Abstract
PURPOSE
Over a 5-year or 10-year period, between 6% and 15% of germline cancer genetic variants undergo reclassification. Up-to-date interpretation can clarify a variant's clinical significance and guide patient management. As the frequency of reclassifications increase, the issue of whether, how, when, and which providers should recontact patients with information about reclassification becomes important. However, the field lacks research evidence and definitive guidance from professional organizations about how providers should recontact patients. We compared the perspectives of US oncologists and cancer genetic counselors (GCs) to describe their practices and views regarding recontact.
MATERIALS AND METHODS
We developed a survey using themes identified from semistructured interviews with oncologists and GCs and administered it in a national sample of oncologists and GCs between July and September 2022.
RESULTS
In total, 634 respondents completed the survey including 349 oncologists and 285 GCs. On frequency of recontacting patients with reclassified results, 40% of GCs reported recontacting often compared with 12.5% of oncologists. Neither group reported recording patient preference for recontact on electronic medical record (EMR). Both groups agreed that all reclassified variants, even those that do not affect clinical management, should be returned to patients. They also reported that recontact via EMR messages, mailed letters, and phone calls from GC assistants were more suitable for downgrades. By contrast, face-to-face meetings and phone calls were preferred for upgrades. Remarkably, oncologists were more likely to endorse face-to-face return of results and were more likely to endorse return through a nongenetics provider compared to GCs.
CONCLUSION
These data on current recontact practices and opinions provide a foundation for developing guidelines with explicit recommendations on patient recontact that can help maximize clinical effect while considering provider preferences for recontact within resource-constrained genomic practice settings.
National survey of US oncologists & GCs answer key questions on patient recontact after variant reclassification.
INTRODUCTION
Over a 5-year or 10-year period, between 6% and 15% of germline cancer genetic variants undergo reclassification.1-5 Up-to-date interpretation can clarify a variant's clinical significance and is facilitated by the availability of updated information about normal human genomic diversity, especially among under-represented minority populations.6 As the frequency of reclassifications increases, issues of whether, how, when, and which providers should recontact patients with information about reclassification becomes important. However, the field lacks research evidence and definitive guidance from professional organizations about how providers should communicate news of variant reclassification to patients.7 As such, clinical decision making in anticipation of and in response to reclassification is left to individual providers and their health care systems. Existing policy documents from US professional organizations' points to consider statements8,9 reflect the need for empirical data on current recontact practices and provider perspectives on best practices to help create more robust practice guidelines.
CONTEXT
Key Objective
This national survey of US oncologists and genetic counselors (GCs) involved in germline cancer genetic testing was designed to understand their practices and views on key questions about patient recontact after variant reclassification.
Knowledge Generated
Oncologists and GCs agreed that all reclassified variants, even those that do not affect clinical management, should be returned to patients but had differing opinions on preferred recontact modalities. Compared with oncologists, GCs are more likely to support recontact over telephone, whereas oncologists prefer face-to-face meetings.
Relevance
The survey results provide a foundation for developing guidelines with explicit recommendations on patient recontact that can help with the clinical delivery of reclassified variants within resource-constrained genomic practice settings.
In European, Australian, and American surveys, providers have expressed concerns about unclear roles and responsibilities in communicating news of reclassification.10-13 This lack of clarity is further complicated by the fact that genetic testing is increasingly offered by specialties such as oncology, which do not have extensive training in hereditary genetics.7,14 Empirical evidence on oncology providers' practices around variant reclassification will be key to developing guidelines that can inform patient care through accurate communication of reclassified variants. Although genetic counselors (GCs) from Canada and the United States have acknowledged their own responsibility in returning reclassified results, they also expressed concern about the liability related to recontact12 and emphasized a need for developing standard operating procedures on how to return these results.15
A persistent question in genomic medicine is whether providers should communicate news of all variant reclassification to patients, regardless of their clinical actionability. For the 5% to 11% of reclassification events that are clinically actionable1-3,6,16 and have the potential to change patient management, there is consensus on the need for recontact. The remaining majority (approximately 90%) that do not affect clinical management but occupy clinical time and resources require consideration. Arguably, all downgraded results should also be communicated to patients as even downgraded variants without a clear clinical utility may have personal utility and communication may provide psychosocial benefits. However, the recontact process is challenged by several barriers including difficulty maintaining up-to-date patient contact information10,13 and lack of resources.17 The increasing use of germline testing, the prevalence of reclassification, and shift in aggregate reclassification toward less clinical certainty,5 combined with a lack of genetics knowledge in interpreting variants among oncologists18 and discomfort with communicating uncertain results to patients,19 underscore the need to understand current practices and providers' opinions on this issue.
Patient-provider communication around reclassification is a complex issue but may be simplified into pre- and post-reclassification stages. Domains related to reinterpretation such as who should initiate it, pay for it, what events should trigger it, and whether consent is required all fall within the prereclassification stage and have been explored in a recent stakeholder survey.12 Postreclassification domains related to concepts such as recontacting patients after reclassification, modes, and timing of recontact, and patient management remains underexplored15 and is the focus of this work. We compared the perspectives of US oncologists in subspecialties that frequently use germline genetic testing and manage patient care after variant reclassification and cancer GCs to describe their practices and views regarding recontact.
MATERIALS AND METHODS
Study Population
To elicit the perspectives of a national sample of oncologists and GCs, we used two separate methods of survey administration. The eligible population of oncologists was identified by an e-mail campaign facilitated by IQVIA, a health data science and clinical research company that maintains a large database of active physicians in the United States. Physicians affiliated with oncology were included; we excluded radiation oncologists as they are not typically involved in ordering genetic tests or recontacting patients with reclassified results. To reach an eligible population of GCs, we disseminated this survey through the National Society of Genetic Counselor's (NSGC) Cancer Special Interest Group (SIG) listserv.
Survey Development
We developed a survey on the basis of semistructured interviews where we identified and explored key themes related to patient recontact after variant reclassification. An interview guide was developed on the basis of relevant literature and author experience (S.M. and B.A.) and used to conduct one-on-one interviews with seven key informant oncologists and GCs recruited from three cancer health care systems. All interviews were audiotaped, transcribed, and analyzed to create survey items. We also derived items from existing surveys on reclassification12 and points to consider statements from the European Society on Human Genetics (ESHG)10 and the American College of Medical Genetics (ACMG).8 The survey was pilot-tested in a sample of seven cancer GCs and their feedback incorporated to develop the final survey. Survey questions were framed around four domains: (1) current practices and experiences with recontacting patients with reclassified variants, (2) opinions on various modes of returning reclassified results, (3) responsibility of recontact, and (4) respondents' demographic information. Questions were accompanied by yes/no, multiple choice, and Likert scale–based response choices.
Survey Administration
Anonymous electronic surveys were administered via Redcap. The initial study invitation e-mail explained the voluntary and anonymous nature of the research. Up to three follow-up e-mails were sent (after 2, 4, and 6 weeks). Respondents were offered $10 US dollars for their participation. The survey was fielded between July and September 2022. Participants provided written informed consent and the study was approved by the University of Texas MD Anderson Cancer Center Institutional Review Board.
Statistical Analysis
Means, standard deviations, and proportions were calculated for continuous and categorical variables. Chi-squared tests were used to compare proportions. Data analysis was performed using R statistical software version R 4.0.1 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Respondent Characteristics
In total, 634 respondents completed the survey including 349 oncologists and 285 GCs; their demographic characteristics are summarized in Table 1. On the basis of the estimated membership of the NSGC Cancer SIG, GC response rate for this survey was 20.4%. The response rate for oncologists cannot be calculated because not everyone in the invited population was eligible for the study. The primary practice setting for most oncologists and GCs were academic medical centers (69.2% and 44.1%, respectively), followed by a community hospital. Overall, GCs were more confident in ordering syndrome-specific, multigene panels, targeted single site, and whole-genome or -exome tests than oncologists (Appendix Fig A1). Both groups were frequently involved in ordering genetic tests, returning results, and interpreting them (Appendix Fig A2).
TABLE 1.
Characteristics of Participating Oncologists, Their Practice Settings, and Patients
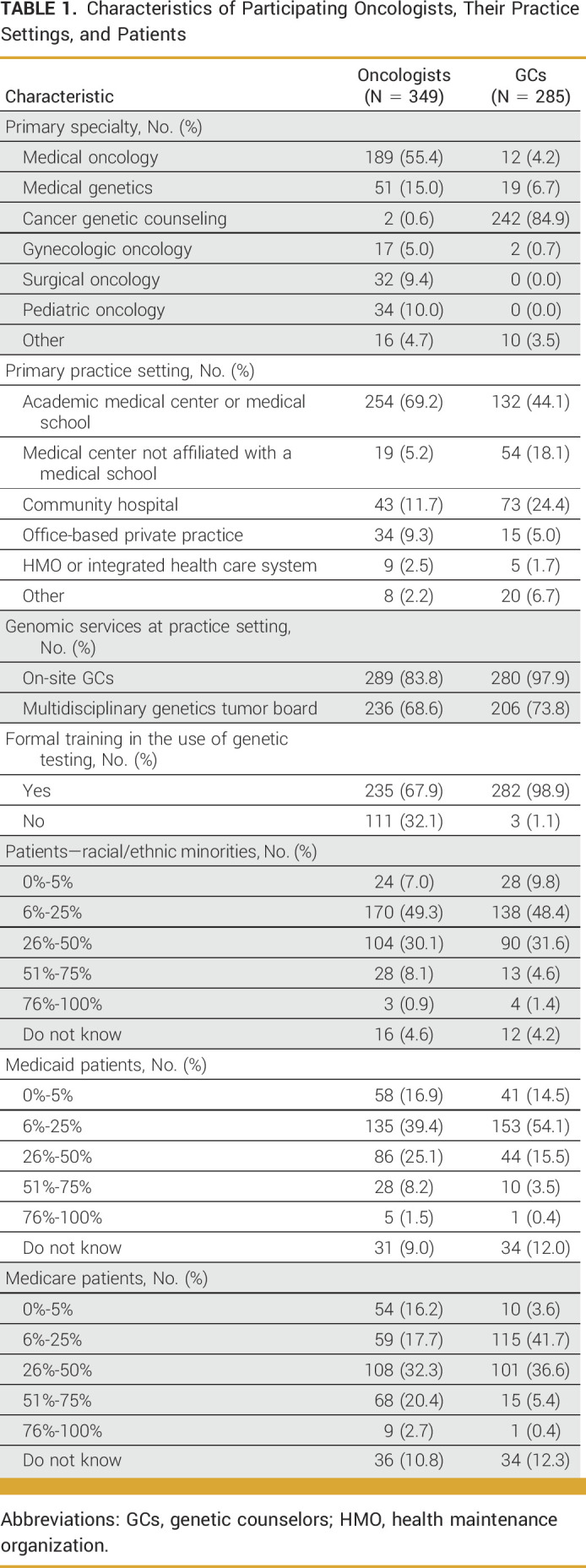
Participants reported that 6% to 25% of their patients were racial/ethnic minorities (Table 1). The patient population served by both oncologists and GCs commonly included those with breast cancer, followed by gastrointestinal cancer and other cancer types (Appendix Fig A3).
Recontact Practices
On the topic of whether patients and/or their family are recontacted when new information about their variant becomes available, both oncologists and GCs commonly responded yes, routinely (50% and 83%, respectively) or yes, occasionally (21% and 14%, respectively). However, oncologists were often unsure or did not know whether recontact occurred (20% v 2% among GCs). Both groups reported recontacting patients or their family members within a week (54% and 48%, respectively) or within 2 to 4 weeks (39% in both groups) of receiving an amended report. Some GCs (11%) reported taking a month or longer to recontact patients. The number of recontact attempts in both groups were widely spread with two attempts being most common among oncologists (33% v 27%, among GCs) and three attempts being most common among GCs (39% v 27%, among oncologists).
Participants were asked what modalities are currently used to return reclassified results with the option of selecting multiple responses. The most frequently selected response among oncologists was phone call with a genetics provider (45.3% for variant downgrades that do not affect management and 51.6% for upgrades that change management), followed by face-to-face meeting for upgrades (50.7%) and electronic medical record (EMR) message and mailed letters for downgrades (27.5% and 26.4%, respectively). The most frequently selected response among GCs for downgrades was mailed letter (70.9%) followed by phone call with clinical genetics provider (49.8%). For upgrades, the most frequently selected response was phone call with a clinical genetics provider (96.8%), followed by face-to-face meeting with clinical genetics provider (60.7%).
When asked about the influence of the 21st Century Cures Act, designed to prevent information blocking, on the practice of returning reclassified results, both oncologists and GCs reported that the Cures Act did not change their recontact practice (46.5% and 60.9%, respectively). However, a few reported that variants are now communicated more promptly than before the Act (10.8% and 7.6%, respectively). Most do not document patient preferences for recontact in EMRs (Fig 1).
FIG 1.
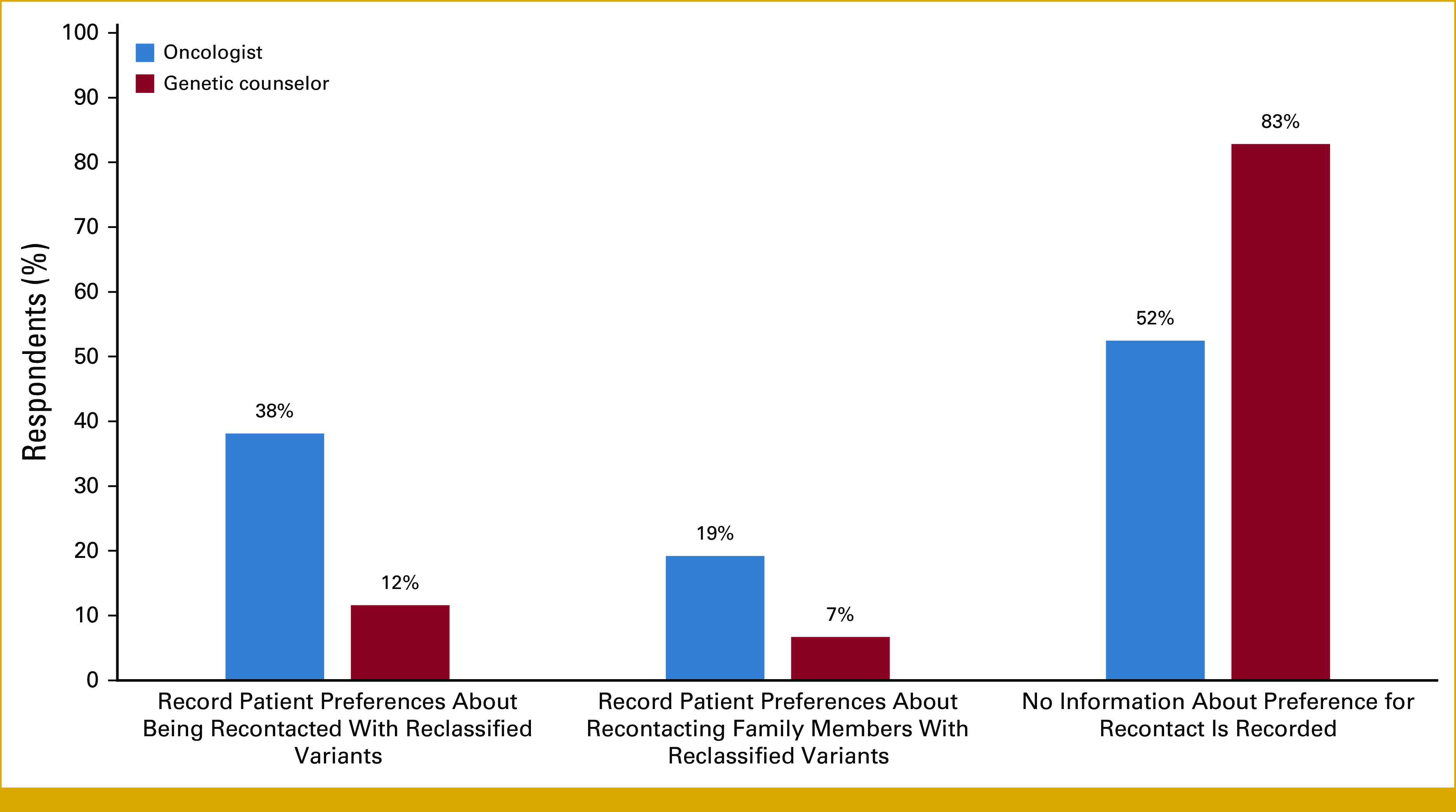
Type of information documented in electronic medical records regarding patient preferences for recontact.
Experience with Variant Reclassification
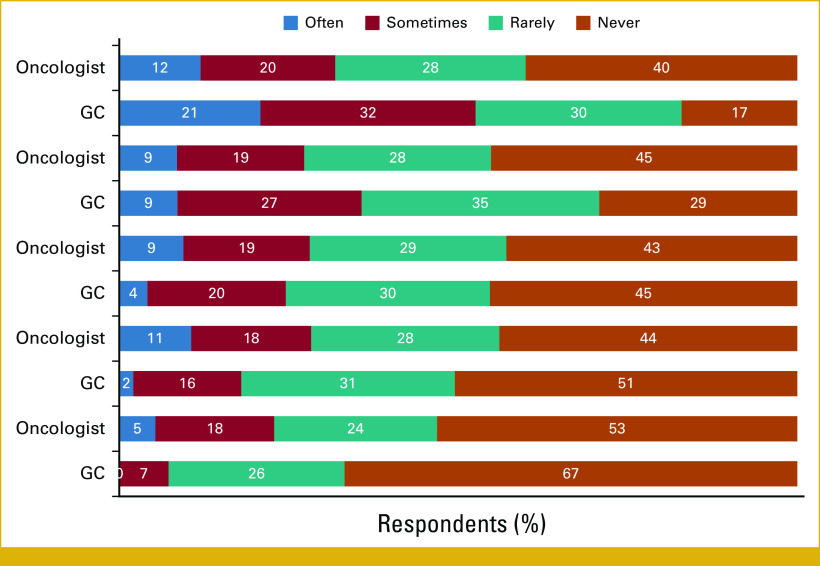
GCs were more likely to discuss reclassified results with patients than oncologists (Fig 2). On frequency of such discussions, 40% of GCs reported discussing this often compared with 12.5% of oncologists. During the last 12 months, most GCs (58%) reported experiencing an increased frequency of receiving reports about variant reclassifications, whereas 70% of oncologists reported a decreased frequency.
FIG 2.
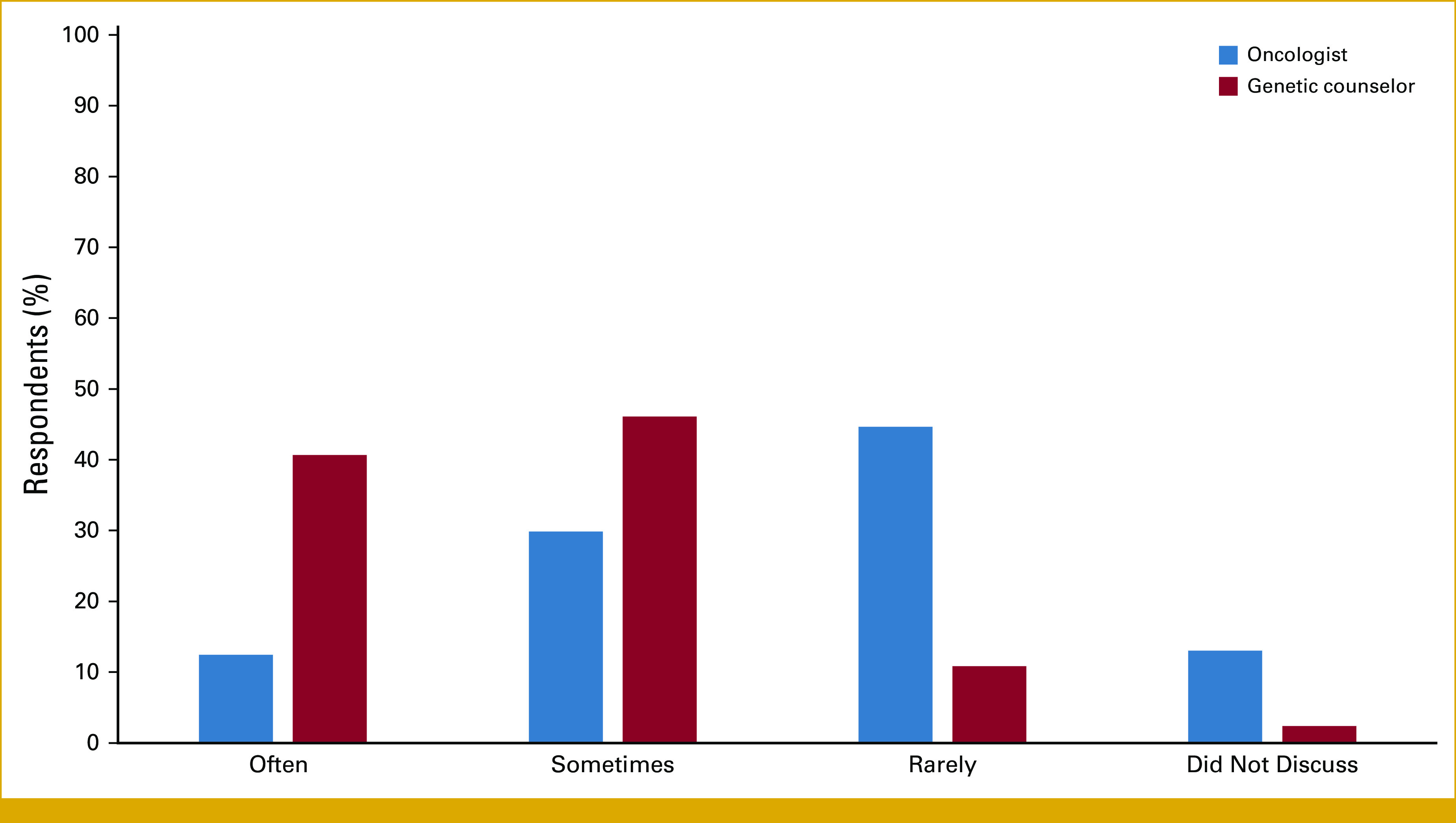
The frequency at which survey respondents discussed reclassified variants with patients in the past 12 months.
Participants were asked about the type of information they considered relevant enough to trigger patient recontact with the option of selecting multiple responses. The most frequently selected response among oncologists was “when there is a change in clinical actionability of the information” (75% v 60% among GCs), whereas among GCs, the most frequently selected response was “any variant reclassification report issued by the genetic testing laboratory” (85% v 53% among oncologists). The least selected option among both oncologists and GCs was “change in analytic validity of the information” (34% and 20%, respectively) or “new phenotype reported in the patient” (35% and 16%, respectively).
Clinical Use of Reclassified Variants
Upon recontact, the type of information discussed with patients by both oncologists and GCs was overwhelmingly “the significance of the information for medical management” (95% and 98%, respectively), followed by “implication of the amended results for patients' family members” (82% and 93%, respectively). Less commonly discussed by both oncologists and GCs was “patients' obligation to provide updated contact information” (25% and 36%, respectively) and “the right to decline recontact” (31% and 8%, respectively).
Most oncologists and GCs reported that reclassified results rarely change a patient's clinical management. When asked to estimate the proportion of all reclassifications that change clinical management in their patients, 0% to 10% was the most endorsed choice (60% of oncologists and 84% of GCs) and many were not sure/did not know whether it changed care (24% of oncologists and 10% of GCs). When reclassifications changed care, results were reported most often to inform prevention/early detection of cancer (12% and 21%, respectively; Appendix Fig A4).
Opinion on Patient Recontact Practices
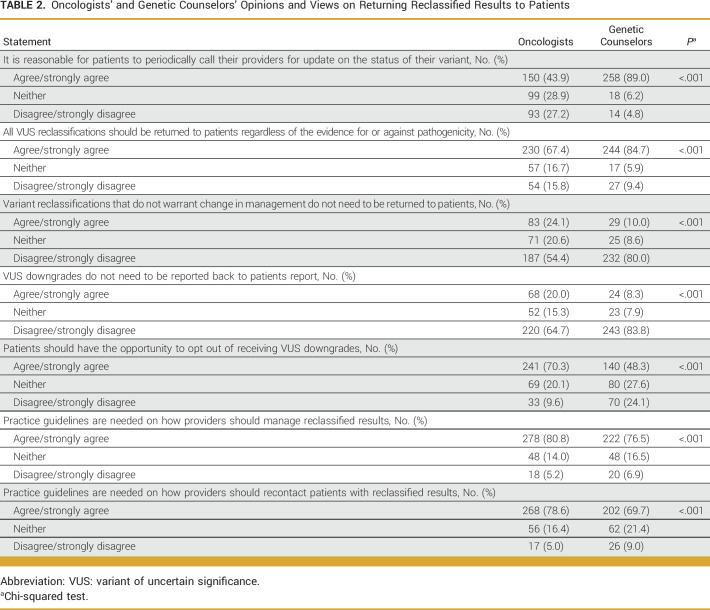
Table 2 summarizes oncologists' and GCs' views and opinions on patient recontact. Respondents strongly endorsed the return of all reclassified variants even when they do not affect clinical management, including variant of uncertain significance (VUS) downgrades. However, they also reported that patients should have the opportunity to opt out of receiving information about VUS downgrades. Both groups reported that patients react positively to news of downgrades that do not change their medical management: they agreed that patients are generally appreciative (69% and 75%, respectively) or are relieved (61% and 65%, respectively). However, more oncologists agreed that their patients feel confused about why they are informed about downgraded variants (45%) than GCs (21%).
TABLE 2.
Oncologists' and Genetic Counselors' Opinions and Views on Returning Reclassified Results to Patients
As shown in Figure 3, both groups reported that recontact decisions should be based on the best interests of patients and their family members (79% oncologists and 82% GCs), whereas patients' personal utility of the reclassified variant was the least endorsed reason (31% oncologists and 44% GCs).
FIG 3.
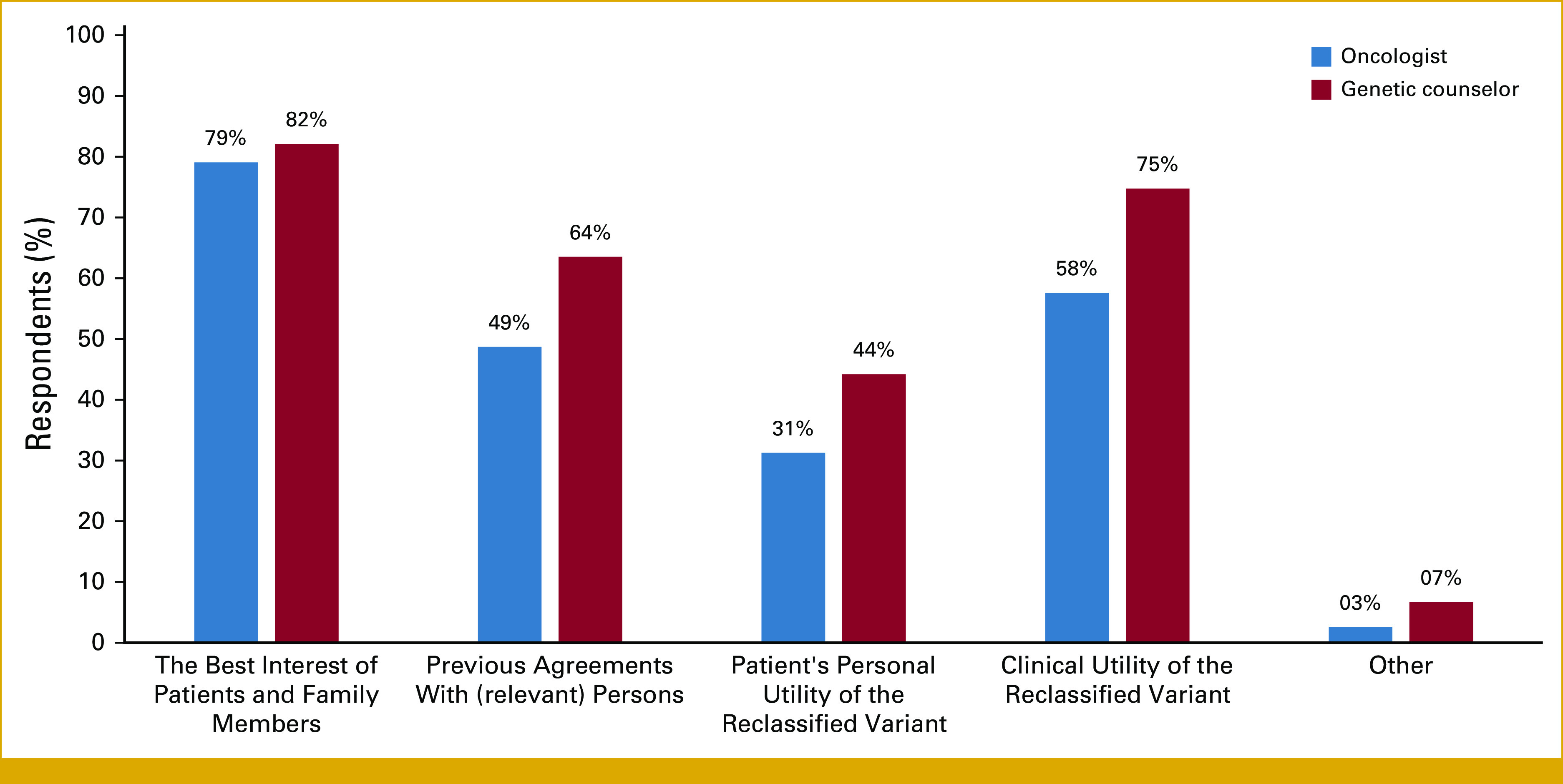
Provider opinion about factors that should be considered in decision making for patient recontact after reclassification.
Opinion on Modality of Patient Recontact
Recognizing that multiple modes of recontact may be necessary for reclassifications that warrant a change in management, respondents were asked about the optimal modality of initiating recontact after reclassification. For variant as well as gene reclassifications (eg, NBN downgrade, RAD51C, RAD51D, and BARD1 upgrade), there was a clear difference in opinion between modalities for returning upgraded and downgraded variants (Fig 4). Both GCs and oncologists agreed that notification via EMR messages, mailed letters, and phone call from GC assistants were more suitable for downgrades. For upgrades, face-to-face meetings and phone calls were preferred. Remarkably, oncologists were more likely to endorse face-to-face return of results and were more likely to endorse return through a nongenetics provider compared with GCs.
FIG 4.
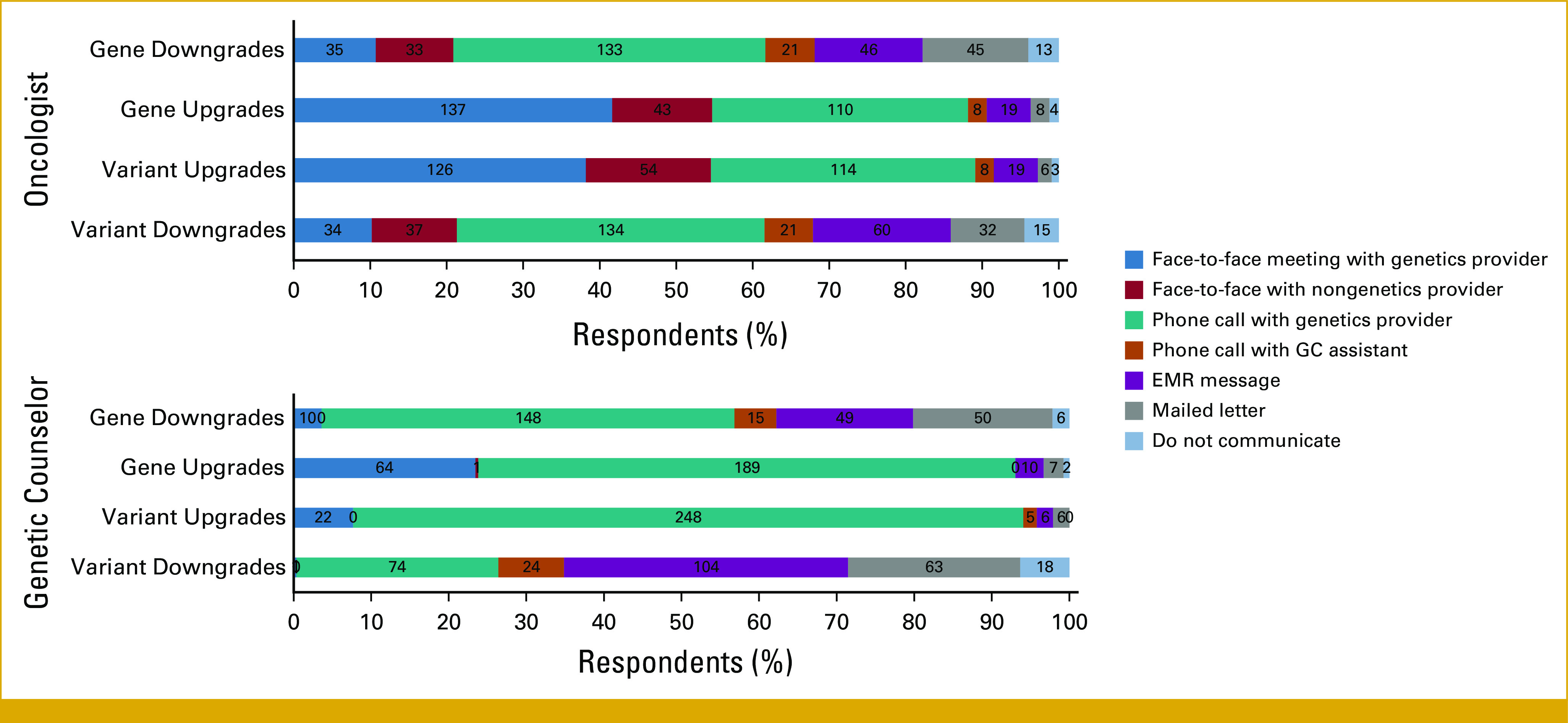
Preferred methods of patient recontact after various types of reclassification. Upgrades refer to upgrades that change management, and downgrades that do not change management. EMR, electronic medical record; GC, genetic counselor.
DISCUSSION
We compared perspectives of oncologists and GCs involved in germline cancer genetic testing on key questions about recontact after variant reclassification to provide data that may inform the development of guidelines for patient recontact. The findings from this study not only provide much needed empirical data on existing recontact practices, but also show differences in preferred recontact practices between oncologists and GCs. Within their practices, GCs have the primary responsibility to recontact patients and often report sharing that responsibility with GC assistants, students, or other support staff. Compared with oncologists, GCs are more likely to support recontact over telephone, whereas oncologists prefer face-to-face meetings.
Interestingly, we found a potential imbalance between providers' duty to recontact, and patients' need to know about reclassification events that do cross the actionability threshold. For example, most oncologists and GCs did not consider personal utility of a reclassified variant alone as an important reason for recontacting patients, yet they expressed strong preference for returning all reclassified results including VUS downgrades that are not clinically actionable. Framework issued by the Cancer Variant interpretation group UK (CanVIG-UK) states that the need for communication with patient/family will depend on perceived significance and robustness of new classification, including proximity of new classification to the actionability threshold.20 Consistent with this framework, participants in this study reported using clinical actionability of the reclassified variant as a triggering event for recontact, yet preferred to return all reclassifications irrespective of whether they cross the actionability threshold. Together with a strong endorsement of patients' opportunity to opt out of receiving VUS downgrades, it is possible that fear of malpractice litigation is contributing to these risk-averse medical practice decisions. Yet, documentation of patient preferences for recontact on EMR,8 as advised in the 2019 ACMG points-to-consider statement8 that could shield providers against such litigation, rarely occur in practice. Instead of relying on previous agreements with patients or their family members, providers preferred to make decisions on the basis of the best interest of the patients and the clinical utility of a variant. This points to a need for duty-to-recontact guidelines that deprioritize communication of reclassifications that do not cross the actionability threshold while allowing use of providers' clinical judgments.
In agreement with the 5% to 11% clinically actionable reclassification rate described in the literature,1-3,6,21 respondents of this study also reported that only 0% to 10% of all reclassifications change patients' clinical management. Time trends in variant reclassification reported by previous studies suggest an increase in reclassifications around 2014/2015 coinciding with the release of ACMG 2015 guidelines and an absolute increase in the number of reclassifications over time because of increasing use of germline tests and number of variants reported. However, we find that GCs, but not oncologists, reported receiving a greater number of amended reports in the last 12 months. The difference in experience between GCs and oncologists likely reflects the numerous downgrades (approximately 90%) that GCs handle and fewer upgrades (approximately 10%) that may require oncologist involvement. Although this reclassification pattern is reassuring for patient care, the need to return reclassified results that do not cross the actionability threshold increases GC workload in an already resource-constrained system. Potential solutions include using low-touch methods such as EMR messages, letters, and e-mails to return results that do not cross the actionability threshold, many of which are already used in practice today. Rigorous assessment of patient outcomes after various modes of returning results may increase the field's confidence in using them more widely.
Within their specialties for both oncologists and GCs, the preferred modalities for returning reclassified results were largely concordant with their current practice of returning results. However, they had different opinions on which disclosure modalities they prefer to initiate recontact. For gene and variant upgrades, oncologists endorsed face-to-face meetings for first initiating recontact (38%-42%), whereas GCs preferred phone calls (69%-85%). Less commonly endorsed methods of recontacting include mailed letters or EMR messages and a very small minority supported the idea of no communication at all. This difference in recontact method between provider types likely represents current genomic medicine practice where GCs are responsible for patient recontact for both downgraded and upgraded variants. By contrast, oncologists are primarily responsible for managing care for the subset of reclassifications that warrant management change. The anticipated increase in volumes of downgraded variants may necessitate low-touch recontact methods, whereas consultation for upgraded variants that warrant management change is more likely to require in-person appointments. Moving forward, patient outcome will be a key determinant in the choice of recontact modalities that the providers use, and different practice settings may require different solutions. For example, volume of reclassifications experienced by individual clinics vary widely with rates between 5% and 33%,3,4,16 which is dramatically different from the 0.6% to 6.4% rate reported from analysis of ClinVar data.5 Different practice settings may need to use different methods of returning reclassified results with consideration of their reclassification volume, patient demographics, and staff availability. Our data from the US confirm recontact preferences reported in a recent Australian survey that mostly included GCs13 and a survey of clinical genetic services from the United Kingdom.10 Scalable, low-touch methods of returning results that are amenable to systemic use already exist.3,13,15 EMR messages, letters, and e-mails offer a convenient middle ground—avoiding the risky option of not returning results at all; but are scalable enough to match today's volume of reclassifications and providers' time constraints. What we lack are outcome data after different methods of recontacting that can increase our confidence that we are not harming patients by using these. Such data are not easy to generate as reclassifications are rare events to begin with, and stratifications by return type are challenging.
This study has many strengths, including the large sample size of oncology providers who represent a diverse range of cancer types providing novel data on recontact practices from a nationwide sample. However, a limitation of the study is the unknown response rate among oncologists, which may lead to bias. The respondents may represent those with the strongest opinions on patient recontact or they may represent those with experiences that deviate from general and thus highlight issues that need to be addressed. To contextualize the practice preferences of oncologists and GCs, it is also important to better understand how the responsibility of returning reclassified results is shared between them in current clinical genetics practice and how the responsibilities might vary by practice setting. Future studies should oversample providers from community oncology settings to understand differences by care settings as well as to better understand settings where most patients with cancer receive care.
Overall, we found important differences and some commonalities in the recontact practices and preferences of oncologists and GCs. This information provide a foundation for developing guidelines with explicit recommendations on patient recontact, which may be used to improve patient-provider communication, while maximizing available clinical resources. It will be important to consider patient preferences for recontact to ensure patient satisfaction and avoid harm. Future research should focus on examining patient outcomes after various modes of reclassification and costs associated with each approach.
Acknowledgments
ACKNOWLEDGMENT
We would like to thank our participants as well as Susan K. Peterson who provided helpful feedback on the design on this survey.
APPENDIX
FIG A1.
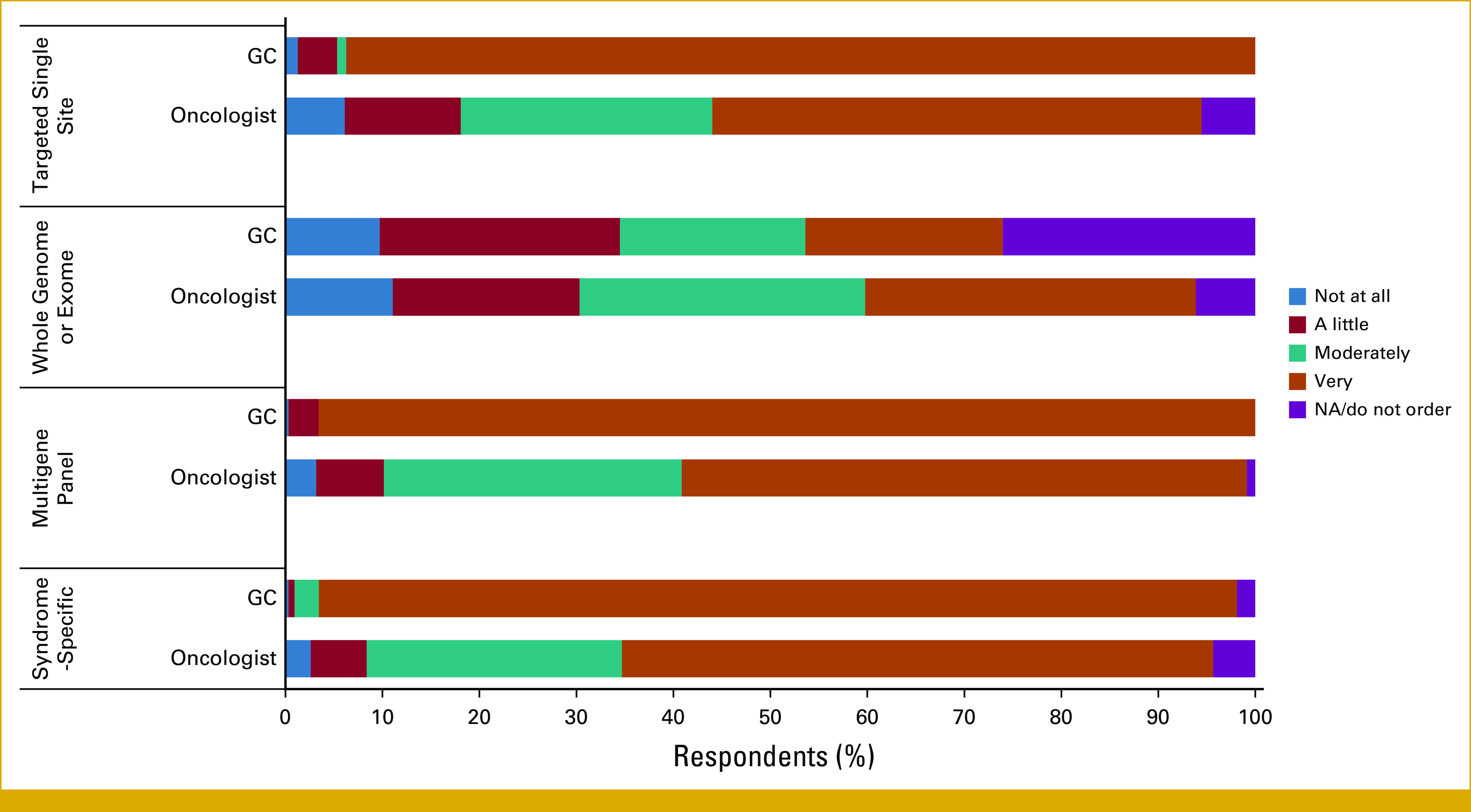
Survey respondents’ confidence in ordering genetic tests. GC, genetic counselor.
FIG A2.
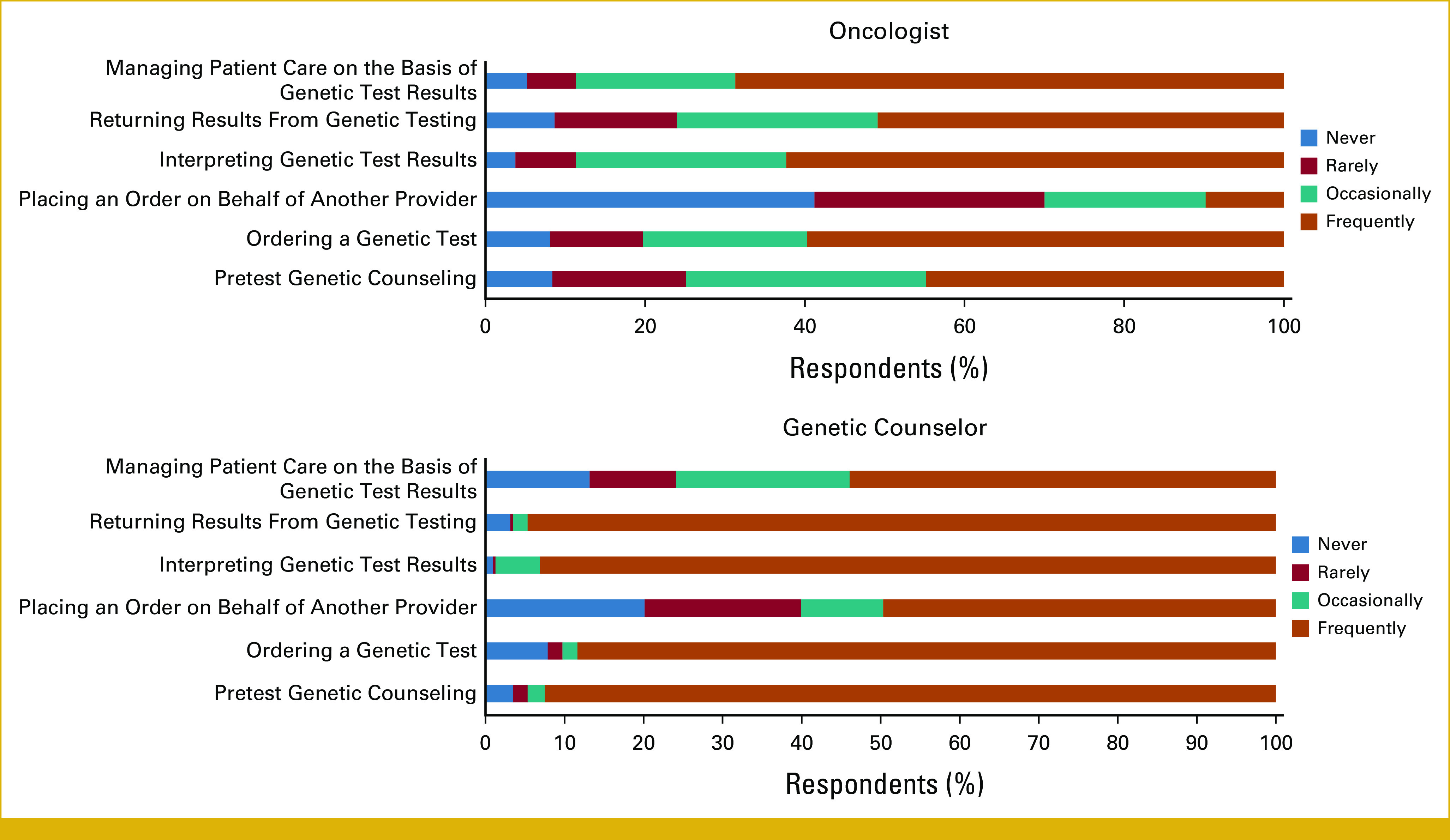
Survey respondents’ involvement in genetic testing processes.
FIG A3.
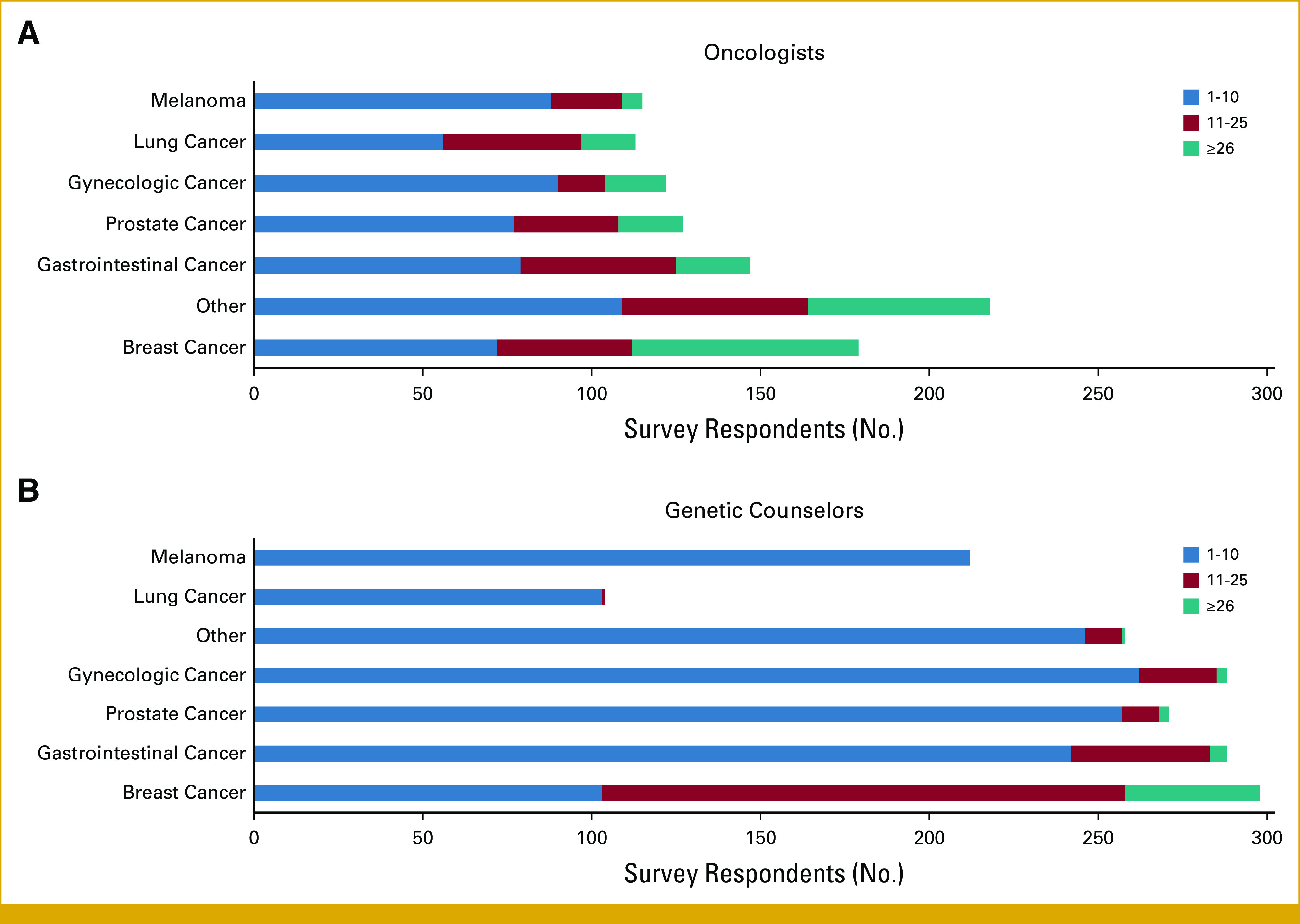
Patient volume and type of patients seen by: (A) oncologists, (B) genetic counselors.
FIG A4.
Clinical use of reclassified results as reported by survey respondents. GC, genetic counselor.
Elenita Davidson
Employment: MD Anderson Cancer Center
Brian Shirts
Consulting or Advisory Role: Constantiam Biosciences
Banu Arun
Research Funding: AstraZeneca (Inst)
Sanjay Shete
Stock and Other Ownership Interests: 23andMe
No other potential conflicts of interest were reported.
DISCLAIMER
The funders had no role in the design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
SUPPORT
Supported in part by a National Cancer Institute Career Development Award (1K99CA256216 to S.M.), a National Cancer Institute through Cancer Center Support Grant (P30CA016672), and AIM Shared Resource.
DATA SHARING STATEMENT
Data are available from the corresponding author upon reasonable request.
AUTHOR CONTRIBUTIONS
Conception and design: Sukh Makhnoon
Financial support: Sukh Makhnoon
Collection and assembly of data: Sukh Makhnoon, Elenita Davidson
Data analysis and interpretation: Sukh Makhnoon, Brian Shirts, Banu Arun, Sanjay Shete
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Elenita Davidson
Employment: MD Anderson Cancer Center
Brian Shirts
Consulting or Advisory Role: Constantiam Biosciences
Banu Arun
Research Funding: AstraZeneca (Inst)
Sanjay Shete
Stock and Other Ownership Interests: 23andMe
No other potential conflicts of interest were reported.
REFERENCES
- 1. Mersch J, Brown N, Pirzadeh-Miller S, et al. Prevalence of variant reclassification following hereditary cancer genetic testing. JAMA. 2018;320:1266–1274. doi: 10.1001/jama.2018.13152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Turner SA, Rao SK, Morgan RH, et al. The impact of variant classification on the clinical management of hereditary cancer syndromes. Genet Med. 2019;21:426–430. doi: 10.1038/s41436-018-0063-z. [DOI] [PubMed] [Google Scholar]
- 3. Muir SM, Reagle R. Characterization of variant reclassification and patient re-contact in a cancer genetics clinic. J Genet Couns. 2022;31:1261–1272. doi: 10.1002/jgc4.1600. [DOI] [PubMed] [Google Scholar]
- 4. Makhnoon S, Levin B, Ensinger M, et al. A multicenter study of clinical impact of variant of uncertain significance reclassification in breast, ovarian, and colorectal cancer susceptibility genes. J Clin Oncol. 2022;40 doi: 10.1002/cam4.5202. suppl 16; abstr 10512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Davidson AL, Kondrashova O, Leonard C, et al. Analysis of hereditary cancer gene variant classifications from ClinVar indicates a need for regular reassessment of clinical assertions. Hum Mutat. 2022;43:2054–2062. doi: 10.1002/humu.24468. [DOI] [PubMed] [Google Scholar]
- 6. Slavin TP, Van Tongeren LR, Behrendt CE, et al. Prospective study of cancer genetic variants: Variation in rate of reclassification by ancestry. J Natl Cancer Inst. 2018;110:1059–1066. doi: 10.1093/jnci/djy027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Appelbaum PS, Parens E, Berger SM, et al. Is there a duty to reinterpret genetic data? The ethical dimensions. Genet Med. 2020;22:633–639. doi: 10.1038/s41436-019-0679-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. David KL, Best RG, Brenman LM, et al. Patient re-contact after revision of genomic test results: Points to consider-a statement of the American College of Medical Genetics and Genomics (ACMG) Genet Med. 2019;21:769–771. doi: 10.1038/s41436-018-0391-z. [DOI] [PubMed] [Google Scholar]
- 9. Deignan JL, Chung WK, Kearney HM, et al. Points to consider in the reevaluation and reanalysis of genomic test results: A statement of the American College of Medical Genetics and Genomics (ACMG) Genet Med. 2019;21:1267–1270. doi: 10.1038/s41436-019-0478-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Carrieri D, Dheensa S, Doheny S, et al. Recontacting in clinical practice: The views and expectations of patients in the United Kingdom. Eur J Hum Genet. 2017;25:1106–1112. doi: 10.1038/ejhg.2017.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sirchia F, Carrieri D, Dheensa S, et al. Recontacting or not recontacting? A survey of current practices in clinical genetics centres in Europe. Eur J Hum Genet. 2018;26:946–954. doi: 10.1038/s41431-018-0131-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Berger SM, Appelbaum PS, Siegel K, et al. Challenges of variant reinterpretation: Opinions of stakeholders and need for guidelines. Genet Med. 2022;24:1878–1887. doi: 10.1016/j.gim.2022.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vora BB, Mountain H, Nichols C, et al. Opinions and experiences of recontacting patients: A survey of Australasian genetic health professionals. J Community Genet. 2022;13:193–199. doi: 10.1007/s12687-021-00570-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Burton H, Alberg C, Stewart A. Mainstreaming genetics: A comparative review of clinical services for inherited cardiovascular conditions in the UK. Public Health Genomics. 2010;13:235–245. doi: 10.1159/000279625. [DOI] [PubMed] [Google Scholar]
- 15. Richardson B, Fitzgerald-Butt SM, Spoonamore KG, et al. Management of amended variant classification laboratory reports by genetic counselors in the United States and Canada: An exploratory study. J Genet Couns. 2022;31:479–488. doi: 10.1002/jgc4.1514. [DOI] [PubMed] [Google Scholar]
- 16. Ha HI, Ryu JS, Shim H, et al. Reclassification of BRCA1 and BRCA2 variants found in ovarian epithelial, fallopian tube, and primary peritoneal cancers. J Gynecol Oncol. 2020;31:e83. doi: 10.3802/jgo.2020.31.e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bombard Y, Mighton C. Recontacting clinical genetics patients with reclassified results: Equity and policy challenges. Eur J Hum Genet. 2019;27:505–506. doi: 10.1038/s41431-018-0313-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rahman B, McEwen A, Phillips JL, et al. Genetic and genomic learning needs of oncologists and oncology nurses in the era of precision medicine: A scoping review. Per Med. 2022;19:139–153. doi: 10.2217/pme-2021-0096. [DOI] [PubMed] [Google Scholar]
- 19. Eccles BK, Copson E, Maishman T, et al. Understanding of BRCA VUS genetic results by breast cancer specialists. BMC Cancer. 2015;15:936. doi: 10.1186/s12885-015-1934-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Loong L, Garrett A, Allen S, et al. Reclassification of clinically-detected sequence variants: Framework for genetic clinicians and clinical scientists by CanVIG-UK (Cancer Variant Interpretation Group UK) Genet Med. 2022;24:1867–1877. doi: 10.1016/j.gim.2022.05.002. [DOI] [PubMed] [Google Scholar]
- 21. Makhnoon S, Bednar EM, Krause KJ, et al. Clinical management among individuals with variant of uncertain significance in hereditary cancer: A systematic review and meta-analysis. Clin Genet. 2021;100:119–131. doi: 10.1111/cge.13966. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available from the corresponding author upon reasonable request.