Abstract
The distribution of ofloxacin (OFLX) along the shaft of each of three hair types, i.e., head, axillary and pubic, was investigated and compared among five healthy male volunteers 1 to 4 months after ingestion of OFLX for 1 or 2 days (total dose, 200 or 600 mg). Five strands of each hair type were sectioned together into successive 0.5-cm lengths starting from the dermal end, over a length of ≤6 cm, and the OFLX concentration in each hair section was measured by high-pressure liquid chromatography with fluorescence detection. The distribution of OFLX along the head hair shaft was narrow, having a single peak even 3 to 4 months after administration, suggesting a rather uniform growth rate among hair strands. On the other hand, the OFLX distribution along axillary or pubic hair shafts tended to be broad, even having two apparent peaks, and the growth rate did not seem uniform. Since axillary hair seemed to stop growing after having gained a length of ≤4 to 5 cm, it was suggested to enter a resting stage after the growth of ≤3 cm over the 2 to 4 months after OFLX incorporation. These findings indicate that head hair is the most suitable for analysis of individual drug use and the larger growth rate and cycle stage variabilities of strands of the other types of hair should be taken into account.
We have so far shown that human head hair is a very useful and suitable biopsy material for tracing back individual drug use from the date of hair sampling for several months, even years, depending on the length of the hair analyzed (14, 21). Head hair incorporates drugs within its structure in proportion to their doses (26) or, to be exact, to their mean concentrations in blood (18), retaining them at the portion which is formed when the drugs are used (16). Thus, it can be said that head hair serves as “tape recording” that stores along its length all information about individual drug use. Especially in the case of antimicrobial chemotherapy, such information about the past drug use of a patient is essential for anticipating the emergence of resistant bacteria and choosing the most suitable medication before starting to treat him or her. Since potential drug-drug interactions relevant to adverse reactions have been reported, for example, for antimicrobial fluoroquinolones coadministered with anti-inflammatory agents, i.e., theophylline and so forth (5, 7), knowledge of the past drug use of a study subject is needed for safe and effective application of drugs.
Fluoroquinolone derivatives, including ofloxacin, norfloxacin, ciprofloxacin, and the other newly developed ones, such as AM-1155, OPC-17116, and Q-35, have also been shown to be detectable in head hair. In addition, their time-sequential use by a subject over the past several months was exactly recorded by their axial distribution along the hair shaft (9–11, 15, 17, 19, 23, 24). It should however, be noted that these favorable characteristics of hair as a tape recording depend greatly on its melanin content (20, 25). For example, ofloxacin, one of the most widely used fluoroquinolones in the world, can be detected in a 2-mm length of a single pigmented hair, even after ingestion of the usual therapeutic dose for only a day (9). This finding could be explained exclusively by its high affinity for melanin in hair. In fact, ofloxacin could hardly be detected in white hair samples collected from persons with grey hair who had previously taken ofloxacin, whereas it was sufficiently quantifiable in black hair samples from the same subjects (20). Due to this high affinity for melanin, however, potential ocular toxicity has been one of the main clinical concerns regarding fluoroquinolones (3, 4), just as with the anti-inflammatory agent chloroquine (2). It has been reported that chloroquine was detectable in nail clippings even 1 year after the cessation of its ingestion. This phenomenon may be attributed to its high affinity for and very slow dissociation from the binding sites in the body, presumably from melanin-containing structures, including melanocytes in the skin (13). Therefore, analysis of fluoroquinolones in human hair seems worthwhile from the viewpoints of both therapeutic monitoring and the clinical toxicology of antimicrobial agents.
Although head hair can provide useful information such as that mentioned above, it seems very important to determine whether other types of hair, such as axillary and pubic hairs, possess the same desirable characteristics as head hair and may substitute for it, especially when a study subject may be bald or getting so bald as to prevent the sampling of head hair. In addition to the usefulness of revealing individual drug use in the past, we have shown that ofloxacin incorporated in hair can serve as a time marker for analysis of other drugs (12) or can be used as an indicator of the growth rate of a single hair (9, 11). Therefore, in the present study, the disposition of ofloxacin, as a model fluoroquinolone derivative, was investigated and compared among head, axillary, and pubic hairs from the same subject. By doing so, the growth characteristics of each hair type have been clearly identified to show whether it is suitable for analysis of individual past use of fluoroquinolones or for the other purposes.
All of the organic solvents and other chemicals used were high-pressure liquid chromatography (HPLC) grade. Ofloxacin tablets (100 mg/tablet; Tarivid; Daiichi Pharmaceutical Co., Ltd., Tokyo, Japan) were purchased from a local supplier. Authentic ofloxacin and 9-fluoro-2,3-dihydro-3-methyl-10-(1- imidazolyl)-7-oxo-7H-pyrido[1,2,3-de]-[1,4]-benzoxazine-6- carboxylic acid (DL-8357), which was used as an internal standard, were kindly donated by Daiichi Pharmaceutical Co., Ltd.
The experimental protocol was reviewed and approved by the local ethics committee. Five healthy male volunteers 24 to 40 years old participated in this study after giving informed consent. We have already shown that the ofloxacin content of hair correlates well with the dose in the range of 100 to 900 mg given over 1 to 3 days (10) and the distribution of ofloxacin along a single hair shaft was sufficiently narrow within this dosage range and administration period (9). Therefore, the volunteers arbitrarily took either 200 mg of ofloxacin once or 100 mg three times daily for 2 consecutive days, i.e., a total of 200 or 600 mg. This treatment protocol also met the recommendation of the ethics committee that healthy subjects be given as little ofloxacin as possible. They had not taken any quinolone derivative within several months prior to the trial. Hair samples of three types were collected two or three times within 4 months, at least twice at 1 to 2 months (earlier samples) and at 3 to 4 months (later samples) after drug administration. Frontal head hair was sampled either by plucking or by cutting several strands at the portion of the scalp closest to the forehead by pulling the hair taut to exclude loose strands that might be in the resting stage and easily shed. There was no substantial difference between these two sampling methods used to collect hairs that were apparently in the growing stage (14). The volunteers also provided axillary and pubic hairs by cutting them closest to the skin in the same manner.
The Shimadzu (Tokyo, Japan) HPLC system used consisted of a liquid chromatograph (LC9A), an automatic injector (SIL-6B), a column oven (CTO-6A), a fluorescence spectrophotometer (RF-5000; wavelengths of 290 nm for excitation and 460 nm for emission), and an integrator (CR4-A). A TSKgel ODS-120T analytical column (250 by 4.6 mm [inside diameter]; Tosoh, Tokyo, Japan) was used. The mobile phase was a mixture of 50 mM phosphate buffer (pH 2.6) and acetonitrile (82:18, vol/vol). The flow rate was 0.8 ml/min.
Details of the procedures used for sample preparation and measurement have been reported elsewhere (10). In brief, hair samples were washed with a 0.1% solution of sodium dodecyl sulfate and distilled water. Five hair strands were sectioned together into successive 5-mm lengths from the dermal end for a length of ≤6 cm, weighed, and dissolved in 0.5 ml of 1 N NaOH by heating at 80°C for 30 min. After the solution was neutralized by addition of 0.5 ml of 1 N HCl, 1 ml of 50 mM phosphate buffer (pH 8.0), 0.05 ml of the internal standard at 2.5 μg/ml, and 5 ml of chloroform were successively added to it. After agitation for 20 min and centrifugation at 1,700 × g for 5 min, the organic layer was transferred to another tube and evaporated under a stream of nitrogen at 40°C. After the residue was dissolved in the HPLC mobile phase, an aliquot (100 μl) was injected into the HPLC system.
Analytical precision and recovery were determined by preparing an ofloxacin solution in 1 M NaOH containing 1 mg of blank hair at concentrations of 10, 40, and 100 ng/ml. Within-run precision was determined by performing the same measurement procedure five times a day. Between-runs precision was determined by performing the same measurement procedure every day for 5 days. The within-run and between-runs precisions thus obtained ranged from 0.35 to 1.21% and from 1.41 to 5.49% (coefficients of variation), respectively. Recovery of ofloxacin from hair ranged from 90.9 to 93.8% within this concentration range. A calibration curve prepared by measuring standard ofloxacin solutions of 1, 2, 4, 10, 20, 40, and 100 ng/ml showed good linearity (r, >0.999).
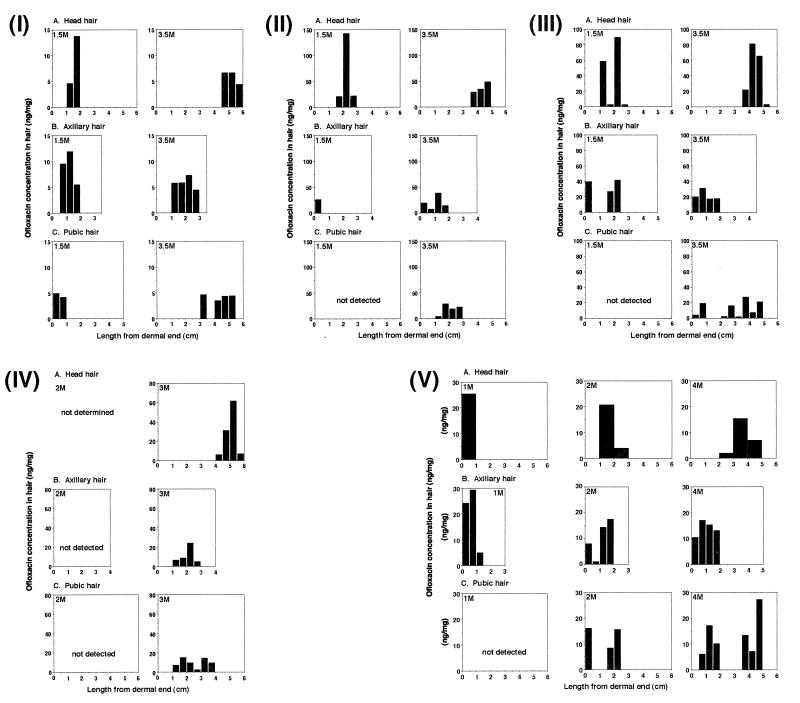
The distributions of ofloxacin along shafts of each of three types of hair, i.e., head, axillary, and pubic, are illustrated for each of five volunteers (subjects 1 to 5 in Fig. 1I to V, respectively). For all subjects who had taken ofloxacin for 1 or 2 days, the drug was detectable in all types of hair, at least when they were sampled 3 to 4 months after drug ingestion. The total dose of ofloxacin was 200 mg only for subject 1, whereas the other four subjects took a total of 600 mg of the drug. Hair sampled just before drug administration was ascertained to contain no ofloxacin. The ofloxacin concentration, even after having been normalized to the dose, apparently varied both among subjects and among the three hair types (Table 1).
FIG. 1.
Ofloxacin distribution along head, axillary, and pubic hairs (upper, middle, and lower panels, respectively) of each of five subjects (I to V). Five strands of each hair type were sectioned together into successive 0.5-cm lengths from the dermal end over the length depicted on the abscissa of each graph, and the ofloxacin in each 0.5-cm length was determined. The height of each black column shows the concentration of ofloxacin in the hair length, starting at the dermal end. (I) Hair samples from subject 1 at 1.5 months (1.5M) and 3.5 months (3.5M) after ingestion of 200 mg of ofloxacin only once. (II) Hair samples from subject 2 at 1.5 and 3.5 months after ingestion of ofloxacin at 300 mg/day for 2 days. (III) Hair samples from subject 3 at 1.5 and 3.5 months after ingestion of ofloxacin at 300 mg/day for 2 days. (IV) Hair samples from subject 4 at 2 and 3 months after ingestion of ofloxacin at 300 mg/day for 2 days. (V) Hair samples from subject 5 at 1, 2, and 4 months after ingestion of ofloxacin at 300 mg/day for 2 days. Head hairs were sectioned into successive 1-cm lengths starting at the dermal end, and the amount of ofloxacin in each section was measured.
TABLE 1.
Mean growth rates and peak ofloxacin concentrations in three types of hair
| Subject no. | Hair growth rate in cm/mo (peak drug concn [ng/mg of hair])
|
|||||
|---|---|---|---|---|---|---|
| Head hair
|
Axillary hair
|
Pubic hair
|
||||
| 1–2 mo | 3–4 mo | 1–2 mo | 3–4 mo | 1–2 mo | 3–4 mo | |
| 1 | 1.0 (41.4)b | 1.5 (19.8)b | 0.83 (35.7)b | 0.54 (21.9)b | 0.33 (14.7)b | 0.93, 1.4a (13.8)b |
| 2 | 1.5 (142.6) | 1.2 (48.8) | 0.17 (26.6) | 0.29 (38.8) | 0 (0) | 0.57 (28.9) |
| 3 | 0.83, 1.5a (89.5) | 1.3 (81.3) | 0.17, 1.3a (41.7) | 0.29 (31.2) | 0 (0) | 0.14, 1.0a (27.4) |
| 4 | NDc | 1.7 (61.7) | 0 (0) | 0.67 (24.1) | 0 (0) | 0.53, 1.1a (15.0) |
| 5 | 1.0 (21.6)d | 0.85 (16.3) | 0.18, 0.75a (17.5)d | 0.25 (17.1) | 0.13, 1.0a (16.0)d | 0.31, 1.1a (27.3)d |
| Mean ± SD | 1.2 ± 0.31e,f (73.8 ± 54.0)f | 1.3 ± 0.32 (45.6 ± 27.7) | 0.49 ± 0.48e (24.3 ± 16.4) | 0.54 ± 0.19 (26.6 ± 8.5) | 0.24 ± 0.39e (6.1 ± 8.4) | 0.79 ± 0.42e (22.5 ± 7.4) |
The growth rate was calculated for each of two peaks of ofloxacin distribution.
The value was normalized to a dose of 600 mg by multiplying the original value by 3 since only subject 1 took a total of 200 mg of ofloxacin instead of 600 mg.
ND, not determined.
The growth rate and peak concentration were calculated for the hair sample collected 2 months after drug ingestion from subject 5.
Values were calculated by using both the first and second peaks.
Values were calculated for all of the five subjects except subject 4, who did not provide a head hair sample at 1 to 2 months after drug ingestion.
The mean growth rate of five hair strands was calculated as the midpoint length of the overall distribution divided by the interval between drug ingestion and hair sampling. For example, it was calculated in subject 1 (Fig. 1I) to be 1.0 and 1.5 cm/month for head hairs sampled 1.5 and 3.5 months after drug ingestion, 0.83 and 0.57 cm/month for 1.5- and 3.5-month axillary hairs, and 0.33 cm/month for 1.5-month pubic hairs, respectively. For 3.5-month pubic hairs, it was calculated to be 0.93 and 1.4 cm/month for the first and second peaks of drug distribution, respectively (Table 1). In our previous study (9), in which the hair growth rate was determined for a single head hair in the growing stage by sectioning it into 2-mm lengths and repeating this kind of measurement with a total of three or four hair strands sampled from the same subject, the mean growth rate (± the standard deviation) was 1.12 ± 0.11 cm/month with an intraindividual coefficient of variation of 4.8 to 18.1%. The values calculated for head hairs in the present study (1.2 ± 0.31 and 1.3 ± 0.32 cm/month for earlier and later samples, respectively; Table 1) are well within this variability range. The head hairs of subject 5 (Fig. 1V) were cut into 1-cm lengths, also showing a single-peak ofloxacin distribution.
As for the earlier samples, ofloxacin was not detected in the pubic hairs collected from four of the five subjects (Fig. 1II to V) and in the axillary hair from one subject (Fig. 1IV), whereas it was detected in all of the head hair examined, except that from one subject, who did not provide the earlier head hair sample (Fig. 1IV). In addition, two ofloxacin distribution peaks appeared to be separately present along the axillary hair shafts of three of the five subjects (Fig. 1II, III, and V) and along the pubic hair shafts of four of the five subjects (Fig. 1I and III to V). These findings show that the growth rate and cycle stage of axillary and pubic hairs of the five subjects varied greatly compared with those of head hair. Since axillary hair seems to stop growing after having gained a length of ≤3 to 5 cm (Fig. 1I, III, and V), it seemed to enter the resting stage after a growth of ≤2 to 3 cm over the 2 to 4 months subsequent to incorporation of ofloxacin.
The present findings indicate that head hair is the most suitable specimen for tracing past use of ofloxacin in a subject back as long as 4 months after drug ingestion. This is because head hairs keep growing at a rather constant rate and the growth rate variability is rather small among hair strands as long as they are in the growing stage. To obtain growing-stage hair, we recommend collection of hair samples by either plucking or cutting several strands at the portion of the scalp closest to the forehead by pulling them taut to exclude loose strands that may be in the resting stage and easily shed (14). In the present study, head hairs were collected as described above and judged to be growing-stage hairs from the results obtained (Fig. 1). On the other hand, it is strongly suggested that caution should be taken when analyzing ofloxacin distribution along axillary and pubic hair shafts to determine past drug use, due to the following growth characteristics. The percentage of resting-stage hair and the variability of the growth rate itself are rather large among the axillary and pubic hairs sampled. In particular, axillary hairs are suggested to enter the resting stage after a growth of ≤2 to 3 cm over 2 to 4 months subsequent to incorporation of ofloxacin into their structures. This can be easily accepted, since we notice that our axillary hairs are usually not longer than several centimeters. Pubic hairs seem to grow continuously to gain lengths of more than 6 cm, but the growth rate variability is large enough to produce two peaks of ofloxacin distribution along their lengths over 3 to 4 months when several hair strands are analyzed together. However, it might be expected from their growth characteristics that pubic hairs could be used instead of head hairs to obtain information about ofloxacin use over several months when a single hair might be analyzed. Finally, information about the past use of a fluoroquinolone by a patient is essential for anticipation of the potential for emergence of resistant bacteria and antimicrobial therapeutic efficacy, as well as to verify patient compliance with drug therapy. Patient noncompliance remains a major clinical problem to be solved, since there have been practically no means to evaluate it. We should be aware that prescription of drugs to a patient does not ensure ingestion of the prescribed drugs. Hair analysis for drugs is expected to become a new way to ensure patient compliance (14).
Fluoroquinolones, including ofloxacin, can be detected in a 2-mm length of a single head hair by employing a conventional HPLC method combined with fluorescence detection, even if the hair sample is collected several months after only 1 day of exposure to the clinically recommended daily dose (9, 11, 15, 17, 23, 24). These desirable characteristics of fluoroquinolones for hair analysis are considered to be due to their high affinity for melanin. Because of this high affinity for melanin, however, potential ocular toxicity has been one of the main clinical concerns about fluoroquinolones (3, 4). We have so far reported that the distribution of nicotine along hair shafts represents time-sequential changes in the smoking behavior of a subject. However, the axial nicotine distribution failed to mark an immediate cessation of smoking, since nicotine tended to tail off along the hair shaft despite smoking cessation, showing a gradual decrease in concentration along the hair shaft (22). This phenomenon could be attributed to the slow dissociation of nicotine from its binding sites, resulting in a prolonged transfer of nicotine from hair bulb cells to the hair matrix. In the case of chloroquine, as can be seen from the report that this agent could be detected in nail clippings as long as 1 year after ingestion (13), its slow dissociation from melanin-containing structures, presumably from the retinal pigmented layer, might lead to prolonged disposition in the retina, resulting in severe impairment of visual function. The present results show that ofloxacin is rapidly released from its binding sites, showing no large tailing-off phenomenon along the hair shaft, in contrast to nicotine and, maybe, chloroquine, as long as head hairs are used for analysis. On the other hand, if only a later sample of axillary or pubic hair had been analyzed, the broad distribution of ofloxacin along the hair shaft, despite a short exposure to the drug, would have led to the misunderstanding that ofloxacin might possess such tailing-off characteristics, resulting in some potentially toxic effects on the retina. Therefore, the disposition characteristics of ofloxacin in different types of hair are quite important from the viewpoint of clinical toxicology, as well as therapeutic drug monitoring.
Detection of the hair portion containing ofloxacin has been shown to serve as a time marker for analyzing other drugs in hair (12). This is also a way to measure the growth rate of each hair strand. Although there have been many different hair evaluation techniques, the ideal one should be noninvasive, easy to perform, and reproducible (27). The phototrichogram is preferred as a noninvasive technique for evaluation of the rate of hair growth, but shaving of an area of, for example, the scalp is needed prior to the start of observation (1). Hair growth is controlled by various factors (2), and shaving or plucking itself may be a stimulus for hair growth or a trigger for hair follicle cells to go from the resting to the growing stage. When a fluoroquinolone is utilized to evaluate the hair growth rate, it is only necessary to collect several to 10 strands, even a single strand, of hair by cutting at the portion nearest to the scalp, instead of plucking the hair, at an arbitrary interval after the administration of 200 to 300 mg of ofloxacin two or three times a day for only 1 day. Moreover, only a single fluoroquinolone administration at the beginning of the control period may be sufficient for evaluation of the effect on the growth rate of some intervention to modify it. Thus, the growth rates can be separately calculated for hairs collected at the end of the control period and for those collected at the end of the treatment period and compared. Since fluoroquinolones may be frequently used by patients with infectious diseases, irrespective of their original disease, or even by otherwise healthy individuals with only minor, for example, urinary, infections, it may even be unnecessary to newly administer some fluoroquinolone to a study subject if the exact date of the fluoroquinolone prescription is evident from the medical record. However, the larger growth rate and cycle stage variabilities among hair strands should be borne in mind when types of hair other than head hair are used for the same purpose. Since axillary or pubic hair enters the resting stage after gaining a length of several centimeters and it may take some time for the portion of the hair into which a drug is incorporated to emerge from the dermal surface, the larger growth rate and cycle stage variabilities may become evident.
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science and Culture of Japan.
REFERENCES
- 1.Courtois M, Loussouarn G, Hourseau C, Grollier J F. Ageing and hair cycles. Br J Dermatol. 1995;132:86–93. doi: 10.1111/j.1365-2133.1995.tb08630.x. [DOI] [PubMed] [Google Scholar]
- 2.Crews S J. Some aspects of retinal drug toxicity. Ophthalmologica. 1969;1–3:232–244. doi: 10.1159/000305820. [DOI] [PubMed] [Google Scholar]
- 3.Fukuda M, Sasaki K. Different iris coloration and uptake of a fluoroquinolone agent into the iris ciliary body of rabbit eyes. Ophthalmic Res. 1994;26:137–140. doi: 10.1159/000267404. [DOI] [PubMed] [Google Scholar]
- 4.Fukuda, M., and K. Sasaki. 1995. Differences between albino and pigmented rabbit eyes in the intraocular pharmacokinetics of sparfloxacin. Drugs 49(Suppl. 2):314–316. [DOI] [PubMed]
- 5.Gillum J G, Israel D S, Polk R E. Pharmacokinetic drug interactions with antimicrobial agents. Clin Pharmacokinet. 1993;25:450–482. doi: 10.2165/00003088-199325060-00005. [DOI] [PubMed] [Google Scholar]
- 6.Ings R M. The melanin binding of drugs and its implications. Drug Metab Rev. 1984;15:1183–1212. doi: 10.3109/03602538409033561. [DOI] [PubMed] [Google Scholar]
- 7.Marchbanks C R. Drug-drug interactions with fluoroquinolones. Pharmacotherapy. 1993;13:23S–28S. [PubMed] [Google Scholar]
- 8.Messenger, A. G. 1993. The control of hair growth: an overview. J. Invest. Dermatol. 101(Suppl. 1):4S–9S. [DOI] [PubMed]
- 9.Miyazawa N, Uematsu T. Analysis of ofloxacin in hair as a measure of hair growth and as a time marker for hair analysis. Ther Drug Monit. 1992;14:525–528. doi: 10.1097/00007691-199212000-00015. [DOI] [PubMed] [Google Scholar]
- 10.Miyazawa N, Uematsu T, Mizuno A, Nagashima S, Nakashima M. Ofloxacin in human hair determined by high performance liquid chromatography. Forensic Sci Int. 1991;51:65–77. doi: 10.1016/0379-0738(91)90206-x. [DOI] [PubMed] [Google Scholar]
- 11.Mizuno A, Uematsu T, Nakashima M. Simultaneous determination of ofloxacin, norfloxacin and ciprofloxacin in human hair by high-performance liquid chromatography and fluorescence detection. J Chromatogr B. 1994;653:187–193. doi: 10.1016/0378-4347(93)e0440-2. [DOI] [PubMed] [Google Scholar]
- 12.Nakano M, Uematsu T, Sato H, Kosuge K, Nishimoto M, Nakashima M. Using ofloxacin as a time marker in hair analysis for monitoring the dosage history of haloperidol. Eur J Clin Pharmacol. 1994;47:195–202. doi: 10.1007/BF00194972. [DOI] [PubMed] [Google Scholar]
- 13.Ofori-Adjei D, Ericsson O. Chloroquine in nail clippings. Lancet. 1985;ii:331. doi: 10.1016/s0140-6736(85)90377-0. [DOI] [PubMed] [Google Scholar]
- 14.Uematsu T. Therapeutic drug monitoring in hair samples. Principles and practice. Clin Pharmacokinet. 1993;25:83–87. doi: 10.2165/00003088-199325020-00001. [DOI] [PubMed] [Google Scholar]
- 15.Uematsu T, Kondo K, Yano S, Yamaguchi T, Umemura K, Nakashima M. Measurement of temafloxacin in human scalp hair as an index of drug exposure. J Pharm Sci. 1994;83:42–45. doi: 10.1002/jps.2600830111. [DOI] [PubMed] [Google Scholar]
- 16.Uematsu T, Kosuge A, Araki S, Ishiye M, Asai Y, Nakashima M. Time course of appearance of ofloxacin in human scalp hair after oral administration. Ther Drug Monit. 1995;17:101–103. doi: 10.1097/00007691-199502000-00017. [DOI] [PubMed] [Google Scholar]
- 17.Uematsu T, Kusajima H, Umemura K, Ishida R, Ohkubo H, Nakashima M. A new antimicrobial quinolone (AM-1155) analysed in hair as an index of drug exposure and as a time-marker. J Pharm Pharmacol. 1993;45:1012–1014. doi: 10.1111/j.2042-7158.1993.tb05651.x. [DOI] [PubMed] [Google Scholar]
- 18.Uematsu T, Matsuno H, Sato H, Hirayama H, Hasegawa K, Nakashima M. Steady-state pharmacokinetics of haloperidol and reduced haloperidol in schizophrenic patients: analysis of factors determining their concentrations in hair. J Pharm Sci. 1992;81:1008–1011. doi: 10.1002/jps.2600811010. [DOI] [PubMed] [Google Scholar]
- 19.Uematsu T, Miyazawa N, Nakashima M. The measurement of ofloxacin in hair as an index of exposure. Eur J Clin Pharmacol. 1991;40:581–584. doi: 10.1007/BF00279974. [DOI] [PubMed] [Google Scholar]
- 20.Uematsu T, Miyazawa N, Okazaki O, Nakashima M. Possible effect of pigment on the pharmacokinetics of ofloxacin and its excretion in hair. J Pharm Sci. 1992;81:45–48. doi: 10.1002/jps.2600810109. [DOI] [PubMed] [Google Scholar]
- 21.Uematsu T, Mizuno A, Kosuge A. Human scalp hair as biopsy material suitable for quantitative analysis in therapeutic drug monitoring. In: Cone E J, Welch M J, Babecki M G B, editors. Hair testing for drugs of abuse: international research on standards and technology. U.S. Washington, D.C: Department of Health and Human Services; 1995. pp. 333–346. [Google Scholar]
- 22.Uematsu T, Mizuno A, Nagashima S, Oshima A, Nakamura M. The axial distribution of nicotine content along hair shaft as an indicator of changes in smoking behaviour: evaluation in a smoking-cessation programme with or without the aid of nicotine chewing-gum. Br J Clin Pharmacol. 1995;39:665–669. doi: 10.1111/j.1365-2125.1995.tb05726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uematsu T, Nakano M, Akiyama H, Nakashima M. The measurement of a new antimicrobial quinolone in hair as an index of drug exposure. Br J Clin Pharmacol. 1993;35:199–203. doi: 10.1111/j.1365-2125.1993.tb05686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uematsu T, Ohsawa Y, Mizuno A, Nakashima M. Analysis of a new fluoroquinolone derivative (Q-35) in human scalp hair as an index of drug exposure and as a time marker in hair. Int J Leg Med. 1994;106:237–243. doi: 10.1007/BF01225412. [DOI] [PubMed] [Google Scholar]
- 25.Uematsu T, Sato R, Fujimori O, Nakashima M. Human scalp hair as evidence of individual dosage history of haloperidol: a possible linkage of haloperidol excretion into hair with hair pigment. Arch Dermatol Res. 1990;282:120–125. doi: 10.1007/BF00493470. [DOI] [PubMed] [Google Scholar]
- 26.Uematsu T, Sato R, Suzuki K, Yamaguchi S, Nakashima M. Human scalp hair as evidence of individual dosage history of haloperidol: method and retrospective study. Eur J Clin Pharmacol. 1989;37:239–244. doi: 10.1007/BF00679777. [DOI] [PubMed] [Google Scholar]
- 27.Van Neste D J J. Hair growth evaluation in clinical dermatology. Dermatology. 1993;187:233–234. doi: 10.1159/000247254. [DOI] [PubMed] [Google Scholar]