Abstract
Artificial intelligence can be indirectly applied to the repair of peripheral nerve injury. Specifically, it can be used to analyze and process data regarding peripheral nerve injury and repair, while study findings on peripheral nerve injury and repair can provide valuable data to enrich artificial intelligence algorithms. To investigate advances in the use of artificial intelligence in the diagnosis, rehabilitation, and scientific examination of peripheral nerve injury, we used CiteSpace and VOSviewer software to analyze the relevant literature included in the Web of Science from 1994–2023. We identified the following research hotspots in peripheral nerve injury and repair: (1) diagnosis, classification, and prognostic assessment of peripheral nerve injury using neuroimaging and artificial intelligence techniques, such as corneal confocal microscopy and coherent anti-Stokes Raman spectroscopy; (2) motion control and rehabilitation following peripheral nerve injury using artificial neural networks and machine learning algorithms, such as wearable devices and assisted wheelchair systems; (3) improving the accuracy and effectiveness of peripheral nerve electrical stimulation therapy using artificial intelligence techniques combined with deep learning, such as implantable peripheral nerve interfaces; (4) the application of artificial intelligence technology to brain-machine interfaces for disabled patients and those with reduced mobility, enabling them to control devices such as networked hand prostheses; (5) artificial intelligence robots that can replace doctors in certain procedures during surgery or rehabilitation, thereby reducing surgical risk and complications, and facilitating postoperative recovery. Although artificial intelligence has shown many benefits and potential applications in peripheral nerve injury and repair, there are some limitations to this technology, such as the consequences of missing or imbalanced data, low data accuracy and reproducibility, and ethical issues (e.g., privacy, data security, research transparency). Future research should address the issue of data collection, as large-scale, high-quality clinical datasets are required to establish effective artificial intelligence models. Multimodal data processing is also necessary, along with interdisciplinary collaboration, medical-industrial integration, and multicenter, large-sample clinical studies.
Keywords: artificial intelligence, artificial prosthesis, medical-industrial integration, brain-machine interface, deep learning, machine learning, networked hand prosthesis, neural interface, neural network, neural regeneration, peripheral nerve
Introduction
Artificial intelligence (AI) can be used to analyze and process data about neural regeneration (Christopher et al., 2020; Williams et al., 2020; Daeschler et al., 2022; Sun et al., 2022; Signaevsky et al., 2022). For example, AI can be used to assess the degree of neuroplasticity and the therapeutic effectiveness of neural stem cells. Research on peripheral nerve injury and repair can also provide valuable data to AI algorithms (Luu et al., 2021), which can, for example, guide the analysis of neural signals and the implementation of neural networks in robotic architectures. Thus, AI systems and research on peripheral nerve injury and repair can reinforce one another to drive medical innovations. AI, which links computer science with neurology, has already been used to assess peripheral nerve injury and repair. For instance, it has been used to develop new research methods and techniques for nerve regeneration (Romeo-Guitart et al., 2018; Luu et al., 2022). Peripheral nerve injury and repair refers to the loss and subsequent regeneration of neurons as the result of an injury or disease. AI can be used to predict the pathological manifestations of the peripheral nervous system and to explore better therapeutic approaches.
Among the various AI technologies that have been applied to peripheral nerve injury and repair, artificial neural networks are commonly used to test the connections and communication between neurons (Koh et al., 2020; Lei and Wu, 2022; Zhao et al., 2022; Khaliq et al., 2023). This technique can be used to understand how neurons might regenerate, leading to the restoration of neural functions after nervous system impairment. In addition, AI can be used to identify and quantitatively assess the morphology and structure of neurons via imaging techniques (Daeschler et al., 2022). AI relies on data imaging to accurately and quantitatively assess neuronal regeneration. Furthermore, machine learning algorithms can be used to determine which genes and pathways are critical for neural regeneration (Stewart et al., 2018; Aberathne et al., 2023). Therefore, AI can be used to enhance our understanding of neural regeneration. Some studies have addressed the intelligent design of nerve guidance conduits with tubular structures made of biomaterials that can guide axonal regeneration from the injured proximal nerve to the distal stump. However, some technical issues and innovation bottlenecks remain. Moreover, AI techniques have not been widely used in clinical practice, except in peripheral nerve injury and repair (Stewart et al., 2020).
Here, we conducted a literature visualization analysis with the goal of identifying research hotspots relevant to AI applications in peripheral nerve injury and repair.
Methods
Search strategy and selection criteria
The first author searched the Web of Science (WOS) Core Collection database for literature regarding AI applications in peripheral nerve injury and repair. The literature search was performed on March 31, 2023.
Search strategy
The search strategies used with the WOS Core Collection are shown in Table 1. We conducted a topic search (TS), including the text in the title, abstract, and keywords. In total, 613 articles were included.
Table 1.
Search strategies for literature on the application of artificial intelligence to peripheral nerve injury and repair in the Web of Science Core Collection database
| Search Strategies | Literature number | |
|---|---|---|
| #1 | TS=(Peripheral Nerv* OR Perineurium OR Epineurium OR Endoneurium OR Peripheral Nerv* Regeneration OR Peripheral Neural Regeneration OR Neurorepair, Peripheral OR neuroprotection, Peripheral OR neurorehabilitation, Peripheral OR Peripheral Nervous System) | 120,129 |
| #2 | TS=(“Artificial Intelligence” OR “Machine Learning” OR “Deep Learning” OR “Natural Language Processing” OR “Robot*” OR “Computer Vision” OR “Expert System” OR “Neural Network” OR “Cognitive Computing” OR “Intelligent System” OR “Autonomous System” OR “Hierarchical Learning” OR “Transfer Learning” OR “Perceptron” OR “Connectionist Model” OR “Knowledge Acquisition (Computer)” OR “Computer Reasoning” OR “AI (Artificial Intelligence)” OR “Knowledge Representation (Computer)”) | 1,218,719 |
| #3 | #1 AND #2 | 613 |
Selection criteria
After reading the document titles and abstracts, documents on AI applications in peripheral nerve injury and repair were retrieved and included in this paper, with no restrictions on article style.
Data analysis software and parameter settings
Visual analysis software
(1) CiteSpace 6.2.R2 Basic software is a JAVA application, developed by Dr. Chaomei Chen from Drexel University. This software enables the visualization and analysis of trends and frontier areas in a scientific field through co-occurrence and co-citation analyses of massive datasets of literature (Chen, 2004; Chen and Song, 2019).
(2) VOSviewer 1.6.18 software is a tool used to generate literature co-citation networks based on web or bibliometric data, which can be used to create co-occurrence and clustering maps (Arruda et al., 2022).
Data export and processing
The WOS Core Collection database was set to contain full records with cited references exported in plain text as TXT files and imported into VOSviewer 1.6.18 software and CiteSpace 6.2.R2 Basic software. This dataset was then used to create scientific knowledge maps, which illustrate scientific knowledge processing and structural relationships, and show trends in subject knowledge domains.
Parameter settings for VOSviewer
We performed country co-occurrence analysis using VOSviewer software, with the minimum number of publications from a country set to 10 articles. This enabled us to visualize the density of research from specific countries and institutional cooperation networks, and to conduct cluster analysis regarding cooperation between countries for publications.
Parameter settings for CiteSpace
The time slicing parameter was set to include articles from January 1994 to March 2023 (CiteSpace 6.2.R2 Basic software can only analyze literature published in the last 30 years), with the default of “1” year as a time zone. Burst Term was selected as the type of subject term used. Keywords and literature or journal were selected as node types for the co-occurrence analysis and co-citation analysis, respectively.
(1) We conducted a literature co-citation analysis to identify the research hotspots in our chosen scientific field (Chen et al., 2010). We created literature co-citation maps using Citespace according to the following selected parameters: an article was defined as a node, the citation frequency was set as the node size, and a connection was established when there was a co-citation relationship between documents. We then conducted a cluster analysis. Co-citation maps can be used to identify “hot” documents that have been frequently cited by other scholars.
(2) We created a journal co-citation analysis map to analyze the distribution of “hot” journals in the field.
(3) We created double-map overlays of journals to analyze the research directions of journals in the field.
(4) Co-occurrence analysis maps of keywords were generated to analyze burst terms in the field.
We used the following settings: Top n = 50 for the threshold setting; Pathfinder to prune the author-institutional collaboration networks; and ‘pruning the merged network’ for the keyword co-occurrence and literature co-citation analysis.
A burst detection algorithm was used to identify innovative topics, as proposed by Goldberg et al. (2002).
Main outcome measures
The integrated visual mapping and bibliometric functions of the WOS Core Collection were used to analyze the number of publications, journals, institutions, countries, keywords, and highly cited articles relevant to the research topic.
Results
Analysis of countries/regions with publications on AI applications in peripheral nerve injury and repair
The VOSviewer 1.6.18 results indicated that there were 18 countries with a minimum of 10 publications on the research topic (Figure 1). There were five clusters (#1–#5), divided by research direction (different colors marked in Figure 1). The five countries with the most publications were the USA, China, Italy, Japan, and Germany, which are the countries with the greatest research output regarding the use of AI in peripheral nerve injury and repair.
Figure 1.
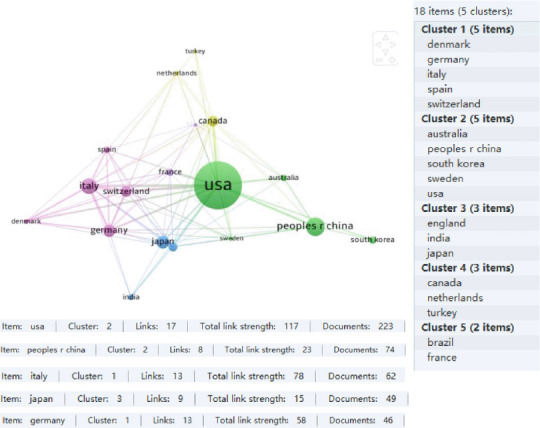
Country/region-based distribution of artificial intelligence research in peripheral nerve injury and repair (data from 613 papers retrieved from the Web of Science Core Collection database).
The figure shows the co-occurrence network of countries with relevant publications. A larger node size represents a higher number of publications and a thicker line represents a greater number of co-authored publications between countries. The link strength represents the number of collaborations.
Analysis of institutions with publications on AI applications in peripheral nerve injury and repair
The CiteSpace 6.2.R2 Basic results showed that of the 613 articles, 19 were published by the Federal Institutes of Technology in Lausanne, Switzerland, 16 were published by Harvard University, USA, and 15 were published by Sant’Anna School of Advanced Studies, Pisa, Italy. Switzerland, USA, and Italy were the three countries with the greatest number of cited articles in this field (Table 2).
Table 2.
The 10 research institutions with the greatest number of publications on the application of artificial intelligence in peripheral nerve injury and repair (data from 613 articles retrieved from the Web of Science Core Collection database)
| Institution | Country | Publications |
|---|---|---|
| Swiss Federal Institutes of Technology Domain | Switzerland | 19 |
| Harvard University | USA | 16 |
| Scuola Superiore Sant’Anna | Italy | 15 |
| Ecole Polytechnique Federale de Lausanne | Switzerland | 12 |
| Aalborg University | Denmark | 11 |
| UDICE-French Research Universities | France | 10 |
| University of Texas System | USA | 10 |
| Harvard Medical School | USA | 9 |
| University of California System | USA | 8 |
| ETH Zurich | Switzerland | 8 |
Analysis of research hotspots and “hot” journals regarding AI applications in peripheral nerve injury and repair based on journal citations
Journal co-citation burst analysis showing the journals with a focus on relevant research hotspots
J COMP NEUROL had the strongest focus on AI applications in peripheral nerve injury and repair, and also followed relevant hotspots for the longest duration (1994–2013). Over the past 5 years (2019–2023), SCI REP-UK, NAT COMMUN, ELIFE, SENSORS-BASEL, ARXIV PREPRINT ARXIV, IEEE ACCESS, PROC CVPR IEEE, and FRONT NEUROL were the main journals with a focus on AI applications in peripheral nerve injury and repair (Figure 2).
Figure 2.
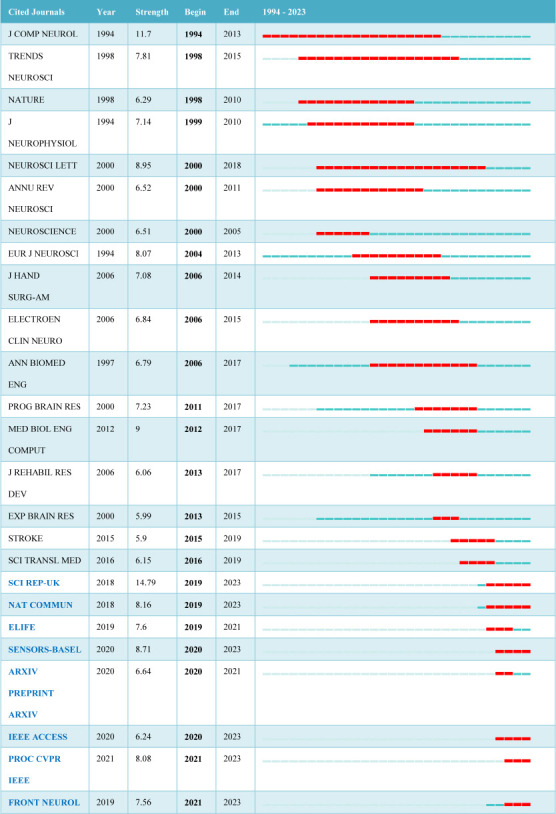
The 25 journals with the strongest citation bursts in terms of the applications of artificial intelligence in peripheral nerve injury and repair from 1994 to 2023 (data from 613 articles retrieved from the Web of Science Core Collection database).
A burst journal is a journal that is suddenly cited many times during a certain period of time. The figure shows the top 25 burst journals identified according to the 613 articles published from 1994–2023. The red squares represent the years in which the burst took place. J COMP NEUROL (1994–2013) is the earliest journal with the longest focus on relevant hotspots in the field, followed by TRENDS NEUROSCI (1998–2015), and NEUROSCI LETT (2000–2018). Journals in blue font are the “hot” journals from 2019 to 2023, including SCI REP-UK, NAT COMMUN, ELIFE, SENSORS-BASEL, ARXIV PREPRINT ARXIV, IEEE ACCESS, PROC CVPR IEEE, and FRONT NEUROL. “Year” represents the earliest year (1994) in which the cited journal literature appeared. “Strength” represents the frequency of citations. “Begin” and “End” represent the beginning and end of citation bursts, respectively.
Most frequently cited journals
The three journals with the most citations regarding AI applications in peripheral nerve injury and repair identified according to the 613 included articles published from 1994–2023 were NATURE, J NEUROSCI, and J NEUROPHYSIOL (Table 3).
Table 3.
The 10 journals with the largest number of citations regarding the application of artificial intelligence in peripheral nerve injury and repair (data from the 613 articles retrieved from the Web of Science Core Collection database)
| Journals | Impact factor | Times cited |
|---|---|---|
| NATURE | 69.503 | 158 |
| J NEUROSCI | 6.709 | 154 |
| J NEUROPHYSIOL | 2.974 | 147 |
| IEEE T BIO-MED ENG | 4.756 | 140 |
| SCIENCE | 63.832 | 139 |
| PLOS ONE | 3.752 | 136 |
| P NATL ACAD SCI USA | 12.778 | 117 |
| IEEE T NEUR SYS REH | 4.528 | 110 |
| SCI REP-UK | 4.997 | 108 |
| J NEUROSCI METH | 2.987 | 108 |
Dual-map overlays showing the research directions of burst journals
According to the basic literature data provided by CiteSpace, the dual-map overlay data were all sourced from Journal Citation Reports (JCR) 2011.
The journal dual-map overlay results indicated that AI remained a “hot” research topic in the field of peripheral nerve injury and repair, receiving attention from NATURE, J NEUROSCI, SCIENCE, etc., during the study period.
We used the Blondel algorithm to create dual-map overlays based on the 613 included articles, as shown in Figure 3, where the left-hand side shows the distribution of the citing journals and the right-hand side shows the distribution of the cited journals (Chen et al., 2014).
Figure 3.
Dual-map overlays of cited/citing journals for 613 articles regarding artificial intelligence applications in peripheral nerve injury and repair from 1994–2033 (data from the Web of Science Core Collection database).
Each publication portfolio was added as one layer of a dual-map overlay over two related, but distinct, global maps of science: one for cited journals and one for citing journals. Each point on the map represents one journal. The left side shows the map of the citing journals, the right side shows the map of the cited journals, and the curves represent citation links, which indicate the citation paths. The length of the ellipse represents the number of authors, and the width of the ellipse represents the number of publications. The two main thick curves (the citation paths) indicate that publications related to NEUROLOGY, SPORT, and OPHTHALMOLOGY (z = 8.469, f = 980) and MOLECULAR BIOLOGY, BIOLOGY, and IMMUNOLOGY (z = 8.144, f = 2060) were strongly associated with publications related to MOLECULAR BIOLOGY, BIOLOGY, GENETICS.
Figure 3 demonstrates that the citing journals mainly focused on two aspects: the fields of neurology, kinesiology, and ophthalmology; or research about molecules, biology, and immunology. The cited journals mainly focused on the latter aspect. Neurology, kinesiology, ophthalmology, molecular biology, immunology, and genetics were more closely linked across journals, and had been “hot” frontiers of AI research in the field of peripheral nerve injury and repair (shown by the two thick curves in Figure 3).
Keyword analysis of research on AI applications in peripheral nerve injury and repair
Keyword cluster analysis
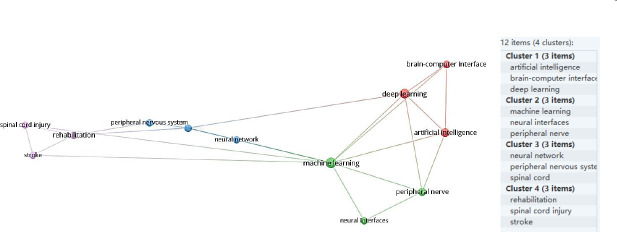
We used VOSviewer 1.6.18 software to conduct the cluster analysis of 613 articles (Figure 4), which involved four clusters:
Figure 4.
Keyword clustering network diagram (data from 613 articles retrieved from the Web of Science Core Collection database).
#1 Artificial intelligence, brain-computer interfaces, deep learning;
#2 Machine learning, neural interfaces, peripheral nerve;
#3 Neural network, peripheral nervous system, spinal cord;
#4 Rehabilitation, spinal cord injury, stroke.
We identified four main research directions regarding the application of AI in the field of peripheral nerve injury and repair:
(1) Brain-computer interfaces and deep learning related technologies;
(2) Machine learning and neural interface related technologies;
(3) Neural networks related to spinal cord injury;
(4) Stroke and spinal cord injury rehabilitation.
Keyword burst analysis
The keyword burst analysis of 613 articles indicated that the research in this field could be mainly divided into two phases: (1) there was a focus on neurostimulation from 2012–2019; (2) deep learning, artificial intelligence, and machine learning were burst words from 2019 until the present (Figure 5).
Figure 5.
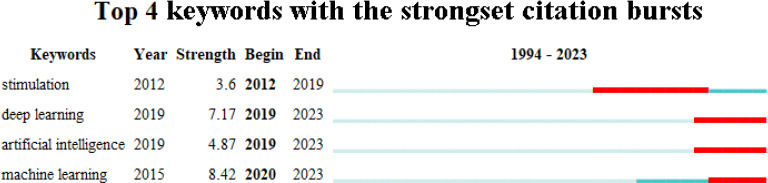
Keyword burst analysis of the application of artificial intelligence in the field of peripheral nerve injury and repair (data from 613 articles retrieved from the Web of Science Core Collection database).
“Year” represents the earliest year (1994) in which the cited journal literature appeared. “Strength” represents the frequency of citations. “Begin” and “End” represent the beginning and end of citation bursts, respectively.
Literature co-citation cluster analysis regarding the application of artificial intelligence to peripheral nerve injury and repair
We obtained a total of 117 clusters, and the literature co-citation network included the largest seven clusters. These seven most representative clusters are detailed in Table 4 and Figure 6.
Table 4.
Clusters of co-cited articles regarding the application of artificial intelligence in peripheral nerve injury and repair (data from 613 articles retrieved from the Web of Science Core Collection database)
| Cluster ID | Size | Silhouette | Label (LLR) |
|---|---|---|---|
| #0 | 37 | 0.988 | Cybernetic hand prostheses |
| #1 | 35 | 0.903 | Ulnar nerve |
| #2 | 32 | 0.988 | Corneal confocal microscopy |
| #3 | 22 | 0.957 | Deep learning-based approaches |
| #5 | 12 | 1.000 | Using coherent anti-Stokes Raman |
| #9 | 10 | 0.975 | Assistive wheelchair system |
| #23 | 5 | 0.984 | Selective recording |
Silhouette refers to the average profile value of the clusters. Generally, clusters with a silhouette score > 0.5 are acceptable while those with a silhouette score > 0.7 have good clustering performance. Size reflects the number of articles contained in the cluster. A larger log likelihood ratio (LLR) value indicates that the clustering label is more representative of the cluster.
Figure 6.
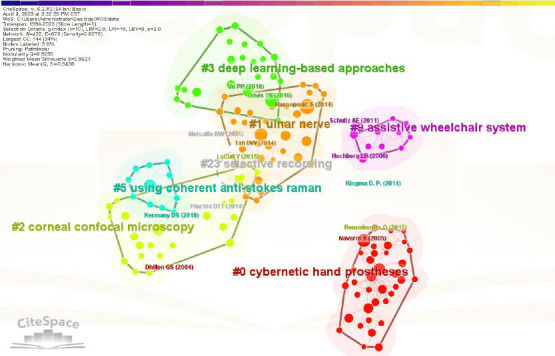
Literature co-citation cluster map regarding the application of artificial intelligence in peripheral nerve injury and repair (data from 613 articles retrieved from the Web of Science Core Collection database).
The block color of a cluster indicates the year in which the co-citation relationship first occurred in this cluster. The literature in the blue block was published earlier than that in the green block, while the literature in the yellow block was published later than that in the green block. Node size represents the citation frequency of the literature (represented by the first author's name and the year of publication). The color of the connecting line represents the time of the first citation, and the citation time colors, read from left to right, indicate the range from 1994 to 2023. There are seven cluster categories in the figure (#0–#3, #5, #9, #23).
Cluster #0: Cybernetic hand prostheses. The main citing article of the cluster is Micera et al. (2008). In recent years, significant progress has been made in the development of highly sensory-neural network prostheses designed to restore sensory-motor functions in patients who have lost these functions owing to traumatic events (amputation). In these cases, one of the main goals is to establish a bidirectional connection between an artificial device (e.g., a robotic hand, arm, or leg) and the nervous system. Several human-machine interfaces have been used. Among them, the interface to the peripheral nervous system, especially to the longitudinal silent intraelectrode, is a promising solution. Micera et al. (2008) described the potential and limitations of such interfaces to control robotic devices.
Cluster #1: The ulnar nerve. The main citing article in the cluster was Vu et al. (2020). The loss of upper extremity motor function can have a devastating impact on quality of life. To restore upper extremity function, researchers and clinicians have developed interfaces that interact directly with the human motor system. Vu et al. (2020) reviewed the details of existing peripheral nerve (ulnar nerve) interfaces and brain-computer interfaces used for upper extremity control over the past 30 years, and highlighted the challenges that remain in translating this technology to clinical practice. In this review, peripheral nerve interfaces and brain-machine interfaces were confirmed to have many similar characteristics, and they can be developed simultaneously. Decoding neural information from both interfaces may lead to new physiological models with the potential to fully restore upper extremity motor function in patients.
Cluster #2: Corneal confocal microscopy. In the past 20 years, corneal confocal microscopy has been increasingly used to image small basal nerve fibers in various peripheral neuropathies and central neurodegenerative diseases. Corneal confocal microscopy has been used to identify subclinical nerve damage and to predict the development of diabetic peripheral neuropathy. Large datasets of 2D corneal nerve images can facilitate the application of AI-based deep learning algorithms. Deep learning algorithms are comparable to manual quantification, but superior to automated quantification in terms of corneal nerve morphology. Recently, end-to-end classification with a 3-class AI model, designed by Alam et al. (2022), was shown to have high sensitivity and specificity in distinguishing healthy volunteers from those with or without peripheral neuropathy. The researchers concluded that AI could be applied to the classification of peripheral neuropathy from neurodegenerative diseases in the central nervous system. These findings indicate that AI has significant potential in improving the diagnostic and prognostic value of corneal confocal microscopy in the treatment of neurodegenerative diseases in the peripheral and central nervous systems.
Cluster #3: Deep learning-based approaches. The main citing article in the cluster was Farina et al. (2021). Robotic limbs can deliver information about the environment with greater precision than biological limbs. However, their actual performance is limited by the current technologies used to connect robotic devices to the body, enabling bidirectional transmission of motor and sensory information between the prosthesis and the user. Farina et al. (2021) considered the direct skeletal attachment of bionic devices via osseointegration, the amplification of neural signals by targeted muscle innervation, the improvement of prosthesis control via implanted muscle sensors and advanced algorithms, and the provision of sensory feedback via electrodes implanted in peripheral nerves, and reported that all of these approaches could be beneficial in creating a new generation of high-performance bionic limbs. These technologies have been preliminarily tested in humans, and mechanical redesigns and appropriate rehabilitation training is expected to contribute to the wider use of bionic limbs in clinical practice.
Cluster #5: Using coherent anti-Stokes Raman spectroscopy. The main citing article in the cluster was Kim et al. (2019). The researchers proposed a new approach to glaucoma diagnosis and localization that relies solely on fundus images, which are analyzed via cutting-edge deep learning techniques. Medinoid was presented as a publicly available prototype of a web application for the computer-aided diagnosis and localization of glaucoma, which finally integrates the most effective predictive model.
Cluster #9: Assistive wheelchair systems. The main citing article in the cluster was Li et al. (2013). With brain-computer interface technology, people with disabilities do not need to use traditional pathways, such as peripheral nerves and muscles, to communicate with the outside environment. Instead, they can communicate using brain activity. Li et al. (2013) designed an electroencephalography-based wheelchair that can be guided by the user’s thoughts without the involvement of other factors. This wheelchair was both accurate and had good performance in operational tests in an actual environment. This technology has great potential for daily applications in people with disabilities.
Cluster #23: Selective recording. The main citing article in the cluster was Sammut et al. (2020). Peripheral nerve interfaces allow researchers to extract motor, sensory, and autonomic information from the nervous system and use it as a control signal in neural prostheses and neuromodulatory systems. Recent studies have attempted to elevate the recording selectivity of peripheral nerve interfaces, including the use of spatiotemporal patterns from multi-contact neural cuff electrodes as input to convolutional neural networks. Before being applied to humans, this methodology must be evaluated in terms of its performance during chronic implantation. Sammut et al. (2020) investigated the performance of a convolutional neural network-based selective recording approach in the presence of encapsulation tissue, which is a common immune response to the implantation of neural interfaces. This factor was simulated via an anatomically accurate computational model of rat sciatic nerves and nerve cuff electrodes. Their study found that a periodic calibration approach may be effective in compensating for alterations in signal recording over time.
In-depth analysis of the most frequently cited literature on the application of artificial intelligence in peripheral nerve injury and repair
Hotspot analysis of the most influential co-cited literature
The cited literature for each node in each cluster was ranked according to co-citation counts, and the 10 articles with the most co-citations are shown in Table 5. Raspopovic et al. (2014) from the BioRobotics Institute at the Scuola Superiore in Sant’Anna, Italy, was the most co-cited article (Citation Counts: 20), and thus the leading “hot” study. This was followed by Davis et al. (2016) from the Department of Bioengineering, University of Utah, USA (Citation Counts 18), and Navarro et al. (2005) from the Department of Cell Biology, Physiology and Immunology, at the Universitat Autònoma de Barcelona, Spain (Citation Counts: 15). These three authors were the most influential scholars in the field of AI applications to peripheral nerve injury and repair.
Table 5.
The 10 most co-cited articles on the application of artificial intelligence in peripheral nerve injury and repair (sorted by co-citation counts) (data from 613 articles retrieved from the Web of Science Core Collection database)
| References | Cluster ID | Label (LLR) | Article highlights | Major Issues | Citation Counts |
|---|---|---|---|---|---|
| Raspopovic et al., 2014 | #1 | Ulnar nerve | By stimulating the median and ulnar nerve fascicles using transversal multichannel intrafascicular electrodes, physiologically appropriate (near-natural) sensory information can be transferred to amputees when decoding different grasping tasks in real time to control a dexterous hand prosthesis, based on the information provided by artificial sensors from a hand prosthesis. This feedback allows the participant to effectively modulate the grasping force of the prosthesis without visual or auditory feedback, thereby providing a key strategy for the near-natural replacement of missing hands. | AI control + electrode stimulation + artificial prosthesis (upper limb) + information feedback | 20 |
| Davis et al., 2016 | #3 | Deep learning-based approaches | A 96-microelectrode array can be implanted in the human peripheral nervous system for up to 1 month. This array can provide intuitive control of a virtual prosthetic hand with extensive sensory feedback. | AI control + electrode implantation in peripheral nerves + information feedback | 18 |
| Navarro et al., 2005 | #0 | Cybernetic hand prostheses | Many neuroprostheses use interfaces with peripheral nerves or muscles for neuromuscular stimulation and signal recording. This review offers a critical overview of existing peripheral interfaces and traces their clinical application in the control of artificial and robotic prostheses. | AI control + artificial prosthesis (upper limb) + information feedback + peripheral nerve interface | 15 |
| Tan et al., 2014 | #1 | Ulnar nerve | Implanted peripheral nerve interfaces in two upper limb amputees provided stable, natural touch sensation in their hands over 1 year. Electrical stimulation using implanted peripheral nerve cuff electrodes that do not penetrate the nerve produced tactile sensation in many locations on the phantom hand, and both subjects had reproducible, stable responses for 16 and 24 months. | AI control + information feedback+peripheral nerve interface | 14 |
| Dhillon et al., 2004 | #0 | Cybernetic hand prostheses | Amputees who underwent elective stump surgery were enrolled. Longitudinal intravascular electrodes were percutaneously inserted and implanted in the amputees’ nerves, and the electrodes were connected to a laptop-controlled amplifier and stimulator system. This study indicates that peripheral nerve interfaces can be used to provide amputees with prosthetic limbs with more natural sensation and control than that provided by current myoelectric and body dynamics control systems. | AI control + electrode implantation in peripheral nerves + information feedback+peripheral nerve interface | 13 |
| Ronneberger et al., 2015 | #2 | Corneal confocal microscopy | A network and training strategy is proposed that relies on a robust use of data augmentation to make more efficient use of the annotated samples available. The architecture consists of a contraction path that captures the context and a symmetric extension path that achieves precise localization. | AI + path localization | 13 |
| Kingma and Ba, 2014 | #5 | Using coherent anti-Stokes Raman | Adam, an algorithm for first-order gradient-based optimization of stochastic objective functions, is introduced. This algorithm is based on adaptive estimates of low-order moments. The method is simple and easy to implement, computationally efficient, has low memory requirements, is insensitive to diagonal remodeling of the gradient, and is available for problems with large-scale data and/or parameters. | AI algorithms | 12 |
| Vu PP et al., 2018 | #3 | Deep learning-based approaches | This study implemented a standard Kalman filter for continuous hand control in non-human primates using intramuscular electromyography from a regenerative peripheral nerve interface (RPNI) and intact muscles. It is the first demonstration of chronic retention electrodes for continuous position control using the Kalman filter. This is an important step forward in providing patients with more natural prosthetic control. | AI control + artificial prosthesis (hands) | 11 |
| Dhillon et al., 2005 | #0 | Cybernetic hand prostheses | This study is the first to demonstrate direct neurofeedback from and direct neural control of an amputee’s artificial arm by implanting electrodes into a single bundle of the amputee’s peripheral nerve stump, through which stimulation can produce graded, discrete sensations of touch or movement, and through which motor neuron activity associated with the tentative movements of the phantom limb can be recorded and used as a hierarchical control signal. | AI control + electrode implantation + information feedback+signal control | 9 |
| Rossini et al., 2010 | #1 | Ulnar nerve | The study evaluated a novel peripheral intraneural multielectrode for multi-movement prosthesis control and sensory feedback, as well as assessing cortical remodeling after the regaining of data streams. | AI control + electrode implantation + information feedback | 9 |
When two (or more) articles were cited as references together by one or more subsequent articles, they were said to be co-cited. Citation Counts refer to the number of co-citations of the literature, and reflect not only the literature impact but also the importance and trending behavior of the research topic, which change over time.
Among the 10 most co-cited publications, there were three publications in Cluster #0, which were focused on AI control + electrode implantation + artificial prosthesis (upper limb) + information feedback + peripheral nerve interface: Navarro et al. (2005), Dhillon et al. (2004, 2005). There were three publications in Cluster #1, focused on AI control + electrode stimulation + artificial prosthesis (upper limbs) + information feedback: Raspopovic et al. (2014), Tan et al. (2014), and Rossini et al. (2010). There was one publication in Cluster #2, which was focused on AI + path localization: Ronneberger et al. (2015); and two publications in Cluster #3: Davis et al. (2016), which focused on AI control + electrode implantation in peripheral nerves + information feedback, and Vu et al. (2018), which focused on AI control + artificial prosthesis (hands). There was one publication in Cluster #5: Kingma and Ba (2014), which focused on AI algorithms.
These were the 10 most influential publications on AI applications to peripheral nerve injury and repair.
Cluster analysis of hotspots in the co-cited literature over the last 5 years
Figure 7 shows a cluster map of literature co-citations for research hotspots in terms of the application of AI to peripheral nerve injury and repair from 2018 to 2022.
Figure 7.
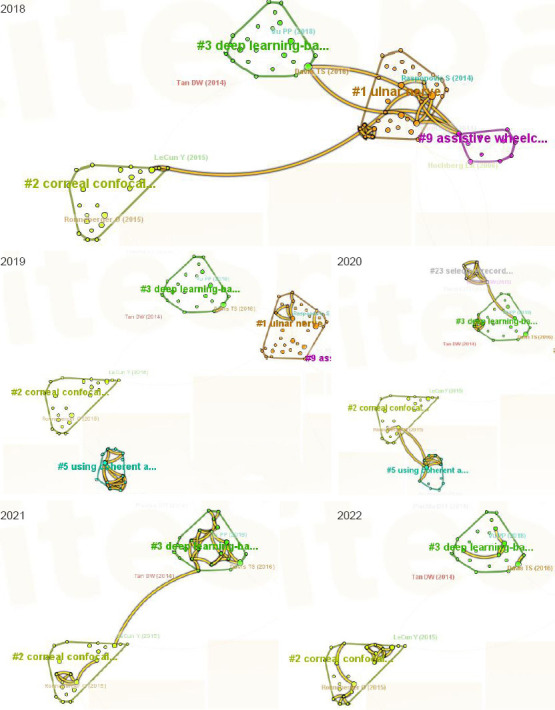
Hotspot cluster analysis of literature co-citations regarding the application of artificial intelligence in peripheral nerve injury and repair from 2018–2022 (data from the Web of Science Core Collection database).
Each block represents a cluster, and each node in the cluster block represents an article. The lines between nodes represent the citation relationships between the various documents. Each illustration indicates the clustering direction of artificial intelligence research in the field of peripheral nerve injury and repair in the past 5 years.
The research hotspots in 2018 could be clustered as follows: #1 the ulnar nerve, #2 corneal confocal microscopy, #3 deep learning-based approaches, and #9 assistive wheelchair systems. Clusters #3, #1, and #9 are closely related. The main research directions can be summarized as the application of deep learning in the repair of peripheral nerve injury and the rehabilitation of peripheral nerve injury, promoted via assistive wheelchair systems. The co-cited articles with high popularity in 2018 were Tan et al. (2014) and Davis et al. (2016).
The research hotspots in 2019 could be clustered as follows: #1 the ulnar nerve, and #5 the use of coherent anti-Stokes Raman spectroscopy. These hotspots are in different fields and were not correlated, indicating that the research hotspots in 2019 were not very prominent.
The research hotspots in 2020 could be clustered as follows: #2 corneal confocal microscopy, #3 deep learning-based approaches, #5 the use of coherent anti-Stokes Raman spectroscopy, and #23 selective recording, with close relationships between #2 and #5, and between #3 and #23.
The research hotspots in 2020–2021 could be clustered as follows: #2 corneal confocal microscopy, and #3 deep learning-based approaches. The two clusters were close but not very close. Collaboration was common in each cluster. The research hotspots were basically the same as in 2020, indicating the presence of similar research hotspots from 2020 to 2023.
The major research directions from 2020 to 2022 could be summarized as the application of AI-based imaging tools in the diagnosis of peripheral nerve diseases and the signal control of peripheral nerve interfaces based on deep learning. Ronneberger et al. (2015) and Vu et al. (2018, 2020) were the co-cited articles with highest popularity in 2020–2022.
Hotspot-based analysis of highly cited articles in the Web of Science Core Collection database
The definition of a ‘highly cited’ article was as follows: By November/December 2022, based on the high citation threshold of the corresponding field and publication year, the citation frequency of the article had entered the top 1% in the academic field of engineering, according to data from the Essential Science Indicators database. Two of the 613 included articles could be classified as highly cited according to the WOS database. Ijspeert et al. (2014) summarized the use of biological robots as physical models for hypothesis testing in the fields of hydrodynamics, biomechanics, neuroscience, and prosthetics, and proposed that their use may facilitate the design of prosthetic devices that more closely follow the principles of human movement compared with previous options. Lee et al. (2019) introduced asynchronously coded electronic skin, which is a neural simulation system that can simultaneously transmit heat-tactile information while maintaining extremely low read latency, even though its array size exceeds 10,000 sensors. The asynchronously coded electronic skin platform is expected to integrate a wide range of skin-like sensors for AI-enhanced autonomous robots, neuroprostheses, and neuromorphic computational metal implants for flexible object manipulation and somaesthesia.
Discussion
Interpretation of visual analysis results
The USA and countries in Europe occupy a leading position in the application of AI to peripheral nerve injury and repair, and the USA is unequivocally dominant in this field. The Ecole Polytechnique Federale de Lausanne in Switzerland, Harvard University in the USA, and Scuola Superiore Sant’Anna in Italy are the three institutions with the largest output of AI research in the field of peripheral nerve injury and repair. NATURE, J NEUROSCI, and J NEUROPHYSIOL are the most authoritative and influential journals with a focus on AI research in the field of peripheral nerve injury and repair. The research directions in this field are mainly focused on neurology, kinematics, ophthalmology, molecular, biology, immunology, and genetics, which are related.
The keyword cluster analysis revealed four major research directions in the application of AI to the field of peripheral nerve injury and repair: (1) brain-computer interface- and deep learning-related technologies; (2) machine learning- and neural interface-related technologies; (3) neural networks related to spinal cord injury; (4) stroke and spinal cord injury rehabilitation. The keyword burst analysis showed that deep learning, AI, and machine learning have become burst terms over the last 5 years.
To further examine the research hotspots in this field, we conducted a cluster analysis of the literature co-citations among the included articles. We obtained seven clusters: cybernetic hand prostheses, the ulnar nerve, corneal confocal microscopy, deep learning-based approaches, the use of coherent anti-Stokes Raman spectroscopy, and assistive wheelchair systems. The literature in these clusters mainly focused on AI control, electrode implantation, artificial prosthesis, information feedback, and peripheral neural interfaces. Further analysis showed that the AI research in the field of peripheral nerve injury and repair mainly focused on AI control, peripheral nerve electrode stimulation, artificial prosthesis (upper limb) control, and information feedback. The ultimate purpose of the research was to use AI to control artificial prostheses, enabling patients with amputation and limb injury to recover limb function more quickly, and to create prosthetic limbs with better control for movement rehabilitation.
We next performed a hotspot cluster analysis of literature co-citations over the past 5 years, and found that the current research hotspots had shifted from the application of deep learning and assisted wheelchair systems in the repair of peripheral nerve injury in 2018 to the application of AI-based diagnostic imaging tools and deep learning-based peripheral nerve interface signal control in the diagnosis of peripheral nerve diseases in 2020–2022. In addition, we identified two highly cited articles from the WOS Core Collection, which describe the application of AI-controlled biological robots and electronic skin in peripheral nerve injury and repair, respectively.
Experts’ views on research hotspots
In recent years, AI technologies have contributed to breakthroughs in peripheral nerve injury and repair, and have positively impacted clinical treatment. First, AI has been widely applied in neuroimaging. For example, deep learning techniques can be used to accurately identify and localize neuroimaging abnormalities, guide neurosurgical localization, and conduct imaging assessments (Kim et al., 2019). Second, AI can facilitate the automated screening and diagnosis of neuromuscular diseases (Navarro et al., 2005). Key signatures can be extracted using machine learning techniques to build efficient classifiers, which can be validated and improved based on big data. Finally, AI has potential applications in the repair of peripheral nerve injuries using 3D printing techniques and biomaterials. For example, artificial nerve graft materials can be generated using 3D printing technology and biomaterials, and further implanted into damaged nerves to promote nerve regeneration and repair (Wen et al., 2021). As AI technology continues to advance, the use of AI in peripheral nerve injury and repair will become more widespread, providing patients with more effective and personalized treatment options.
Integration of research hotspots regarding AI applications in peripheral nerve injury and repair
By combining the above literature visualization and metrological results with an in-depth understanding of highly cited literature and discussions with experts, the authors identified the following research hotspots in the application of AI to peripheral nerve injury and repair over the last 5 years:
(1) Imaging analysis of peripheral nerves: Diagnosis, classification, and prognosis assessments of peripheral nerve injury can be performed using neuroimaging and AI techniques, such as corneal confocal microscopy and the use of coherent anti-Stokes Raman spectroscopy. Neuroimaging can provide in-depth information about the structure and function of a patient’s brain and nervous system.
(2) Movement control and rehabilitation: Artificial neural networks and machine learning algorithms can be used to study movement control and rehabilitation following peripheral nerve injury. When developing wearable technologies, machine learning and AI techniques can be used to monitor and predict individual activities and needs in real time. Artificial neural networks enable the recoding of neural control to help restore normal motor function in stiff and paralytic parts of the body.
(3) Peripheral nerve electrical stimulation: AI techniques can be used to improve the accuracy and effectiveness of peripheral nerve electrical stimulation, which has most commonly been used in ulnar nerve research. Using electrodes implanted in blood vessels or at the ends of injured peripheral nerves, deep learning can be applied to peripheral nerve electrical stimulation, which can facilitate the repair of injured nerves.
(4) Brain-computer interfaces: AI techniques, applied to brain-computer interfaces, can enable people with disabilities and reduced mobility to control machines or other devices, such as cybernetic hand prostheses.
(5) Intelligent robots: AI can drive robots to replace doctors in certain key steps during surgery or rehabilitation, thereby reducing surgical risk and the likelihood of complications as well as facilitating postoperative recovery.
(6) Peripheral nerve remodeling and regeneration: Using artificial neural networks and biocomputing, seed cells can be applied to heal skin or tissue, contributing to the restoration of tissue with broken or missing nerves.
(7) Peripheral nerve grafting: Nerve grafting techniques can be used for peripheral nerve repair and tissue regeneration. An AI analyzer can perform “real-time” assessments of pathological nervous systems in afferent neuronal populations to better process the complex data needed for successful nerve grafting. It is important to note that patients undergoing allogeneic neural stem cell transplantation must weigh the risks and benefits, and receive treatment under a physician’s supervision. They should be fully informed of possible risks and side effects (including immune rejection, infection, and wound bleeding) before treatment and undergo regular check-ups and monitoring during treatment.
(8) Peripheral nervous information processing: Machine learning technology and big data analyses are used for the processing and interpretation of neural signals, enabling a deeper understanding that could drive therapeutic approaches.
Citespace and VOSviewer softwares are two machine learning-based algorithms that could not accurately identify research hotspots based on the obtained data alone. Therefore, the research hotspots were identified by combining the visual data with expert opinions. The research hotspots presented in the visual data were largely consistent with those reviewed by the experts, and the above research hotspots were integrated with relatively reliable results. These aforementioned research directions, which closely integrate AI with peripheral nerve injury and repair, will help to improve the efficacy of nerve repair and rehabilitation, and provide more effective treatment solutions.
Study limitations
We only searched one authoritative database, the WOS Core Collection, for relevant literature, and the number of studies was small, with only 613 articles included. We did not search other English language databases. This was mainly because the Citespace and VOSviewer software can only recognize exported literature data from certain databases. In particular, the Citespace software uses a relatively simple algorithm to identify the co-occurrence of keywords and literature, and the algorithm is not very precise for large-scale data. In addition, given restrictions regarding the article length, details about the specific research methods included in the literature were not fully presented, and only an overview of the perspectives in the literature was described. The keywords included in the 613 articles were searched precisely to ensure that the included literature met the research objectives of the scientific field.
Challenges in relevant AI research
Although AI has shown great potential in the field of peripheral nerve injury and repair, there remain some issues to be addressed in the future.
(1) Data deficiency and imbalance: Both the quality and quantity of data are critical factors when training an AI model. However, in the field of peripheral nerve injury and repair, data deficiency and imbalance are often encountered because of the difficulty and cost of acquiring human data. In addition, multiple types of nerve injury may be involved. This leads to more complex data types and affects the reliability of the results.
(2) Accuracy and reproducibility: In some cases, especially when the data is similar to medical images, the accuracy of AI algorithms may be compromised. This problem may lead to incorrect diagnostic results and failure of treatment plans. In addition, low transparency and/or integrity of data processing may lead to difficulties in reproducing results.
Medical ethical issues and challenges
The application of AI in the medical field involves a series of ethical and moral issues. The following are several medical ethical issues related to AI:
(1) Privacy: Medical data contains a large amount of sensitive information, such as personal medical history, genetic information, and imaging data. When using AI technology for medical data analysis, it is necessary to ensure that patients’ personal privacy information is fully protected, and also to ensure the security of the data.
(2) Transparency of neural networks: Neural networks in AI technology arise from black-box algorithms that are not good at explaining the decision-making processes they produce, also known as “transparency multipliers.” This means that when using AI technology, researchers and patients may not be able to understand how the machine makes decisions, which can undermine people’s sense of trust and protection of their rights and interests.
(3) Responsible use: Medical AI technologies must be used responsibly to avoid human or algorithmic errors, misunderstandings, and erroneous results, as well as biases caused by the data used by the algorithm, such as that related to race or sex.
(4) Impact on patients, doctors, and society: Medical AI technologies can have an impact on patients, doctors, and society. For example, if machine learning algorithms can accurately diagnose diseases or predict future health risks, this could have a positive impact on the digital reform of healthcare and patient care. Conversely, AI misuse or inappropriate use could lead to major social controversies and other negative consequences.
In conclusion, AI should only be applied following serious considerations of medical ethics, including privacy, safety, transparency, fairness, responsible use, and impact on patients and society. To better address these issues, researchers need to draw on knowledge from related fields such as ethics, law, and policy to formulate ethical principles and norms.
Future prospects
The following topics are critical in the future application of AI in the field of peripheral nerve injury and repair.
(1) Improvement of data quality and quantity: Future developments of AI need to address the issue of data collection. More large-scale, high-quality datasets are needed to train AI models, and a variety of experimental tools are needed to generate relevant datasets when studying biomedicine.
(2) Multimodal data processing: Current research on peripheral nerve injury and repair requires the integration of a wide range of different types of information, including peripheral nerve imaging, transcriptomic data, and functional information. Integrating the different types of datasets and making them available to AI algorithms for learning and decision making is an important challenge.
(3) Interdisciplinary collaboration: The application of AI requires interdisciplinary collaboration, integrating expertise from different fields such as clinical medicine, science and engineering, and computer science. Researchers should learn from each other and try to merge ideas and methods from different disciplines to expand the use of AI in the field of peripheral nerve injury and repair.
(4) Conducting clinical trials: There is a paucity of large-sample clinical trials in this field; therefore, future prospective, multicenter, large-sample, randomized controlled clinical trials are needed for further validation.
(5) Clinical application of AI and machine learning in peripheral nerve injury: AI and machine learning have the potential to improve, and potentially streamline and accelerate, clinical trials through more efficient recruitment and matching of participants, as well as more comprehensive data analysis. In addition, by matching historical data to the inclusion criteria of a target trial, it will be possible to create a synthetic control group. AI and machine learning can also be used to better predict and understand possible adverse events and patient subgroups. AI appears to generate “synthetic patients” that can be used to simulate diagnostic or treatment outcomes. However, the use of AI and machine learning applications and interventions introduces a series of uncertainties that should be addressed in clinical trial protocols and reports.
(6) Acceptable use of ChatGPT in conducting research on peripheral nerve injury and repair: ChatGPT, which has been a “hot” topic recently, is an AI chat tool based on natural language comprehension technology. ChatGPT can help researchers and clinicians process and analyze vast amounts of data related to peripheral nerve injury repair. For example, ChatGPT can assist clinicians in diagnosing and treating patients, help them to better understand research findings regarding nerve regeneration, and assist in the development and implementation of treatment plans in clinical practice. Diagnosing diseases and recommending treatment plans is a promising application of ChatGPT. However, users without clinical experience may have difficulty with the distinction between fact and fiction. Caution should be taken to avoid improper use of ChatGPT, such as relying on machines to complete all analyses and medical decisions without regard to expertise. In addition, privacy issues and data security issues must be considered to protect patients’ private information and rights when using ChatGPT. Overall, there is a strong link between ChatGPT and the repair of peripheral nerve injury, but ethical and legal regulations need to be strictly followed in the application process with full consideration of the protection of life and health.
Footnotes
Funding: This study was supported by the Capital’s Funds for Health Improvement and Research, No. 2022-2-2072 (to YG).
Conflicts of interest: The authors declare that there are no conflicts of interest associated with this manuscript.
Data availability statement: No additional data are available.
C-Editors: Zhao M; S-Editors: Wang J, Li CH; L-Editors: Koke S, Song LP; T-Editor: Jia Y
References
- 1.Aberathne I, Kulasiri D, Samarasinghe S. Detection of Alzheimer's disease onset using MRI and PET neuroimaging:longitudinal data analysis and machine learning. Neural Regen Res. 2023;18:2134–2140. doi: 10.4103/1673-5374.367840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alam U, Anson M, Meng Y, Preston F, Kirthi V, Jackson TL, Nderitu P, Cuthbertson DJ, Malik RA, Zheng Y, Petropoulos IN. Artificial intelligence and corneal confocal microscopy:the start of a beautiful relationship. J Clin Med. 2022;6199;11 doi: 10.3390/jcm11206199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allen J. Photoplethysmography and its application in clinical physiological measurement. Physiol Meas. 2007;28:R1–39. doi: 10.1088/0967-3334/28/3/R01. [DOI] [PubMed] [Google Scholar]
- 4.Arruda H, Silva ER, Lessa M, Proença D, Jr, Bartholo R. VOSviewer and Bibliometrix. J Med Libr Assoc. 2022;110:392–395. doi: 10.5195/jmla.2022.1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen C. Searching for intellectual turning points:progressive knowledge domain visualization. Proc Natl Acad Sci U S A. 2004;101:5303–5310. doi: 10.1073/pnas.0307513100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen C, Ibekwe-SanJuan F, Hou J. The structure and dynamics of cocitation clusters:a multiple-perspective cocitation analysis. J Am Soc Inf Sci Technol. 2010;61:1386–1409. [Google Scholar]
- 7.Chen C, Dubin R, Kim M. Emerging trends andnew developments in regenerative medicine:a scientometric update (2000-2014) Exp Opin Biol Ther. 2014;14:1295–1317. doi: 10.1517/14712598.2014.920813. [DOI] [PubMed] [Google Scholar]
- 8.Chen C, Song M. Visualizing a field of research:a methodology of systematic scientometric reviews. PLoS One. 2019;14:e0223994. doi: 10.1371/journal.pone.0223994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christopher M, Bowd C, Belghith A, Goldbaum MH, Weinreb RN, Fazio MA, Girkin CA, Liebmann JM, Zangwill LM. Deep learning approaches predict glaucomatous visual field damage from oct optic nerve head en face images and retinal nerve fiber layer thickness maps. Ophthalmology. 2020;127:346–356. doi: 10.1016/j.ophtha.2019.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daeschler SC, Bourget MH, Derakhshan D, Sharma V, Asenov SI, Gordon T, Cohen-Adad J, Borschel GH. Rapid, automated nerve histomorphometry through open-source artificial intelligence. Sci Rep. 2022;5975;12 doi: 10.1038/s41598-022-10066-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis TS, Wark HA, Hutchinson DT, Warren DJ, O'Neill K, Scheinblum T, Clark GA, Normann RA, Greger B. Restoring motor control and sensory feedback in people with upper extremity amputations using arrays of 96 microelectrodes implanted in the median and ulnar nerves. J Neural Eng. 2016;13:036001. doi: 10.1088/1741-2560/13/3/036001. [DOI] [PubMed] [Google Scholar]
- 12.Dhillon GS, Lawrence SM, Hutchinson DT, Horch KW. Residual function in peripheral nerve stumps of amputees:implications for neural control of artificial limbs. J Hand Surg Am. 2004;29:605–615. doi: 10.1016/j.jhsa.2004.02.006. discussion 616-618. [DOI] [PubMed] [Google Scholar]
- 13.Dhillon GS, Horch KW. Direct neural sensory feedback and control of a prosthetic arm. IEEE Trans Neural Syst Rehabil Eng. 2005;13:468–472. doi: 10.1109/TNSRE.2005.856072. [DOI] [PubMed] [Google Scholar]
- 14.Farina D, Vujaklija I, Brånemark R, Bull AMJ, Dietl H, Graimann B, Hargrove LJ, Hoffmann KP, Huang HH, Ingvarsson T, Janusson HB, Kristjánsson K, Kuiken T, Micera S, Stieglitz T, Sturma A, Tyler D, Weir RFF, Aszmann OC. Toward higher-performance bionic limbs for wider clinical use. Nat Biomed Eng. 2023;7:473–485. doi: 10.1038/s41551-021-00732-x. [DOI] [PubMed] [Google Scholar]
- 15.Goldberg D, McCouch, Kleinberg J. Constructing comparative genome maps with unresolved marker order. Pac Symp Biocomput. 2002;7:139–150. [PubMed] [Google Scholar]
- 16.Ijspeert AJ. Biorobotics:using robots to emulate and investigate agile locomotion. Science. 2014;346:196–203. doi: 10.1126/science.1254486. [DOI] [PubMed] [Google Scholar]
- 17.Khaliq F, Oberhauser J, Wakhloo D, Mahajani S. Decoding degeneration:the implementation of machine learning for clinical detection of neurodegenerative disorders. Neural Regen Res. 2023;18:1235–1242. doi: 10.4103/1673-5374.355982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim M, Han JC, Hyun SH, Janssens O, Van Hoecke S, Kee C, De Neve W. Medinoid:computer-aided diagnosis and localization of glaucoma using deep learning. Appl Sci. 2019;3064;9 [Google Scholar]
- 19.Kingma D, Ba J. Adam:A method for stochastic optimization. Comput Sci. 2014 DOI:10.48550/arXiv.1412.6980. [Google Scholar]
- 20.Koh RGL, Balas M, Nachman AI, Zariffa J. Selective peripheral nerve recordings from nerve cuff electrodes using convolutional neural networks. J Neural Eng. 2020;17:016042. doi: 10.1088/1741-2552/ab4ac4. [DOI] [PubMed] [Google Scholar]
- 21.Lee WW, Tan YJ, Yao H, Li S, See HH, Hon M, Ng KA, Xiong B, Ho JS, Tee BCK. A neuro-inspired artificial peripheral nervous system for scalable electronic skins. Sci Robot. 2019;4:eaax2198. doi: 10.1126/scirobotics.aax2198. [DOI] [PubMed] [Google Scholar]
- 22.Lei X, Wu Y. Vibration and trajectory tracking control of engineering mechanical arm based on neural network. Comput Intell Neurosci. 2022;2022:4461546. doi: 10.1155/2022/4461546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li J, Liang J, Zhao Q, Li J, Hong K, Zhang L. Design of assistive wheelchair system directly steered by human thoughts. Int J Neural Syst. 2013;23:1350013. doi: 10.1142/S0129065713500135. [DOI] [PubMed] [Google Scholar]
- 24.Luu DK, Nguyen AT, Jiang M, Xu J, Drealan MW, Cheng J, Keefer EW, Zhao Q, Yang Z. Deep learning-based approaches for decoding motor intent from peripheral nerve signals. Front Neurosci. 2021;15:667907. doi: 10.3389/fnins.2021.667907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luu DK, Nguyen AT, Jiang M, Drealan MW, Xu J, Wu T, Tam WK, Zhao W, Lim BZH, Overstreet CK, Zhao Q, Cheng J, Keefer EW, Yang Z. Artificial intelligence enables real-time and intuitive control of prostheses via nerve interface. IEEE Trans Biomed Eng. 2022;69:3051–3063. doi: 10.1109/TBME.2022.3160618. [DOI] [PubMed] [Google Scholar]
- 26.Micera S, Navarro X, Carpaneto J, Citi L, Tonet O, Rossini PM, Carrozza MC, Hoffmann KP, Vivó M, Yoshida K, Dario P. On the use of longitudinal intrafascicular peripheral interfaces for the control of cybernetic hand prostheses in amputees. IEEE Trans Neural Syst Rehabil Eng. 2008;16:453–472. doi: 10.1109/TNSRE.2008.2006207. [DOI] [PubMed] [Google Scholar]
- 27.Navarro X, Krueger TB, Lago N, Micera S, Stieglitz T, Dario P. A critical review of interfaces with the peripheral nervous system for the control of neuroprostheses and hybrid bionic systems. J Peripher Nerv Syst. 2005;10:229–258. doi: 10.1111/j.1085-9489.2005.10303.x. [DOI] [PubMed] [Google Scholar]
- 28.Raspopovic S, Capogrosso M, Petrini FM, Bonizzato M, Rigosa J, Di Pino G, Carpaneto J, Controzzi M, Boretius T, Fernandez E, Granata G, Oddo CM, Citi L, Ciancio AL, Cipriani C, Carrozza MC, Jensen W, Guglielmelli E, Stieglitz T, Rossini PM, Micera S. Restoring natural sensory feedback in real-time bidirectional hand prostheses. Sci Transl Med. 2014;6:222ra19. doi: 10.1126/scitranslmed.3006820. [DOI] [PubMed] [Google Scholar]
- 29.Romeo-Guitart D, Forés J, Herrando-Grabulosa M, Valls R, Leiva-Rodríguez T, Galea E, González-Pérez F, Navarro X, Petegnief V, Bosch A, Coma M, Mas JM, Casas C. Neuroprotective drug for nerve trauma revealed using artificial intelligence. Sci Rep. 2018;1879;8 doi: 10.1038/s41598-018-19767-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ronneberger O, Fischer P, Brox T. U-Net: Convolutional networks for biomedical image segmentation. international conference on medical image computing and computer-assisted intervention. Springer: Cham; 2015. doi:10.1007/978-3-319-24574-4_28. [Google Scholar]
- 31.Rossini PM, Micera S, Benvenuto A, Carpaneto J, Cavallo G, Citi L, Cipriani C, Denaro L, Denaro V, Di Pino G, Ferreri F, Guglielmelli E, Hoffmann KP, Raspopovic S, Rigosa J, Rossini L, Tombini M, Dario P. Double nerve intraneural interface implant on a human amputee for robotic hand control. Clin Neurophysiol. 2010;121:777–783. doi: 10.1016/j.clinph.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 32.Sammut S, Koh RGL, Zariffa J. Impact of encapsulation tissue growth on selective recording in nerve cuff electrodes:a simulation study. Annu Int Conf IEEE Eng Med Biol Soc. 2020;2020:3444–3447. doi: 10.1109/EMBC44109.2020.9176736. [DOI] [PubMed] [Google Scholar]
- 33.Signaevsky M, Marami B, Prastawa M, Tabish N, Iida MA, Zhang XF, Sawyer M, Duran I, Koenigsberg DG, Bryce CH, Chahine LM, Mollenhauer B, Mosovsky S, Riley L, Dave KD, Eberling J, Coffey CS, Adler CH, Serrano GE, White CL, 3rd, et al. Antemortem detection of Parkinson's disease pathology in peripheral biopsies using artificial intelligence. Acta Neuropathol Commun. 2022;10:21. doi: 10.1186/s40478-022-01318-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stewart CE, Kan CFK, Stewart BR, Sanicola HW, 3rd, Jung JP, Sulaiman OAR, Wang D. Machine intelligence for nerve conduit design and production. J Biol Eng. 2020;14:25. doi: 10.1186/s13036-020-00245-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun JH, Huang M, Fang Z, Li TX, Wu TT, Chen Y, Quan DP, Xu YY, Wang YM, Yang Y, Zou JL. Nerve bundle formation during the promotion of peripheral nerve regeneration:collagen VI-neural cell adhesion molecule 1 interaction. Neural Regen Res. 2022;17:1023–1033. doi: 10.4103/1673-5374.324861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tan DW, Schiefer MA, Keith MW, Anderson JR, Tyler J, Tyler DJ. A neural interface provides long-term stable natural touch perception. Sci Transl Med. 2014;6:257ra138. doi: 10.1126/scitranslmed.3008669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vu PP, Irwin ZT, Bullard AJ, Ambani SW, Sando IC, Urbanchek MG, Cederna PS, Chestek CA. Closed-loop continuous hand control via chronic recording of regenerative peripheral nerve interfaces. IEEE Trans Neural Syst Rehabil Eng. 2018;26:515–526. doi: 10.1109/TNSRE.2017.2772961. [DOI] [PubMed] [Google Scholar]
- 38.Vu PP, Chestek CA, Nason SR, Kung TA, Kemp SWP, Cederna PS. The future of upper extremity rehabilitation robotics:research and practice. Muscle Nerve. 2020;61:708–718. doi: 10.1002/mus.26860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wen ZQ, Yu MJ, Wu WS, Zeng ZM, Zheng KM. Application progress of 3D printing technology in cranial nerve disease. Yixue Zongshu. 2021;27:3871–3875. [Google Scholar]
- 40.Williams BM, Borroni D, Liu R, Zhao Y, Zhang J, Lim J, Ma B, Romano V, Qi H, Ferdousi M, Petropoulos IN, Ponirakis G, Kaye S, Malik RA, Alam U, Zheng Y. An artificial intelligence-based deep learning algorithm for the diagnosis of diabetic neuropathy using corneal confocal microscopy:a development and validation study. Diabetologia. 2020;63:419–430. doi: 10.1007/s00125-019-05023-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao C, Huang WJ, Feng F, Zhou B, Yao HX, Guo YE, Wang P, Wang LN, Shu N, Zhang X. Abnormal characterization of dynamic functional connectivity in Alzheimer's disease. Neural Regen Res. 2022;17:2014–2021. doi: 10.4103/1673-5374.332161. [DOI] [PMC free article] [PubMed] [Google Scholar]