Abstract
Background
Heart rate variability (HRV) is one piece among a complex network of adaptations existent in athletes that help them gain a better understanding of their own physiology. Sympathovagal balance is one of the spectral components of HRV analysis and is used to assess the frequently changing oscillations of a healthy heart, which can help in gauging the response of cardiac function towards physiological stress during exercise. This index is extensively used in appraising cardiac autonomic modulation. An evaluation of body composition in athletes has become a critical consideration when tracking HRV, as it helps practitioners understand the role of the autonomic nervous system (ANS) in obesity. The body shape index (BSI), which is based on waist circumference (WC), is an anthropometric parameter with decent predictive ability when measuring centripetal obesity. In this regard, the current study is an attempt to unravel the relationship between BSI and sympathovagal balance during exercise performed on two different instruments (treadmill and ergometer) by elite and amateur athletes.
Methods
It was an observational case-control study that included 30 elite and 120 amateur athletes. Symptom-limited exercise testing was performed by athletes on a motorized treadmill and ergometer in the sports physiology laboratory of a rural medical college in central India. Different anthropometric parameters like BSI and body surface area (BSA) were also recorded. Short-term HRV extracted from electrocardiogram (ECG) recordings was obtained using the Power Lab system and HRV analysis by LabChart software.
Results
The sympathovagal ratio, i.e., ratio of low frequency (LF) to high frequency (HF) in elite and amateur male populations showed a higher value than that in females, indicating a dominant sympathetic response in the males. There was a significant (p=0.042) positive correlation (r=0.24) between BSI and LF/HF Ratio in amateur females during treadmill exercise, whereas a significant (p=0.049) negative correlation (r=-0.27) was obtained in amateur males during ergometer exercise. Hence, increased weight and BSI were found to be associated with high sympathetic dominance, indicating a sympathovagal imbalance.
Conclusion
We attempted to explore the interaction between BSI and LF/HF during exercise performed on two different instruments (treadmill and ergometer) by elite and amateur athletes, which can help in testing the response of cardiac function to stress experienced during exercise. The study's uniqueness stems from discovering the relationship between BSI and HRV and how this relationship impacts sports performance. BSI measurement in athletes, both elite and amateur, allows for the assessment and forecasting of potential autonomic activity under exercise-induced stress by linking HRV with BSI.
Keywords: body shape index, heart rate variability, ergometer, treadmill, elite athletes, amateur athlete
Introduction
Not all humans are genetically adapted for the sustained, extreme aerobic efforts that athletes endure. Specifically, elite athletes tend to have superior cardiovascular physiology when compared to the average person [1]. Athletes nowadays have access to formerly unthinkable metrics and statistics to help them make decisions about their preparation for a competition, performance, and recovery strategies. In the contemporary, fast-evolving arena of athlete analytics, heart rate variability (HRV) continues to be a primarily employed tool, particularly for elite athletes [2]. The physiological basis of changing oscillations in a healthy heart, which is manifested as HRV, is considered to be an outcome of an individual’s autonomic nervous system (ANS). Frequency domain (or spectral analysis) measures like sympathovagal balance denote the contribution of various frequencies to the overall variability of the signal [3].
Driven by ease, the accurate interpretation and clinical applications of HRV metrics have drastically increased, making the use of HRV more common and widespread. It has now become compulsory and necessary for athletes to track their HRV data by taking measurements first thing in the morning [4]. While the avenues for upgrading athletic performance are abundant, so are the potential pitfalls. This has led to an increase in the availability and use of HRV metrics in the wide-ranging athletic population and may be particularly useful to elites in comparison to amateurs. Elite athletes generally exhibit a greater resting HRV than healthy individuals [5,6].
The use of HRV in monitoring the physiology of athletes is in its early days. It has become critical to evaluate body composition in athletes when tracking HRV in order to better understand the role of the ANS in obesity. Thus, an understanding of the different variables and the complex physiological interactions that produce differing patterns of HRV in athletes can help improve the interpretation of the data as well as give insights into improving their performance in terms of cardiovascular efficiency. Body shape index (BSI), which is derived from waist circumference (WC, independently from BMI), is an anthropometric parameter that is considered to be a better index of an individual’s body composition and has a decent predictive ability with respect to abdominal obesity.
Although some research has been performed in the past to determine the HRV indices pre- and post-exercise or in the resting state, there is a lack of literature on the same parameters in athletes during exercise [7,8]. Previous studies have investigated HRV indices in athletes but not with reference to amateur and elite athletes [9,10].
Due to scant data on body composition indices such as BSI in athletes and conflicting results in past research on HRV among athletes, this study was planned to unravel the relationship between BSI and sympathovagal balance during exercise performed on two different instruments (treadmill and ergometer) by elite and amateur athletes and to investigate the extent to which ANS contributes to obesity.
Materials and methods
Study design and setting
This was an observational, cross-sectional study. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for the case-control study were used for reporting and preparing the manuscript. The study was carried out in the sports physiology laboratory in the department of physiology of a medical college in central India.
Study participants
A total of 150 participants were recruited for the study. The sample size was estimated using OpenEpi 3.01 statistical software (Centers for Disease Control and Prevention (CDC), Atlanta) with the assumptions as the confidence level of 95%, alpha of 0.05, and power of study as 80%. To give the study more credibility and authenticity the case-to-control ratio was kept at 1:4. Hence, there were 30 cases and 120 controls. In this study, only pure athletes were recruited and were further classified into elite and amateur ones [11]. The elite athletes were considered as cases and amateur ones as controls in order to compare the anthropometric and cardiovascular parameters between them. They were grouped on the basis of the selection criteria.
Each participant in this study provided informed consent, or they chose not to. MGIMS/IEC/PHY/127/2017 was approved by the Mahatma Gandhi Institute of Medical Sciences' Institutional Ethics Committee for Research on Human Subjects.
Selection criteria
As per the definition of an athlete by the American Heart Association, “a participant in an organized team or individual sports that place a high importance on excellence and performance, regular competition against other athletes, and involve some type of systematic training (typically intensive)”, subjects were chosen after proper inquiry and history taking [11].
Further, the subjects who were deemed as athletes as per the selection criteria were classified into elite ones and others were considered as amateur ones. Elite athletes were those who competed at the collegiate level, and at the national level, won medals, were seasoned professionals who held records, and trained frequently [12].
Athletes between the age of 18-40 years and those who gave informed written consent were included in the study. Asthma, emphysema, chronic obstructive pulmonary disease, chronic hypertension, diabetes, musculoskeletal diseases, cardiovascular conditions, diagnosed psychiatric disorders, subjects experiencing musculoskeletal pain on the day of the test, and those who refused to consent were among the diseases that were excluded from the study (exclusion criteria). Only after assessment and clearance by the institutional ethics committee did the study get underway.
Data sources and measurement of variables
Anthropometric Variables
The subjects' age was recorded in years. The individuals' standing height (in cm) was measured while they were barefoot and stood with their heels together. Weight (in kg) was measured when the subject was standing, wearing just light clothing, and barefoot. Over bare skin or light knickers, WC was measured using a measuring tape.
The individual was in a standing position when measurement sites were taken. In the horizontal plane, the WC was measured midway between the iliac crest and the lower border of the ribs. Two measurements were taken and recorded to the closest 0.5 cm. A third measurement was done if there was more than 2 cm of difference between the first two. It was determined what was closest in terms of the two metrics. The Phoenix Height Weight Body Mass Index machine (Model: PBMI-200) measured BMI [13].
WC was used to create the BSI, which is considered to be a superior index. BSI was calculated using the formula below [13].
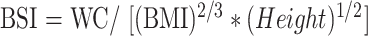
Body surface area (BSA) was calculated using the formula below [13].

Heart Rate Variability
HRV was recorded using the Power Lab system and analyzed by the LabChart software by AD Instruments, Australia. This system gave live wireless electrocardiogram (ECG) along with a recording of HRV parameters while the subject was maximally exercising on the treadmill and cycle ergometer. Fast Fourier transformation is used to extract frequency domain (or spectrum analysis) estimates of HRV from time domain data, which show the relative contributions of various frequencies to the signal's overall variability [14]. Very low frequency (VLF, 0.04 Hz), low frequency (LF, 0.04-0.15 Hz), and high frequency (HF, 0.15-0.4 Hz) spikes are the most often seen. According to conventional wisdom, HF power represents parasympathetic activity while LF power represents sympathetic activity [9]. The ratio of LF to HF is thought to be a reflection of sympathovagal balance [15]. HRV recordings were separately done when the subject was exercising on the motorized treadmill and cycle ergometer (Aerofit AF 101, Nityasach Fitness Pvt Ltd, Mumbai, India).
Statistical analysis
The collected data were input into an Excel spreadsheet, where the mean and standard deviation were calculated. The SPSS (Statistical Package for Social Sciences, Version 20.0, SPSS Inc, Chicago, Illinois, USA) was used to analyze the data. Using Pearson correlation, the study's variables were correlated. The information was normally distributed. The t-test was used to determine the significance of the correlation coefficient value "r" between two quantitative variables, which might be positive (direct correlation) or negative (inverse correlation). The significance threshold remained at p< 0.05.
Results
Out of 120 amateur athletes, 50 (41.7%) were males and 70 (58.3%) were females. Of a total of 30 elite athletes, 17 (56%) were males and 13 (44%) were females. Male elite athletes had a mean age of 20.94 ± 2.75, while female elite athletes had a mean age of 22.17 ± 3.30. Amateur males had a mean age of 21.06 ± 3.02 while amateur females had a mean age of 22.50 ± 6.25. Tables 1, 2 list various demographic information along with anthropometric and cardiovascular readings of the study participants. As per the tables, it can be deduced that there was a statistically significant difference among males in the mean values of BMI (p=0.021) and BSI (p=0.012). However, there was no statistically significant difference in the remaining parameters in both the groups and the genders.
Table 1. Descriptive statistics of the male subjects.
BSA: Body surface area; BSI: Body shape index; LF: Low frequency; HF: High frequency; WC: Waist circumference; HR: Heart rate
| Parameter | Elite Males (n = 17) | Amateur Males (n = 50) | ||||||
| Mean | Standard Deviation | 95% confidence interval for mean | Mean | Standard Deviation | 95% confidence interval for mean | |||
| Lower bound | Upper bound | Lower bound | Upper bound | |||||
| Height (m) | 1.75 | 0.04 | 1.73 | 1.76 | 1.7 | 0.07 | 1.68 | 1.72 |
| Resting HR (beats/min) | 80.18 | 8.09 | 76.33 | 84.02 | 84.06 | 10.87 | 81.05 | 87.07 |
| Weight (kg) | 65.65 | 10.68 | 60.57 | 70.72 | 68.02 | 11.04 | 64.96 | 71.08 |
| WC (cm) | 84 | 5.83 | 81.23 | 86.77 | 82.24 | 18.42 | 77.14 | 87.34 |
| BMI (kg/m²) | 21.5 | 2.83 | 20.16 | 22.84 | 23.66 | 4.32 | 22.46 | 24.85 |
| BSA (m²) | 1.79 | 0.14 | 1.73 | 1.86 | 1.78 | 0.13 | 1.74 | 1.82 |
| BSI | 0.083 | 0.003 | 0.08 | 0.085 | 0.077 | 0.016 | 0.073 | 0.082 |
| LF/HF Ratio (Treadmill) | 1.3 | 1.05 | 0.8 | 1.8 | 0.87 | 1.26 | 0.52 | 1.22 |
| LF/HF Ratio (Ergometer) | 2.77 | 2.25 | 1.23 | 0.63 | 2.79 | 4.91 | 1.43 | 4.16 |
Table 2. Descriptive statistics of the female subjects.
BSA: Body surface area; BSI: Body shape index; LF: Low frequency; HF: High frequency; WC: Waist circumference; HR: Heart rate
| Parameter | Elite Females (n = 13) | Amateur Females (n = 70) | ||||||
| Mean | Standard Deviation | 95% confidence interval for mean | Mean | Standard Deviation | 95% confidence interval for mean | |||
| Lower bound | Upper bound | Lower bound | Upper bound | |||||
| Height (m) | 1.62 | 0.07 | 1.58 | 1.65 | 1.61 | 0.04 | 1.6 | 1.63 |
| Resting HR (beats/min) | 84.17 | 13.86 | 76.32 | 92.01 | 89.5 | 10.75 | 81.05 | 87.07 |
| Weight (kg) | 57 | 8.63 | 52.11 | 61.89 | 58.91 | 8.43 | 55.99 | 61.83 |
| WC (cm) | 85.08 | 6.88 | 81.19 | 88.98 | 87.41 | 8.41 | 84.49 | 90.32 |
| BMI (kg/m²) | 21.82 | 3 | 20.13 | 23.52 | 22.59 | 3.13 | 21.51 | 23.68 |
| BSA (m²) | 1.6 | 0.13 | 1.52 | 1.67 | 1.62 | 0.11 | 1.58 | 1.65 |
| BSI | 0.086 | 0.004 | 0.084 | 0.088 | 0.087 | 0.007 | 0.084 | 0.089 |
| LF/HF Ratio (Treadmill) | 0.55 | 0.26 | 0.4 | 0.69 | 0.71 | 1.1 | 0.33 | 1.09 |
| LF/HF Ratio (Ergometer) | 0.95 | 0.84 | 0.48 | 1.43 | 1.31 | 1.14 | 0.91 | 1.7 |
Further correlation analysis was also performed, primarily between sympathovagal balance and BSI, in elite and amateur male and female athletes. As the recordings were taken using a treadmill and ergometer, two separate datasets were obtained, as depicted in Tables 3, 4. A statistically weak significant positive relationship (r=0.24, p=0.042) was found between the two parameters in amateur females on treadmill exercise and a weak negative correlation (p=-0.27, p=0.049) which was statistically significant was obtained among amateur males upon ergometer exercise. There was a strong negative correlation between LF/HF ratio and BSI in elite female athletes but it did not reach statistical significance.
Table 3. Correlation of parameters obtained on treadmill .
BSI: Body shape index; S: Significant; NS: Not significant; LF: Low frequency; HF: High frequency
| Study group | BSI (Mean ± Standard Deviation) | LF/HF | r value | P value |
| Amateur males | 0.077 ± 0.016 | 0.87 ± 1.26 | -0.10 | 0.48(NS) |
| Elite males | 0.083 ± 0.003 | 1.30 ± 1.05 | 0.107 | 0.662 (NS) |
| Amateur females | 0.087 ± 0.007 | 0.71 ± 1.10 | 0.24 | 0.042 (S) |
| Elite females | 0.086 ± 0.004 | 0.55 ± 0.26 | - 0.11 | 0.696 (NS) |
Table 4. Correlation of parameters obtained on ergometer.
BSI: Body shape index; S: Significant; NS: Not significant; LF: Low frequency; HF: High frequency
| Study group | BSI (Mean ± Standard Deviation) | LF/HF | r value | P value |
| Amateur males | 0.077 ± 0.016 | 2.79 ± 4.91 | - 0.27 | 0.049 (S) |
| Elite males | 0.083 ± 0.003 | 2.77 ± 2.25 | 0.13 | 0.595 (NS) |
| Amateur females | 0.087 ± 0.007 | 1.31 ± 1.14 | -0.11 | 0.357 (NS) |
| Elite females | 0.086 ± 0.004 | 0.95 ± 0.94 | -0.42 | 0.119 (NS) |
Discussion
HRV indices, particularly sympathovagal balance, are viewed as measures of intricate cardiac autonomic modulations of an athletic heart. Alterations of HR are known to contribute to improvements in predicting performance, enhancing training and competition strategies, and refining the science of sports physiology. Earlier research implicating HRV to sports has focused majorly on endurance sports. Lundstrom et al. found that greater HRV scores indicated better cardiovascular adaptations due to higher training intensities, favoring HRV as a measure to optimize individualized training in elite runners [1].
In spite of this, published data on professional athletes are uncommon; the majority of HRV research conducted so far has involved trained or recreational participants [16-18]. Elite athletes may react differentially to training pressures and subsequent recovery due to genetics and training experience [19,20]. Therefore, top athletes may use HRV in a number of ways as the data has the potential to improve their competitive performance to the fullest.
There is a widespread misperception among sports physiologists that there is a direct linear link between vagal-related indices of HRV and the parasympathetic impact on heart rate (HR) when using HRV to determine autonomic health. Actually, a quadratic connection exists [21]. This implies that vagal-related HRV indices are decreased at both low (high HR) and high (low HR) levels of vagal tone. For instance, elite athletes who have received proper training typically have both a low resting HR and elevated HRV indices [22]. However, many athletes with low resting heart rates have also been found to have lower HRV [23]. The primary mechanism is probably myocyte-level acetylcholine receptor saturation: elevated vagal tone may result in persistent parasympathetic regulation of the sinus node, which may lower HRV [24].
In the current study, during exercise, the HF (vagal) component increased significantly, in elite athletes whereas the LF component and the LF/HF ratio decreased as compared to amateur counterparts which reflects parasympathetic dominance in the elite athletes. This finding goes in agreement to the well documented fact that exercise leads to an increase in heart rate to meet increased metabolic demands, while frequency HRV measures decrease dramatically [10,25,26].
Owing to the wealth of information and metrics available to athletes, there is growing interest in physiological forecasting, or predicting results or projecting outcomes based on a number of variables that may be obtained via wireless HRV technology during exercise. This unique modality of HRV measurement was a major strength of this study. According to Treff et al., this technique may also prove to help in enhancing the overall performance of athletes by quantifying stress and recovery [27]. Highly trained elite athletes returned to baseline more quickly than amateur athletes in the current study as well.
As per the existing literature exploring HRV as a way to assess cardiometabolic risk, the sensitivity of HRV to body composition indices has been well-established [28]. So it is reasonable enough to assume that elite and amateur athletes may likewise experience an impact on their HRV due to an index like BSI. It has also been noted in amateur athletes that there were improvements in body composition and strength when training was guided by HRV. With regard to BSI values, the elite and amateur females did not differ significantly from each other while the amateur males had lower BSI as compared to elite males. However, the female athletes had a higher BSI than their counterparts [28].
The sympatho-vagal ratio in the elite and amateur male population showed a higher value than that in females indicating a dominant sympathetic response. There was a weak significant positive correlation between BSI and LF/HF Ratio in amateur females on treadmill exercise whereas a weakly significant negative correlation was obtained in amateur males on ergometer exercise.
Like every research project, this one had some limitations as well. The sample size was limited due to the brief duration of this short-term research. The age range was narrowed in order to accomplish the achievable target group of athletes. The data was collected at a specific point in time as it wasn’t a longitudinal study. This design does not allow for causal relationships to be established or changes in readings over time to be examined. HRV testing has several disadvantages as well, such as the time commitment and participation of the individual throughout the test. Recruiting interested volunteers for this kind of study was extremely challenging. Hence, prescribing them or guiding them to get their autonomic and anthropometric parameters tested at a regular basis would pave way for further progress and outcomes in the field.
Conclusions
To conclude, the use of the HRV index like sympathovagal balance represents an inexpensive alternative to evaluate and predict possible autonomic activity during exercise. It may help improve the performance of both the elite and the amateur athletes. This article further highlights that HRV monitoring along with BSI measurement in elites is required to understand their unique individual HRV fingerprint. This study demonstrated how increases and decreases in HRV relate to variations in BSI in elite and amateur athletes.
Acknowledgments
The data are stored as de-identified participant data, which are available on reasonable request, with Prashanth A (prashantheck@gmail.com). The authors acknowledge all the participants of the study.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained or waived by all participants in this study. Institutional Ethics Committee for Research on Human Subjects of Mahatma Gandhi Institute of Medical Sciences, Wardha issued approval MGIMS/IEC/PHY/127/2017
Animal Ethics
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
References
- 1.Practices and applications of heart rate variability monitoring in endurance athletes. Lundstrom CJ, Foreman NA, Biltz G. Int J Sports Med. 2023;44:9–19. doi: 10.1055/a-1864-9726. [DOI] [PubMed] [Google Scholar]
- 2.Heart rate variability (HRV) as a tool for diagnostic and monitoring performance in sport and physical activities. Makivić B, Nikić Djordjević M, Willis MS. https://vsdiagnostics.com/wp-content/uploads/2021/12/Heart_Rate_Variability_HRV_as_a_Tool_for.pdf J Exerc Physiol Online. 2013;16:103–131. [Google Scholar]
- 3.A quantitative systematic review of normal values for short-term heart rate variability in healthy adults. Nunan D, Sandercock GR, Brodie DA. Pacing Clin Electrophysiol. 2010;33:1407–1417. doi: 10.1111/j.1540-8159.2010.02841.x. [DOI] [PubMed] [Google Scholar]
- 4.Training adaptation and heart rate variability in elite endurance athletes: opening the door to effective monitoring. Plews DJ, Laursen PB, Stanley J, Kilding AE, Buchheit M. Sports Med. 2013;43:773–781. doi: 10.1007/s40279-013-0071-8. [DOI] [PubMed] [Google Scholar]
- 5.Heart rate variability indexes as a marker of chronic adaptation in athletes: a systematic review. da Silva VP, de Oliveira NA, Silveira H, Mello RG, Deslandes AC. Ann Noninvasive Electrocardiol. 2015;20:108–118. doi: 10.1111/anec.12237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Monitoring athletic training status through autonomic heart rate regulation: a systematic review and meta-analysis. Bellenger CR, Fuller JT, Thomson RL, Davison K, Robertson EY, Buckley JD. Sports Med. 2016;46:1461–1486. doi: 10.1007/s40279-016-0484-2. [DOI] [PubMed] [Google Scholar]
- 7.An overview of heart rate variability metrics and norms. Shaffer F, Ginsberg JP. Front Public Health. 2017;5:258. doi: 10.3389/fpubh.2017.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heart-rate variability—more than heart beats? Ernst G. Front Public Health. 2017;5:240. doi: 10.3389/fpubh.2017.00240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cardiac autonomic responses during exercise and post-exercise recovery using heart rate variability and systolic time intervals—a review. Michael S, Graham KS, Davis GM Oam. Front Physiol. 2017;8:301. doi: 10.3389/fphys.2017.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Influence of body shape index and heart rate variability in athletes as assessed during treadmill exercise. Sharma S, Bokariya P, Kothari R, Srivastava Srivastava, S S. J Clin Prev Cardiol. 2022;11:90–96. [Google Scholar]
- 11.Athlete or non-athlete? This is the question in body composition. Campa F, Coratella G. Front Physiol. 2021;12:814572. doi: 10.3389/fphys.2021.814572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.What does ‘elite’ mean in sport and why does it matter. Williams A, Day S, Stebbings G, Erskine R. https://bases.org.uk/imgs/51_article_p6527.pdf Sport Exerc Scientist. 2017:6. [Google Scholar]
- 13.Exploring the relationship between the indices of body composition with grip strength performance and peak VO2. Sushmitha S, Kothari R, Mittal G, et al. Cureus. 2023;15:0. doi: 10.7759/cureus.40874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology the North American Society of Pacing Electrophysiology. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- 15.The LF/HF ratio does not accurately measure cardiac sympatho-vagal balance. Billman GE. Front Physiol. 2013;4:26. doi: 10.3389/fphys.2013.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Influence of short-term endurance exercise training on heart rate variability. Lee CM, Wood RH, Welsch MA. Med Sci Sports Exerc. 2003;35:961–969. doi: 10.1249/01.MSS.0000069410.56710.DA. [DOI] [PubMed] [Google Scholar]
- 17.Dose-response relationship of autonomic nervous system responses to individualized training impulse in marathon runners. Manzi V, Castagna C, Padua E, et al. Am J Physiol Heart Circ Physiol. 2009;296:0–40. doi: 10.1152/ajpheart.00054.2009. [DOI] [PubMed] [Google Scholar]
- 18.Monitoring endurance running performance using cardiac parasympathetic function. Buchheit M, Chivot A, Parouty J, Mercier D, Al Haddad H, Laursen PB, Ahmaidi S. Eur J Appl Physiol. 2010;108:1153–1167. doi: 10.1007/s00421-009-1317-x. [DOI] [PubMed] [Google Scholar]
- 19.What makes champions? A review of the relative contribution of genes and training to sporting success. Tucker R, Collins M. Br J Sports Med. 2012;46:555–561. doi: 10.1136/bjsports-2011-090548. [DOI] [PubMed] [Google Scholar]
- 20.Using recovery modalities between training sessions in elite athletes: does it help? Barnett A. Sports Med. 2006;36:781–796. doi: 10.2165/00007256-200636090-00005. [DOI] [PubMed] [Google Scholar]
- 21.Dissociation of heart rate variability from parasympathetic tone. Goldberger JJ, Ahmed MW, Parker MA, Kadish AH. Am J Physiol. 1994;266:0–7. doi: 10.1152/ajpheart.1994.266.5.H2152. [DOI] [PubMed] [Google Scholar]
- 22.Relationship of heart rate variability to parasympathetic effect. Goldberger JJ, Challapalli S, Tung R, Parker MA, Kadish AH. Circulation. 2001;103:1977–1983. doi: 10.1161/01.cir.103.15.1977. [DOI] [PubMed] [Google Scholar]
- 23.Saturation of high-frequency oscillations of R-R intervals in healthy subjects and patients after acute myocardial infarction during ambulatory conditions. Kiviniemi AM, Hautala AJ, Seppänen T, Mäkikallio TH, Huikuri HV, Tulppo MP. Am J Physiol Heart Circ Physiol. 2004;287:0–7. doi: 10.1152/ajpheart.00433.2004. [DOI] [PubMed] [Google Scholar]
- 24.The high frequency component of heart rate variability reflects cardiac parasympathetic modulation rather than parasympathetic 'tone'. Hedman AE, Hartikainen JE, Tahvanainen KU, Hakumäki MO. Acta Physiol Scand. 1995;155:267–273. doi: 10.1111/j.1748-1716.1995.tb09973.x. [DOI] [PubMed] [Google Scholar]
- 25.Spectral analysis of heart rate variability during exercise in trained subjects. Pichon AP, de Bisschop C, Roulaud M, Denjean A, Papelier Y. Med Sci Sports Exerc. 2004;36:1702–1708. doi: 10.1249/01.mss.0000142403.93205.35. [DOI] [PubMed] [Google Scholar]
- 26.The use of heart rate variability measures to assess autonomic control during exercise. Sandercock GR, Brodie DA. Scand J Med Sci Sports. 2006;16:302–313. doi: 10.1111/j.1600-0838.2006.00556.x. [DOI] [PubMed] [Google Scholar]
- 27.The integration of training and off-training activities substantially alters training volume and load analysis in elite rowers. Treff G, Leppich R, Winkert K, Steinacker JM, Mayer B, Sperlich B. Sci Rep. 2021;11:17218. doi: 10.1038/s41598-021-96569-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heart rate variability: a cardiometabolic risk marker with public health implications (Article in French) Marsac J. https://pubmed.ncbi.nlm.nih.gov/24672989/ Bull Acad Natl Med. 2013;197:175–186. [PubMed] [Google Scholar]


