Abstract
In Asia, a few countries have a long and established history of collaborative clinical trials successfully formed national children's cancer study groups, but many still do not have such groups. The process of forming national children's cancer groups is fraught with many hurdles, which varies among the countries. One of the basic requirements for running clinical trials is an affordable health care system in which most of the children with cancer can receive the proposed treatment. The health insurance coverage for children with cancer varies from <20% to as high as 100% among Asian countries, and the operation of clinical trials must also be adjusted accordingly. Shortage of research personnel is common, including medical, nursing, research coordinators, and data managers. The establishment of the Asian Pediatric Hematology and Oncology Group aims to provide a good platform for promotion of international clinical trials in the Asian countries.
INTRODUCTION
Cancer is an important cause of morbidity and mortality in children. It is estimated that globally there are 413,000 new cases of childhood cancer annually in 2020.1 With the advancement of anticancer treatment and supportive care, the survival rate of childhood cancer is now more than 80% in high-income countries (HICs). However low-income countries (LICs) and middle-income countries (MICs) have a survival of only 10%-60% for childhood cancers, and these countries constituted the majority of cancer cases in children globally. Asia with 48 countries is the largest continent with the most population worldwide; the continent consists of HICs, MICs, and LICs. The treatment outcome of childhood cancers from different countries in Asia varies greatly. Establishment of collaborative clinical trial groups has a long history in North America and European countries.2 The clinical trial groups initially started as multicenter study groups in a country and later developed into national children's cancer study groups, and some also extended further to multinational study groups. The clinical trials groups have performed numerous clinical trials in various oncology conditions. These activities bring remarkable achievement in improving survival outcome, setting clinical guidelines, and promoting cost-effective treatment approaches.
CONTEXT
Key Objective
The paper studied the current status of clinical trial activities of pediatric oncology in Asia and the challenges the groups were facing. There was little information on the trial activities in pediatric oncology in Asia, but the continent has the largest patient load of childhood cancer.
Knowledge Generated
The greatest challenges encountered in clinical research were shortage of manpower and funding support. Lack of designated research personnel in the background of extremely busy clinical setting is a major hurdle for successful clinical trials.
Relevance
The information on low-income countries was scarce and they require much support to develop an affordable health care system before moving towards clinical trials. The Asian Pediatric Hematology Oncology Group may be a platform for establishing clinical trials in Asia in the future.
In Asia, a few countries have a long and established history of collaborative clinical trials, and some of them successfully formed national children's cancer study groups. The process of forming national children's cancer groups encountered many hurdles which varied among the countries. One of the basic requirements for running clinical trials is an affordable health care system in which most of the children with cancer can receive the proposed treatment. The health care system and financial support from the government for care of children with cancer varies in Asian countries, and the operation of clinical trials also has to be adjusted accordingly. The newly formed Asian Pediatric Hematology and Oncology Group (APHOG) with representation from many Asian countries aims at promoting clinical trials in Asia.3 As part of its initial activity, APHOG wanted to understand the history and functioning of national study groups in Asia, which may help some other countries to establish their national groups, and also learn the ways to overcome hurdles of running collaborative clinical trials. This study is based on the presentations of national children's cancer study groups in a APHOG scientific meeting in October 2021.
METHODS
The coauthors retrospectively collected the information for their own countries, and he/she was also the representative of the national groups and provided the information of health care system of their countries. The national representatives contacted the study coordinators for the details of the studies or from the publications. The national group representatives reported the challenges they encountered according to a suggested format with closed-ended and open-ended questions. Each national group provided a brief history of the formation of the national group and also the number of institutions or members (Table 1). The achievement of national group was described regarding the number of clinical trials conducted or organized and the approximate percentage of affected children recruited into the clinical trials in their countries. The health care system of each country was briefly discussed, and the type of coverage was elaborated, such as national insurance or private insurance or out-of-pocket payment. We also illustrated the major challenges in conducting clinical trials across these national groups.
TABLE 1.
Background Information
Chinese Children's Cancer Group
Chinese Children’s Cancer Group (CCCG) is the official clinical study group of the China Anti-Cancer Association (CACA). CCCG was established in 1992, and up to December 2021, there were 78 member institutions with 1,775 individual members. This group has organized various types of clinical trials with 10-50 institutions participating in the trials. The spectrum of cancers in these trials included hematolymphoid and solid tumors.4-8 CCCG has formed a total of eight subspecialty study groups namely medical oncology, surgical oncology, nursing, pathology, imaging, radiotherapy, prevention, and new technology development study groups.
Most of the completed CCCG studies were retrospective studies without funding support. Recently, a prospective clinical trial with 20 participating institutions on ALL, CCCG-ALL-2015 protocol, was conducted. More than 7,600 patients with newly diagnosed ALL were recruited over 5 years. The current protocol, CCCG-ALL-2020, is recruiting patients from 24 institutions. Other ongoing CCCG clinical trials were of smaller scale and included AML, non-Hodgkin lymphoma (NHL), neuroblastoma, rhabdomyosarcoma, and other solid tumors. These trials received limited funding support from either government or foundations.
Regarding the health care system in China, each provincial government covers 50%-100% medical expenses for pediatric patients with cancer, depending on the economic development of the provinces. In recent years, more families purchase private insurance services for their children. For patients who do not have insurance, part of the medical expense is covered by self-payment or donations from individual contributors and charity organizations. Overall, about 70%-80% of the patients can receive standard care although some of them are still under heavy financial pressure. However, the most recently developed new and expensive treatments, such as chimeric antigen receptor (CAR)-T cell therapy, and some novel target therapies, including monoclonal antibodies, are not affordable for most families. In addition, most of the molecular genetic diagnostic tests such as panel next generation sequencing or whole exome sequencing are provided by the private laboratory and has to be paid by the families.
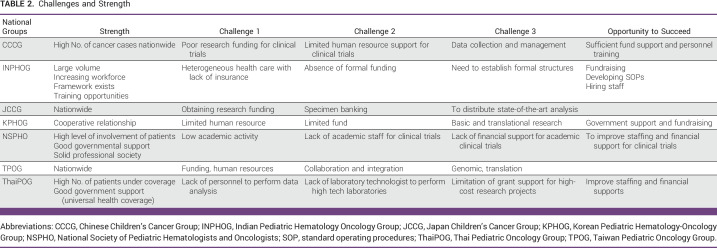
In China, the following difficulties to conduct nationwide clinical trials are encountered. First, China is a huge country with cities at different stages of economic development; participating institutions are mostly restricted to those with better resources. Funding support for clinical trials is the most important hurdle to overcome. Second, the investigators are under immense workload of the clinical service. Personnel involved such as project coordinators, clinical research assistants, and data managers are not regularly employed as staff in hospitals. Third, abandonment still happens because some families cannot afford expensive treatment for cancers (Table 2). There is a trend of improving the research environment for the nationwide clinical trials in pace with the rapid economic development in China.
TABLE 2.
Challenges and Strength
Indian Pediatric Hematology Oncology Group
Indian Pediatric Hematology Oncology Group (INPHOG previously known as InPOG) was established in September 2008 and has been active since January 2015. The mission has been to improve the outcomes of children with cancer in India through collaborative research. The objective is to promote regionally relevant pediatric cancer research, including multicenter clinical trials in India to generate evidence in the local population and to improve outcomes. The group has been active exclusively in the childhood cancer space, and more recently in July 2019, it has also incorporated collaborative research related to benign hematological disorders in children which is reflected in the new name INPHOG.
INPHOG operates through 19 disease-specific and seven transdisciplinary subcommittees.9 There are currently a portfolio of 31 studies of which seven have completed recruitment and 16 are currently recruiting. Notable among these include randomized controlled trials in acute lymphoblastic leukemia (pulse v continuous steroid induction and mitoxantrone v doxorubicin in delayed intensification) and AML (induction with or without etoposide) (Table 3). Since 2015, 10,017 children with cancer have been enrolled with 69.3% in observational studies and 30.7% in interventional studies from 114 institutions across the country.10,11 This would imply that <5% of children with cancer treated in India got enrolled on to collaborative clinical studies. The work so far has led to 15 publications in peer-reviewed journals. Only four of these 23 studies (which have completed recruitment or are recruiting) have had formal partial or total funding (60,000,000 Indian Rupee or 820,000 US dollars [USD]). The remaining majority have used existing human resources and infrastructure, coupled with support from not-for-profit organizations.
TABLE 3.
Ongoing Clinical Trials
The main rate limiting factor has been the absence of funding. Till recently, INPHOG which was established under the aegis of the Pediatric Hematology and Oncology chapter of the Indian Academy of Pediatrics (PHO-IAP) did not have a separate entity and legal status, thus limiting the ability to raise funds and enter into formal agreements with organizations and collaborators. It lacked logistical support in terms of clinical research assistants, data managers, statisticians, research nurses, clinical trial management systems, etc. In December 2021, INPHOG Research Foundation was registered as a not-for-profit separate legal entity to build its administrative, governance, and fundraising structure (Table 2). It now plans to put build capacity and put systems in place to further enhance collaborative research in childhood cancer and benign hematological disorders in India. There is also a need to develop own administrative capacity with a dedicated central office of INPHOG providing human resource management, resource mobilization, and monitoring research conduct.12 At the same time, INPHOG is keen to engage with international efforts such as APHOG and SIOP to collaborate in larger global studies.
Japan Children's Cancer Group
Japan Children's Cancer Group (JCCG) was established in 2014 and currently has 204 participating facilities. To our knowledge, to date, 25 clinical trials have been completed for hematologic malignancies and 17 for solid tumors, with 36 trials under follow-up or enrollment.13-17 Percentages of children enrolled vary by diseases, including 90% for ALL, 78% for AML, 93% for neuroblastoma, and 42% for brain tumors. Some of the ongoing clinical trials testing new agents or treatment approaches are shown in Table 3. Japan has a universal health insurance system, meaning that a public insurance system covers everyone. Individuals pay around 30% of their medical expenses. Furthermore, in the case of pediatric patients with cancer, this 30% contribution is also waived. If the household income is high, there is a partial copayment, but it is up to a maximum of 130 USD per month.
The major challenges to conduct clinical trials in the group are listed. First, shortage of supporting staff is a common problem. Many participating facilities and physicians require significant extra-administrative effort to conduct the clinical trials. Second, financial resources are most challenging; the funding mainly coming from members' dues and donations supports the JCCG's operations. Funds for conducting clinical trials are financed separately for each trial and are separated from the JCCG's funds. It is difficult to obtain additional funds to conduct clinical trials because the funding from commercial companies is limited (Table 2). National organizations have a system to provide funds for trials under government support; however, it is very competitive. In addition, the funding duration is rather short, and the number of years offered is limited to 3 years. Therefore, funding may become insufficient in the middle period of the clinical trials.
Korean Pediatric Hematology-Oncology Group
Korean Pediatric Hematology-Oncology Group (KPHOG) is the official clinical study group of the Korean Society of Pediatric Hematology-Oncology (KSPHO). KSPHO was established in September in 1993 and KPHOG in 2014. There are a total of 55 institutions joining KSPHO, but only about 15 centers are actively participating to KPHOG. KPHOG has 13 committees including nine oncology, two benign hematology, one hematopoietic stem cell transplantation, and one registry, led by each committee chair. Most KPHOG studies have been retrospective because of limited resources, and six articles have been published in peer-reviewed journals in the recent 2 years.18-21 Currently, four multicenter prospective clinical trials are ongoing for childhood cancers including three ALL studies participated by three institutions and one AML study by four institutions. Although the numbers of participating centers are small, these include about 40% and 50% of patients in Korea, respectively. One multicenter clinical trial was completed and published in recent 5 years.22
Regarding the health care system in Korea, the National Health Insurance Service covers 95% of medical expenses in patients with cancer but only for approved practices. Most people also purchase various kinds of private insurance services, and donations from individual contributors and social organizations are also available. Therefore, practically every pediatric patient with cancer in Korea can receive standard care even if they are in deep financial strain.
KPHOG has been facing many difficulties in performing nationwide multicenter clinical trials. Professionals in the big 5 hospitals in Seoul, the capital of Korea, are taking care of approximately 80% of all pediatric patients with cancer in Korea. Because of highly efficient public transportation along with unlimited access to any hospital, most families with cancer prefer to go to a big hospital in Seoul. Nonetheless, there are only three or four faculty members in each big 5 center, which put them under heavy clinical service burden. Given this situation, the majority of pediatric hemato-oncologists do not have enough time and resources to run basic and translational research. In addition to human resource issues, limited financial support or donation for KPHOG studies from the government, companies, or individual contributors has made well-designed nationwide multicenter clinical trials difficult (Table 2). Recently, a large donation has just been donated by a big corporation in Korea to support pediatric patients with cancer.
Limited human resource remains the biggest challenge; however, with anticipated government support and fundraising campaigns, this could encourage more people to get interested in pediatric hematology-oncology and thus more manpower to support better care for children with cancer.
National Society of Pediatric Hematologists and Oncologists (Russian Federation)
The National Society of Pediatric Hematologists and Oncologists (NSPHO, Russian Federation) was founded in 2010 by the major institutions in pediatric hematology/oncology. There are now 1,150 members from 84 institutions in NSPHO across Russia which covers 98% of specialists working in the field of pediatric hematology/oncology in Russia. Twenty-three clinical trials in pediatric hematology-oncology have been conducting currently, for both malignant and nonmalignant diseases (Table 3). For ALL trials, there are both the Russian MB protocol and iBFM trial. NSPHO also participates in SIOP-RTSG protocol and international HSCT trials.23,24 These trials are participated either multicenter or single center in Russia.
The Government of the Russian Federation provides funding that covers the treatment of all children with hematological and oncological diseases across the country. Coverage includes those modalities recommended by guidelines set by two pathways: (1) clinical guidelines and trials led by the National centers and NSPHO and (2) clinical guidelines prepared by NSPHO on the basis of approved and completed clinical trials. Fifty-three approved clinical guidelines and current clinical trials cover 95% of patients today. Other patients are treated with experimental schemes. It is obligatory to receive recommendation from the national center and/or study committee to start new treatment.
The challenges that NSPHO encountered include additional human resources to cover the operation of clinical trials and scientific activity. In addition, improvement of nursing manpower and expertise and improvement of financial support for clinical trials are required. Such improvements would increase the number of trials and academic activities of NSPHO in the future.
Thai Pediatric Oncology Group
Thai Pediatric Oncology Group (ThaiPOG) was established in 2000 and currently has 71 participating facilities and 155 members. So far 12 clinical studies have been completed, five in hematological malignancies and seven in solid tumors, and four trials are now under follow-up or recruitment.25-29 Percentages of patients enrolled in the trials vary by diseases, ranging from 10% to 90%.
Thailand has a universal health coverage system, which covers 92% of the payment for childhood cancers; the remaining 6% are covered by government officer services and 2% out of families' own pocket.
To conduct clinical trials in Thailand, the challenges encountered include lack of personnel to take care of data collection and analysis in nonmedical school centers and lack of laboratory technologists and laboratories to perform some of the state-of-the-art investigations. Grant support is also limited for the high-cost research projects. Doctors in some service hospitals have high workload and just have limited time to take care of research activity (Table 2).
Taiwan Pediatric Oncology Group
Taiwan Pediatric Oncology Group (TPOG) was established in 1988 and currently has 28 participating institutions. The group has enrolled 10,511 patients from 1988 to 2020 into clinical studies. TPOG has been focusing on childhood cancer research. To our knowledge, to date, 21 clinical trials have been completed, 10 in hematologic malignancies and 11 in solid tumors.30-34 There are now 21 ongoing trials under active enrollment or follow-up. About 90% of children with cancer in Taiwan are recruited to TPOG trials (according to 2017 National Registry), which captured 95% of ALL, 95% of AML, 98% of NHL, and nearly 100% for Wilms' tumor, retinoblastoma, osteosarcoma, and neuroblastoma.
Taiwan launched the National Health Insurance (NHI) System in 1995, which is a compulsory individual social insurance plan run by the Government. The NHI delivers universal coverage, covering most expenditures related to medical care. Patients pay only fixed copayments for clinic visits, which is independent of personal income. Hospitalization incurs additional copayments, depending on the service items, usually accounting for 5%-10% of the total medical expenses. Catastrophic illnesses, such as cancer and certain chronic health conditions, had additional waivers for copayments related to the corresponding illness. Since 2002, Taiwan NHI Payment System has changed from fee-for-service to a global budget payment. The revenue base is capped, and premiums are infrequently raised which was regulated by the politicians. Because of capping of the Global Budget, the Drug Pricing System had been so tight or harsh, leading to the adapting of generic drugs. In addition, the Payment System becomes a major rate limiting factor for introducing newly marketed medical devices and drugs in timely manner. Those items not listed in the National Institutes of Health Payment will have to be shouldered by the patient's family totally.
Major challenges to conduct clinical trials in the group are related to lack of human resource and financial constraints. Data managers and contracted statisticians are sponsored by nonprofit organizations. TPOG does not have a clinical trial management system, and the administrative infrastructures are incomplete. There is shortage of trial research assistants, research nurses, and resources to monitor research activities. Infrastructural, hardware, software, and data managers/statisticians are financially supported by a nonprofit charity organization, mainly the Childhood Cancer Foundation (CCF) Taiwan. CCF Taiwan raises funding through public donations to support cancer research. There are many hurdles and limitations to apply for the highly competitive government grants for clinical trials related to childhood cancers. So far, TPOG has not received any government funding support to conduct clinical trials (Table 2).
DISCUSSION
About 50% of childhood cancers are found in Asia, but there is wide variation in the 5-year childhood cancer survival rates across Asia, from 28.8% in Southeast Asia to 53.8% in East Asia.1,35 In 2018, the WHO announced the Global Initiative for Childhood Cancer, with the goal of achieving a 60% survival rate for all children with cancer by 2030 through the effort in mobilizing numerous regional and national stakeholders.36 To achieve a high survival rate for children with cancer, there should be a robust system of providing good quality care to these children from diagnosis to specific anticancer treatment and supportive care. Clinical guidelines have been established in some countries at national level, which take into consideration the local situation and resources. The clinical guidelines are very useful to frontline clinical teams for formulating an appropriate care plan for each individual patient with cancer, especially in LICs and MICs. To have further improvement in the clinical care of children with cancer, clinical trials under multicenter setting is necessary. The WHO Global Initiative for Childhood Cancer CureAll Framework recommends as a priority action to invest in cancer research infrastructure and participate in collaborative research networks.37 The local trials can ask specific questions relevant to the local situation, such as how best to use the specific anticancer agents in a cost-effective approach on the basis of limited available resources. In western countries, there is a long history of conducting large multicenter clinical trials, either national or international. The support for operation of these clinical trial groups may come from government or large charity organizations.
In Asia, there is great variability in the development of clinical trial groups. Some have been successful in conducting prospective randomized controlled trials which introduces significant impact on the clinical practice.4,14 Owing to various reasons, some trial groups mainly conducted retrospective studies to review the outcome of standard treatment or report on specific issues related to a treatment protocol, such as complications or late effects.19,25,38 Recognizing challenges in accessing care, INPHOG has a subcommittee focused on multicenter research in access to care and is enrolling patients in extensive multicenter studies that are examining out-of-pocket expenditure, pathways to diagnosis, and the effects of COVID-19 on treatment access. The reasons leading to the slow development of clinical trial groups are multifactorial, and some are due to local regulatory issues. Another important contributing factor is the financial model of the health care delivery service. From this report, some of the countries have national insurance scheme in place thus patients can be managed with a standard clinical path, from diagnosis to specific treatment. However, the rate of insurance coverage also varies from 70% to nearly 100%. To introduce new and expensive treatment modalities, this has to be included in the insurance framework, so the cost may be reimbursed. Asian clinical trial groups have difficulties in participating in new drug trials especially for phase I and II studies. Only limited Asian centers have been invited to participate in the global studies and majority of them are phase III or IV studies. The formation of a strong clinical trial structure among Asian countries for multinational studies may facilitate the paradigm shift in the decision making of the pharmaceutical industry.
To establish well-organized clinical trial structure, it requires local leadership for formation of a structured clinical trial body. The members of the group should design research studies of resource-conscious adapted treatment regimens and prioritization of stepwise infrastructure building. Japan is taking a lead in the research organization with well-organized study group structure and carefully designed and conduct of the studies. For MICs such as India, there is a long history of clinical work and optimizing care using adapted treatment protocols. In recent years, the Indian pediatric oncology community has also started building clinical trial infrastructure and collaborative trials, for example, INPHOG. The steps toward retrospective studies to prospective studies including randomized control study depend on the socioeconomic development and a dedicated team of researchers. There are positive signs of development in clinical researches for many countries in recent years, but they also face many hurdles and challenges in the road ahead.
In this report, many national groups bring up some common challenges. Human resource is one of the major hurdles, from clinicians to supporting staff. Clinical investigators are essential for operation of clinical trials; however, many countries are facing shortage of medical and nursing manpower to support clinical trials. The health care teams are very busy with the heavy clinical workload while the number of trained personnel is limited. Many countries do not have regular postings for data managers, project coordinators, or research nurses. The data management workload has to be shared with the clinical teams who are already under very heavy clinical duties. Some groups have been successful to obtain funding from research grants or nongovernment organizations to support the additional staff for the clinical trials, but such short-term support may not be sustainable. The hospitals or government should consider allocating extra resources to support and facilitate clinical teams to conduct clinical trials. National funding for children's cancer collaborative groups in the country would be the most cost-effective approach in the operation.
The creation of regional pediatric cancer units would strengthen the group's research productivity. However, it cannot be a one-size-fits-all approach to increase the collaboration between international pediatric cancer clinical trial groups, understanding of local stakeholders, and needs to form partnerships, that is, needs assessments. A model of this improvement in outcomes, with its endogenous formation within Africa, demonstrates that engaging local stakeholders in the creation of infrastructure and protocols is a critical component of forming sustainable collaborative groups. The creation of regional pediatric cancer units by the French-African Pediatric Oncology Group (GFAOP) is a cornerstone of the group's research productivity and is evidence that careful mapping of available cancer care resources may mitigate some challenges of operating in resource-poor areas.39 Many twinning relationships were based on the development of a single adapted treatment regimen, which enabled subsequent infrastructure building and the formation of a new cooperative group. Twinning initiatives need not only be focused on clinical trial development as building foundational cancer databases and registries are equally essential for improving care of children with cancers. Multinational clinical trial for childhood cancers has yet to be developed in Asia because of the factors discussed above. A future target is also to have wider representation from other countries in Asia including LICs. In the absence of national collaborative research groups, the newly formed APHOG will be an ideal platform to promote clinical trials in the Asian continent.
In conclusion, in the past few decades, there is remarkable development in clinical trial groups among some Asian countries. There is great room for improvement among existing clinical trial groups but requires the support from international community and governments. The formation of APHOG is collaborative platform across Asian countries to enhance the clinical trial activities in the continent. The challenges of running clinical trials need to be addressed, and greater funding support for research activities is in urgent need.
Chi-kong Li
Consulting or Advisory Role: Amgen Asia
Speakers' Bureau: Amgen (Europe)
Ramandeep Singh Arora
Employment: Max Healthcare
Keon Hee Yoo
Stock and Other Ownership Interests: CELLnLife
Honoraria: Kyowa Kirin
Consulting or Advisory Role: Affyxell therapeutics
Godfrey C.F. Chan
Leadership: Xellera Therapeutics, Pangenia
Consulting or Advisory Role: Xellera Therapeutics, Pangenia
Speakers' Bureau: Xellera Therapeutics, Pangenia
Patents, Royalties, Other Intellectual Property: Xellera Therapeutics, Pangenia
Alice Yu
Leadership: OPKO Health
Stock and Other Ownership Interests: OPKO Health/GeneDx
Consulting or Advisory Role: OBI Pharma
Research Funding: OBI Pharma
Patents, Royalties, Other Intellectual Property: Globo H-Diphtheria toxoid vaccine for cancer therapy, NKT stimulatory phenyl-glycolipids for cancer therapy and vaccine adjuvant, cancer targeting peptides, Methods for suppressing cancer by inhibition of TMCC3
No other potential conflicts of interest were reported.
AUTHOR CONTRIBUTIONS
Conception and design: Chi-kong Li, Purna Kurkure, Bow Wen Chen, Yasuhiro Okamoto, Bharat Agarwal, Godfrey C.F. Chan, Hiroki Hori, Muhammad Saghir Khan, Alice Yu, Akira Nakagawara
Financial support: Akira Nakagawara
Administrative support: Akira Nakagawara
Provision of study materials or patients: Chi-kong Li, Bow Wen Chen, Yasuhiro Okamoto, Panya Seksarn, Keon Hee Yoo, Rashmi Dalvi, Akira Nakagawara
Collection and assembly of data: Purna Kurkure, Ramandeep Singh Arora, Bow Wen Chen, Kirill Kirgizov, Panya Seksarn, Yongmin Tang, Keon Hee Yoo, Rashmi Dalvi, Muhammad Saghir Khan, Akira Nakagawara
Data analysis and interpretation: Purna Kurkure, Ramandeep Singh Arora, Keon Hee Yoo, Muhammad Saghir Khan, Akira Nakagawara
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/go/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Chi-kong Li
Consulting or Advisory Role: Amgen Asia
Speakers' Bureau: Amgen (Europe)
Ramandeep Singh Arora
Employment: Max Healthcare
Keon Hee Yoo
Stock and Other Ownership Interests: CELLnLife
Honoraria: Kyowa Kirin
Consulting or Advisory Role: Affyxell therapeutics
Godfrey C.F. Chan
Leadership: Xellera Therapeutics, Pangenia
Consulting or Advisory Role: Xellera Therapeutics, Pangenia
Speakers' Bureau: Xellera Therapeutics, Pangenia
Patents, Royalties, Other Intellectual Property: Xellera Therapeutics, Pangenia
Alice Yu
Leadership: OPKO Health
Stock and Other Ownership Interests: OPKO Health/GeneDx
Consulting or Advisory Role: OBI Pharma
Research Funding: OBI Pharma
Patents, Royalties, Other Intellectual Property: Globo H-Diphtheria toxoid vaccine for cancer therapy, NKT stimulatory phenyl-glycolipids for cancer therapy and vaccine adjuvant, cancer targeting peptides, Methods for suppressing cancer by inhibition of TMCC3
No other potential conflicts of interest were reported.
REFERENCES
- 1.Major A, Palese M, Ermis E, et al. Mapping pediatric oncology clinical trial collaborative groups on the global stage. JCO Glob Oncol. [DOI] [PMC free article] [PubMed]
- 2. Atun R, Bhakta N, Denburg A, et al. Sustainable care for children with cancer: A Lancet Oncology Commission. Lancet Oncol. 2020;21:e185–e224. doi: 10.1016/S1470-2045(20)30022-X. [DOI] [PubMed] [Google Scholar]
- 3. Nakagawara A. Asian Pediatric Hematology and Oncology Group (APHOG) and SIOP Asia: Two wheels of a cart. Pediatr Hematol Oncol J. 2020;5:140–144. [Google Scholar]
- 4. Shen S, Chen X, Cai J, et al. Effect of dasatinib vs imatinib in the treatment of pediatric Philadelphia chromosome-positive acute lymphoblastic leukemia: A randomized clinical trial. JAMA Oncol. 2020;6:358–366. doi: 10.1001/jamaoncol.2019.5868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tang J, Yu J, Cai J, et al. Prognostic factors for CNS control in children with acute lymphoblastic leukemia treated without cranial irradiation. Blood. 2021;138:331–343. doi: 10.1182/blood.2020010438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yang W, Cai J, Shen S, et al. Pulse therapy with vincristine and dexamethasone for childhood acute lymphoblastic leukaemia (CCCG-ALL-2015): An open-label, multicentre, randomised, phase 3, non-inferiority trial. Lancet Oncol. 2021;22:1322–1332. doi: 10.1016/S1470-2045(21)00328-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liu A, Zhen Z, Yang Q, et al. Treatment barriers and clinical outcome of children with medulloblastoma in China: A report from the Chinese Children’s Cancer Group (CCCG) Neurooncol Adv. 2021;3:1–10. doi: 10.1093/noajnl/vdab134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang D, Kaweme N, Duan P, et al. Upfront treatment of pediatric high-risk neuroblastoma with chemotherapy, surgery, and radiotherapy Combination: The CCCG-NB-2014 protocol. Front Oncol. 2021;11:745794. doi: 10.3389/fonc.2021.745794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Arora RS, Bakhshi S. Indian Pediatric Oncology Group (InPOG)—Collaborative research in India comes of age. Pediatr Hematol Oncol J. 2016;1:13–17. [Google Scholar]
- 10. Arora RS, Raj R, Mahajan A, et al. Collaborative cancer research: Progress report from the Indian Pediatric Oncology Group. Lancet Child Adolescent Health. 2021;5:239–240. doi: 10.1016/S2352-4642(21)00056-0. [DOI] [PubMed] [Google Scholar]
- 11. Roy P, Narula G, Arora B, et al. Implementation of risk adapted therapeutic strategy for childhood acute lymphoblastic leukaemia—interim report of the pilot InPOG-ALL-15-01 study. Pediatr Hematol Oncol J. 2018;3:S19. [Google Scholar]
- 12. Arora RS, Mahajan A, Dinand V, et al. InPOG-HL-15-01—Challenges and lessons learnt in setting up the first collaborative multicentre prospective clinical trial in childhood cancer in India. Pediatr Hematol Oncol J. 2020;5:166–170. [Google Scholar]
- 13. Koh K, Kato M, Saito AM, et al. Phase II/III study in children and adolescents with newly diagnosed B-cell precursor acute lymphoblastic leukemia: Protocol for a nationwide multicenter trial in Japan. Jpn J Clin Oncol. 2018;48:684–691. doi: 10.1093/jjco/hyy071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tomizawa D, Tanaka S, Hasegawa D, et al. Evaluation of high-dose cytarabine in induction therapy for children with de novo acute myeloid leukemia: A study protocol of the Japan Children's Cancer Group multi-center seamless phase II-III randomized trial (JPLSG AML-12) Jpn J Clin Oncol. 2018;48:587–593. doi: 10.1093/jjco/hyy061. [DOI] [PubMed] [Google Scholar]
- 15. Tsurusawa M, Mori T, Kikuchi A, et al. Improved treatment results of children with B-cell non-Hodgkin lymphoma: a report from the Japanese Pediatric Leukemia/Lymphoma Study Group B-NHL03 study. Pediatr Blood Cancer. 2014;61:1215–1221. doi: 10.1002/pbc.24975. [DOI] [PubMed] [Google Scholar]
- 16. Hishiki T, Matsumoto K, Ohira M, et al. Japan Childhood Cancer Group Neuroblastoma Committee (JNBSG). Results of a phase II trial for high-risk neuroblastoma treatment protocol JN-H-07. Int J Clin Oncol. 2018;23:965–973. doi: 10.1007/s10147-018-1281-8. [DOI] [PubMed] [Google Scholar]
- 17. Hiyama E, Ueda Y, Onitake Y, et al. A cisplatin plus pirarubicin-based JPLT2 chemotherapy for hepatoblastoma: Experience and future of the Japanese Study Group for Pediatric Liver Tumor (JPLT) Pediatr Surg Int. 2013;29:1071–1075. doi: 10.1007/s00383-013-3399-0. [DOI] [PubMed] [Google Scholar]
- 18. Park JE, Noh OK, Lee Y, et al. Loss of heterozygosity at chromosome 16q is a negative prognostic factor in Korean pediatric patients with favorable histology Wilms tumor: A report of the Korean Pediatric Hematology Oncology Group (K-PHOG) Cancer Res Treat. 2020;52:438–445. doi: 10.4143/crt.2019.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee JM, Choi JY, Hong KT, et al. Clinical characteristics and treatment outcomes in children, adolescents, and young-adults with Hodgkin's lymphoma: A KPHOG Lymphoma Working-party, multicenter, retrospective study. J Korean Med Sci. 2020;35:e3933. doi: 10.3346/jkms.2020.35.e393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Park M, Han JW, Hahn SM, et al. Atypical teratoid/rhabdoid tumor of the central nervous system in children under the age of 3 Years. Cancer Res Treat. 2021;53:378–388. doi: 10.4143/crt.2020.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Park KM, Yoo KH, Kim SK, et al. Clinical characteristics and treatment outcomes of childhood acute promyelocytic leukemia in Korea: A nationwide multicenter retrospective study by Korean Pediatric Oncology Study Group. Cancer Res Treat. 2022;54:269–276. doi: 10.4143/crt.2021.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kang HJ, Hong KT, Lee JW, et al. Improved outcome of a reduced toxicity-fludarabine, cyclophosphamide, plus antithymocyte globulin conditioning regimen for unrelated donor transplantation in severe aplastic anemia: Comparison of 2 multicenter prospective studies. Biol Blood Marrow Transplant. 2016;22:1455–1459. doi: 10.1016/j.bbmt.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 23. Kirgizov KI, Kogan SA, Erdomaeva YA, et al. Russian experience in pediatric hematology-oncology: Collaboration of the national society and national centers. Pediatr Hematol Oncol J. 2020;5:156–161. [Google Scholar]
- 24. Karachunskiy A, Tallen G, Roumiantseva J, et al. Reduced vs. standard dose native E. coli-asparaginase therapy in childhood acute lymphoblastic leukemia: Long-term results of the randomized trial Moscow-Berlin 2002. J Cancer Res Clin Oncol. 2019;145:1001–1012. doi: 10.1007/s00432-019-02854-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pakakasama S, Veerakul G, Sosothikul D, et al. Late effects in survivors of childhood acute lymphoblastic leukemia: A study from Thai Pediatric Oncology Group. Int J Hematol. 2010;91:850–854. doi: 10.1007/s12185-010-0594-9. [DOI] [PubMed] [Google Scholar]
- 26. Wiangnon S, Veerakul G, Nuchprayoon I, et al. Childhood cancer incidence and survival 2003-2005, Thailand: Study from the Thai Pediatric Oncology Group. Asian Pac J Cancer Prev. 2011;12:2215–2220. [PubMed] [Google Scholar]
- 27. Seksarn P, Wiangnon S, Veerakul G, et al. Outcome of childhood acute lymphoblastic leukemia treated using the Thai national protocols. Asian Pac J Cancer Prev. 2015;16:4609–4614. doi: 10.7314/apjcp.2015.16.11.4609. [DOI] [PubMed] [Google Scholar]
- 28. Pongtanakul B, Sirachainan N, Surapolchai P, et al. Pediatric primary central nervous system tumors registry in Thailand under National Health Security Office schemes. J Neurooncol. 2020;149:141–151. doi: 10.1007/s11060-020-03582-w. [DOI] [PubMed] [Google Scholar]
- 29. Suwannaying K, Monsereenusorn C, Rujkijyanont P, et al. Treatment outcomes among high-risk neuroblastoma patients receiving non-immunotherapy regimen: Multicenter study on behalf of the Thai Pediatric Oncology Group. Pediatr Blood Cancer. 2022:e29757. doi: 10.1002/pbc.29757. [DOI] [PubMed] [Google Scholar]
- 30. Yang YL, Jiang TH, Chen SH, et al. Treatment outcomes of pediatric acute myeloid leukemia: A retrospective analysis from 1996 to 2019 in Taiwan. Nat Sci Rep. 2021;11:5893. doi: 10.1038/s41598-021-85321-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chen SH, Chen JS, Jou ST, et al. Outcome and prognosis of anaplastic large cell lymphoma in children: A report from the Taiwan Pediatric Oncology Group. Leuk Lymphoma. 2019;60:1942–1949. doi: 10.1080/10428194.2018.1562182. [DOI] [PubMed] [Google Scholar]
- 32. Yeh TC, Liang DC, Hou JY, et al. Treatment of childhood acute lymphoblastic leukemia with delayed first intrathecal therapy and omission of prophylactic cranial irradiation: Results of the TPOG-ALL-2002 study. Cancer. 2018;124:4538–4547. doi: 10.1002/cncr.31758. [DOI] [PubMed] [Google Scholar]
- 33. Chen SH, Hung IJ, Yang CP, et al. Clinical features and long-term outcomes of bilateral Wilms tumor treated with Taiwan Pediatric Oncology Group protocols: A single center report. Asia Pac J Clin Oncol. 2016;12:300–307. doi: 10.1111/ajco.12501. [DOI] [PubMed] [Google Scholar]
- 34. Liang DC, Yang CP, Lin DT, et al. Long-term results of Taiwan Pediatric Oncology Group studies 1997 and 2002 for childhood acute lymphoblastic leukemia. Leukemia. 2010;24:397–405. doi: 10.1038/leu.2009.248. [DOI] [PubMed] [Google Scholar]
- 35. Ward ZJ, Yeh JM, Bhakta N, et al. Global childhood cancer survival estimates and priority-setting: A simulation-based analysis. Lancet Oncol. 2019;20:972–983. doi: 10.1016/S1470-2045(19)30273-6. [DOI] [PubMed] [Google Scholar]
- 36.WHO Global Initiative for Childhood Cancer: An Overview. World Health Organization; 2020. https://www.who.int/docs/default-source/documents/health-topics/cancer/who-childhood-cancer-overview-booklet.pdf?sfvrsn=83cf4552_1&download=true [Google Scholar]
- 37.CureAll framework WHO global initiative for childhood cancer: Increasing access, advancing quality, saving lives. https://apps.who.int/iris/handle/10665/347370
- 38. Rahiman EA, Bakhshi S, Pushpam D, et al. Outcome and prognostic factors in childhood B non-Hodgkin lymphoma from India: Report by the Indian Pediatric Oncology Group (InPOG-NHL-16-01 study) Pediatr Hematol Oncol. 2022;39:391–405. doi: 10.1080/08880018.2021.2002485. [DOI] [PubMed] [Google Scholar]
- 39. Harif M, Barsaoui S, Benchekroun S, et al. Treatment of B-cell lymphoma with LMB modified protocols in Africa—Report of the French-African Pediatric Oncology Group (GFAOP) Pediatr Blood Cancer. 2008;50:1138–1142. doi: 10.1002/pbc.21452. [DOI] [PubMed] [Google Scholar]