Abstract
There is an increasing volume of nano-enabled materials in the market. Once composites containing nano-additives are disposed of, weathering could deteriorate their structures, releasing nanoparticles and risking exposure of humans and aquatic organisms. Composite degradation due to environmental aging continues, including structural deterioration resulting in cracking, fragmentation, and release of microplastics and nano-additives to the environment. This research aims to study the degradation and release of initially embedded nanomaterials (NMs) from composites and their toxicity. The molecular interaction of carbon nanotube (CNT)/polymer composites is critical for modifying the polymer properties. This study investigated the interactions of functional multiwalled carbon nanotube (MWCNT) composites which affect their release during accelerated weathering processes. Different epoxy–MWCNT composites were prepared by filling a polymer with pure MWCNTs and MWCNTs functionalized with acid () and amine () groups. The physical and chemical changes of aged composites were characterized by gravimetric analysis, contact angle measurements, FTIR, SEM, and laser confocal microscopy. A loss of hydrophobicity was observed for composite surfaces long before surface cracks materialized. Released polymer fragments and nanoparticles were analyzed in wash water using TEM, FTIR and Raman spectroscopy. The environmental risks for long-term use of CNT–polymer composites and the influence of fillers on the extent of chemical photodegradation depended on the combination of polymer and fillers. If nanoparticles are released from the matrix, the high surface-to-volume ratio and reactivity of NMs make them highly dynamic in environmental systems. Exposure to these released NMs could negatively affect human health and the environment. This study provides fragmentation and CNT particle release data that could describe how molecular-level interactions between functionalized CNTs and epoxy polymers affect the aging and release of CNTs. A toxicity assessment based on a reactive oxygen species (ROS) formation assay and MTS assay for cell viability and activity of the released polymer and CNT fragments and leachate showed moderate levels of cytotoxicity of released materials as compared to pristine epoxy plates.
1. Introduction
Over the past two decades, commercial products have been incorporated into engineered nanomaterials (ENMs). Combining nanoparticles with polymers has been extensively used to improve the polymers’ mechanical performance, fire or heat resistance, water or air impermeability, and optical and electrical characteristics as well as expanding their application.1,2 Adding 0.5% to 5% nanosized particles improves mechanical strength, electrical and thermal conductivities significantly, automotive and increasingly used for medical products, and environmental applications.3,4
The predominant focus of nanomaterial implication research efforts has been defining the fate, transport, and toxic properties of pristine or “as manufactured” nanomaterials. Composite materials are made from phases where one or more of the phases are at nano-scale. Over the past three decades, the production and use of nanocomposite polymer materials have increased rapidly.5,6 The incorporation of carbon nanotubes into epoxy composites has the effect of improving electrical conductivity at low percolation levels. The molecular interaction of CNT/polymer composites is critical in modifying the polymer properties. The use of non-ionic surfactants to disperse CNTs impacts interfacial properties affecting glass transition temperatures.7 Efficient dispersion of CNTs into a polymer matrix is necessary since the dispersion affects the alignment and control of the CNTs in the matrix. The hydrophobic part of amphiphilic molecules interacts preferentially with the CNT surface and helps to disperse the CNTs in the polymer matrix.8
Epoxy-based materials are widely used for coatings, electronics insulation, and waterproofing applications due to their physical and chemical properties, such as excellent adhesion, electrical insulation, and heat resistance, along with their strong mechanical properties.9 Composites are enhanced by mixing nanomaterials, such as CNTs, carbon nanofibers, graphene, graphene oxide and nano-carbon black, with epoxy, which improves the composite’s physicochemical properties.10 CNTs are considered typical additives for polymer modification due to their mechanical strength and large aspect ratio. Extensive efforts have been made to use nano-fillers in epoxy–nanomaterial composites with improved properties such as decreased composite weight, better thermal stability and increased mechanical strength.11–13 Epoxy-based composites are made by adding nanomaterials to epoxy resins through catalytic homopolymerization or with a wide range of co-reactants. Furthermore, depending on the functionalization methods, CNTs may have different functional groups, including , , and . Since epoxy resins are reactive polymers, various functional groups on the added CNTs may react with epoxy resin and affect the polymer’s microenvironment and hence the curing process.14,15
Thermoplastic nanocomposites play a significant role in the fast-growing area of nanotechnology such as being a matrix material in fibrous composites for the aerospace and wind turbine industries. Epoxies are used for a wide range of applications because of their high specific strength, stiffness, corrosion resistance and chemical compatibility with reinforcing fibers. The molecular structure of a cured epoxy is amorphous. Due to this amorphous structure, there exists significant free volume/space in the molecular structure, which is greater than the specific volume found in a crystalline state at a given temperature. The volume of nanocomposites used for non-structural applications, such as packaging, is growing.16,17 Compared to their conventional micro and macro, or neat counterparts, low amounts of nano-fillers (i.e., nanomaterials) exhibit superior property enhancements.18–20 Nanoparticles (NPs) are increasingly incorporated in various polymeric materials with favorable matrix–filler interaction, resulting in improved physical, chemical, and electrical properties.21 At the same time, the mobility of nano-fillers is much higher than that of conventional micro or macro composite fillers because of their comparable time scale of motion with polymer chains.22,23 Various studies have indicated that the release of nanoparticles (NPs) during their use or at the end of their useful life from their immobilized matrix may pose a risk to human health and the environment.24,25 Therefore, it is essential to understand the relationship between the inherent characteristics of nanocomposite products and the likelihood of the release and implication of NPs throughout the product’s life cycle.26 To quantify the amount and form of released NPs from a polymer matrix, we need to understand better the nanofiller and polymer interaction and the polymer aging processes.
There is growing scientific evidence that free NPs can cross cellular barriers and that exposure to some of these NPs may lead to oxidative damage and inflammatory reactions.27 Also, increasing the use of nanomaterials in volume and types for food packaging may cause concern where the release of NPs from the package would migrate to food or the environment. Predicting structural changes in the polymer matrix and the potential of the release of NPs from the polymer composite matrix is needed to develop environmentally benign nanocomposites.28,29
Using existing information, we developed a protocol to determine and quantify the release of nanomaterials induced by weathering and assess their reproducibility, transferability, and sensitivity toward different materials and uses.24,30,31 Recent studies have shown that weathering protocols change the environmental weathering of different polymeric nanocomposites filled with carbonaceous NMs, such as MWCNTs, graphene, carbon black and inorganic NPs of , , kaolin, , -phthalocyanines.30,32 Their study has also explained how dry or wet weathering protocols affect the aging and release characteristics of nanocomposites. Previous studies by Zepp et al. have shown that the polymer matrix is the most significant factor in nanocomposite aging. Wet weathering is more realistic than dry weathering, but dry weathering seems to provide a more controlled release of material than wet weathering.32,33 Several methods have been reported where CNTs were dispersed through functionalization, strong acids, solvents, and surfactants or by the addition of amphiphilic molecules.3 Curing agents are added in epoxy–nanomaterial resins for crosslinking, and the curing is done at high temperatures. Various techniques have been used to explain molecular interactions of the system for phase separation and curing characteristics of epoxy with CNTs, including the glass transition temperature and viscometry.34,35 Therefore, it is of great interest to investigate the effects of the CNT fillers with different functional groups on the property changes of epoxy–CNT composites for longterm applications.
This study focuses on how weathering of nanocomposites degrades nanocomposites during service or at the end of their useful life. Nano-enabled epoxy composites were tested in an accelerated weathering chamber, which simulates environmental weathering conditions during warm and wet seasons. We collected wash-water rinse solutions from individual sample plates simulating rain during aging. UV radiation has enough energy to break the carbon and oxygen bonds in polymers and to build volatile fragments. Accelerated aging of nano-enabled composites changes the physical, surface, and chemical properties resulting in the polymer degradation and the leaching of polymer moieties, such as phenolic compounds from epoxy, and the release of filler nanoparticles and fragments.
This study aims to investigate if the embedded nanomaterials in the nanocomposites could be released into the environment. The changes in the structural properties affecting the composite breakdown and possible release of nanoparticles caused by the brittling of the matrix material were analyzed. To provide insight into the stability and kinetics of structural changes of UV weathering, measurement of the light emitted through chemoluminescence was performed to determine the concentration of the excited polymer matrix elements. Results were compared with those from other characterization techniques for the surfaces of aged plates and released polymer fragments. The toxicity of released materials provides additional insight into the risk of polymer aging and nano-release. The results of this study provide a basis for the impacts of nano-additives on epoxy composites and increase our understanding of plastic aging, and the risks of nanomaterial release into the environment.
2. Experimental section
2.1. Materials
Epoxy plates were made using resin transfer moulding of Epon 862 (bisphenol F epoxy) and the curing agent EpiCure W (Miller-Stephenson Chemical Company, Inc.) to produce dimensionally accurate composites with high surface quality. The molecular structures of the resin and curing agent are shown in Scheme S1.† For the synthesis of the polymer, pyrrole (, ≥98%), ammonium persulfate (APS, , 98%) and -toluenesulfonic acid (PTSA, , ≥98.5%) were obtained from Sigma-Aldrich, St. Louis, MO. A high-speed mixer at 900 rpm was used for 30 minutes to minimize the aggregation of nanomaterials and form homogeneous mixtures (reaction components and nanoadditives are shown in ESI† Fig. S1 and Table S1). The mixtures were cast into a 30 cm × 30 cm rectangular mold, and adequate defoaming was carried out to cure the epoxy under controlled isothermal conditions for one hour at 100 °C and 2 h at 120 °C. For the weathering studies, four types of epoxy plates (5 cm × 5 cm × 3 mm thick (±0.1 mm)) were cut to make test samples using pure epoxy, and epoxy composites with 1 wt% neat MWCNTs, , and (Fig. S2†).
2.2. Environmental aging of epoxy–nanocomposites
Laboratory accelerated aging tests were carried out with the unfilled epoxy and three types of epoxy composites filled with neat and functionalized CNTs under controlled and reproducible conditions to achieve repeatability. A climactic test chamber simulating solar aging was equipped with a xenon lamp (Suntest XLS+, Atlas Material Testing Technology LLC) for controlling broad spectrum lighting and moisture conditions. We followed the ISO 4892–2:2013 method for exposing specimens to xenon-arc light in the presence of moisture to reproduce environmental weathering effects in shorter time frames.30,32,36 The xenon arc-lamp has a UV irradiance (300–400 nm) of 60 ± 2 W m−2 and black standard temperature of 65 ± 3 °C. The chamber temperature was kept at 40 ± 3 °C and the relative humidity was maintained at 50% ± 10%. Additional details of similar experimental protocols are available in the literature.24,28,29,37 The daily cumulative (simulated) solar irradiance was recorded, and the actual exposure time was calculated using accumulated annualized radiant exposure data. Weathering cycling included 111 min of irradiance with 9 min of simulated rain. This cycle was repeated until the total irradiance time reached an equivalent of 12 months of simulated environmental aging. The lamp emits a solar light flux of 700 W m−2 and wavelength of 200–800 nm and the black substrate temperature was 65 °C. The system monitors humidity and heat in the chamber during the aging study. Since the light intensity (irradiance) was varied in the chamber by ±10%, the sample position was changed daily. Moreover, the release of bisphenol A (BPA) and F (BPF), halogenated phenolic by-products, and carbon nanotubes was monitored periodically during the aging process. None of the control coupons were exposed in the chamber; instead they were soaked with warm water to test for the release of organic or inorganic material.
2.3. Collection of released fragments and leachates
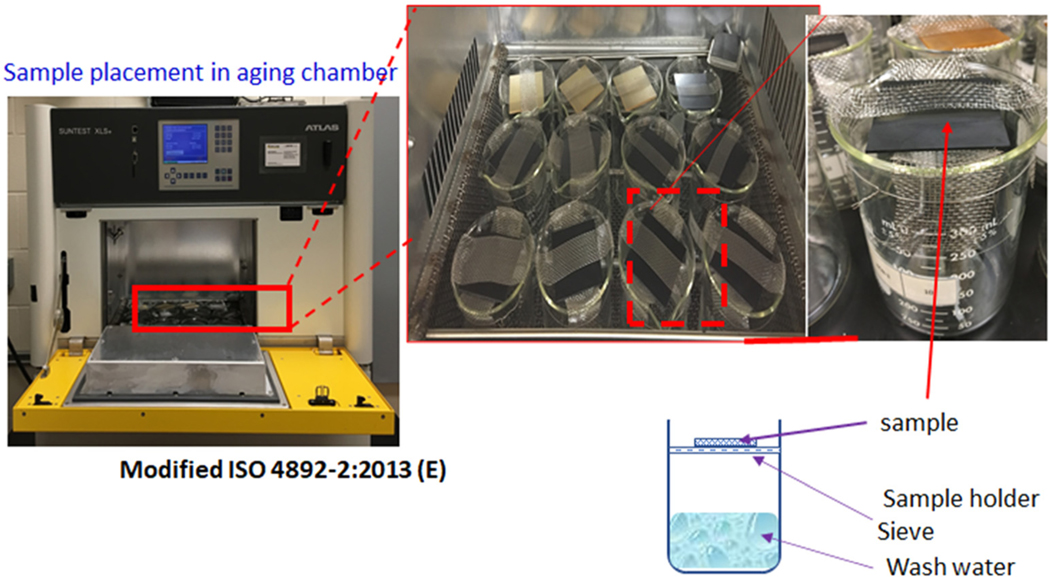
Each plate was placed on a stainless-steel sieve over a 250 mL beaker to collect spray water (simulated rain), thus rinsing the plates during the aging process and creating wash water (Fig. 1 and S3†). Milli-Q-grade water (type 1) (resistivity – ) was used for the spray water. The wash water was collected from each beaker every 24 h and transferred to purge bottles, where ultrapure was bubbled through the wash water to concentrate the leachate (Fig. S2, ESI†). At the end of the aging tests, the plates were sonicated in type 1 grade water. This method allowed us to recover all released polymer fragments and other non-volatile chemicals released from the epoxy matrix.
Fig. 1.
Experimental set-up and sample position in the aging chamber rotated daily to ensure even spraying and solar exposure. Weathering involved a solar irradiance of 700 W m−2, total irradiance of 6588 MJ m−2, and black substance temperature of 65 °C. A cycle of weathering was 111 min of solar light illumination and 9 minutes of rain spray.
2.4. Physical characterization of aged materials
Changes in physical and chemical properties of the epoxy and epoxy–MWCNT composites during the weathering process were examined. The changes in the sample thickness and mass were monitored. Contact angles of water droplets on the surface of pristine and aged composites were measured with a drop shape analyzer (DSA25E, KRÜSS GmbH). Mass variation of samples was observed by weighing samples before and at selected time intervals during the aging process. The mass variation results of aged samples were calculated by using eqn (1):
2.5. Fourier transform infrared spectroscopy (FT-IR)
The photo-initiated oxidation of polymers in the composites, including aesthetic changes such as discoloration accompanied by physical deterioration, was also investigated. Due to the combined effect of UV light, moisture and free radicals, the surface chemistry of the composites changed.
The chemical changes were investigated using an ATR-FTIR spectrometer (Cary 610 with a single point mapping FTIR microscope) and with an attenuated total reflectance (ATR) attachment between 4000 and 500 cm−1 with a resolution of 2 cm−1. The analysis conditions included 32 scans. IR spectroscopy was the most useful tool to detect the group of carbonyl compounds absorbing light in the 1710–1735 cm−1 region, which includes aldehydes (1735 cm−1) and ketones (1720 cm−1).38,39 Surface chemical bonds that absorb UV light of varying frequencies and different functional groups in the epoxy were detected by the absorption of IR light at a wavelength of 3500–500 cm−1.31 FTIR analysis in ATR mode was performed on neat epoxy and the three CNT-composites before and periodically during the aging tests at multiple locations on the exposed sides of the plates.
2.6. Scanning electron microscopy (SEM)
SEM is a powerful visualization tool to characterize morphologies of composites. SEM allows three-dimensional high-resolution images that describe the sample surface’s morphological changes during weathering. The surface of non-conductive samples must be coated with a thin layer of gold by ion-sputtering to reveal structural details such as cracks, swelling, and surface erosions. A JEOL SEM (JSM6490V) was used at 3–5 kV to investigate the aging effects on the morphology of the different types of epoxy–CNT composites, including surface changes.
2.7. Release fragment analysis with transmission electron microscopy (TEM)
Released nanomaterials from aged epoxy composites were investigated by collecting wash water samples prepared following the leaching protocol mentioned earlier in this section, and drying a single droplet of collected water on a TEM grid (FCF400-Cu, Electron Microscopy Sciences). Grids prepared in this manner were then observed using a JEM 2100 (JEOL, USA) transmission electron microscope. Dynamic light scattering (DLS) analysis (NanoBrook Omni, Brookhaven Instrument Corporation) and UV-visible spectroscopy (Spectra max plus, Molecular Devices) were also conducted on the concentrated wash-water solutions without sample preparation or treatment.
Transmission electron microscopy (TEM) was used to investigate the release of nanomaterials and fragments from the aged epoxy–CNT composites. The collected and concentrated wash water samples were used to analyze the released material contained during the simulated three month and 12 month exposure testing, based on an aging duration of 613 h and 2616 h, respectively. The final aged materials were sonicated using a laboratory sonicator (Branson 2800, Branson Ultrasonics Co., Danbury, CT, USA) for 30 min to study the materials released from the aged samples. A single droplet of supernatant was dried on a copper TEM grid (LC352-Cu, Electron Microscopy Sciences). For the analysis, a high-resolution TEM (HR-TEM, JEM-2010F, JEOL, Japan) was operated with a field emission electron gun at 200 kV.
2.8. Raman spectroscopy
Raman spectroscopy is a nondestructive technique for the characterization of carbon-based materials such as carbon black, carbon nanotubes, and graphene-based materials (i.e., graphene and graphene oxide). Raman spectroscopy of CNTs or graphene based composite materials has been used to evaluate the state of dispersion of CNTs and the polymer–filler interactions reflected by shifts or width changes of the peaks. Raman data were collected at the NanoWorld Center, Department of Chemical and Environmental Engineering, University of Cincinnati. Samples were analyzed using a Renishaw inVia™ Raman microscope that uses an Ar-ion laser with a 514 nm/50 mW wavelength/power configuration. The laser spot size is ~1 μm2 and operated at 100% with an exposure time of 10 s. The samples were placed on a stage perpendicular to the laser inlet. Spots were found using the internal microscope at a 500× magnification. The shroud was then closed, and samples were exposed to the laser recording wavenumber intensities from 100–3200 cm−1. The system was calibrated using a single crystal silicon wafer with a peak at 520 cm−1.
2.9. Toxicity determination of aged–released nano- or micro-fragments and chemicals
Toxicity measurements were compared on paired samples (0 h to 12 months simulated aging) of epoxy and epoxy with different types of MWCNTs. Toxicity was determined using two different assays: reactive oxygen species (ROS) formation assay and MTS assay for cell viability and cell activity, as described elsewhere.31 Human alveolar epithelial cells (A549; CCL-185, ATCC) were cultured using Dulbecco’s modified Eagle medium (DMEM, Gibco), supplemented with 10% FBS (Hi-Clone), 1% sodium pyruvate (Gibco), and 1% penicillin–streptomycin–neomycin (PSN, Gibco) and grown at 37 °C in a 5% CO2 atmosphere.
Epoxy–MWCNT composites, with no aging and maximum aging, were suspended in cell culture media at a concentration of 12.5 mg mL−1. The mass of suspended particles was calculated based on the rinse volume, bubbling volume reduction, and weight loss of each plate and confirmed by taking the dry weight of the suspended samples. The particle suspensions were sonicated as described above and then serially diluted to a 2 mg mL−1 final concentration during cell exposure. A control sample with just a medium was also added for the sonication and toxicity tests. ROS formation was determined using the dichlorofluorescein (DCF) assay. The conversion of (2′,7′-dichlorodihydrofluorescein, Molecular Probes) to fluorescent DCF signals the presence of ROS. For each test, 2 × 104 A549 cells were seeded per well of a 96-well plate in a volume of 200 μL and grown for 1 day, after which the medium was replaced by 100 μL of 50 μM in Hanks’ Balanced Salt Solution (HBSS). Thereafter, the cells were incubated for 60 min at 37 °C and 5% , followed by washing with prewarmed HBSS. Cells were then exposed to 100 μL of suspensions of all samples. Peroxynitrite donor 3-morpholinosydnonimine (Sin-1, Sigma-Aldrich) was used as a positive control, as it generates both superoxide anions and nitric oxide that spontaneously form peroxynitrite, a potent oxidant. Fluorescence intensities were measured after 2 h using a fluorescence microplate reader (Molecular Devices) at an excitation wavelength of 485 nm and an emission wavelength of 528 nm. Fluorescence values were blank, corrected and normalized.
The MTS assay, measuring cell viability and cell activity, was performed using a CellTiter 96 AQueous One solution (Promega) containing 3-(4,5-dimethylthiazol-2-yl)-5-(3carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium inner salt [MTS] as the tetrazolium compound and an electron coupling reagent (phenazine ethosulfate; PES). To perform the assay, 8 × 103 A549 cells were seeded in 200 μL of complete cell culture medium per well of a 96 well plate and grown for 1 day. Afterwards, cells were exposed to 200 μL of suspensions of all samples or the positive control CdSO4 (100 μM) for 24 h, at which point the medium was replaced by 120 μL of MTS working solution. The plate was incubated for 60 min at 37 °C and 5% before the optical density was measured at 490 nm in a microplate reader (Molecular Devices).
3. Results and data analysis
3.1. Gravimetric analysis and changes in surface hydrophobic properties
To reduce the effects of water uptake in the aging plates, the samples were taken out of the weathering chamber at the end of the UV light exposure cycle before water spray started.
The weight (mass) loss percentage experienced by the neat and MWCNT epoxy composite plates during 2616 h of accelerated weathering in the UV test chamber is depicted in Fig. 2. From the twelve plates at the beginning of the test, four were taken out after 613 h and then another four were taken out after 1226 h to investigate the morphological changes of the samples by weathering. There is a slow and steady mass loss that continued throughout the test period. The changes in the thickness of the plates taken at six locations of each plate at selected aging periods are shown in Fig. 3a. The initial weight loss may be attributed to the desorption of the residual moisture and volatiles and may not be attributed to polymer degradation.40 The epoxy composites with and functionalized MWCNTs showed slightly more mass and thickness changes at longer exposure periods, which appears related to polymer chain scission. The spray-washing used to simulate rain during the aging of epoxy and epoxy-composites may have caused some mass loss, where the wet aging process further oxidized and weakened the surface of the aged samples and led to the loss of microplastic fragments and nanofibers from the samples during aging. There was increasing discoloration of unfilled epoxy, as shown in images at selected aging times (Fig. S4†).
Fig. 2.
Weight changes of neat and MWCNT-modified epoxy plate samples during weathering.
Fig. 3.
The physical impact of weathering of epoxy samples: (a) change in sample plates’ thickness during the weathering and (b) increase in hydrophilic properties based on changes of contact angle during weathering.
3.2. Weathering induced changes in surface hydrophobicity
The hydrophobicity of epoxy composites is a measure of the material’s ability to repel water. Thus, a higher hydrophobicity gives a low wettability, and it is an important factor for the stability and outdoor performance of epoxy applications. The virgin surfaces of all the plates were hydrophobic, and there was little measurable difference in contact angles for both nano-filled and unfilled epoxy. Initially, all the materials had contact angles higher than 100°, where the epoxy–neat CNT composite had the highest contact angle (110° ± 2°), and the epoxy composites with amine or acid-modified CNTs had slightly lower contact angles (Fig. 3b). These results agree with literature values41 since these contact angles were all greater than 90°, meaning they were also hydrophobic. The changes in hydrophobicity levels are directly linked to the polarity of the material, which in turn can be described by the surface energy. The stability of hydrophobicity of the different epoxy composites depends on the changes in the chemical bonds in the polar backbone of the epoxy due to environmental stresses. The decrease in contact angle is higher for the nanocomposites, especially that with , where the surfaces have turned completely hydrophilic, and a continuous water film can form in the presence of moisture. When the epoxy nanocomposites with MWCNTs and MWCNT derivatives were prepared, their color became black due to the filler’s MWCNT color. Darker bodies absorb more light energy resulting in increased surface oxidation, where , benzene ring, and phenyl bonds were effectively degraded or severed producing more functional groups. This resulted in the decreased hydrophobicity of samples by the weathering, and similar results of the formation of functional groups on the surface of aged polymer nanocomposites was reported in previous studies.24,31,32,42 The surface wettability and decrease in contact angle are affected by the chemical functional groups and increased surface roughness.43 The low hydrophobicity of aged nano-filled epoxy is consistent with earlier studies that reported that extended aging results in the formation of surface polar functional groups and radicals and the accumulation of functionalized MWCNTs on the surface of aged epoxy surfaces.44–46 The results of FTIR analysis in section 3.3 confirmed the chemical changes of the epoxy nanocomposites’ surface by the weathering.
The study shows that the degradation of epoxy composites grows with an increase in UV exposure. Prolonged weathering results from free radical oxidation between water molecules and reactive oxygen species and the polymer chain. Hydrophobicity decreased sharply with water uptake, resulting in changes in surface chemistry. UV-aged polymer surfaces became hydrophilic due to chemical modification of the surface due to an increase in oxidized compounds, carbonyls and other double bond moieties.41 Neat epoxy retained some hydrophobic properties after an extended exposure period, similar to previously reported results.32
3.3. Chemical changes in epoxy composites during weathering
Epoxy resins are susceptible to ultraviolet (UV) light damage and their durability is reduced significantly when exposed to the outdoor environment.47 However, additives such as carbon black nanoparticles have been shown to improve the UV degradation resistance of epoxy.48 The degradation of epoxy-nanocomposites resulted in the physicochemical changes of the exposed surfaces. Because of this, a combination of environmental stresses, including exposure to UV light, moisture and heat, has an aging effect on the polymers. The changes in the chemical functional groups of the exposed surface during weathering were monitored using attenuated total reflectance-FTIR spectra collected at different selected times. The results of the LC-QTOF-MS and UV-vis spectroscopy analysis were used to monitor polymer chain scission caused by the aging process at the micro level (Fig. S7–S9†). High molecular weight epoxy polymers of bisphenol A have been shown to change color, indicating that chain scission and cross-linking occurred simultaneously (Fig. S1 and S5†).49 ATR spectra were acquired prior to weathering and after 613, 1226 and 2616 hours of UV exposure to evaluate the overall effects of weathering on the polymer, and as a baseline for designing a weathering test matrix. FTIR spectra of aged plates were compared with those of unaged neat and nanomaterial doped epoxy samples. Fig. 4 shows comparisons between spectra acquired before weathering and at selected exposure periods. The FTIR absorption peaks were identified as those associated with the chemical structure of the crosslinked epoxy–amine film.
Fig. 4.
Surface FTIR analysis of pristine and aged epoxy-composites after selected aging periods for composites made of (a) , (b) epoxy , and (c) .
For the neat and nanomaterial-added epoxy samples, the characteristic band of near 1730 cm−1 increased with the decrease of near 2922 cm−1 and 2851 cm−1, which indicates that bonds were oxidized, and saturated carbonyls formed.50,51 Weathering exposure resulted in a continuous decrease in peak intensity and width for all observed peaks. The intensity of carbonyl and bonds did not appear to have obvious changes from 1000 h to 3000 h of the aging time. Additionally, the intensity of characteristic absorption bands of benzene rings near 1606 cm−1, 1508 cm−1 and 1456 cm−1 decreased with the weathering because of the destruction of the benzene ring structure and the formation of chemical species .52 Epoxy wafers are sensitive to UV degradation, and after only 500 hours of exposure, a significant decrease in peak height was observed. Longer weathering completely broke down the polymer structure, indicated by the disappearance of absorption peaks. The intensity of the absorption band at 1178 cm−1, corresponding to the symmetric stretching vibration of –phenyl, decreased with the aging process. The results showed that some of the –phenyl bonds were destroyed.51
3.4. Measuring weathering-caused changes in the surface morphology
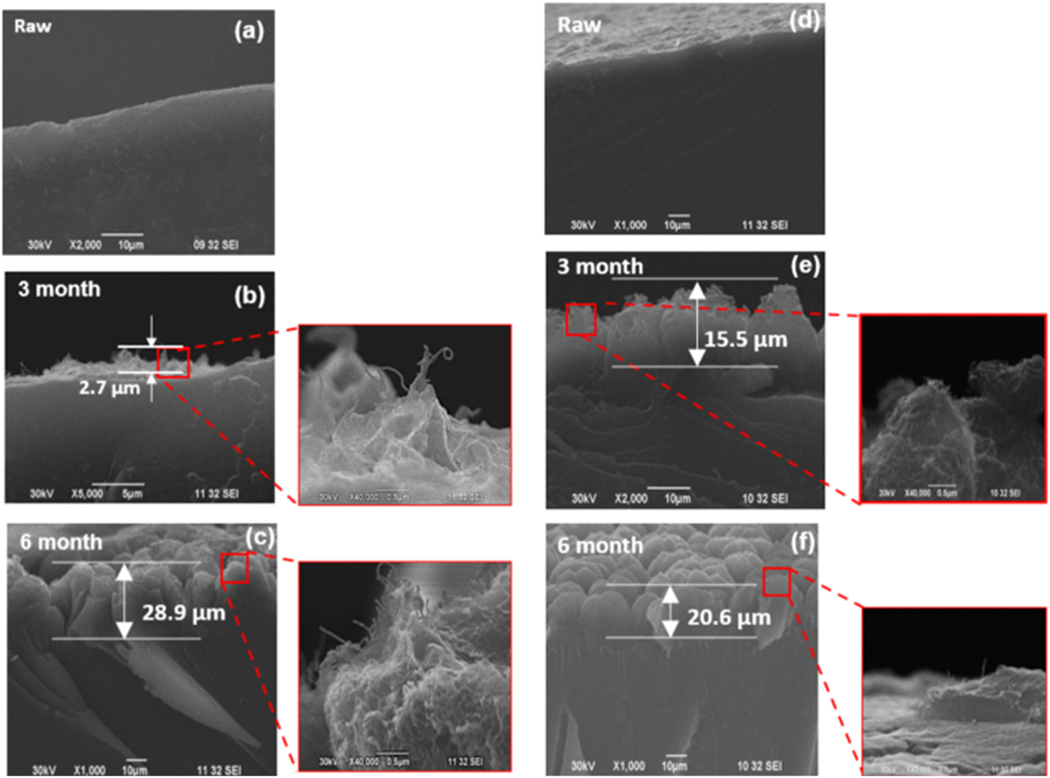
The surfaces of exposed and unexposed samples of neat and MWCNT-composites subjected to accelerated weathering were examined by scanning electron microscopy (SEM). Before exposure to accelerated weathering, the unweathered samples showed clean and smooth surfaces without cracks. However, due to weathering, very rough surfaces formed for all aged samples were observed. Also, embedded MWCNTs in the composites were found on the surface of aged epoxy nanocomposites due to surface oxidation caused by the weathering (Fig. 5).
Fig. 5.
SEM images of the exposed surface morphology of neat epoxy and epoxy with different functionalized MWCNT composites before and after weathering.
The weathering damage of the polymer occurs at the macroscopic and microscopic levels, where surface oxidation forms shallow cracks at the onset of the aging process. Thus, changes in the chemical, physical and mechanical properties of epoxy from environmental aging lead to mass loss and micro-cracking during use. This loss of material and chemical changes in the polymer have major economic and environmental implications. A mismatch in the thermal expansion coefficients due to resin and fiber shrinkage gives rise to localized stresses and damage, eventually leading to interface micro-cracks. Weathering causes stress cracking, a common cause of material failure in amorphous polymers such as epoxy.53 Previous studies have shown that due to the oxidation of the polymer surface, it becomes brittle, resulting in deformations such as micro-cracks, which can be ~30 nm in diameter.54 These surface micro-cracks are typically undetected. They become broader and deeper with increased aging, resulting in declining physical and mechanical properties of the material, including a loss of elasticity and material failure. Previous studies reported similar structures of micro-cracks and matrix erosion in epoxy–carbon nanofiber nanocomposites by UV photooxidation and water condensation.55
In this study, SEM analysis allowed for the observation of the surface morphology of the neat and nanomaterial added composites exposed to accelerated aging (Fig. 6 and 7). Surface microcracks gradually deepened leading to overall material degradation. Pure discoloration and damage of the polymer can propagate locally causing a rapid deterioration of the physical properties of the polymer (Fig. S1†). The impacts of weathering on the neat and CNT modified epoxy samples were also investigated to determine the degree to which crack formation depended on the composite’s matrix material. Fig. 6(a) to (f) show images of cross-sectioned samples of unaged and aged pristine epoxy and epoxy filled with MWCNTs. The impacts of the aging process were evident in all epoxy samples from the presence of obviously rougher surfaces and cracks in the aged samples. Similar changes due to surface aging are shown in Fig. 7(a) to (f) for the and epoxy samples. The primary toughening mechanism in the nanocomposites is the formation of many microcracks and the increase in the fractured surface is due to crack deflection. Previous studies have shown that nanoparticles in epoxy induce various fracture mechanisms, such as crack deflections and plastic deformation. At the same time, the toughness and strength of the material are improved.56 Moreover, in cases of nanocomposites reinforced with nanoclays, MWCNTs, carbon black, graphene, and SiO2, surface cracks and matrix erosion were observed, resulting in the deformation of materials by weathering.24,31,32 Functionalized MWCNTs are expected to influence the deformation of epoxy on the micro-scale and retard the formation of larger cracks. Fig. S6† shows images of cracks widening with extended weathering. They were obtained using a Zyglo fluorescent penetrant dye (, laser confocal microscopy, 40×).
Fig. 6.
SEM images of cross-sectioned pure epoxy sample (a) unaged, (b) 3 months aged, and (c) 6 months aged, and epoxy–neat MWCNT composites (d) unaged, (e) 3 months aged, and (f) 6 months aged. Higher magnifications (right) show the surface layer and embedded MWCNTs.
Fig. 7.
SEM images of cross-sectioned composite (a) unaged, (b) 3 months aged, and (c) 6 months aged, and composite (d) unaged, (e) 3 months aged, and (f) 6 months aged. Higher magnifications (right) show the surface layer and embedded MWCNTs.
3.5. Measuring and imaging of fragment releases and transmission electron microscopy
In addition to TEM analysis for this nano-release study, dynamic light scattering (DLS) was used to measure the particle size distribution of released materials from the aged epoxy–MWCNT composites. As seen in Fig. 9, the released particle size distribution from samples decreased from several hundred nanometers to a few nanometers after filtering using 0.45 μm syringe filters (Whatman™ GD/X PVDF syringe filters), indicating that both microplastics and carbon nanotubes are released together, and the presence of nanomaterials in the samples was hidden by microplastic particles. The shapes of the profiles and the average diameter of the DLS curves show the overall behavior of the polymer resulting from photodegradation changes with time. After filtering, we effectively observed the presence of released nanomaterials from the aged composites. Released nanoparticles were detected in all aged epoxy–MWCNT composites. Although some of the aged samples showed nanoparticles, in the sample aged for 756 h, there were few released CNTs detected over extended aging times (Fig. S10†).
Fig. 9.
Raman spectroscopic characterization of (a) neat MWCNTs showing main bands with a 514 nm laser, (b) released fragment samples after 1226 h exposure and (c) released fragment samples after 2616 h from the different epoxy–MWCNT composites.
Long term weathering caused the degradation of the epoxy and the release of polymer molecule fragments. Although the release of polymeric fragments and nanofillers from photodegraded composites was previously studied, the role of functionalized MWCNTs in the leachate water collected during weathering was not investigated (ESI† Fig. S6 to S9). The release of micro-fragments and MWCNTs from aged epoxy composites was studied here.
All the wash water samples collected from individual sample beakers during the accelerated weathering (see Fig. 1) were concentrated by bubbling N2 and then analyzed by TEM. Unlike previous studies where aged samples were immersed in surfactant water solution and sonicated to release loose particles, released fragments and leachates were collected over the entire aging period.24,30,42 Representative TEM images of leaching solutions of plates weathered for 613 h and 1226 h with the wet protocol show the release of fragments from a few nanometers to several micrometers in size from the aged epoxy–MWCNT composites (Fig. 8). The first row shows fragments derived from neat epoxy without a filler, which contains macro-scale fragments; most of the plates were disintegrated and had to be removed from the weathering chamber. MWCNTs were released or were protruding from (but still embedded in) polymer fragment samples after 613 h, while free MWCNTs were observed from samples after 1226 h. A similar release of free and embedded MWCNTs was reported in prior studies.31,32,42 A previous study showed that particle release slows down after the first polymer surface layer oxidizes, slowing the aging process.42
Fig. 8.
Transmission electron microscopy images of released polymer fragments and added CNTs from wash water samples collected after 613 h and 1226 h of aging.
3.6. Raman spectroscopy analysis of released fragments
Conventionally, SEM and TEM imaging techniques are often used to study polymer composite fragment dispersions because of their ability to provide direct visualization of nanomaterials. However, they provide only a qualitative understanding of the structure of released materials. Raman spectroscopy could provide valuable structural information about samples while preserving their integrity. Raman spectra were obtained for the control samples of three replicate samples of MWCNT with a 514 nm AR-ion laser for excitation (Fig. 9).
MWCNT has a typical Raman spectrum that includes a G band (~1585 cm−1), for the bond stretching mode of bonds in the lattice, a G′ band (~2700 cm−1), for most sp2 carbon materials, and a D band (~1350 cm−1). Studies have shown that the output of 514 nm laser excitation is most prominent in the 2500 nm region.57 The D-band is the most intense feature of MWCNTs when using 514 nm excitation, and therefore was chosen as the analysis target to assess the detection limit of Raman spectroscopy for the released fragments with MWCNT nanocomposite loading in the samples. The Raman spectra of neat MWCNTs are shown in Fig. 9a. The main peaks are the G band at 1580 cm−1 (inplane vibration of bonds), the D band at 1350 cm−1 (presence of disorder in carbon) and the G′ band at 2698 cm−1 (overtone of the D band). Raman spectra of released fragment samples after 1000 h and 3000 h exposure are shown in Fig. 9b and c, respectively. Wash water samples from the composite show little detected nanomaterials, which could mean that interacts more with the epoxy. Samples from epoxy with neat CNTs released the most detected nanomaterials which was more apparent after 3000 h weathering. The Raman band of the functionalized NTs shifted to a higher wavenumber, suggesting that inter-tube interaction was lower than the physical interaction with the polymer (Table S1†).
The D-band and G-band peak position could be associated with the electronic and vibrational density of the state since they originate from the “resonance phenomenon”.58 The interaction of epoxy molecules with MWCNTs may alter the vibrational density of state and could eventually affect the vibrational modes of MWCNTs.59 The tangential displacement G-band is also sensitive to the charge that is exchanged between the CNTs and the adsorbed molecule. Hence, the shift in the G-band to a higher wavenumber is observed for epoxy with the acid and amine-functionalized CNTs, where the modified MWCNTs could be due to the interaction between the epoxy modifier fragment molecules and MWCNTs. Previous studies have reported a similar shift to higher wavelengths in the Raman spectroscopic peaks of pyrene-modified MWCNTs.60 Furthermore, a shift to a higher wavenumber can also arise due to weaker inter-tube interactions, suggesting the “de-agglomeration” of MWCNTs in the presence of the modifier molecules.
3.7. Measuring the toxicity of released nanomaterials and leachates
The wash water collected from the bottom of aging sample plates was collected, transferred to bottles, and gradually evaporated by bubbling nitrogen (Fig. S7†). Nontarget analysis of the final concentrated samples with LC-QTOF-MS was performed to identify components in the leachate and UV-vis spectroscopy showed phenolic and aromatic compounds, polymer fragments, and MWCNTs (Fig. S8 and S9 and Table S2†) similar to recent studies.61 As shown in Fig. S8 in the ESI,† different pollutants were released and remained in the leachate during the weathering process.
A comparative animal inhalation exposure toxicity study involving generated dust from the mechanical abrasion of epoxy composites with and without MWCNTs, and MWCNT–epoxy showed similar pulmonary responses, but there were differences in liver histology.62,63 However, high doses of dust were required to elicit a response. Saber et al.’s toxicity study suggests that the exposure concentration is vital to the deposited dose in the pulmonary region. In our study, toxicity assessments were done for paired samples of unaged (0 h) and fully aged wash water, concentrated on both epoxy and epoxy–MWCNTs. Two common toxicity measurements are the formation of reactive oxygen species (ROS) in cells exposed to toxins and the effects on cell activity and viability.31,64–66
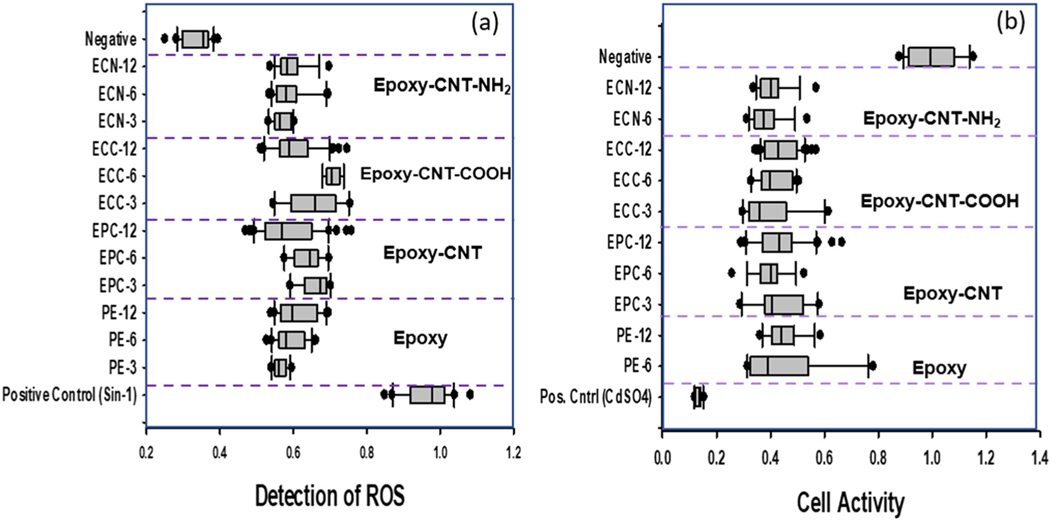
The cytotoxicity and in vitro biocompatibility of composite materials used for medical devices are tested following ISO 10993–5.67 Although inhalation is not the primary exposure route for degraded and leached nanoparticles, we tested the toxicity of non-aged and aged samples on A549 alveolar epithelial cells because these cells respond to damaging reactive oxygen species (ROS) via redox cycling.42,68 We measured the production of intracellular ROS on all samples, using Sin-1 as a positive control, which generates both superoxide ions and nitric oxide, spontaneously forming peroxynitrite, a potent oxidant. As shown in Fig. 10a, all aged samples produced significant levels of ROS, suggesting that the degradation did not increase radical oxygen species (ROS) with radical oxides as toxins. Light irradiation and water spray created some interaction with the epoxy resin and produced degradation products. Previous studies have shown that water cuts the crosslink chains by hydrolysis reaction and reacts with amine groups to form and bonds.69 The release of particle fragments, MWCNTs, and leachates increased with the aging of materials. For the samples with the pristine MWCNT and acid-functionalized MWCNT additives, the detected ROS increased for aged samples. The epoxy samples with no additive show similar organic leachates, which could be partially responsible for cytotoxicity, and low toxicity levels to cell viability assay.
Fig. 10.
Intracellular formation of reactive oxygen species (ROS) and cell activity monitoring of A549 cells exposed to wash water leachate of minimum aged (3 month) and aged samples (maximum aged sample) of epoxy and epoxy–MWCNTs. Each assay had a positive control and a negative control. (a) Levels of ROS produced as measured in fluorescence units in the various samples. Sin-1 served as a positive control. (b) Cell viability and activity as determined by the production of formazan, after 24 h exposure to the various samples. served as a positive control (sample names: PE = neat epoxy, EPC = epoxy with CNTs, ECC = epoxy with acid functionalized MWCNTs, and ECN = amine functionalized MWCNT added epoxy, the numbers refer to the aging times equivalent to 3, 6, and 12 months).
Further toxicity testing was done using the MTS assay, which assesses cell activity and viability. MTS is a compound that is bioreduced by cells into formazan, a colored product that can be detected by optical density. In this study, cells were exposed to leachate and released water samples for 24 h and compared to non-exposed cells. (negative control) (Fig. 10b). The assay indicated that while the positive control, CdSO4, reduced cell activity, all of the wash water paired aged and non-aged samples did not significantly affect cell activity. This reduction of cell activity was lower compared to the negative control. Although there have been studies analyzing MWCNT composites,70,71 there have been limited studies to date evaluating the toxicity of epoxy and epoxy–MWCNT leachate composites.72,73 The generation of ROS is critical to the cellular reactions involving MWCNTs. The A549 assay used for this study has shown to be a reliable and reproducible indicator for many toxicity studies.74–76 Our results show that Sin-1 can be used as a qualitative control to induce H2DCF processing in a cellular and cell-free environment. The wash water samples from both filled and unfilled epoxy plates did not show a significant difference in the generation of ROS (Fig. 10(a)). This might be because the polymer leachate and fragments cause more ROS generation than the release of embedded MWCNTs. The cell activity test and comparison with pristine epoxy shown in Fig. 10(b) indicate the type of functionalization of MWCNT released and the weather period are less significant than the negative control samples. MWCNTs showed an inhibited MTS assay, which indicates the negative impact of MWCNTs on cell proliferation and metabolic activities.77 The non-aged and aged samples in this study produce degraded products that may cause toxicity. Changes to the functional groups or other surface properties of the same nanoparticles can also affect toxicity levels, Fig. 10(a).
4. Conclusion
The present paper examines the accelerated weathering mechanism of epoxy polymer and the role of neat and modified MWCNT additives. The main factors affecting the degradation of polymers are the polymer matrix and the UV irradiation dose. Photo-initiated free radicals oxidize the epoxy matrix, changing its physical and surface properties, such as a rapid decrease in contact angle of the aged surface, which appeared early, and the plates became brittle and cracked. Cracking increased and deepened with UV dose gradually. Epoxy loses some of its toughness after UV exposure, and fragments and cracks form under extended aging. The neat epoxy plate shows discoloration due to oxidation or elimination of functional groups along the polymer chain. Polymer fragmentation also occurred. Weathering of epoxy–CNT composites released phenolic and aromatic compounds, nanomaterials, and polymer fragments. CNT–epoxy degradation is not significantly influenced by the addition of functionalized CNTs. However, the functional modification of CNTs could modulate the properties of released nano-additives. Epoxy with acid-functionalized CNTs showed less free release than those with non-functionalized and amine-functionalized CNTs. The released material from the neat epoxy and CNT–epoxy composite showed moderate cytotoxicity – based on ROS generation, cell activity, and viability tests. The toxicity appears more related to released organic components than nanomaterials released from the resin. Future studies might focus on the toxicity of the different released materials and leachates to determine a quantitative understanding of the risk.
Supplementary Material
Environmental significance.
Nano-sized particles (NPs) are added to many products to increase quality. The risks versus benefits of nanoadditive products – such as composites – and the potential for release over their life cycle should be evaluated to determine the exposure levels and legislated in favor of public health. The influence of NP interactions with polymer matrices on their environmental release has not been well studied. The current paper focuses on some analytical techniques suitable for evaluating the effects of weathering on the detection and characterization of NPs, including Fourier transform infrared spectroscopy (FT-IR), optical microscopy, contact angle measurements, gravimetric analysis, confocal microscopy, transmission electron microscopy (TEM), scanning electron microscopy (SEM) and Raman spectroscopy. The study also includes the toxicity of released particles and organic compounds.
Acknowledgements
This paper has been subjected to a U.S. EPA review and has been approved for publication. Note that approval through this review process does not signify that the contents necessarily reflect the views of the Agency. Any mention of trade names, manufacturers, or products does not imply an endorsement by the U.S. Government or the U.S. EPA. The U. S. EPA and its employees do not endorse any commercial products, services, or enterprises. CH acknowledges the partial support from the Korea Environmental Industry & Technology Institute (KEITI) through the Measurement and Risk Assessment Program for Management of Microplastics Project, funded by the Korea Ministry of Environment (MOE) (grant number 2020003110005) and the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. 2021R1A2C1093183).
Footnotes
Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2en01014c
Conflicts of interest
There are no conflicts to declare.
References
- 1.Sun X, et al. , Developing polymer composite materials: carbon nanotubes or graphene?, Adv. Mater, 2013, 25(37), 5153–5176. [DOI] [PubMed] [Google Scholar]
- 2.Lim D-S, An J-W and Lee HJ, Effect of carbon nanotube addition on the tribological behavior of carbon/carbon composites, Wear, 2002, 252(5–6), 512–517. [Google Scholar]
- 3.Sulong AB, et al. , Wear behavior of functionalized multiwalled carbon nanotube reinforced epoxy matrix composites, J. Compos. Mater, 2006, 40(21), 1947–1960. [Google Scholar]
- 4.Hollertz R, et al. , Improvement of toughness and electrical properties of epoxy composites with carbon nanotubes prepared by industrially relevant processes, Nanotechnology, 2011, 22(12), 125702. [DOI] [PubMed] [Google Scholar]
- 5.Lvov Y, Guo B. and Fakhrullin RF, Functional polymer composites with nanoclays, Royal Society of Chemistry, 2016. [Google Scholar]
- 6.Ajayan PM, Schadler LS and Braun PV, Nanocomposite science and technology, John Wiley & Sons, 2006. [Google Scholar]
- 7.Tkalya EE, et al. , The use of surfactants for dispersing carbon nanotubes and graphene to make conductive nanocomposites, Curr. Opin. Colloid Interface Sci, 2012, 17(4), 225–232. [Google Scholar]
- 8.Sahoo NG, et al. , Polymer nanocomposites based on functionalized carbon nanotubes, Prog. Polym. Sci, 2010, 35(7), 837–867. [Google Scholar]
- 9.Wei H, et al. , Adhesion and cohesion of epoxy-based industrial composite coatings, Composites, Part B, 2020, 193, 108035. [Google Scholar]
- 10.Liu S, et al., A review of extending performance of epoxy resins using carbon nanomaterials, Composites, Part B, 2018, 136, 197–214. [Google Scholar]
- 11.Gilbert EN, Hayes BS and Seferis JC, Nano-alumina modified epoxy based film adhesives, Polym. Eng. Sci, 2003, 43(5), 1096–1104. [Google Scholar]
- 12.Wang J, et al. , Effect of amino-functionalization of multiwalled carbon nanotubes on the dispersion with epoxy resin matrix, J. Appl. Polym. Sci, 2006, 100(1), 97–104. [Google Scholar]
- 13.Fidelus J, et al. , Thermo-mechanical properties of randomly oriented carbon/epoxy nanocomposites, Composites, Part A, 2005, 36(11), 1555–1561. [Google Scholar]
- 14.Wang L. and Wong C, Epoxy-additive interaction studies of thermally reworkable underfills for flip-chip applications, in 1999 Proceedings. 49th Electronic Components and Technology Conference (Cat. No. 99CH36299), IEEE, 1999. [Google Scholar]
- 15.Baller J, et al. , Interactions between silica nanoparticles and an epoxy resin before and during network formation, Polymer, 2009, 50(14), 3211–3219. [Google Scholar]
- 16.Hu Y, et al. , Novel micro-nano epoxy composites for electronic packaging application: Balance of thermal conductivity and processability, Compos. Sci. Technol, 2021, 209, 108760. [Google Scholar]
- 17.De Azeredo HM, Nanocomposites for food packaging applications, Food Res. Int, 2009, 42(9), 1240–1253. [Google Scholar]
- 18.Friedrich K, Fakirov S. and Zhang Z, Polymer composites: from nano-to macro-scale, Springer Science & Business Media, 2005. [Google Scholar]
- 19.Idumah CI, Hassan A. and Ihuoma DE, Recently emerging trends in polymer nanocomposites packaging materials, Polym.-Plast. Technol. Mater, 2019, 58(10), 1054–1109. [Google Scholar]
- 20.Hatzigrigoriou N. and Papaspyrides C, Nanotechnology in plastic food-contact materials, J. Appl. Polym. Sci, 2011, 122(6), 3719–3738. [Google Scholar]
- 21.Jancar J, et al. , Current issues in research on structure– property relationships in polymer nanocomposites, Polymer, 2010, 51(15), 3321–3343. [Google Scholar]
- 22.Ilie N, Rencz A. and Hickel R, Investigations towards nanohybrid resin-based composites, Clin. Oral Investig, 2013, 17(1), 185–193. [DOI] [PubMed] [Google Scholar]
- 23.Dasari A, Yu Z-Z and Mai Y-W, Fundamental aspects and recent progress on wear/scratch damage in polymer nanocomposites, Mater. Sci. Eng., R, 2009, 63(2), 31–80. [Google Scholar]
- 24.Han C, et al. , Environmental aging and degradation of multiwalled carbon nanotube reinforced polypropylene, Carbon, 2018, 129, 137–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Souza VGL and Fernando AL, Nanoparticles in food packaging: Biodegradability and potential migration to food —A review, Food Packag. Shelf Life, 2016, 8, 63–70. [Google Scholar]
- 26.Iavicoli I, et al. , Nanotechnology in agriculture: Opportunities, toxicological implications, and occupational risks, Toxicol. Appl. Pharmacol, 2017, 329, 96–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adjei IM, Sharma B. and Labhasetwar V, Nanoparticles: cellular uptake and cytotoxicity, Adv. Exp. Med. Biol, 2014, 73–91. [DOI] [PubMed] [Google Scholar]
- 28.Harper S, et al. Measuring nanomaterial release from carbon nanotube composites: review of the state of the science, J. Phys.: Conf. Ser, 2015, 617, 012026. [Google Scholar]
- 29.Kingston C, et al. , Release characteristics of selected carbon nanotube polymer composites, Carbon, 2014, 68, 33–57. [Google Scholar]
- 30.Wohlleben W, et al. , NanoRelease: Pilot interlaboratory comparison of a weathering protocol applied to resilient and labile polymers with and without embedded carbon nanotubes, Carbon, 2017, 113, 346–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Han C, et al. , Evaluating Weathering of Food Packaging Polyethylene-Nano-clay Composites: Release of Nanoparticles and their Impacts, NanoImpact, 2018, 9, 61–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zepp R, et al. , Fragmentation of polymer nanocomposites: modulation by dry and wet weathering, fractionation, and nanomaterial filler, Environ. Sci.: Nano, 2020, 7(6), 1742–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nowack B, et al. , Potential release scenarios for carbon nanotubes used in composites, Environ. Int, 2013, 59, 1–11. [DOI] [PubMed] [Google Scholar]
- 34.Gomez CM and Bucknall CB, Blends of poly (methyl methacrylate) with epoxy resin and an aliphatic amine hardener, Polymer, 1993, 34(10), 2111–2117. [Google Scholar]
- 35.Tikhani F, et al. , Curing kinetics and thermal stability of epoxy composites containing newly obtained nano-scale aluminum hypophosphite (AlPO2), Polymer, 2020, 12(3), 644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuvshinnikova O, Boven G. and Pickett JE, Weathering of aromatic engineering thermoplastics: Comparison of outdoor and xenon arc exposures, Polym. Degrad. Stab, 2019, 160, 177–194. [Google Scholar]
- 37.Wang J, Schlagenhauf L. and Setyan A, Transformation of the released asbestos, carbon fibers and carbon nanotubes from composite materials and the changes of their potential health impacts, J. Nanobiotechnol, 2017, 15(1), 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barbosa APC, et al. , Accelerated aging effects on carbon fiber/epoxy composites, Composites, Part B, 2017, 110, 298–306. [Google Scholar]
- 39.Ollier-Durbault V. and Gosse B, Photo-oxidation and electrical aging of anhydride-cured epoxy resins, IEEE Trans. Dielectr. Electr. Insul, 1998, 5(6), 935–943. [Google Scholar]
- 40.Ozcelik O, Aktas L. and Altan M, Thermo-oxidative degradation of graphite/epoxy composite laminates: Modeling and long-term predictions, eXPRESS Polym. Lett, 2009, 3(12), 797–803. [Google Scholar]
- 41.Hameed N, et al. , Hydrogen bonding interactions, crystallization, and surface hydrophobicity in nanostructured epoxy/block copolymer blends, J. Polym. Sci., Part B: Polym. Phys, 2010, 48(7), 790–800. [Google Scholar]
- 42.Han C, et al. , Polypropylene–MWCNT composite degradation, and release, detection and toxicity of MWCNTs during accelerated environmental aging, Environ. Sci.: Nano, 2019, 6(6), 1876–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang X, Kumagai S. and Yoshimura N, Contamination performances of silicone rubber insulator subjected to acid rain, IEEE Trans. Dielectr. Electr. Insul, 1998, 5(6), 909–916. [Google Scholar]
- 44.Hsieh HS and Jafvert CT, Reactive oxygen species generation and dispersant-dependent electron transfer through single-walled carbon nanotubes in water, Carbon, 2015, 89, 361–371. [Google Scholar]
- 45.Chen C-Y and Jafvert CT, The role of surface functionalization in the solar light-induced production of reactive oxygen species by single-walled carbon nanotubes in water, Carbon, 2011, 49(15), 5099–5106. [Google Scholar]
- 46.Suresh B, et al. , Mechanical and surface properties of low-density polyethylene film modified by photo-oxidation, Polym. J, 2011, 43(4), 398–406. [Google Scholar]
- 47.Amin M. and Salman M, Aging of polymeric insulators (an overview), Rev. Adv. Mater. Sci, 2006, 13(2), 93–116. [Google Scholar]
- 48.Ghasemi-Kahrizsangi A, et al. , Improving the UV degradation resistance of epoxy coatings using modified carbon black nanoparticles, Prog. Org. Coat, 2015, 85, 199–207. [Google Scholar]
- 49.Kelleher P. and Gesner B, Photo-oxidation of phenoxy resin, J. Appl. Polym. Sci, 1969, 13(1), 9–15. [Google Scholar]
- 50.Pei Y-M, et al. , Thermal-oxidative aging of DGEBA/EPN/ LMPA epoxy system: Chemical structure and thermal–mechanical properties, Polym. Degrad. Stab, 2011, 96(7), 1179–1186. [Google Scholar]
- 51.Wolfrum J, Eibl S. and Lietch L, Rapid evaluation of long-term thermal degradation of carbon fibre epoxy composites, Compos. Sci. Technol, 2009, 69(3–4), 523–530. [Google Scholar]
- 52.Ohno S, et al. , Thermal degradation of IM7/BMI5260 composite materials: characterization by X-ray photoelectron spectroscopy, Mater. Sci. Eng., A, 2000, 293(1–2), 88–94. [Google Scholar]
- 53.Maxwell A, et al. , Review of accelerated ageing methods and lifetime prediction techniques for polymeric materials, 2005. [Google Scholar]
- 54.Arnold J, Environmental stress crack initiation in glassy polymers, Trends Polym. Sci, 1996, 12(4), 403–408. [Google Scholar]
- 55.Kumar BG, Singh RP and Nakamura T, Degradation of carbon fiber-reinforced epoxy composites by ultraviolet radiation and condensation, J. Compos. Mater, 2002, 36(24), 2713–2733. [Google Scholar]
- 56.Wetzel B, et al. , Epoxy nanocomposites–fracture and toughening mechanisms, Eng. Fract. Mech, 2006, 73(16), 2375–2398. [Google Scholar]
- 57.Piao Y, et al. , Comparative study of multiwall carbon nanotube nanocomposites by Raman, SEM, and XPS measurement techniques, Compos. Sci. Technol, 2021, 208, 108753. [Google Scholar]
- 58.Poyekar AV, et al. , Influence of noncovalent modification on dispersion state of multiwalled carbon nanotubes in melt-mixed immiscible polymer blends, ACS Appl. Mater. Interfaces, 2014, 6(14), 11054–11067. [DOI] [PubMed] [Google Scholar]
- 59.Hussain S, et al. , Spectroscopic investigation of modified single wall carbon nanotube (SWCNT), J. Mod. Phys, 2011, 2(06), 538. [Google Scholar]
- 60.Zhang Y, et al. , Spectroscopic evidence and molecular simulation investigation of the π–π interaction between pyrene molecules and carbon nanotubes, J. Nanosci. Nanotechnol, 2007, 7(7), 2366–2375. [DOI] [PubMed] [Google Scholar]
- 61.Luft A, et al. , Nontarget analysis via LC-QTOF-MS to assess the release of organic substances from polyurethane coating, Environ. Sci. Technol, 2017, 51(17), 9979–9988. [DOI] [PubMed] [Google Scholar]
- 62.Saber AT, et al. , Epoxy composite dusts with and without carbon nanotubes cause similar pulmonary responses, but differences in liver histology in mice following pulmonary deposition, Part. Fibre Toxicol, 2015, 13(1), 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Du J, et al. , Understanding the toxicity of carbon nanotubes in the environment is crucial to the control of nanomaterials in producing and processing and the assessment of health risk for human: a review, Environ. Toxicol. Pharmacol, 2013, 36(2), 451–462. [DOI] [PubMed] [Google Scholar]
- 64.Schlagenhauf L, et al. , Carbon Nanotubes Released from an Epoxy-Based Nanocomposite: Quantification and Particle Toxicity, Environ. Sci. Technol, 2015, 49(17), 10616–10623. [DOI] [PubMed] [Google Scholar]
- 65.Mikkelsen L, et al. , Cytotoxicity, oxidative stress and expression of adhesion molecules in human umbilical vein endothelial cells exposed to dust from paints with or without nanoparticles, Nanotoxicology, 2013, 7(2), 117–134. [DOI] [PubMed] [Google Scholar]
- 66.Johnston HJ, et al. , A critical review of the biological mechanisms underlying the in vivo and in vitro toxicity of carbon nanotubes: The contribution of physico-chemical characteristics, Nanotoxicology, 2010, 4(2), 207–246. [DOI] [PubMed] [Google Scholar]
- 67.Li W, Zhou J. and Xu Y, Study of the in vitro cytotoxicity testing of medical devices, Biomed. Rep, 2015, 3(5), 617–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jacobsen NR, et al. , Genotoxicity, cytotoxicity, and reactive oxygen species induced by single-walled carbon nanotubes and C60 fullerenes in the FE1-Muta™ Mouse lung epithelial cells, Environ. Mol. Mutagen, 2008, 49(6), 476–487. [DOI] [PubMed] [Google Scholar]
- 69.Xiao G, Delamar MA and Shanahan M, Irreversible interactions between water and DGEBA/DDA epoxy resin during hygrothermal aging, J. Appl. Polym. Sci, 1997, 65(3), 449–458. [Google Scholar]
- 70.Pulskamp K, Diabaté S. and Krug HF, Carbon nanotubes show no sign of acute toxicity but induce intracellular reactive oxygen species in dependence on contaminants, Toxicol. Lett, 2007, 168(1), 58–74. [DOI] [PubMed] [Google Scholar]
- 71.Reddy ARN, et al. , Multi wall carbon nanotubes induce oxidative stress and cytotoxicity in human embryonic kidney (HEK293) cells, Toxicology, 2010, 272(1–3), 11–16. [DOI] [PubMed] [Google Scholar]
- 72.Sahle-Demessie E, et al. , Aging and release of pristine and functionalized carbon nanotubes from epoxy-nanocomposites during accelerated weathering, Abstracts of Papers, American Chemical Society Meeting, Boston, MA, 2018, ENVR-293. [Google Scholar]
- 73.Froggett SJ, et al. , A review and perspective of existing research on the release of nanomaterials from solid nanocomposites, Part. Fibre Toxicol, 2014, 11(1), 1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Davoren M, et al. , In vitro toxicity evaluation of single walled carbon nanotubes on human A549 lung cells, Toxicol. In Vitro, 2007, 21(3), 438–448. [DOI] [PubMed] [Google Scholar]
- 75.Fabian E, et al. , Tissue distribution and toxicity of intravenously administered titanium dioxide nanoparticles in rats, Arch. Toxicol, 2008, 82(3), 151–157. [DOI] [PubMed] [Google Scholar]
- 76.Yehia HN, et al. , Single-walled carbon nanotube interactions with HeLa cells, J. Nanobiotechnol, 2007, 5, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wörle-Knirsch J, Pulskamp K. and Krug H, Oops they did it again! Carbon nanotubes hoax scientists in viability assays, Nano Lett., 2006, 6(6), 1261–1268. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.