Biologists have forever sought to understand how species arise and persist. Historically, species that rarely interbreed, or are reproductively isolated, were considered the norm, while those with incomplete reproductive isolation were considered less common. Over the last few decades, advances in genomics have transformed our understanding of the frequency of gene flow between species and with it our ideas about reproductive isolation in nature. These advances have uncovered a rich and often complicated history of genetic exchange between species — demonstrating that such genetic introgression is an important evolutionary process widespread across the tree of life (Figure 1).
Figure 1. Examples of species that have experienced introgression.

(A) Heliconius butterflies (photo: iNaturalist/Mike Melton). (B) Helianthus sunflowers (photo: iNaturalist/Grace Stark). (C) Brewer’s yeasts (Saccharomyces cerevisiae; photo: Quinn K Langdon). (D) Humans (Homo sapiens; photo: mizmareck/Flickr). (E) Snowshoe hares (Lepus americanus; photo: Van Alstine). (F) Fire ants (Solenopsis sp.; photo: iNaturalist/ Eric Blomberg). (G) Darwin’s finches (Geospiza species). (H) African Great Lake cichlids (family Cichlidae; photo: Catherine E Wagner). (I) Anopheles gambiae mosquitos (photo: CDC/Wikicommons).
If matings between members of two species produce offspring that are at least partially viable and fertile, such hybrid offspring might reproduce with members of one (or both) of their parental species producing backcrossed offspring. If these backcrossed offspring continue to reproduce with the same parental species, this can result over time in the lasting transfer of DNA from one of the species into the genome of the other (Figure 2A). This process is known as ‘introgression’. Introgression differs from other processes that may produce similar genetic patterns, for example incomplete lineage sorting, because it describes the incorporation of the DNA from one species into another (Figure 2B).
Figure 2. The integration of segments of DNA through introgression.

(A) The process of introgression occurs through hybridization and subsequent backcrossing. An initial hybridization event between two distinct species — blue and red — produces an F1 hybrid. If this F1 hybrid backcrosses into the blue species and their offspring subsequently breed with the blue species as well, this will result in introgression of the red species’ DNA into the blue species’ genome. (B) The results of this introgression event are shown in the phylogeny (the red arrow pointing from the lineage of the red species into the lineage of the blue species), phenotypes, and genotypes of the group of organisms. The red species and the outgroup are unaffected by this unidirectional introgression event.
The extent of introgression
Examples of identified introgression events are distributed widely across the tree of life (Figure 1). From introgression of genes underpinning wing color patterns in Heliconius butterflies (Figure 1A) and genes allowing sunflowers to thrive in harsh environments (Figure 1B) to extensive ancient introgression of metabolic pathways in yeast (Figure 1C), evidence for introgression events is now ubiquitous in nature. In fact, the continued discovery of both recent and more ancient introgression events (even in organisms where it was unexpected due to strong hybrid inviability in these species today) has upended the traditional thinking that reproductive barriers prevent the movement of genes between species. It now seems that hybridization and introgression have occurred quite commonly in the history of many species — a view long-held for single-celled eukaryotes and plants but now widely accepted in animals as well. One of the best studied examples of ancient introgression is in our own species, Homo sapiens, where sequencing of ancient and modern genomes has revealed the introgression of DNA from archaic hominins — Neanderthals and Denisovans — approximately 2,000 generations ago (Figure 1D).
From the many examples that have now been identified, it has become clear that in most cases introgression does not occur evenly across the genome. It seems that certain regions of the genome introgress more or less readily than others (Figure 3). Studies in a variety of organisms, including humans, Drosophila and Xiphophorus swordtail fishes, have found that introgressed ancestry is rapidly purged in the early generations after hybridization. The causes of this pattern are still being unraveled, but genome-wide analyses have provided important clues. The density of genes and the frequency at which recombination events occur varies across the genome, and these two factors appear to be correlated with the retention or loss of introgressed DNA (Figure 3A,B). In regions with high gene density, introgressed DNA is observed less frequently, presumably because its retention could interfere with gene function. Additionally, regions of the genome with low recombination rates experience less introgression because recombination is not frequent enough to uncouple genes that are harmful in hybrids from the rest of the introgressed DNA segment. Interestingly, many species differ in the organization of the genome in ways that impact this process: some species have more compact genomes with higher gene density or have lower overall recombination rates, exacerbating this effect. For instance, lower rates of recombination lead to more rapid and complete purging of introgressed DNA in Drosophila species compared to humans.
Figure 3. Potential outcomes after an initial introgression event.
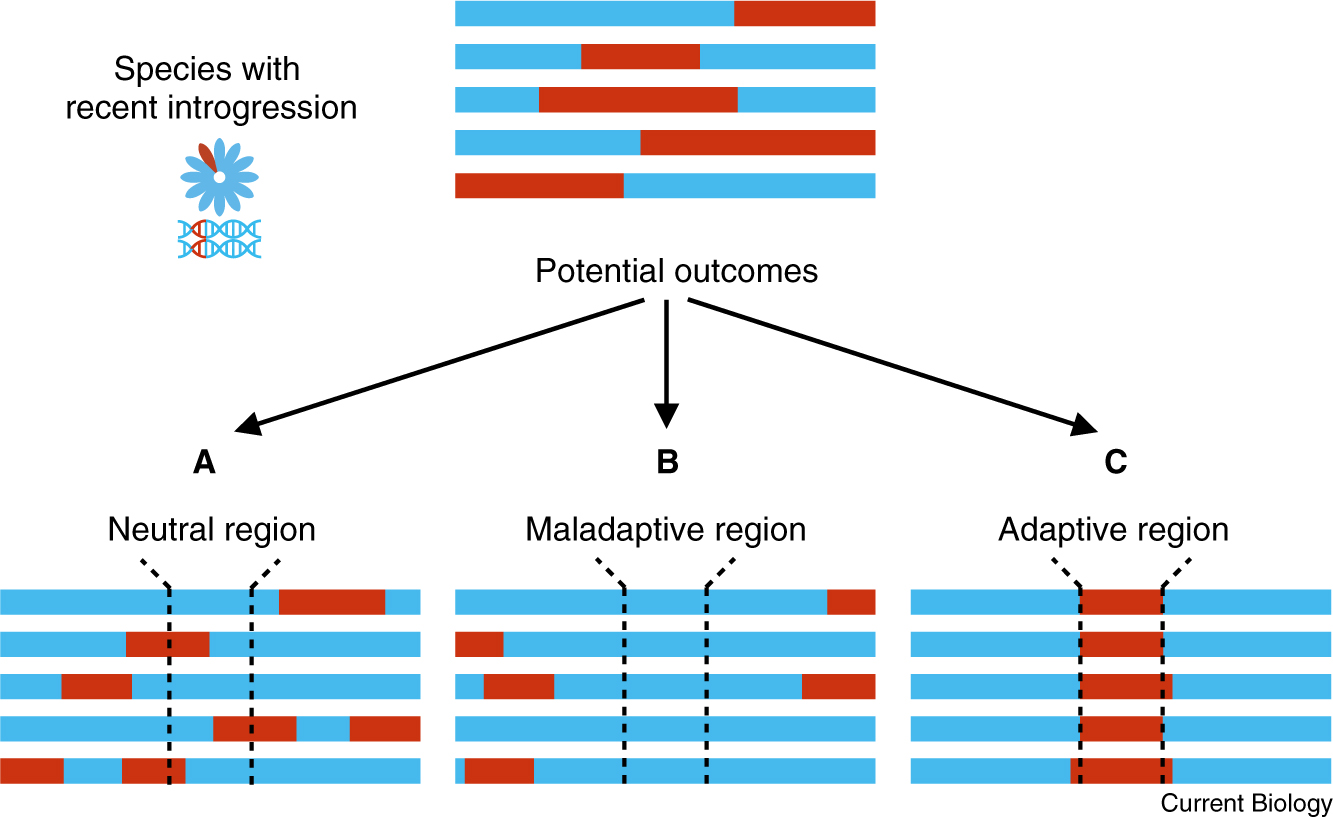
After initial introgression of DNA segments from the red species into the blue species occurs, there are different potential outcomes in the genome. Each bar represents a schematic of part of the genome with the color representing the ancestry of origin and the dashed lines denoting a particular region of interest. (A) If introgression occurs in a neutral region of the genome (e.g., a region with low gene density), the introgressed segments will be broken into smaller pieces by recombination and their frequencies will be influenced by genetic drift over time. (B) If introgression is maladaptive in a particular region of the genome (e.g., a region with high gene density or one containing loci involved in genetic incompatibilities between genes from red and blue ancestry), the introgressed segments are likely to be purged from the population. (C) Finally, if an allele in an introgressed region provides an adaptive advantage (e.g., an ecological advantage), the introgressed segments containing that allele will likely increase in frequency in the population.
Genes involved in hybrid incompatibilities — those that evolved within the genetic background of one species and are harmful in other genetic backgrounds — can act as local roadblocks for introgression in the genome. Incompatible genotype combinations are unlikely to introgress and instead lead to regions of the genome that appear ‘resistant’ to introgression, as there is strong selective pressure to purge the incompatible genes after initial hybridization (Figure 3B). To date, only a handful of genetic incompatibilities have been dissected down to the gene level. For example, in swordtail fishes, the interaction between the genes xmrk and cd97 causes hybrids to develop melanoma, while a mitochondrial–nuclear interaction between ndufs5, ndufa13 and mitochondrially encoded genes cause lethality in hybrids.
Introgression in adaptation and speciation
Despite the potential negative consequences of introgression, the movement of adaptive alleles between species via introgression can also be an important route to adaptation (Figure 3C). Instead of waiting for a beneficial mutation to arise, gene flow can instead introduce variation that has been ‘pre-tested’ by selection, allowing species to evolve rapidly. For instance, alleles causing brown winter coat color in snowshoe hares (Figure 1E), early flowering time in sunflowers or serpentine soil tolerance in Arabidopsis have introgressed from closely related species, which has facilitated adaptation to new environments. Groups of linked loci contained in chromosomal inversions, so-called ‘supergenes’, can also introgress adaptively between species, as was found for colony social organization in fire ants (Figure 1F) and wing color patterns in Heliconius butterflies. Additionally, introgression has played a key role in fueling some of the most striking adaptive radiations in nature, including Darwin’s finches (Figure 1G), African Great Lake cichlids (Figure 1H) and Heliconius butterflies. Researchers working on these systems have proposed that introgression may have triggered adaptive radiation by creating a multitude of evolutionary novel combinations of alleles on which selection could act.
Approaches for detecting introgression
Which genetic signals indicate that introgression has occurred between two species? In the past two decades there have been major technical and theoretical advances in identifying introgression in the genome both at the global (whole-genome) and local (specific genomic region) level. Detecting introgression at either scale usually requires genome sequencing data from both parental species and from the resulting introgressed offspring.
New methods for global ancestry analysis have allowed researchers to identify previously unknown cases of introgression and even to estimate the proportion of the genome that has moved between species. However, especially in the cases of introgression discussed above, sometimes it is important to have a more detailed picture of which pieces of the genome have moved from one species to another. While many approaches exist, some of the most sensitive approaches involve local ancestry inference, where statistical frameworks are used to infer which segments of the genome originated from a given parental species. Two commonly used methods for local ancestry inference are hidden Markov models (HMMs) and conditional random fields (CRFs). Based on sites in the genome that differ in state or frequency between two species, these methods leverage the spatial arrangement of such sites and recombination probabilities between them, among other parameters, to infer the probability that a given region of the genome is introgressed.
An important consideration in detecting local introgression in the genome is how distantly in the past the initial introgression event occurred. This is because over time, recombination will fragment the pieces of DNA derived from introgression into smaller and smaller segments (e.g., Figure 3A). Recent introgression is more obvious because introgressed DNA segments remain long and unbroken, and there has been little time for new mutations, making these segments easier to detect and characterize. Because the size of the introgressed pieces get smaller with many generations of backcrossing, recombination and selection, and because mutations continue to arise over time, it becomes more challenging to detect DNA fragments from older introgression events. In such cases, researchers often rely on genome-wide signals and comparisons to expected phylogenetic patterns in the absence of gene flow. However, it is important to keep in mind how other processes could generate similar signals (most commonly incomplete lineage sorting, whereby individual gene trees may differ from overall species trees). Method development in this area continues apace. In particular, recent advances in machine learning have shown promise for identifying introgression in the genome.
Introgression and changing environment
Environmental changes have influenced patterns of introgression and hybridization throughout the evolutionary history of organisms. For example, the locations of many existing hybrid zones — geographic regions where hybridization between species occurs — appear to coincide with shifts in species’ distributions that occurred after the last glacial maximum, providing strong support for the idea that environmental change can trigger hybridization. In recent years, the dramatic ways in which humans have changed the habitats of species around the globe has provided an opportunity to directly observe the connection between environmental change and introgression in real time. Environmental changes often result in strong selective pressures on organisms (e.g., through pesticide use or release of pollutants), which can rapidly select for introgressed loci that increase fitness in this changed environment. Human-driven environmental changes have selected for introgressed loci contributing to rodenticide resistance in house mice, insecticide resistance in mosquitos (Figure 1I) and industrial pollution tolerance in Gulf killifish. In the latter two cases, populations became resistant in less than 20 generations after initial introgression. Intriguingly, in some cases, human-driven changes in species distributions are likely to have caused the initial hybridization events that resulted in eventual adaptive introgression.
In addition, changes in climate have drastically altered the pressures that many species face, and introgression has the potential to play a positive or negative role as species adapt to these changes. For instance, adaptive introgression could lead to the movement of alleles from species that are adapted to hotter temperatures into a species that is less heat tolerant. Such introgression could allow the originally less heat tolerant species to adapt to higher temperatures. However, changes in climate could also lead to sweeping negative consequences as a result of introgression. With species moving to new areas to try to keep pace with changes in the environment, there is the potential for maladaptive introgression from these new arrivals into the original inhabitants of the area. This is of particular concern if resident species are outnumbered by the new arrivals, leading to genetic swamping where hybridization and introgression drive genetic replacement of the original inhabitants. Although it is unclear if human-mediated changes in climate and habitat disturbance are currently leading to an increase in hybridization and introgression in nature, continued changes over time are likely to exacerbate the already present patterns.
Over the last two decades, researchers have made great strides in understanding introgression across the tree of life and characterizing its distribution across the genome. But many key questions about its ecological and evolutionary consequences remain: How common is adaptive introgression? How frequent was introgression from now extinct lineages? How effective is introgression as a mechanism of evolutionary rescue in threatened species? The development of new methods to work with genomic sequencing data will provide new and interesting discoveries about introgression to answer these, and other, important questions in the future.
Footnotes
DECLARATION OF INTERESTS
The authors declare no competing interests.
FURTHER READING
- Arnold ML, and Kunte K (2017). Adaptive genetic exchange: a tangled history of admixture and evolutionary innovation. Trends Ecol. Evol. 32, 601–611. [DOI] [PubMed] [Google Scholar]
- Edelman NB, and Mallet J (2021). Prevalence and adaptive impact of introgression. Annu. Rev. Genet. 55, 265–283. [DOI] [PubMed] [Google Scholar]
- Gower G, Picazo PI, Fumagalli M, and Racimo F (2021). Detecting adaptive introgression in human evolution using convolutional neural networks. eLife 10, e64669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison RG, and Larson EL (2014). Hybridization, introgression, and the nature of species boundaries. J. Hered. 105, 795–809. [DOI] [PubMed] [Google Scholar]
- Hibbins MS, and Hahn MW (2022). Phylogenomic approaches to detecting and characterizing introgression. Genetics 220, iyab173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin SH, and Jiggins CD (2017). Interpreting the genomic landscape of introgression. Curr. Opin. Genet. Dev. 47, 69–74. [DOI] [PubMed] [Google Scholar]
- Moran BM, Payne C, Langdon Q, Powell DL, Brandvain Y, and Schumer M (2021). The genomic consequences of hybridization. eLife 10, e69016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravinet M, Faria R, Butlin RK, Galindo J, Bierne N, Rafajlovic M, Noor MAF, Mehlig B, and Westram AM (2017). Interpreting the genomic landscape of speciation: a road map for finding barriers to gene flow. J. Evol. Biol. 30, 1450–1477. [DOI] [PubMed] [Google Scholar]
- Sankararaman S (2020). Methods for detecting introgressed archaic sequences. Curr. Opin. Genet. Dev. 62, 85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SA, and Larson EL (2019). Insights from genomes into the evolutionary importance and prevalence of hybridization in nature. Nat. Ecol. Evol. 3, 170–177. [DOI] [PubMed] [Google Scholar]


