Abstract
Local ischemia often causes a series of inflammatory reactions when both brain immune cells and the peripheral immune response are activated. In the human body, the gut and lung are regarded as the key reactional targets that are initiated by brain ischemic attacks. Mucosal microorganisms play an important role in immune regulation and metabolism and affect blood-brain barrier permeability. In addition to the relationship between peripheral organs and central areas and the intestine and lung also interact among each other. Here, we review the molecular and cellular immune mechanisms involved in the pathways of inflammation across the gut-brain axis and lung-brain axis. We found that abnormal intestinal flora, the intestinal microenvironment, lung infection, chronic diseases, and mechanical ventilation can worsen the outcome of ischemic stroke. This review also introduces the influence of the brain on the gut and lungs after stroke, highlighting the bidirectional feedback effect among the gut, lungs, and brain.
Keywords: enteric glia cells, gut microbiota, gut-brain axis, immune response, inflammation, ischemic stroke, lung-brain axis, microglia
Introduction
Ischemic stroke is an acute cerebrovascular disease caused by cerebral artery occlusion. Although this disease has a great impact on public health, its exact etiological and pathological process are not directed and precise (Pluta et al., 2021). Currently, researchers believe that a complex series of neuropathological events such as excitotoxicity, oxidative stress, neuroinflammation, apoptosis, immune response, acidosis, and mitochondrial damage result in brain damage in ischemic stroke (Wang et al., 2021a, 2022b; Zhu et al., 2022; Zhu and Wan, 2023). Severe changes in local circulation lead to the accumulation of cell debris, and extensive infiltration of immune cells in the brain tissue also induces the secondary progression of cell injury. However, there is no single answer to the mechanisms of ischemic stroke, because of which there is considerable ambiguity regarding the direction of stroke treatment (Xiong et al., 2018). Despite alternative treatments such as thrombus removal to allow tissue reperfusion, the limited treatment window and inadequate response lead to the poor prognosis of many patients.
Ischemia of the local brain tissue has been regarded to cause significant inflammation and has been studied in the past (Cuenca-López et al., 2010). Rather than concentrating on restricted inflammation alone, recent studies are now carrying out systematic level analyses, because of increasing concerns about the relationship between the central nervous system (CNS) and the enteric nervous system. Once inflammation occurs in the human body, the whole immune system of the relative tissue of the organism undergoes activation and rapidly participates in the inflammatory immune response.
From the perspective of the brain, resident glial cells act as the main immune defense by eliminating injured or necrotic parts (Singh et al., 2016), while the intestinal tract and lung also assist the process via additional pathways, by translocation of immune cells and changing the central response through some metabolites (Wang et al., 2021b). This notion follows the popular concept of the gut-brain axis and lung-brain axis found by scholars. Therefore, this review attempts to rationalize the basic understanding of the immune mechanism of ischemic stroke and summarize the detailed role of the gut-brain axis and lung-brain axis in the inflammatory-immune response.
Search Strategy
The articles used in this review were retrieved though an electronic search of the PubMed database from 2013 to 2023. The search was performed using the following key terms: Ischemic Stroke (MeSH Terms) AND ((Brain-Gut axis (MeSH Terms) OR lung (All fields)). To achieve a comprehensive search, we did not restrict the search terms for inflammation or immunity. We expanded the search range by changing the words ischemic stroke to brain (All fields) or nervous system (All fields). The results were further screened based on the title and abstract, which contained immune response or inflammation-related information.
The Immune Mechanism of Ischemic Stroke
Targeting the immune system
Recently, a series of relatively completed studies and reviews have highlighted the pathological process of cerebral ischemia with respect to the immune system (Gerganova et al., 2022; Wang and van de Pavert, 2022; Wicks et al., 2022). The core issues addressed are increased permeability of the blood-brain barrier (BBB) and subsequent cytoplasmic and nuclear events (Di Napoli et al., 2011). The brief problems caused by these two events include the production of inflammatory mediators, activation and infiltration of brain-resident and peripheral immune cells, and the release of damage-associated molecular patterns (DAMPs) by dying neurons, which contribute to the formation of an inflammatory environment (Zeng et al., 2022). The above-mentioned inflammatory cascades are always activated immediately after the pathological process of ischemic stroke and present for several days or weeks. To better understand the role of the gut-brain axis, we briefly emphasize on the different characteristics of each immune phase in ischemic stroke (Figure 1).
Figure 1.
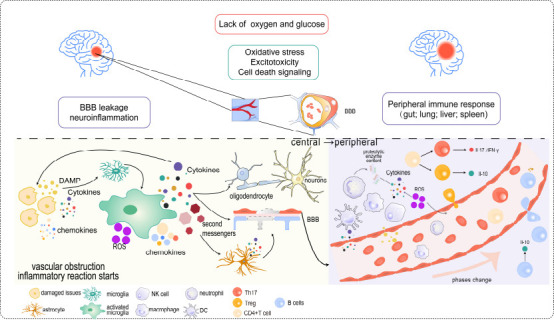
BBB leakage, neuroinflammation, and related peripheral signaling pathways of immune response involved in ischemic stroke.
Because of lacking oxygen and glucose, the brain initiates a series of reactions such as oxidative stress. These reactions will cause BBB leakage and subsequent neuroinflammation. DAMPs released by damaged issues will promote the activation of microglia and stimulate them to release various elements such as cytokines, chemokines, second messengers, and ROS. Such materials have different effects at different times when triggering the peripheral immune cells to respond. Created with Adobe Illustrator 2019. BBB: Blood-brain barrier; DAMP: damage-associated molecular patterns; IFN-γ: interferon-γ; IL-17: interleukin-17; IL-10: interleukin-10; ROS: reactive oxygen species.
Innate immune response
DAMP, brain antigens, microglia and astrocytes initiate immune response by recognizing dsDNA and oxidative stress as the cytokines (Xu et al., 2019; DeLong et al., 2022). Normally, microglia dynamically supervise changes in brain homeostasis and can be activated to eliminate cellular debris and release abundant cytokines, neurotransmitters, or other molecules to save both neurons and synapse functions (Borst et al., 2021). Previous studies have fully distinguished the roles between M1 and M2 phenotypes, and suggested that M2 microglia are the earliest call type to be activated when the stroke occurs (Sumbria et al., 2012; Li et al., 2018; Umahara et al., 2018). While some studies have confirmed the proinflammatory role of M1 microglia at the beginning of ischemic stroke, it should be made clear that such discrepancy probably depends on the primary ischemia condition, reperfusion time, lesion location, and research time (Neher et al., 2013; Alawieh et al., 2018). Additionally, another essential role of microglia is the interaction with endothelial cells, astrocytes, and oligodendrocytes (Xu et al., 2020). Activated microglia will induce A1-reactive astrocytes in vitro and in vivo by releasing interleukin (IL)-1α, tumor necrosis factor-α (TNF-α), and complement component subunit 1q (Liddelow and Barres, 2017). This study also showed that astrocytes reduce brain damage and downregulate neuroinflammation, as it is vitally important to reconstruct the BBB and maintain the stability of central neurons (Pekny et al., 2016). Other studies have reported that astrocytes interact with microglia by secreting chemokines to stimulate the microglia and participate in its phenotype alteration and later ischemic inflammation (Inose et al., 2015).
The subacute phase of ischemic stroke is more complicated than innate one. Damaged neurons release IL-4, and via interferon regulatory factor 4 signaling, myeloid cells recruited from the periphery in the acute period transition from a naive condition to anti-inflammatory state (Zhao et al., 2015, 2017). However, a few days after the initial injury, some neurons will also promote the production of proinflammatory monocyte-derived macrophages and then activate proapoptotic signals by releasing ATP and Fas ligands (Meng et al., 2016). In particular, microglia can directly or indirectly damage neural structures in the chronic phase by interacting with various adhesion molecules that migrate to the ischemic injury site including the immunoglobulin superfamily (e.g., intercellular cell adhesion molecule-1) and inflammatory cytokines produced by white blood cells (Murray et al., 2013; Gülke et al., 2018). Furthermore, astrocytes will promote or hinder functional rehabilitation and regeneration of neuronal axons (Xu et al., 2020).
Adaptive immune response
Some brain-derived antigens such as S100b belong to the mutagenic family and are so named because of the solubility in 100% saturated solution of ammonium sulfate at neutral pH. S100b can stimulate the production of T and B cells to increase the amount of proinflammatory factors in acute ischemic stroke (Choi et al., 2021). Additionally, T cells infiltrate the brain 3–4 days after infiltration by monocytes and neutrophils; in rodent models of stroke, regulatory T cells (Tregs) accumulate within 24 hours (Yilmaz et al., 2006). When facing new antigens such as myelin basic protein, sensitized circulating T cells will mainly participate in the response, and different kinds of T cells will play distinct roles (Bornstein et al., 2001). For instance, Th1 plays a vital role in ischemic stroke by releasing pro-inflammatory cytokines (IL-2, IL-12, TNF-α). While Th17 secretes IL-17 to promote inflammation, Th2 can secrete IL-4, IL-5, IL-10, and IL-13 to play an important role in neuro-protection (Wu et al., 2021). By contrast, Tregs secrete IL-10 to hinder the production of pro-inflammatory cytokines and have profound influences on inflammation resolution and stroke recovery (Wu et al., 2021).
Similar to T cells, B cells also migrate to the brain after ischemic stroke. A deficiency of B cells is known to worsen functional outcomes and histological damage, especially after transient cerebral ischemia (Ren et al., 2011). A study even showed that a lack of B cells led to an increase in activated microglial neutrophils, macrophages, and T cells to further facilitate the proinflammatory state (Ren et al., 2011). One beneficial function of B cells is the release of IL-10. However, further studies are needed regarding the role of B cells, particularly in the different phases of ischemia. As B cells change, they alter the T-cell response in stroke recovery. Additionally, DAMP-mediated activation of Toll-like receptors (TLRs) also stimulates B cells by increasing the expression of costimulatory molecules to further promote activation and autoimmunity (Zera and Buckwalter, 2020).
Notably microglia also engaged in the promotion of T-cell differentiation, and distinct M1/M2 microglia will lead to opposite effects, which have been well documented in previous reviews (Coughlan et al., 2005; Park et al., 2006). Astrocytes also have tight interactions with T cells, and they can secrete IL-15, which increases the concentration of CD8+ T cells and natural killer (NK) cells to worsen brain injury (Lee et al., 2018b). In addition to IL-15, astrocytes also increase the levels of IL-17 (Ito et al., 2019), IL-33, and chemokines with the CC motif 1 (CCL1). IL-33 is thought to promote the proliferation of Tregs, and CCL1 levels increase when combined with chemokines with the CC motif 8 Tregs that secrete a vascular endothelial-derived growth factor ligand in the chronic phase of stroke. Tregs also regulate IL-6 and the signal transducer of activators of transcription pathways and ameliorates neurological deficits (Ito et al., 2019).
Extra immune cells
With knowledge of the roles of the common brain resident glia and peripheral innate and adaptive immune cells (DeLong et al., 2022), a recent study are focusing on the innate lymphoid cell (ILC) family that have been shown to migrate towards ischemic stroke lesions and elicit protective and recovery functions (Wang et al., 2023). This family includes the NK cells and ILC type 1, 2, and 3 cells, and the study speculated that through BBB, C-X-C motif chemokine12-C-X-C chemokine receptor type 4 axis expressed by ischemia brain will mainly recruit NK and ILC1s to move to stroke region and improve experiment models’ motor movement (Wang et al., 2023). By contrast, IL-17A secreted by ILC will promote lesion enlargement, and a study revealed that IL-10 will restrict that signaling (Piepke et al., 2021).
Another new study reported that CD8+CD122+CD49dlo T regulatory-like cells (CD8+ TRLs) and its function of earliest infiltration in the animal brain to weaken inflammation after ischemic stroke (Cai et al., 2022); furthermore, its primary role is to suppress the proliferation of effector T lymphocytes and proinflammatory effects to maintain the immune homeostasis. This study also explored the function of CD8+ TRLs, which is implemented by releasing IL-10 and research discovered a material named epidermal growth factor-like transforming growth factor will assist the function of IL-10 (Cai et al., 2022).
Immune mechanism through the gut-brain axis
In current years, scientists have found that the gut-brain axis displays a vital link between the gut and cerebral ischemia (Pluta et al., 2021). Previously, researchers found that the brain, which has neural circuits, neurotransmitters, and receptors, can influence the movement, secretion, and immune function of the intestinal tract, and in stroke or transient brain ischemia patients, there is considerable dysbiosis of the gut microbiota. Remarkably, after a stroke, up to 50% of patients will experience gastrointestinal complications, including constipation, gastrointestinal bleeding, fecal incontinence, and dysphagia, which are associated with poor stroke prognosis (Camara-Lemarroy et al., 2014).
From the immune aspect, the intestinal tract is rich in immune cells that accounts for more than 70–80% of the whole-body related immune system (Li et al., 2019). Researchers are interested in multiple functions of gut microbes such as regulating brain development, immune regulation, antagonism, anticancer activity, and physiological nutrition (Cerf-Bensussan and Gaboriau-Routhiau, 2010; Human Microbiome Project Consortium, 2012). Specific to the gut-brain axis, studies have focused on the following mechanisms: the immune system, the gut microbiota metabolism pathway, the intestinal mucosal barrier and BBB, the neuroanatomical pathway, and the hypothalamic-pituitary-adrenal (HPA) axis neuroendocrine pathway (Wang et al., 2022a). Notably, all these pathways could interact and commonly act from an immune point of view, so deciphering the basic mechanisms of inflammatory signaling along the gut-brain axis is critical to our understanding of neuroimmune communication and should aid the development of immunomodulatory therapies for gastrointestinal and neurological diseases (Figure 2).
Figure 2.
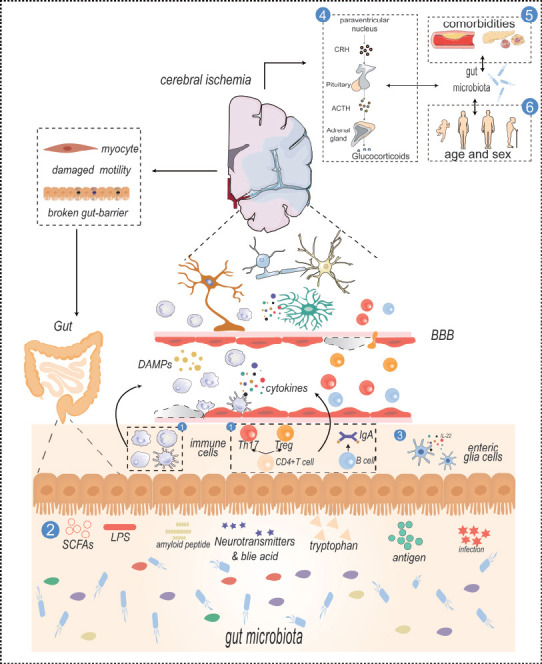
The gut-brain axis in ischemic stroke.
① Immune cells including NK cells, neutrophils, and adaptive immune cells stimulated by cytokines or brain-derived antigens will circulate in the blood and cross the BBB to take part in neuroinflammation. ② Gut metabolites, except LPS which is a membrane component of gut microbiota, SCFAs, and tryptophan are generated by specific gut microbiomes that contribute to various effects like the activation of astrocytes or microglia. ③ Gut enteric cells release IL-22 to form a bidirectional communication and maintain the gut barrier integrity. ④ HPA axis plays an important role in ischemic stroke. Gut microbiota can affect the release of hormones, and hormones can also affect the composition of gut microbiota. ⑤ and ⑥ Comorbidities, age, sex, plaque formation in atherosclerosis, and insulin resistance in diabetes are related to gut microbiota, and the composition of gut microbiota is also different in different ages and sex. These factors are related to the onset of ischemic stroke. The prominent influences of interfering gut myocytes and gut barrier caused by the brain are drawn on the left. Created with Adobe Illustrator 2019. ACTH: Adrenocorticotropic hormone; BBB: blood-brain barrier; CRH: corticotropin-releasing hormone; DAMP: damage-associated molecular pattern; HPA: hypothalamic-pituitary-adrenal; IgA: immunoglobulin A; IL-22: interleukin-22; LPS: lipopolysaccharide; NK cells: natural killer cells; SCFA: short-chain fatty acid; Th17: T helper type 17 cell; Treg: regulatory T cell.
Immune system
In the intestinal tract, the first-line of immune cells are the macrophages, neutrophils, dendritic cells (DCs), NK cells, and ILCs (types 1–3). While the kinds of adaptive immune cells depend on DC-dependent antigen presentation, some T and B cells also migrate to the brain through these processes (Rojas et al., 2019). The activation and action of all these cells are secondary to the stimulation of the central inflammatory response in ischemic stroke. From the perspective of the immune system, the gut microbiota plays a great role in the maturation of the intestinal mucosal immune system by promoting the formation of gut-associated lymphoid tissue, IgA-producing B cells, T-helper (Th) 17 cells, and innate lymphoid cells (Camara-Lemarroy et al., 2020; Pröbstel et al., 2020). The acute response of the intestinal tract after stroke onset and the subsequent chronic process are described in the following sections:
Acute progressive stage
Under ischemic stroke, the rapidly progressive inflammatory response triggers the peripheral immune response, and the intermediate bridge involves the damaged tissue, brain glial cells (especially microglia), and a series of cytokines, DAMPs, and chemokines released by them. Studies have found that a subset of meningeal NK cells in the gut produce interferon (IFN)-γ, and these cells stimulate brain astrocytes that express lysosomal protein LAMP1 and the death receptor ligand TRAIL (Giovannoni and Quintana, 2020; Sanmarco et al., 2021), along with circulating immune cells moving to the damaged area and upregulating and activating adhesion molecule family-activated interactions between lymphocytes, leukocytes, endothelial cells, and platelets. Neutrophils, as the most important leukocytes, produce inflammatory cytokines such as IL-1β, IFN-γ, IL-17, matrix metalloproteinase-9, and C-C motif chemokine 2 (Qin et al., 2022). They can also generate ROS, initiate the complement system, and mediate their high proteolytic activity to cause direct or indirect damage to the neural structures (Nathan, 2006). The whole process is inseparable from the role of the gut microbiome. Recent studies have suggested that gut microbes have the following influence. First, they change circulating cytokines and cause brain inflammatory reactions. Many studies have shown that both the intestinal microbiota and mucosal cells can regulate the activation of immune molecules that affect the CNS, including the proinflammatory factors IL-8 and IL-1 and the anti-inflammatory factors IL-10 and TGF-β (Shi et al., 2017; Kayama et al., 2020). Second, they shape the maturation and homeostasis of the microglia, which is important for the regulation of CNS development, such as myelination and neurogenesis in the adult brain and maintenance, and play an immune surveillance role in the brain by participating in information transmission and clearing cellular debris (Abdel-Haq et al., 2019). When microglia are lacking, their function in the phagocytosis of amyloid and tau protein that accumulates after ischemia is restricted (Endres et al., 2022). Third, as an important component of gram-negative microbes, lipopolysaccharide (LPS) combines with lipopolysaccharide or other microbial-related molecules with Toll-like receptors, which are one of the intestinal mucosal pattern recognition receptors (Siebler et al., 2008). This process activates some immune cells such as DCs, neutrophils, and macrophages, and then produces proinflammatory cytokines like IL-1a, IL-1b, TNF-a, and IL-6, to cross the BBB and affect brain function (Camara-Lemarroy et al., 2020). These conditions will further influence the outcome of the brain if it experiences an ischemic attack. Fourth, under the influence of the gut microbiota, some immune cells in the intestinal tract will migrate to the brain and play reverse roles to exacerbate inflammatory outcomes (Wastyk et al., 2021).
Chronic phase
Apart from brain-resident immune cells, there is still a wide repertoire of leukocytes in the meningeal layers such as T cells, B cells, and type 2 innate lymphoid cells (Alves de Lima et al., 2020). Although the core function of meningeal immune cells has not been clarified, gut microbiota have been shown to influence the migration of these cells to the brain. An earlier study showed that these cells participate in response to injury or infection. Meningeal T cells can assist cognitive function by releasing IL-4 and IFN-γ in the CNS (Alves de Lima et al., 2020). They communicate with astrocytes to induce Th1 and Th17 cell responses through the secretion of IL-12 and IL-23 or to restrict Th17 cell activity and stimulate IL-10-producing T cells through the secretion of IL-27 (Giovannoni and Quintana, 2020).
Ischemic stroke alters the gut microbiota composition, and this environment leads to the inner-gut proliferation of Th1 and Th17 cells accompanied by the migration of monocytes and intestine-derived T cells to the ischemic brain tissue, thereby aggravating neuroinflammation (Singh et al., 2016). Normally, CD4+ T cells are the main lymphocytes in the intestinal tract and mediate various kinds of host immune protection and homeostasis, and such responses are realized though the differentiation of T cells into Th1, Th2, Th17, and Tregs which are induced by the metabolites of gut microbiome (Shale et al., 2013). Furthermore, different kinds of immune T cells act differently, and conversion between T cells, such as Th17 cells and Tregs, is represented by relevant chemokine or cytokine expression. Low expression levels of TGF-β, IL-23, or IL-6 induce the differentiation of naive T cells into Th17 cells, while high expression levels of TGF-β induce Tregs (Gagliani et al., 2015). In this process, gut microbes are phagocytosed by DCs, which in turn are stimulated to release cytokines. Some bacteria will also promote CD8+ T cell intestinal microbes and reduce some adaptive cells such as Th17 cells. This process may lead to immune defects, but at the same time, it will definitely reduce the rates of some diseases, such as autoimmune conditions, which may play a role in the reduction of ischemic stroke (Stubbe et al., 2013). Specific cytokine combinations, biogeography, and the microbial environment are the three main ways that microbiota influence the formation of T cell subsets. For example, by expressing a critical regulator of the Th1 differentiation program, T-bet stimulates Th1 cells to secrete IFN-γ under the influence of IL-12, IL-18, and IL-23; by contrast, Th2 cells are formed under the influence of IL-4 or IL-5, express GATA-binding protein 3, and secrete IL-5 and IL-13 (Geva-Zatorsky et al., 2017). In addition, the majority of microorganisms that have the ability to invade epithelial cells can be phagocytosed by DCs, which can also stimulate DCs to release inflammatory cytokines such as TNF-a and IL-6 and bind to OX40 (a tumor necrosis factor receptor) and IL-12. These signals preferentially polarize Th1 cells (Brown et al., 2019). γδT cells are a group of cells with primitive innate immune functions that are predominantly located on the surface of the intestinal epithelium (Prinz et al., 2013). Following an imbalance in the gut microbiota, γδT cells can secrete large amounts of IL-17, which can cause chemokine production by peripheral monocytes and neutrophils and then exacerbate ischemic brain injury (Shichita et al., 2009). Effector T cells can induce enterogenous ischemic brain injury, but Tregs can exert adverse effects (Liesz et al., 2015). Tregs play a neuroprotective role by secreting IL-10 to inhibit IL-17+ γδT cells (Liesz et al., 2009). However, Tregs seem to not enter the brain parenchyma during the acute stroke phase, indicating that the beneficial effects of Tregs are achieved on the peripheral immune system instead of damaged brain tissue (Li et al., 2013). The detailed and exact mechanism of how the brain motivates peripheral immune cells to move to the CNS still needs to be explored.
The function of B cells cannot be neglected when intestinal stimulators such as antigens occur in the gut, as they promote the differentiation of B cells into plasma cells that secrete immunoglobulin A and control luminal microbes to prevent association (Rojas et al., 2019). When facing an attack, massive intestinal IgA-positive plasma cells will relocate into the brain and spinal cord to relieve neuroinflammation (Pröbstel et al., 2020). Although studies have found that this process is mediated by IL-10, the detailed signals have not been identified. Anatomically, researchers regard them as adjacent to dural venous sinuses to protect the CNS from the invasiveness of peripheral pathogens (Fitzpatrick et al., 2020).
Gut metabolites
Microbes in the gut synthesize neurotransmitters, short-chain fatty acids, and bile acids that enter the bloodstream, similar to brain cells and microglia in the CNS. Here, we have listed five terms of metabolites largely studied by recent researches.
Short-chain fatty acids (SCFAs), mainly acetate, propionate, and butyrate, are produced by specific microbes. SCFAs have a significant effect on the CNS in the following ways: First, SCFAs bind to the G protein-coupled receptors (e.g., GPR41 and GPR43) and act as signal molecules to activate specialized immune cells (Kim et al., 2013). The process involves a response to an acute infection or abnormal regulatory mechanism; consequently, this results in increased intestinal permeability and absorption of active metabolites (Sorboni et al., 2022). Second, SCFAs attenuate neuroinflammation by inhibiting the translocation of LPS to brain tissue. Third, SCFAs also regulate the apoptosis of neurocytes and cerebral neurotransmitters and modulate immunoinflammatory responses mediated by the microglia. Fourth, such materials may be absorbed into circulation and cross the BBB to exert their biological effects (Dang and Marsland, 2019; Sorboni et al., 2022). Differences in bacterial species are associated with SCFA production (Table 1).
Table 1.
Changes in the intestinal flora in relevant animal and clinical stroke models
| Type | Model | Species | Changes in microbiota | Reference |
|---|---|---|---|---|
| IS | MCAO | Mice | ↑ Bacteroidetes | Singh et al., 2016 |
| ↑ Peptococcaceae; | Houlden et al., 2016 | |||
| ↓ Prevotellaceae | ||||
| ↑ Anaerotruncus, Alistipes | Stanley et al., 2018 | |||
| ↑ Actinobacteria, Proteobacteria, Verrucomicrobia, Synergistetes, Erysipelotrichaceae, Butyricimonas, Desulfovibrio | Chen et al., 2019c | |||
| ↑ Muribaculaceae, Bacteroidaceae, Lachnospiraceae; | Shen et al., 2023 | |||
| ↓ Lactobacillaceae, Veillonellaceae, Erysipelotrichaceae | ||||
| ↑ Proteobacteria; | Zhang et al., 2023b | |||
| ↓ Firmicutes, Verrucomicrobia, Akkermansia | ||||
| Monkeys | ↑ Prevotella; | Chen et al., 2019e | ||
| ↓ Faecalibacterium, Streptococcus, Lactobacillus, Oscillospira | ||||
| Gerbils | ↑ Clostridium, Desulfovibrio, Oscillospira; | Ryuk et al., 2022 | ||
| ↓ Lactobacillus, Bifidobacterium, Allobaculum | ||||
| Transient forebrain | Gerbils | ↑ Lachanospiracae, Allobaculum; | Zhang et al., 2021b | |
| ischemia | ↓ Lactobacillus, Akkermansia | |||
| Sequencing of fecal | Humans | ↓ Bacteroidetes | Yin et al., 2015 | |
| specimens | ||||
| IS+HT | MCAO | Mice | ↑ Proteobacteria, Actinobacteria | Huang et al., 2022 |
| CI | Sequencing of fecal specimens | Humans | ↑ Ruminococcaceae, Christensenellaceae, Lachnospiraceae | Li et al., 2019 |
| AIS | Sequencing of fecal specimens | Humans | ↑ Atopobium; | Yamashiro et al., 2017 |
| ↓ Lactobacillus sakei | ||||
| ↑ Enterobacteriaceae, Ruminococcaceae, Veillonellaceae, Lachnospiraceae; | Xu et al., 2021a | |||
| ↓ Bacteroidaceae, Prevotellaceae |
AIS: Acute ischemic stroke; CI: cerebral ischemic stroke; HT: hemorrhagic transformation; IS: ischemic stroke; MCAO: middle cerebral artery occlusion.
Amyloid peptides: Some Enterobacter species may produce amyloid peptides, and this material is associated with forming brain amyloids (Lundmark et al., 2005). Amyloids released by microbes will stimulate the nucleation of amyloid, and this accumulation can cause an inflammatory response that will also be triggered by microbial endotoxins and its ability to promote the formation of amyloid fibrils (Asti and Gioglio, 2014). This amyloid aggregation is reduced by the intestinal microflora-produced SCFAs. Another essential molecule, gram-negative LPS, in turn supports amyloid deposition in the brain and negatively affects cognition. It is unclear how bacterial amyloid protein cooperates with other neuropathologic mechanisms after cerebral ischemia, such as posttranslational tau alterations, β-amyloid peptide production, neuroinflammation, and cerebrovascular degeneration (Jang et al., 2018). However, it is clear that after ischemia, bacterial amyloids influence the nucleation of amyloid accumulation in the brain, participate in brain gliosis, increase immunoreactivity, and control brain inflammation; these observations have been verified both in vivo and in vitro (Lee et al., 2018a). Therefore, when the numbers and types of bacterial flora change, different levels of bacterial amyloids as well as metabolites will accordingly change the development of neurodegeneration in the postischemic brain.
Trimethylamine N-oxide (TMAO): As a by-product of intestinal microflora, TMAO has been thought to be closely related to stroke, but its functions still need to be verified. Additionally, there may be a subtle relationship between the number of proinflammatory monocytes and the concentration of TMAO in the serum (Komaroff, 2018). In additional, a study displayed that the cutC gene in microbes is used to promote TMAO production and enlarge the infarct area after stroke (Zhu et al., 2021).
Tryptophan: Tryptophan from the diet is metabolized by intestinal microbes into aryl hydrocarbon receptor agonists and then combines with their receptor to activate microglia and promote TGF-α and vascular endothelial growth factor B expression, thereby stimulating astrocyte pathogenic activity (Scott et al., 2020). A previous study showed the essential impact of astrocytes; some metabolites such as indole-3-aldehydes and indole-3-propionic acid, which are produced by the gut microbiota, resolve tryptophan and in turn regulate astrocyte activity by activating astrocyte aromatic hydrocarbon receptors (Rothhammer et al., 2018). Furthermore, another study showed that the initiation of aryl hydrocarbon receptor through kynurenine pathways will cause worse acute brain damage (Cuartero et al., 2014).
LPS: This is recognized as a main component of bacteria and circulates from the damaged gut barrier, arrives in the brain, activates microglia, and initiates the inflammatory response. LPS also increases the expression of TLR4 and CD14 and then promotes the release of many inflammatory cytokines and antigens in the brain (Yang et al., 2020). Additionally, it is said that antigens will in turn activate TLR4 and form a positive feedback loop. However, some studies have shown that LPS decreases the activation of brain microglia and delays and decreases immune cells and their relevant inflammatory cytokines IL-6, IL-β, and TNF-a (Matcovitch-Natan et al., 2016).
Intestinal barrier and enteric glia cells
The intestinal barrier is formed by three structures: a mucus layer, an epithelial barrier and a gut vascular barrier, and once these barriers are injured or their permeability increased, some pathogens, toxins, and food particles will pass through the physical barrier into the blood stream and reach the brain (Pellegrini et al., 2023). Therefore, the interaction between the intestinal tract and brain is largely controlled by the barriers.
Furthermore, gut microbiota plays an essential role in the contact between intestinal barrier on luminal part (Pellegrini et al., 2023). Gut microbiota plays a significant role in the homeostasis of the barrier, as changes in gut microbiota composition will lead to leakage of the intestinal epithelial barrier, the cells of which are tightly bound together by intercellular tight junctions, and then the permeability of the barrier will increase (Logsdon et al., 2018). Along with the translocation of some intestinal bacteria that could promote TLR4 activation and the initiation of inflammatory processes in the brain from the gut to the whole body, this process will lead to the swelling of astrocytes and release proinflammatory factors, all of which are relevant to the development of progressive cognitive impairment and dementia (Farhadi et al., 2003).
From the tissue side, the intestinal barrier makes contacts with the enteric nervous system, with enteric neurons and enteric glia cells (EGCs) (Pellegrini et al., 2023). A recent review has summarized that EGCs play essential roles in intestine immune homeostasis when it forms a bidirectional communication with immune cells (Seguella and Gulbransen, 2021). Additionally, EGCs are activated with enteric neurons to drive neuroinflammation through modulating gastrointestinal reflexes (Seguella and Gulbransen, 2021). Studies have also revealed that when responding to pro-inflammatory mediators, EGCs can secrete proteins like S100β and subsequently induce neuronal damage, while EGCs also produce pro- or anti-inflammatory cytokines to interact with immune cells (Chow and Gulbransen, 2017; Seguella and Gulbransen, 2021). Although many scientists have studied the roles of EGCs in both innate and adaptive immune responses; for instance, (i) EGCs secrete neurotrophins to stimulate IL-22 production and induce group 3 innate lymphoid cells, (ii) they share vagal anti-inflammatory signals to modulate local immune cells and maintain gut barrier integrity, and (iii) they influence T cells to display immunosuppressive effects, wherein the aspect of glial-driven immune responses needs to be researched upon in future (Ibiza et al., 2016; Langness et al., 2017).
Neuroendocrine pathway
Now, it is clear that the stimulators of the HPA axis are some environmental stressors or peripheral responses to gut inflammation that are integrated into the brain and coordinate with the adrenal gland to release glucocorticoids (Cryan et al., 2019). Glucocorticoids have the ability to both restore homeostasis and assist with GI dysfunction by altering intestinal permeability, motility, mucus production, enteric immune cell activity, gut function and environment, and microbial composition. The main role of the intestinal microbiome is to modulate the HPA axis, as a lack of microbiota will in turn enhance HPA activity in response to moderate stressors (Cryan et al., 2019).
The impact of comorbidities
Given that gut microbiota can not only influence ischemic stroke but also interfere with stroke risk factors such as diabetes and atherosclerosis (Liu et al., 2007), studies have indicated the influence between the comorbidity—diabetes and ischemic stroke—from the perspective of the gut-brain axis.
Thus far, there is a relevant mechanism showing the complicated pathways among intestinal microflora, intracranial atherosclerosis, and innate and adaptive immune cells with cerebral injury after ischemic stroke (de la Cuesta-Zuluaga et al., 2018).
In epidemiological terms, diabetic patients have a 1.8–6 times higher risk of ischemic attack (Bäckhed et al., 2005). It is clear that hyperglycemia is another risk factor independently associated with ischemic stroke, and the key factor is insulin resistance. It has been found that an imbalance of the microbiota in the gut will lead to lower insulin levels, cause insulin resistance, and subsequently increase blood glucose levels (Qin et al., 2012). Once the microbiota show dysbiosis, it will initiate an inflammatory reaction, and some inflammatory factors will lead to insulin resistance by affecting the insulin signaling pathway. The chronic inflammatory response is a vital pathogenic characteristic of insulin resistance. The introduction of LPS into the circulation increases the production of proinflammatory cytokines that induce serine phosphorylation of insulin receptor substrate 1 in the muscle and adipose tissue, blocking insulin signaling pathways and leading to insulin resistance (Saad et al., 2016).
Age and sex dependence
Compared with young adults, older people have different patterns of gut microbes: the variety of bacteria and probiotics as well as SCFAs decrease, but facultative anaerobic bacteria increase, and dominant species change (Ling et al., 2022). A stroke pathology study revealed that aged mice (20 months) have reduced infarct volumes, but usually these animals have much worse functional outcomes than young mice (2.5 months) (Shin et al., 2015). One possible association is that in aged individuals’ bodies, there probably exists a “primed” inflammatory environment as well as chronic systemic inflammation, which leads to the exacerbation of the poststroke inflammatory response. This is just one aspect of the influence of aging on stroke. It is well known that substantial synaptogenesis and neural development occur in the first 3 years of childhood development. At the same time, the gut microbiota also undergoes a process of maturation and forms a similar structure to the adult intestinal microbiota (Yatsunenko et al., 2012). Although there are fewer types of gut microbes in the early years of the human body, the types and numbers of gut microbiota need to increase to resist disturbances with increasing age, and interactions between the host and gut microbiota have occurred since long in the process of growth and development. Hence, it is evident that in younger children, interruption of the intestinal microbiota may be involved in its association with the brain, in turn promoting CNS diseases.
Influences focusing on the intestinal tract after ischemic stroke
Another aspect of the brain-gut axis focuses on the influences caused by cerebral ischemia. Normally, the tract is innervated by both intrinsic and extrinsic nerves, wherein the latter one includes neurons of the sympathetic and parasympathetic system (Uesaka et al., 2016). A study showed that these efferent fibers will maintain gut microbiota as well as the integrity of the mucosal barrier and affect its tight junctions (Crapser et al., 2016). Another previous review introduced some pathways of the interactions between the brain and gut such as the autonomic nervous system, CNS, stress system (HPA-axis), corticotropin-releasing factor system, and the intestinal responses involving the intestinal barrier, luminal microbiota, and intestinal immune response (Cryan et al., 2019). Additionally, current studies have come up with the gut connectome which mainly focus not only on the direct effects of CNS such as using stress mediator-induced virulence genes and the secretion, motility, permeability, and immunological defense of the enteric nervous system to interrupt gut microbiota but also on the indirect modulation of the intestinal barrier induced by the autonomic system (Osadchiy et al., 2019). Currently, the study about diseases of the gastrointestinal tract gather in inflammatory bowel disease (Fairbrass et al., 2022) and irritable bowel syndrome (Jacobs et al., 2021), the bidirectional communication between brain and gut are getting increasing attentions.
Same to above mention, a clinical trial has revealed an accompany of cerebral ischemic diseases and gastrointestinal symptoms and gut dysbiosis. This study also revealed the gut influences caused by the ischemic brain, which are listed as follows (Zeng et al., 2019). (1) Cerebral ischemia will disturb the intestinal microbiota and gut metabolites, as reported by an experiment that showed that ischemic stroke is associated with less Butyricicoccus, Lactobacillus, Prevotella, Meganonas, and Alloprevotella, but more of opportunistic pathogens such as Proteus, Alistipes, Klebsiella, Fusobacterium, Haemophilus, Shuttleworthia, and Papillibacter (Chen et al., 2019d). (2) Stroke will cause increased gut permeability and a leakage of microbiota that can translocate to the lung, brain, and liver to provoke systemic immune response and whole-body infection (Stanley et al., 2016). (3) Acute brain injury also induces severe gastrointestinal paralysis and damages intestinal motility which will cause the microbiota antigen to be presented to gut immune cells to initiate whole body and neuronal inflammation (Candelario-Jalil and Paul, 2021). (4) An ischemic stroke will repeatedly lead to former affects (gut dysbiosis, intestinal motility, and impaired intestinal barrier function) though the catecholaminergic stress response in the gut (Iadecola et al., 2020). (5) The effects induced by cerebral ischemia display age-related differences. Animal experiments indicated that young stroke models will change the gut microbes compare to aged models (Spychala et al., 2018). Another research formally identified that though stroke can cause indifferent alteration in both young and aged mice, the aged animals tend to be more susceptible to sepsis and immune dysfunction (Crapser et al., 2016). In summary, considering hypoxia-ischemia contributes to neurofunctional and gastrointestinal disorders, diseases other than inflammatory bowel disease, such as gut microbiota dysbiosis, necrotizing enterocolitis, and tumorigenesis of the colorectal carcinoma are also cause for concern (Yang et al., 2022). Hence, it is essential to further illustrate the interaction between brain tissues and the gastrointestinal tract.
Immunological mechanisms of the brain-lung axis
The pulmonary complications of ischemic stroke have been largely studied in recent years, and many studies have highlighted the pathological mechanisms of respiratory environment interrupting the outcome of ischemic stroke (Pelosi et al., 2011; Hannawi et al., 2013; Smith et al., 2015). In the following sections, we have listed the immune or immune-related interactions between the brain and lung.
The influences to CNS caused by the lung
Early in 1998, Johnston et al. suggested after-stroke pneumonia will cause brain injury. Recently, a study discovered respiratory infection will alter the permeability of the BBB to large molecules and immune cells and induce CNS inflammation (Bohmwald et al., 2021). The influences to central nervous system caused by the lung involve that rats with chronic pulmonary infection showed invasion of the CNS by increased circulating inflammatory markers such as TNF-α and matrix metalloproteinase-9 to exacerbate neuronal cell death (Chen et al., 2019a, b). Acute lung injury (ALI) also led to brain dysfunction and lung tumors create several antigens to attack brain and caused undifferentiated autoimmune encephalitis (Bickenbach et al., 2009; Kazarian and Laird-Offringa, 2011), which indicated immune system plays an essential role in the lung communicating with the brain tissue.
Respiratory tract infections are a prominent inflammatory response of the body and it will increase the risk of ischemic stroke (Violi et al., 2014). A previous review reported that the activation of tissue factor and platelet are the key steps for activating the clotting system and pro-inflammatory stimulus, and LPS induction is necessary for this process (Aras et al., 2004; Berthet et al., 2012). From the aspect of the risk factors of ischemic stroke, recent studies have focused on chronic obstructive pulmonary disease (COPD) (Austin et al., 2016) and ambient air pollution (Verhoeven et al., 2021; Kulick et al., 2023). COPD is known to directly and independently increase the risk of stroke due to systemic inflammation and increase oxidative stress so that promoting cerebral vascular dysfunction (Austin et al., 2016). A current Rotterdam study included patients with COPD or restrictive lung functions and showed more lacunar infarcts and poor global cognitive function (Xiao et al., 2022). Moreover, the dual risk factors of aging and smoking also produce vascular impairments indirectly driven by the NADPH oxidases (Iida et al., 2008; Mayhan et al., 2008). The effects of ambient air pollution involve the local and subsequent global inflammation contributed by inhalation pollutants and autonomic respiratory reflex arcs which has been elucidated in previous studies (Verhoeven et al., 2021; Kulick et al., 2023).
As patients with severe brain damage are often put on mechanical ventilation (MV) for airway protection, and the influences acted by MV on cerebral ischemia brain have been studied for more than 10 years (Pelosi and Rocco, 2011). Except for ventilator-associated lung injury, regardless of previous lung diseases, MV will interrupt the brain though induced neutrophil infiltration and release inflammatory markers to increase neuron activations in many CNS areas to activate brain responses, alter regional cerebral perfusion, and activate the autonomic system (Pelosi et al., 2005). A recent study also indicated that MV provokes the deleterious effect on the hippocampus though a TRPV4-ATP-P2X signaling in lung tissue to cause MV-related cognitive disorders (González-López et al., 2019). Notably, with respect to other CNS diseases, scientists have significantly discovered the brain-lung interaction engaging in the lung microbes which induced LPS to cross the BBB and influence the brain-resident microglia to initiate type I interferon signaling pathways to restrict experimental autoimmune encephalomyelitis in animal models (Hosang et al., 2022). Such results provide a promising direction for future studies related to the lung microbiota and its immune roles in ischemic stroke.
The influences to lung caused by the CNS
Heuer noted that acute intracranial hypertension will augment lung injury and pointed out the interactive role (Heuer et al., 2011). A recent review has settled the crosstalk between the brain and lung and made a detailed introduction of the complications after acute brain damages, which mainly concentrated on ventilator-associated pneumonia, acute respiratory distress syndrome, and neurogenic pulmonary edema (Mrozek et al., 2020). To understand the lung influences that caused brain damage, particularly in cerebral ischemia, a summary involving the immune mechanisms related to neuroanatomy, endocrinology, HPA-axis, metabolites (Santos Samary et al., 2016), aging, and MV follows.
Neuroanatomical pathway: Stroke can directly destroy the vagus nerve and damage its endings distributed in the lung (Li et al., 2023). Besides the function of swallowing, the vagus nerve also participates in cholinergic anti-inflammatory pathways to reduce systemic inflammation, while also inducing immunosuppression in the lung and increasing the risk of bacterial pneumonia (Li et al., 2023).
Immune pathway: BBB destruction is the key event after ischemic stroke and it mediates the release of some necrotic substances such as high mobility group box 1—receptor for advanced glycation end signal—to inhibit the peripheral immune response and cause post-stroke-associated pneumonia (Kim et al., 2018). In addition, injured brain tissue will initiate proinflammatory response with producing abundant inflammatory cytokines (IL-1, IL-6, IL-8, TNF) (Ott et al., 1994). In post-ischemia stroke experiment, animal models displayed diffuse alveolar damage and ultrastructural damage and the protein levels of proinflammatory mediators were documented among lung, brain and plasma, but the phagocytic ability of macrophages reduced (Samary et al., 2018).
Endocrine pathway and sympathetic nervous system regulation: In addition to the immune reaction, the maintenance of whole immune response and the two-hit mechanism is incomplete without significant stimulation of the sympathetic nervous system and endocrine reaction. Initial sympathetic discharge stimulates systemic inflammatory response (Mascia, 2009) and cooperates with the release of catecholamines to create a systemic inflammatory environment (first hit) that will make the lung more susceptible to “the second hit” such as sepsis, MV, or surgery (Mascia, 2009). Complete pathways and their effects have been illustrated in previous reviews, which have highlighted the release of the neurotransmitter norepinephrine and peripheral lymphopenia to cause impaired monocytes and shift from Th1 to Th2 (Prass et al., 2003; Walter et al., 2013). Though this process will protect the brain from further injury, pneumonia will neglect the shift and cover Th1 autoimmune response against CNS antigens (Prass et al., 2003; Winklewski et al., 2014).
HPA axis: Due to the effects of stress and inflammatory response after trauma, the HPA axis in acute brain injuries is involved in pulmonary dysfunction. Once the HPA axis is activated by inflammatory mediators such as IL-6, it will protect the peripheral organs by initiating the compensatory anti-inflammatory response syndrome throughout the body to delete inflammatory responses (Offner et al., 2002). Furthermore, endogenous glucocorticoids stimulate anti-infectious immunity (Rhen and Cidlowski, 2005).
Aging: Through lung imaging, scientists have revealed impaired pulmonary intravascular neutrophil function after stroke, and this fact is exacerbated by aging, which results in worse neutrophil antibacterial responses and increased risk of stroke-associated infection (Wen et al., 2022).
The effects caused by SARS-CoV-2 infection
A study identified that severe acute respiratory syndrome coronavirus (SARS-CoV-2) could reach the brain through the lung epithelium (Iroegbu et al., 2020). In past pandemics, researches have indicated that the SARS-CoV-2 infection will largely increase the risk of ischemic stroke (Beyrouti et al., 2020), resulting in poorer prognosis and higher mortality than infection with other respiratory viruses. In recent years, scientists have proposed various reasons and pathways to explain the mechanisms including activating HPA axis led by upregulated cytokines, SARS-CoV-2-related hypercoagulability, bacteremia induced by the usage of MV (Huang et al., 2020; Wang et al., 2020), and the molecules hypoxia-inducible factor-1a (Francistiová et al., 2021). While based on the study of damaged tricompartmental model of lung parenchyma oxygenation (alveolus, bronchial artery, and pulmonary artery) in SARS-CoV-2 infection, a case study reported that some pulmonary vein clots forming in the special pulmonary intravascular thrombotic lesions may embolize to the brain, thereby broadening the recognition of the fundamentals of ischemic stroke occurring with SARS-CoV-2 infection (Meaney et al., 2022). By contrast, other research has shown that the development of stroke will increase the risk of SARS-CoV-2 infection though promoting the expression of its receptor angiotensin-converting enzyme (ACE) 2 in the lung (Singh et al., 2021). Taken together, SARS-CoV-2 infection enhances the understanding of the dual interaction between respiratory system disorders and cerebral ischemia, and the responses of the lung may be more profound than those of the gastrointestinal system. Figure 3 presents a brief summary of the gut-brain-lung axis.
Figure 3.
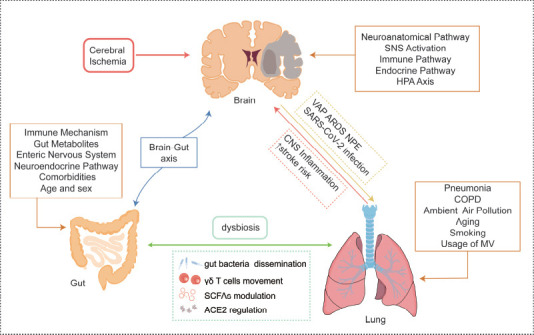
The lung-brain axis and gut-lung axis in ischemic stroke.
Ischemic stroke will cause a series of pulmonary complications though neuroanatomical and other pathways. In turn, the lung influenced by pneumonia and COPD and many other issues will in the end lead to CNS inflammation and increase the risk of stroke and poor outcomes. The above section has introduced the brain-gut axis’ different mechanisms, and the interaction between the gut and lung involves gut microbiota translocation, γδ T cells movement, and metabolite changes such as SCFAs and ACE2. Created with Adobe Illustrator 2019. ACE2: Angiotensin-converting enzyme 2; ARDS: acute respiratory distress syndrome; CNS: central nervous system; COPD: chronic obstructive pulmonary disease; MV: mechanical ventilation; NPE: neurogenic pulmonary edema; SCFAs; short-chain-fatty acids; VAP: ventilator-associated pneumonia.
Gut-Lung Interaction
As patients with inflammatory bowel disease often show an increased prevalence of COPD, and respiratory infection always accompanies gastrointestinal symptoms, the gut-lung axis in ischemic stroke and other diseases have been studied to reveal several mechanisms including alteration of gut/lung mucosa barrier permeability, bacterial translocation and abundance changes, movement of T cells, phenotype polarization of local macrophages, metabolites (SCFAs) and ACE2 receptors (Mahadeva et al., 2000; Ekbom et al., 2008).
Studies have shown that in both ischemic and hemorrhagic stroke, disruption of intestinal structure and increased barrier permeability will cause gut bacteria to disseminate to the lung and induce gut-derived lung infection (Wen et al., 2019; Zhang et al., 2021a). Another study showed significant alteration in the tight junctions of intestinal structure with downregulated zonula occludens-1, a key protein of the tight junction complex, and production of TNF-a that was more obvious in aged mice (Wen et al., 2019). Such effects finally lead to prominent differences that increase microbiota cultivated in the lung compared to other organs like the liver or spleen (Wen et al., 2019). These findings will further clarify the suitable intervention timing of secondary pulmonary infection in stroke patients (Zhang et al., 2021a).
Another mechanism involves inhibition of γδ T cells movement. This will alleviate the effects led by social isolation to cerebral ischemia and stroke-associated pneumonia from small intestine. Because it will limit γδ T cells to release the pro-inflammatory cytokines such as IL-17A, IL-22 and facilitate dysfunction of the gut-lung axis (Xie et al., 2023). Additionally, LPS will aggravate the immune damage of the lung when dysregulating intestinal microflora; increased immune response to local LPS and, in turn, LPS-induced lung injury will provoke a low-grade gut inflammation in acute injury. Such roles of LPS will be inhibited by mesenchymal stem cells to decrease the CD8+ T-cells and enhance barrier function (Xu et al., 2021b).
Moreover, gut microbiome metabolite SCFAs have also been reviewed to play dual roles in the lung and are distributed from the intestine to other organs throughout the portal venous system, and SCFAs also become the potent modulators for host immune response among the gut and the lung and the CNS (Ney et al., 2023). The COVID-19 pandemic resulted in extensive research on the functions of ACE2, wherein the link between gut dysbiosis and pulmonary hypertension was hypothesized to cause leaky gut and pathological events in the lung (Oliveira et al., 2020; Sharma et al., 2020b). Considering some probiotics may express ACE2, there is a potential advantage of the lung interrupting the intestine and influencing the gut transporters and communication between gut epithelial and its microbiota (Sharma et al., 2020a).
In addition, diseases from other systems such as nontuberculous mycobacterial infection (Kim et al., 2022), cancer (Lu et al., 2021), bronchopulmonary dysplasia (Ran et al., 2021), and toxic exposure (Zhang et al., 2023a) have identified the current knowledge regarding the interaction and bi-directional responses between the gut and the lung.
Perspectives
Based on the above detailed review and expectation from clinical practice, we go on to list three dimensions concentrating on the different phases of ischemic stroke on the brain-gut axis or brain-lung axis.
Key open questions: therapeutic time windows are now being considered to improve clinical outcome (Zhang et al., 2023b). First, enhancing the control of the risk factors at pre-stroke and post-stroke; second, reducing infarct size and complications at the acute phase; and third, preventing recurrence and promoting recovery at the subacute phase of stroke. These three main points provide practical ideas for future studies. When it comes to the brain-lung axis, studies related to lung microbiomes and stroke is limited and needs further research.
Potential targets and route: Considering the current studies focusing on the roles of EGCs in the regeneration of the enteric nervous system and its relative feasibility compared to microglia cells, the application of enteric glial cells in central nervous system regeneration after ischemic stroke may be a promising field. Furthermore, the dietary therapy targeted regulating gut microbiome and its metabolites, and the usage of antibiotics and prebiotics are waiting for more convincing results. Apart from diet and medication, the latest study has discovered electroacupuncture and induced pluripotent stem cell-derived small extracellular vesicles (Zhang et al., 2023b) modulating the gut microbiome to have significant and accurate effects on ischemic stroke, which provoke more physical or cellular strategies for substantial benefits. Other measures focusing on triggering receptor expressed on myeloid cells 1 and natural materials targeting SCFAs are all potential fields (Roselli and Huber-Lang, 2019; Guo et al., 2023). As for the lung-brain axis, early detection of acute lung injury through clinical signs and measurements of extravascular pulmonary fluid as well as brain injury by measuring specific markers can help optimize ventilation settings and even contribute to the overall clinical management of critically ill patients. A recent study identified that molecules used in the lung can restrict brain injury after ischemia reperfusion and systemic inflammation activation, which indicates lung inflammation is the potential target for stroke treatment.
Key hurdles: Focusing on the BBB leakage and subsequent pathological procedures, past studies neglect the occurrence of other barriers like the gut vascular barrier and choroid plexus vascular barrier that provide potentially attractive targets (Carloni and Rescigno, 2022). Furthermore, the replicability of previous study results focusing on fecal transplant gavage or intestinal flora transplantation still remain the core problems for scientists, and researchers are also required to differentiate between aged persons and an aged gut microbiome. Some hurdles arise from the brain-lung axis contain the effects of anesthetic used on model bronchus, ischemic models (local or diffuse), results deduced to human, indirect effects of topical drugs on intracranial pressure and so on. The hurdles existing in the brain-lung axis include the effects of anesthetic used on model bronchus, ischemic models (local or diffuse), results deduced to human, and indirect effects of topical drugs on intracranial pressure.
In conclusion, there are close relationships between ischemic stroke and the gut or lung from the perspective of immune responses, gut microbiome, and lung sympathetic nervous system activation. Promotion of systemic inflammation is the core aspect that regulates the interaction between the brain and two peripheral organs. The dependent association between the brain and other organs provide requires continuous exploration. In the future, we look forward to more studies on the brain and peripheral tissues, especially the intestine and lung, of ischemic stroke, and hope that more effective treatment measures can be studied and applied to clinical practice to improve the clinical outcome of patients.
This review has some limitations. First, this review only focuses on the single role of the immune mechanism in disease progression, ignoring the influence of other mechanisms on immune function, thus resulting in the contents of this article being superficial, without in-depth details to explore the specific molecular pathways among the brain, gut, and lung. In addition, this article does not comprehensively summarize the current treatment measures of the gut-brain axis and lung-brain axis, the role between different measures, efficacy, and clinical significance, which have a direct significance for clinical practice. This article only briefly introduces some current targets and research gaps. In addition, in the brain-lung axis section, we did not make a detailed distinction between ischemic stroke and hemorrhagic stroke, rather only generalized the immune mechanisms underlying ischemic stroke.
Footnotes
Funding: This work was supported by the National Natural Science Foundation of China, No. 82204663; and the Natural Science Foundation of Shandong Province, No. ZR2022QH058 (both to TZ).
Conflicts of interest: The authors declare that there are no conflicts of interest associated with this manuscript.
Data availability statement: All relevant data are within the paper.
C-Editor: Zhao M; S-Editors: Yu J, Li CH; L-Editors: Yu J, Song LP; T-Editor: Jia Y
References
- 1.Abdel-Haq R, Schlachetzki JCM, Glass CK, Mazmanian SK. Microbiome-microglia connections via the gut-brain axis. J Exp Med. 2019;216:41–59. doi: 10.1084/jem.20180794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alawieh A, Langley EF, Tomlinson S. Targeted complement inhibition salvages stressed neurons and inhibits neuroinflammation after stroke in mice. Sci Transl Med. 2018;10:eaao6459. doi: 10.1126/scitranslmed.aao6459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alves de Lima K, Rustenhoven J, Kipnis J. Meningeal immunity and its function in maintenance of the central nervous system in health and disease. Annu Rev Immunol. 2020;38:597–620. doi: 10.1146/annurev-immunol-102319-103410. [DOI] [PubMed] [Google Scholar]
- 4.Aras O, Shet A, Bach RR, Hysjulien JL, Slungaard A, Hebbel RP, Escolar G, Jilma B, Key NS. Induction of microparticle- and cell-associated intravascular tissue factor in human endotoxemia. Blood. 2004;103:4545–4553. doi: 10.1182/blood-2003-03-0713. [DOI] [PubMed] [Google Scholar]
- 5.Asti A, Gioglio L. Can a bacterial endotoxin be a key factor in the kinetics of amyloid fibril formation? J Alzheimers Dis. 2014;39:169–179. doi: 10.3233/JAD-131394. [DOI] [PubMed] [Google Scholar]
- 6.Austin V, Crack PJ, Bozinovski S, Miller AA, Vlahos R. COPD and stroke:are systemic inflammation and oxidative stress the missing links? Clin Sci (Lond) 2016;130:1039–1050. doi: 10.1042/CS20160043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bäckhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- 8.Berthet J, Damien P, Hamzeh-Cognasse H, Arthaud CA, Eyraud MA, Zéni F, Pozzetto B, McNicol A, Garraud O, Cognasse F. Human platelets can discriminate between various bacterial LPS isoforms via TLR4 signaling and differential cytokine secretion. Clin Immunol. 2012;145:189–200. doi: 10.1016/j.clim.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 9.Beyrouti R, Adams ME, Benjamin L, Cohen H, Farmer SF, Goh YY, Humphries F, Jäger HR, Losseff NA, Perry RJ, Shah S, Simister RJ, Turner D, Chandratheva A, Werring DJ. Characteristics of ischaemic stroke associated with COVID-19. J Neurol Neurosurg Psychiatry. 2020;91:889–891. doi: 10.1136/jnnp-2020-323586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bickenbach J, Zoremba N, Fries M, Dembinski R, Doering R, Ogawa E, Rossaint R, Kuhlen R. Low tidal volume ventilation in a porcine model of acute lung injury improves cerebral tissue oxygenation. Anesth Analg. 2009;109:847–855. doi: 10.1213/ane.0b013e3181ad5769. [DOI] [PubMed] [Google Scholar]
- 11.Bohmwald K, Soto JA, Andrade-Parra C, Fernández-Fierro A, Espinoza JA, Ríos M, Eugenin EA, González PA, Opazo MC, Riedel CA, Kalergis AM. Lung pathology due to hRSV infection impairs blood-brain barrier permeability enabling astrocyte infection and a long-lasting inflammation in the CNS. Brain Behav Immun. 2021;91:159–171. doi: 10.1016/j.bbi.2020.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bornstein NM, Aronovich B, Korczyn AD, Shavit S, Michaelson DM, Chapman J. Antibodies to brain antigens following stroke. Neurology. 2001;56:529–530. doi: 10.1212/wnl.56.4.529. [DOI] [PubMed] [Google Scholar]
- 13.Borst K, Dumas AA, Prinz M. Microglia:Immune and non-immune functions. Immunity. 2021;54:2194–2208. doi: 10.1016/j.immuni.2021.09.014. [DOI] [PubMed] [Google Scholar]
- 14.Brown EM, Kenny DJ, Xavier RJ. Gut microbiota regulation of T cells during inflammation and autoimmunity. Annu Rev Immunol. 2019;37:599–624. doi: 10.1146/annurev-immunol-042718-041841. [DOI] [PubMed] [Google Scholar]
- 15.Cai W, Shi L, Zhao J, Xu F, Dufort C, Ye Q, Yang T, Dai X, Lyu J, Jin C, Pu H, Yu F, Hassan S, Sun Z, Zhang W, Hitchens TK, Shi Y, Thomson AW, Leak RK, Hu X, et al. Neuroprotection against ischemic stroke requires a specific class of early responder T cells in mice. J Clin Invest. 2022;132:e157678. doi: 10.1172/JCI157678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Camara-Lemarroy CR, Ibarra-Yruegas BE, Gongora-Rivera F. Gastrointestinal complications after ischemic stroke. J Neurol Sci. 2014;346:20–25. doi: 10.1016/j.jns.2014.08.027. [DOI] [PubMed] [Google Scholar]
- 17.Camara-Lemarroy CR, Silva C, Greenfield J, Liu WQ, Metz LM, Yong VW. Biomarkers of intestinal barrier function in multiple sclerosis are associated with disease activity. Mult Scler. 2020;26:1340–1350. doi: 10.1177/1352458519863133. [DOI] [PubMed] [Google Scholar]
- 18.Candelario-Jalil E, Paul S. Impact of aging and comorbidities on ischemic stroke outcomes in preclinical animal models:A translational perspective. Exp Neurol. 2021;335:113494. doi: 10.1016/j.expneurol.2020.113494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carloni S, Rescigno M. Unveiling the gut-brain axis:structural and functional analogies between the gut and the choroid plexus vascular and immune barriers. Semin Immunopathol. 2022;44:869–882. doi: 10.1007/s00281-022-00955-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cerf-Bensussan N, Gaboriau-Routhiau V. The immune system and the gut microbiota:friends or foes? Nat Rev Immunol. 2010;10:735–744. doi: 10.1038/nri2850. [DOI] [PubMed] [Google Scholar]
- 21.Chen HJ, Poran A, Unni AM, Huang SX, Elemento O, Snoeck HW, Varmus H. Generation of pulmonary neuroendocrine cells and SCLC-like tumors from human embryonic stem cells. J Exp Med. 2019a;216:674–687. doi: 10.1084/jem.20181155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen J, Yan Y, Yuan F, Cao J, Li S, Eickhoff SB, Zhang J. Brain grey matter volume reduction and anxiety-like behavior in lipopolysaccharide-induced chronic pulmonary inflammation rats:a structural MRI study with histological validation. Brain Behav Immun. 2019b;76:182–197. doi: 10.1016/j.bbi.2018.11.020. [DOI] [PubMed] [Google Scholar]
- 23.Chen R, Wu P, Cai Z, Fang Y, Zhou H, Lasanajak Y, Tang L, Ye L, Hou C, Zhao J. Puerariae Lobatae Radix with chuanxiong Rhizoma for treatment of cerebral ischemic stroke by remodeling gut microbiota to regulate the brain-gut barriers. J Nutr Biochem. 2019c;65:101–114. doi: 10.1016/j.jnutbio.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 24.Chen R, Xu Y, Wu P, Zhou H, Lasanajak Y, Fang Y, Tang L, Ye L, Li X, Cai Z, Zhao J. Transplantation of fecal microbiota rich in short chain fatty acids and butyric acid treat cerebral ischemic stroke by regulating gut microbiota. Pharmacol Res. 2019d;148:104403. doi: 10.1016/j.phrs.2019.104403. [DOI] [PubMed] [Google Scholar]
- 25.Chen Y, Liang J, Ouyang F, Chen X, Lu T, Jiang Z, Li J, Li Y, Zeng J. Persistence of gut microbiota dysbiosis and chronic systemic inflammation after cerebral infarction in cynomolgus monkeys. Front Neurol. 2019e;10:661. doi: 10.3389/fneur.2019.00661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choi YH, Laaker C, Hsu M, Cismaru P, Sandor M, Fabry Z. Molecular mechanisms of neuroimmune crosstalk in the pathogenesis of stroke. Int J Mol Sci. 2021;22:9486. doi: 10.3390/ijms22179486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chow AK, Gulbransen BD. Potential roles of enteric glia in bridging neuroimmune communication in the gut. Am J Physiol Gastrointest Liver Physiol. 2017;312:G145–G152. doi: 10.1152/ajpgi.00384.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coughlan T, Gibson C, Murphy S. Modulatory effects of progesterone on inducible nitric oxide synthase expression in vivo and in vitro. J Neurochem. 2005;93:932–942. doi: 10.1111/j.1471-4159.2005.03068.x. [DOI] [PubMed] [Google Scholar]
- 29.Crapser J, Ritzel R, Verma R, Venna VR, Liu F, Chauhan A, Koellhoffer E, Patel A, Ricker A, Maas K, Graf J, McCullough LD. Ischemic stroke induces gut permeability and enhances bacterial translocation leading to sepsis in aged mice. Aging (Albany NY) 2016;8:1049–1063. doi: 10.18632/aging.100952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cryan JF, O'Riordan KJ, Cowan CSM, Sandhu KV, Bastiaanssen TFS, Boehme M, Codagnone MG, Cussotto S, Fulling C, Golubeva AV, Guzzetta KE, Jaggar M, Long-Smith CM, Lyte JM, Martin JA, Molinero-Perez A, Moloney G, Morelli E, Morillas E, O'Connor R, et al. The microbiota-gut-brain axis. Physiol Rev. 2019;99:1877–2013. doi: 10.1152/physrev.00018.2018. [DOI] [PubMed] [Google Scholar]
- 31.Cuartero MI, Ballesteros I, de la Parra J, Harkin AL, Abautret-Daly A, Sherwin E, Fernández-Salguero P, Corbí AL, Lizasoain I, Moro MA. L-kynurenine/aryl hydrocarbon receptor pathway mediates brain damage after experimental stroke. Circulation. 2014;130:2040–2051. doi: 10.1161/CIRCULATIONAHA.114.011394. [DOI] [PubMed] [Google Scholar]
- 32.Cuenca-López MD, Brea D, Segura T, Galindo MF, Antón-Martínez D, Agulla J, Castillo J, Jordán J. Inflammation as a therapeutic agent in cerebral infarction:cellular inflammatory response and inflammatory mediators. Rev Neurol. 2010;50:349–359. [PubMed] [Google Scholar]
- 33.Dang AT, Marsland BJ. Microbes, metabolites, and the gut-lung axis. Mucosal Immunol. 2019;12:843–850. doi: 10.1038/s41385-019-0160-6. [DOI] [PubMed] [Google Scholar]
- 34.de la Cuesta-Zuluaga J, Corrales-Agudelo V, Velásquez-Mejía EP, Carmona JA, Abad JM, Escobar JS. Gut microbiota is associated with obesity and cardiometabolic disease in a population in the midst of Westernization. Sci Rep. 2018;8:11356. doi: 10.1038/s41598-018-29687-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DeLong JH, Ohashi SN, O'Connor KC, Sansing LH. Inflammatory responses after ischemic stroke. Semin Immunopathol. 2022;44:625–648. doi: 10.1007/s00281-022-00943-7. [DOI] [PubMed] [Google Scholar]
- 36.Di Napoli M, Elkind MS, Godoy DA, Singh P, Papa F, Popa-Wagner A. Role of C-reactive protein in cerebrovascular disease:a critical review. Expert Rev Cardiovasc Ther. 2011;9:1565–1584. doi: 10.1586/erc.11.159. [DOI] [PubMed] [Google Scholar]
- 37.Ekbom A, Brandt L, Granath F, Löfdahl CG, Egesten A. Increased risk of both ulcerative colitis and Crohn's disease in a population suffering from COPD. Lung. 2008;186:167–172. doi: 10.1007/s00408-008-9080-z. [DOI] [PubMed] [Google Scholar]
- 38.Endres M, Moro MA, Nolte CH, Dames C, Buckwalter MS, Meisel A. Immune pathways in etiology, acute phase, and chronic sequelae of ischemic stroke. Circ Res. 2022;130:1167–1186. doi: 10.1161/CIRCRESAHA.121.319994. [DOI] [PubMed] [Google Scholar]
- 39.Fairbrass KM, Lovatt J, Barberio B, Yuan Y, Gracie DJ, Ford AC. Bidirectional brain-gut axis effects influence mood and prognosis in IBD:a systematic review and meta-analysis. Gut. 2022;71:1773–1780. doi: 10.1136/gutjnl-2021-325985. [DOI] [PubMed] [Google Scholar]
- 40.Farhadi A, Banan A, Fields J, Keshavarzian A. Intestinal barrier:an interface between health and disease. J Gastroenterol Hepatol. 2003;18:479–497. doi: 10.1046/j.1440-1746.2003.03032.x. [DOI] [PubMed] [Google Scholar]
- 41.Fitzpatrick Z, Frazer G, Ferro A, Clare S, Bouladoux N, Ferdinand J, Tuong ZK, Negro-Demontel ML, Kumar N, Suchanek O, Tajsic T, Harcourt K, Scott K, Bashford-Rogers R, Helmy A, Reich DS, Belkaid Y, Lawley TD, McGavern DB, Clatworthy MR. Gut-educated IgA plasma cells defend the meningeal venous sinuses. Nature. 2020;587:472–476. doi: 10.1038/s41586-020-2886-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Francistiová L, Klepe A, Curley G, Gulya K, Dinnyés A, Filkor K. Cellular and molecular effects of SARS-CoV-2 linking lung infection to the brain. Front Immunol. 2021;12:730088. doi: 10.3389/fimmu.2021.730088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gagliani N, Amezcua Vesely MC, Iseppon A, Brockmann L, Xu H, Palm NW, de Zoete MR, Licona-Limón P, Paiva RS, Ching T, Weaver C, Zi X, Pan X, Fan R, Garmire LX, Cotton MJ, Drier Y, Bernstein B, Geginat J, Stockinger B, et al. Th17 cells transdifferentiate into regulatory T cells during resolution of inflammation. Nature. 2015;523:221–225. doi: 10.1038/nature14452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gerganova G, Riddell A, Miller AA. CNS border-associated macrophages in the homeostatic and ischaemic brain. Pharmacol Ther. 2022;240:108220. doi: 10.1016/j.pharmthera.2022.108220. [DOI] [PubMed] [Google Scholar]
- 45.Geva-Zatorsky N, Sefik E, Kua L, Pasman L, Tan TG, Ortiz-Lopez A, Yanortsang TB, Yang L, Jupp R, Mathis D, Benoist C, Kasper DL. Mining the human gut microbiota for immunomodulatory organisms. Cell. 2017;168:928–943.e11. doi: 10.1016/j.cell.2017.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Giovannoni F, Quintana FJ. The role of astrocytes in CNS inflammation. Trends Immunol. 2020;41:805–819. doi: 10.1016/j.it.2020.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.González-López A, López-Alonso I, Pickerodt PA, von Haefen C, Amado-Rodríguez L, Reimann H, Niendorf T, Kuebler W, Albaiceta GM, Francis RCE, Spies CD. Lung purinoceptor activation triggers ventilator-induced brain injury. Crit Care Med. 2019;47:e911–e918. doi: 10.1097/CCM.0000000000003977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gülke E, Gelderblom M, Magnus T. Danger signals in stroke and their role on microglia activation after ischemia. Ther Adv Neurol Disord. 2018;11:1756286418774254. doi: 10.1177/1756286418774254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guo HH, Shen HR, Tang MZ, Sheng N, Ding X, Lin Y, Zhang JL, Jiang JD, Gao TL, Wang LL, Han YX. Microbiota-derived short-chain fatty acids mediate the effects of Dengzhan Shengmai in ameliorating cerebral ischemia via the gut-brain axis. J Ethnopharmacol. 2023;306:116158. doi: 10.1016/j.jep.2023.116158. [DOI] [PubMed] [Google Scholar]
- 50.Hannawi Y, Hannawi B, Rao CP, Suarez JI, Bershad EM. Stroke-associated pneumonia:major advances and obstacles. Cerebrovasc Dis. 2013;35:430–443. doi: 10.1159/000350199. [DOI] [PubMed] [Google Scholar]
- 51.Heuer JF, Pelosi P, Hermann P, Perske C, Crozier TA, Brück W, Quintel M. Acute effects of intracranial hypertension and ARDS on pulmonary and neuronal damage:a randomized experimental study in pigs. Intensive Care Med. 2011;37:1182–1191. doi: 10.1007/s00134-011-2232-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hosang L, Canals RC, van der Flier FJ, Hollensteiner J, Daniel R, Flügel A, Odoardi F. The lung microbiome regulates brain autoimmunity. Nature. 2022;603:138–144. doi: 10.1038/s41586-022-04427-4. [DOI] [PubMed] [Google Scholar]
- 53.Houlden A, Goldrick M, Brough D, Vizi ES, Lénárt N, Martinecz B, Roberts IS, Denes A. Brain injury induces specific changes in the caecal microbiota of mice via altered autonomic activity and mucoprotein production. Brain Behav Immun. 2016;57:10–20. doi: 10.1016/j.bbi.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huang Q, Di L, Yu F, Feng X, Liu Z, Wei M, Luo Y, Xia J. Alterations in the gut microbiome with hemorrhagic transformation in experimental stroke. CNS Neurosci Ther. 2022;28:77–91. doi: 10.1111/cns.13736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Iadecola C, Buckwalter MS, Anrather J. Immune responses to stroke:mechanisms, modulation, and therapeutic potential. J Clin Invest. 2020;130:2777–2788. doi: 10.1172/JCI135530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ibiza S, García-Cassani B, Ribeiro H, Carvalho T, Almeida L, Marques R, Misic AM, Bartow-McKenney C, Larson DM, Pavan WJ, Eberl G, Grice EA, Veiga-Fernandes H. Glial-cell-derived neuroregulators control type 3 innate lymphoid cells and gut defence. Nature. 2016;535:440–443. doi: 10.1038/nature18644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Iida H, Iida M, Takenaka M, Fukuoka N, Dohi S. Rho-kinase inhibitor and nicotinamide adenine dinucleotide phosphate oxidase inhibitor prevent impairment of endothelium-dependent cerebral vasodilation by acute cigarette smoking in rats. J Renin Angiotensin Aldosterone Syst. 2008;9:89–94. doi: 10.3317/jraas.2008.012. [DOI] [PubMed] [Google Scholar]
- 60.Inose Y, Kato Y, Kitagawa K, Uchiyama S, Shibata N. Activated microglia in ischemic stroke penumbra upregulate MCP-1 and CCR2 expression in response to lysophosphatidylcholine derived from adjacent neurons and astrocytes. Neuropathology. 2015;35:209–223. doi: 10.1111/neup.12182. [DOI] [PubMed] [Google Scholar]
- 61.Iroegbu JD, Ifenatuoha CW, Ijomone OM. Potential neurological impact of coronaviruses:implications for the novel SARS-CoV-2. Neurol Sci. 2020;41:1329–1337. doi: 10.1007/s10072-020-04469-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ito M, Komai K, Mise-Omata S, Iizuka-Koga M, Noguchi Y, Kondo T, Sakai R, Matsuo K, Nakayama T, Yoshie O, Nakatsukasa H, Chikuma S, Shichita T, Yoshimura A. Brain regulatory T cells suppress astrogliosis and potentiate neurological recovery. Nature. 2019;565:246–250. doi: 10.1038/s41586-018-0824-5. [DOI] [PubMed] [Google Scholar]
- 63.Jacobs JP, Gupta A, Bhatt RR, Brawer J, Gao K, Tillisch K, Lagishetty V, Firth R, Gudleski GD, Ellingson BM, Labus JS, Naliboff BD, Lackner JM, Mayer EA. Cognitive behavioral therapy for irritable bowel syndrome induces bidirectional alterations in the brain-gut-microbiome axis associated with gastrointestinal symptom improvement. Microbiome. 2021;9:236. doi: 10.1186/s40168-021-01188-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jang SE, Lim SM, Jeong JJ, Jang HM, Lee HJ, Han MJ, Kim DH. Gastrointestinal inflammation by gut microbiota disturbance induces memory impairment in mice. Mucosal Immunol. 2018;11:369–379. doi: 10.1038/mi.2017.49. [DOI] [PubMed] [Google Scholar]
- 65.Johnston KC, Li JY, Lyden PD, Hanson SK, Feasby TE, Adams RJ, Faught RE, Jr, Haley EC., Jr Medical and neurological complications of ischemic stroke:experience from the RANTTAS trial. RANTTAS Investigators. Stroke. 1998;29:447–453. doi: 10.1161/01.str.29.2.447. [DOI] [PubMed] [Google Scholar]
- 66.Kayama H, Okumura R, Takeda K. Interaction between the microbiota, epithelia, and immune cells in the intestine. Annu Rev Immunol. 2020;38:23–48. doi: 10.1146/annurev-immunol-070119-115104. [DOI] [PubMed] [Google Scholar]
- 67.Kazarian M, Laird-Offringa IA. Small-cell lung cancer-associated autoantibodies:potential applications to cancer diagnosis, early detection, and therapy. Mol Cancer. 2011;10:33. doi: 10.1186/1476-4598-10-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim ID, Lee H, Kim SW, Lee HK, Choi J, Han PL, Lee JK. Alarmin HMGB1 induces systemic and brain inflammatory exacerbation in post-stroke infection rat model. Cell Death Dis. 2018;9:426. doi: 10.1038/s41419-018-0438-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim MH, Kang SG, Park JH, Yanagisawa M, Kim CH. Short-chain fatty acids activate GPR41 and GPR43 on intestinal epithelial cells to promote inflammatory responses in mice. Gastroenterology. 2013;145:396–406.e1-10. doi: 10.1053/j.gastro.2013.04.056. [DOI] [PubMed] [Google Scholar]
- 70.Kim YJ, Lee JY, Lee JJ, Jeon SM, Silwal P, Kim IS, Kim HJ, Park CR, Chung C, Han JE, Choi JW, Tak EJ, Yoo JH, Jeong SW, Kim DY, Ketphan W, Kim SY, Jhun BW, Whang J, Kim JM, et al. Arginine-mediated gut microbiome remodeling promotes host pulmonary immune defense against nontuberculous mycobacterial infection. Gut Microbes. 2022;14:2073132. doi: 10.1080/19490976.2022.2073132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Komaroff AL. The microbiome and risk for atherosclerosis. JAMA. 2018;319:2381–2382. doi: 10.1001/jama.2018.5240. [DOI] [PubMed] [Google Scholar]
- 72.Kulick ER, Kaufman JD, Sack C. Ambient air pollution and stroke:an updated review. Stroke. 2023;54:882–893. doi: 10.1161/STROKEAHA.122.035498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Langness S, Kojima M, Coimbra R, Eliceiri BP, Costantini TW. Enteric glia cells are critical to limiting the intestinal inflammatory response after injury. Am J Physiol Gastrointest Liver Physiol. 2017;312:G274–G282. doi: 10.1152/ajpgi.00371.2016. [DOI] [PubMed] [Google Scholar]
- 74.Lee CYD, Daggett A, Gu X, Jiang LL, Langfelder P, Li X, Wang N, Zhao Y, Park CS, Cooper Y, Ferando I, Mody I, Coppola G, Xu H, Yang XW. Elevated TREM2 gene dosage reprograms microglia responsivity and ameliorates pathological phenotypes in Alzheimer's disease models. Neuron. 2018a;97:1032–1048.e5. doi: 10.1016/j.neuron.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee GA, Lin TN, Chen CY, Mau SY, Huang WZ, Kao YC, Ma RY, Liao NS. Interleukin 15 blockade protects the brain from cerebral ischemia-reperfusion injury. Brain Behav Immun. 2018b;73:562–570. doi: 10.1016/j.bbi.2018.06.021. [DOI] [PubMed] [Google Scholar]
- 76.Li C, Chen W, Lin F, Li W, Wang P, Liao G, Zhang L. Functional two-way crosstalk between brain and lung:the brain-lung axis. Cell Mol Neurobiol. 2023;43:991–1003. doi: 10.1007/s10571-022-01238-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li M, Chen S, Shi X, Lyu C, Zhang Y, Tan M, Wang C, Zang N, Liu X, Hu Y, Shen J, Zhou L, Gu Y. Cell permeable HMGB1-binding heptamer peptide ameliorates neurovascular complications associated with thrombolytic therapy in rats with transient ischemic stroke. J Neuroinflammation. 2018;15:237. doi: 10.1186/s12974-018-1267-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li N, Wang X, Sun C, Wu X, Lu M, Si Y, Ye X, Wang T, Yu X, Zhao X, Wei N, Wang X. Change of intestinal microbiota in cerebral ischemic stroke patients. BMC Microbiol. 2019;19:191. doi: 10.1186/s12866-019-1552-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li P, Gan Y, Sun BL, Zhang F, Lu B, Gao Y, Liang W, Thomson AW, Chen J, Hu X. Adoptive regulatory T-cell therapy protects against cerebral ischemia. Ann Neurol. 2013;74:458–471. doi: 10.1002/ana.23815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liddelow SA, Barres BA. Reactive astrocytes:production, function, and therapeutic potential. Immunity. 2017;46:957–967. doi: 10.1016/j.immuni.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 81.Liesz A, Suri-Payer E, Veltkamp C, Doerr H, Sommer C, Rivest S, Giese T, Veltkamp R. Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nat Med. 2009;15:192–199. doi: 10.1038/nm.1927. [DOI] [PubMed] [Google Scholar]
- 82.Liesz A, Hu X, Kleinschnitz C, Offner H. Functional role of regulatory lymphocytes in stroke:facts and controversies. Stroke. 2015;46:1422–1430. doi: 10.1161/STROKEAHA.114.008608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ling Z, Liu X, Cheng Y, Yan X, Wu S. Gut microbiota and aging. Crit Rev Food Sci Nutr. 2022;62:3509–3534. doi: 10.1080/10408398.2020.1867054. [DOI] [PubMed] [Google Scholar]
- 84.Liu M, Wu B, Wang WZ, Lee LM, Zhang SH, Kong LZ. Stroke in China:epidemiology, prevention, and management strategies. Lancet Neurol. 2007;6:456–464. doi: 10.1016/S1474-4422(07)70004-2. [DOI] [PubMed] [Google Scholar]
- 85.Logsdon AF, Erickson MA, Rhea EM, Salameh TS, Banks WA. Gut reactions:how the blood-brain barrier connects the microbiome and the brain. Exp Biol Med (Maywood) 2018;243:159–165. doi: 10.1177/1535370217743766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lu H, Gao NL, Tong F, Wang J, Li H, Zhang R, Ma H, Yang N, Zhang Y, Wang Y, Liang Z, Zeng H, Chen WH, Dong X. Alterations of the human lung and gut microbiomes in non-small cell lung carcinomas and distant metastasis. Microbiol Spectr. 2021;9:e0080221. doi: 10.1128/Spectrum.00802-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lundmark K, Westermark GT, Olsén A, Westermark P. Protein fibrils in nature can enhance amyloid protein A amyloidosis in mice:Cross-seeding as a disease mechanism. Proc Natl Acad Sci U S A. 2005;102:6098–6102. doi: 10.1073/pnas.0501814102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mahadeva R, Walsh G, Flower CD, Shneerson JM. Clinical and radiological characteristics of lung disease in inflammatory bowel disease. Eur Respir J. 2000;15:41–48. doi: 10.1183/09031936.00.15104100. [DOI] [PubMed] [Google Scholar]
- 89.Mascia L. Acute lung injury in patients with severe brain injury:a double hit model. Neurocrit Care. 2009;11:417–426. doi: 10.1007/s12028-009-9242-8. [DOI] [PubMed] [Google Scholar]
- 90.Matcovitch-Natan O, Winter DR, Giladi A, Vargas Aguilar S, Spinrad A, Sarrazin S, Ben-Yehuda H, David E, Zelada González F, Perrin P, Keren-Shaul H, Gury M, Lara-Astaiso D, Thaiss CA, Cohen M, Bahar Halpern K, Baruch K, Deczkowska A, Lorenzo-Vivas E, Itzkovitz S, et al. Microglia development follows a stepwise program to regulate brain homeostasis. Science. 2016;353:aad8670. doi: 10.1126/science.aad8670. [DOI] [PubMed] [Google Scholar]
- 91.Mayhan WG, Arrick DM, Sharpe GM, Sun H. Age-related alterations in reactivity of cerebral arterioles:role of oxidative stress. Microcirculation. 2008;15:225–236. doi: 10.1080/10739680701641421. [DOI] [PubMed] [Google Scholar]
- 92.Meaney JFM, O'Donnell JS, Bridgewood C, Harbison J, McGonagle D. Perspective:The case for acute large vessel ischemic stroke in COVID-19 originating within thrombosed pulmonary venules. Stroke. 2022;53:2411–2419. doi: 10.1161/STROKEAHA.121.038056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Meng HL, Li XX, Chen YT, Yu LJ, Zhang H, Lao JM, Zhang X, Xu Y. Neuronal soluble Fas ligand drives M1-microglia polarization after cerebral ischemia. CNS Neurosci Ther. 2016;22:771–781. doi: 10.1111/cns.12575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mrozek S, Gobin J, Constantin JM, Fourcade O, Geeraerts T. Crosstalk between brain, lung and heart in critical care. Anaesth Crit Care Pain Med. 2020;39:519–530. doi: 10.1016/j.accpm.2020.06.016. [DOI] [PubMed] [Google Scholar]
- 95.Murray KN, Buggey HF, Denes A, Allan SM. Systemic immune activation shapes stroke outcome. Mol Cell Neurosci. 2013;53:14–25. doi: 10.1016/j.mcn.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 96.Nathan C. Neutrophils and immunity:challenges and opportunities. Nat Rev Immunol. 2006;6:173–182. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- 97.Neher JJ, Emmrich JV, Fricker M, Mander PK, Théry C, Brown GC. Phagocytosis executes delayed neuronal death after focal brain ischemia. Proc Natl Acad Sci U S A. 2013;110:E4098–4107. doi: 10.1073/pnas.1308679110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ney LM, Wipplinger M, Grossmann M, Engert N, Wegner VD, Mosig AS. Short chain fatty acids:key regulators of the local and systemic immune response in inflammatory diseases and infections. Open Biol. 2023;13:230014. doi: 10.1098/rsob.230014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Offner PJ, Moore EE, Ciesla D. The adrenal response after severe trauma. Am J Surg. 2002;184:649–653. doi: 10.1016/s0002-9610(02)01101-7. discussion 653-654. [DOI] [PubMed] [Google Scholar]
- 100.Oliveira AC, Richards EM, Raizada MK. Pulmonary hypertension:pathophysiology beyond the lung. Pharmacol Res. 2020;151:104518. doi: 10.1016/j.phrs.2019.104518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Osadchiy V, Martin CR, Mayer EA. Gut microbiome and modulation of CNS function. Compr Physiol. 2019;10:57–72. doi: 10.1002/cphy.c180031. [DOI] [PubMed] [Google Scholar]
- 102.Ott L, McClain CJ, Gillespie M, Young B. Cytokines and metabolic dysfunction after severe head injury. J Neurotrauma. 1994;11:447–472. doi: 10.1089/neu.1994.11.447. [DOI] [PubMed] [Google Scholar]
- 103.Park EM, Cho S, Frys KA, Glickstein SB, Zhou P, Anrather J, Ross ME, Iadecola C. Inducible nitric oxide synthase contributes to gender differences in ischemic brain injury. J Cereb Blood Flow Metab. 2006;26:392–401. doi: 10.1038/sj.jcbfm.9600194. [DOI] [PubMed] [Google Scholar]
- 104.Pekny M, Pekna M, Messing A, Steinhäuser C, Lee JM, Parpura V, Hol EM, Sofroniew MV, Verkhratsky A. Astrocytes:a central element in neurological diseases. Acta Neuropathol. 2016;131:323–345. doi: 10.1007/s00401-015-1513-1. [DOI] [PubMed] [Google Scholar]
- 105.Pellegrini C, Fornai M, D'Antongiovanni V, Antonioli L, Bernardini N, Derkinderen P. The intestinal barrier in disorders of the central nervous system. Lancet Gastroenterol Hepatol. 2023;8:66–80. doi: 10.1016/S2468-1253(22)00241-2. [DOI] [PubMed] [Google Scholar]
- 106.Pelosi P, Severgnini P, Chiaranda M. An integrated approach to prevent and treat respiratory failure in brain-injured patients. Curr Opin Crit Care. 2005;11:37–42. doi: 10.1097/00075198-200502000-00006. [DOI] [PubMed] [Google Scholar]
- 107.Pelosi P, Rocco PR. The lung and the brain:a dangerous cross-talk. Crit Care. 2011;15:168. doi: 10.1186/cc10259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pelosi P, Ferguson ND, Frutos-Vivar F, Anzueto A, Putensen C, Raymondos K, Apezteguia C, Desmery P, Hurtado J, Abroug F, Elizalde J, Tomicic V, Cakar N, Gonzalez M, Arabi Y, Moreno R, Esteban A Ventila Study Group. Management and outcome of mechanically ventilated neurologic patients. Crit Care Med. 2011;39:1482–1492. doi: 10.1097/CCM.0b013e31821209a8. [DOI] [PubMed] [Google Scholar]
- 109.Piepke M, Clausen BH, Ludewig P, Vienhues JH, Bedke T, Javidi E, Rissiek B, Jank L, Brockmann L, Sandrock I, Degenhardt K, Jander A, Roth V, Schädlich IS, Prinz I, Flavell RA, Kobayashi Y, Renné T, Gerloff C, Huber S, et al. Interleukin-10 improves stroke outcome by controlling the detrimental interleukin-17A response. J Neuroinflammation. 2021;18:265. doi: 10.1186/s12974-021-02316-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pluta R, Januszewski S, Czuczwar SJ. The role of gut microbiota in an ischemic stroke. Int J Mol Sci. 2021;22:915. doi: 10.3390/ijms22020915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Prass K, Meisel C, Höflich C, Braun J, Halle E, Wolf T, Ruscher K, Victorov IV, Priller J, Dirnagl U, Volk HD, Meisel A. Stroke-induced immunodeficiency promotes spontaneous bacterial infections and is mediated by sympathetic activation reversal by poststroke T helper cell type 1-like immunostimulation. J Exp Med. 2003;198:725–736. doi: 10.1084/jem.20021098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Prinz I, Silva-Santos B, Pennington DJ. Functional development of γδT cells. Eur J Immunol. 2013;43:1988–1994. doi: 10.1002/eji.201343759. [DOI] [PubMed] [Google Scholar]
- 113.Pröbstel AK, Zhou X, Baumann R, Wischnewski S, Kutza M, Rojas OL, Sellrie K, Bischof A, Kim K, Ramesh A, Dandekar R, Greenfield AL, Schubert RD, Bisanz JE, Vistnes S, Khaleghi K, Landefeld J, Kirkish G, Liesche-Starnecker F, Ramaglia V, et al. Gut microbiota-specific IgA(+. B cells traffic to the CNS in active multiple sclerosis. Sci Immunol. 2020;5:eabc7191. doi: 10.1126/sciimmunol.abc7191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Qin C, Yang S, Chu YH, Zhang H, Pang XW, Chen L, Zhou LQ, Chen M, Tian DS, Wang W. Signaling pathways involved in ischemic stroke:molecular mechanisms and therapeutic interventions. Signal Transduct Target Ther. 2022;7:215. doi: 10.1038/s41392-022-01064-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, Liang S, Zhang W, Guan Y, Shen D, Peng Y, Zhang D, Jie Z, Wu W, Qin Y, Xue W, Li J, Han L, Lu D, Wu P, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490:55–60. doi: 10.1038/nature11450. [DOI] [PubMed] [Google Scholar]
- 116.Ran X, He Y, Ai Q, Shi Y. Effect of antibiotic-induced intestinal dysbacteriosis on bronchopulmonary dysplasia and related mechanisms. J Transl Med. 2021;19:155. doi: 10.1186/s12967-021-02794-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ren X, Akiyoshi K, Dziennis S, Vandenbark AA, Herson PS, Hurn PD, Offner H. Regulatory B cells limit CNS inflammation and neurologic deficits in murine experimental stroke. J Neurosci. 2011;31:8556–8563. doi: 10.1523/JNEUROSCI.1623-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids--new mechanisms for old drugs. N Engl J Med. 2005;353:1711–1723. doi: 10.1056/NEJMra050541. [DOI] [PubMed] [Google Scholar]
- 119.Rojas OL, Pröbstel AK, Porfilio EA, Wang AA, Charabati M, Sun T, Lee DSW, Galicia G, Ramaglia V, Ward LA, Leung LYT, Najafi G, Khaleghi K, Garcillán B, Li A, Besla R, Naouar I, Cao EY, Chiaranunt P, Burrows K, et al. Recirculating intestinal iga-producing cells regulate neuroinflammation via IL-10. Cell. 2019;176:610–624.e18. doi: 10.1016/j.cell.2018.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Roselli F, Huber-Lang M. TREM1-ors shake the brain and gut after stroke. Nat Immunol. 2019;20:950–952. doi: 10.1038/s41590-019-0443-9. [DOI] [PubMed] [Google Scholar]
- 121.Rothhammer V, Borucki DM, Tjon EC, Takenaka MC, Chao CC, Ardura-Fabregat A, de Lima KA, Gutiérrez-Vázquez C, Hewson P, Staszewski O, Blain M, Healy L, Neziraj T, Borio M, Wheeler M, Dragin LL, Laplaud DA, Antel J, Alvarez JI, Prinz M, et al. Microglial control of astrocytes in response to microbial metabolites. Nature. 2018;557:724–728. doi: 10.1038/s41586-018-0119-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ryuk JA, Ko BS, Moon NR, Park S. Protection against neurological symptoms by consuming corn silk water extract in artery-occluded gerbils with reducing oxidative stress, inflammation, and post-stroke hyperglycemia through the gut-brain axis. Antioxidants (Basel) 2022;11:168. doi: 10.3390/antiox11010168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Saad MJ, Santos A, Prada PO. Linking gut microbiota and inflammation to obesity and insulin resistance. Physiology (Bethesda) 2016;31:283–293. doi: 10.1152/physiol.00041.2015. [DOI] [PubMed] [Google Scholar]
- 124.Samary CS, Ramos AB, Maia LA, Rocha NN, Santos CL, Magalhães RF, Clevelario AL, Pimentel-Coelho PM, Mendez-Otero R, Cruz FF, Capelozzi VL, Ferreira TPT, Koch T, de Abreu MG, Dos Santos CC, Pelosi P, Silva PL, Rocco PRM. Focal ischemic stroke leads to lung injury and reduces alveolar macrophage phagocytic capability in rats. Crit Care. 2018;22:249. doi: 10.1186/s13054-018-2164-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sanmarco LM, Wheeler MA, Gutiérrez-Vázquez C, Polonio CM, Linnerbauer M, Pinho-Ribeiro FA, Li Z, Giovannoni F, Batterman KV, Scalisi G, Zandee SEJ, Heck ES, Alsuwailm M, Rosene DL, Becher B, Chiu IM, Prat A, Quintana FJ. Gut-licensed IFNγ(+) NK cells drive LAMP1(+)TRAIL(+) anti-inflammatory astrocytes. Nature. 2021;590:473–479. doi: 10.1038/s41586-020-03116-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Santos Samary C, Pelosi P, Leme Silva P, Rieken Macedo Rocco P. Immunomodulation after ischemic stroke:potential mechanisms and implications for therapy. Crit Care. 2016;20:391. doi: 10.1186/s13054-016-1573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Scott SA, Fu J, Chang PV. Microbial tryptophan metabolites regulate gut barrier function via the aryl hydrocarbon receptor. Proc Natl Acad Sci U S A. 2020;117:19376–19387. doi: 10.1073/pnas.2000047117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Seguella L, Gulbransen BD. Enteric glial biology, intercellular signalling and roles in gastrointestinal disease. Nat Rev Gastroenterol Hepatol. 2021;18:571–587. doi: 10.1038/s41575-021-00423-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Shale M, Schiering C, Powrie F. CD4(+) T-cell subsets in intestinal inflammation. Immunol Rev. 2013;252:164–182. doi: 10.1111/imr.12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sharma RK, Stevens BR, Obukhov AG, Grant MB, Oudit GY, Li Q, Richards EM, Pepine CJ, Raizada MK. ACE2 (angiotensin-converting enzyme 2) in cardiopulmonary diseases:ramifications for the control of SARS-CoV-2. Hypertension. 2020a;76:651–661. doi: 10.1161/HYPERTENSIONAHA.120.15595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sharma RK, Oliveira AC, Yang T, Karas MM, Li J, Lobaton GO, Aquino VP, Robles-Vera I, de Kloet AD, Krause EG, Bryant AJ, Verma A, Li Q, Richards EM, Raizada MK. Gut pathology and its rescue by ACE2 (angiotensin-converting enzyme 2) in hypoxia-induced pulmonary hypertension. hypertension. 2020b;76:206–216. doi: 10.1161/HYPERTENSIONAHA.120.14931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Shen J, Guo H, Liu S, Jin W, Zhang ZW, Zhang Y, Liu K, Mao S, Zhou Z, Xie L, Wang G, Hao H, Liang Y. Aberrant branched-chain amino acid accumulation along the microbiota-gut-brain axis:crucial targets affecting the occurrence and treatment of ischaemic stroke. Br J Pharmacol. 2023;180:347–368. doi: 10.1111/bph.15965. [DOI] [PubMed] [Google Scholar]
- 133.Shi N, Li N, Duan X, Niu H. Interaction between the gut microbiome and mucosal immune system. Mil Med Res. 2017;4:14. doi: 10.1186/s40779-017-0122-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Shichita T, Sugiyama Y, Ooboshi H, Sugimori H, Nakagawa R, Takada I, Iwaki T, Okada Y, Iida M, Cua DJ, Iwakura Y, Yoshimura A. Pivotal role of cerebral interleukin-17-producing gammadeltaT cells in the delayed phase of ischemic brain injury. Nat Med. 2009;15:946–950. doi: 10.1038/nm.1999. [DOI] [PubMed] [Google Scholar]
- 135.Shin JA, Jeong SI, Kim M, Yoon JC, Kim HS, Park EM. Visceral adipose tissue inflammation is associated with age-related brain changes and ischemic brain damage in aged mice. Brain Behav Immun. 2015;50:221–231. doi: 10.1016/j.bbi.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 136.Siebler J, Galle PR, Weber MM. The gut-liver-axis:endotoxemia, inflammation, insulin resistance and NASH. J Hepatol. 2008;48:1032–1034. doi: 10.1016/j.jhep.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 137.Singh V, Roth S, Llovera G, Sadler R, Garzetti D, Stecher B, Dichgans M, Liesz A. Microbiota dysbiosis controls the neuroinflammatory response after stroke. J Neurosci. 2016;36:7428–7440. doi: 10.1523/JNEUROSCI.1114-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Singh V, Beer A, Kraus A, Mang F, Zhang X, Xue J, Hagemann N, Hermann DM, Gunzer M. Stroke increases the expression of ACE2, the SARS-CoV-2 binding receptor, in murine lungs. Brain Behav Immun. 2021;94:458–462. doi: 10.1016/j.bbi.2021.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Smith CJ, Bray BD, Hoffman A, Meisel A, Heuschmann PU, Wolfe CD, Tyrrell PJ, Rudd AG. Can a novel clinical risk score improve pneumonia prediction in acute stroke care?A UK multicenter cohort study. J Am Heart Assoc. 2015;4:e001307. doi: 10.1161/JAHA.114.001307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sorboni SG, Moghaddam HS, Jafarzadeh-Esfehani R, Soleimanpour S. A comprehensive review on the role of the gut microbiome in human neurological disorders. Clin Microbiol Rev. 2022;35:e0033820. doi: 10.1128/CMR.00338-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Spychala MS, Venna VR, Jandzinski M, Doran SJ, Durgan DJ, Ganesh BP, Ajami NJ, Putluri N, Graf J, Bryan RM, McCullough LD. Age-related changes in the gut microbiota influence systemic inflammation and stroke outcome. Ann Neurol. 2018;84:23–36. doi: 10.1002/ana.25250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Stanley D, Mason LJ, Mackin KE, Srikhanta YN, Lyras D, Prakash MD, Nurgali K, Venegas A, Hill MD, Moore RJ, Wong CH. Translocation and dissemination of commensal bacteria in post-stroke infection. Nat Med. 2016;22:1277–1284. doi: 10.1038/nm.4194. [DOI] [PubMed] [Google Scholar]
- 143.Stanley D, Moore RJ, Wong CHY. An insight into intestinal mucosal microbiota disruption after stroke. Sci Rep. 2018;8:568. doi: 10.1038/s41598-017-18904-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Stubbe T, Ebner F, Richter D, Engel O, Klehmet J, Royl G, Meisel A, Nitsch R, Meisel C, Brandt C. Regulatory T cells accumulate and proliferate in the ischemic hemisphere for up to 30 days after MCAO. J Cereb Blood Flow Metab. 2013;33:37–47. doi: 10.1038/jcbfm.2012.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sumbria RK, Boado RJ, Pardridge WM. Brain protection from stroke with intravenous TNFαdecoy receptor-Trojan horse fusion protein. J Cereb Blood Flow Metab. 2012;32:1933–1938. doi: 10.1038/jcbfm.2012.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Uesaka T, Young HM, Pachnis V, Enomoto H. Development of the intrinsic and extrinsic innervation of the gut. Dev Biol. 2016;417:158–167. doi: 10.1016/j.ydbio.2016.04.016. [DOI] [PubMed] [Google Scholar]
- 147.Umahara T, Uchihara T, Hirokawa K, Hirao K, Shimizu S, Hashimoto T, Terasi H, Hanyu H. Time-dependent and lesion-dependent HMGB1-selective localization in brains of patients with cerebrovascular diseases. Histol Histopathol. 2018;33:215–222. doi: 10.14670/HH-11-914. [DOI] [PubMed] [Google Scholar]
- 148.Verhoeven JI, Allach Y, Vaartjes ICH, Klijn CJM, de Leeuw FE. Ambient air pollution and the risk of ischaemic and haemorrhagic stroke. Lancet Planet Health. 2021;5:e542–e552. doi: 10.1016/S2542-5196(21)00145-5. [DOI] [PubMed] [Google Scholar]
- 149.Violi F, Cangemi R, Calvieri C. Pneumonia, thrombosis and vascular disease. J Thromb Haemost. 2014;12:1391–1400. doi: 10.1111/jth.12646. [DOI] [PubMed] [Google Scholar]
- 150.Walter U, Kolbaske S, Patejdl R, Steinhagen V, Abu-Mugheisib M, Grossmann A, Zingler C, Benecke R. Insular stroke is associated with acute sympathetic hyperactivation and immunodepression. Eur J Neurol. 2013;20:153–159. doi: 10.1111/j.1468-1331.2012.03818.x. [DOI] [PubMed] [Google Scholar]
- 151.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wang J, Zhang H, He J, Xiong X. The role of the gut microbiota in the development of ischemic stroke. Front Immunol. 2022a;13:845243. doi: 10.3389/fimmu.2022.845243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wang L, Wang L, Wang H, Zhu T. Investigation into the potential mechanism and molecular targets of Fufang Xueshuantong capsule for the treatment of ischemic stroke based on network pharmacology and molecular docking. Front Pharmacol. 2022b;13:949644. doi: 10.3389/fphar.2022.949644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wang L, Zhu T, Xu HB, Pu XP, Zhao X, Tian F, Ding T, Sun GB, Sun XB. Effects of notoginseng leaf triterpenes on small molecule metabolism after cerebral ischemia/reperfusion injury assessed using MALDI-MS imaging. Ann Transl Med. 2021a;9:246. doi: 10.21037/atm-20-4898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wang S, van de Pavert SA. Innate lymphoid cells in the central nervous system. Front Immunol. 2022;13:837250. doi: 10.3389/fimmu.2022.837250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wang S, de Fabritus L, Kumar PA, Werner Y, Ma M, Li D, Siret C, Simic M, Li B, Kerdiles YM, Hou L, Stumm R, van de Pavert SA. Brain endothelial CXCL12 attracts protective natural killer cells during ischemic stroke. J Neuroinflammation. 2023;20:8. doi: 10.1186/s12974-023-02689-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wang XQ, Li H, Li XN, Yuan CH, Zhao H. Gut-brain axis:possible role of gut microbiota in perioperative neurocognitive disorders. Front Aging Neurosci. 2021b;13:745774. doi: 10.3389/fnagi.2021.745774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wastyk HC, Fragiadakis GK, Perelman D, Dahan D, Merrill BD, Yu FB, Topf M, Gonzalez CG, Van Treuren W, Han S, Robinson JL, Elias JE, Sonnenburg ED, Gardner CD, Sonnenburg JL. Gut-microbiota-targeted diets modulate human immune status. Cell. 2021;184:4137–4153.e14. doi: 10.1016/j.cell.2021.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wen SW, Shim R, Ho L, Wanrooy BJ, Srikhanta YN, Prame Kumar K, Nicholls AJ, Shen SJ, Sepehrizadeh T, de Veer M, Srikanth VK, Ma H, Phan TG, Lyras D, Wong CHY. Advanced age promotes colonic dysfunction and gut-derived lung infection after stroke. Aging Cell. 2019;18:e12980. doi: 10.1111/acel.12980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wen SW, Shim R, Hall P, Bedo J, Wilson JL, Nicholls AJ, Hickey MJ, Wong CHY. Lung imaging reveals stroke-induced impairment in pulmonary intravascular neutrophil function, a response exacerbated with aging. J Immunol. 2022;208:2019–2028. doi: 10.4049/jimmunol.2100997. [DOI] [PubMed] [Google Scholar]
- 161.Wicks EE, Ran KR, Kim JE, Xu R, Lee RP, Jackson CM. The translational potential of microglia and monocyte-derived macrophages in ischemic stroke. Front Immunol. 2022;13:897022. doi: 10.3389/fimmu.2022.897022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Winklewski PJ, Radkowski M, Demkow U. Cross-talk between the inflammatory response, sympathetic activation and pulmonary infection in the ischemic stroke. J Neuroinflammation. 2014;11:213. doi: 10.1186/s12974-014-0213-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wu Y, Li J, Shou J, Zhang W, Chen C. Diverse functions and mechanisms of regulatory T cell in ischemic stroke. Exp Neurol. 2021;343:113782. doi: 10.1016/j.expneurol.2021.113782. [DOI] [PubMed] [Google Scholar]
- 164.Xiao T, Wijnant SRA, van der Velpen I, Terzikhan N, Lahousse L, Ikram MK, Vernooij MW, Brusselle GG, Ikram MA. Lung function impairment in relation to cognition and vascular brain lesions:the Rotterdam Study. J Neurol. 2022;269:4141–4153. doi: 10.1007/s00415-022-11027-9. [DOI] [PubMed] [Google Scholar]
- 165.Xie B, Zhang Y, Han M, Wang M, Yu Y, Chen X, Wu Y, Hashimoto K, Yuan S, Shang Y, Zhang J. Reversal of the detrimental effects of social isolation on ischemic cerebral injury and stroke-associated pneumonia by inhibiting small intestinal γδT-cell migration into the brain and lung. J Cereb Blood Flow Metab. 2023 doi: 10.1177/0271678X231167946. doi:10.1177/0271678X231167946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Xiong XY, Liu L, Yang QW. Refocusing neuroprotection in cerebral reperfusion era:new challenges and strategies. Front Neurol. 2018;9:249. doi: 10.3389/fneur.2018.00249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Xu K, Gao X, Xia G, Chen M, Zeng N, Wang S, You C, Tian X, Di H, Tang W, Li P, Wang H, Zeng X, Tan C, Meng F, Li H, He Y, Zhou H, Yin J. Rapid gut dysbiosis induced by stroke exacerbates brain infarction in turn. Gut. 2021a doi: 10.1136/gutjnl-2020-323263. doi:10.1136/gutjnl-2020-323263. [DOI] [PubMed] [Google Scholar]
- 168.Xu P, Zhang X, Liu Q, Xie Y, Shi X, Chen J, Li Y, Guo H, Sun R, Hong Y, Liu X, Xu G. Microglial TREM-1 receptor mediates neuroinflammatory injury via interaction with SYK in experimental ischemic stroke. Cell Death Dis. 2019;10:555. doi: 10.1038/s41419-019-1777-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Xu S, Lu J, Shao A, Zhang JH, Zhang J. Glial cells:role of the immune response in ischemic stroke. Front Immunol. 2020;11:294. doi: 10.3389/fimmu.2020.00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Xu Y, Zhu J, Feng B, Lin F, Zhou J, Liu J, Shi X, Lu X, Pan Q, Yu J, Zhang Y, Li L, Cao H. Immunosuppressive effect of mesenchymal stem cells on lung and gut CD8(+) T cells in lipopolysaccharide-induced acute lung injury in mice. Cell Prolif. 2021b;54:e13028. doi: 10.1111/cpr.13028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Yamashiro K, Tanaka R, Urabe T, Ueno Y, Yamashiro Y, Nomoto K, Takahashi T, Tsuji H, Asahara T, Hattori N. Gut dysbiosis is associated with metabolism and systemic inflammation in patients with ischemic stroke. PLoS One. 2017;12:e0171521. doi: 10.1371/journal.pone.0171521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Yang J, Wise L, Fukuchi KI. TLR4 cross-talk with NLRP3 inflammasome and complement signaling pathways in Alzheimer's disease. Front Immunol. 2020;11:724. doi: 10.3389/fimmu.2020.00724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Yang Z, Wei F, Zhang B, Luo Y, Xing X, Wang M, Chen R, Sun G, Sun X. Cellular immune signal exchange from ischemic stroke to intestinal lesions through brain-gut axis. Front Immunol. 2022;13:688619. doi: 10.3389/fimmu.2022.688619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, Magris M, Hidalgo G, Baldassano RN, Anokhin AP, Heath AC, Warner B, Reeder J, Kuczynski J, Caporaso JG, Lozupone CA, Lauber C, Clemente JC, Knights D, Knight R, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486:222–227. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Yilmaz G, Arumugam TV, Stokes KY, Granger DN. Role of T lymphocytes and interferon-gamma in ischemic stroke. Circulation. 2006;113:2105–2112. doi: 10.1161/CIRCULATIONAHA.105.593046. [DOI] [PubMed] [Google Scholar]
- 176.Yin J, Liao SX, He Y, Wang S, Xia GH, Liu FT, Zhu JJ, You C, Chen Q, Zhou L, Pan SY, Zhou HW. Dysbiosis of gut microbiota with reduced trimethylamine-N-oxide level in patients with large-artery atherosclerotic stroke or transient ischemic attack. J Am Heart Assoc. 2015;4:e002699. doi: 10.1161/JAHA.115.002699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Zeng J, Bao T, Yang K, Zhu X, Wang S, Xiang W, Ge A, Zeng L, Ge J. The mechanism of microglia-mediated immune inflammation in ischemic stroke and the role of natural botanical components in regulating microglia:A review. Front Immunol. 2022;13:1047550. doi: 10.3389/fimmu.2022.1047550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Zeng X, Gao X, Peng Y, Wu Q, Zhu J, Tan C, Xia G, You C, Xu R, Pan S, Zhou H, He Y, Yin J. Higher risk of stroke is correlated with increased opportunistic pathogen load and reduced levels of butyrate-producing bacteria in the gut. Front Cell Infect Microbiol. 2019;9:4. doi: 10.3389/fcimb.2019.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Zera KA, Buckwalter MS. The local and peripheral immune responses to stroke:implications for therapeutic development. Neurotherapeutics. 2020;17:414–435. doi: 10.1007/s13311-020-00844-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Zhang H, Huang Y, Li X, Han X, Hu J, Wang B, Zhang L, Zhuang P, Zhang Y. Dynamic Process of Secondary Pulmonary Infection in Mice With Intracerebral Hemorrhage. Front Immunol. 2021a;12:767155. doi: 10.3389/fimmu.2021.767155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Zhang L, Zhang Y, Jiang X, Mao L, Xia Y, Fan Y, Li N, Jiang Z, Qin X, Jiang Y, Liu G, Qiu F, Zhang J, Zou Z, Chen C. Disruption of the lung-gut-brain axis is responsible for cortex damage induced by pulmonary exposure to zinc oxide nanoparticles. Toxicology. 2023a;485:153390. doi: 10.1016/j.tox.2022.153390. [DOI] [PubMed] [Google Scholar]
- 182.Zhang Q, Deng P, Chen S, Xu H, Zhang Y, Chen H, Zhang J, Sun H. Electroacupuncture and human iPSC-derived small extracellular vesicles regulate the gut microbiota in ischemic stroke via the brain-gut axis. Front Immunol. 2023b;14:1107559. doi: 10.3389/fimmu.2023.1107559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Zhang T, Ryu MS, Wu X, Yang HJ, Jeong SJ, Seo JW, Jeong DY, Park S. Alleviation of neuronal cell death and memory deficit with chungkookjang made with bacillus amyloliquefaciens and bacillus subtilis potentially through promoting gut-brain axis in artery-occluded gerbils. Foods. 2021b;10:2697. doi: 10.3390/foods10112697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Zhao SC, Wang C, Xu H, Wu WQ, Chu ZH, Ma LS, Zhang YD, Liu F. Age-related differences in interferon regulatory factor-4 and -5 signaling in ischemic brains of mice. Acta Pharmacol Sin. 2017;38:1425–1434. doi: 10.1038/aps.2017.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Zhao X, Wang H, Sun G, Zhang J, Edwards NJ, Aronowski J. Neuronal interleukin-4 as a modulator of microglial pathways and ischemic brain damage. J Neurosci. 2015;35:11281–11291. doi: 10.1523/JNEUROSCI.1685-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Zhu T, Wang L, Wang LP, Wan Q. Therapeutic targets of neuroprotection and neurorestoration in ischemic stroke:Applications for natural compounds from medicinal herbs. Biomed Pharmacother. 2022;148:112719. doi: 10.1016/j.biopha.2022.112719. [DOI] [PubMed] [Google Scholar]
- 187.Zhu T, Wan Q. Pharmacological properties and mechanisms of Notoginsenoside R1 in ischemia-reperfusion injury. Chin J Traumatol. 2023;26:20–26. doi: 10.1016/j.cjtee.2022.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Zhu W, Romano KA, Li L, Buffa JA, Sangwan N, Prakash P, Tittle AN, Li XS, Fu X, Androjna C, DiDonato AJ, Brinson K, Trapp BD, Fischbach MA, Rey FE, Hajjar AM, DiDonato JA, Hazen SL. Gut microbes impact stroke severity via the trimethylamine N-oxide pathway. Cell Host Microbe. 2021;29:1199–1208.e5. doi: 10.1016/j.chom.2021.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]


