Abstract
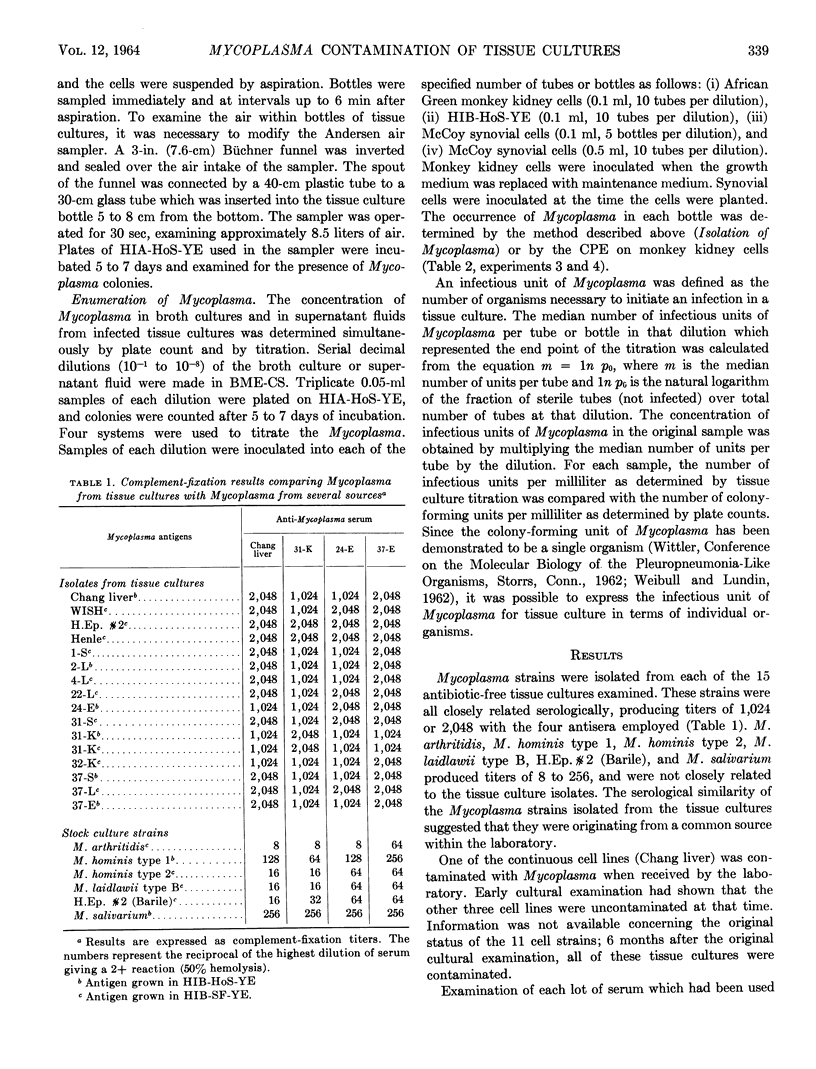
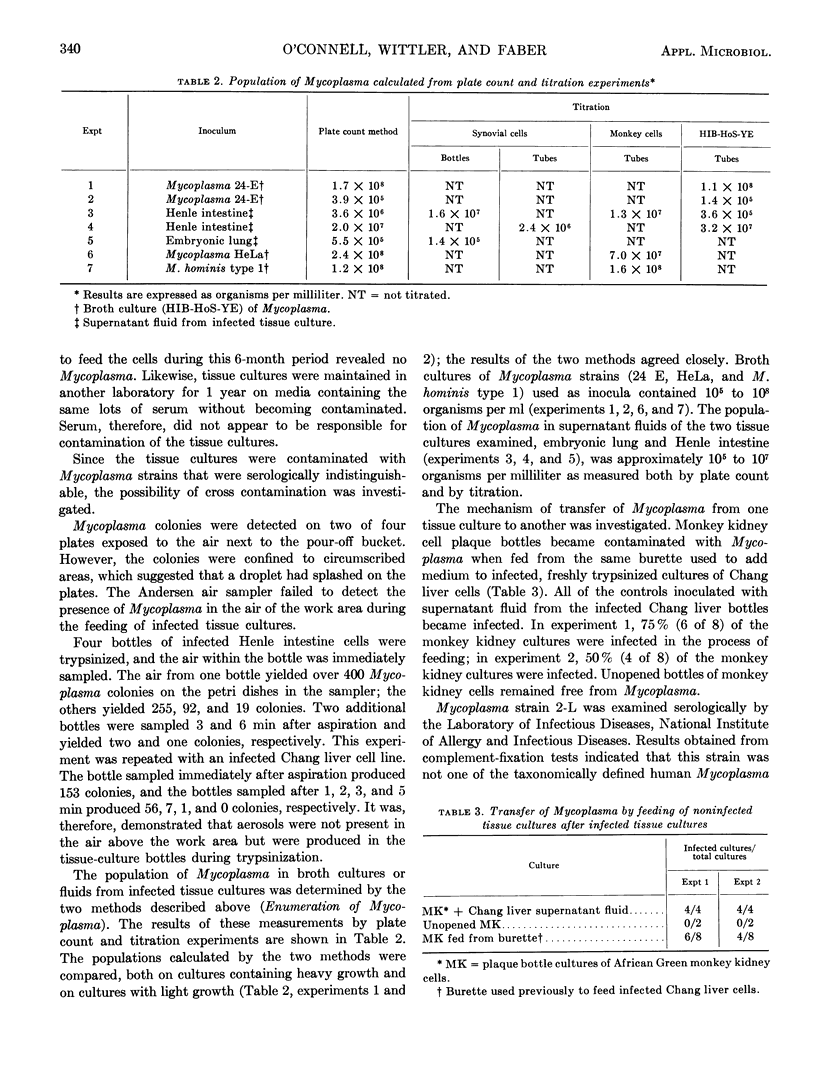
Mycoplasma isolates were cultured from 15 antibiotic-free cell cultures obtained from a single laboratory. Complement-fixation tests showed that these isolates were antigenically related to each other but were unrelated to M. hominis type 1, M. hominis type 2, M. arthritidis, M. laidlawii type B, Mycoplasma sp. H.Ep. #2 (Barile), or M. salivarium. Examination of serum used to feed the infected cell lines revealed no Mycoplasma. Infection resulting from cross-contamination by a single Mycoplasma strain from one cell culture to another was investigated. Although the organisms were not found in the air over the work area, aerosols containing these contaminants were produced in tissue culture bottles during the trypsinization of cell monolayers. The minimal infectious dose of Mycoplasma for tissue cultures was measured, and it was determined that one organism was capable of initiating an infection in a tissue culture. The pattern of contamination and the small dose required for infection indicated that Mycoplasma contamination was spread from one tissue culture to another via aerosols. It was demonstrated that Mycoplasma can be transferred from one cell culture to another through the use of a common burette for dispensing medium.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSEN A. A. New sampler for the collection, sizing, and enumeration of viable airborne particles. J Bacteriol. 1958 Nov;76(5):471–484. doi: 10.1128/jb.76.5.471-484.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARILE M. F., MALIZIA W. F., RIGGS D. B. Incidence and detection of pleuropneumonia-like organisms in cell cultures by fluorescent antibody and cultural procedures. J Bacteriol. 1962 Jul;84:130–136. doi: 10.1128/jb.84.1.130-136.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANOCK R. M., HAYFLICK L., BARILE M. F. Growth on artificial medium of an agent associated with atypical pneumonia and its identification as a PPLO. Proc Natl Acad Sci U S A. 1962 Jan 15;48:41–49. doi: 10.1073/pnas.48.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRAEMER P. M., DEFENDI V., HAYFLICK L., MANSON L. A. Mycoplasma (PPLO) strains with lytic activity for murine lymphoma cells in vitro. Proc Soc Exp Biol Med. 1963 Feb;112:381–387. doi: 10.3181/00379727-112-28052. [DOI] [PubMed] [Google Scholar]
- MCCARTY K. S., WOODSON B., AMSTEY M., BROWN O. ARGININE AS A PRECURSOR OF PYRIMIDINES IN STRAIN L-929 FIBROBLASTS INFECTED WITH PLEUROPNEUMONIA-LIKE ORGANISMS. J Biol Chem. 1964 Feb;239:544–549. [PubMed] [Google Scholar]
- NELSON J. B. The behavior of murine PPLO in HeLa cell cultures. Ann N Y Acad Sci. 1960 Jan 15;79:450–457. doi: 10.1111/j.1749-6632.1960.tb42710.x. [DOI] [PubMed] [Google Scholar]
- O'CONNELL R. C., WITTLER R. G., FABER J. E. Serological study of a pleuropneumonia-like organism and an associated Corynebacterium species. Antonie Van Leeuwenhoek. 1961;27:321–331. doi: 10.1007/BF02538462. [DOI] [PubMed] [Google Scholar]
- POLLOCK M. E., KENNY G. E., SYVERTON J. T. Isolation and elimination of pleuropneumonia-like organisms from mammalian cell cultures. Proc Soc Exp Biol Med. 1960 Oct;105:10–15. doi: 10.3181/00379727-105-25992. [DOI] [PubMed] [Google Scholar]
- POWELSON D. M. Metabolism of animal cells infected with mycoplasma. J Bacteriol. 1961 Aug;82:288–297. doi: 10.1128/jb.82.2.288-297.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIBULL C., LUNDIN B. M. Size and shape of pleuropneumonia-like organisms grown in liquid media. J Bacteriol. 1962 Sep;84:513–519. doi: 10.1128/jb.84.3.513-519.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]


