Keywords: blood-brain barrier, cognition, hippocampus, interneurons, long-term potentiation, microglial cell, neuroinflammation, spatial memory, temporal epilepsy, treadmill exercise
Abstract
Epilepsy frequently leads to cognitive dysfunction and approaches to treatment remain limited. Although regular exercise effectively improves learning and memory functions across multiple neurological diseases, its application in patients with epilepsy remains controversial. Here, we adopted a 14-day treadmill-exercise paradigm in a pilocarpine injection-induced mouse model of epilepsy. Cognitive assays confirmed the improvement of object and spatial memory after endurance training, and electrophysiological studies revealed the maintenance of hippocampal plasticity as a result of physical exercise. Investigations of the mechanisms underlying this effect revealed that exercise protected parvalbumin interneurons, probably via the suppression of neuroinflammation and improved integrity of blood-brain barrier. In summary, this work identified a previously unknown mechanism through which exercise improves cognitive rehabilitation in epilepsy.
Introduction
Epilepsy is one of the most common neurological diseases, affecting more than 70 million people worldwide (Fiest et al., 2017; Thijs et al., 2019). In addition to the repeated seizures and bouts of unconsciousness, epilepsy frequently leads to cognitive deficits, which severely affect patient quality of life (Sen et al., 2020). The major neurophysiological feature of epilepsy is the imbalance between excitation and inhibition across multiple brain regions, leading to hyperexcitability and hypersynchrony of the neuronal network in the brain (Devinsky et al., 2013; Qi et al., 2022). Neural circuit homeostasis is normally achieved via excitatory-inhibitory balance. In addition to excitatory glutamatergic neurons, parvalbumin (PV)-interneurons have also been reported to be linked with epilepsy pathogenesis (Jiang et al., 2016). Fast-spiking PV-interneurons are critical for the generation of gamma oscillations, which are needed for information processing across brain regions (Kann, 2016). In human brain slices, patients with temporal lobe epilepsy showed a decreased density of PV-positive chandelier cell boutons (Alhourani et al., 2020), supporting the hypothesis of PV-interneuron dysfunction in epilepsy.
Physical exercise is an effective means to reduce the long-term risk of psychiatric and neurological diseases (Chekroud et al., 2018; Liu et al., 2022; Cheng et al., 2023; Tan et al., 2023). However, its potential value in epilepsy treatment remains highly debated. In the past, clinicians have recommended that patients restrict their activity due to safety considerations (Pimentel et al., 2015). These suggestions may somehow neglect the nature of seizure activity under an exercise paradigm, and recent views tend to agree that moderate physical exercise can improve the mental status and efficacy of drug treatments (Cavalcante et al., 2021). When tempting to determine the mechanism through which exercise affects epilepsy treatment, several independent studies have highlighted the role of brain-derived neurotrophic factor (de Almeida et al., 2017, 2018; Lin et al., 2019); however, these studies paid little attention to interneurons. Interestingly, regular exercise has been shown to protect hippocampal PV-interneurons under different disease models (Rizzo et al., 2021; Wang et al., 2021a). We thus hypothesized that daily exercise can improve epilepsy-related cognitive deficits by modulating PV-interneuron activity.
To test this hypothesis, we generated a mouse model of temporal lobe epilepsy with acute injections of pilocarpine (Curia et al., 2008). Using this model, we tested the effect of a 2-week treadmill-exercise paradigm (1 hour daily) on cognitive function, specifically memory. We also investigated the neural substrate for any changes in memory capability, including long-term potentiation (LTP) of hippocampal CA1 neurons, microglial activation, neuroinflammation, and the integrity of the blood-brain barrier (BBB).
Methods
Experimental animals
All animals used in this experiment were purchased from Guangdong Yancheng Biological Co., Ltd. (Guangzhou, Guangdong, China, Animal License SCXK (Yue) 2019-0010). Because estrogen levels can affect seizure episodes in mice (Wang et al., 2021b), we selected 5- to 6-week-old male ICR mice with approximate body weights of 30 g for the experiment. During the experiment, we adopted a normal 12-hour dark/light (lights on at 8 a.m.) circadian cycle, a temperature of 22 ± 2°C, a relative humidity of 60 ± 10%, and ad libitum food and water. The experimental procedures were approved by the Experimental Animal Use Committee of Jinan University (approved on February 27, 2019 under approval No. 2019393). All experiments were designed and reported according to the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines (Percie du Sert et al., 2020).
Adult ICR mice were randomly divided into control and model groups. All epilepsy model mice received an injection of scopolamine (1 mg/kg, intraperitoneal, Sigma, St. Louis, MO, USA), followed after 30 minutes by an injection of pilocarpine (300 mg/kg, intraperitoneal, Wako, Osaka, Japan) to induce five bouts of acute epilepsy at Racine grade III or above. Lastly, we administered diazepam to terminate the acute status epilepticus (Racine, 1972). The control group was injected twice with an equal volume (0.1 mL) of normal saline at the matched time points. We subdivided the model mice into an epilepsy-only (Pilo) group and an epilepsy-with-exercise (Pilo+Ex) group. Two days after acute pilocarpine injection, mice in the Pilo+Ex group began to receive 1 hour of exercise (10 m/min) on the treadmill apparatus (Dyets, Wuxi, China) every day for 14 days. The treadmill velocity was set at 10 m/min, which can reach approximately 70–80% maximum cardiac load, and has been employed by most similar studies (Bhambhani et al., 1997). We also used a fourth group of mice (Pilo+PLX) whose normal mouse diet was changed to a special diet containing 0.12% PLX5622 (Sigma) on the second day after pilocarpine injection, and which lasted until day 14 after Pilo injection.
Evans blue assay
The animals in each group were injected with 2% Evans blue (Sigma) through their tail veins. One hour later, the mice were anesthetized by intraperitoneal injection of 10% pentobarbital sodium (100 mg/kg; Sigma). After saline perfusion, the brain tissue was weighed, fixed in formamide, homogenized with a sonicator (Sonics, Newtown, CT, USA), and incubated at 60°C for 24 hours. After centrifugation, the supernatant was measured for absorbance at 620 nm using a spectrophotometer (METASH, Shanghai, China). The standard curve was used to determine the content of Evans blue in the brain tissue.
Behavioral tests
Open-field test
The aim of the open-field test was to evaluate the general motor behavior and anxiety levels of the animal. On the 18th day after modeling, each test mouse was put into the corner of the arena inside a square box (50 cm × 50 cm × 40 cm). Then, the mouse was allowed to move freely for 5 minutes. A computerized video-tracking system (Ethovision XT; Noldus, Wageningen, the Netherlands) was connected to an infrared camera, and software calculated the total distance moved during the 5-minute period (Sturman et al., 2018).
Novel object recognition test
The novel object recognition test is commonly used for evaluating the working memory of the mouse. This assay was also performed on 18th day after modeling. In the learning phase, two identical objects (2× object A) were put into a 50 cm × 50 cm box, and the distance between the two objects was 25 cm. A mouse was put into the box in the center between the two objects and allowed to explore freely for 5 minutes while video was recorded throughout. The test phase began after 1 hour. In this phase, we replaced one object A with a new object (object B), which had the same texture and identical size as object A, but had different shapes. Because mice will spend more time exploring new objects (object B), the relative amount of time spent exploring the two objects can tell us whether or not a mouse recognizes (i.e., remembers) object A. In our test phase, we recorded video as mice explored the two objects for 5 minutes. A mouse was said to approach an object if they probed with their nose or mouth within 2–3 cm distance of the object (Lueptow, 2017). We counted the number of approaches to object A and object B, and calculated a recognition index (time spent exploring novel object B/total time in exploring object A and object B) as the preference toward the novel object during the test phase.
Y-maze test
The Y-maze assay is another commonly used behavioral test for spatial memory function. This assay was performed on the 18th day after modeling. Each arm of the Y-maze was 35 cm long and 10 cm wide, and the angle between each arm was 120°. One of the arms was randomly selected as the “start arm”, another was the “familiar arm”, and the last one was the “novel arm”. The novel arm had a shutter that blocked its entrance. Each mouse was first allowed to freely explore the start arm and the familiar for 10 minutes. After 1 hour, the same mouse was placed in the Y-maze for a 5-minute test phase in which the shutter of the novel arm was opened so that it could also be freely explored. We recorded the latency of the mouse to enter the novel arm and how long it stayed in that arm. Like the novel object recognition test, this test relies on the mouse’s tendency to explore the unfamiliar, provided they can remember the familiar (Kraeuter et al., 2019).
Enzyme-linked immunosorbent assay
On the 18th day after modeling, mice were anesthetized, and the hippocampus was isolated and rinsed three times with pre-cooled 1× phosphate buffer saline (PBS). The tissue was mixed with 200 μL protein extract (Radioimmunoprecipitation assay buffer: protease inhibitor: phosphatase inhibitor = 100: 1: 1) and was completely homogenized using a sonicator. Tissue lysates were then incubated on ice for 30 minutes and were centrifuged. The supernatant was quantified using the bicinchoninic acid kit (Beyotime, Shanghai, China) according to the protein standard curve. The concentration of cytokines, including tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6), was measured using enzyme-linked immunosorbent assay kits (J&L Biological, Shanghai, China) according to the manuals’ instructions.
Immunofluorescence staining
On the 18th day after modeling, mice were anesthetized and perfused with normal saline followed by 4% paraformaldehyde. The skull was opened to remove the whole brain tissue, which was put into 4% paraformaldehyde for 24 hours of fixation. Gradient (15% and 30%) sucrose solutions were used to dehydrate the brain, which was then sectioned into 40 μm slices with a sliding microtome (Leica, Wetzlar, Germany). For immunofluorescence staining, the hippocampal slices were rinsed in 1× PBS and incubated in a 500-μL blocking buffer for 2 hours. Brain slices were then transferred to the blocking solution containing primary antibody (PV, mouse, 1:1000, Sigma, Cat# P3088, RRID: AB_477329; NeuN, rabbit, 1:1000, Abcam, Cambridgeshire, UK, Cat# ab177487, RRID: AB_2532109; ionized calcium-binding adapter molecule 1 [Iba-1], rabbit, 1:1000, WAKO, Osaka, Japan, Cat# 019-19741, RRID: AB_839504; or CD68, rat, 1:1000, Abcam, Cat# ab53444, RRID: AB_869007) at 4°C overnight. Brain slices were then washed with 1× PBS and transferred to diluted secondary antibody (Donkey Anti-Rabbit [Alexa Fluor® 647], 1:1000, Abcam, Cat# ab150075, RRID: AB_2752244; Donkey Anti-Rabbit [Alexa Fluor® 555], 1:1000, Invitrogen, Carlsbad, CA, USA, Cat# A32794, RRID: AB_2762834; Donkey Anti-Mouse [Alexa Fluor® 555], 1:1000, Invitrogen, Cat# A31570, RRID: AB_2536180; Donkey Anti-Rat [Alexa Fluor® 488, or mCherry conjugated), 1:1000, Invitrogen, Cat# A21208, RRID: AB_2535794; Goat Anti-Rabbit [Alexa Fluor® 488, or mCherry conjugated), 1:1000, Abcam, Cat# ab150077, RRID: AB_2630356; Goat Anti-Rabbit [Alexa Fluor® 594, or GFP conjugated), 1:1000, Abcam, Cat# ab150080, RRID: AB_2650602; or Goat Anti-Mouse [Alexa Fluor® 488, or mCherry conjugated), 1:1000, Abcam, Cat# ab150113, RRID: AB_2576208) for 2 hours at room temperature. After washing, brain slices were spread on an adhesive glass slide, and mounted with 4′,6-diamidino-2-phenylindole-containing mounting medium (Thermo Fisher Scientific, Belmont, MA, USA). Finally, a fluorescent microscope (Zeiss, Batenfuburg Prefecture, Germany) was used for image acquisition and the analysis of cell density aided performed by ImageJ (NIH, Bethesda, MA, USA).
Morphological analysis of microglia
Morphological characteristics (synaptic length and number of intersections) of microglia in the hippocampus were evaluated by Sholl analysis. First, a single microglia skeleton was drawn using Neurolucida360 (MBF Bioscience, Williston, ND, USA) for each hippocampus image using a Zeiss LSM700 confocal laser microscope at 20× zoom, and then Sholl analysis was performed using Neurolucida Explorer (MBF Bioscience). Concentric circles were drawn with the cell bodies of microglia cells as the center, with a starting radius of 10 μm, increasing outward by 10 μm at a time.
Electrophysiological recording
On the 18th day after modeling, electrophysiological recording was performed. Artificial cerebrospinal fluid (aCSF; comprising 126 mM NaCl, 2.5 mM KCl, 1.25 mM NaH2PO4, 10 mM glucose, 26 mM NaHCO3, 2 mM CaCl2, and 2 mM MgCl2) was added with 5 μM γ-aminobutyric acid (GABA) during the perfusion. The aCSF was prepared in a 33°C water bath for 1 hour and was continuously perfused with oxygen. The slicing solution (comprising 85 mM NaCl, 75 mM sucrose, 2.5 mM KCl, 1.25 mM NaH2PO4, 25 mM glucose, 24 mM NaHCO3, 0.5 mM CaCl2, and 4 mM MgCl2) was prepared on ice and was bubbled with oxygen for 1 hour.
Mice were first anesthetized with isoflurane for 5 minutes (0.50%; RWD, Shenzhen, China) and quickly decapitated. The whole brain was immediately extracted and placed in a VibroTome (Leica) to prepare 400-μm thick coronal sections that included the hippocampus. The slices were quickly transferred to the aCSF, and after incubation for 1 hour, electrophysiological experiments were performed using the AXON patch-clamp system (Molecular Devices, San Jose, CA, USA). The paired-pulse ratio (PPR) was calculated as the ratio of evoked excitatory potentials between two consecutive stimuli. During LTP electrophysiological recording, the recording electrode was placed in the CA1 radiation layer (using aCSF as the internal buffer of the electrode). The stimulating electrode was placed to the left side of the recording electrode to stimulate the Schaeffer collateral, and the field excitatory postsynaptic potential (EPSP) was measured. Before measuring the baseline, input-output curves were drawn to determine the stimulus intensity up to half of the maximum field EPSP slope for subsequent experiments and LTP was recorded 10 minutes after high-frequency stimulation. All field EPSP slopes were measured between 30–60 minutes after recording had begun. The experimental results were processed with Clampfit 10.4 (Molecular Devices).
Quantitative polymerase chain reaction
On the 18th day after modeling, total RNA was extracted from hippocampal tissue using an RNA extraction kit, according to the manufacturer’s instructions. RNA purity was measured with a spectrophotometer (METASH, Shanghai, China). A total of 1 μg RNA was added to the in vitro reverse-transcription system (Sangon Biotech, Shanghai, China) to produce complementary DNA, and was stored at –20°C. A SYBR Green kit (Takara, Beijing, China) was used for the quantitative polymerase chain reaction (PCR), using the Gapdh gene as the internal reference. The relative gene expression was analyzed using the 2–ΔΔCt method (Arocho et al., 2006). Primer sequences were as follows: Claudin-5-forward, 5′-GCAAG GTGTA TGAAT CTGTG CT-3′; reverse, 5′-GTCAA GGTAA CAAAG AGTGC CA-3′; Gapdh-forward, 5′-AGGTC GGTGT GAACG GATTT G-3′; reverse, 5′-TGTAG ACCAT GTAGT TGAGG TCA-3′. The PCR parameters were as follows: 95°C × 30 seconds (95°C × 5 minutes, plus 60°C × 30 seconds); 40 cycles; ending with 95°C × 30 seconds, 60°C × 30 seconds, 95°C × 15 seconds. The expression level was normalized against Gapdh gene.
Statistical analysis
All results were processed using GraphPad Prism (version 8.0.0 for Windows, GraphPad Software, San Diego, CA, USA, www.graphpad.com). All data are presented as mean ± standard error of the mean (SEM). Student’s t-test was used for comparing two groups, and a one-way analysis of variance (ANOVA) was used to compare among three groups, followed by Tukey’s post hoc comparisons. For the analysis of two-factors, two-way ANOVA was performed in conjunction with Tukey’s post hoc comparisons. The significance level was set as α = 0.05.
Results
Treadmill exercise relieves memory deficits and improves hippocampal neural transmission in the epilepsy mouse model
Epilepsy is frequently associated with cognitive dysfunction (Braun, 2017), including poor memory. We investigated whether or not regular exercise helps improve memory impairments by making a mouse model of epilepsy, allowing the model mice to use an exercise treadmill for two weeks (1 per day; Figure 1A), and then evaluating their memory function. An open-field assay showed no change in total distance (Figure 1B) covered, suggesting intact locomotor activity after daily exercise. We used a novel object recognition test to test short-term object memory (Figure 1C). The recognition index showed that the Pilo group exhibited impaired object discrimination, while the Pilo+Ex group exhibited higher object recognition index compared to Pilo group (one-way ANOVA, P = 0.0142; Figure 1D). In a second assay employing the Y-maze (Figure 1E), we again saw evidence of impaired memory (longer latency to enter the novel arm and shorter durations exploring it) in the Pilo group and improvements (in terms of latency and preference for the novel arm) in the Pilo+Ex group (one-way ANOVA, P = 0.0008; Figure 1F; one-way ANOVA, P = 0.0002; Figure 1G). These results collectively suggest the retention of normal short-term memory function in the epileptic mice after regular physical exercise.
Figure 1.
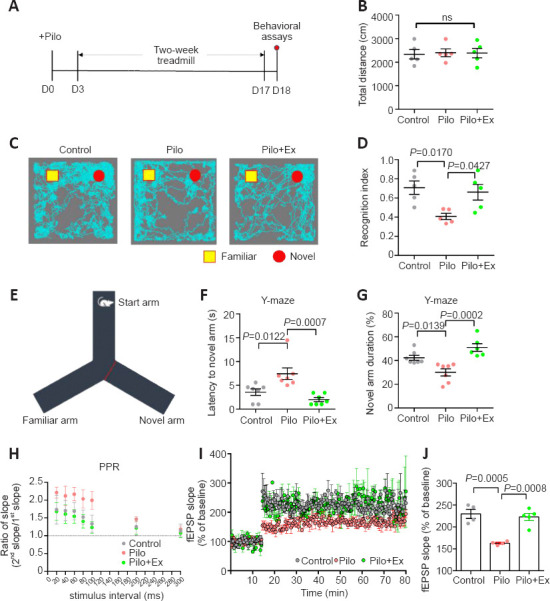
Physical exercise helps maintain memory function and synaptic plasticity in a muse model of epilepsy.
(A) Experimental schedules. (B) No significant change of total distance in the open field (one-way ANOVA followed by Tukey’s post hoc comparisons; F(2,12) = 0.03448, P = 0.9662). (C) Example animal movement paths in the novel object recognition task. (D) Pilocarpine reduced mouse preference toward the novel object, and exercise prevented this from happening (one-way ANOVA followed by Tukey’s post hoc comparisons; F(2,12) = 6.193, P = 0.0142). n = 5 mice in each group in B–D. (E) Schematic illustration of the Y-maze task. (F) The Pilo group showed longer latency to enter the novel arm, and exercise training decreased this latency (one-way ANOVA followed by Tukey’s post hoc comparisons; F(2,18) = 10.97, P = 0.0008). (G) The Pilo group displayed decreased preference for the novel arm, while this effect was absent in the Pilo+Ex group (one-way ANOVA followed by Tukey’s post hoc comparisons; F(2,18) = 14.08, P = 0.0002). n = 8 mice in each group in F and G. (H) Paired-pulse ratio was elevated in the Pilo group when stimulus intervals were short, and this hyperexcitability was missing after exercise in the Pilo+Ex group (two-way ANOVA with group factor; F(2,129) = 9.834, P = 0.0001). n = 10 neurons from three mice in each group. (I) Neurons in brain slices from the model group could not maintain LTP. Neurons in brain slices obtained from the Pilo+Ex group displayed normal LTP (two-way ANOVA with group factor; F(2,1471) = 289.1, P < 0.0001). (J) The fEPSP slope showed similar trends as it was abnormal in slices from the Pilo group and normal in slices from the Pilo+Ex group (one-way ANOVA followed by Tukey’s post hoc comparisons; F(2,10) = 20.41, P = 0.0003). n = 4 mice in each group in I and J. All data are presented as mean ± SEM. ANOVA: Analysis of variance; Ex: exercise; LTP: long-term potentiation; ns: no significant difference; Pilo: pilocarpine; PPR: paired-pulse ratio.
To find the neural substrate of these behavioral improvements, we focused on plasticity of hippocampal CA1 neurons because the CA1 is a critical region involved in memory-related cognition (Klausberger and Somogyi, 2008). We calculated PPRs to test the short-term plasticity of CA1 neurons, as the ratio of the second pulse to the first pulse usually correlates with LTP under a paired stimulation with a fixed interval paradigm (Kleschevnikov et al., 1997). In our assay, the PPR in Pilo group increased with shorter stimulation intervals (Figure 1H), suggesting elevated excitability in the epilepsy model. However, physical exercise decreased PPRs in the Pilo group (two-way ANOVA, P = 0.0001; Figure 1H). We also measured LTP in CA1 slices. Neurons in the CA1 of the Pilo group exhibited impaired LTP maintenance, while those from the Pilo+Ex group showed enhanced LTP strength obtained from control group CA1 neurons (Figure 1I and J). Therefore, regular exercise effectively improved hippocampal memory function to baseline levels.
Physical exercise relieves neuroinflammation and prevents PV-interneuron depopulation in the mouse model of epilepsy
Hippocampal synaptic plasticity is under tight control of PV-interneurons (Seo et al., 2021), which have been reported to be the phagocytotic target of microglial cells (Crapser et al., 2020). Since epilepsy frequently induces neuroinflammation and microglial activation (Jiang et al., 2021), we next investigated the potential involvement of microglial cells and PV-interneurons in the exercise effect. Immunofluorescence staining showed that the density of PV-interneurons in the dentate gyrus (DG) and CA1 regions of hippocampus were markedly lower in the Pilo group than in controls. In contrast, PV-interneuron density was increased in the Pilo+Ex group (Figure 2A–C). Moreover, we found that compared with controls, the density of microglial cells was higher in the Pilo group, and the microglial population was decreased in the Pilo+Ex group (Figure 2D and E).
Figure 2.
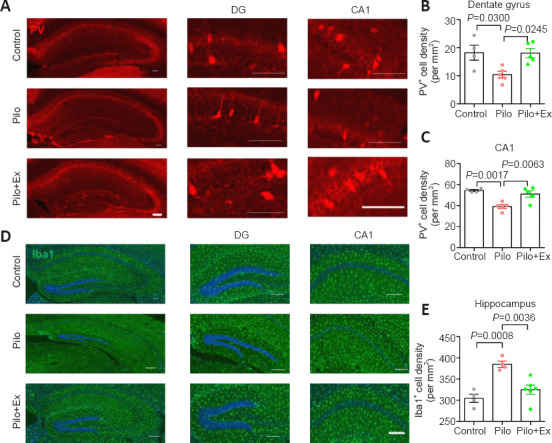
Exercise suppresses neuroinflammation and restores PV-interneurons in the mouse model of epilepsy.
(A) Immunofluorescence images of PV-interneurons (mCherry, red) in hippocampal DG and CA1. (B) PV-interneuron density in the DG was lower in the Pilo group than in controls, and higher in the Pilo+Ex group than in the Pilo group (one-way analysis of variance followed by Tukey’s post hoc comparisons, F(2,11) = 6.365, P = 0.0146). (C) PV-interneurons in the CA1 were also preserved after treadmill exercise (one-way ANOVA followed by Tukey’s post hoc comparisons; F(2,11) = 12.96, P = 0.0013). (D) Immunofluorescence staining of microglial cells by Iba-1 (green, Alexa Fluor® 594). Scale bars: 100 μm in A and D. (E) Microglial population was larger than normal after pilocarpine treatment and this effect was suppressed after exercise (one-way ANOVA followed by Tukey’s post hoc comparisons; F(2,11) = 14.93, P = 0.0007). All data are presented as mean ± SEM (n = 4, 5, and 5 mice in control, Pilo, and Pilo+Ex groups, respectively). ANOVA: Analysis of variance; DG: dentate gyrus; Ex: exercise; Iba-1: ionized calcium-binding adapter molecule 1; Pilo: pilocarpine; PV: parvalbumin.
The reactivity of microglial populations has been characterized by the presence of lysosomal protein CD68 (Bernal et al., 2002). We found that CD68 levels were elevated in Pilo group, and this was significantly alleviated in Pilo+Ex group (Figure 3A and B). We also performed a morphometric study of individual microglial cell dendrites (Figure 3C), and analysis showed decreased dendritic complexity in the model mice, as well as the improvement of cellular morphology in the model mice that were allowed to exercise (Figure 3D and E). The morphometric data and the lower levels of inflammation after exercise (indexed by levels of inflammatory cytokines: interleukin-6 and tumor necrotic factor-α; Figure 3F and G) converged to support the conclusion that neuroinflammation improved as a result of treadmill exercise.
Figure 3.
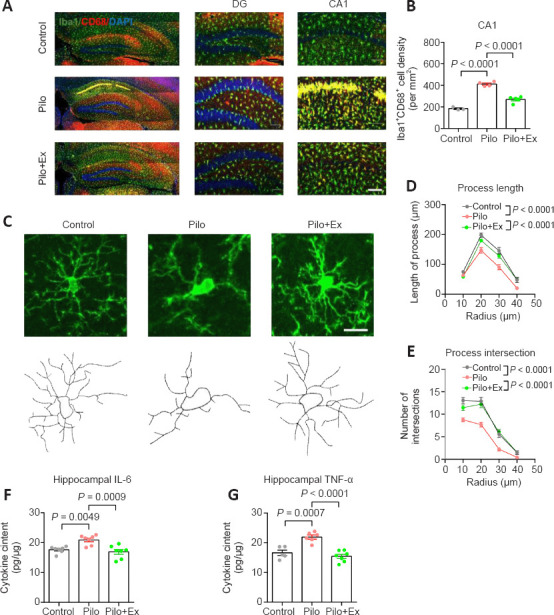
Activation of microglial cells and neuroinflammation in the mouse model of epilepsy with regular exercise.
(A) Immunofluorescence staining of microglial cells and their reactivity via Iba-1 (GFP, green) co-labeling with CD68 (mCherry, red). Scale bar: 100 μm. (B) The activated microglial population was significantly suppressed after regular exercise (one-way ANOVA followed by Tukey’s post hoc comparisons; F(2,9) = 87.29, P < 0.0001. n = 3, 4 and 5 mice in control, Pilo, and Pilo+Ex groups, respectively). (C) Upper panels, fluorescent images of representative microglial cells. Lower panels, reconstructions of cellular process contours. Scale bar: 20 μm. (D) The total microglia process length was smaller than normal in neurons from the Pilo group, and this effect was absent in neurons from the Pilo+Ex group (two-way ANOVA with respect to the interaction between group and radius factor; F(6,343) = 2.923, P = 0.0086). (E) Microglial process intersected fewer arbitrary concentric circles in neurons from the Pilo group, and those effects were absent from the Pilo+Ex group (two-way ANOVA with respect to the interaction effect; F(6,343) = 3.230, P = 0.0042). N = 20 cells from four mice in each group in D and E. (F) IL-6 concentration in the hippocampus was elevated in the Pilo group and not elevated in the Pilo+Ex group (one-way ANOVA followed by Tukey’s post hoc comparisons; F(2,18) = 11.24, P = 0.0007). (G) TNF-α levels in the Pilo+Ex groups were also lower than those in the Pilo group (one-way ANOVA followed by Tukey’s post hoc comparisons, F(2,18) = 21.96, P < 0.0001). n = 7 mice per group in F and G. All data are presented as mean ± SEM. ANOVA: Analysis of variance; DG: dentate gyrus; Ex: exercise; GFP: green fluorescent protein; Iba-1: ionized calcium-binding adapter molecule 1; IL-6 interleukin-6; Pilo: pilocarpine; TNF-α: tumor necrosis factor-α.
To further illustrate the role of neuroinflammation in the depopulation of PV-interneurons during epilepsy, we fed mice PLX5622, an inhibitor of colony-stimulating factor 1 receptor, to eliminate microglial cells (Walter and Crews, 2017). Two-week oral administration of PLX5622 effectively decreased the density of microglial cells in hippocampus (Figure 4A–C). As a consequence, PV-interneuron density was markedly elevated in Pilo group (Figure 4D; one-way ANOVA, P < 0.0001; Figure 4E; one-way ANOVA, P = 0.0007; Figure 4F), supporting the role of microglial cells in depleting the hippocampus of PV-interneurons. Moreover, behavioral assays showed that PLX5622 treatment improved memory performance in Pilo treated mice (one-way ANOVA, P = 0.1846; Figure 4G; one-way ANOVA, P = 0.0005; Figure 4H; one-way ANOVA, P = 0.0003; Figure 4I; one-way ANOVA, P < 0.0001; Figure 4J), highlighting the participation of neuroinflammation in the pathogenesis of epilepsy-associated memory deficits.
Figure 4.
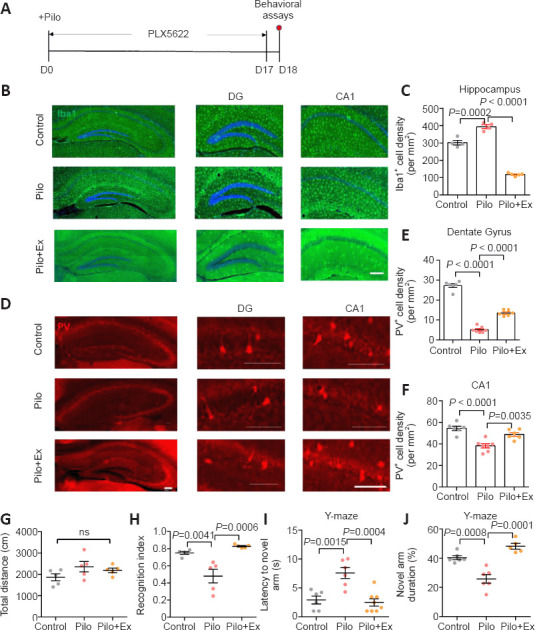
Neuroinflammation leads to PV-interneuron loss and memory deficits in the mouse model of epilepsy with regular exercise.
(A) Experimental schedules. (B) Immunofluorescence staining of microglial cells by Iba-1 (GFP, green). (C) The microglial population was largely eliminated by PLX5622 treatment (one-way ANOVA followed by Tukey’s post hoc comparisons; F(2,11) = 6.365, P = 0.0146). Immunofluorescence images of PV-interneurons (mCherry, red) in hippocampal DG and CA1. Scale bar: 100 μm in B and D. (E) PV-interneuron density in the DG was partially elevated in Pilo group by microglial ablation (one-way ANOVA followed by Tukey’s post hoc comparisons; F(2,16) = 252.9, P < 0.0001). (F) PV-interneurons in the CA1 were also preserved by PLX5622 treatment (one-way ANOVA followed by Tukey’s post hoc comparisons; F(2,16) = 18.52, P < 0.0001). N = 5, 7, and 6 mice in control, Pilo, and Pilo+PLX groups, respectively. (G) No significant change in total distance was observed in the open-field test (one-way ANOVA followed by Tukey’s post hoc comparisons; F(2,12) = 1.951, P = 0.1846). (H) Microglial ablation led to better memory of the familiar object (one-way ANOVA followed by Tukey’s post hoc comparisons; F(2,12) = 15.16, P = 0.0005). n = 5 mice in each group in G and H. (I) PLX5622 treatment led to shorter latency to enter the novel arm (one-way ANOVA followed by Tukey’s post hoc comparisons; F(2,17) = 13.97, P = 0.0003). (J) Preference for the novel arm was preserved by microglial ablation (one-way ANOVA followed by Tukey’s post hoc comparisons; F(2,17) = 26.13, P < 0.0001). n = 6, 6, and 8 mice in control, Pilo, and Pilo+PLX groups in I and J. All data are presented as mean ± SEM. ANOVA: Analysis of variance; DG: dentate gyrus; Ex: exercise; GFP: green fluorescent protein; Iba-1: ionized calcium-binding adapter molecule 1; Pilo: pilocarpine; PV: parvalbumin.
Exercise helps maintain the integrity of the BBB in the mouse model of epilepsy
Lastly, to further investigate the neuroinflammation mechanism in this epilepsy model, we examined the permeability of the BBB, whose leakage amplifies the severity of neuroinflammation (Takata et al., 2021). After intravenous injection of Evans blue dye, the Pilo group of mice consistently showed higher concentrations of dye in brain tissue than did the control group (Figure 5A and B), suggesting that the BBB is compromised in this epilepsy model. Conversely, exercise improved BBB function, as evidenced the lower amounts of dye in the brain (Figure 5C). Additionally, we further assessed the structural integrity of the BBB by quantifying expression levels of Claudin-5 (Cldn5), a critical component of the vascular barrier (Yang et al., 2021). We found that pilocarpine treatment significantly decreased Claudin-5 levels, and that either regular exercise or PLX5622 increased its expression levels (Figure 5D). PLX5622 treatment also reduced the reactivity of microglial cells, as evidenced by lower CD68 fluorescent-signal intensity (Figure 5E and F). In summary, our molecular, electrophysiological, and behavioral data converged to show that hippocampal plasticity and memory function improved in a mouse model of epilepsy after 2 weeks of regular exercise.
Figure 5.
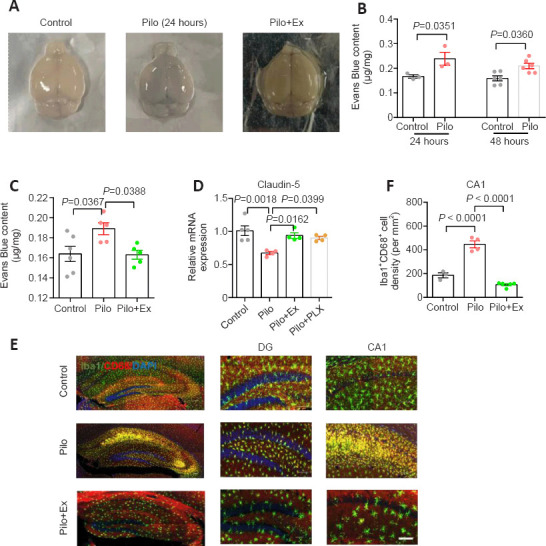
Exercise training maintained BBB integrity and relieved neuroinflammation in the mouse model of epilepsy.
(A) Sample images of the whole brain stained with Evans blue. (B) Increased concentration of Evans blue dye was observed at 24 and 48 hours after pilocarpine injection (Student’s t-test, n = 3 mice per group in 24 hours, and 6 mice per group in 48 hours). (C) Exercise training restored BBB function, as evidenced by less Evans blue leakage (one-way ANOVA followed by Tukey’s post hoc comparisons; F(2,13) = 5.132, P = 0.0028. n = 6, 5, and 5 mice in control, Pilo, and Pilo+Ex groups, respectively). (D) Expression of Cldn5 (Caludin-5) was lower than normal in the epilepsy model mice, and this trend was prevented by regular exercise or PLX5622 administration (one-way ANOVA followed by Tukey’s post hoc comparisons; F(3,13) = 8.133, P = 0.0026. n = 5, 4, 4, and 4 animals in control, Pilo, Pilo+Ex, and Pilo+PLX5 groups, respectively). (E) Immunofluorescence staining of microglial cells and their reactivity via Iba-1 (GFP, green) co-labeling with CD68 (mCherry, red). Scale bar: 100 μm. (F) The population of activated microglial was smaller in model mice after PLX5622 treatment than when only pilocarpine was administered (one-way ANOVA followed by Tukey’s post hoc comparisons; F(2,9) = 82.83, P < 0.0001. n = 3, 4, and 5 mice in control, Pilo and Pilo+PLX groups, respectively). All data are presented as mean ± SEM. ANOVA: Analysis of variance; BBB: blood-brain barrier; Ex: exercise; GFP: green fluorescent protein; Iba-1: ionized calcium-binding adapter molecule 1; Pilo: pilocarpine.
Discussion
Cognitive deficits are prominent symptoms in patients with epilepsy (Sen et al., 2020; Chen et al., 2023), while few effective drugs are currently available. Our work identified treadmill exercise as a potential intervention that protected normal learning and memory functions in our mouse model of epilepsy. Interrogation of neural mechanisms identified an unchanged PV-interneuron population as a key factor that allowed the retention of synaptic plasticity in the hippocampus.
For the treatment of epilepsy, currently available drugs primarily target specific ion channels to attenuate the hyperexcitation and hypersynchrony of neuronal network activity (Manford, 2017). However, drug-resistance is prevalent and largely impedes pharmacological treatments, thus calling for alternative treatment approaches (Thijs et al., 2019). Neurosurgery has been used for drug-resistant focal epilepsy, and neuromodulation or deep brain stimulation devices are also being adopted (Sen et al., 2020; Ryvlin et al., 2021). Beyond these invasive approaches that attempt to relieve refractory epilepsy, lifestyle interventions are worth further investigation. Here, we tested the effect of physical exercise on memory function using a mouse model of epilepsy. The positive effect of exercise intervention has been widely accepted across numerous psychiatric or neurological diseases. In patients with epilepsy, however, motor activity is frequently restricted due to safety concerns. Nevertheless, there are a growing number of clinical trials that have tested the effect of regular exercise in patients with epilepsy. In recent reports, physical exercise was shown to improve cognitive function (Popp et al., 2021), functional connectivity across brain regions (Koirala et al., 2017), and overall health conditions (Zhang et al., 2022). Our work thus supports these human cohort studies and supports the application of exercise rehabilitation in cases of epilepsy.
To investigate the potential neural substrate underlying this effect of exercise, we focused on modulation of inhibitory neurotransmission. After focal epilepsy emerges, PV-interneurons act as the “inhibitory barrier” that impedes its propagation (Cammarota et al., 2013). Increasing PV-interneuron depolarization could thus limit epileptiform activity (Călin et al., 2021). In fact, abnormal PV-interneurons activity has been identified as a factor in epilepsy pathogenesis. For example, the ablation of voltage-gated Cav2.1 Ca2+ channels in PV-interneurons impairs fast spiking and leads to generalized seizures (Rossignol et al., 2013). PV-positive chandelier cell boutons have been reported to be fewer than normal in human brain slices from patients with temporal lobe epilepsy (Alhourani et al., 2020), supporting the hypothesis that PV-interneurons are dysfunctional in epilepsy. As a result, studies have proposed a neuromodulatory approach that targets GABAergic transmission as a potential treatment for epilepsy. One study reported that the direct activation of GABAergic interneurons prevented motor seizures in a kainite-induced model of epilepsy (Jiang et al., 2018), and another study found that cannabidiol restored hippocampal PV-interneuron function in a model of temporal lobe epilepsy (Khan et al., 2018). In contrast to these other studies, our current study shows that regular exercise acts as a non-pharmaceutical, indirect treatment that achieves the same outcome; increased PV-interneuron inhibitory transmission. The relatively low cost, plus fewer adverse effects of physical exercise, make it a promising strategy for cognitive rehabilitation in patients with epilepsy.
In the current study, we found regular exercise effectively protected PV-interneurons in the DG, and led to normal LTP in CA1 neurons. Indeed, the anatomical and functional evidence connecting the DG and CA1 has been previously demonstrated as some GABAergic neurons in the DG have projections to the contralateral CA1, providing output for information processing (Buckmaster et al., 2002; Yuan et al., 2017). Therefore, the effect of regular exercise on PV-interneurons helped to reshape the activity of local hippocampal circuits, aiding in the improvement of memory disfunctions. Similar alleviation of epilepsy via hippocampal PV-interneurons has been recently supported by another study in which an optogenetic approach was developed to maintain PV-interneuron activity, which reduced the probability of epileptiform activity (Călin et al., 2021).
Interestingly, activated microglia seem to preferentially impair PV-interneuron function. In one of our ongoing studies, similar phenotypes have been replicated using a mouse model of neurodegenerative disease (data unpublished). Currently, two major hypotheses have been proposed. (i) The relatively high energy requirement (Ruden et al., 2021): Under normal physiological conditions, the fast-spiking feature of PV-interneurons confers a high energy demand (Kann et al., 2014), making them the primary target when cellular metabolism is disrupted, as it is by pilocarpine (Lee et al., 2012). (ii) The disruption of the extracellular matrix around PV-interneurons: Perineuronal nets usually surround PV-interneurons to help them maintain normal function (Burket et al., 2021). Pilocarpine is known to affect the extracellular matrix of PV-interneurons in the hippocampus (Brenneke et al., 2004), and thus it is possible that the epilepsy model compromises perineuronal nets, leading to PV-interneuron damage.
Epilepsy is associated with the activation of neuroinflammation, which aggravates neuronal damage via the complement pathway (Jiang et al., 2021). In a mouse model of audiogenic epilepsy, PV-interneurons showed dystrophy, in association with microglial activation plus elevated interleukin-6 and tumor necrotic factor-α levels (Yeung et al., 2018). Regular exercise was recently found to relieve neuroinflammation. For example, running exercise repressed hippocampal microglia proliferation in a mouse model of chronic stress (Xiao et al., 2021). Specifically, microglial phagocytosis can be modulated by regular training (Li et al., 2022). Because microglial cells eliminate interneurons from the brain network (Denizet et al., 2017; Crapser et al., 2020), we further proposed that exercise training would modulate microglial cell activation to protect the PV-interneuron population, and thus maintain normal hippocampal function.
The current study has certain limitations and weakness. Exercise involves the adaptive change of multiple organs throughout the body. Therefore, a peripheral-central pathway is likely to be needed for transducing the body exercise into a healthier brain. We did not perform related studies to explain why treadmill-exercise training helps to improve the BBB integrity. We can speculate that it involves certain cytokines and exerkines, which can be inspected by proteomics or metabolomics studies. Moreover, the potential role of peripheral-derived cytokines, in addition to brain-secreted molecules, is worth further investigation.
In summary, our work identified a previously unrecognized role of physical exercise in remodeling excitatory-inhibitory balance within the hippocampus of a mouse model of epilepsy. Regular exercise maintained the integrity of the BBB and suppressed neuroinflammation, contributing to the preservation of PV-interneurons and normal memory function. Our results provide empirical evidence that regular exercise can help to improve cognition in epilepsy patients.
Additional file: Open peer review report 1 (77.9KB, pdf) .
Footnotes
Funding: This study was supported by STI2030-Major Projects, No. 2022ZD0207600 (to LZ), the National Natural Science Foundation of China, Nos. 82171446 (to JY), U22A20301 (to KFS), 32070955 (to LZ), Guangdong Basic and Applied Basic Research Foundation, No. 2023B1515040015 (to LZ), and Science and Technology Program of Guangzhou of China, No. 202007030012 (to KFS and LZ).
Conflicts of interest: All authors declare no conflict of interests.
Data availability statement: All data supporting the findings of this article has been reported in the text and figures. For any further request of experimental data please contact the lead correspondence (Li Zhang, zhangli@jnu.edu.cn).
Open peer reviewer: Jonathan M. Borkum, University of Maine, USA.
P-Reviewer: Borkum JM; C-Editor: Zhao M; S-Editors: Yu J, Li CH; L-Editors: Yu J, Song LP; T-Editor: Jia Y
References
- 1.Alhourani A, Fish KN, Wozny TA, Sudhakar V, Hamilton RL, Richardson RM. GABA bouton subpopulations in the human dentate gyrus are differentially altered in mesial temporal lobe epilepsy. J Neurophysiol. 2020;123:392–406. doi: 10.1152/jn.00523.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arocho A, Chen B, Ladanyi M, Pan Q. Validation of the 2-DeltaDeltaCt calculation as an alternate method of data analysis for quantitative PCR of BCR-ABL P210 transcripts. Diagn Mol Pathol. 2006;15:56–61. doi: 10.1097/00019606-200603000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Bernal F, Graus F, Pifarré A, Saiz A, Benyahia B, Ribalta T. Immunohistochemical analysis of anti-Hu-associated paraneoplastic encephalomyelitis. Acta Neuropathol. 2002;103:509–515. doi: 10.1007/s00401-001-0498-0. [DOI] [PubMed] [Google Scholar]
- 4.Bhambhani Y, Buckley S, Maikala R. Physiological and biomechanical responses during treadmill walking with graded loads. Eur J Appl Physiol Occup Physiol. 1997;76:544–551. doi: 10.1007/s004210050288. [DOI] [PubMed] [Google Scholar]
- 5.Braun KP. Preventing cognitive impairment in children with epilepsy. Curr Opin Neurol. 2017;30:140–147. doi: 10.1097/WCO.0000000000000424. [DOI] [PubMed] [Google Scholar]
- 6.Brenneke F, Schachner M, Elger CE, Lie AA. Up-regulation of the extracellular matrix glycoprotein tenascin-R during axonal reorganization and astrogliosis in the adult rat hippocampus. Epilepsy Res. 2004;58:133–143. doi: 10.1016/j.eplepsyres.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Buckmaster PS, Yamawaki R, Zhang GF. Axon arbors and synaptic connections of a vulnerable population of interneurons in the dentate gyrus in vivo. J Comp Neurol. 2002;445:360–373. doi: 10.1002/cne.10183. [DOI] [PubMed] [Google Scholar]
- 8.Burket JA, Webb JD, Deutsch SI. Perineuronal nets and metal cation concentrations in the microenvironments of fast-spiking, parvalbumin-expressing GABAergic interneurons:relevance to neurodevelopment and neurodevelopmental disorders. Biomolecules. 2021;1235;11 doi: 10.3390/biom11081235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Călin A, Ilie AS, Akerman CJ. Disrupting epileptiform activity by preventing parvalbumin interneuron depolarization block. J Neurosci. 2021;41:9452–9465. doi: 10.1523/JNEUROSCI.1002-20.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cammarota M, Losi G, Chiavegato A, Zonta M, Carmignoto G. Fast spiking interneuron control of seizure propagation in a cortical slice model of focal epilepsy. J Physiol. 2013;591:807–822. doi: 10.1113/jphysiol.2012.238154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cavalcante BRR, Improta-Caria AC, Melo VH, De Sousa RAL. Exercise-linked consequences on epilepsy. Epilepsy Behav. 2021;121:108079. doi: 10.1016/j.yebeh.2021.108079. [DOI] [PubMed] [Google Scholar]
- 12.Chekroud SR, Gueorguieva R, Zheutlin AB, Paulus M, Krumholz HM, Krystal JH, Chekroud AM. Association between physical exercise and mental health in 1▪2 million individuals in the USA between 2011 and 2015: a cross-sectional study. Lancet Psychiatry. 2018;5:739–746. doi: 10.1016/S2215-0366(18)30227-X. [DOI] [PubMed] [Google Scholar]
- 13.Chen J, Wang Y, Chen C, Zhang Q, Wang S, Wang Y, Fang J, Wang Y. Activation of medial septum cholinergic neurons restores cognitive function in temporal lobe epilepsy. Neural Regen Res. 2023;18:2459–2465. doi: 10.4103/1673-5374.371369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng X, Mao GP, Hu WJ, Yu ZR, Xu YY, Chen W, Li X, Zeng XL, Zhang WW, Chen JW, Wan Y, Wang L. Exercise combined with administration of adipose-derived stem cells ameliorates neuropathic pain after spinal cord injury. Neural Regen Res. 2023;18:1841–1846. doi: 10.4103/1673-5374.361533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crapser JD, Spangenberg EE, Barahona RA, Arreola MA, Hohsfield LA, Green KN. Microglia facilitate loss of perineuronal nets in the Alzheimer's disease brain. EBioMedicine. 2020;58:102919. doi: 10.1016/j.ebiom.2020.102919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Curia G, Longo D, Biagini G, Jones RS, Avoli M. The pilocarpine model of temporal lobe epilepsy. J Neurosci Methods. 2008;172:143–157. doi: 10.1016/j.jneumeth.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Almeida AA, Gomes da Silva S, Lopim GM, Vannucci Campos D, Fernandes J, Cabral FR, Arida RM. Resistance exercise reduces seizure occurrence, attenuates memory deficits and restores BDNF signaling in rats with chronic epilepsy. Neurochem Res. 2017;42:1230–1239. doi: 10.1007/s11064-016-2165-9. [DOI] [PubMed] [Google Scholar]
- 18.de Almeida AA, Gomes da Silva S, Lopim GM, Vannucci Campos D, Fernandes J, Cabral FR, Arida RM. Physical exercise alters the activation of downstream proteins related to BDNF-TrkB signaling in male Wistar rats with epilepsy. J Neurosci Res. 2018;96:911–920. doi: 10.1002/jnr.24196. [DOI] [PubMed] [Google Scholar]
- 19.Denizet M, Cotter L, Lledo PM, Lazarini F. Sensory deprivation increases phagocytosis of adult-born neurons by activated microglia in the olfactory bulb. Brain Behav Immun. 2017;60:38–43. doi: 10.1016/j.bbi.2016.09.015. [DOI] [PubMed] [Google Scholar]
- 20.Devinsky O, Vezzani A, Najjar S, De Lanerolle NC, Rogawski MA. Glia and epilepsy:excitability and inflammation. Trends Neurosci. 2013;36:174–184. doi: 10.1016/j.tins.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 21.Fiest KM, Sauro KM, Wiebe S, Patten SB, Kwon CS, Dykeman J, Pringsheim T, Lorenzetti DL, Jetté N. Prevalence and incidence of epilepsy:A systematic review and meta-analysis of international studies. Neurology. 2017;88:296–303. doi: 10.1212/WNL.0000000000003509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang GT, Shao L, Kong S, Zeng ML, Cheng JJ, Chen TX, Han S, Yin J, Liu WH, He XH, Liu YM, Gongga L, Peng BW. Complement C3 aggravates post-epileptic neuronal injury via activation of TRPV1. Neurosci Bull. 2021;37:1427–1440. doi: 10.1007/s12264-021-00750-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang X, Lachance M, Rossignol E. Involvement of cortical fast-spiking parvalbumin-positive basket cells in epilepsy. Prog Brain Res. 2016;226:81–126. doi: 10.1016/bs.pbr.2016.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang X, Lupien-Meilleur A, Tazerart S, Lachance M, Samarova E, Araya R, Lacaille JC, Rossignol E. Remodeled cortical inhibition prevents motor seizures in generalized epilepsy. Ann Neurol. 2018;84:436–451. doi: 10.1002/ana.25301. [DOI] [PubMed] [Google Scholar]
- 25.Kann O. The interneuron energy hypothesis:Implications for brain disease. Neurobiol Dis. 2016;90:75–85. doi: 10.1016/j.nbd.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 26.Kann O, Papageorgiou IE, Draguhn A. Highly energized inhibitory interneurons are a central element for information processing in cortical networks. J Cereb Blood Flow Metab. 2014;34:1270–1282. doi: 10.1038/jcbfm.2014.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khan AA, Shekh-Ahmad T, Khalil A, Walker MC, Ali AB. Cannabidiol exerts antiepileptic effects by restoring hippocampal interneuron functions in a temporal lobe epilepsy model. Br J Pharmacol. 2018;175:2097–2115. doi: 10.1111/bph.14202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klausberger T, Somogyi P. Neuronal diversity and temporal dynamics:the unity of hippocampal circuit operations. Science. 2008;321:53–57. doi: 10.1126/science.1149381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kleschevnikov AM, Sokolov MV, Kuhnt U, Dawe GS, Stephenson JD, Voronin LL. Changes in paired-pulse facilitation correlate with induction of long-term potentiation in area CA1 of rat hippocampal slices. Neuroscience. 1997;76:829–843. doi: 10.1016/s0306-4522(96)00342-9. [DOI] [PubMed] [Google Scholar]
- 30.Koirala GR, Lee D, Eom S, Kim NY, Kim HD. Altered brain functional connectivity induced by physical exercise may improve neuropsychological functions in patients with benign epilepsy. Epilepsy Behav. 2017;76:126–132. doi: 10.1016/j.yebeh.2017.06.021. [DOI] [PubMed] [Google Scholar]
- 31.Kraeuter AK, Guest PC, Sarnyai Z. The Y-maze for assessment of spatial working and reference memory in mice. Methods Mol Biol. 2019;1916:105–111. doi: 10.1007/978-1-4939-8994-2_10. [DOI] [PubMed] [Google Scholar]
- 32.Lee EM, Park GY, Im KC, Kim ST, Woo CW, Chung JH, Kim KS, Kim JS, Shon YM, Kim YI, Kang JK. Changes in glucose metabolism and metabolites during the epileptogenic process in the lithium-pilocarpine model of epilepsy. Epilepsia. 2012;53:860–869. doi: 10.1111/j.1528-1167.2012.03432.x. [DOI] [PubMed] [Google Scholar]
- 33.Li C, Wang Y, Xing Y, Han J, Zhang Y, Zhang A, Hu J, Hua Y, Bai Y. Regulation of microglia phagocytosis and potential involvement of exercise. Front Cell Neurosci. 2022;16:953534. doi: 10.3389/fncel.2022.953534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin XY, Cui Y, Wang L, Chen W. Chronic exercise buffers the cognitive dysfunction and decreases the susceptibility to seizures in PTZ-treated rats. Epilepsy Behav. 2019;98:173–187. doi: 10.1016/j.yebeh.2019.07.032. [DOI] [PubMed] [Google Scholar]
- 35.Liu Y, Hu PP, Zhai S, Feng WX, Zhang R, Li Q, Marshall C, Xiao M, Wu T. Aquaporin 4 deficiency eliminates the beneficial effects of voluntary exercise in a mouse model of Alzheimer's disease. Neural Regen Res. 2022;17:2079–2088. doi: 10.4103/1673-5374.335169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lueptow LM. Novel object recognition test for the investigation of learning and memory in mice. J Vis Exp. 2017:55718. doi: 10.3791/55718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Manford M. Recent advances in epilepsy. J Neurol. 2017;264:1811–1824. doi: 10.1007/s00415-017-8394-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Percie du Sert N, Hurst V, Ahluwalia A, Alam S, Avey MT, Baker M, Browne WJ, Clark A, Cuthill IC, Dirnagl U, Emerson M, Garner P, Holgate ST, Howells DW, Karp NA, Lazic SE, Lidster K, MacCallum CJ, Macleod M, Pearl EJ, et al. The ARRIVE guidelines 2.0:Updated guidelines for reporting animal research. PLoS Biol. 2020;18:e3000410. doi: 10.1371/journal.pbio.3000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pimentel J, Tojal R, Morgado J. Epilepsy and physical exercise. Seizure. 2015;25:87–94. doi: 10.1016/j.seizure.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 40.Popp JL, Szaflarski JP, Kaur M, Martin RC, Brokamp GA, Terry DM, Diggs MD, Allendorfer JB. Relationships between cognitive function, seizure control, and self-reported leisure-time exercise in epilepsy. Epilepsy Behav. 2021;118:107900. doi: 10.1016/j.yebeh.2021.107900. [DOI] [PubMed] [Google Scholar]
- 41.Qi Y, Cheng H, Wang Y, Chen Z. Revealing the precise role of calretinin neurons in epilepsy:we are on the way. Neurosci Bull. 2022;38:209–222. doi: 10.1007/s12264-021-00753-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- 43.Rizzo FR, Guadalupi L, Sanna K, Vanni V, Fresegna D, De Vito F, Musella A, Caioli S, Balletta S, Bullitta S, Bruno A, Dolcetti E, Stampanoni Bassi M, Buttari F, Gilio L, Mandolesi G, Centonze D, Gentile A. Exercise protects from hippocampal inflammation and neurodegeneration in experimental autoimmune encephalomyelitis. Brain Behav Immun. 2021;98:13–27. doi: 10.1016/j.bbi.2021.08.212. [DOI] [PubMed] [Google Scholar]
- 44.Rossignol E, Kruglikov I, van den Maagdenberg AM, Rudy B, Fishell G. CaV 2.1 ablation in cortical interneurons selectively impairs fast-spiking basket cells and causes generalized seizures. Ann Neurol. 2013;74:209–222. doi: 10.1002/ana.23913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ruden JB, Dugan LL, Konradi C. Parvalbumin interneuron vulnerability and brain disorders. Neuropsychopharmacology. 2021;46:279–287. doi: 10.1038/s41386-020-0778-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ryvlin P, Rheims S, Hirsch LJ, Sokolov A, Jehi L. Neuromodulation in epilepsy:state-of-the-art approved therapies. Lancet Neurol. 2021;20:1038–1047. doi: 10.1016/S1474-4422(21)00300-8. [DOI] [PubMed] [Google Scholar]
- 47.Sen A, Jette N, Husain M, Sander JW. Epilepsy in older people. Lancet. 2020;395:735–748. doi: 10.1016/S0140-6736(19)33064-8. [DOI] [PubMed] [Google Scholar]
- 48.Seo HJ, Park JE, Choi SM, Kim T, Cho SH, Lee KH, Song WK, Song J, Jeong HS, Kim DH, Kim BC. Inhibitory neural network's impairments at hippocampal CA1 LTP in an aged transgenic mouse model of Alzheimer's disease. Int J Mol Sci. 2021;22:698. doi: 10.3390/ijms22020698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sturman O, Germain PL, Bohacek J. Exploratory rearing:a context- and stress-sensitive behavior recorded in the open-field test. Stress. 2018;21:443–452. doi: 10.1080/10253890.2018.1438405. [DOI] [PubMed] [Google Scholar]
- 50.Takata F, Nakagawa S, Matsumoto J, Dohgu S. Blood-brain barrier dysfunction amplifies the development of neuroinflammation:understanding of cellular events in brain microvascular endothelial cells for prevention and treatment of BBB dysfunction. Front Cell Neurosci. 2021;15:661838. doi: 10.3389/fncel.2021.661838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tan XF, Ding ZM, Guo CG, Sun P. Advances in exercise modulation of mitochondrial function to improve myocardial ischemia-reperfusion injury. Zhongguo Zuzhi Gongcheng Yanjiu. 2023;27:2242–2248. [Google Scholar]
- 52.Thijs RD, Surges R, O'Brien TJ, Sander JW. Epilepsy in adults. Lancet. 2019;393:689–701. doi: 10.1016/S0140-6736(18)32596-0. [DOI] [PubMed] [Google Scholar]
- 53.Walter TJ, Crews FT. Microglial depletion alters the brain neuroimmune response to acute binge ethanol withdrawal. J Neuroinflammation. 2017;14:86. doi: 10.1186/s12974-017-0856-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang J, Tang J, Liang X, Luo Y, Zhu P, Li Y, Xiao K, Jiang L, Yang H, Xie Y, Zhang L, Deng Y, Li J, Tang Y. Hippocampal PGC-1α-mediated positive effects on parvalbumin interneurons are required for the antidepressant effects of running exercise. Transl Psychiatry. 2021a;11:222. doi: 10.1038/s41398-021-01339-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang Z, Xie R, Yang X, Yin H, Li X, Liu T, Ma Y, Gao J, Zang Z, Ruan R, Li Y, Huang K, Chen Q, Shen K, Lv S, Zhang C, Yang H, Warner M, Gustafsson JA, Liu S, et al. Female mice lacking ERβdisplay excitatory/inhibitory synaptic imbalance to drive the pathogenesis of temporal lobe epilepsy. Theranostics. 2021b;11:6074–6089. doi: 10.7150/thno.56331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xiao K, Luo Y, Liang X, Tang J, Wang J, Xiao Q, Qi Y, Li Y, Zhu P, Yang H, Xie Y, Wu H, Tang Y. Beneficial effects of running exercise on hippocampal microglia and neuroinflammation in chronic unpredictable stress-induced depression model rats. Transl Psychiatry. 2021;11:461. doi: 10.1038/s41398-021-01571-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang Z, Lin P, Chen B, Zhang X, Xiao W, Wu S, Huang C, Feng D, Zhang W, Zhang J. Autophagy alleviates hypoxia-induced blood-brain barrier injury via regulation of CLDN5 (claudin 5) Autophagy. 2021;17:3048–3067. doi: 10.1080/15548627.2020.1851897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yeung RK, Xiang ZH, Tsang SY, Li R, Ho TYC, Li Q, Hui CK, Sham PC, Qiao MQ, Xue H. Gabrb2-knockout mice displayed schizophrenia-like and comorbid phenotypes with interneuron-astrocyte-microglia dysregulation. Transl Psychiatry. 2018;8:128. doi: 10.1038/s41398-018-0176-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yuan M, Meyer T, Benkowitz C, Savanthrapadian S, Ansel-Bollepalli L, Foggetti A, Wulff P, Alcami P, Elgueta C, Bartos M. Somatostatin-positive interneurons in the dentate gyrus of mice provide local- and long-range septal synaptic inhibition. Elife. 2017;6:e21105. doi: 10.7554/eLife.21105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang CQ, Li HY, Wan Y, Bai XY, Gan L, Sun HB. Effect of different physical activity training methods on epilepsy:A protocol for systematic review and meta-analysis. Medicine (Baltimore) 2022;101:e29085. doi: 10.1097/MD.0000000000029085. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


