The reasons for the emergence and spread of vancomycin-resistant enterococci are not yet fully understood. Nevertheless, various hypotheses and proposals which are being vigorously and extensively debated have been advanced (1–7, 10, 11, 15, 17, 19). The use of avoparcin, another member of the glycopeptide class of antibiotics, as a growth-promoting agent in the production of food animals is often cited as playing a role in the spread of glycopeptide-resistant microorganisms (1, 18, 20). Although it is well recognized that vancomycin resistance is more prevalent in the United States than in Europe, it has not been explained why avoparcin usage fails to correlate with the different epidemiologies of resistance between the two continents; avoparcin was never approved for use in animals in the United States, in contrast to its broad use as a growth-promoting agent in Europe (5, 7, 10).
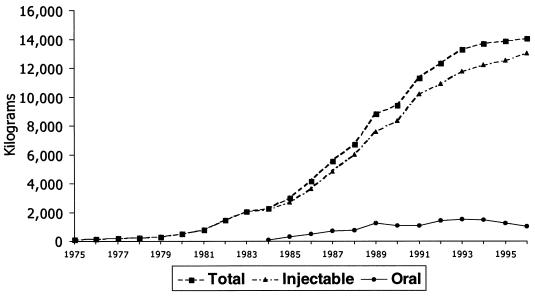
In order to find correlations and explanations, several studies have attempted to document the clinical usage of vancomycin, but these studies have necessarily been relatively limited in the scope of their survey (11, 12, 14, 16). The present report provides a much more complete description of the overall usage pattern of vancomycin over a period of more than 20 years (Fig. 1). The data for the years 1975 to 1983 were compiled from the Lilly database during the period in which Lilly was the sole supplier of vancomycin. The data for the years 1984 to 1996 have been obtained from IMS International.
FIG. 1.
Usage of vancomycin (in kilograms) in the United States, France, Italy, Germany, United Kingdom, and The Netherlands.
Vancomycin use began to accelerate in the early 1980s, beginning a trend that further accelerated when oral formulations of vancomycin became available in the mid-1980s. However, it should be noted that oral use of vancomycin long preceded the appearance of commercial oral formulations, because the injectable formulation could simply be administered in an oral manner (9, 13). Orally administered vancomycin is not absorbed and is used to treat intestinal infections, especially those associated with Clostridium difficile. There is continuing debate concerning the respective roles of parenteral versus oral modes of vancomycin administration as risk factors for the development and spread of vancomycin resistance (11, 15). Vancomycin usage continued to rise rapidly worldwide throughout the 1980s and early 1990s. The slight decline after 1994 reflects the beginning of attempts to restrict vancomycin use in response to concerns about the spread of vancomycin-resistant bacteria (8).
A more-detailed account of vancomycin usage by both the intravenous and oral formulations over the past 12 years within several major markets is presented in Table 1. These data were obtained from IMS International. First, it is readily apparent that greater quantities of vancomycin were used in the United States than in the major European countries. Second, use of the oral formulation increased during the 1980s.
TABLE 1.
Yearly vancomycin usage (all suppliers, all forms) in the United States and major European markets
| Year | Vancomycin usage (kg) per yra
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| United States
|
France
|
Germany
|
Italy
|
United Kingdom
|
Netherlands
|
|||||||
| Inj. | Oral | Inj. | Oral | Inj. | Oral | Inj. | Oral | Inj. | Oral | Inj. | Oral | |
| 1984 | 1,900 | 100 | 200 | 0 | 21 | 0 | 26 | 0 | 42 | 5 | 9 | 0 |
| 1985 | 2,300 | 300 | 300 | 0 | 26 | 0 | 29 | 0 | 45 | 5 | 8 | 0 |
| 1986 | 3,200 | 500 | 300 | 0 | 40 | 0 | 43 | 0 | 55 | 9 | 13 | 1 |
| 1987 | 4,300 | 700 | 300 | 24 | 84 | 0 | 69 | 0 | 70 | 11 | 19 | 2 |
| 1988 | 5,300 | 700 | 380 | 36 | 145 | 0 | 83 | 0 | 79 | 14 | 15 | 4 |
| 1989 | 6,800 | 800 | 400 | 430 | 165 | 0 | 105 | 0 | 112 | 16 | 17 | 6 |
| 1990 | 7,200 | 1,000 | 650 | 57 | 213 | 0 | 168 | 0 | 116 | 17 | 30 | 6 |
| 1991 | 8,781 | 1,013 | 828 | 47 | 245 | 0 | 211 | 2 | 121 | 23 | 33 | 5 |
| 1992 | 9,355 | 1,335 | 798 | 59 | 354 | 0 | 212 | 20 | 144 | 34 | 39 | 6 |
| 1993 | 9,984 | 1,380 | 975 | 64 | 350 | 0 | 261 | 28 | 181 | 42 | 45 | 8 |
| 1994 | 10,152 | 1,308 | 1,086 | 65 | 371 | 0 | 375 | 33 | 207 | 64 | 47 | 8 |
| 1995 | 10,186 | 1,093 | 1,066 | 59 | 509 | 0 | 471 | 40 | 259 | 61 | 54 | 9 |
| 1996 | 10,312 | 888 | 1,165 | 57 | 629 | 0 | 553 | 45 | 301 | 48 | 52 | 8 |
Inj., injectable.
One conclusion from these data is that the high level of vancomycin use in the United States relative to the amount used in Europe correlates well with the greater prevalence of vancomycin resistance found in the United States relative to that found in Europe. In view of these data, the need to invoke a second mechanism for the spread of vancomycin-resistant bacteria in humans due to avoparcin use in Europe remains open to debate. Concern regarding the high level of vancomycin usage in U.S. hospitals and its role in the development and spread of vancomycin-resistant organisms has already been expressed (6).
Acknowledgments
The assistance of Blair Jarratt and Judy Shutt at IMS International is gratefully appreciated by the authors.
REFERENCES
- 1.Aarestrup F M, Ahrens P, Madsen M, Pallesen L V, Poulsen R L, Westh H. Glycopeptide susceptibility among Danish Enterococcus faecium and Enterococcus faecalis isolates of animal and human origin and PCR identification of genes within the VanA cluster. Antimicrob Agents Chemother. 1996;40:1938–1940. doi: 10.1128/aac.40.8.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cook R R. Antimicrobial resistance—use in veterinary and human medicine. J Antimicrob Chemother. 1997;39:435–439. doi: 10.1093/jac/39.3.435. [DOI] [PubMed] [Google Scholar]
- 3.Coque T M, Tomayko J F, Ricke S C, Okhyusen P C, Murray B E. Vancomycin-resistant enterococci from nosocomial, community, and animal sources in the United States. Antimicrob Agents Chemother. 1996;40:2605–2609. doi: 10.1128/aac.40.11.2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Devriese L A, Ieven M, Goossens H, Vandamme P, Pot B, Hommez J, Haesebrouck F. Presence of vancomycin-resistant enterococci in farm and pet animals. Antimicrob Agents Chemother. 1996;40:2285–2287. doi: 10.1128/aac.40.10.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donnelly J P, Voss A, Witte W, Murray B E. Does the use in animals of antimicrobial agents, including glycopeptide antibiotics, influence the efficacy of antimicrobial therapy in humans? J Antimicrob Chemother. 1996;37:389–390. doi: 10.1093/jac/37.2.389. [DOI] [PubMed] [Google Scholar]
- 6.Edmond M B, Ober J F, Weinbaum D L, Pfaller M A, Hwang T, Sanford M D, Wenzel R P. Vancomycin-resistant Enterococcus faecium bacteremia: risk factors for infection. Clin Infect Dis. 1995;20:1126–1133. doi: 10.1093/clinids/20.5.1126. [DOI] [PubMed] [Google Scholar]
- 7.Hayes P W, Gustafson R H, Lotgering I F K, Mudd A J. Reply. J Antimicrob Chemother. 1996;37:390–392. [Google Scholar]
- 8.Hospital Infection Control Practice Advisory Committee (HICPAC) Recommendations for preventing the spread of vancomycin resistance. Infect Control Hosp Epidemiol. 1995;16:105–113. doi: 10.1086/647066. [DOI] [PubMed] [Google Scholar]
- 9.Keighley M R B, Burdon D W, Arabi Y. Randomised controlled trial of vancomycin for pseudomembranous colitis and postoperative diarrhoea. Br Med J. 1978;2:1667–1669. doi: 10.1136/bmj.2.6153.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leclercq R, Courvalin P. Resistance to glycopeptides in enterococci. Clin Infect Dis. 1997;24:545–556. doi: 10.1093/clind/24.4.545. [DOI] [PubMed] [Google Scholar]
- 11.Luber A D, Jacobs R A, Jordan M, Guglielmo B J. Relative importance of oral versus intravenous vancomycin exposure in the development of vancomycin-resistant enterococci. J Infect Dis. 1996;173:1292–1293. doi: 10.1093/infdis/173.5.1292. [DOI] [PubMed] [Google Scholar]
- 12.Maki D G, Bohn J M, Stolz S M, Kroncke G M, Archer C W, Myerowitz P D. Comparative study of cefazolin, cefamandole, and vancomycin for surgical prophylaxis in cardiac and vascular operations. J Thor Cardiovasc Surg. 1992;104:1423–1434. [PubMed] [Google Scholar]
- 13.Mogg G A G. Antibiotic-associated colitis: a review of 66 cases. Br J Surg. 1979;66:738–742. doi: 10.1002/bjs.1800661017. [DOI] [PubMed] [Google Scholar]
- 14.Payne N R, Schilling C G, Steinberg S. Selecting antibiotics for nosocomial bacterial infections in patients requiring neonatal intensive care. Neonatal Network. 1994;13:41–51. [PubMed] [Google Scholar]
- 15.Shay D K, Jarvis W R, Montecalvo M. Reply. J Infect Dis. 1996;173:1293–1294. [Google Scholar]
- 16.Swartz M N. Hospital-acquired infections: diseases with increasingly limited therapies. Proc Natl Acad Sci USA. 1994;91:2420–2427. doi: 10.1073/pnas.91.7.2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van den Bogaard A E. Antimicrobial resistance—relation to human and animal exposure to antibiotics. J Antimicrob Chemother. 1997;40:453–454. doi: 10.1093/jac/40.3.453. [DOI] [PubMed] [Google Scholar]
- 18.Van den Bogaard A E, Mertens P, London N H, Stobberingh E E. High prevalence of colonization with vancomycin- and pristinamycin-resistant enterococci in healthy humans and pigs in The Netherlands: is the addition of antibiotics to animal feeds to blame? J Antimicrob Chemother. 1997;40:454–456. doi: 10.1093/jac/40.3.454. [DOI] [PubMed] [Google Scholar]
- 19.Van der Auwera P, Pensart N, Korten V, Murray B E, Leclercq R. Influence of oral glycopeptides on the fecal flora of human volunteers: selection of highly glycopeptide-resistant enterococci. J Infect Dis. 1996;173:1129–1136. doi: 10.1093/infdis/173.5.1129. [DOI] [PubMed] [Google Scholar]
- 20.Witte W. Impact of antibiotic use in animal feeding on resistance of bacterial pathogens in humans. CIBA Found Symp. 1997;207:61–75. doi: 10.1002/9780470515358.ch5. [DOI] [PubMed] [Google Scholar]