Abstract
Background:
Efficiency of randomised clinical trials (RCTs) of acute respiratory distress syndrome (ARDS) depends on the fraction of deaths attributable to ARDS (AFARDS) to which interventions are targeted. Estimates of AFARDS in subpopulations of ARDS could improve design of ARDS trials.
Methods:
We performed a matched case-control study using the LUNG-SAFE cohort. Primary outcome was intensive care unit mortality. We used nearest neighbour propensity score matching without replacement to match ARDS to non-ARDS populations. We derived two separate AFARDS estimates by matching patients with ARDS to patients with non-acute hypoxaemic respiratory failure (non-AHRF) and to patients with AHRF with unilateral infiltrates only (AHRF-UL). We also estimated AFARDS in subgroups based on severity of hypoxaemia, number of lung quadrants involved, and hyper- versus hypo-inflammatory phenotypes. Additionally, we derived AFAHRF estimates by matching patients with AHRF to non-AHRF controls, and AFAHRF-UL estimates by matching patients with AHRF-UL to non-AHRF controls.
Results:
Estimated AFARDS was 20.9%(95%CI 10.5–31.4%) when compared to AHRF -UL controls and 38.0%(95%CI 34.4%−41.6%) compared to non-AHRF controls. Within subgroups, estimates for AFARDS compared to AHRF-UL controls were highest in patients with severe hypoxaemia (41.1%(95%CI 25.2–57.1%)), in those with four quadrant involvement on chest radiography (28.9%(95%CI 13.4–44.3%)), and in the hyperinflammatory sub-phenotype (26.8%(95%CI 6.9–46.7%)). Estimated AFAHRF was 33.8%(95%CI 30.5%−37.1%) compared to non-AHRF controls. Estimated AFAHRF-UL was 21.3%(12.8–29.7%) compared to non-AHRF controls
Conclusions:
Overall AFARDS mean values were between 20.9%−38.0%, with higher AFARDS seen with severe hypoxaemia, four quadrant involvement on chest radiography, and hyperinflammatory ARDS.
Keywords: Acute Respiratory distress syndrome, Attributable fraction, randomised clinical trials
Introduction
Acute respiratory distress syndrome (ARDS) refers to acute hypoxaemic respiratory failure (AHRF) occurring within one week of a known clinical insult, with bilateral opacities on chest radiography that are not fully explained by effusions, lobar/lung collapse, or nodules[1]. Treatments with biological plausibility[2] and strong supporting pre-clinical evidence, when tested within randomised clinical trials (RCTs)[3], often report statistically indeterminate results (i.e., uncertainty highlighted by non-significant results of two-tailed tests, rather than proof of no difference between treatments (negative)[4]). Addressing this issue remains a major clinical and methodological challenge[2].
There are several explanations for statistically indeterminate RCT results, aside from testing of ineffective treatments. First, as ARDS is a heterogeneous syndrome, RCTs may consist of participants who either benefit, have no effect, or are harmed by the tested intervention. This explanation is supported by observations that distinct sub-phenotypes of ARDS respond differentially to treatments[5–11]. Second, there are several design issues within ARDS RCTs. This explanation is supported by observations that sample size calculations overestimate control arm event rates, and the expected average treatment effect[12].
In this manuscript, we explore another explanation – variation in the excess fraction of mortality attributable to ARDS (AFARDS)[13, 14] in RCT participants. Patients with ARDS may die from ARDS (i.e., AFARDS) or with ARDS (i.e., death may be due to risk factors like comorbidities and/or other organ dysfunction during critical illness). If we explicitly link the eligibility criteria of ARDS RCTs to AFARDS estimates (I.e., the excess proportion of deaths from ARDS), then efficiency of ARDS RCTs would be increased from the generic predictive and prognostic enrichment alongside increase in average treatment effect. We hypothesised that AFARDS will vary by severity of hypoxaemia as per the Berlin ARDS definitions[1, 15], by number of quadrants affected on chest radiography[16], and by sub-phenotype. Our hypothesis was informed by the following observations: first, two small cohort studies (eTable-1) indicated that AFARDS ranges between 15% and 37%[17, 18]; second, in the Berlin ARDS definitions, ARDS outcomes worsened with increasing severity of hypoxaemia[1]. Third, the hyperinflammatory ARDS sub-phenotype had higher mortality and greater treatment effect within RCTs [5–11]. Recently, these ARDS sub-phenotypes were identified within the LUNG-SAFE cohort using machine learning models [19], are available as predefined categories within the LUNG-SAFE dataset, and currently there are no AFARDS estimates for them. Given recent proposals to include AHRF patients (including those with unilateral infiltrates[20]) within an expanded ARDS case definition[21], we also compared AFAHRF and AFARDS.
Methods
Data source
Our data source was the well described LUNG-SAFE (Large observational study to Understand the Global impact of Severe Acute respiratory FailurE) dataset. We summarise key elements of the LUNG-SAFE study design and data collection in eMethods-1. National coordinators and site investigators of the LUNG-SAFE study are listed in the online supplement. AHRF was defined as concurrent presence of: (a) arterial oxygen tension: inspired fraction of oxygen (PaO2:FiO2) ratio ≤ 300 mmHg; (b) new pulmonary parenchymal abnormalities (either unilateral or bilateral) on chest radiography; and (c) ventilatory support with continuous positive airway pressure or expiratory positive airway pressure or positive end expiratory pressure ≥ 5 cmH2O. The diagnosis of ARDS in LUNG-SAFE studies was made by a computer algorithm in the analysis phase of the study using the “raw” data that made up the various components of the Berlin ARDS definition[15].
Study population
Selection criteria of AHRF/ARDS cohort reported in this manuscript were described previously by Pham et al[20]. We defined four populations for our matched cohort study, after excluding patients with congestive heart failure: (1) ARDS – AHRF patients who met the Berlin ARDS criteria, (2) AHRF – patients who met criteria for AHRF (and therefore includes all ARDS patients), (3) AHRF patients with unilateral infiltrates (AHRF-UL) - met criteria for AHRF but not ARDS, and (4) Non-AHRF controls - patients receiving non-invasive or invasive ventilation who did not meet criteria for AHRF (Figure-1). The LUNG-SAFE study collected only the following variables for non-AHRF controls - age, sex, ICU length of stay and ICU mortality.
Figure-1. Flowchart of patients screened and included in the models used to generate overall and subpopulation estimates of AFARDS, AFAHRF and AFAHRF-UL.
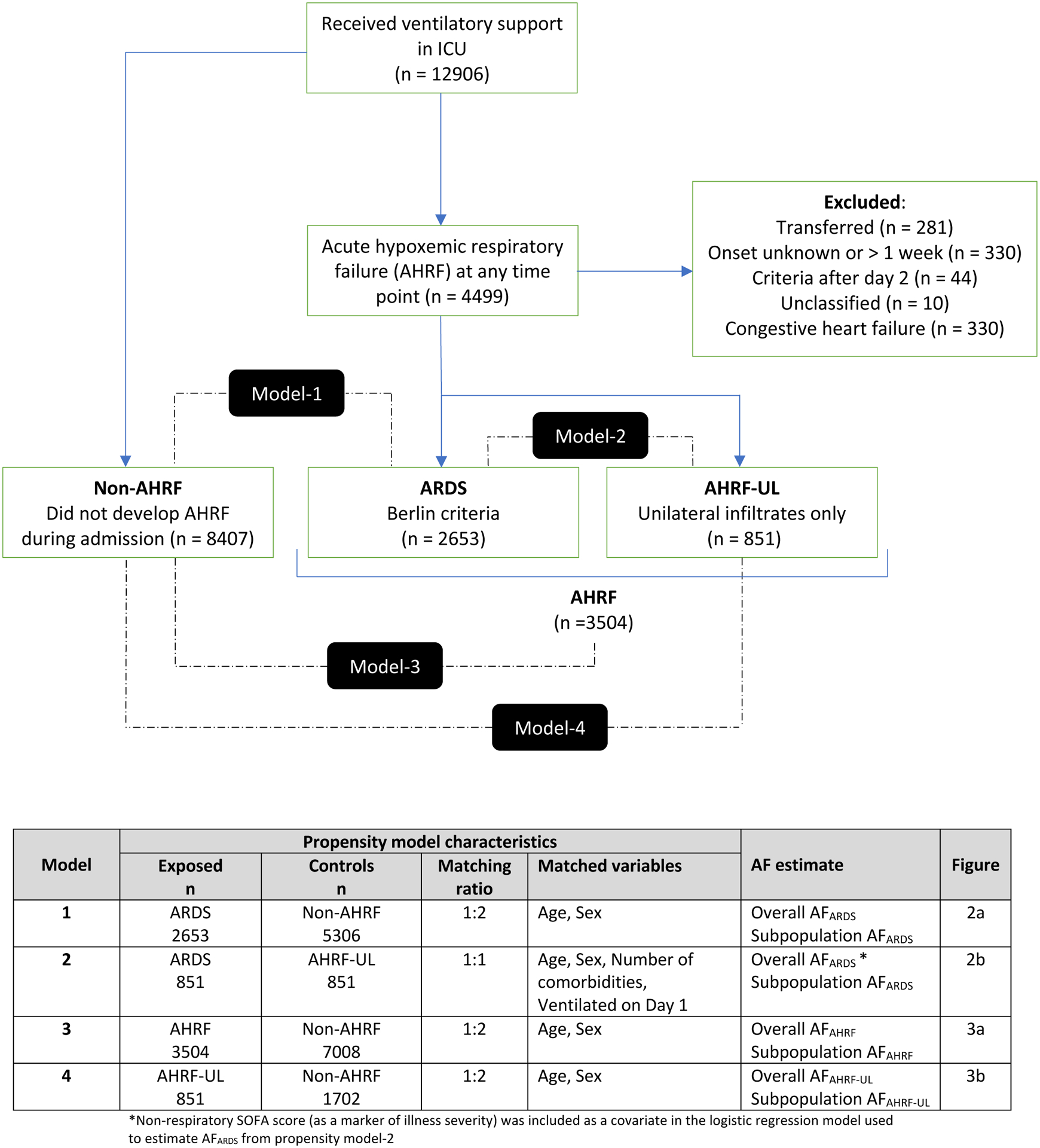
AF is the proportion of individuals with the outcome of interest e.g. death that can be attributed to the exposure e.g. ARDS. For example, AFARDS= [(Deaths in ARDS – Deaths in non-ARDS)/Deaths in ARDS].
Comparisons used to generate overall estimates for AFARDS, AFAHRF, and AFAHRF-UL are shown in the black rectangles. Further details on each model, and the AF estimates generated are provided in the table. To generate subpopulation estimates, analysis was stratified by severity of hypoxaemia, maximum number of quadrants involved in the first 48 hours, and ARDS sub-phenotype.
ARDS: acute respiratory distress syndrome; AF: attributable fraction; AHRF: acute hypoxaemic respiratory failure; AHRF-UL: acute hypoxaemic respiratory failure with unilateral infiltrates only
ARDS sub-phenotypes have recently been assigned in the LUNG-SAFE cohort using a clinical classifier model with a limited selection of predictor variables[19] (eMethods-2). Of note, this model used optimised probability cutoffs and did not use latent class analysis to assign sub-phenotypes. Patients without ARDS do not have sub-phenotype allocation There were no patients who had unilateral infiltrates without AHRF, to act as controls for AFAHRF range estimates, similar to the AFARDS range estimates we report.
Analyses framework
The primary exposure was either ARDS, or AHRF, or AHRF-UL. The primary outcome was ICU mortality, as one of the most reported outcomes in ARDS RCTs[3]. Pre-defined enrichment categories within the primary exposures were severity of hypoxaemia, number of quadrants affected on chest radiography, and sub-phenotypes. Due to the low proportion of missing data (e-Table 2a), without any discernible pattern of missingness, these data were assumed to be missing at random, and complete-case analyses were used for all models.
Estimation of AFARDS requires careful selection of controls and consideration of potential confounding variables. We estimated propensity scores for the exposures using logistic regression. We used nearest neighbour matching without replacement to match exposed patients to controls. With this approach the mortality in the control groups within each prespecified ARDS severity category (severity of hypoxaemia, radiology, and sub-phenotype) would also vary based on matching, enabling estimation of variation in AFARDS within these categories, along with AFARDS range.
Propensity score models and scenarios
Model-1 scenario: AFARDS could be reduced with treatment to mortality seen in ICU patients of similar age and sex without AHRF (one ARDS patient was matched to two non-AHRF controls).
Model-2 scenario: AFARDS could be reduced with treatment to mortality seen in ICU patients with AHRF after accounting for variables commonly considered as part of eligibility criteria in ARDS RCTs at the time of randomisation such as age, sex, number of comorbidities, receipt of invasive mechanical ventilation, and illness severity (one ARDS patient was matched to one AHRF-UL control).
Model-3 scenario: AFAHRF could be reduced with treatment to mortality seen in ICU patients of similar age and sex without AHRF (one AHRF patient was matched to two non-AHRF controls)
Model-4 scenario: AFAHRF-UL could be reduced with treatment to mortality seen in ICU patients of similar age and sex without AHRF scenario (one AHRF-UL patient was matched to two non-AHRF controls).
Additional rationale for matching methods, covariate selection and assessment of each of four different propensity score models are available in Figure-1, eMethods-3, and eFigure-1. We used four separate logistic regression models to estimate AF (AFARDS, AFAHRF, AFAHRF-UL).
Estimation of AFARDS range
We used model-1, and model-2 to estimate the variation in AFARDS by severity of hypoxaemia categories (mild (PaO2:FiO2 >200mmHg); moderate (PaO2:FiO2 100–200mmHg), or severe (PaO2:FIO2 <100mmHg)); the number of quadrants involved radiographically in the first 48 hours after ICU admission (two, three, or four), and the hyperinflammatory vs hypoinflammatory ARDS subphenotypes within LUNG-SAFE dataset[19].
Estimation of AFAHRF
We used model-3 to estimate the variation in AFAHRF by severity of hypoxaemia categories (mild, moderate, severe), and the number of quadrants involved radiographically in the first 48 hours after ICU admission (two, three, or four).
Estimation of AFAHRF-UL
We used model-4 to estimate the variation in AFAHRF-UL by severity of hypoxaemia categories (mild, moderate, severe), and the number of quadrants involved radiographically in the first 48 hours after ICU admission (one, two).
Simulating AFARDS based sample size estimates for different enrichment subgroups
To illustrate how AFARDS can influence sample size estimation, we compared predicted sample size estimates from 28 published ARDS RCTs[22–49] that used mortality as primary outcome (identified in our previous systematic review[3]) to simulated sample size estimates. We simulated sample size calculations for the scenario where AFARDS = 100%, and for estimates of AFARDS from Model-1 stratified by severity of hypoxaemia (mild, moderate, or severe), maximum number of quadrants involved on chest radiography at 48 hours (two, three, or four), and (c) sub-phenotype of ARDS (hyperinflammatory or hypoinflammatory). For all simulations, RCT control event rate was fixed at 40%, alpha at 0.05, and power at 0.80.
Sensitivity analysis
We report the following sensitivity analyses; (i) unmatched analyses in all 4 models to assess how matching - and therefore, exclusion of controls - affected overall and subpopulation estimates; and (ii) used hospital mortality as outcome measure in Model-2 - instead of ICU mortality - to assess how choice of mortality timepoint affected overall and subpopulation estimates for AFARDS.
χ2 test was used to assess linear trends in ICU mortality across subpopulations, and to assess relationship between enrichment categories. Reported p-values are two-sided and p values less than 0.05 were considered statistically significant. All analyses were performed using R version 3.4.2 (AF[50], Matchit[51], and tidyverse[52] packages).
Results
Among 12906 admissions who received ventilatory support in the ICU, we identified 3504 eligible AHRF patients; 2653 met the Berlin ARDS criteria, and 851 patients had AHRF-UL (Figure-1). Missing data are summarised in eTable-2a. Baseline characteristics and outcomes for non-AHRF patients are summarised in eTable-2b. Patients with mild hypoxaemia most often had two quadrant infiltrates, whilst patients with severe hypoxaemia had four quadrant infiltrates (eTable-3a). There was no association between severity of hypoxaemia and ARDS subphenotype (eTable-3b).
Model-1 scenario
Model-1 scenario compared 2653 patients with ARDS matched to 5306 non-AHRF controls. Patients with ARDS had higher ICU mortality compared to controls (34.8% vs 13.8%) (Table-1). Significant linear trends in mortality were seen with severity of hypoxaemia category (mild 28.6% vs 13.2%; moderate 33.2% vs 14.6%; severe 43.0% vs 13.1%; X2 = 32.7; p<0.001); and with increase in number of quadrants involved (two quadrants 27.7% vs 14.4%; three quadrants 34.7% vs 14.7%; four quadrants 40.6% vs 12.7%; X2 = 90.9; p<0.001). The ICU mortality was higher for hyperinflammatory (51.7% vs 12.4%) and hypoinflammatory (28.0% vs 14.4%) ARDS, compared with non-AHRF controls (X2 = 413.07; p<0.001).
Table 1.
Baseline characteristics of ARDS, AHRF, AHRF-UL and corresponding propensity matched control populations to derive ARDS/AHRF attributable fraction.
| Model-1 AFARDS | Model-2 AFARDS | Model-3 AFAHRF | Model-4 AFAHRF-UL | |||||
|---|---|---|---|---|---|---|---|---|
| ARDS (N = 2653) | Non-AHRF (N = 5306) | ARDS (N = 851) | AHRF-UL (N = 851) | AHRF (N = 3504) | Non-AHRF (N = 7008) | AHRF-UL (N = 851) | Non-AHRF (N = 1702) | |
| Age (years) Mean (SD) | 61.5 (16.8) | 61.4 (16.8) | 63.0 (16.2) | 63.0 (16.9) | 61.8 (16.8) | 62.0 (16.8) | 63.0 (16.9) | 63.0 (16.8) |
| Sex Female n (%) | 1014 (38.2) | 2028 (38.2) | 297 (34.9) | 301 (35.4) | 1315 (37.5) | 2664 (38.0) | 301 (35.4) | 599 (35.2) |
| BMI Mean (SD) | 27.4 (8.6) | - | 26.9 (7.3) | 26.7 (6.8) | 27.3 (8.2) | - | 26.7 (6.8) | - |
| Number of comorbidities n (%) | ||||||||
| 0 | 1053 (39.7) | - | 330 (38.8) | 322 (37.8) | 1375 (39.2) | - | 322 (37.8) | - |
| 1 | 937 (35.3) | - | 302 (35.5) | 302 (35.5) | 1239 (35.4) | - | 302 (35.5) | - |
| 2 or more | 663 (25.0) | - | 219 (25.7) | 227 (26.7) | 890 (25.4) | - | 227 (26.7) | - |
| Risk factor for ARDS n (%) | ||||||||
| Pneumonia | 1574 (59.3) | - | 510 (59.9) | 433 (50.9) | 2007 (57.3) | - | 433 (50.9) | - |
| Extrapulmonary sepsis | 421 (15.9) | - | 141 (16.6) | 115 (13.5) | 536 (15.3) | - | 115 (13.5) | - |
| Aspiration | 386 (14.5) | - | 114 (13.4) | 157 (18.4) | 543 (15.5) | - | 157 (18.4) | - |
| Pancreatitis | 57 (2.1) | - | 16 (1.9) | 11 (1.3) | 68 (1.9) | - | 11 (13) | - |
| Pulmonary vasculitis | 12 (0.5) | - | 2 (0.2) | 0 (0) | 12 (0.3) | - | 0 (0.0) | - |
| Trauma | 108 (4.1) | - | 37 (4.3) | 48 (5.6) | 156 (4.5) | - | 48 (5.6) | - |
| Inhalation | 69 (2.6) | - | 28 (3.3) | 22 (2.6) | 91 (2.6) | - | 22 (2.6) | - |
| Pulmonary contusion | 82 (3.1) | - | 28 (3.3) | 34 (4.0) | 116 (3.3) | - | 34 (4.0) | - |
| Burns | 7 (0.3) | - | 3 (0.4) | 2 (0.2) | 9 (0.3) | - | 2 (0.2) | - |
| Non-cardiogenic shock | 201 (7.6) | - | 75 (8.8) | 52 (6.1) | 253 (7.2) | - | 52 (6.1) | - |
| Drowning | 2 (0.1) | - | 1 (01) | 0 (0) | 2 (0.1) | - | 0 (0.0) | - |
| Drug overdose | 47 (1.8) | - | 11 (1.3) | 22 (2.6) | 69 (2.0) | - | 22 (2.6) | - |
| Transfusion-related | 107 (4.0) | - | 34 (4.0) | 27 (3.2) | 134 (3.8) | - | 27 (3.2) | - |
| Other | 67 (2.5) | - | 17 (2.0) | 45 (5.3) | 112 (3.2) | - | 45 (5.3) | - |
| No risk factor for ARDS | 229 (8.6) | - | 79 (9.3) | 0 (0) | 229 (6.5) | - | 0 (0.0) | - |
| PaO2:FiO2 ratio on day 1 Mean (SD) * | 159 (67.4) | - | 161 (67.6) | 188 (63.6) | 166 (67.8) | - | 188 (63.6) | - |
| Severity of hypoxemia on day 1 n (%) | ||||||||
| Mild (200–300) | 760 (28.6) | - | 249 (29.3) | 377 (44.3) | 1137 (32.4) | - | 377 (44.3) | - |
| Moderate (100–200) | 1263 (47.6) | - | 400 (47.0) | 380 (44.7) | 1643 (46.9) | - | 380 (44.7) | - |
| Severe (<100) | 626 (23.6) | - | 200 (23.5) | 93 (10.9) | 719 (20.5) | - | 93 (10.9) | - |
| Maximum number of quadrants involved in first 48 hours n (%) * | ||||||||
| 1 | - | - | - | 598 (70.2) | 482 (13.8) | - | 482 (70.2) | - |
| 2 | 1045 (39.4) | - | 351 (41.2) | 236 (27.7) | 1281 (36.6) | - | 236 (27.7) | - |
| 3 | 615 (23.2) | - | 214 (25.1) | - | 624 (17.8) | - | - | - |
| 4 | 993 (37.4) | - | 286 (33.6) | - | 1000 (28.5) | - | - | - |
| Subphenotype of ARDS n (%) * | ||||||||
| Hypoinflammatory | 1966 (74.1) | - | 616 (72.4) | - | - | - | - | - |
| Hyperinflammatory | 687 (25.9) | - | 235 (27.6) | - | - | - | - | - |
| SOFA score day 1 Mean (SD) | 9.39 (4.08) | - | 9.55 (4.19) | 8.69 (3.97) | 9.2 (4.1) | - | 8.69 (3.97) | - |
| Non respiratory SOFA score day 1 * | 6.48 (3.96) | - | 6.65 (4.06) | 6.04 (3.87) | 6.4 (4.0) | - | 6.04 (3.87) | - |
| Ventilated on day 1 n (%) | 2151 (81.1) | - | 700 (82.3) | 698 (82.0) | 2849 (81.3) | - | 698 (82.0) | - |
| Duration of invasive mechanical ventilation Mean (SD) * | 12.0 (12.9) | - | 12.2 (12.8) | 10.6 (12.6) | 11.7 (12.9) | - | 10.6 (12.6) | - |
| ICU LOS Mean (SD) | 14.2 (14.0) | 6.2 (8.2) | 12.7 (13.8) | 6.2 (8.2) | 13.8 (14.0) | 6.2 (8.2) | 12.7 (13.8) | 6.2 (7.9) |
| ICU mortality n (%) | 906 (34.2) | 730 (13.8) | 293 (34.4) | 206 (24.2) | 1112 (31.7) | 1000 (14.3) | 206 (24.2) | 247 (14.5) |
| Hospital LOS Mean (SD) * | 23.1 (20.8) | - | 23.6 (20.7) | 22.6 (20.7) | 22.9 (20.8) | - | 22.6 (20.7) | - |
| Hospital mortality n (%) * | 1044 (39.4) | - | 342 (40.2) | 259 (30.4) | 1303 (37.3) | - | 259 (30.4) | - |
AFARDS in Model-1 was estimated by matching 2653 ARDS patients to non-AHRF controls that were propensity score balanced on age and sex in a 1:2 ratio. AFARDS in Model-2 was estimated by matching 851 ARDS patients to AHRF-UL controls in a 1:1 ratio and propensity score balanced on age, sex, number of comorbidities, and ventilation status on day 1. AFAHRF in Model-3 was estimated by matching 3504 AHRF cases to non-AHRF controls that were propensity score balanced on age and sex in a 1:2 ratio. AFAHRF-UL in Model-4 was estimated by matching 851 AHRF-UL cases to non-AHRF controls that were propensity score balanced on age and sex in a 1:2 ratio.
Denotes variable with missing data. Further details of missing data are available in eTable-2a
Post matching standardised mean differences (SMDs) for the matching covariates in each model are shown in eTable-4 and were all < 0.05; empirical cumulative density function plots for matching covariates were also consistent with good balance (eFigure-2). Propensity score overlap following matching is shown for each model in eFigure-3.
ARDS: acute respiratory distress syndrome; AHRF: acute hypoxaemic respiratory failure; AHRF-UL: acute hypoxaemic respiratory failure with unilateral infiltrates only; Std Diff: standardised difference; SD: standard deviation; BMI: Body Mass Index; SOFA: sequential organ failure assessment; ICU: intensive care unit; LOS: length of stay; NMV: non-matched variable.
Model-2 scenario
Model-2 scenario compared 851 patients with ARDS matched to 851 AHRF-UL controls. Patients with ARDS had higher ICU mortality (34.4% vs 24.2%; p<0.001), with the higher control arm mortality compared to model-1 reflecting the differences in matching variables between the models (Table-1). Similar to model-1, significant linear trends in mortality were seen with severity of hypoxaemia (mild 28.9% vs 19.3%; moderate 32.8% vs 28.5%; severe 44.5% vs 22.0%; X2 = 58.3; p < 0.001), and with increase in the number of quadrants in patients with ARDS compared with AHRF-UL controls, the absolute differences were lower compared with model-1 (mild 28.9% vs 19.3%; moderate 32.8% vs 28.5%; severe 44.5% vs 22.0%; X2 = 51.5; p<0.001). The ICU mortality was higher for hyperinflammatory (50.6% vs 26.8%) and hypoinflammatory (28.2% vs 23.2%) ARDS subphenotypes, compared with matched non-AHRF controls (X2 = 147.27; p<0.001).
Range of AFARDS and categories using model-1, and model-2
The AFARDS ranges between 38.0% (95% CI 34.4% - 41.6%) from model-1(Figure-2a) and 20.9% (95% CI 10.5 – 31.4%) from model-2 (Figure-2b).
Figure-2. Overall and subpopulation estimates of AFARDS.
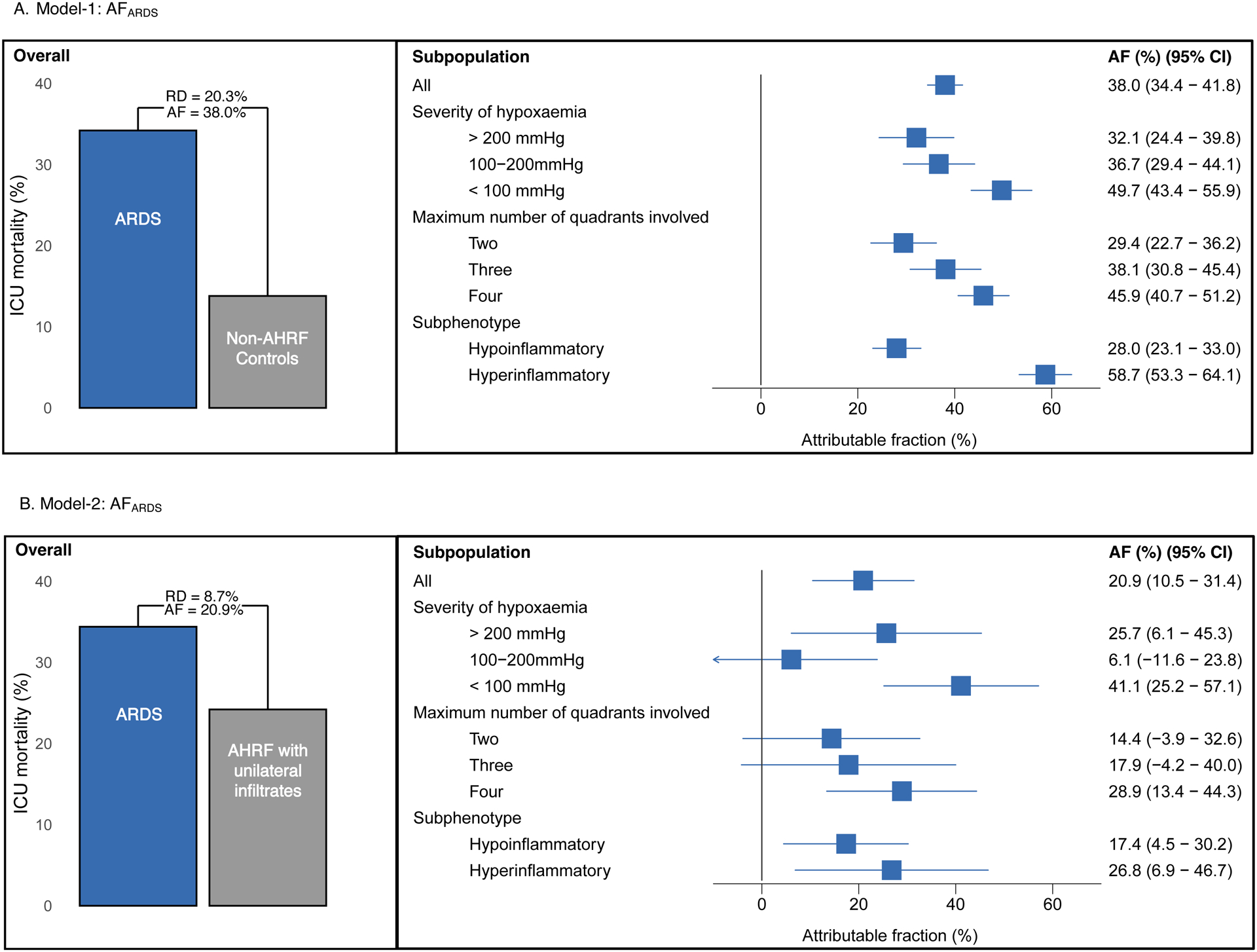
Figure-2a. The fraction of deaths attributable to the ARDS exposure was ascertained using proportions. Propensity for ARDS logistic regression models were used to derive estimates for AFARDS in model-1. Bar graph shows the mortality difference between ARDS population compared with propensity matched non-AHRF controls (Model-1). Analysis was then stratified by severity of hypoxemia, maximum number of quadrants involved in the first 48 hours, and ARDS hypo/hyper-inflammatory subphenotype. Subpopulation AF ARDS estimates from model-1 are shown in the forest plot.
Figure-2b. Propensity for ARDS logistic regression models were then used to derive estimates for AFARDS in model-2. Bar graph shows the mortality difference between ARDS population compared with propensity matched controls who had AHRF with unilateral infiltrates (Model-2). Analysis was also stratified by severity of hypoxaemia, maximum number of quadrants involved in the first 48 hours, and ARDS hypo/hyper-inflammatory subphenotype. Subpopulation AFARDS estimates from model-2 are shown in the forest plot. AF: attributable fraction; ARDS: acute respiratory distress syndrome; AHRF: acute hypoxemic respiratory failure; CI: confidence interval; RD: risk difference.
AFARDS varies by severity of hypoxaemia
In model-1, AFARDS increased with worsening severity of hypoxaemia (mild = 32.1% (95 % CI 24.4 – 39.8%), moderate = 36.7% (95% CI 29.4 – 44.1%), severe = 49.7% (95% CI 43.4 – 55.9%)) (Figure-2a).
Model-2 also highlighted increase in AFARDS with worsening severity of hypoxaemia (mild = 25.7% (95 % CI 6.1 – 45.3%), moderate = 6.1% (95% CI −11.6 – 23.8%), and severe = 41.1% (95% CI 25.2 – 57.1%) (Figure-2b)).
AFARDS varies by number of quadrants involved on chest radiography
In model-1, AFARDS increased with number of quadrants involved on chest radiography (two = 29.4% (95 % CI 22.7 – 36.2%), three = 38.1% (95% CI 30.8 – 45.4%), four = 45.9% (95% CI 40.7 – 51.2%)) (Figure 2a).
Model-2 also highlighted increase in AFARDS with number of quadrants involved on chest radiography (two = 14.4% (95 % CI −3.9 – 32.6%), three = 17.9% (95% CI −4.2 – 40.0%), four = 28.9% (95% CI 13.4 – 44.3%) (Figure-2b)).
AFARDS was higher in hyperinflammatory subphenotype
In model-1, AFARDS was higher in hyperinflammatory subphenotype (hyperinflammatory= 58.7% (95% CI 53.3 – 64.1%) vs hypoinflammatory = 28.0% (95 % CI 23.1 – 33.0%)) (Figure-2a).
Model-2 also highlighted higher AFARDS the hyperinflammatory subphenotype ((hyperinflammatory= 28.6% (95% CI 6.9 – 46.7%) vs hypoinflammatory 17.4% (95% CI 4.5 – 30.2%) (Figure-2b).
Model-3 scenario estimating AFAHRF
Model-3 scenario compared 3504 patients with AHRF matched to 7008 non-AHRF controls. Patients with AHRF had higher ICU mortality compared with non-AHRF controls (31.7% vs 14.1%).
The estimated AFAHRF from model-3 was 33.8% (95% CI 30.5% - 37.1%), which is lower than the AFARDS from model-1 and higher than the AFARDS from model-2. These differences were reflected in the severity of hypoxaemia and radiography categories (Figure-3a).
Figure-3. Overall and subpopulation estimates of AFAHRF and AFAHRF-UL.
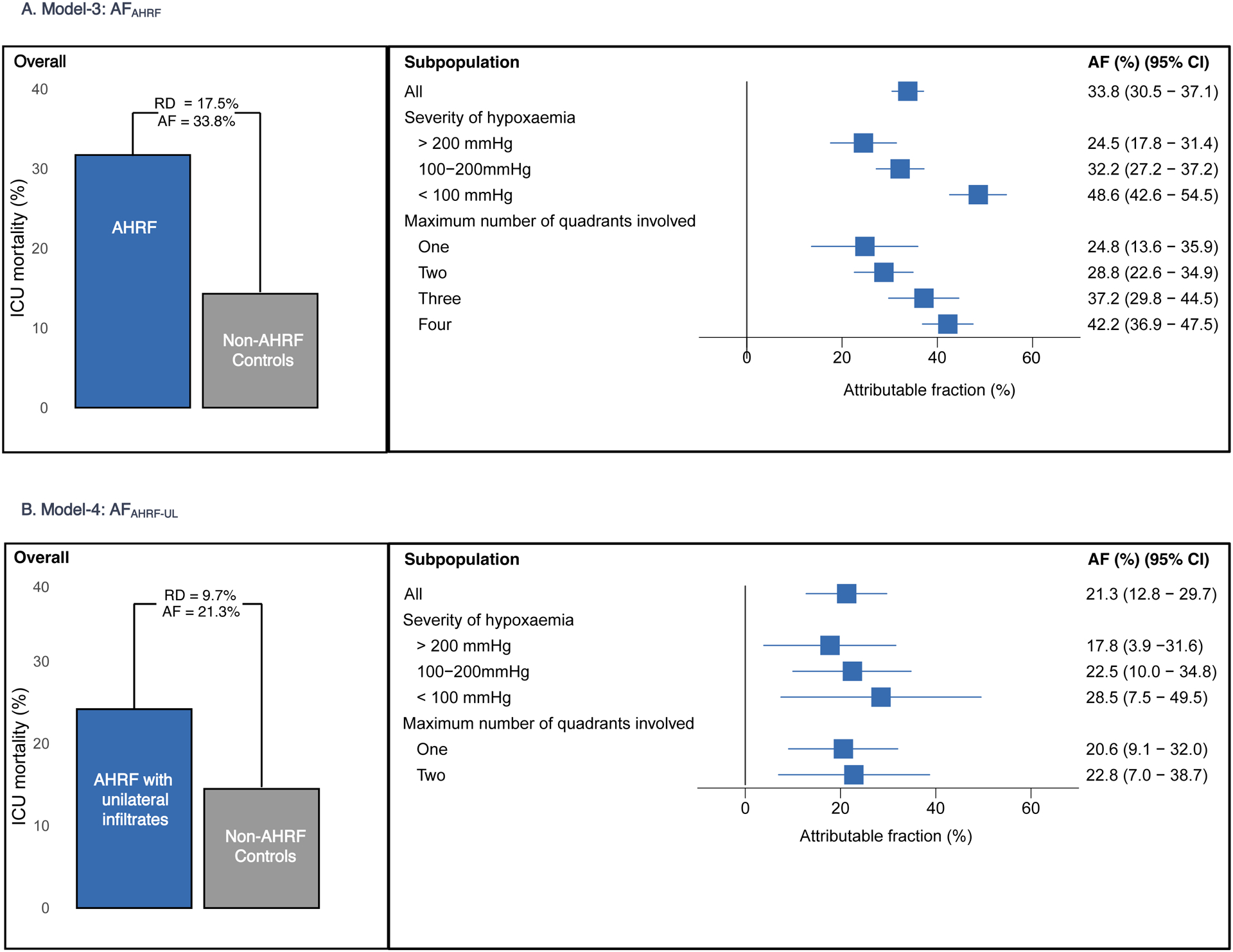
Figure-3a. Bar graphs show the mortality difference between AHRF population compared with propensity matched non-AHRF controls. AFAHRF estimates stratified by severity of hypoxaemia and maximum number of quadrants involved in the first 48 hours are shown in the forest plot.
Figure-3b. Bar graphs show the mortality difference between AHRF-UL population compared with propensity matched non-AHRF controls. AFAHRF-UL estimates stratified by severity of hypoxemia and maximum number of quadrants involved in the first 48 hours are shown in the forest plot.
AF: attributable fraction; AHRF: acute hypoxemic respiratory failure; AHRF-UL: acute hypoxemic respiratory failure with unilateral infiltrates only; CI: confidence interval; RD: risk difference.
Model-4 scenario estimating AFAHRF-UL
Model-4 scenario compared 851 patients with AHRF-UL matched to 1702 non-AHRF controls. Patients with AHRF-UL had higher ICU mortality (24.2% vs 14.5%). The estimate of AFAHRF-UL was 21.3% (95% CI 12.8% - 29.7%), which was which lower than the AFARDS from model-1 and comparable to model-2 (Figure-3b). In patients with unilateral, two-quadrant involvement, who would be excluded from ARDS RCTs, the estimate of AFAHRF-UL was 22.8% (95% CI 7.0% - 38.7%), which was comparable to AFARDS from model-2.
Sample size requirements for ARDS RCTs change with estimated AFARDS
As the AFARDS increases in a RCT population, the sample size required will decrease for pre-specified alpha, beta, and risk reduction combinations. For example, from our current work, sample size estimates were lower for severe hypoxaemia compared to mild or moderate hypoxaemia, and lower for four quadrant radiographic involvement, compared with two or three quadrant radiographic involvement, and lower for hyperinflammatory sub-phenotype, compared with hypo-inflammatory sub-phenotype (Figure-4).
Figure-4. Illustrative examples of sample size calculations for different AFARDS scenarios.
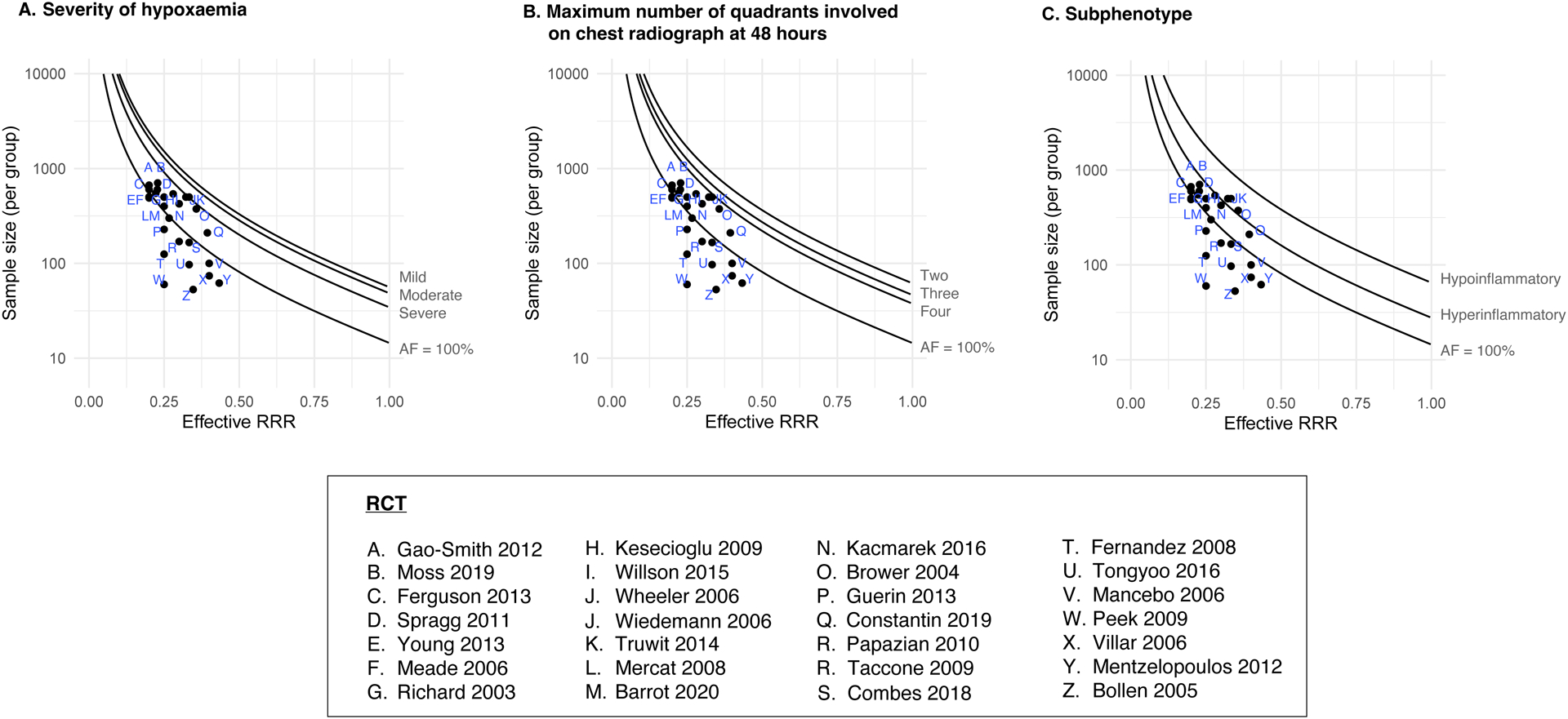
These curves illustrate the AFARDS principle. Each curve represents the sample sizes required for different AF estimates (when control event rate is fixed at 40%). We show the estimates of AFARDS from Model-1, stratified by (a) severity of hypoxaemia, (b) maximum number of quadrants involved on chest radiography at 48 hours, and (c) sub-phenotype of ARDS. We contrast these against the common assumption that AFARDS is expected to be 100%.
Dot plots represent ARDS RCTs with mortality as primary outcome identified previously in our systematic review[12]; they correspond to the actual RRR used for sample size estimation and sample size per group in these RCTs. Median (IQR) control group mortality used for sample size calculations in these RCTs was 45.0% (33.3% - 52.5%) and RRR was 29.0% (24.5% - 33.3%). Most trials aimed for 80% power and 5% alpha. The sample size per group varied between 53 to 704 patients.
RCTs above a curve will have an adequate sample size to detect the predicted RRR.
RRR; relative risk reduction, AF; attributable fraction, ARDS: acute respiratory distress syndrome.
Sensitivity analyses
Overall unmatched estimates of AFARDS, AFAHRF and AFAHRF-UL were consistent with overall estimates from matched analyses. In the unmatched analyses of model-1, model- 3 and model-4 - which led to an increase in number of controls – trends within enrichment categories were no longer significant. In the unmatched analysis of model-2 - which led to an increase in number of exposed patients with ARDS – trends within enrichment categories were consistent with the matched analysis (Table-2).
Table 2.
Sensitivity analysis - Overall and subpopulation estimates of AFARDS, AFAHRF and AFAHRF-UL
| Model 1: AFARDS (non- AHRF controls) | Model 2: AFARDS (AHRF-UL controls) | Model 3: AFAHRF (non- AHRF controls) | Model 4: AFAHRF-UL (non-AHRF controls) | ||
|---|---|---|---|---|---|
| Sensitivity analysis | Unmatched analysis | Unmatched analysis | Matched analysis - hospital mortality as outcome | Unmatched analysis | Unmatched analysis |
| Patients with ARDS/AHRF | 2653 | 2653 | 851 | 3504 | 851 |
| Controls | 8407 | 851 | 851 | 8407 | 8407 |
| Overall AF | 25.2% (22.7 – 27.6%) | 22.3% (14.4 – 30.1%) | 17.7% (7.7 – 27.6%) | 26.5% (23.9 – 29.1%) | 5.9% (4.1 – 7.7%) |
| PaO2/FiO2 ratio (mmHg) | |||||
| >200 | 8.9% (4.5 – 13.4%) | 10.7% (−1.6 – 23.0%) | 25.8% (7.6 – 43.9%) | 10.6% (6.3 – 15.0%) | 2.5% (=2.5 – 7.5%) |
| 100–200 | 17.2% (13.4 – 21.1%) | 23.6% (13.2 – 34.0%) | 3.1% (−13.7 – 19.9%) | 18.9% (15.2 – 22.7%) | 3.6% (1.3 – 8.5%) |
| <100 | 14.5% (10.7 – 18.5%) | 33.9% (25.9 – 41.9%) | 35.0% (18.9 – 51.2%) | 15.6% (11.7 – 19.5%) | 1.6% (−3.5% - 6.7%) |
| Maximum number of quadrants involved in first 48 hours | |||||
| One | - | - | - | 3.9% (−1.0 – 8.8%) | 3.9% (−1.0 – 8.8%) |
| Two | 11.3% (7.0 – 15.6%) | 9.7% (−2.9 – 22.4%) | 12.0% (−5.3 – 29.4%) | 12.8% (8.6 – 17.0%) | 2.3% (−2.7 – 7.3%) |
| Three | 10.8% (6.6 – 15.1%) | 20.0% (9.7 – 30.2%) | 18.3% (−1.8 – 38.5%) | 10.9% (6.6% - 15.1%) | - |
| Four | 18.8% (15.1 – 22.4%) | 36.4% (28.2 – 44.6%) | 22.3% (7.0 – 37.6%) | 18.7% (15.1 – 22.4%) | - |
| Subphenotype | |||||
| Hypoinflammatory | 18.3% (14.4 – 22.1%) | 21.3% (10.2 – 32.4%) | 14.1% (1.9 – 26.3%) | - | - |
| Hyperinflammatory | 19.4% (15.9 – 23.0%) | 31.9% (22.4 – 41.5%) | 25.9% (7.4 – 44.4%) | - | - |
Our primary analysis used matching without replacement with ICU mortality as the primary outcome measure. We repeated the analysis without matching in all four models to examine how matching – and therefore selection of controls, affected AF estimates. The matched analysis was also conducted using hospital mortality (instead of ICU mortality) as the outcome measure in Model-2.
AF: attributable fraction; CI; confidence interval; ARDS: acute respiratory distress syndrome; AHRF: acute hypoxaemic respiratory failure; AHRF-UL: acute hypoxaemic respiratory failure with unilateral infiltrates only
Overall estimate of AFARDS in model-2 was lower when hospital mortality was used as the outcome measure - instead of ICU mortality; subpopulation estimates were also consistently lower, but overall trends within enrichment categories remained consistent (Table-2).
Discussion
Using the LUNG-SAFE database, we report mean estimates of AFARDS between 20.9% to 38.0%. We observed a dose-response increase in AFARDS with severity of hypoxaemia and with quadrants of radiographic involvement. AFARDS was higher in the hyperinflammatory compared with the hypoinflammatory subphenotype of ARDS. Our results are consistent with previous work on AFARDS[17, 18] and we have extended these previous works by modelling distinct clinical scenarios, including the value of incorporating patients with AHRF in the extended ARDS case definitions[21]. Of note, our AFARDS estimates are consistent with a very different approach using marginal structural models, reported by Torres and colleagues[17].
We focused on several a priori defined ARDS subpopulations that have the potential for enrichment in clinical trials[53]. From our previous work, higher all-cause control-arm mortality does not necessarily generate larger average treatment effects in ARDS RCTs[12], making us hypothesise that ARDS-specific enrichment subgroups may outperform generic illness severity based prognostic enrichment.
Enrichment strategy, whether prognostic or predictive, is a trade-off between population prevalence, feasibility, and expected treatment effect[54]. In the LUNG-SAFE cohort, 23.6% of patients had severe ARDS, 36.7% had four-quadrant involvement, and 36.4% were hyperinflammatory ARDS subphenotype. Severe hypoxaemia is a potentially implementable enrichment criteria for ARDS RCTs, by using the approach highlighted within the Kigali modification of the ARDS definitions[55, 56]. Further, in previous RCTs of prone positioning and extracorporeal support[30, 57, 58] enriching on severe hypoxaemia has shown promise. Another element of the AHRF-ARDS debate is the inter- and intra-observer reliability of chest radiology, and its feasibility in resource limited settings. Whilst acknowledging this debate[59], four-quadrant involvement appears to be an enrichment marker for high AFARDS.
Another enrichment strategy linked to precision medicine is the subphenotyping of ARDS, which would require either measuring discriminant biomarkers with near patient testing, or implementation of machine learning-derived classifier models incorporating clinically available data. Similar subphenoptypes have been reported in non-ARDS populations including COVID-19[6], AHRF and sepsis, which potentially broadens the implications of our findings[60]. For illustration, we compared the sample size estimations from 28 ARDS RCTs[22–49], that used mortality as a primary outcome, for different AFARDS scenarios (Figure-4), which suggests that previous ARDS RCTs may lack sensitivity under the key assumption that only AFARDS deaths are affected by the tested treatment.
Our findings also lead us to consider how our work informs the debate on the need for distinction between ARDS and AHRF. Specifically, the estimate of AFAHRF-UL for patients with unilateral, two-quadrant involvement, who would be excluded currently from ARDS RCTs, was comparable to AFARDS from model-2. Currently ARDS is conceptualised as a subset of AHRF; exclusion of AHRF patients with similar AF and overlapping biology[61] from the overall definition has implications for future RCTs and generalisability to clinical practice. Future research should explore the impact of including these populations in ARDS/AHRF RCTs.
Our analysis has strengths and limitations. We used the LUNG-SAFE dataset - a large multinational cohort recruited from 459 ICUs that was prospectively designed to enrol and follow up patients with AHRF and which underwent systematic validation after data collection. Our assessment of enrichment categories used inclusion criteria that would be immediately applicable to inform design of ARDS RCTs. Despite the use of propensity score methods, residual confounding remains a concern, given the available characteristics of controls and because we have not accounted for differences in mortality between study site and countries. Although we have not accounted for risk factors for ARDS, type of comorbidity, potential worsening (or improvement) over time in hypoxaemia, and geographic variations in usual care/ outcomes in these analyses, eligibility criteria in ARDS RCTs seldom stipulate these covariates[3]. The risk factor of ARDS limitation is explicit, as the LUNG-SAFE cohort did not collect ARDS risk factors for non-AHRF controls. Our primary and sensitivity analyses focus on mortality; the impact on other outcomes used in ARDS RCTs (such as ventilator free days) needs to be assessed. An implicit assumption in these models is that the putative treatment for ARDS/AHRF has no effect on non-ARDS/non-AHRF patients’ mortality. This assumption would not bias AF-ARDS estimates, as the control groups in ARDS RCTs would either not receive the intervention or those who do, will be analysed as crossovers / intention to treat framework.
Conclusions
ARDS is associated with excess mortality in critically ill patients. Our results highlight generic enrichment populations based on commonly used ARDS RCT eligibility criteria such as severity of hypoxaemia, and number of quadrants involved in chest radiography. We show that hyperinflammatory ARDS sub-phenotype has higher attributable fraction.
Supplementary Material
Key messages.
What is already known on this topic: The excess mortality - or attributable fraction (AF)-due to acute respiratory distress syndrome (ARDS) has been estimated to range between 15–37%. We do not know how this varies by severity of hypoxaemia, radiographic findings, and ARDS sub-phenotype.
What this study adds: We observed a dose-response increase in AFARDS with severity of hypoxaemia, quadrants of radiographic involvement, and that AFARDS was higher in the hyperinflammatory compared with the hypoinflammatory sub-phenotype of ARDS.
How this study might affect research, practice or policy: We highlight ARDS subpopulations that can inform enrichment options in randomised clinical trials.
Funding statement
The LUNG-SAFE study was funded and supported by the European Society of Intensive Care Medicine (ESICM), Brussels, Belgium, by St Michael’s Hospital, Toronto, Canada, and by the University of Milan-Bicocca, Monza, Italy. ESICM provided support in data collection and study coordination. ESICM, St Michael’s Hospital, Toronto, and University of Milan-Bicocca had no role in the design and conduct of the study; management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the current manuscript for publication.
Competing Interest
CS is funded by UKRI [MR/S035753/1 and MR/X005070/1] and the National Institute for Health and Care Research [NIHR133788]. Work in her research group is supported by GlaxoSmithKline, the Wellcome Trust and the Cambridge NIHR Biomedical Research Centre, and she has received consultancy fees from Abbvie, Sanofi and GlaxoSmithKline. CC is funded by National Institutes of Health R35-HL140026 and reports grant funding from Roche-Genentech and Quantum Leap Healthcare collaborative, in addition to the National Institutes of Health, and consulting fees from Vasomune, Gen1e Life Sciences, Cellenkos, and Janssen.
EF reports personal fees from ALung Technologies, Aerogen, Baxter, GE Healthcare, Inspira, and Vasomune outside the submitted work.
JL is funded by a Future Research Leaders Award (16-FRL-3845) from Science Foundation Ireland and reports receiving consulting fees from Baxter Inc and from Glaxosmithkline. MS-H is funded by a clinician scientist fellowship from the National Institute for Health Research [CS-2016-16-011] and reports receiving grants from the NIHR, MRC, EME, HTA, Huo Foundation, and highlights industry support for TRAITS research programme - a Chief Scientists Office, Scotland funded time critical precision medicine in adult critically ill patients (TRAITS Programme).
Footnotes
Ethics approval
All participating ICUs individually obtained local ethics committee approval and obtained either patient consent or ethics committee waiver of consent in the original LUNG SAFE study. Research ethics board approval - St. Michael’s Hospital (one of two lead sites) REB# 13–384.
Data sharing
Requests for access to the LUNG-SAFE dataset should be submitted to research@esicm.org
Disclaimer
The views expressed in this publication are those of the authors and not necessarily those of the National Health Service, the National Institute for Health and Care Research, or the Department of Health and Social Care
References
- 1.Force ADT, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS: Acute respiratory distress syndrome: the Berlin Definition. JAMA 2012, 307(23):2526–2533. [DOI] [PubMed] [Google Scholar]
- 2.Matthay MA, Zemans RL, Zimmerman GA, Arabi YM, Beitler JR, Mercat A, Herridge M, Randolph AG, Calfee CS: Acute respiratory distress syndrome. Nat Rev Dis Primers 2019, 5(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saha R, Assouline B, Mason G, Douiri A, Summers C, Shankar-Hari M: Impact of differences in acute respiratory distress syndrome randomised controlled trial inclusion and exclusion criteria: systematic review and meta-analysis. Br J Anaesth 2021, 127(1):85–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sackett DL: Superiority trials, non-inferiority trials, and prisoners of the 2-sided null hypothesis. Evid Based Med 2004, 9(2):38–39. [Google Scholar]
- 5.Shankar-Hari M, Santhakumaran S, Prevost AT, Ward JK, Marshall T, Bradley C, Calfee CS, Delucchi KL, Sinha P, Matthay MA et al. : Defining phenotypes and treatment effect heterogeneity to inform acute respiratory distress syndrome and sepsis trials: secondary analyses of three RCTs. 2021, 8:10. [PubMed] [Google Scholar]
- 6.Sinha P, Calfee CS, Cherian S, Brealey D, Cutler S, King C, Killick C, Richards O, Cheema Y, Bailey C et al. : Prevalence of phenotypes of acute respiratory distress syndrome in critically ill patients with COVID-19: a prospective observational study. The Lancet Respiratory medicine 2020, 8(12):1209–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sinha P, Delucchi KL, Thompson BT, McAuley DF, Matthay MA, Calfee CS, Network NA: Latent class analysis of ARDS subphenotypes: a secondary analysis of the statins for acutely injured lungs from sepsis (SAILS) study. Intensive Care Med 2018, 44(11):1859–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calfee CS, Delucchi KL, Sinha P, Matthay MA, Hackett J, Shankar-Hari M, McDowell C, Laffey JG, O’Kane CM, McAuley DF et al. : Acute respiratory distress syndrome subphenotypes and differential response to simvastatin: secondary analysis of a randomised controlled trial. The Lancet Respiratory medicine 2018, 6(9):691–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calfee CS, Janz DR, Bernard GR, May AK, Kangelaris KN, Matthay MA, Ware LB: Distinct molecular phenotypes of direct vs indirect ARDS in single-center and multicenter studies. Chest 2015, 147(6):1539–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Famous KR, Delucchi K, Ware LB, Kangelaris KN, Liu KD, Thompson BT, Calfee CS, Network A: Acute Respiratory Distress Syndrome Subphenotypes Respond Differently to Randomized Fluid Management Strategy. Am J Respir Crit Care Med 2017, 195(3):331–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calfee CS, Delucchi K, Parsons PE, Thompson BT, Ware LB, Matthay MA, Network NA: Subphenotypes in acute respiratory distress syndrome: latent class analysis of data from two randomised controlled trials. The Lancet Respiratory medicine 2014, 2(8):611–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saha R, Assouline B, Mason G, Douiri A, Summers C, Shankar-Hari M: The impact of sample size mis-estimations on the interpretation of acute respiratory distress syndrome trials: systematic review and meta-analysis. CHEST. [DOI] [PubMed] [Google Scholar]
- 13.Sinclair JC, Haynes RB: Selecting participants that raise a clinical trial’s population attributable fraction can increase the treatment effect within the trial and reduce the required sample size. J Clin Epidemiol 2011, 64(8):893–902. [DOI] [PubMed] [Google Scholar]
- 14.Benichou J: A review of adjusted estimators of attributable risk. Stat Methods Med Res 2001, 10(3):195–216. [DOI] [PubMed] [Google Scholar]
- 15.Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, Gattinoni L, van Haren F, Larsson A, McAuley DF et al. : Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA 2016, 315(8):788–800. [DOI] [PubMed] [Google Scholar]
- 16.Warren MA, Zhao Z, Koyama T, Bastarache JA, Shaver CM, Semler MW, Rice TW, Matthay MA, Calfee CS, Ware LB: Severity scoring of lung oedema on the chest radiograph is associated with clinical outcomes in ARDS. Thorax 2018, 73(9):840–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Torres LK, Hoffman KL, Oromendia C, Diaz I, Harrington JS, Schenck EJ, Price DR, Gomez-Escobar L, Higuera A, Vera MP et al. : Attributable mortality of acute respiratory distress syndrome: a systematic review, meta-analysis and survival analysis using targeted minimum loss-based estimation. Thorax 2021, 76(12):1176–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Auriemma CL, Zhuo H, Delucchi K, Deiss T, Liu T, Jauregui A, Ke S, Vessel K, Lippi M, Seeley E et al. : Acute respiratory distress syndrome-attributable mortality in critically ill patients with sepsis. Intensive Care Med 2020, 46(6):1222–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maddali MV, Churpek M, Pham T, Rezoagli E, Zhuo H, Zhao W, He J, Delucchi KL, Wang C, Wickersham N et al. : Validation and utility of ARDS subphenotypes identified by machine-learning models using clinical data: an observational, multicohort, retrospective analysis. The Lancet Respiratory Medicine 2022, 10(4):367–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pham T, Pesenti A, Bellani G, Rubenfeld G, Fan E, Bugedo G, Lorente JA, Fernandes ADV, Van Haren F, Bruhn A et al. : Outcome of acute hypoxaemic respiratory failure: insights from the LUNG SAFE Study. Eur Respir J 2021, 57(6). [DOI] [PubMed] [Google Scholar]
- 21.Matthay MA, Thompson BT, Ware LB: The Berlin definition of acute respiratory distress syndrome: should patients receiving high-flow nasal oxygen be included? The Lancet Respiratory medicine 2021, 9(8):933–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barrot L, Asfar P, Mauny F, Winiszewski H, Montini F, Badie J, Quenot J-P, Pili-Floury S, Bouhemad B, Louis G et al. : Liberal or Conservative Oxygen Therapy for Acute Respiratory Distress Syndrome. New England Journal of Medicine 2020, 382(11):999–1008. [DOI] [PubMed] [Google Scholar]
- 23.Bollen CW, van Well GT, Sherry T, Beale RJ, Shah S, Findlay G, Monchi M, Chiche JD, Weiler N, Uiterwaal CS et al. : High frequency oscillatory ventilation compared with conventional mechanical ventilation in adult respiratory distress syndrome: a randomized controlled trial [ISRCTN24242669]. Crit Care 2005, 9(4):R430–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brower RG, Lanken PN, MacIntyre N, Matthay MA, Morris A, Ancukiewicz M, Schoenfeld D, Thompson BT, National Heart L, Blood Institute ACTN: Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med 2004, 351(4):327–336. [DOI] [PubMed] [Google Scholar]
- 25.Combes A, Hajage D, Capellier G, Demoule A, Lavoué S, Guervilly C, Da Silva D, Zafrani L, Tirot P, Veber B et al. : Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome. New England Journal of Medicine 2018, 378(21):1965–1975. [DOI] [PubMed] [Google Scholar]
- 26.Constantin J-M, Jabaudon M, Lefrant J-Y, Jaber S, Quenot J-P, Langeron O, Ferrandière M, Grelon F, Seguin P, Ichai C et al. : Personalised mechanical ventilation tailored to lung morphology versus low positive end-expiratory pressure for patients with acute respiratory distress syndrome in France (the LIVE study): a multicentre, single-blind, randomised controlled trial. The Lancet Respiratory Medicine 2019, 7(10):870–880. [DOI] [PubMed] [Google Scholar]
- 27.Ferguson ND, Cook DJ, Guyatt GH, Mehta S, Hand L, Austin P, Zhou Q, Matte A, Walter SD, Lamontagne F et al. : High-frequency oscillation in early acute respiratory distress syndrome. N Engl J Med 2013, 368(9):795–805. [DOI] [PubMed] [Google Scholar]
- 28.Fernandez R, Trenchs X, Klamburg J, Castedo J, Serrano JM, Besso G, Tirapu JP, Santos A, Mas A, Parraga M et al. : Prone positioning in acute respiratory distress syndrome: a multicenter randomized clinical trial. Intensive Care Med 2008, 34(8):1487–1491. [DOI] [PubMed] [Google Scholar]
- 29.Gao Smith F, Perkins GD, Gates S, Young D, McAuley DF, Tunnicliffe W, Khan Z, Lamb SE: Effect of intravenous β−2 agonist treatment on clinical outcomes in acute respiratory distress syndrome (BALTI-2): a multicentre, randomised controlled trial. Lancet (London, England) 2012, 379(9812):229–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guerin C, Reignier J, Richard JC, Beuret P, Gacouin A, Boulain T, Mercier E, Badet M, Mercat A, Baudin O et al. : Prone positioning in severe acute respiratory distress syndrome. N Engl J Med 2013, 368(23):2159–2168. [DOI] [PubMed] [Google Scholar]
- 31.Kacmarek RM, Villar J, Sulemanji D, Montiel R, Ferrando C, Blanco J, Koh Y, Soler JA, Martínez D, Hernández M et al. : Open Lung Approach for the Acute Respiratory Distress Syndrome. Crit Care Med 2016, 44(1):32–42. [DOI] [PubMed] [Google Scholar]
- 32.Kesecioglu J, Beale R, Stewart TE, Findlay GP, Rouby JJ, Holzapfel L, Bruins P, Steenken EJ, Jeppesen OK, Lachmann B: Exogenous natural surfactant for treatment of acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med 2009, 180(10):989–994. [DOI] [PubMed] [Google Scholar]
- 33.Mancebo J, Fernández R, Blanch L, Rialp G, Gordo F, Ferrer M, Rodríguez F, Garro P, Ricart P, Vallverdú I et al. : A Multicenter Trial of Prolonged Prone Ventilation in Severe Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med 2006, 173(11):1233–1239. [DOI] [PubMed] [Google Scholar]
- 34.Meade MO, Cook DJ, Guyatt GH, Slutsky AS, Arabi YM, Cooper DJ, Davies AR, Hand LE, Zhou Q, Thabane L et al. : Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end-expiratory pressure for acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA 2008, 299(6):637–645. [DOI] [PubMed] [Google Scholar]
- 35.Mentzelopoulos SD, Malachias S, Zintzaras E, Kokkoris S, Zakynthinos E, Makris D, Magira E, Markaki V, Roussos C, Zakynthinos SG: Intermittent recruitment with high-frequency oscillation/tracheal gas insufflation in acute respiratory distress syndrome. Eur Respir J 2012, 39(3):635–647. [DOI] [PubMed] [Google Scholar]
- 36.Mercat A, Richard JC, Vielle B, Jaber S, Osman D, Diehl JL, Lefrant JY, Prat G, Richecoeur J, Nieszkowska A et al. : Positive end-expiratory pressure setting in adults with acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA 2008, 299(6):646–655. [DOI] [PubMed] [Google Scholar]
- 37.National Heart L, and Blood Institute PETAL Clinical Trials Network, Moss M, Huang DT, Brower RG, Ferguson ND, Ginde AA, Gong MN, Grissom CK, Gundel S, Hayden D, Hite RD, Hou PC, Hough CL, Iwashyna TJ, Khan A, Liu KD, Talmor D, Thompson BT, Ulysse CA, Yealy DM, Angus DC.: Early Neuromuscular Blockade in the Acute Respiratory Distress Syndrome. New England Journal of Medicine 2019, 380(21):1997–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Papazian L, Forel JM, Gacouin A, Penot-Ragon C, Perrin G, Loundou A, Jaber S, Arnal JM, Perez D, Seghboyan JM et al. : Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med 2010, 363(12):1107–1116. [DOI] [PubMed] [Google Scholar]
- 39.Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, Hibbert CL, Truesdale A, Clemens F, Cooper N et al. : Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet 2009, 374(9698):1351–1363. [DOI] [PubMed] [Google Scholar]
- 40.Richard C, Warszawski J, Anguel N, Deye N, Combes A, Barnoud D, Boulain T, Lefort Y, Fartoukh M, Baud F et al. : Early use of the pulmonary artery catheter and outcomes in patients with shock and acute respiratory distress syndrome: a randomized controlled trial. JAMA 2003, 290(20):2713–2720. [DOI] [PubMed] [Google Scholar]
- 41.Spragg RG, Taut FJ, Lewis JF, Schenk P, Ruppert C, Dean N, Krell K, Karabinis A, Gunther A: Recombinant surfactant protein C-based surfactant for patients with severe direct lung injury. Am J Respir Crit Care Med 2011, 183(8):1055–1061. [DOI] [PubMed] [Google Scholar]
- 42.Taccone P, Pesenti A, Latini R, Polli F, Vagginelli F, Mietto C, Caspani L, Raimondi F, Bordone G, Iapichino G et al. : Prone positioning in patients with moderate and severe acute respiratory distress syndrome: a randomized controlled trial. JAMA 2009, 302(18):1977–1984. [DOI] [PubMed] [Google Scholar]
- 43.Tongyoo S, Permpikul C, Mongkolpun W, Vattanavanit V, Udompanturak S, Kocak M, Meduri GU: Hydrocortisone treatment in early sepsis-associated acute respiratory distress syndrome: results of a randomized controlled trial. Crit Care 2016, 20(1):329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Truwit JD, Bernard GR, Steingrub J, Matthay MA, Liu KD, Albertson TE, Brower RG, Shanholtz C, Rock P, Douglas IS et al. : Rosuvastatin for sepsis-associated acute respiratory distress syndrome. N Engl J Med 2014, 370(23):2191–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Villar J, Ferrando C, Martínez D, Ambrós A, Muñoz T, Soler JA, Aguilar G, Alba F, González-Higueras E, Conesa LA et al. : Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. The Lancet Respiratory Medicine 2020, 8(3):267–276. [DOI] [PubMed] [Google Scholar]
- 46.Wheeler AP, Bernard GR, Thompson BT, Schoenfeld D, Wiedemann HP, deBoisblanc B, Connors AF Jr., Hite RD, Harabin AL, National Heart L et al. : Pulmonary-artery versus central venous catheter to guide treatment of acute lung injury. N Engl J Med 2006, 354(21):2213–2224. [DOI] [PubMed] [Google Scholar]
- 47.Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, deBoisblanc B, Connors AF Jr., Hite RD, Harabin AL, National Heart L et al. : Comparison of two fluid-management strategies in acute lung injury. N Engl J Med 2006, 354(24):2564–2575. [DOI] [PubMed] [Google Scholar]
- 48.Willson DF, Truwit JD, Conaway MR, Traul CS, Egan EE: The Adult Calfactant in Acute Respiratory Distress Syndrome Trial. Chest 2015, 148(2):356–364. [DOI] [PubMed] [Google Scholar]
- 49.Young D, Lamb SE, Shah S, MacKenzie I, Tunnicliffe W, Lall R, Rowan K, Cuthbertson BH, Group OS: High-frequency oscillation for acute respiratory distress syndrome. N Engl J Med 2013, 368(9):806–813. [DOI] [PubMed] [Google Scholar]
- 50.Dahlqwist E, Magnusson PKE, Pawitan Y, Sjolander A: On the relationship between the heritability and the attributable fraction. Hum Genet 2019, 138(4):425–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ho DE, Imai K, King G, Stuart EA: MatchIt: Nonparametric Preprocessing for Parametric Causal Inference. Journal of Statistical Software 2011, 42(8):1 – 28. [Google Scholar]
- 52.Wickham H, Averick M, Bryan J, Chang W, McGowan L, François R, Grolemund G, Hayes A, Henry L, Hester J et al. : Welcome to the Tidyverse. Journal of Open Source Software 2019, 4(43). [Google Scholar]
- 53.Ware LB, Matthay MA, Mebazaa A: Designing an ARDS trial for 2020 and beyond: focus on enrichment strategies. Intensive Care Med 2020, 46(12):2153–2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shankar-Hari M, Rubenfeld GD: Population enrichment for critical care trials: phenotypes and differential outcomes. Curr Opin Crit Care 2019, 25(5):489–497. [DOI] [PubMed] [Google Scholar]
- 55.Riviello ED, Kiviri W, Twagirumugabe T, Mueller A, Banner-Goodspeed VM, Officer L, Novack V, Mutumwinka M, Talmor DS, Fowler RA: Hospital Incidence and Outcomes of the Acute Respiratory Distress Syndrome Using the Kigali Modification of the Berlin Definition. Am J Respir Crit Care Med 2016, 193(1):52–59. [DOI] [PubMed] [Google Scholar]
- 56.Vercesi V, Pisani L, van Tongeren PSI, Lagrand WK, Leopold SJ, Huson MMA, Henwood PC, Walden A, Smit M, Riviello ED et al. : External confirmation and exploration of the Kigali modification for diagnosing moderate or severe ARDS. Intensive Care Med 2018, 44(4):523–524. [DOI] [PubMed] [Google Scholar]
- 57.Goligher EC, Tomlinson G, Hajage D, Wijeysundera DN, Fan E, Juni P, Brodie D, Slutsky AS, Combes A: Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome and Posterior Probability of Mortality Benefit in a Post Hoc Bayesian Analysis of a Randomized Clinical Trial. JAMA 2018, 320(21):2251–2259. [DOI] [PubMed] [Google Scholar]
- 58.Combes A, Hajage D, Capellier G, Demoule A, Lavoue S, Guervilly C, Da Silva D, Zafrani L, Tirot P, Veber B et al. : Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome. N Engl J Med 2018, 378(21):1965–1975. [DOI] [PubMed] [Google Scholar]
- 59.Rubenfeld GD, Caldwell E, Granton J, Hudson LD, Matthay MA: Interobserver variability in applying a radiographic definition for ARDS. Chest 1999, 116(5):1347–1353. [DOI] [PubMed] [Google Scholar]
- 60.Heijnen NFL, Hagens LA, Smit MR, Cremer OL, Ong DSY, van der Poll T, van Vught LA, Scicluna BP, Schnabel RM, van der Horst ICC et al. : Biological Subphenotypes of Acute Respiratory Distress Syndrome Show Prognostic Enrichment in Mechanically Ventilated Patients without Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med 2021, 203(12):1503–1511. [DOI] [PubMed] [Google Scholar]
- 61.Garcia-de-Acilu M, Marin-Corral J, Vazquez A, Ruano L, Magret M, Ferrer R, Masclans JR, Roca O: Hypoxemic Patients With Bilateral Infiltrates Treated With High-Flow Nasal Cannula Present a Similar Pattern of Biomarkers of Inflammation and Injury to Acute Respiratory Distress Syndrome Patients. Crit Care Med 2017, 45(11):1845–1853. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


