Abstract
BACKGROUND:
Preeclampsia is a hypertensive disorder of pregnancy characterized by chronic placental ischemia and suppression of proangiogenic proteins, causing oxidative stress, hypertension, and maternal systemic organ damage. The transcription factor, PPARγ (peroxisome proliferator-activated receptor-γ) promotes healthy trophoblast differentiation but is dysregulated in the preeclampsia placenta. Our study identifies the beneficial impact of Rosiglitazone-mediated PPARγ-activation in the stressed preeclampsia placenta.
METHODS:
We used first trimester placentas, preeclamptic and preterm control placentas, and human trophoblast cell lines to study PPARγ activation.
RESULTS:
Induction of PPARγ activates cell growth and antioxidative stress pathways, including the gene, heme oxygenase 1 (Hmox1). Protein expression of both PPARγ and HO1 (heme oxygenase 1) are reduced in preeclamptic placentas, but Rosiglitazone restores HO1 signaling in a PPARγ-dependent manner.
CONCLUSIONS:
Restoring disrupted pathways by PPARγ in preeclampsia offers a potential therapeutic pathway to reverse placental damage, extending pregnancy duration, and reduce maternal sequelae. Future research should aim to understand the full scope of impaired PPARγ signaling in the human placenta and focus on compounds for safe use during pregnancy to prevent severe perinatal morbidity and mortality.
Keywords: HO1, hypertension, placenta, PPARγ, preeclampsia, rosiglitazone
NOVELTY AND RELEVANCE.
What Is New?
PPARγ (peroxisome proliferator-activated receptor-γ) and HO1 (heme oxygenase 1) expression are reduced in preeclamptic placental villi and is recapitulated in the in vitro BeWo model of hypoxia-ischemia placental injury. Rosiglitazone is capable of restoring deficient HO1 signaling within placental villi in a PPARγ-dependent manner.
What Is Relevant?
There is a lack of pharmacological treatments for preeclampsia, which directly target the disease-causing perturbations from the placenta. Rosiglitazone has significant beneficial effects in the diseased-preeclamptic placenta in addition to increasing HO1 expressions.
Clinical/Pathophysiological Implications?
Rosiglitazone has been shown to significantly improve the diseased-preeclamptic placenta; thus, future research should investigate the use of Rosiglitazone for short-term use in women with preeclampsia, especially in those who are at a high risk of progressing to severe preeclampsia.
Preeclampsia is a serious hypertensive disorder of pregnancy that remains a leading cause of perinatal morbidity and mortality worldwide. Without a prenatal cure or effective treatment to prolong pregnancy when preeclampsia is diagnosed before term, women may require delivery of the fetus prematurely along with the diseased placenta.1,2 Multiorgan impairment, due to elevated peripheral vascular resistance and low cardiac output, typifies early onset severe preeclampsia.3,4 Compelling evidence indicates that abnormal uteroplacental vascular development in the first trimester mediates pathological development of the placental villi in the second trimester. This consequently results in impaired secretion of proangiogenic proteins, such as PlGF (placental growth factor) and HO1 (heme oxygenase 1) followed by an exponential rise in the soluble vascular endothelial growth factor decoy protein, sFLT1 (soluble fms-like tyrosine kinase 1), into maternal blood. This progressive circulatory imbalance of placental-derived proteins directly impairs endothelial function leading to elevated systemic vascular resistance, ultimately causing maternal hypertension and organ damage.5 It would, therefore, be advantageous to understand the molecular signaling mechanisms within placental villi that are disrupted early in the clinically asymptomatic phase of the disease, which ultimately may cause either stillbirth or preterm delivery from severe preeclampsia. This approach has the potential to identify therapeutic options for effective disease prevention.
The transcription factor, peroxisome PPARγ (proliferator activated-receptor-γ) regulates genes in several cellular pathways including cell differentiation, oxidative stress, nutrient balance, and anti-inflammatory pathways.6 PPARγ has important roles in human placental development and function, such as promoting normal villous trophoblast differentiation and turnover that is required for normal expression and secretion of PlGF into maternal blood.6,7 PPARγ expression is decreased in placental villi obtained at delivery of pregnancies affected by severe preeclampsia.1 Further, animal-based studies indicate that reduced placental activity and expression of PPARγ is likely implicated in the development of preeclampsia.8–10 Importantly, animal studies show that aberrant trophoblast differentiation and function in pathological conditions can be restored when deficient PPARγ signaling is activated by Rosiglitazone.7,10–12
Major disruptions to HO1 expression and activity are implicated in several diseases, including cardiovascular and metabolic disease, and especially in the preeclampsia placenta.13,14 The reduction of placental HO1 expression in preeclampsia is a major contributor to exacerbated oxidative stress, endothelial dysfunction, a proinflammatory state, and immune imbalances in the placenta and are systemic features of women with preeclampsia.13,14 HO1 is an inducible cytoprotective molecule that initiates the metabolism of heme into free iron, carbon monoxide, and biliverdin/bilirubin. HO1 and its metabolites have a significant impact on maintaining cytoprotection from oxidative stress and excessive inflammation. Effective tissue expression of HO1 may be necessary to maintain normal blood pressure and prevent cardiovascular disease through maintaining endothelial relaxation and vasodilation.14 Thus, induction of this molecule may serve an important role in decreasing the risk of hypertension and endothelial dysfunction in women at risk of developing preeclampsia.
Several studies have highlighted the importance of targeting HO1 for restoring normal placental function in placental villi derived from women with preeclampsia via the ability for PPARγ to enhance tissue HO1 expression.15,16 PPARγ activation has been shown to upregulate HO1 and inhibit inflammation during lung injury17 and in mouse models of asthma.18 McCarthy et al19 also show that activating PPARγ via Rosiglitazone in a placental-hypoxia rat model reversed the preeclampsia phenotype by decreasing hypertension and increasing the expression of HO1.
While animal models show that PPARγ activation can increase HO1, it is unclear if this occurs in the human placenta and if the underlying mechanism is responsive to PPARγ-stimulation. In this study, we hypothesized that a PPARγ-HO1 axis can be enhanced in preeclampsia to restore elements of normal placental function. Our study began with RNA sequencing of Rosiglitazone-treated placental-derived cell-based model oxidative stress. While we discovered several altered genes and pathways, HO1 was discovered to be an important PPARγ target. We used human placental tissues from first trimester, preterm controls, and preeclampsia pregnancies and treated with Rosiglitazone to determine whether this pathway is altered in the human placenta. We activated PPARγ via Rosiglitazone and inhibited PPARγ via siRNA in our in vitro oxidative-stress model to understand how changes in PPARγ expression influenced HO1. Our data highlight the potential of PPARγ-activation as a potential therapeutic intervention for women at risk of developing severe preeclampsia.
METHODS
Data Availability
The authors declare that all supporting data are available within the article and its Supplemental Material.20,21
First Trimester Placental Tissue Collection and Explant Culture
First trimester placental tissues were obtained with written informed consent from healthy pregnant women undergoing elective termination of pregnancy during 2013 to 2017. The Institutional Review Board of the Wayne State University approved all consent forms and protocols used in this study, which abide by the National Institutes of Health research guidelines.
Statistical Analysis
All statistical analysis was performed with GraphPad Prism 7.0 software. The mRNA and protein expressions were normalized to housekeeping genes or protein. Data from tissues or cells treated with either dimethylsulfoxide and Rosiglitazone or PPARγ-siRNA and scramble siRNA were normalized to housekeeping genes or protein, then relative expression or secretion values for each tissue or biological replicate set were subsequently normalized to respective dimethylsulfoxide (set equal to 1) or scramble siRNA control (set equal to 1). ELISA data was normalized based on protein concentration. Groups were analyzed by student t test, after determination if samples are normally distributed. F-test was applied to determine variances between groups, which determined the parameters for the t test. P<0.05 is considered significant and is indicated with (*) on each graph. Bar plot data are reported as mean±SEM. All sample numbers are reported as per group.
RESULTS
Rosiglitazone Restores Expression of Multiple Dysregulated Transcripts, Including HO1 Isoforms, Under Hypoxia-Reoxygenation Conditions
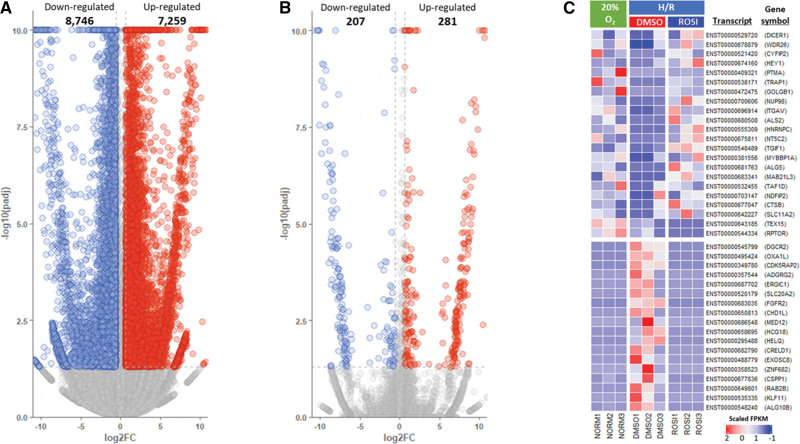
To elucidate the molecular mechanisms underlying cellular stress in hypoxia/reoxygenation (H/R) conditions similar to that of the preeclampsia placenta, we conducted RNA sequencing to compare the transcriptomes of BeWo cells under normoxia (20% O2) and H/R conditions, with or without exposure to Rosiglitazone. Using a fold change threshold of >1.8 (abs [log2 Fold Change] >0.6) and an adjusted P value (false discovery rate) of <0.05, we identified 8746 downregulated and 7259 upregulated transcripts in BeWo cells under H/R conditions (Figure 1A). Further analysis revealed that 207 transcripts were downregulated, and 281 transcripts were upregulated in Rosiglitazone-treated cells compared with vehicle-treated controls under H/R conditions (Figure 1B). Several of the upregulated transcripts, such as HEY1 (Hairy/enhancer-of-split related with YRPW motif protein 1), TRAP1 (tumor necrosis factor receptor-associated protein 1), ITGAV (Integrin alpha-V/beta-6), TGIF1 (TG-interacting factor 1), and ALG5 (ALG5 Dolichyl-Phosphate Beta-Glucosyltransferase), were involved in cell growth, proliferation, and migration (Figure 1C). Gene ontology analysis revealed that transcripts regulated by Rosiglitazone were significantly enriched in a variety of biological processes, including cell growth, differentiation, and apoptosis/death (Figure 2A). Furthermore, we observed that 2 major HO1 transcripts, ENST00000216117 encoding HMOX1-201 and ENST00000678411 encoding HMOX1-206, were significantly upregulated (Figure 2B) in stressed cells exposed to Rosiglitazone, suggesting that Rosiglitazone promotes the production of key angiogenic factors such as HO1 and contributes to the inhibition of cellular stress in hypoxic conditions.
Figure 1.
RNA sequencing of Rosiglitazone treatment during hypoxia/reoxygenation (H/R) in BeWo cells. Volcano (A) depicts the differentially expressed genes in BeWo cells under H/R compared with normoxia (20% O2) and (B) show differential gene expression profiles of Rosiglitazone-treated cells under H/R conditions relative to drug vehicle (dimethylsulfoxide)-exposed cells. C, A heatmap visualizes the differences of gene isoform expression profiles between the various conditions. The results suggest that Rosiglitazone is capable of rescuing the perturbed gene isoforms in BeWo cells under H/R conditions.
Figure 2.
Cellular changes induced by Rosiglitazone. A, Bubble plots visualizing the top 15 enriched gene ontology (GO) functions of differentially expressed gene isoforms related to Rosiglitazone in BeWo cells under hypoxia/reoxygenation (H/R) conditions, with a focus on biological processes (BP). B, Significantly increased expression of 2 HO1 (heme oxygenase 1) isoforms (ENST00000216117; HMOX1-201 and ENST00000678411; HMOX1-206) in BeWo cells exposed to Rosiglitazone under H/R conditions.
Cultured Preeclamptic Placentas Exhibit Reduced Protein Expression of PPARγ and HO1 and Reduced HO1 Secretion Compared With Control Placentas
We further assessed how HO1 expression changes in human placentas. We identified a significant reduction of PPARγ protein expression in preeclampsia placentas compared with PTC (0.47±0.13 versus 1.03±0.11 relative expression values; P=0.01, n=7; Figure 3A). We observed a significant reduction of HO1 protein expression (0.79±0.1 versus 0.34±0.06 relative expression values; P=0.0045, n=7; Figure 3B) and a significant reduction of HO1 secretion (as measured by ELISA) in preeclampsia compared with PTC (99±8.2 pg/mL versus 141.0±16 pg/mL; P=0.0476, n=7; Figure 3C).
Figure 3.
PPARγ (peroxisome proliferator-activated receptor-γ) and HO1 (heme oxygenase 1) are reduced in the preeclamptic (PE) placenta. Cultured human PE placentas exhibit significant less protein expression of PPARγ (A) and HO1 (B) and as well secrete significantly less HO1 (C) compared with preterm controls (PTC; n=7). Protein expression was normalized to β-actin, followed by a student t test to determine significant differences between groups; *P<0.05, **P<0.01, n=7.
Cell-Based Model of Ischemia-Reperfusion Injury Shows a Reduction of PPARγ and HO1 Protein Expression and HO1 Secretion
BeWo cells were cultured for 18 hours in hypoxia (1.5% O2) followed by 18 hours of normoxia (20% O2; H/R) to mimic the ischemia-reperfusion injury that occurs in preeclampsia.22 PPARγ protein expression was significantly reduced during H/R conditions compared with normoxia (0.37±0.12 versus 0.9±0.08 relative expression values; P=0.026, n=5; Figure 4A). In addition, HO1 protein expression was significantly reduced in H/R compared with normoxia (0.54±0.09 versus 0.9±0.09 relative expression values; P=0.045, n=7; Figure 4B). HO1 secretion was measured by ELISA and was significantly reduced in H/R compared with normoxia (1681±129 pg/mL versus 2666±170 pg/mL; P=0.01, n=5; Figure 4C). These data suggest the BeWo H/R model can recapitulate the in vitro reduction of PPARγ and HO1 during ischemic-reperfusion conditions.
Figure 4.
In vitro ischemia-reperfusion causes a reduction of PPARγ (peroxisome proliferator-activated receptor-γ) and HO1 (heme oxygenase 1). BeWo exposed to hypoxia/reoxygenation (H/R) mimics the reduced PPARγ (A) and HO1 protein expression (B) and HO1 secretion (C) that was previously observed in the preeclamptic placenta (n=3). Protein expression was normalized to β-actin, followed by a student t test to determine significant differences between groups; * P<0.05, **P<0.01. NT indicates no treatment.
Rosiglitazone Rescues HO1 Expression and Secretion in the Preeclamptic Placenta and in a Cell-Based Model of Ischemia-Reperfusion Injury
To test if PPARγ activation influences HO1 expression and secretion, we treated preeclampsia placentas, first trimester placental explants, and BeWo cells cultured in H/R conditions with Rosiglitazone or vehicle. Rosiglitazone-treated preeclampsia placentas show a significant increase of Hmox1 gene expression (1.213±0.17 versus 1 relative expression values; n=6, P=0.0069; Figure 5A) and HO1 protein expression (1.7±0.07 versus 1 relative expression values; n=7, P=0.01; Figure 5A), and an increase in HO1 protein secretion (1.16±0.02 versus 1 relative expression values; n=9, P=0.017; Figure 5A). BeWo cultured in H/R conditions and treated with Rosiglitazone mimicked a significant increase of Hmox1 gene expression (2.15±0.02 versus 1±0.03 relative expression values; n=6, P=0.004; Figure 5B), protein expression (1.25±0.05 versus 1±0.02 relative expression values; n=6, P=0.009; Figure 5B), and protein secretion (1.67±0.2 versus 1±0.01 relative expression values; n=6, P=0.025; Figure 5B). An overnight culture of first trimester placentas treated with Rosiglitazone also caused an increase of HO1 protein expression (1.75±0.32 versus 1 relative expression values; n=4, P<0.05; Figure 5C) and secretion into the culture media (1.45±0.22 versus 1 relative expression values; n=4, P<0.05; Figure 5C). Immunohistochemical staining of first trimester explants treated with Rosiglitazone shows an increase of HO1 production in the syncytiotrophoblast layer of the first trimester explant compared with the vehicle control (Figure 5D).
Figure 5.
Rosiglitazone (Rosi) restores HO1 (heme oxygenase 1) expression in preeclamptic placentas, first trimester placentas, and during in vitro ischemia-reperfusion injury. PPARγ (peroxisome proliferator-activated receptor-γ) induction by Rosi significantly increases in Hmox1 gene and HO1 protein expression and secretion in the preeclamptic placenta (A, n=6). BeWo exposed to hypoxia/reoxygenation (H/R) show a significant increase in Hmox1 gene expression, HO1 protein expression, and secretion when treated with Rosi (B, n=6). First trimester explants treated with Rosi also show a significant increase in HO1 protein expression and secretion (C, n=4). Moreover, a qualitative assessment of HO1 staining revealed strong HO1 production in the syncytiotrophoblast layer of the first trimester placenta (D) when treated with Rosi. Relative mRNA and protein expression were determined by normalization to housekeeping genes or protein. ELISA data were normalized to total protein content or semidry tissue weight. Relative expression values were normalized to vehicle (set equal to 1) and subsequent statistical analysis was performed by student t test to determine significant differences between groups; *P<0.05, **P<0.01.
HO1-Induction Is PPARγ Dependent
We questioned if HO1 expression is directly influenced by PPARγ. To test this, PPARγ expression was reduced via siRNA in BeWo cells and expression of PPARγ and HO1 were measured by quantitative PCR and Western blotting. PPARγ-siRNA treatment led to a 90% reduction of PPARγ gene expression (0.09±0.01 versus 1 relative expression values; n=3, P<0.0001; Figure 6A) and a 53% reduction of PPARγ protein expression (0.47±0.01 versus 1 relative expression values; n=3, P=0.0004; Figure 6A). The siRNA-mediated reduction of PPARγ led to a 44% reduction of Hmox1 gene expression (0.56±0.06 versus 1 relative expression values; n=3, P=0.0029; Figure 6B) and a 40% reduction of HO1 protein expression (0.6±0.1 versus 1 relative expression values; n=3, P=0.0285; Figure 6B). We next treated the PPARγ-silenced cells with Rosiglitazone to determine whether the previously observed upregulation of HO1 by Rosiglitazone occurs in a PPARγ-dependent manner. In the scramble siRNA control cells, Rosiglitazone significantly increased HO1 protein expression compared with vehicle-treated scramble siRNA cells (1.17±0.13 versus 0.77±0.02 relative expression values; n=3, P=0.0416; Figure 6C). In the PPARγ-siRNA-treated cells, Rosiglitazone did not change HO1 expression in comparison to the vehicle control (0.43±0.03 versus 0.56±0.07 relative expression values; n=3, P=0.17; Figure 6C). HO1 protein expression was significantly reduced in the PPARγ siRNA-Rosiglitazone-treated cells in comparison to the vehicle-treated scramble siRNA control cells (0.43±0.03 versus 0.77±0.02 relative expression values; n=3, P=0.011; Figure 6C) and Rosiglitazone (0.43±0.03 versus 1.17±0.13 relative expression values, n=3, P=0.0053; Figure 6C).
Figure 6.
siRNA-mediated reduction of PPARγ (peroxisome proliferator-activated receptor-γ) significantly decreased HO1 (heme oxygenase 1) expression that were not rescued by Rosiglitazone (Rosi). PPARγ siRNA caused a significant reduction of PPARγ gene and protein expression (A, n=3) and HO1 gene and protein expression (B, n=3) when compared with the scramble siRNA control. Scramble siRNA (Scr)-treated cells were simultaneously treated with Rosi and caused a significant increase in HO1 protein expression in comparison to the Scr-treated vehicle control (C, n=3). In the PPARγ-silenced cells, Rosi could not increase HO1 protein expression when compared with vehicle-treated PPARγ-silenced cells (C, n=3). HO1 protein expression was significantly reduced in both vehicle and Rosi-treated PPARγ-silenced cells in comparison to the Rosi and vehicle-treated Scr-treated cells, which collectively suggests a PPARγ-dependent mechanism for HO1 regulation. Relative mRNA and protein expression were determined by normalization to housekeeping genes or protein. Relative expression values for sample sets were normalized to Scr control (set equal to 1) and subsequent statistical analysis was performed by student t test to determine significant differences between groups; *P<0.05, **P<0.01, ***P<0.0001. siPPARγ indicates PPARγ siRNA.
DISCUSSION
We used RNA sequencing methods to detail the molecular changes provided by Rosiglitazone treatment in an in vitro oxidative-stress model to recapitulate the placental stress conditions that occur in preeclampsia placental villi. Rosiglitazone effects cell growth, proliferation, and migration pathways and increased expression of HO1, which is a key cytoprotective and proangiogenic molecule whose expression is reduced in preeclampsia placental villi. The key findings of our study are 3-fold: first, PPARγ and HO1 expression are both reduced in the placental villi of pregnancies complicated by preeclampsia; second, these same observations are recapitulated in the in vitro BeWo cell model of hypoxia-ischemia placental injury; and lastly, Rosiglitazone is capable of restoring deficient HO1 signaling within placental villi in a PPARγ-dependent manner. Collectively, these findings have important implications for both the treatment and prevention of severe preeclampsia.
The strengths of our approach are that we studied this PPARγ-HO1 axis in clinically relevant human tissues. Severe preeclampsia is clearly mediated by a dysregulated trophoblast surface of the floating placental villi that directly contact maternal blood, because explanted preeclampsia villi have analogous abnormal secretion patterns of both PlGF and sFLT11,23,24 as is observed in maternal blood.25,26 Since severe preeclampsia is a placental disease that begins in the late first and early second trimesters, it is therefore critical to show that HO1 expression within placental villi at this early gestational age is indeed PPARγ-responsive.
The most common underlying placental disease mediating severe preeclampsia is described as maternal vascular malperfusion,27,28 and is characterized by reduced placental mass, diseased maternal spiral arterioles, and abnormal differentiation of the placental villous surface into syncytial knots that hypersecrete sFLT1.23,29,30 The abnormal secretion of proteins released from the diseased placenta into maternal circulation can cause damage to the maternal endothelium leading to high blood pressure and organ damage. PPARγ normally regulates villous trophoblast differentiation in the placenta and its perturbations in preeclampsia are thought to directly contribute to the aberrant secretion of proteins, such as HO1, that lead to the endothelial dysfunction. HO1 is an important target in preeclampsia due to its roles in regulating cytoprotection from oxidative stress, excessive inflammation, and maintaining endothelial relaxation and vasodilation,14 which are perturbed in preeclampsia. Identifying women at risk of preeclampsia and further targeting the upstream pathways regulating these abnormally secreted proteins, such as HO1, may pose an opportunity to dampen maternal sequelae.
Most patients at risk of developing severe preeclampsia exhibit abnormal uterine artery Doppler waveforms and low maternal circulating PlGF.31,32 Our ability to identify women at high risk of developing the disease, many weeks ahead of any abnormal clinical signs, affords clinicians a therapeutic opportunity to either prevent the disease entirely or to at least convert it into a milder entity occurring near term, when delivery will produce a satisfactory outcome for both mother and fetus. This time between identification of at-risk individuals and overt disease expression is characterized by progressive ischemia-reperfusion injury to the placental villi, which can now be identified in vivo by magnetic resonance oximetry.33 Progressive placental injury may lead to areas of placental infarction, thereby further reducing PlGF secretion and mediating fetal growth restriction.27
Currently, our limited ability to intervene effectively to prevent preeclampsia from developing in high-risk women is based on low-dose aspirin.34 Subsequent prospective research demonstrates the effectiveness of this strategy in a high-throughput setting in the United Kingdom with confirmed high rates of aspirin compliance.35 However, it is conceptually hard to imagine that low-dose aspirin could prevent the most severe forms of preeclampsia that currently require preterm delivery. To date, no large-scale aspirin prevention studies have captured placental pathology. Because no evidence has suggested that PlGF/sFLT1 levels are influenced by maternal aspirin ingestion, it is likely that aspirin mediates its favorable actions via independent anti-inflammatory pathways. Likewise, the mechanisms of action of the antidiabetic drug metformin, shown in secondary analyses to reduce preeclampsia in obese women at risk of gestational diabetes,36 are unknown in vivo, although in vitro metformin is capable of suppressing sFLT1 secretion by preeclampsia placental villi.37,38
Therapeutic strategies that are based on repressing sFLT1 may hold less potential than maintaining effective production of proangiogenic growth factors (ie, PlGF, HO1, and many others) for several reasons. Serial maternal blood sampling studies reveal repression of PlGF occurs well before elevation of sFLT1 production.31,39 Attempts to reduce exponentially elevated levels of sFLT1 from maternal blood of women with established disease have been shown to be ineffective, because sFLT1 levels rebound quickly after maternal plasma ultrafiltration. Less dramatic and far more cost-effective is the administration of oral metformin to women requiring antihypertensive therapy for severe preeclampsia before 34 weeks of gestation. In the recent large-scale randomized control trial from South Africa, metformin safely extended pregnancy duration by a median of 10 days, which conferred important neonatal benefits.37
The ability of adjunctive therapies to confer clinically meaningful benefits, in addition to aspirin, in earlier stages of high-risk pregnancies remains uncertain. A recent prospective study considered women at high-risk for recurrent severe placenta-mediated preeclampsia disease and who had commenced aspirin prophylaxis by 12 weeks and the incidence of preterm delivery at <34+0 weeks was 9%.40 At 16 weeks of gestation, low circulating PlGF was more predictive of preterm delivery than abnormal artery Doppler,40 because some placental diseases causing severe preeclampsia are not associated with an impaired uteroplacental circulation.41 Restoration of deficient PlGF secretion in the early second trimester of this niche group of women at highest risk of severe preeclampsia and stillbirth may be an appropriate next step to evaluate the potential of HO1-augmentation to deliver improved outcomes. A similar pilot observational strategy at this same gestational age, using low-molecular-weight heparin was recently demonstrated to restore deficient levels of PlGF via the non-anticoagulant actions of low-molecular-weight heparin.42
Despite these significant efforts, there still persists a great need to treat women suspected of severe preeclampsia to improve pregnancy outcomes. The recently established Food and Drug Administration approval for measuring PlGF/sFLT1 levels in the blood to detect women at risk for progressing to sever preeclampsia43 further emphasizes the need for therapeutic intervention that directly impacts placental function to restore this protein balance. Our group previously showed lower levels of PlGF secreted from preeclampsia tissues when compared with control placentas, which were increased when the preeclampsia placentas were treated with Rosiglitazone.24 While this data was not statistically significant, it included a mixed population of late-term and severe preeclampsia that warrants additional follow-up. Our former study showing that Rosiglitazone-mediated activation of PPARγ decreases sFLT1 in preeclampsia placenta, also largely suggests Rosiglitazone can mediate the GCM1-syncytin 1 regulatory axis of PlGF.44 With this compelling evidence, more studies are still required to elucidate any direct or indirect effects of PPARγ activation on PlGF expression in the preeclampsia placenta.
With the cumulative evidence showing the ability for Rosiglitazone to restore the expression of disease-causing molecules, Rosiglitazone should be considered as an alternative approach in this high-risk population of women, which can be identified via abnormal uterine artery Doppler waveforms and low maternal circulating PlGF/high circulating sFLT1. We can so far rely on the limited case reports using Rosiglitazone treatment during pregnancy45–47 and in multiple animal studies that result in healthy human and murine pregnancy outcomes. Specifically, in a study by McCarthy et al, they showed that activation of PPARγ by Rosiglitazone in the hypoxia-induced rat model of preeclampsia can improve placental function.10 This preeclampsia phenotype was achieved by performing surgical clamping of the uterine artery to simulate placental hypoxia. These rats developed hypertension, decreased proangiogenic protein secretion, and endothelial dysfunction. Rosiglitazone decreased blood pressure and significantly increased secretion of vasodilatory proteins to improve endothelial health.
Our studies spotlight Rosiglitazone’s effect on therapeutically targetable mechanisms that can increase placental function and hold potential to reduce the maternal disease burden. Indeed, there is potential for Rosiglitazone to be a groundbreaking treatment for those with preeclampsia, but future studies to assess the effect of Rosiglitazone on fetal development in vivo and verifying these results in large-scale clinical trials is still needed.
PERSPECTIVES
Preeclampsia poses a major threat to the long-term maternal-fetal health if the disease progresses to severe features and requires preterm delivery. Severe preeclampsia is clearly mediated by the dysregulated trophoblast, yet there are not any established therapeutic interventions that directly improve placental functions in these patients. Our study aimed to address the molecular changes provided by Rosiglitazone-mediated activation of PPARγ in the human preeclampsia placenta and during cellular stress in an in vitro oxidative-stress model. Our data highlights PPARγ’s beneficial actions upon activation in preeclampsia, through the increase in cell growth, proliferation, and migration pathways and increased expression of antioxidant molecules such as HO1 that occurs in a PPARγ-dependent manner. Our study highlights the need for therapeutic intervention and encourages future researchers to re-evaluate the therapeutic potential of Rosiglitazone in the potential treatment of preeclampsia.
ARTICLE INFORMATION
Author Contributions
All authors listed contributed to this article; B. Grimaldi, S. Drewlo, and J.C. Kingdom contributed to conceptualization; B. Grimaldi contributed to methodology, formal analysis, and writing—original draft preparation, major editing, and formalizing; B. Grimaldi, H.-R. Kohan-Ghadr, P. Nandi, C.D. Halari, J.C. Kingdom, and S. Drewlo contributed to writing—review and editing; H.-R. Kohan-Ghadr, S. Drewlo, and J.C. Kingdom contributed to supervision; and S. Drewlo contributed to funding acquisition. All authors have read and agreed to the published version of the article.
Sources of Funding
This research was supported by National Heart Lung and Blood Institute under grant number R01-HL128628-01 awarded to S. Drewlo; Department of Obstetrics, Gynecology and Reproductive Biology at Michigan State University College of Human Medicine; Research support for B. Grimaldi’s work in this publication was provided by the Eunice Kennedy Shriver National Institute of Child Health and Human Development Health under Award Number T32HD087166 awarded to Michigan State University AgBio Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health Support.
Disclosures
None.
Supplementary Material
Nonstandard Abbreviations and Acronyms
- HO1
- heme oxygenase 1
- H/R
- hypoxia/reoxygenation
- PlGF
- placental growth factor
- PPARγ
- peroxisome proliferator-activated receptor-γ
- sFLT1
- soluble fms-like tyrosine kinase 1
For Sources of Funding and Disclosures, see page 2394 & 2395.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/HYPERTENSIONAHA.123.21645.
REFERENCES
- 1.Armistead B, Kadam L, Siegwald E, McCarthy FP, Kingdom JC, Kohan-Ghadr H-R, Drewlo S. Induction of the PPARγ (Peroxisome Proliferator-Activated Receptor γ)-GCM1 (Glial Cell Missing 1) Syncytialization Axis Reduces sFLT1 (Soluble fms-Like Tyrosine Kinase 1) in the preeclamptic placenta. Hypertension. 2021;78:230–240. doi: 10.1161/HYPERTENSIONAHA.121.17267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ackerman CM, Platner MH, Spatz ES, Illuzzi JL, Xu X, Campbell KH, Smith GN, Paidas MJ, Lipkind HS. Severe cardiovascular morbidity in women with hypertensive diseases during delivery hospitalization. Am J Obstet Gynecol. 2019. 220:582 e1–582 e11. doi: 10.1016/j.ajog.2019.02.010 [DOI] [PubMed] [Google Scholar]
- 3.McLaughlin K, Audette MC, Parker JD, Kingdom JC. Mechanisms and clinical significance of endothelial dysfunction in high-risk pregnancies. Can J Cardiol. 2018;34:371–380. doi: 10.1016/j.cjca.2018.01.006 [DOI] [PubMed] [Google Scholar]
- 4.Baczyk D, Dunk C, Huppertz B, Maxwell C, Reister F, Giannoulias D, Kingdom JCP. Bi-potential behaviour of cytotrophoblasts in first trimester chorionic villi. Placenta. 2006. 27:367–374. doi: 10.1016/j.placenta.2005.03.006 [DOI] [PubMed] [Google Scholar]
- 5.Benton SJ, Leavey K, Grynspan D, Cox BJ, Bainbridge SA. The clinical heterogeneity of preeclampsia is related to both placental gene expression and placental histopathology. Am J Obstet Gynecol. 2018;219:604.e1–604.e25. doi: 10.1016/j.ajog.2018.09.036 [DOI] [PubMed] [Google Scholar]
- 6.Barak Y, Nelson MC, Ong ES, Jones YZ, Ruiz-Lozano P, Chien KR, Koder A, Evans RM. PPAR gamma is required for placental, cardiac, and adipose tissue development. Mol Cell. 1999;4:585–595. doi: 10.1016/s1097-2765(00)80209-9 [DOI] [PubMed] [Google Scholar]
- 7.Levytska K, Drewlo S, Baczyk D, Kingdom J. PPAR-γ regulates trophoblast differentiation in the BeWo cell model. PPAR Res. 2014;2014:637251. doi: 10.1155/2014/637251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCarthy FP, Drewlo S, English FA, Kingdom J, Johns EJ, Kenny LC, Walsh SK. Evidence implicating peroxisome proliferator-activated receptor-gamma in the pathogenesis of preeclampsia. Hypertension. 2011;58:882–887. doi: 10.1161/HYPERTENSIONAHA.111.179440 [DOI] [PubMed] [Google Scholar]
- 9.McCarthy FP, Drewlo S, English FA, Kingdom J, Johns EJ, Kenny LC, Walsh SK. Peroxisome proliferator activated receptor gamma critically regulates the risk of preeclampsia in rodent gestation. Reprod Sci. 2011;18:285a–285a. doi: 10.1161/HYPERTENSIONAHA.111.179440 [Google Scholar]
- 10.McCarthy FP, Drewlo S, Kingdom J, Johns EJ, Walsh SK, Kenny LC. Peroxisome proliferator-activated receptor-gamma as a potential therapeutic target in the treatment of preeclampsia. Hypertension. 2011;58:280–286. doi: 10.1161/HYPERTENSIONAHA.111.172627 [DOI] [PubMed] [Google Scholar]
- 11.Kohan-Ghadr HR, Kilburn BA, Kadam L, Johnson E, Kolb BL, Rodriguez-Kovacs J, Hertz M, Armant DR, Drewlo S. Rosiglitazone augments antioxidant response in the human trophoblast and prevents apoptosis†. Biol Reprod. 2019;100:479–494. doi: 10.1093/biolre/ioy186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parast MM, Yu H, Ciric A, Salata MW, Davis V, Milstone DS. PPARgamma regulates trophoblast proliferation and promotes labyrinthine trilineage differentiation. PLoS One. 2009;4:e8055. doi: 10.1371/journal.pone.0008055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Consoli V, Sorrenti V, Grosso S, Vanella L. Heme oxygenase-1 signaling and redox homeostasis in physiopathological conditions. Biomolecules. 2021;11:589. doi: 10.3390/biom11040589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ozen M, Zhao H, Lewis DB, Wong RJ, Stevenson DK. Heme oxygenase and the immune system in normal and pathological pregnancies. Front Pharmacol. 2015;6:84. doi: 10.3389/fphar.2015.00084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi AM, Alam J. Heme oxygenase-1: function, regulation, and implication of a novel stress-inducible protein in oxidant-induced lung injury. Am J Respir Cell Mol Biol. 1996;15:9–19. doi: 10.1165/ajrcmb.15.1.8679227 [DOI] [PubMed] [Google Scholar]
- 16.Kadam L, Gomez-Lopez N, Mial TN, Kohan-Ghadr H-R, Drewlo S. Rosiglitazone regulates TLR4 and rescues HO-1 and NRF2 expression in myometrial and decidual macrophages in inflammation-induced preterm birth. Reprod Sci. 2017;24:1590–1599. doi: 10.1177/1933719117697128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang G, Han D, Zhang Y, Xie X, Wu Y, Li S, Li M. A novel hypothesis: up-regulation of HO-1 by activation of PPARγ inhibits HMGB1-RAGE signaling pathway and ameliorates the development of ALI/ARDS. J Thorac Dis. 2013;5:706–710. doi: 10.3978/j.issn.2072-1439.2013.08.69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu J, Zhu Y-T, Wang G-Z, Han D, Wu Y-Y, Zhang D-X, Liu Y, Zhang Y-H, Xie X-M, Li S-J. The PPARγ agonist, rosiglitazone, attenuates airway inflammation and remodeling via heme oxygenase-1 in murine model of asthma. Acta Pharmacol Sin. 2015;36:171–178. doi: 10.1038/aps.2014.128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCarthy FP, Drewlo S, Kingdom J, Johns EJ, Walsh SK, Kenny LC. Peroxisome proliferator-activated receptor-γ as a potential therapeutic target in the treatment of preeclampsia. Hypertension. 2011;58:280–286. doi: 10.1161/HYPERTENSIONAHA.111.172627 [DOI] [PubMed] [Google Scholar]
- 20.Li B, Dewey CN. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinf. 2011;12:323. doi: 10.1186/1471-2105-12-323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu T, Hu E, Xu S, Chen M, Guo P, Dai Z, Feng T, Zhou L, Tang W, Zhan L. Clusterprofiler 4.0: a universal enrichment tool for interpreting omics data. Innovation (Camb). 2021;2:100141. doi: 10.1016/j.xinn.2021.100141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kohan-Ghadr HR, Kilburn BA, Kadam L, Johnson E, Kolb BL, Rodriguez-Kovacs J, Hertz M, Armant DR, Drewlo S. Rosiglitazone augments antioxidant response in the human trophoblast and prevents apoptosisdagger. Biol Reprod. 2019;100:479–494. doi: 10.1093/biolre/ioy186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Brien M, Baczyk D, Kingdom JC. Endothelial dysfunction in severe preeclampsia is mediated by soluble factors, rather than extracellular vesicles. Sci Rep. 2017;7:5887. doi: 10.1038/s41598-017-06178-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grimaldi B, Kohan-Ghadr HR, Drewlo S. The potential for placental activation of PPARγ to improve the angiogenic profile in preeclampsia. Cells. 2022;11:3514. doi: 10.3390/cells11213514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rana S, Powe CE, Salahuddin S, Verlohren S, Perschel FH, Levine RJ, Lim K-H, Wenger JB, Thadhani R, Karumanchi SA. Angiogenic factors and the risk of adverse outcomes in women with suspected preeclampsia. Circulation. 2012;125:911–919. doi: 10.1161/CIRCULATIONAHA.111.054361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zeisler H, Hund M, Verlohren S. The sFlt-1:PlGF ratio in women with suspected preeclampsia. N Engl J Med. 2016;374:1785–1786. doi: 10.1056/NEJMc1602338 [DOI] [PubMed] [Google Scholar]
- 27.McLaughlin K, Snelgrove JW, Audette MC, Syed A, Hobson SR, Windrim RC, Melamed N, Carmona S, Kingdom JC. Placental growth factor testing in clinical practice: evidence from a Canadian tertiary maternity referral centre. Hypertension. 2021;77:2057. doi: 10.1161/HYPERTENSIONAHA.121.17047 [DOI] [PubMed] [Google Scholar]
- 28.Kingdom JC, Audette MC, Hobson SR, Windrim RC, Morgen E. A placenta clinic approach to the diagnosis and management of fetal growth restriction. Am J Obstet Gynecol. 2018;218(2S):S803–S817. doi: 10.1016/j.ajog.2017.11.575 [DOI] [PubMed] [Google Scholar]
- 29.Rajakumar A, Cerdeira AS, Rana S, Zsengeller Z, Edmunds L, Jeyabalan A, Hubel CA, Stillman IE, Parikh SM, Karumanchi SA. Transcriptionally active syncytial aggregates in the maternal circulation may contribute to circulating soluble fms-like tyrosine kinase 1 in preeclampsia. Hypertension. 2012;59:256–264. doi: 10.1161/HYPERTENSIONAHA.111.182170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taché V, LaCoursiere DY, Saleemuddin A, Parast MM. Placental expression of vascular endothelial growth factor receptor-1/soluble vascular endothelial growth factor receptor-1 correlates with severity of clinical preeclampsia and villous hypermaturity. Hum Pathol. 2011;42:1283–1288. doi: 10.1016/j.humpath.2010.11.018 [DOI] [PubMed] [Google Scholar]
- 31.Myers JE, Kenny LC, McCowan LME, Chan EHY, Dekker GA, Poston L, Simpson NAB, North RA; SCOPE consortium. Angiogenic factors combined with clinical risk factors to predict preterm pre-eclampsia in nulliparous women: a predictive test accuracy study. BJOG. 2013;120:1215–1223. doi: 10.1111/1471-0528.12195 [DOI] [PubMed] [Google Scholar]
- 32.Ashwal E, McLaughlin K, Melamed N, Ravichandran A, Ellul K, Hobson SR, Windrim RC, Kingdom JC. Predictive accuracy of early mid-trimester placental markers for recurrence of placenta-mediated pregnancy complications. Ultrasound Obstet Gynecol. 2023;61:418–420. doi: 10.1002/uog.26082 [DOI] [PubMed] [Google Scholar]
- 33.Ho AEP, Hutter J, Jackson LH, Seed PT, Mccabe L, Al-Adnani M, Marnerides A, George S, Story L, Hajnal JV. T2* placental magnetic resonance imaging in preterm preeclampsia: an observational cohort study. Hypertension. 2020;75:1523–1531. doi: 10.1161/HYPERTENSIONAHA.120.14701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rolnik DL, Wright D, Poon LC, O’Gorman N, Syngelaki A, de Paco Matallana C, Akolekar R, Cicero S, Janga D, Singh M. Aspirin versus placebo in pregnancies at high risk for preterm preeclampsia. N Engl J Med. 2017;377:613–622. doi: 10.1056/NEJMoa1704559 [DOI] [PubMed] [Google Scholar]
- 35.Guy GP, Leslie K, Diaz Gomez D, Forenc K, Buck E, Khalil A, Thilaganathan B. Implementation of routine first trimester combined screening for pre-eclampsia: a clinical effectiveness study. BJOG. 2021;128:149–156. doi: 10.1111/1471-0528.16361 [DOI] [PubMed] [Google Scholar]
- 36.Syngelaki A, Nicolaides KH, Balani J, Hyer S, Akolekar R, Kotecha R, Pastides A, Shehata H. Metformin versus placebo in obese pregnant women without diabetes mellitus. N Engl J Med. 2016;374:434–443. doi: 10.1056/NEJMoa1509819 [DOI] [PubMed] [Google Scholar]
- 37.Cluver CA, Hiscock R, Decloedt EH, Hall DR, Schell S, Mol BW, Brownfoot F, Kaitu’u-Lino TJ, Walker SP, Tong S. Use of metformin to prolong gestation in preterm pre-eclampsia: randomised, double blind, placebo controlled trial. BMJ. 2021;374:n2103. doi: 10.1136/bmj.n2103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hastie R, Bergman L, Walker SP, Kaitu’u-Lino T, Hannan NJ, Brownfoot F, Schell S, Harper A, Cannon P, Cluver CA, et al. Associations between soluble fms-like tyrosine kinase-1 and placental growth factor and disease severity among women with preterm eclampsia and preeclampsia. J Am Heart Assoc. 2022;11:e024395. doi: 10.1161/JAHA.121.024395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bujold E. Optimal screening for preeclampsia in the first trimester of pregnancy. Hypertension. 2022;79:323–324. doi: 10.1161/HYPERTENSIONAHA.121.18421 [DOI] [PubMed] [Google Scholar]
- 40.Ashwal E, McLaughlin K, Melamed N, Ravichandran A, Ellul K, Hobson SR, Windrim RC, Kingdom JC. Predictive accuracy of early mid-trimester placental markers for recurrence of placenta-mediated pregnancy complications. Ultrasound Obstet Gynecol. 2022;61:418. doi: 10.1002/uog.26082 [DOI] [PubMed] [Google Scholar]
- 41.Agrawal S, Parks WT, Zeng HD, Ravichandran A, Ashwal E, Windrim RC, Hobson SR, Melamed N, Kingdom JC. Diagnostic utility of serial circulating placental growth factor levels and uterine artery Doppler waveforms in diagnosing underlying placental diseases in pregnancies at high risk of placental dysfunction. Am J Obstet Gynecol. 2022;227:618.e1–618.e16. doi: 10.1016/j.ajog.2022.05.043 [DOI] [PubMed] [Google Scholar]
- 42.McLaughlin K, Nadeem L, Wat J, Baczyk D, Lye SJ, Kingdom JC. Low molecular weight heparin promotes transcription and release of placental growth factor from endothelial cells. Am J Physiol Heart Circ Physiol. 2020;318:H1008–H1017. doi: 10.1152/ajpheart.00109.2020 [DOI] [PubMed] [Google Scholar]
- 43.FDA Roundup: May 19, 2023. U.S. Food and Drug Administration; 2023. [Google Scholar]
- 44.Chang M, Mukherjea D, Gobble RM, Groesch KA, Torry RJ, Torry DS. Glial cell missing 1 regulates placental growth factor (PGF) gene transcription in human trophoblast. Biol Reprod. 2008;78:841–851. doi: 10.1095/biolreprod.107.065599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yaris F, Yaris E, Kadioglu M, Ulku C, Kesim M, Kalyoncu NI. Normal pregnancy outcome following inadvertent exposure to rosiglitazone, gliclazide, and atorvastatin in a diabetic and hypertensive woman. Reprod Toxicol. 2004;18:619–621. doi: 10.1016/j.reprotox.2004.02.014 [DOI] [PubMed] [Google Scholar]
- 46.Kalyoncu NI, Yaris F, Ulku C, Kadioglu M, Kesim M, Unsal M, Dikici M, Yaris E. A case of rosiglitazone exposure in the second trimester of pregnancy. Reprod Toxicol. 2005;19:563–564. doi: 10.1016/j.reprotox.2004.11.003 [DOI] [PubMed] [Google Scholar]
- 47.Haddad GF, Jodicke C, Thomas MA, Williams DB, Aubuchon M. Case series of rosiglitazone used during the first trimester of pregnancy. Reprod Toxicol. 2008;26:183–184. doi: 10.1016/j.reprotox.2008.08.001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that all supporting data are available within the article and its Supplemental Material.20,21