm the diagnosis incisional biopsy was done, after taken patient's consent.
Histopathology
Histopathological examination of H and E section revealed, overlying stratified squamous epithelium showing dysplastic features in the basal layer [Figure 4]. Underlying connective tissue stroma consists of numerical follicles of odontogenic epithelium. Peripheral cells of follicles resemble ameloblast-like cells with palisading nuclei. The central cells are stellate reticulum like, and few follicles represent squamous metaplasia with extensive keratinization [Figure 5]. Many follicles are showing features of dysplasia like cellular pleomorphism, nuclear pleomorphism, increased N/C ratio, multiple nucleoli, and increased and bizarre mitotic figures [Figure 6].
Figure 4.

The H and E stained tissue section shows epithelium and connective tissue stroma with numerous follicles (10×)
Figure 5.

Photomicrograph showing odontogenic epithelial island with peripheral ameloblast-like cells and centrally stellate reticulum-like cells. Also seen is acanthomatous change (squamous metaplasia with keratin pearl formation) within the follicle. Increased N/C ratio also evident (40×)
Figure 6.

Photomicrograph showing an area of the slide which is significant as in this area epithelial cells show numerous dysplastic features such as prominent nucleoli, hyperchromatic nucleus, altered cell shape as well as nucleus shape, increased N/C ratio, aberrant mitotic figures are also seen. (40×)
Overall features suggestive of—ex-AC.
To confirm the diagnosis immunohistochemistry was planned. Recent molecular diagnosis revealed that BRAF (V600E) activating mutation in genetic aberration results in the formation of AMs. BRAF (V600E) mutation might cause to confirm the expansion of SOX2-positive cells. Hence, SOX2 markers were used in the diagnosis of AC. In this case, immunohistochemical marker-SOX2 confirmed the diagnosis of AC [Figure 7].
Figure 7.
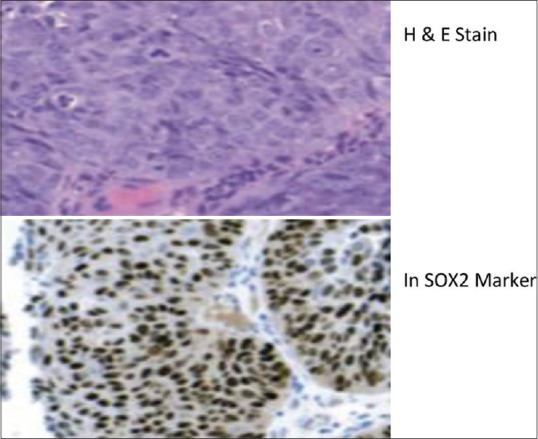
H&E stain and SOX2 Marker
The first case, peripheral acanthomatous ameloblastoma that was diagnosed 1 year ago, was an expansive lesion, and the second one, AC, was destructive.
The treatment advised was complete surgical resection with adjuvant radiation and chemotherapy. Unfortunately, the patient did not undergo treatment strategy because of his poor economic condition and illiteracy. After 3 months, again patient reported with a complaint of enlarged growth with pain [Figure 8]. Still, he did not ready for surgical treatment. After 7 months, his relatives confirmed his demise.
Figure 8.
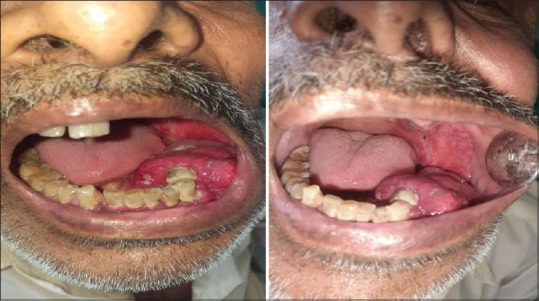
Inra-oral pic of the patient after 3 months
DISCUSSION
The tumor can be defined as a swelling of the tissue that does not imply a neoplastic process.[6] As the name implies, these lesions are exclusively found within the jaw. Odontogenic lesions derived from epithelial, mesenchymal, or ectomesenchyme elements of tooth-forming apparatus. A molecular or genetic mutation alters the development and hence progression of these lesions occurs. Among the odontogenic tumors, AM is one of the most common benign odontogenic neoplasms of the jaws, affecting mainly the posterior region of the mandible. AM may represent mostly multilocular sometimes unilocular osteolytic lesions that frequently produce cortical expansion. If paresthesia, pain, irregular borders, and invasion of adjacent structures are present, then a malignant lesion should be suspected of its malignant counterpart.[7] Ameloblastic neoplasms represent a spectrum of entities that arise from odontogenic epithelium. Its diagnosis is based on histologic features, including ameloblastic differentiation, mitotic rate, nuclear/cellular pleomorphism, and the presence or absence of angiolymphatic/perineural invasion. Differential diagnosis of AC includes primary intraosseous squamous cell carcinoma and clear cell odontogenic carcinoma in which both lesions can exclude based on histologic features as primary intraosseous squamous cell carcinoma exhibits moderately to poorly differentiated squamous epithelial cells with variable keratinization and clear cell odontogenic carcinoma exhibits nests and islands of clear cells, also cytological atypia is absent, all these features are absent in AC.
Malignant counterpart of AM
According to the classification of odontogenic tumors in 2005, there were two malignant counterparts of AM—metastatic AM and AC. Although in the 2017 classification, metastatic carcinoma was no longer counted as its malignant counterpart due to the absence of characteristics of malignancy except its nature or metastasizing. In the recent 2022 classification of odontogenic lesions, only AC was considered as its malignant counterpart. AC is one of the most common malignant odontogenic neoplasms found in the head and neck region.[8] This neoplasm shows histologic features of AM with marked cytologic atypia. In general, ameloblastic neoplasms are generally categorized into AB, AC, and intermediate-grade atypical ameloblastoma. AC could develop de novo (primary type) or in existing AM (secondary type, dedifferentiated) according to the recent World Health Organization classification of these tumors. The most probable cause of this case of ex-ameloblastoma is incomplete removal of ameloblastic tissue during surgical excision. ACs are rare, with <120 cases reported in the English literature. Metastasis in the vital tissues is the main cause behind the poor prognosis of this neoplasm. Patients of various ages can be affected, but the disease most commonly occurs during the fourth to sixth decade of life. It shows male predominance. It is more common in the mandible, (around 80%), particularly the posterior mandible. Rare reports of maxillary involvement. It presents rapid growth, pain, perforation of the cortical plate, soft tissue infiltration, and/or lower lip paresthesia which raises an alarming sign of malignancy. Lymph node involvement has been reported as a dominant sign of metastasis to the lungs, cervical lymph nodes, and other distant sites. Often shows tooth apex resorption and cortical expansion with perforation.
Therefore both the lesions AM and AC present a diagnostic conundrum because of the overlapping of histopathologic features. Eventually, other intraosseous carcinomas of the jaws should be ruled out. Thus, a marker that would highlight these worrisome areas in a pre-existing ameloblastic lesion and in a definitive AC would be of substantial assistance in facilitating the diagnosis. Some immunohistochemical markers correlating with the diagnosis of AC have been identified such as CK19, Ki67, and SOX2 play a crucial role.
Recent molecular diagnosis revealed that BRAF (V600E) activating mutation in genetic aberration results in the formation of AMs. BRAF (V600E) mutation might cause to confirm the expansion of SOX2-positive cells.[4] SOX2 is a transcription factor of the SOX family involved in multiple tumor cell functions, such as the assistance in tumor cell proliferation, the ability to inhibit apoptosis, enhance cell invasion and migration, and accelerate tumor stem cell populations.[4] Hence its expression plays an important role in tumor diagnosis, especially in the case of AC.
The management of AC in the head and neck region includes surgical resection along with radical neck dissection is advised.[9] Complete removal of the jaw with 2 cm of normal bone margin is recommended for disease-free surgical margins which also results in a recurrence rate below 15%. Radiotherapy and chemotherapy are also advised.
CONCLUSION
AC is a rare odontogenic malignant tumor that includes features of malignancy histopathologically along with features essential for AM in its primary site. AC should be considered as an alarming sign diagnostically when patients present with pain, rapid growth, and paresthesia. Due to malignant neoplasm, metastasis to other vital organs such as the lung, liver, lymph nodes, bone, and brain might cause death and should be ruled out. AC has a poor prognosis with only 5 years of survival after surgical treatment. Hence, surgical treatment and long-term follow-up are necessary for the proper management of this neoplasm. The present case of ex-ameloblastoma, in which the patient was earlier diagnosed with peripheral AM, reinforces the rarity of AC and its spectrum.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Manchanda AS, Narang RS, Nagi RS. Ameloblastic carcinoma: A case report and evaluation. J Oral Maxillofac Pathol. 2022;26(Suppl 1):S63–7. doi: 10.4103/jomfp.jomfp_378_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Niu Z, Li Y, Chen W, Zhao J, Zheng H, Deng Q, et al. Study on clinical and biological characteristics of ameloblastic carcinoma. Orphanet J Rare Dis. 2020;15:316. doi: 10.1186/s13023-020-01603-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aoki T, Akiba T, Kondo Y, Sasaki M, Kajiwara H, Ota Y. The use of radiation therapy in the definitive management of ameloblastic carcinoma: A case report. Oral Surg Oral Med Oral Pathol Oral Radiol. 2019;127:e56–60. doi: 10.1016/j.oooo.2018.09.009. [DOI] [PubMed] [Google Scholar]
- 4.Lei Y, Jaradat JM, OwoshoA, Adebiyi KE, Lybrand KS, Neville BW, et al. Evaluation of SOX2 as a potential marker for ameloblastic carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;117:608–16.e1. doi: 10.1016/j.oooo.2014.01.017. [DOI] [PubMed] [Google Scholar]
- 5.Tseng C-H, Lu P-H, Wang Y-P, Chang JYF. Enrichment of SOX2-positive cells in BRAF V600E mutated and recurrent ameloblastoma. J Pers Med. 2022;12:77. doi: 10.3390/jpm12010077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tomar U, Tomar S, Singhal M, Lall AB, Prabhat KB, Bhardwaj A. Oncepts of biphasic/bimorphic lesions in oral and maxillofacial tumors. J Pharm Negat Results. 2022;13:2033–35. [Google Scholar]
- 7.Kumaran PS, Anuradha V, Gokkulakrishnan S, Thambiah L, Jagadish AK, Satheesh G. Ameloblastic carcinoma: A case series. J Pharm Bioall Sci. 2014;6(Suppl 1):S208–11. doi: 10.4103/0975-7406.137473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shrikaar M, Suwasini S, Chaterjee K, Sinha S. Maxillary ameloblastic carcinoma: A diagnostic conundrum. J Oral Maxillofac Pathol. 2021;25:159–62. doi: 10.4103/jomfp.JOMFP_71_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gunaratne DA, Coleman HG, Lim L, Morgan GJ. Ameloblastic carcinoma. Am J Case Rep. 2015;16:415–9. doi: 10.12659/AJCR.893918. [DOI] [PMC free article] [PubMed] [Google Scholar]


